Abstract
The early cortical primordium develops from a sheet of neuroepithelium that is flanked by distinct signaling centers. Of these, the hem and the antihem are positioned as longitudinal stripes, running rostro-caudally along the medial and lateral faces, respectively, of each telencepahlic hemisphere. In this review we examine the similarities and differences in how these two signaling centers arise, their roles in patterning adjacent tissues, and the cells and structures they contribute to. Since both the hem and the antihem have been identified across many vertebrate phyla, they appear to be part of an evolutionary conserved set of mechanisms that play fundamental roles in forebrain development.
Keywords: Patterning, Induction, Hippocampal organizer, Hem, Antihem
1. Defining the hem and antihem: position, molecular expression domains and signaling molecules
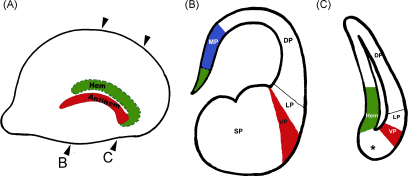
Neuroepithelium that gives rise to the cerebral cortex is flanked by the hem medially, and the antihem laterally ([1,5]; Fig. 1A). Therefore, these two structures are separated by the expanse cortical neuroepithelium (Fig. 1B) except at the extreme caudal pole of the telencephalon, where they almost meet [5], separated by a small domain of cortical neuroepithelium ([2]; Fig. 1C).
Fig. 1.
The positions of the hem and the antihem in the dorsal telencephalon. (A) A schematic of an E12.5 telencephalic hemisphere viewed from the lateral face, showing the antihem (red). The hem is schematized on the medial face (green). Rostral is to the left. (B) and (C) are schematics representing mid-level and caudal sections of such a hemisphere, showing the medial pallium (MP) which includes the hem (green) and the hippocampal primordium (blue), the dorsal pallium (DP), lateral pallium (LP), ventral pallium in red (VP/antihem), and subpallium (SP). Asterisk denotes DP tissue present between the hem and antihem at extreme caudal levels, which is the source of the amygdaloid nucleus nLOT2. Modified from Remedios et al. [2] [www.nature.com/neuro/index.html]. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
Along the medio-lateral axis, the telencephalic neuroepithelium can be divided into four different types of pallial tissue based on gene expression patterns (Fig. 1). The medial pallium (MP) contains the hem and the hippocampal primordium; the dorsal pallium (DP) corresponds to the neocortical primordium; the lateral pallium (LP) is thought to give rise to the piriform cortex; and the ventral pallium (VP), which together with the LP, contributes to specific components of the claustroamygdaloid complex [3,4]. The ventricular zone of the VP is identified as the antihem [5]. An adjacent subpallial region, the dLGE, is also thought to contribute to the amygdaloid complex ([4,6]). The VP and the dLGE lie on either side of the pallial–subpallial boundary (PSB; [7]). A prominent pallisade of radial glial fibers delineates the PSB, originating in a region of the ventricular zone termed the “corticostriatal junction” [8], and extending up to the pial surface in the region of the piriform cortex and amygdala.
Dbx1, a transcription factor, is restricted to the VP ventricular zone [4,7,9]. This exclusive expression of Dbx1, as well as an enriched expression of the secreted frizzled related gene sFrp2, serves to delineate the antihem from adjacent domains [1,5,7,10]. The VP and the adjacent dLGE both share an enriched expression of Pax6. In the VP, this expression is limited to the ventricular zone (the antihem), whereas in the dLGE Pax6 expression extends into the mantle [7].
The hem and the antihem were proposed to be important embryonic signaling centers on the basis of their locations, flanking the cortical neuroepithelium, and their enriched expression of several different types of signaling molecules. The hem expresses signaling molecules of the Wnt family, Wnt2b, 3a, and 5a. Several members of the Bmp gene family are expressed in broader domains that include the adjacent choroid plexus and/or hippocampal primordium [11,12]. The antihem expresses epidermal growth factor (EGF) family members, a fibroblast growth factor Fgf7, as well as a Wnt signaling inhibitor Sfrp2 [1,5]. Of the several EGF family members, ligands Tgfα, Nrg1 and Nrg3 are concentrated at the antihem. Egf is itself expressed throughout the ventral neuroepithelium, but is not concentrated at the PSB [5,13]. Sfrp2 is intensely expressed in the antihem, and more weakly in the rest of the telencephalic neuroepithelium [1,5]. Members of this family bind directly to Wnts and act as Wnt antagonists [14–16].
In the subsequent sections, we will review the mechanisms that regulate the positions of the hem and the antihem, and how these positions enable the signaling centers to control the structural organization of different brain structures.
2. Specification of the hem and the antihem
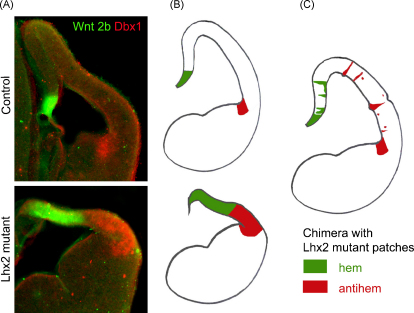
Along the rostro-caudal axis, the antihem is more pronounced rostrally, appearing at levels anterior to the hem. In contrast, the hem is seen from mid-levels, and is most prominent caudally, persisting at levels where the antihem is no longer present [5,12,17]. These positions parallel the graded expression of developmental control molecules in the telencephalon: Pax6 is expressed in a rostrolateral (high) to caudo-medial (low) gradient whereas Lhx2 and Emx2 show the opposite gradients [18,19]. Pax6 is required for the specification of the antihem ([1,5]). Lhx2 suppresses both hem and antihem fates, and both structures are expanded in the Lhx2 mutant [17,20,21]. The hem and the antihem are non-cortical in that they do not contribute to the hippocampus or the neocortex. However, cells of the prospective cortical primordium take on either hem or antihem fate in the absence of Lhx2, revealing a fundamental commonality between these two fates. Studies using embryonic stem cell chimeras have demonstrated that Lhx2 null cells become hem if located medially, and antihem if located laterally ([17]; Fig. 2). It is unknown how this positional control of hem versus antihem fate choice is regulated. Attractive candidates are early-expressing transcription factors that are themselves graded in expression, such as Pax6 and Emx2 [22], or Foxg1, which suppresses hem fate, and appears to be required for lateral fates including that of the antihem [23,24]. In the dorsal telencephalon of the Foxg1 mutant, medial fates are expanded and lateral fates are missing [24]. In mosaic embryos created by tamoxifen-induced gene disruption of Lhx2, medially located Lhx2 null patches do not express Pax6 or Foxg1, whereas laterally located Lhx2 null patches express both these genes [17]. While this is entirely consistent with the requirement of Pax6 and Foxg1 for antihem fate, it still does not explain how these differences between medial and lateral Lhx2 null patches is brought about in the first place. This remains a fundamental open question: to understand the early patterning of the telencephalon into distinct signaling centers flanking a territory of “responding” tissue, the cortical neuroepithelium.
Fig. 2.
Lhx2 suppresses hem and antihem fate in a position-dependent manner. (A) Hem marker Wnt2b (green) and antihem marker Dbx1 (red) reveal these structures separated by the cortical neuroepithelium in a control E12.5 brain. In the Lhx2 mutant both the hem and the antihem are expanded and there is no intervening cortical neuroepithelium. (B) Schematics representing these data. (C) In a chimeric brain with Lhx2 null clusters scattered amidst wild-type neuroepithelium, the medial null patches take on hem identity whereas lateral patches differentiate into antihem. From Mangale et al. [17] [www.sciencemag.org]. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
3. Molecular mechanisms that act to position and specify the cortical hem
Several molecular models have been proposed to explain the position of the cortical hem in the telencephalon. These studies seek to explain the mechanisms which define the rostro-caudal as well as the medio-lateral boundaries of this signaling center. Fgfs expressed by the anterior neural ridge (ANR) are fundamental regulators of mid-line patterning [25]. They activate midline expressing transcription factors and repress Lhx2 and help establish the mid-line domain within the telencephalon prior to invagination. At the same time, Bmps from the roof plate restrict the extent of the Fgf expression and are themselves repressed by the Fgfs [26–28]. Fgfs also repress Wnt genes whose expression defines the cortical hem [27]. This cross regulation between two groups of secreted signals helps to define a caudo-medial position for the hem.
This domain is further refined by cross-regulatory interactions between transcription factors Emx2 and Pax6 [29]. In particular, Emx2 appears to specify a caudo-medial domain in the telencephalon which contains the cortical hem as well as the hippocampus. Emx2 may act by restricting the anterior region of Fgf gene expression [27]. Furthermore, Emx2 functions as an effector of the canonical Wnt signaling from the hem to regulate proliferation within the caudo-medial region [30]. Thus Emx2 appears to act at two stages: to establish the domain where hem induction will occur, and later, to mediate the effects of hem signaling during further development of this region.
While these mechanisms serve to define the rostro-caudal extent of the hem, other mechanisms act to define the medio-lateral boundaries of the hem with the choroid plexus and the cortex. An early acting regulatory mechanism involving transcription factors of the bHLH family regulates the hem–choroid plexus boundary [31]. The hem and choroid plexus are defined by the differential expression of Hes and Ngn genes. At the time of boundary formation, Hes genes are enriched in the putative choroid plexus region, possibly as a result of the direct activation of these genes by Bmps from the roof plate. At the same time, this region downregulates Ngn gene expression, which continues to be maintained in the adjacent cortical hem. This downregulation of Ngn expression is important in establishing the choroid plexus fate and therefore delineating the hem–choroid plexus boundary [31].
4. Molecular mechanisms that act to position and specify the antihem
Three transcription factors, Pax6, Tlx, and Gsh2, are known to regulate the specification and positioning of the antihem. Its location at the PSB makes the antihem vulnerable to perturbations that disrupt dorsoventral patterning in the telencephalon.
The PSB is severely affected in the Pax6 mutant. There is a ventralization of the pallial neuroepithelium of the Pax6 mutant telencephalon, such that the VP and LP now express subpallial markers Mash1, Gsh2 and Dlx2 ([32,33,1,7,34,35]). Tlx mutants exhibit a similar, but less severe phenotype than Pax6 mutants [36]. Tlx is expressed throughout the neuroepithelium, high at the lateral sulcus and on both sides of the PSB. As in the case of the Pax6 mutant, the Tlx mutant too exhibits LGE characteristics at the expense of those of the VP [36]. In contrast, the Gsh2 mutant displays the opposite phenotype, one in which pallial gene expression signatures are seen in subpallial domains such as the dLGE [7,35]. A detailed analysis of the interactions of Pax6 and Gsh2 reveals a cross-repressive mechanism, wherein Pax6 is required to induce VP-specific markers, and Gsh2 is necessary to suppress the expression of these genes in the dLGE, thereby restricting them to the VP [37].
5. “Organizer” functions
5.1. Hem
The cortical hem was considered to be analogous to the dorsal signaling center of the spinal cord, the roof plate, which also secretes Wnt and Bmp family molecules [12,38]. Bmp signaling from the roof plate is responsible for patterning adjacent neuronal fates [39], and ablation of the roof plate causes loss of specific neuronal populations [40]. A similar role for the cortical hem was proposed [12]. Supporting this hypothesis, the entire hippocampus is missing when the hem is deleted [41], or when a particular hem-specific signaling molecule, Wnt3a, is disrupted [42]. When components of the Wnt signaling cascade Lef1 [43] or Lrp6 [44] are disrupted, the dentate precursor pool is diminished and does not mature or migrate properly. But highly reduced cell populations of the dentate precursors were detected in each mutant [43,44]. Therefore, these studies were not able to separate a role for Wnt signaling in the expansion of the precursor population from one in which they act to specify of hippocampal cell fates [45].
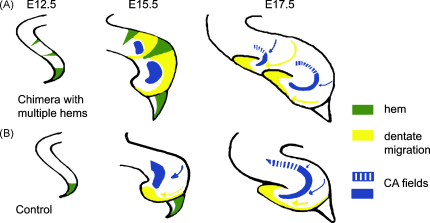
The role of the cortical hem has also been tested in explant culture experiments in which the hem was either removed, or transplanted to ectopic locations of medial telencephalic preparations [46]. However, the age of the tissue used was E12.5, apparently too late for either perturbation to have any effect on hippocampal specification. Indeed, the authors concluded that the fine details of hippocampal field specification must have occurred by E12.5, even though overt differentiation of hippocampal fields occurs much later, from E15.5 [46]. Definitive evidence of the role of the cortical hem came from chimeras in which Lhx2 null cells, surrounded by wild-type cortical neuroepithelium, differentiated into ectopic hem tissue [17]. An ectopic hippocampus formed adjacent to each patch of hem, with spatially correct induction and positioning of multiple hippocampal fields (Fig. 3). This consolidated the cortical hem as an organizer for the hippocampus.
Fig. 3.
Ectopic hem induces and organizes multiple hippocampal fields. (A) and (B) are schematic representations of chimeric and control brains, respectively. The normal hem is seen at the medial extreme of the E12.5 telencephalic neuroepithelium. In the chimeras, Lhx2 mutant clusters form ectopic patches of hem in the medial telencephalon. By E15.5, control brains display markers for the hippocampal CA fields as well as for dentate granule cells. Both cell types originate in neuroepithelium and migrate away (blue and yellow arrows) to form the characteristic morphology of the Ammon's horn and the dentate gyrus by E17.5. In chimeric brains, CA and dentate cells are induced in appropriate spatial order adjacent to each ectopic hem, with the dentate granule cells immediately adjacent to the hem. By E17.5, the chimeras have assembled distinct dentate gyri and CA fields forming a double hippocampus. Modified from [17] [www.sciencemag.org]. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
Which signaling molecules from the hem are critical for ectopic hippocampal induction? The literature strongly supports a role of Wnt signaling for this role. The Wnt3a, Lef1, and Lrp6 mutant studies all indicate that Wnt signaling is necessary for hippocampal development [42–44]. Furthermore, ectopic activation of Lef1 upregulated some hippocampal field markers in lateral neuroepithelium, demonstrating that Wnt signaling is sufficient for this process [47]. In contrast, Bmp signaling has not been implicated in hippocampal development. The Bmpr1a mutant, which lacks the telencephalic choroid plexus, appears to form a hippocampus [48]. In terms of regulating telencephalic neuronal development, Bmp signaling appears to act at earlier stages, including specifying the extreme medial fate of the choroid plexus [48–50]; causing cell death [11], and regulating the expression of Lhx2 itself [21]. Furthermore, Bmp signaling is implicated in constraining the Fgf8 domain, which in turn limits the domain of Wnt expression in the medial telencephalon [27]. Thus early actions of Bmp signaling may set in motion events which permit the formation of the cortical hem, which in turn induces the hippocampus. How signals from the hem bring about the specification of distinct hippocampal field identities remains an important open question.
An important issue is how the hem can direct not only the specification, but also the structural organization of multiple hippocampal fields. A clue comes from examining the radial glial palisade associated with the dentate migration. The organization of this palisade is thought to guide the dentate cells from their origin at the ventricular zone adjacent to the hem to their final location where they form the blades of the dentate gyrus [51]. Mangale et al. [17] report additional radial glial pallisades associated with ectopic hem tissue, which appear to guide distinct migratory streams terminating at each dentate gyrus (Fig. 3). The organization of the radial glial scaffolding itself is dependent on Wnt signaling [44]. Thus each patch of hem may be responsible for orienting the scaffolding adjacent to it, which would then guide the ectopically induced dentate cells to form an ectopic gyrus.
5.2. Antihem
In contrast to the organizer function of the hem, such a role for the antihem has yet to be identified. Nonetheless, the loss of the antihem is known to correlate with severe disruption of the radial glial pallisade at the PSB, raising a strong parallel with the role of the hem in organizing the hippocampal radial glia. Tlx mutants have fewer radial glial fibers at the PSB which do not appear to fasciculate to form a palisade [36]. Pax6 is itself required for the differentiation of radial glia in the forebrain [52]. Not surprisingly, the radial glial palisade at the PSB is disrupted in the Pax6 mutant [33,53,54]. Although markers identified the radial glial progenitor cell population, the fascicle itself could not be distinguished. Furthermore, interneurons produced within the subpallium were detected in greater numbers in the Pax6 mutant cortex, suggesting that some feature of the normal PSB serves to restrict the tangential migration of interneurons [54]. Finally, Pax6 mutant also displays profound defects in thalamocortical and corticofugal axon pathfinding. The underlying cause of this defect was suggested to be a combination of structural abnormalities and alterations in the expression of specific pathfinding molecules of the Semaphorin family at the Pax6 mutant PSB [55].
Which other signaling molecules might mediate some of these defects? Nrg1, which is concentrated in the antihem, has been shown to be essential for the formation and maintenance of radial glial cells [56,57]. Drawing parallels with the hem, Wnt signaling might also regulate the radial glial pallisades organized by the antihem. Wnt7b is expressed adjacent to the antihem, in the dLGE [1]. The expression of the Wnt antagonist Sfrp2 in the antihem may lead to a concentration of the Wnt signal to the subpallial side of the PSB [1], providing a positional signal to the radial glial pallisade.
Together, these studies support an integral role for the antihem in mediating axon guidance and cell migration, that of interneurons into the cortex as well as that of the derivatives of the lateral telencephalon, such as the olfactory cortex, the claustrum, and the amygdala. The latter role is likely to arise from the regulation of the radial glial pallisade at the PSB.
6. Derivatives: similarities and differences between the hem and the antihem
The hem was suggested to produce Cajal–Retzius cells [58] and this was demonstrated using genetic techniques to fate map the hem lineage [41,59]. The antihem also gives rise to Cajal–Retzius cells, as does the septum [9]. It is not at all clear why this earliest-born cell population has multiple origins. An attractive hypothesis proposes that this diversity of Cajal–Retzius cell progenitor zones may correlate with or regulate the development of cytoarchitectonic differences between the neocortex, olfactory cortex, and the hippocampus [9].
Reelin expression is a common feature to all these different types of Cajal–Retzius cells. Cajal–Retzius cells from the hem and antihem, but not those from the septum, express calretinin [9]. The hem lineage Cajal–Retzius cells express p73 [60], but those from the antihem and septum do not [9]. Dbx1 expression, in contrast, marks cells from the antihem and the septum, but not those from the hem [9]. This unique combination of markers was used to selectively ablate specific sub populations, to examine possible functional roles arising from this diversity of Cajal–Retzius cell subtypes [9]. When antihem and septum derived Cajal–Retzius cells were ablated by expressing DTA (Diphtheria toxin) via the Dbx1 locus, a significant loss of reelin expression was seen in the septum and pirifom cortex at E11.5, but this was compensated for by E14.5, presumably by Cajal–Retzius cells from other sources. However, there was a gross reduction in the thickness of the lateral cortex. This defect was selective for the lateral region, since the cingulate cortex appeared normal, indicating an important role for the antihem-derived Cajal–Retzius cells in regional cortical development [9].
A surprising, counterintuitive result came from experiments in which hem-derived Cajal–Retzius cells were ablated by expressing DTA via Wnt3a locus [41]. This caused a massive and near-complete depletion of Cajal–Retzius cells overlying the neocortex, which was apparently not rescued by migration of Cajal–Retzius cells from other sources. Despite this, neocortical lamination was unaffected [41]. Similarly, in p73 mutants, which also display a loss of cortical Cajal–Retzius cells, neocortical lamination was normal except for the absence of the hippocampal fissure, which may be due to the loss of p73 itself [60]. Thus the precise role of hem-derived Cajal–Retzius cells continues to be elusive.
In addition to the production of Cajal–Retzius cells, the hem and the antihem also make unique contributions to the telencephalon. The hem produces the epithelial component of the choroid plexus, a secretory non-neuronal tissue that produces cerebrospinal fluid [61]. This is likely to be controlled by Bmp signaling [48] and by cross suppression between the Ngn and Hes genes [31]. The antihem is a major contributor of excitatory, pallial-derived cells of the amygdaloid complex. Gene expression studies [3,4,6] and genetic lineage tracing of the Dbx1 lineage [62] indicates that the lateral and basomedial nuclei of the amygdaloid complex arise from the VP/antihem. The LP is thought to give rise to the basolateral nucleus which positions itself in between the two VP derived nuclei, and together these form the basolateral complex of the amygdala [4]. Mechanisms that disrupt the antihem also affect these structures, which are consequently greatly shrunken or missing in the Pax6 [6] and the Tlx mutants [36]. In contrast, consistent with the antihem being spared in the Lhx2 mutant, these structures are specified in the absence of Lhx2 [63]. The radial glial pallisade at the PSB is likely to participate in the migration of these cells to their final destinations, and indeed, such migrations have been visualized using GFP electroporation [64]. However, radial glia-independent type of “chain migration” has also been reported at the PSB [65]. Furthermore, migration of the basolateral complex of the amygdala does not seem to share mechanistic parallels with that in the cerebral cortex. Reelin is required for all neocortical cells to migrate to their appropriate destinations [66]. Cells of the superficial layers of the cortex require Cdk5 to migrate past the deep layers [67,68]. In contrast, cell migration of the basolateral amygdala is normal in the Reelin and the Cdk5 mutants [2] suggesting that the mechanisms that regulate the assembly of the intricate structural complexity of the amygdaloid complex are far from simple, and as yet poorly understood.
7. Evolutionary perspectives
Both the hem and the antihem are evolutionarily ancient, having been identified in several vertebrate phyla. The hem has been identified in birds [69] and also in reptiles [70]. The antihem appears earlier, and was an important discovery as a ventral pallial territory in amphibians [71–73].
The positions of the hem and the antihem on the medial and lateral edges of the pallium, respectively, therefore preceded the expansion of the pallium in mammals. This motivates the speculation that the interactions of the hem and the antihem may have played a role in stimulating the expansion of the dorsal pallium. At the caudal telencephalon of the mouse embryo, where these two signaling centers almost meet (Fig. 1C), the intervening tissue produces an unusual stream of migrating cells, the caudal amygdaloid stream, that forms the nucleus of the lateral olfactory tract (layer 2; nLOT2). This is the only component of the amygdaloid complex that originates in the dorsal pallium, in contrast to other nuclei that arise from the lateral or ventral pallium [2]. The nLOT2 is also unique in its dependence on two modern mechanisms for cell migration: Cdk5 and Reelin. When either of these mechanisms are disrupted, the nLOT2 is selectively affected, whereas the rest of the amygdaloid complex is unperturbed [2]. Since the nLOT has itself been identified in reptiles [74], it too predates the appearance of laminated neocortex, derived from the mammalian dorsal pallium. The requirements of migration of the nLOT2 may in fact have presaged the Cdk5 and Reelin dependence of the mammalian neocortex. Whether the hem or the antihem regulate any aspect of the nLOT2 specification or migration is unknown, but their juxtaposition on either side of the nLOT2 primordium places them as potentially significant not only in the development, but also in the evolution of the cortex.
Acknowledgements
Work in S. Tole's lab is supported by a Swarnajayanti Fellowship (Dept. of Science and Technology, Govt. of India), grants from the Department of Biotechnology, and the Department of Science and Technology, Govt. of India. S. Tole is a sabbatical visitor at Stanford University supported by a sabbatical award from the Wellcome Trust. L. Subramanian is the recipient of a Kanwal Rekhi Career Development Award from the TIFR Endowment Fund.
References
- 1.Kim A.S., Anderson S.A., Rubenstein J.L., Lowenstein D.H., Pleasure S.J. Pax-6 regulates expression of SFRP-2 and Wnt-7b in the developing CNS. J Neurosci. 2001;21:RC132. doi: 10.1523/JNEUROSCI.21-05-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Remedios R., Huilgol D., Saha B., Hari P., Bhatnagar L., Kowalczyk T. A stream of cells migrating from the caudal telencephalon reveals a link between the amygdala and neocortex. Nat Neurosci. 2007;10:1141–1150. doi: 10.1038/nn1955. [DOI] [PubMed] [Google Scholar]
- 3.Puelles L., Kuwana E., Puelles E., Bulfone A., Shimamura K., Keleher J. Pallial and subpallial derivatives in the embryonic chick and mouse telencephalon, traced by the expression of the genes Dlx-2, Emx-1, Nkx-2.1, Pax-6, and Tbr-1. J Comp Neurol. 2000;424:409–438. doi: 10.1002/1096-9861(20000828)424:3<409::aid-cne3>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 4.Medina L., Legaz I., González G., De Castro F., Rubenstein J.L., Puelles L. Expression of Dbx1, Neurogenin 2, Semaphorin 5A, Cadherin 8, and Emx1 distinguish ventral and lateral pallial histogenetic divisions in the developing mouse claustroamygdaloid complex. J Comp Neurol. 2004;474:504–523. doi: 10.1002/cne.20141. [DOI] [PubMed] [Google Scholar]
- 5.Assimacopoulos S., Grove E.A., Ragsdale C.W. Identification of a Pax6-dependent epidermal growth factor family signaling source at the lateral edge of the embryonic cerebral cortex. J Neurosci. 2003;23:6399–6403. doi: 10.1523/JNEUROSCI.23-16-06399.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tole S., Remedios R., Saha B., Stoykova A. Selective requirement of Pax6, but not Emx2, in the specification and development of several nuclei of the amygdaloid complex. J Neurosci. 2005;25:2753–2760. doi: 10.1523/JNEUROSCI.3014-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yun K., Potter S., Rubenstein J.L. Gsh2 and Pax6 play complementary roles in dorsoventral patterning of the mammalian telencephalon. Development. 2001;128:193–205. doi: 10.1242/dev.128.2.193. [DOI] [PubMed] [Google Scholar]
- 8.Molnár Z., Butler A.B. The corticostriatal junction: a crucial region for forebrain development and evolution. Bioessays. 2002;24:530–541. doi: 10.1002/bies.10100. [DOI] [PubMed] [Google Scholar]
- 9.Bielle F., Griveau A., Narboux-Nême N., Vigneau S., Sigrist M., Arber S. Multiple origins of Cajal–Retzius cells at the borders of the developing pallium. Nat Neurosci. 2005;8:1002–10012. doi: 10.1038/nn1511. [DOI] [PubMed] [Google Scholar]
- 10.Vyas A., Saha B., Lai E., Tole S. The Paleocortex is specified in mice in which dorsal telencephalic patterning is severely disrupted. J Comp Neurol. 2003:466. doi: 10.1002/cne.10900. [DOI] [PubMed] [Google Scholar]
- 11.Furuta Y., Piston D.W., Hogan B.L. Bone morphogenetic proteins (BMPs) as regulators of dorsal forebrain development. Development. 1997;124:2203–2212. doi: 10.1242/dev.124.11.2203. [DOI] [PubMed] [Google Scholar]
- 12.Grove E.A., Tole S., Limon J., Yip L., Ragsdale C.W. The hem of the embryonic cerebral cortex is defined by the expression of multiple Wnt genes and is compromised in Gli3 deficient mice. Development. 1998;125:2315–2325. doi: 10.1242/dev.125.12.2315. [DOI] [PubMed] [Google Scholar]
- 13.Kornblum H.I., Hussain R.J., Bronstein J.M., Gall C.M., Lee D.C., Seroogy K.B. Prenatal ontogeny of the epidermal growth factor receptor and its ligand, transforming growth factor alpha, in the rat brain. J Comp Neurol. 1997;380:243–261. doi: 10.1002/(sici)1096-9861(19970407)380:2<243::aid-cne7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 14.Rattner A., Hsieh J.C., Smallwood P.M., Gilbert D.J., Copeland N.G., Jenkins N.A. A family of secreted proteins contains homology to the cysteine-rich ligand-binding domain of frizzled receptors. Proc Natl Acad Sci USA. 1997;94:2859–2863. doi: 10.1073/pnas.94.7.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawano Y., Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003;116:2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- 16.Ladher R.K., Church V.L., Allen S., Robson L., Abdelfattah A., Brown N.A. Cloning and expression of the Wnt antagonists Sfrp-2 and Frzb during chick development. Dev Biol. 2000;218:183–198. doi: 10.1006/dbio.1999.9586. [DOI] [PubMed] [Google Scholar]
- 17.Mangale V.S., Hirokawa K.E., Satyaki P.R., Gokulchandran N., Chikbire S., Subramanian L. Lhx2 selector activity specifies cortical identity and suppresses hippocampal organizer fate. Science. 2008;319:304–309. doi: 10.1126/science.1151695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bishop K.M., Goudreau G., O’Leary D.D. Regulation of area identity in the mammalian neocortex by Emx2 and Pax6. Science. 2000;288:344–349. doi: 10.1126/science.288.5464.344. [DOI] [PubMed] [Google Scholar]
- 19.Nakagawa Y., O’Leary D.D. Combinatorial expression patterns of LIM-homeodomain and other regulatory genes parcellate developing thalamus. J Neurosci. 2001;21:2711–2725. doi: 10.1523/JNEUROSCI.21-08-02711.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bulchand S., Grove E.A., Porter F.D., Tole S. LIM-homeodomain gene Lhx2 regulates the formation of the cortical hem. Mech Dev. 2001;100:165–175. doi: 10.1016/s0925-4773(00)00515-3. [DOI] [PubMed] [Google Scholar]
- 21.Monuki E.S., Porter F.D., Walsh C.A. Patterning of the dorsal telencephalon and cerebral cortex by a roof plate-Lhx2 pathway. Neuron. 2001;32:591–604. doi: 10.1016/s0896-6273(01)00504-9. [DOI] [PubMed] [Google Scholar]
- 22.Muzio L., DiBenedetto B., Stoykova A., Boncinelli E., Gruss P., Mallamaci A. Emx2 and Pax6 control regionalization of the pre-neuronogenic cortical primordium. Cereb Cortex. 2002;2:129–139. doi: 10.1093/cercor/12.2.129. [DOI] [PubMed] [Google Scholar]
- 23.Dou C.L., Li S., Lai E. Dual role of brain factor-1 in regulating growth and patterning of the cerebral hemispheres. Cereb Cortex. 1999;9:543–550. doi: 10.1093/cercor/9.6.543. [DOI] [PubMed] [Google Scholar]
- 24.Muzio L., Mallamaci A. Foxg1 confines Cajal–Retzius neuronogenesis and hippocampal morphogenesis to the dorsomedial pallium. J Neurosci. 2005;25:4435–4441. doi: 10.1523/JNEUROSCI.4804-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okada T., Okumura Y., Motoyama J., Ogawa M. FGF8 signaling patterns the telencephalic midline by regulating putative key factors of midline development. Dev Biol. 2008;320:92–101. doi: 10.1016/j.ydbio.2008.04.034. [DOI] [PubMed] [Google Scholar]
- 26.Ohkubo Y., Chiang C., Rubenstein J.L. Coordinate regulation and synergistic actions of BMP4, SHH and FGF8 in the rostral prosencephalon regulate morphogenesis of the telencephalic and optic vesicles. Neuroscience. 2002;111:1–17. doi: 10.1016/s0306-4522(01)00616-9. [DOI] [PubMed] [Google Scholar]
- 27.Shimogori T., Banuchi V., Ng H.Y., Strauss J.B., Grove E.A. Embryonic signaling centers expressing BMP, WNT and FGF proteins interact to pattern the cerebral cortex. Development. 2004;131:5639–5647. doi: 10.1242/dev.01428. [DOI] [PubMed] [Google Scholar]
- 28.Storm E.E., Garel S., Borello U., Hebert J.M., Martinez S., McConnell S.K. Dose-dependent functions of Fgf8 in regulating telencephalic patterning centers. Development. 2006;133:1831–1844. doi: 10.1242/dev.02324. [DOI] [PubMed] [Google Scholar]
- 29.Kimura J., Suda Y., Kurokawa D., Hossain Z.M., Nakamura M., Takahashi M. Emx2 and Pax6 function in cooperation with Otx2 and Otx1 to develop caudal forebrain primordium that includes future archipallium. J Neurosci. 2005;25:5097–5108. doi: 10.1523/JNEUROSCI.0239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muzio L., Soria J.M., Pannese M., Piccolo S., Mallamaci A. A mutually stimulating loop involving emx2 and canonical Wnt signalling specifically promotes expansion of occipital cortex and hippocampus. Cereb Cortex. 2005;15:2021–2028. doi: 10.1093/cercor/bhi077. [DOI] [PubMed] [Google Scholar]
- 31.Imayoshi I., Shimogori T., Ohtsuka T., Kageyama R. Hes genes and neurogenin regulate non-neural versus neural fate specification in the dorsal telencephalic midline. Development. 2008;135:2531–2541. doi: 10.1242/dev.021535. [DOI] [PubMed] [Google Scholar]
- 32.Stoykova A., Fritsch R., Walther C., Gruss P. Forebrain patterning defects in small eye mutant mice. Development. 1996;122:3453–3465. doi: 10.1242/dev.122.11.3453. [DOI] [PubMed] [Google Scholar]
- 33.Stoykova A., Götz M., Gruss P., Price J. Pax6-dependent regulation of adhesive patterning, R-cadherin expression and boundary formation in developing forebrain. Development. 1997;124:3765–3777. doi: 10.1242/dev.124.19.3765. [DOI] [PubMed] [Google Scholar]
- 34.Stoykova A., Treichel D., Hallonet M., Gruss P. Pax6 modulates the dorsoventral patterning of the mammalian telencephalon. J Neurosci. 2000;20:8042–8050. doi: 10.1523/JNEUROSCI.20-21-08042.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toresson H., Potter S.S., Campbell K. Genetic control of dorsal-ventral identity in the telencephalon: opposing roles for Pax6 and Gsh2. Development. 2000;127:4361–4371. doi: 10.1242/dev.127.20.4361. [DOI] [PubMed] [Google Scholar]
- 36.Stenman J., Yu R., Evans R., Campbell K Tlx and Pax6 co-operate genetically to establish the pallio-subpallial boundary in the embryonic mouse telencephalon. Development. 2003;130:1113–1122. doi: 10.1242/dev.00328. [DOI] [PubMed] [Google Scholar]
- 37.Carney R.S., Cocas L.A., Hirata T., Mansfield K., Corbin J.G. Differential regulation of telencephalic pallial–subpallial boundary patterning by Pax6 and Gsh2. Cerebral Cortex Epub. 2008 doi: 10.1093/cercor/bhn123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chizhikov V.V., Millen K.J. Roof plate-dependent patterning of the vertebrate dorsal central nervous system. Dev Biol. 2005;277:287–295. doi: 10.1016/j.ydbio.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 39.Liem K.F., Jr., Tremml G., Jessell T.M. A role for the roof plate and its resident TGFbeta-related proteins in neuronal patterning in the dorsal spinal cord. Cell. 1997;91:127138. doi: 10.1016/s0092-8674(01)80015-5. [DOI] [PubMed] [Google Scholar]
- 40.Lee K.J., Dietrich P., Jessell T.M. Genetic ablation reveals that the roof plate is essential for dorsal interneuron specification. Nature. 2000;403:734–740. doi: 10.1038/35001507. [DOI] [PubMed] [Google Scholar]
- 41.Yoshida M., Assimacopoulos S., Jones K.R., Grove E.A. Massive loss of Cajal–Retzius cells does not disrupt neocortical layer order. Development. 2006;133:537–545. doi: 10.1242/dev.02209. [DOI] [PubMed] [Google Scholar]
- 42.Lee S.M., Tole S., Grove E., McMahon A.P. A local Wnt-3a signal is required for development of the mammalian hippocampus. Development. 2000;127:457–467. doi: 10.1242/dev.127.3.457. [DOI] [PubMed] [Google Scholar]
- 43.Galceran J., Miyashita-Lin E.M., Devaney E., Rubenstein J.L., Grosschedl R. Hippocampus development and generation of dentate gyrus granule cells is regulated by LEF1. Development. 2000;127:469–482. doi: 10.1242/dev.127.3.469. [DOI] [PubMed] [Google Scholar]
- 44.Zhou C.J., Zhao C., Pleasure S.J. Wnt signaling mutants have decreased dentate granule cell production and radial glial scaffolding abnormalities. J Neurosci. 2004;24:121–126. doi: 10.1523/JNEUROSCI.4071-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li G., Pleasure S.J. Morphogenesis of the dentate gyrus: what we are learning from mouse mutants. Dev Neurosci. 2005;27:93–99. doi: 10.1159/000085980. [DOI] [PubMed] [Google Scholar]
- 46.Tole S., Grove E.A. Detailed field pattern is intrinsic to the embryonic mouse hippocampus early in neurogenesis. J Neurosci. 2001;21:1580–1589. doi: 10.1523/JNEUROSCI.21-05-01580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Machon O., Backman M., Machonova O., Kozmik Z., Vacik T., Andersen L. A dynamic gradient of Wnt signaling controls initiation of neurogenesis in the mammalian cortex and cellular specification in the hippocampus. Dev Biol. 2007;311:223–237. doi: 10.1016/j.ydbio.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 48.Hébert J.M., Mishina Y., McConnell S.K. BMP signaling is required locally to pattern the dorsal telencephalic midline. Neuron. 2002;35:1029–1041. doi: 10.1016/s0896-6273(02)00900-5. [DOI] [PubMed] [Google Scholar]
- 49.Panchision D.M., Pickel J.M., Studer L., Lee S.-H., Turner P.A., Hazel T.G. Sequential actions of BMP receptors control neural precursor cell production and fate. Genes Dev. 2001;15:2094–2110. doi: 10.1101/gad.894701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng X., Hsu C.M., Currle D.S., Hu J.S., Barkovich A.J., Monuki E.S. Central roles of the roof plate in telencephalic development and holoprosencephaly. J Neurosci. 2006;26:7640–7649. doi: 10.1523/JNEUROSCI.0714-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rickmann M., Amaral D.G., Cowan W.M. Organization of radial glial cells during the development of the rat dentate gyrus. J Comp Neurol. 1987;264:449–479. doi: 10.1002/cne.902640403. [DOI] [PubMed] [Google Scholar]
- 52.Götz M., Stoykova A., Gruss P. Pax6 controls radial glia differentiation in the cerebral cortex. Neuron. 1998;21:1031–1044. doi: 10.1016/s0896-6273(00)80621-2. [DOI] [PubMed] [Google Scholar]
- 53.Hirata T., Nomura T., Takagi Y., Sato Y., Tomioka N., Fujisawa H. Mosaic development of the olfactory cortex with Pax6-dependent and -independent components. Brain Res Dev Brain Res. 2002;136:17–26. doi: 10.1016/s0165-3806(02)00304-8. [DOI] [PubMed] [Google Scholar]
- 54.Chapouton P., Gärtner A., Götz M. The role of Pax6 in restricting cell migration between developing cortex and basal ganglia. Development. 1999;126:5569–5579. doi: 10.1242/dev.126.24.5569. [DOI] [PubMed] [Google Scholar]
- 55.Jones L., López-Bendito G., Gruss P., Stoykova A., Molnár Z. Pax6 is required for the normal development of the forebrain axonal connections. Development. 2002;129:5041–5052. doi: 10.1242/dev.129.21.5041. [DOI] [PubMed] [Google Scholar]
- 56.Anton E.S., Marchionni M.A., Lee K.F., Rakic P. Role of GGF/neuregulin signaling in interactions between migrating neurons and radial glia in the developing cerebral cortex. Development. 1997;124:3501–3510. doi: 10.1242/dev.124.18.3501. [DOI] [PubMed] [Google Scholar]
- 57.Schmid R.S., McGrath B., Berechid B.E., Boyles B., Marchionni M., Sestan N. Neuregulin 1-erbB2 signaling is required for the establishment of radial glia and their transformation into astrocytes in cerebral cortex. Proc Natl Acad Sci USA. 2003;100:4251–4256. doi: 10.1073/pnas.0630496100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meyer G., Perez-Garcia C.G., Abraham H., Caput D. Expression of p73 and Reelin in the developing human cortex. J Neurosci. 2002;22:4973–4986. doi: 10.1523/JNEUROSCI.22-12-04973.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takiguchi-Hayashi K., Sekiguchi M., Ashigaki S., Takamatsu M., Hasegawa H., Suzuki-Migishima R. Generation of reelin-positive marginal zone cells from the caudomedial wall of telencephalic vesicles. J Neurosci. 2004;24:2286–2295. doi: 10.1523/JNEUROSCI.4671-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meyer G., Cabrera Socorro A., Perez Garcia C.G., Martinez Millan L., Walker N., Caput D. Developmental roles of p73 in Cajal–Retzius cells and cortical patterning. J Neurosci. 2004;24:9878–9887. doi: 10.1523/JNEUROSCI.3060-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Louvi A., Yoshida M., Grove E.A. The derivatives of the Wnt3a lineage in the central nervous system. J Comp Neurol. 2007;504:550–569. doi: 10.1002/cne.21461. [DOI] [PubMed] [Google Scholar]
- 62.Hirata T., Li P., Lanuza G.M., Cocas L.A., Huntsman M.M., Corbin J.G. Identification of distinct telencephalic progenitor pools for neuronal diversity in the amygdala. Nat Neurosci. 2009;12:141–149. doi: 10.1038/nn.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Remedios R., Subramanian L., Tole S. LIM genes parcellate the embryonic amygdala and regulate its development. J Neurosci. 2004;24:6986–6990. doi: 10.1523/JNEUROSCI.0001-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soma M., Aizawa H., Ito Y., Maekawa M., Osumi N., Nakahira E. Development of the mouse amygdala as revealed by enhanced green fluorescent protein gene transfer by means of in utero electroporation. J Comp Neurol. 2009;513:113–128. doi: 10.1002/cne.21945. [DOI] [PubMed] [Google Scholar]
- 65.Carney R.S., Alfonso T.B., Cohen D., Dai H., Nery S., Stoica B. Cell migration along the lateral cortical stream to the developing basal telencephalic limbic system. J Neurosci. 2006;26:11562–11574. doi: 10.1523/JNEUROSCI.3092-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Caviness V.J. Development of neocortical afferent systems: studies in the reeler mouse. Neurosci Res Program Bull. 1982;20:560–569. [PubMed] [Google Scholar]
- 67.Ohshima T., Ward J.M., Huh C.G., Longenecker G., Veeranna, Pant H.C. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc Natl Acad Sci USA. 1996;93:11173–11178. doi: 10.1073/pnas.93.20.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gilmore E.C., Ohshima T., Goffinet A.M., Kulkarni A.B., Herrup K. Cyclin-dependent kinase 5-deficient mice demonstrate novel developmental arrest in cerebral cortex. J Neurosci. 1998;18:6370–6377. doi: 10.1523/JNEUROSCI.18-16-06370.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garda A.L., Puelles L., Rubenstein J.L., Medina L. Expression patterns of Wnt8b and Wnt7b in the chicken embryonic brain suggest a correlation with forebrain patterning centers and morphogenesis. Neuroscience. 2002;113:689–698. doi: 10.1016/s0306-4522(02)00171-9. [DOI] [PubMed] [Google Scholar]
- 70.Cabrera-Socorro A., Hernandez-Acosta N.C., Gonzalez-Gomez M., Meyer G. Comparative aspects of p73 and Reelin expression in Cajal–Retzius cells and the cortical hem in lizard, mouse and human. Brain Res. 2007;1132:59–70. doi: 10.1016/j.brainres.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 71.Smith-Fernandez A.S., Pieau C., Reperant J., Boncinelli E., Wassef M. Expression of the Emx-1 and Dlx-1 homeobox genes define three molecularly distinct domains in the telencephalon of mouse, chick, turtle and frog embryos: implications for the evolution of telencephalic subdivisions in amniotes. Development. 1998;125:2099–2111. doi: 10.1242/dev.125.11.2099. [DOI] [PubMed] [Google Scholar]
- 72.Moreno N., Bachy I., Rétaux S., González A. LIM-homeodomain genes as developmental and adult genetic markers of Xenopus forebrain functional subdivisions. J Comp Neurol. 2004;472:52–72. doi: 10.1002/cne.20046. [DOI] [PubMed] [Google Scholar]
- 73.Brox A., Puelles L., Ferreiro B., Medina L. Expression of the genes Emx1, Tbr1, and Eomes (Tbr2) in the telencephalon of Xenopus laevis confirms the existence of a ventral pallial division in all tetrapods. J Comp Neurol. 2004;474:562–577. doi: 10.1002/cne.20152. [DOI] [PubMed] [Google Scholar]
- 74.Martinez-Garcia F., Olucha F.E., Teruel V., Lorente M.J., Schwerdtfeger W.K. Afferent and efferent connections of the olfactory bulbs in the lizard Podarcis hispanica. J Comp Neurol. 1991;305:337–347. doi: 10.1002/cne.903050214. [DOI] [PubMed] [Google Scholar]