Abstract
Platelet endothelial cell adhesion molecule-1 (PECAM-1) inhibits platelet response to collagen and may also inhibit two other major platelet agonists ADP and thrombin although this has been less well explored. We hypothesized that the combined effect of inhibiting these three platelet activating pathways may act to significantly inhibit thrombus formation. We demonstrate a negative relationship between PECAM-1 surface expression and platelet response to cross-linked collagen related peptide (CRP-XL) and ADP, and an inhibitory effect of PECAM-1 clustering on platelet response to CRP-XL, ADP and thrombin. This combined inhibition of multiple signaling pathways results in a marked reduction in thrombus formation.
Keywords: Platelets, PECAM-1, Collagen, ADP, Thrombin, Thrombus
1. Introduction
Platelet endothelial cell adhesion molecule-1 (PECAM-1, CD31), an immunoreceptor tyrosine-based inhibitory motif (ITIM) containing protein plays a largely activatory role regulating integrin activation in leukocytes and endothelial cells [1–5]. It is also expressed at high levels on platelets (5000–20 000 copies per cell [6]) where, upon homophilic ligation or following stimulation with collagen or thrombin, it becomes tyrosine phosphorylated (Tyr663 and Tyr686) by Src-family kinases [7–10] facilitating the binding of Src-homology 2 domain-containing signaling proteins [8,11].
Reports of the role of PECAM-1 in platelets have been inconsistent. Initially characterized as co-stimulatory for ADP-mediated platelet activation [12], we and others have shown that clustering or ligation of PECAM-1 inhibits signaling downstream of collagen thereby inhibiting platelet aggregation and thrombus formation in vitro [13,14]. While these effects have been consistently demonstrated, their magnitude is modest [15] and might be considered mechanisms for fine tuning platelet reactivity.
Reports of PECAM-1 deficient mouse platelets are also complex and differ depending on the agonist used. Hyper-responsiveness to collagen [16] and increased thrombus formation [17] have been reported while aggregation in response to PAR-4 stimulation or ADP remains unaltered, as does static adhesion [18]. Furthermore impaired outside-in signaling through αIIbβ3 has been demonstrated in knock-out mice [18], which, together with the known role of PECAM-1 in the up regulation of integrin activity in leukocytes and endothelial cells, suggests that platelet PECAM-1 may act positively to enhance integrin mediated outside-in signaling.
In this study we demonstrate, in humans, a negative relationship between the surface expression of PECAM-1 and variation in platelet response to stimulation with cross-linked collagen related peptide (CRP-XL) and ADP, and an inhibitory effect of PECAM-1 receptor clustering on platelet response to CRP-XL, ADP and thrombin. The combined effect of this small but significant inhibition of multiple agonist stimulation pathways is a more marked reduction in thrombus formation.
2. Materials and methods
2.1. Ethics
Eighty-nine healthy subjects were recruited, to study the association between PECAM-1 expression and platelet function from the National Health Service Blood and Transplant blood donor clinic in Cambridge after gaining informed written consent. The study was approved by the Huntingdon Research Ethics Committee. Blood from all other subjects was taken after informed written consent approved by the University of Reading Research Ethics Committee.
2.2. Reagents
CRP-XL (monomeric sequence GCI[GPO]10GCOG) was prepared as described previously [19], thrombin and ADP were from Sigma Chemical Co. Ltd. (Poole, Dorset, UK). Thrombin receptor activation peptide (TRAP, SFLLRN) was from Bachem (St. Helens, UK). Where indicated, specific inhibitors of subsidiary activation pathways where added to ensure pathway specific responses were measured; indomethacin, apyrase, and hirudin (Sigma). Gly-pro-arg-pro peptide (GPRP) (Sigma) was added where thrombin was present to prevent clotting. PECAM-1 monoclonal antibody (mAb) AB468 and appropriate isotype control AB600 (Autogen Bioclear, Wilts, UK) were dialyzed to remove azide. mAb IV.3 was purified from hybridoma cell culture medium and F(ab′) fragments generated using the Fab Preparation Kit (Thermo Fisher Scientific, Northumberland, UK). Digestion of the F(ab′) fragments were confirmed by separating fragments by SDS–PAGE under reducing condition and visualized using Coomassie stain. Efficacy of the F(ab′) was determined by it’s ability to block activation of the low-affinity IgG receptor FcγRIIA by cross-linked whole mAb IV.3. PE/Cy5-CD62P was from BD Biosciences (Oxford, UK), and fibrinogen was from Sigma and FITC labeled in house. FITC-anti-fibrinogen (Dako Ltd., Ely, UK) or PE-anti-CD62P (Bristol Institute for Transfusion Science, Bristol, UK). Hepes buffered saline (HBS; 0.14 M NaCl, 5 mM KCl, 1 mM MgSO4, 10 mM HEPES (sodium salt), pH7.4) was used for all dilutions.
2.3. Blood collection
Blood was drawn from the antecubital fossa vein via a 21-gauge butterfly needle and a vacuette tube (Greiner Bio-One, Stonehouse, UK), and was used within 10 min of donation. The first 3 mL of blood were discarded and subsequent samples were taken into 0.105 M sodium citrate.
2.4. PECAM-1 cross-linking
PECAM-1 cross-linking was performed prior to platelet stimulation as follows. Platelets, in citrated whole blood or platelet rich plasma (PRP), were incubated with a saturating concentration of anti-PECAM-1 or isotype control (10 μg/mL) for 10 min followed by an excess of cross-linking antibody, goat-anti-mouse IgG antibody (20 μg/mL, Sigma) for 10 min.
2.5. Calcium flux [Ca2+]i
Indomethacin (10 μM), hirudin (10 U/mL) (not thrombin stimulated samples), apyrase (4 U/mL) (not ADP stimulated samples) and GPRP (0.5 mg/mL) (thrombin stimulated samples only) were added to PRP with an equal volume of HBS containing Fluo-4 NW dye (Molecular Probes, Paisley, UK) and probenecid (2.5 mM) and incubated at 37 °C for 30 min. Platelets were stimulated with ADP (10−5M), thrombin (1U/mL) or CRP-XL (5 μg/mL). [Ca2+]i was measured using a Fluoroskan Ascent plate reader (Thermo Labsystems, Basingstoke, UK) with excitation at 485 nm, and emission measured at 538 nm.
2.6. Flow cytometry
Whole blood flow cytometry was carried out as previously described [20]. Briefly, citrated whole blood was added to HBS containing pathway specific inhibitors as appropriate (described above). Samples were incubated for 20 min at room temperature with either CRP-XL, thrombin, ADP, or TRAP, and FITC-labeled fibrinogen (in excess) and PE/Cy5-anti-CD62P. Reactions were stopped by 100-fold dilution in 0.2% formyl saline. Data for 5000 platelets were recorded as percentage of cells positive for the activation marker.
2.7. Association between PECAM-1 expression and platelet function
Platelet reactivity was analyzed by whole blood flow cytometry in 89 healthy blood donors taken from the Bloodomics Platelet Functional Cohort, as described previously [20]. Platelets were stimulated, in the presence of pathway specific inhibitors as appropriate (described above), with ADP (10−7 M), TRAP (2 × 10−6 M) and CRP-XL (0.1 μg/mL) and binding of FITC-anti-fibrinogen and PE-anti-CD62P were measured by flow cytometry. To measure the surface expression of PECAM-1 (mouse CD31 mAb (Serotec, Oxford, UK)), αIIbβ3 (RFGP56, A.H. Goodall, University of Leicester), GPVI (HY101, M.L. Kahn, University of Pennsylvannia) and GPIa (15D7, H. Deckmyn, Katholieke Universiteit Leuven), antibodies were incubated with PRP for 30 min. Platelets were then washed, incubated with FITC-goat-anti-mouse secondary antibody (Dako, Ely, UK) for 30 min, and analyzed by flow cytometry.
2.8. Thrombus formation in vitro
Thrombus formation in citrated whole blood under arterial flow conditions was performed as previously described [21,22]. Briefly, whole blood loaded with 3,3′-dihexyloxacarbocyanine iodide was perfused through collagen coated (100 μg/mL) glass micro-capillaries (shear rate = 1000 s−1), followed by Tyrodes–Hepes buffer. Images were captured at 2 μm intervals in the Z-plane within five randomly-selected fields of view. Thrombi were then visualized using a Leica DMIRE2 inverted confocal microscope (using NPLANL20_/0.4 objective lens with 0- to 2-mm correction). Thrombus volume calculated from Z-series images captured and analyzed using TCS SP2 software (Leica). Protein was subsequently eluted from the slides by perfusion with 200 μL Tris-buffered saline containing NP40 (1% vol/vol) and quantified using the BCA Protein assay (Thermo Fisher Scientific, Cramlington, UK).
2.9. Statistics
PECAM-1 cross-linking modulation of platelet response was analyzed by repeat measure ANOVA. Paired t-test was used to analyse the peak value, final value and total (area under the curve) [Ca2+]i. The association between PECAM-1 surface expression and platelet response was tested using linear regression. All analysis was carried out using SPSS version 15 (SPSS Inc., Chicago, USA).
3. Results
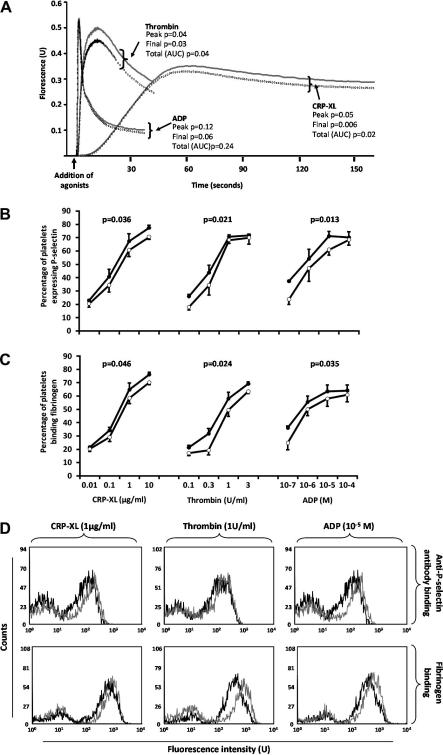
The inhibition, by PECAM-1, of platelet response to GPVI stimulation, has been demonstrated by us and others [13,14], although some debate surrounds the issue of whether PECAM-1 modulates signaling stimulated by other platelet agonists. We therefore explored this in more detail by measuring platelet reactivity to pathway specific activation by CRP-XL, thrombin and ADP in the presence of cross-linked anti-PECAM-1 antibody which we have previously shown to cause PECAM-1 phosphorylation [13], or isotype control. Following PECAM-1 cross-linking total platelet [Ca2+]i reduced by 7.5 ± 2.0% in response to CRP-XL, and 11.2 ± 3.3% in response to thrombi. Similarly, peak [Ca2+]i was reduced by 6.2 ± 2.2% and 10.0 ± 2.8%, and final [Ca2+]i reduced by 8.8 ± 1.3% and 14.9 ± 4.0%, respectively (P < 0.05, in all cases). Neither total nor peak [Ca2+]i in response to ADP were affected by PECAM-1 cross-linking (Fig. 1a). PECAM-1 cross-linking did not initiate [Ca2+]i in the absence of agonist (data not shown). Furthermore PECAM-1 cross-linking, across a range of CRP-XL concentrations inhibited P-selectin exposure by 11.2% (range: 2.7–20.8%, P = 0.036) and fibrinogen binding to αIIbβ3 by 9.9% (range: 3.3–17.4%, P = 0.046) (Fig. 1B and C). Similar magnitudes of inhibition were seen in response to both thrombin and ADP (Fig. 1B and C). The mean inhibition of P-selectin exposure in response to thrombin was 8.1% (range: 0.8–14.9%, P = 0.021) and in response to ADP was 14.9% (range: 3.9–22.7%, P = 0.013). Fibrinogen binding was inhibited by 9.3% (range: 6.1–16.1%, P = 0.024) in response to thrombin and 10.7% (range: 2.8–24.4%, P = 0.035) in response to ADP.
Fig. 1.
Inhibitory effect of PECAM-1 cross-linking on platelet function. (A) [Ca2+]i in response to agonists following anti-PECAM-1 antibody (dotted line) and the isotype control (solid line) cross-linking (mean of four individuals). (B) P-selectin expression and (C) fibrinogen binding to platelets in response to stimulation following anti-PECAM-1 antibody (open circles) and the isotype control (full circles) cross-linking. All points are the mean ± S.E. of five individuals. P-values compare the difference between the two curves over the entire concentration range. (D) Representative flow cytometry histograms showing P-selectin expression and fibrinogen binding to platelets in response to stimulation following anti-PECAM-1 antibody (black line) and the isotype control (gray line) cross-linking.
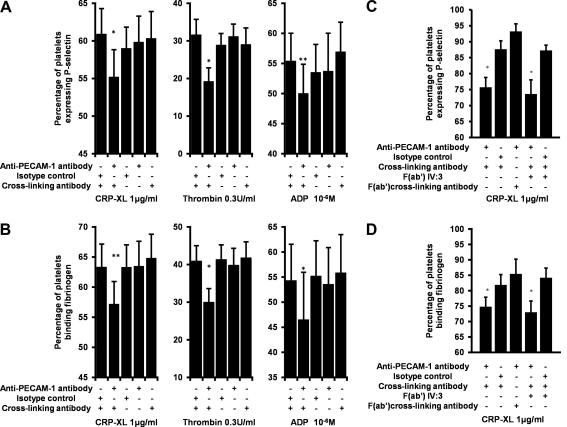
The inhibitory effect seen here was specific for cross-linked anti-PECAM-1 antibody. The level of stimulation achieved in the presence of the cross-linked isotype control was not significantly different to that seen in the absence of antibodies (data not shown) and no other combination of antibodies, apart from cross-linked anti-PECAM-1 antibody, caused any significant change to platelet activation (Fig. 2). Platelet response was not inhibited if incubated with the PECAM-1 antibody and F(ab′) fragments of the cross-linking antibody (Fig. 2). Furthermore, pre-incubation with IV.3 F(ab′) fragments, to block the low-affinity receptor for IgG FcγRIIA, did not alter the inhibitory effect of PECAM-1 cross-linking (Fig. 2). Therefore the inhibition of platelet reactivity by PECAM-1 is not a generic antibody effect. As with [Ca2+]i no combination of antibodies caused any platelet activation in the absence of agonists (data not shown).
Fig. 2.
Inhibitory effect of PECAM-1 is not a generic antibody effect. (A and C) P-selectin expression and (B and D) fibrinogen binding to platelets in response to stimulation is only inhibited by cross-linking anti-PECAM-1 antibodies, and (A and B) not with any other combination of the anti-PECAM-1, isotype control, cross-linking antibodies or (C and D) F(ab′) of the cross-linking antibody. Nor (C and D) was this inhibition attenuated by pre-incubation with IV.3 F(ab′) fragments. (A and B) stimulation occurred in the presence of appropriate agonists. (C and D) No inhibitors were used. All bars are the mean ± S.E. of four individuals. ∗P < 0.05, ∗∗P < 0.01. P-values compare the difference between the samples incubated with cross-linked anti-PECAM-1 and all other points.
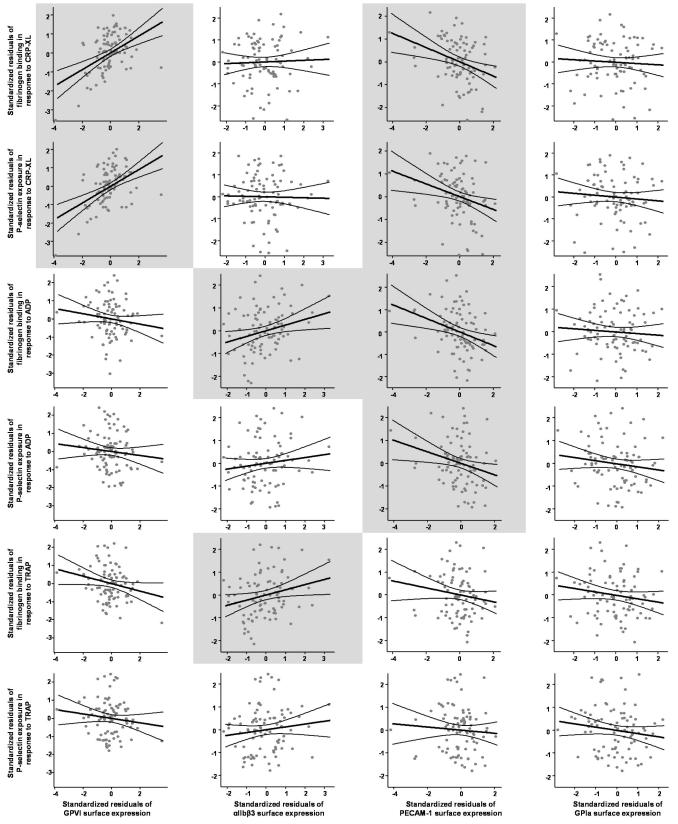
If PECAM-1 function is important for the regulation of platelet function, variation in the surface expression of the protein should impact on platelet reactivity. To test this we examined the relationship between surface expression of PECAM-1 on unactivated platelets and its association with P-selectin exposure and fibrinogen binding to platelets in response to CRP-XL, ADP and TRAP, in 89 healthy subjects [20]. Surface expression of PECAM-1 was negatively correlated with P-selectin expression and fibrinogen binding in response to CRP-XL (P = 0.009 and 0.003, respectively) and ADP (P = 0.017 and 0.003, respectively) although not to TRAP (Table 1). While the strength of this association is modest, accounting for between 6% and 10% of the variation in platelet response to CRP-XL and ADP, it was of a similar magnitude to the positive association seen between platelet reactivity and GPVI or αIIbβ3 surface expression. Associations of this magnitude are entirely expected in this context given that platelet activation is a multi-factorial process requiring the integration of multiple signaling steps. Variation in any of these steps will have a moderate impact on the final level of response. It is important here to separate out the information being given by the r2 value and the P-value. r2 is the proportion of variation in platelet function explained by the regression model and gives information about the strength of the association, while the P-value gives information on whether that association is likely to have occurred by chance. Therefore given an appropriate sample size it is entirely possible, as is the case here, to identify weak associations that are statistically significant. The negative association identified here confirms our findings of an inhibitory role for PECAM-1 in CRP-XL and ADP induced platelet activation.
Table 1.
Association between PECAM-1 surface expression and platelet reactivity. The linear regression analysis showing the association (positive (bold) and negative (bold italic)) between surface expression of four platelet surface molecules and platelet reactivity. In line with previous reports surface expression of GPVI is positively associated with platelet response to CRP-XL [25] accounting for approximately 20% of the variation in platelet response to this agonist. Surface expression of αIIbβ3 was also positively correlated with platelet fibrinogen binding in response to ADP and TRAP. Surface expression of PECAM-1 showed a negative association with both fibrinogen binding and P-selectin expression in response to CRP-XL and ADP. GPVI and αIIbβ3 expression were included as confounding variables where indicated. As previously reported time of day was found to affect platelet response to CRP-XL in this study and was included, where indicated, as a confounding factor [20]. Mean platelet volume was strongly correlated with PECAM-1 expression (P = 0.00003, r2 = 0.18) and was also included as a confounding variable as indicated.
| Agonist | Measured response | Percentage of positive platelets. Median (min–max) | Platelet surfaces molecules |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GPVI |
αIIbβ3 |
PECAM-1 |
Gpla |
|||||||
| Receptor surface expression. Median fluorescence intensity (U). Median (min–max) | ||||||||||
| 12.0 (5.2–24.9 |
172.5 (56.1–272.9) |
31.5 (11.7–49.0) |
16.5 (7.0–27.0) |
|||||||
| r2 | P-value | r2 | P-value | r2 | P-value | r2 | P-value | |||
| CRP-XL (0.1 μg/mL) | Fibrinogen binding | 39.0% (3.2–78.2) | 0.200a | 0.00001a | 0.001a,b | 0.74a,b | 0.097a,b,d | 0.003a,b,d | 0.003a,b | 0.595a,b |
| P-selectin expression | 56.0% (3.9–90.7) | 0.211a | 0.000005a | 0.0004a,b | 0.847a,b | 0.075a,b,d | 0.009a,b,d | 0.007a,b | 0.431a,b | |
| ADP (1 × 10−7 M) | Fibrinogen binding | 26.5% (2.4–73.7) | 0.021 | 0.178 | 0.061 | 0.02 | 0.094c,d | 0.003c,d | 0.005c | 0.511c |
| P-selectin expression | 11.3% (5.2–26.7) | 0.012 | 0.306 | 0.016 | 0.245 | 0.064d | 0.017d | 0.019 | 0.205 | |
| TRAP (2 × 10−6 M) | Fibrinogen binding | 12.2% (2.1–68.8) | 0.042 | 0.053 | 0.051 | 0.034 | 0.024d | 0.148c,d | 0.023c | 0.161c |
| P-selectin expression | 20.8% (6.3–62.0) | 0.015 | 0.251 | 0.015 | 0.25 | 0.005d | 0.519d | 0.02 | 0.191 | |
Confounding variables:
Times of day.
GPVI surface expression.
αIIbβ3 surface expression.
Mean platelet volume.
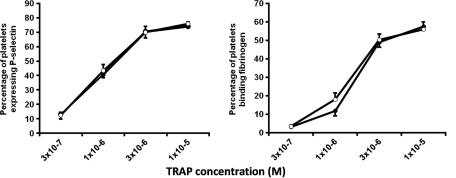
There is a clear dichotomy in these results. While PECAM-1 cross-linking inhibited platelet response to thrombin (Fig. 1), PECAM-1 expression showed no relationship with platelet activation following stimulation by TRAP in the association studies (Table 1). Subsequent investigation established that PECAM-1 cross-linking did not inhibit either fibrinogen binding or P-selectin expression in response to TRAP (Fig. 3). These results support the findings of the association study, however, a discrepancy remains between the inhibitory effect of PECAM-1 on thrombin activation of platelets and its lack of effect on TRAP stimulation. This may stem from the involvement of glycoprotein (GP) Ibα in thrombin [23] but not TRAP (which is PAR-1 specific) induced platelet activation and may indicate a role for PECAM-1 in mediating the action of GPIbα during thrombin stimulation of platelets. PECAM-1 has previously been shown to be involved in GPIbα mediated activation of platelets, becoming tyrosine phosphorylated upon vWF binding to GPIbα, and PECAM-1 deficient mice show enhanced aggregation in response to vWF [24]. Thus it is not inconceivable that stimulation of PECAM-1 inhibits the action of GPIbα causing reduced thrombin induced platelet activation.
Fig. 3.
Inhibitory effect of PECAM-1 cross-linking does not reduce TRAP induced platelet activation. P-selectin exposure and fibrinogen binding to platelets in response to TRAP (comparing the entire concentration range, P = 0.566 and 0.489, respectively). Cross-linked anti-PECAM-1 antibody (open circles) and the isotype control (full circles). All data are given as the mean ± S.E. of four individuals.
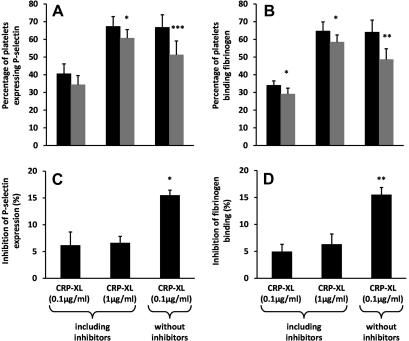
During thrombus formation platelets are exposed to a variety of stimuli simultaneously, including collagen, thrombin and ADP. It is, therefore, possible that the small inhibitory effects in multiple pathways, that we have described so far, interact resulting in a greater inhibition of platelet response and thrombus formation. This could be seen in the flow cytometry assay when comparing the response of platelets to CRP-XL in the presence and absence of inhibitors of endogenous thrombin, ADP, and thromboxane. PECAM-1 cross-linking had a greater inhibitory effect on CRP-XL (0.1 μg/mL) stimulation in the absence of hirudin, apyrase and indomethacin, thereby allowing secondary activation pathways to play a role, than in the presence of these inhibitors; fibrinogen binding was reduced by 15.5 ± 2.7% compared to 4.9 ± 2.8%, P = 0.002, and P-selectin expression was reduced by 15.5 ± 1.8% compared to 6.2 ± 4.9%, P = 0.01, respectively (Fig. 4). A similar increase in inhibition was seen when comparing the effect of PECAM-1 cross-linking on platelets stimulated with CRP-XL (1 μg/mL) in the presence of inhibitors or CRP-XL (0.1 μg/mL) in their absence. While both treatments gave comparable levels of platelet activation with the isotype control, the level of inhibition resulting from PECAM-1 cross-linking was significantly greater in the absence of inhibitors (Fig. 4).
Fig. 4.
The inhibitory effects of PECAM-1 on multiple activation pathways interact to enhance the inhibition of platelet activation. (A and B) P-selectin exposure and fibrinogen binding to platelets in response to CRP-XL in the presence or absence of inhibitors of endogenous thrombin, ADP, and thromboxane. P-values compare cross-linked anti-PECAM-1 antibody (gray bar) and the isotype control (black bar) treated samples. (C and D) Inhibition of platelet response following PECAM-1 cross-linking. P-values compare the level of inhibition in the absence of inhibitors with that seen in their presence. All data are given as the mean ± S.E. of four individuals. ∗P < 0.05, ∗∗P < 0.005, ∗∗∗P < 0.0005.
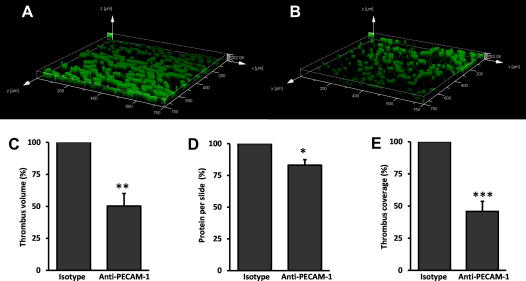
To identify the magnitude of the inhibitory effect of PECAM-1 in conditions that are more reflective of in vivo processes, thrombi were formed under arterial flow conditions in collagen coated micro-capillary slides. Following PECAM-1 cross-linking total thrombus volume was reduced by 49.8 ± 9.9% (P = 0.002) compared to treatment with the isotype control, and the area of the glass capillary covered by thrombi was similarly reduced by 54.1 ± 7.9% (P = 0.0005) (Fig. 5). The total amount of protein eluted from the slides following thrombus formation also decreased in the presence of cross-linked PECAM-1 by 16.9 ± 4.3% (Fig. 5D).
Fig. 5.
Inhibitory effect of PECAM-1 cross-linking on thrombus formation. (A and B) Representative images showing thrombus formation on collagen coated slides following cross-linking of the isotype control or anti-PECAM-1 antibodies, respectively. (C) Mean volume, (D) the total amount of protein eluted from the slides, and (E) coverage following thrombus formation following anti-PECAM-1 antibody or isotype control cross-linking. To correct for inter-individual variation in thrombus formation all data are expressed as a percentage of the isotype control. Data are the mean ± S.E. of seven individuals (C and E) and 3 individuals (D). ∗P < 0.05, ∗∗P < 0.005, ∗∗∗P < 0.0005.
4. Discussion
The inhibitory action of PECAM-1 on collagen induced platelet activation has been well documented. However, the impact of PECAM-1 activation on platelet response to other agonists has been less well explored. In this study we confirm the inhibitory effect of PECAM-1 on GPVI signaling, and go further to show inhibition of thrombin and ADP induced platelet activation. In line with other reports PECAM-1 cross-linking exerts a relatively small effect corresponding to 5–15% inhibition in platelet function. Similarly variation in the surface expression of PECAM-1 accounted for 6–10% of the variation in fibrinogen binding and P-selectin expression in response to stimulation. To put this into context, we also show that 20–21% of the variation in platelet response to CRP-XL is accounted for by variation in the surface expression of GPVI (Table 1). Clearly the inhibitory effect of PECAM-1 is subtle, and can even be overcome at the highest concentrations of agonists (Fig. 1B and C: thrombin 1U/mL, and ADP 10−4M). However, because PECAM-1 inhibits multiple activation pathways it is likely to be physiologically important. Therefore during thrombus formation the impact of PECAM-1 is more substantial than that seen on individual pathways in isolation. It is worth noting that, while the two systems are not strictly analogous, the inhibitory effect on thrombus formation of cross-linking PECAM-1 was of a similar magnitude to the increase in thrombus formation previously reported in the absence of PECAM-1 in knock-out mice [17].
The negative relationship between PECAM-1 surface expression and platelet response to stimuli provides independent confirmation of the physiological effect of PECAM-1. The specificity of this negative association between PECAM-1 and platelet responses is supported by the observation that GPVI and αIIbβ3 showed positive correlations, while GPIa expression did not exhibit any association. The magnitude of this PECAM-1 association is similar to that resulting from variation in GPVI or αIIbβ3, and is entirely expected given that platelet response requires the integration of a large number of proteins and varies widely throughout the population [20]. Furthermore, the impact of PECAM-1 on platelet function may be underestimated in these studies because the reduced platelet–platelet interaction in flow cytometric assays limits homophilic ligation.
In conclusion, we have demonstrated that the activation of PECAM-1, through cross-linking, inhibits platelet response to the collagen mimetic, CRP-XL, ADP and thrombin, and have confirmed the inhibitory action of PECAM-1, by showing an association between increased surface expression of PECAM-1 and decreased platelet response. The combination of a relatively small inhibitory effect on multiple signaling pathways results in a more dramatic inhibition of thrombus formation than may have been previously appreciated.
Acknowledgements
This study was supported by the British Heart Foundation (RG/05/007 and FS/07/018), 6th Framework Programme of the European Union (LSHM-CT-2004-503485) and a grant from the National Institute of Health Research of England to SFG and WHO and the Cambridge Biomedical Research Centre. The authors wish to thank the staff of the Cambridge BioResource at NHSBT Cambridge for obtaining donor samples and Dr Nicolas Raynal (Department of Biochemistry, University of Cambridge) for the CRP-XL.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.febslet.2009.10.037.
Appendix A. Supplementary data
Supplementary Fig. S1.
Association between PECAM-1 surface expression and platelet reactivity. A graphical representation of the linear regression analysis summarised in Table 1 showing the association between surface expression of four platelet surface molecules and plate reactivity (light gray – P < 0.05). In line with previous reports surface expression of GPVI is positively associated with platelet response to CRP-XL [25] accounting for approximately 20% of the variation in platelet response to this agonist. Surface expression of αIIbβ3 was also positively correlated with platelet fibrinogen binding in response to ADP and TRAP. Surface expression of PECAM-1 showed a negative association with both fibrinogen binding and P-selectin expression in response to CRP-XL and ADP. GPVI and αIIbβ3 expression, time of day, and mean platelet volume were includes as confounding variables, as indicated in Table 1.
References
- 1.Muller W.A., Weigl S.A., Deng X., Phillips D.M. PECAM-1 is required for transendothelial migration of leukocytes. J. Exp. Med. 1993;178:449–460. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiba R., Nakagawa N., Kuroasawa K., Tanaka Y., Saito Y., Iwamoto I. Ligation of CD31(PECAM-1) on endothelial cells increases adhesive function of avb3 integrin and enhances b1 integrin mediated adhesion of eosinophilis to endothelial cells. Blood. 1999;94:1319–1329. [PubMed] [Google Scholar]
- 3.Piali L., Albelda S.M., Baldwin H.S., Hammel P., Gisler R.H., Imhof B.A. Murine platelet endothelial cell adhesion molecule (PECAM-1/CD31) modulates b2 integrins on lymphokine-activated killer cells. Eur. J. Immunol. 1993;23:2464–2471. doi: 10.1002/eji.1830231013. [DOI] [PubMed] [Google Scholar]
- 4.Zhao T.M., Newman P.J. Integrin activation by regulated dimerization and oligomerization of platelet endothelial cell adhesion molecule (PECAM)-1 from within the cell. J. Cell Biol. 2001;152:65–73. doi: 10.1083/jcb.152.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berman M.E., Xie Y., Muller W.A. Roles of platelet endothelial cell adhesion molecule-1 (PECAM- 1, CD31) in natural killer cell transendothelial migration and beta(2) integrin activation. J. Immunol. 1996;156:1515–1524. [PubMed] [Google Scholar]
- 6.Novinska M.S., Rathore V., Newman D.K., Newman P.J. Pecam-1. In: Michelson A.D., editor. Platelets. Academic Press; 2007. pp. 221–230. [Google Scholar]
- 7.Cicmil M., Thomas J.M., Sage T., Barry F.A., Leduc M., Bon C., Gibbins J.M. Collagen, convulxin, and thrombin stimulate aggregation-independent tyrosine phosphorylation of CD31 in platelets. Evidence for the involvement of Src family kinases. J. Biol. Chem. 2000;275:27339–27347. doi: 10.1074/jbc.M003196200. [DOI] [PubMed] [Google Scholar]
- 8.Edmead C.E., Crosby D.A., Southcott M., Poole A.W. Thrombin induced association of SHP-2 with multiple tyrosine phosphorylated proteins in human platelets. FEBS Lett. 1999;459:27–32. doi: 10.1016/s0014-5793(99)01209-0. [DOI] [PubMed] [Google Scholar]
- 9.Sagawa K., Kimura T., Swieter M., Siraganian R.P. The protein-tyrosine phosphatase SHP-2 associates with tyrosine-phosphorylated adhesion molecule PECAM-1 (CD31) J. Biol. Chem. 1997;272:31086–31091. doi: 10.1074/jbc.272.49.31086. [DOI] [PubMed] [Google Scholar]
- 10.Jackson D.E., Kupcho K.R., Newman P.J. Characterization of phosphotyrosine binding motifs in the cytoplasmic domain of platelet/endothelial cell adhesion molecule-1 (PECAM-1) that are required for the cellular association and activation of the protein-tyrosine phosphatase, SHP-2. J. Biol. Chem. 1997;272:24868–24875. doi: 10.1074/jbc.272.40.24868. [DOI] [PubMed] [Google Scholar]
- 11.Jackson D.E., Ward C.M., Wang R., Newman P.J. The protein-tyrosine phosphatase SHP-2 binds platelet/endothelial cell adhesion molecule-1 (PECAM-1) and forms a distinct signaling complex during platelet aggregation. J. Biol. Chem. 1997;272:6986–6993. doi: 10.1074/jbc.272.11.6986. [DOI] [PubMed] [Google Scholar]
- 12.Varon D., Jackson D.E., Shenkman B., Dardik R., Tamarin I., Savion N., Newman P. Platelet/endothelial cell adhesion molecule-1 serves as a co-stimulatory agonist receptor that modulates integrin dependent adhesion and aggregation of human platelets. Blood. 1998;91:500–507. [PubMed] [Google Scholar]
- 13.Cicmil M., Thomas J.M., Leduc M., Bon C., Gibbins J.M. PECAM-1 signalling inhibits the activation of human platelets. Blood. 2002;99:137–144. doi: 10.1182/blood.v99.1.137. [DOI] [PubMed] [Google Scholar]
- 14.Jones K.L., Hughan S.C., Dopheide S.M., Farndale R.W., Jackson S.P., Jackson D.E. Platelet endothelial cell adhesion molecule-1 is a negative regulator of platelet-collagen interactions. Blood. 2001;98:1456–1463. doi: 10.1182/blood.v98.5.1456. [DOI] [PubMed] [Google Scholar]
- 15.Dhanjal T.S., Ross E.A., Auger J.M., McCarty O.J., Hughes C.E., Senis Y.A., Buckley C.D., Watson S.P. Minimal regulation of platelet activity by PECAM-1. Platelets. 2007;18:56–67. doi: 10.1080/09537100600881396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patil S., Newman D.K., Newman P.J. Platelet endothelial cell adhesion molecule-1 serves as an inhibitory receptor that modulates platelet responses to collagen. Blood. 2001;97:1727–1732. doi: 10.1182/blood.v97.6.1727. [DOI] [PubMed] [Google Scholar]
- 17.Falati S. Platelet PECAM-1 inhibits thrombus formation in vivo. Blood. 2006;107:535–541. doi: 10.1182/blood-2005-04-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wee J.L., Jackson D.E. The Ig-ITIM superfamily member PECAM-1 regulates the “outside-in” signaling properties of integrin {alpha}IIb{beta}3 in platelets. Blood. 2005;106:3816–3823. doi: 10.1182/blood-2005-03-0911. [DOI] [PubMed] [Google Scholar]
- 19.Morton L.F., Hargreaves P.G., Farndale R.W., Young R.D., Barnes M.J. Integrin alpha 2 beta 1-independent activation of platelets by simple collagen-like peptides: collagen tertiary (triple-helical) and quaternary (polymeric) structures are sufficient alone for alpha 2 beta 1-independent platelet reactivity. Biochem. J. 1995;306:337–344. doi: 10.1042/bj3060337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones C.I. Mapping the platelet profile for functional genomic studies and demonstration of the effect size of the GP6 locus. J. Thromb. Haemostasis. 2007;5:1756–1765. doi: 10.1111/j.1538-7836.2007.02632.x. [DOI] [PubMed] [Google Scholar]
- 21.Jones S. Peripheral tachykinins and the neurokinin receptor NK1 are required for platelet thrombus formation. Blood. 2008;111:605–612. doi: 10.1182/blood-2007-07-103424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tucker, K. L. et al. (2008). A dual role for integrin linked kinase in platelets: regulating integrin function and {alpha}-granule secretion. Blood, blood-2008-03-148502. [DOI] [PMC free article] [PubMed]
- 23.De Candia E., Hall S.W., Rutella S., Landolfi R., Andrews R.K., De Cristofaro R. Binding of thrombin to glycoprotein Ib accelerates the hydrolysis of Par-1 on intact platelets. J. Biol. Chem. 2001;276:4692–4698. doi: 10.1074/jbc.M008160200. [DOI] [PubMed] [Google Scholar]
- 24.Rathore V., Stapleton M.A., Hillery C.A., Montgomery R.R., Nichols T.C., Merricks E.P., Newman D.K., Newman P.J. PECAM-1 negatively regulates GPIb/V/IX signaling in murine platelets. Blood. 2003;102:3658–3664. doi: 10.1182/blood-2003-06-1888. [DOI] [PubMed] [Google Scholar]
- 25.Jones C.I. A functional genomics approach reveals novel quantitative trait loci associated with platelet signaling pathways. Blood. 2009;114:1405–1416. doi: 10.1182/blood-2009-02-202614. [DOI] [PubMed] [Google Scholar]