Abstract
Perioperative visual loss (POVL), a rare, but devastating complication, can follow non-ocular surgery. Highest rates of visual loss are with cardiac and spine surgery. The main causes of visual loss after non-ocular surgery are retinal vascular occlusion and ischaemic optic neuropathy. This review updates readers on the incidence, suspected risk factors, diagnosis, and treatment of POVL due to these conditions.
Keywords: complications, neurological; complications, neuropathy; eye, intraocular pressure; eye, pupil; surgery, spinal
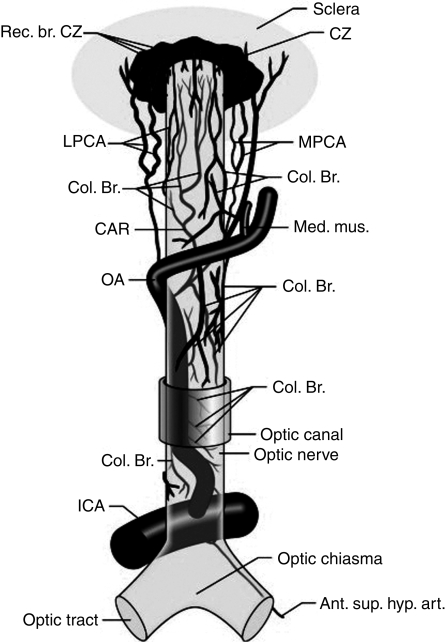
Visual loss after anaesthesia and surgery is a rare, unexpected, and devastating complication. Clinicians have suspected that incidence is increasing, particularly with spinal fusion surgery; however, recent data suggest that this may not be the case. This review will update readers on the incidence, suspected risk factors, diagnosis, and treatment of perioperative visual loss (POVL), in the setting of non-ocular surgery. The causes of POVL are primarily retinal vascular occlusion (RVO) and ischaemic optic neuropathy (ION). The relevant anatomy of the ocular circulation has been reviewed elsewhere and is summarized in Figure 1.80 Other sources are glycine toxicity during transurethral resection of the prostate, acute angle closure glaucoma, cortical blindness, and expansion of an intraocular sulphur hexafluoride vitrectomy bubble by administration of nitrous oxide. As these are less common, the reader is referred for details to a recent more inclusive review.80
Fig 1.
Origin, course, and branches of the ophthalmic artery, including the posterior ciliary arteries, seen from above. Ant. sup. hyp. art., anterior superior hypophyseal artery; CAR, central retinal artery; Col. Br., collateral branches; CZ, circle of Zinn and Haller; ICA, internal carotid artery; LPCA, lateral posterior ciliary artery; MPCA, medial posterior ciliary artery; OA, ophthalmic artery; Rec. br., recurring branches. Figure reproduced with permission from Hayreh SS. ‘The central artery of the retina. Its role in the blood supply of the optic nerve.’ Br J Ophthalmol 1963; 47: 651–63 (later reproduced in Pillanut and colleagues77).
Occurrence of POVL
Perioperative ION is rare, occurring, overall, in ∼1/60 000–1/125 000 anaesthetics.84,97 But most reports in the literature are case reports and small case series. Recently, Shen and colleagues examined the POVL prevalence in the US Nationwide Inpatient Sample (NIS) in the eight most commonly performed surgical procedures, excluding obstetrics and gynaecological surgery, the largest patient population studied to date. The highest frequencies were: spine (3.09 per 10 000, 0.03%) and cardiac surgery (8.64 per 10 000, 0.086%). In contrast, it was 0.12 per 10 000 after appendectomy. The yearly rates of POVL in the procedures studied by Shen and colleagues89 have been decreasing in the 10-yr period from 1996 to 2005. Patil and colleagues74 found an overall rate of 0.094% in spine surgery discharges in the NIS. In a previous, smaller case series, Stevens and colleagues93 found four ION cases in 3450 spine surgeries (0.1%), and two cases in 3300 patients after spine surgery were reported at another hospital (0.06%).81 Chang and Miller19 reviewed 14 102 spine surgery procedures in one hospital, identifying four ION cases (0.028%). After cardiac surgery, incidence was estimated as high as 1.3% in one study,88 but 0.06% and 0.113% in two more recent larger retrospective studies.52,72
Types of POVL
Retinal ischaemia: branch and central retinal artery occlusion
Central retinal artery occlusion (CRAO) decreases blood supply to the entire retina, whereas branch retinal arterial occlusion (BRAO) affects a portion. These most commonly result in the perioperative period from improper patient positioning and external compression of the eye. Pressure within the orbit can also be increased internally after retrobulbar haemorrhage, associated with vascular injuries during sinus or nasal surgery. Retinal microemboli, common during open-heart surgery, constitute another potential cause.10,11 Hypotension seems to be a rare cause of retinal ischaemia; the incidence was three cases in 27 930 deliberate hypotensive anaesthetic procedures.63
Central retinal artery occlusion
Improper head positioning or its unintended movement when on a horseshoe headrest is a hazard that can place the eye in contact with the headrest. In a review of published cases of CRAO after spine surgery, Kumar and colleagues57 described signs and symptoms that included unilateral vision loss; no light perception; afferent pupil defect; periorbital, eyelid oedema, or both; chemosis; proptosis; ptosis; paraesthesias of the supraorbital region; hazy/cloudy cornea; and corneal abrasion. Loss of eye movements, ecchymosis, or other trauma near the eye was also reported. Most did not have imaging studies, but early orbital computed tomography (CT) or magnetic resonance imaging (MRI) showed proptosis and extraocular muscle swelling in some patients.57 Macular/retinal oedema, cherry red spot, or attenuated retinal vessels were typical. Four patients in another report who sustained external compression had retinal pigmentary alterations, suggesting simultaneous choroidal circulatory ischaemia.17,51
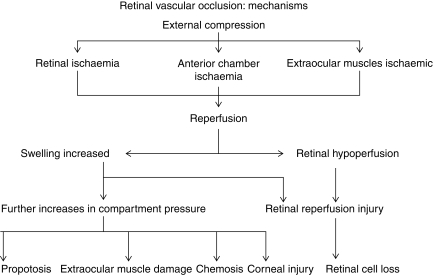
Hollenhorst and colleagues replicated human findings in monkeys by elevating intraocular pressure (IOP) to 200 mm Hg for 60 min together with hypotension. Monkey eyes demonstrated retinal oedema and dilated vascular channels, followed 4 months later by retinal structural damage and optic nerve axonal loss due to retrograde degeneration after death of retinal ganglion cells.49 Bui and colleagues13 documented that during acute increases in IOP in the rat, changes in visual function assessed by electroretinography progressed from inner to outer retina (i.e. retinal ganglion cells) were the most sensitive. In monkeys, Hayreh and Weingast44 found that irreversible retinal damage followed ∼100 min of ischaemia. However, these studies surgically occluded the central retinal artery; RAO caused by external compression and increased IOP may produce a greater degree of ischaemia and correspondingly shorter retinal survival times. In rodents, when the retinal artery was occluded by increasing IOP, periods of retinal ischaemia as short as 20 min have produced damage.28 Mechanisms of injury with increased IOP from external compression are summarized in Figure 2.
Fig 2.
Mechanisms of retinal ischaemia. Modified with permission from Roth.80
Correct use of modern head-positioning devices such as square or circular foam headrests with cutouts for the eyes and also a mirror to view the eyes (i.e. Prone View, Dupaco, Inc., Oceanside, CA, USA) should prevent compression. But, unilateral RAO was described in a prone-positioned patient with his head in a square foam headrest and goggles covering the eyes. The goggles apparently moved and compressed one eye.85
Ischaemic ocular compartment syndrome, more typically found with retrobulbar haemorrhage after nasal sinus surgery,40,47 was reported in a patient undergoing spine surgery in the prone position, believed to be related to positioning with direct pressure on the eye.62 It is an acute ophthalmological emergency, requiring prompt decompression to relieve the increased IOP. The patient's head had been positioned on a silicone headrest for 8 h during which he received only 1 litre of crystalloids. After operation, he had unilateral ocular pain, no light perception in the eye, facial oedema, 4 mm left proptosis, and a tight orbit. There was corneal oedema with a large abrasion, mid-dilated and fixed pupil, advanced cataract, pale optic nerve, retinal haemorrhages, and loss of eye movements. This syndrome is not the same as ION after spine surgery but may be similar to early descriptions of ocular compression by Hollenhorst and colleagues.49 CRAO has also been reported after neck and nasal/sinus surgery.75 BRAO has resulted from direct injection into the local arterial circulation of various drugs into the head and neck region.16
Branch retinal artery occlusion
BRAO is mainly due to emboli or vasospasm. Retinal fluorescein angiography reveals microemboli during cardiopulmonary bypass. In animal studies, mechanisms include non-perfusion, vascular leakage and spasm, red blood cell sludging, and haemorrhage, blocked by perfluorocarbon priming.46 BRAO usually leads to permanent partial visual field loss.
Prognosis and treatment
Prognosis is poor and treatment is generally inadequate. There are some treatment strategies that can be attempted. Ocular massage can lower IOP and possibly dislodge an embolus, if present, to more peripheral arterial branches, and can be instituted by the anaesthesiologist.103 I.V. acetazolamide can be administered to increase retinal blood flow, and 5% CO2 in oxygen inhaled to enhance dilation and increase O2 delivery.2 A preliminary study showed that fibrinolysis via the ophthalmic artery within 6–8 h after spontaneous CRAO was associated with improved visual outcome. An ongoing multicentre study is testing efficacy.30 Localized application of hypothermia to the affected eye is a simple technique that decreased injury in animal studies after ischaemia,29 and it is probably reasonable to institute in humans because of minimal risk.
Prevention
In patients positioned prone for surgery, a foam headrest should be used. Placing head in pins is an obviously more invasive technique. Ensure that eyes are properly positioned and checked intermittently by palpation or visualization. Of course, documentation on the record of eye checks will assist in verifying that anaesthesia personnel followed correct procedures should an issue arise. For cervical spine surgery with the patient in prone position, the horseshoe headrest should not be used because of the greater risk of head movement caused by the surgeon, with compression of the eye.
No data from human studies established the exact frequency of eye checks when patient is prone. On the basis of results in rodent models of elevated IOP, it is advisable to examine eyes for position change and absence of external compression at least every 20 min. For most procedures in which the patient is prone, it is reasonable to use any of the commercially available square foam headrests. It is not advisable to cover the eyes with goggles when the head is positioned prone in a square foam headrest. With a transparent surgical table head piece, a mirror can enable indirect viewing of the eyes. A foam headrest with a mirror attachment allows eyes to be viewed easily during surgery.
Ischaemic optic neuropathy
ION has been reported after a wide variety of surgical procedures, mostly in adults, less often in children,55 with most cases after cardiothoracic surgery,91,95 instrumented spinal fusion operations,20,48,61,69,70 head and neck surgery,56,87 and nose or sinus surgery.15,64 However, a recent study using the NIS found that patients <18 yr of age had the highest risk of POVL, which appears to be mostly due to higher risk of cortical blindness.89 The reasons for this disconcerting finding are not clear. There are also reports concerning vascular surgery, general surgery and urology operations, Caesarean section and gynaecologic surgery, and liposuction.12,22,39,66,97,98,102
Lack of controlled studies, absence of an animal model, and poorly defined pathology and risk factors limit our understanding. Specific mechanism and location of the vascular insult remain uncertain.5 A rodent model for anterior ION (AION) has been described; its relevance to perioperative ION is not known.9 Most knowledge is from individual case reports and case collections; the largest and best described single series is the ASA Postoperative Visual Loss Registry.61 There has been one case–control study in spine surgery patients,70 two in cardiac surgery,72,88 and one that included patients undergoing a variety of surgical procedures. A summary of individual case reports from 1968 to 2002 is available elsewhere.80 Case series and reviews have been published involving all types of surgery,33,86 and case–control studies50 in spine surgery (case series;20,48,61 case–control study70), cardiac surgery (case series;52 case–control72,88), and in trauma patients.24
Patient characteristics
Most cases occurring after spine surgery are posterior ION (PION) and usually bilateral.48,61,101 AION has been more frequently reported after cardiac surgery. Visual loss was typically within the first 24–48 h, frequently noted when the patient awoke after surgery. Later onset has been described, particularly in sedated patients who remained mechanically ventilated after operation.48,61,80 There is usually painless visual loss, afferent pupil defect or non-reactive pupil, no light perception, or visual field deficit. Colour vision is decreased or absent. In AION, altitudinal visual field deficit may be present. AION typically has optic disc oedema and haemorrhages upon symptom onset; in PION, the optic disc appears normal despite visual loss. Optic atrophy develops over subsequent weeks to months. MRI frequently shows no abnormalities,78 although some reports described nerve enlargement or perineural enhancement suggestive of oedema.96 Visual evoked potentials are abnormal.31
Possible pathogenic factors
Possible factors are: prone positioning, lengthy spinal fusion surgery, decreased arterial pressure, blood loss, anaemia or haemodilution, change in venous haemodynamics, cerebrospinal fluid flow in the optic nerve (including effects of patient positioning and perioperative fluid resuscitation), abnormal optic nerve blood flow autoregulation, optic nerve blood supply anatomic variation, low cup-to-disc ratio, use of vasopressors, systemic vascular risk factors including hypertension, diabetes, atherosclerosis, hyperlipidaemia, smoking, sleep-apnoea syndrome, and hypercoaguability.14,37,67,80,101 One or more risk factors are often present in a patient in an unpredictable fashion, with some degree of hypotension or anaemia and fluid resuscitation. Many patients with ION after spine surgery have been relatively healthy before operation. Hypotension, blood loss, lengthy surgery, and large amounts of fluid administration seem to occur frequently in many patients undergoing complex spine surgery.14,20,48,70,86 Possibly, combinations of these factors, perhaps together with abnormal autoregulation in the posterior optic nerve, prothrombotic tendencies, and other patient-specific factors, lead to decreased oxygen delivery to the optic nerve sufficient to cause ischaemic injury. Possible roles of most of these factors will be discussed below.
Prone positioning
IOP increased in prone position and was influenced by operating table position (head up or down). But correlation between perioperative IOP and visual outcome or visual function changes has not been reported.21,73 In anaesthetized patients, IOP increased on initial prone positioning relative to supine [27 (2) vs 13 (1) mm Hg].21 After 5 h, IOP remained elevated to 40 (2) mm Hg. Time-dependent IOP increase has also been reported in laparoscopic radical prostatectomy with patients in steep head down position (case reports of ION have recently appeared concerning this surgery as well).7,98 Although these data suggest that ocular perfusion pressure might decline even during normotension, results should be considered in the context that the largest IOP increases were near the time patients were awakening. Moreover, there was no supine group to control for the effects of fluid administration. These results are valuable and they are concerning, but further studies are needed to fully evaluate their significance. It is advisable to position a patient's head level or above the heart, where possible, and in a neutral position during spine surgery performed with the patient in the prone position.
External pressure on the eye
Many cases of ION have been reported in which it was impossible for pressure to have been placed on the eye, including with the head in pin head holders20,59 or where the head was turned and the affected eye upward.82 Although we showed that high IOP decreased retinal and choroidal blood flows in cats,83 Geijer and Bill35 specifically measured the impact of graded IOP increases on blood flow in the retina and optic nerve in monkeys. When IOP increased but calculated perfusion pressure was >40 cm H2O, there were small effects upon retinal blood flow and in the prelaminar optic nerve. But with calculated perfusion pressure <40 cm H2O, retinal and prelaminar flows were proportional to the perfusion pressure. At very high IOP, blood flow stopped in the retina and in the prelaminar area, but flow in the retrolaminar region increased. Results indicate that high IOP redistributes blood flow preferentially to the retrolaminar optic nerve. Therefore, increased IOP would not result in ION without also causing retinal damage. Further support is that sustained increases in IOP significantly decreased both retinal and choroidal blood flows, and even small increases in IOP damaged the retinal ganglion cells, which are sensitive to pressure alterations.13,83
Length of surgery and hypotension
Surgery was longer in patients with postoperative blindness after spine surgery vs unaffected patients.4,70 Intraoperative hypotension is not always present in patients with ION, and because some degree of hypotension frequently occurs in anaesthetized patients, hypotension itself might not be responsible1,26,59,88 but is mentioned in case reports.12,53 Hypotension may play a role either because of anatomic circulatory variation or abnormal autoregulation and an inability to adequately compensate for decreased perfusion pressure. There is a lack of data in the literature to quantify what level of hypotension is potentially dangerous.4
In a survey of 24 cases of CRAO and ION after spine surgery, deliberate hypotension was used in only two.20 In a retrospective case–control study of patients undergoing spinal fusion, levels of hypotension and anaemia were equivalent in patients who developed ION after spinal surgery compared with those who did not.70 Holy and colleagues50 showed similar results in a mixed patient population in a large single-institution study. ION patients in the ASA Postoperative Visual Loss Registry, the largest series after spine surgery, showed wide variation in arterial pressure decreases intraoperatively; lowest systolic arterial pressures were >90 mm Hg in 33% of the patients, whereas in 20%, lowest systolic arterial pressures were ≤80 mm Hg. About 57% of the patients had systolic or mean arterial pressure 20–39% below baseline, and 40–49% below preoperative baseline in 25% of the patients. Deliberate hypotension was used in 27%.61 Patil and colleagues74 reported a higher odds ratio for visual loss in spine surgery patients with a discharge code for ‘hypotension’ using administrative data from the NIS. However, NIS data do not reveal the level of hypotension, nor its timing, that is, whether it was present intraoperatively or after operation. Important considerations and limitations when using these types of databases are discussed elsewhere.89,90
In a retrospective single-institution case–control study of 602 open-heart surgery patients over 2 yr, lowest systemic perfusion pressure intraoperatively was no different between affected and normal patients.88 A more recent series by Holy and colleagues50 showed similar results, but their series included other surgical procedures which complicates interpretation of the specific results with cardiac surgery. Nuttall and colleagues72 in a case–control study did not report arterial pressures during cardiopulmonary bypass. There were no differences in pre- or post-bypass arterial pressures between patients with ION and unaffected patients. In open-heart surgery, other factors such as hypothermia and systemic inflammatory response syndrome may be involved in ION, but these mechanisms have not been studied.
Blood loss
With uncontrolled haemorrhage where blood volume is not maintained, decreased oxygen delivery to the optic nerve could result in either AION or PION.42 Just how low or for how long the haemoglobin concentration must decrease to lead to this complication is not known. Myers and colleagues’ study reported higher intraoperative blood loss in patients with visual loss after spine surgery compared with those without. They determined that lowest levels of haematocrit did not differ between patients with ION and those unaffected.70 A similar result has been reported by Holy and colleagues.50 Nuttall and colleagues72 found a somewhat different result in cardiac surgery. Lower minimum postoperative haemoglobin associated weakly with ION (odds ratio, 1.9; P<0.047). Also, 13 of the 17 patients with ION had minimum postoperative haemoglobin <8.5 g dl−1 compared with 14 of the 34 controls. The type of ION was not specified, and the sample size was small with numerous statistical comparisons. Spine and open-heart surgery procedures and patients differ, so that it is doubtful that the results can be extended to surgery other than cardiac. Some authors have suggested that allowing haemoglobin to decrease, common in anaesthesia practice, may be putting patients at increased risk;12,101 however, whether practice should be changed in surgical procedures such as spine or heart surgery—or in any operative procedure—remains controversial. Perioperative blood transfusion practice, based on ASA practice guidelines,3 suggests that transfusion is not generally required for haemoglobin values >8.0 g dl−1. The Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists, after reviewing the available evidence base for these practices in cardiac surgery, have issued a recent, similar clinical practice guideline.32 POVL is not mentioned in either guideline.
Haemodilution
Haemodilution is commonly present in surgical patients due to current transfusion practices.3 There have been studies of its impact upon the optic nerve in animals. Blood flow in the optic nerve head in minipigs, measured by laser Doppler, was maintained with isovolumic haemodilution (30% decrease in haematocrit). Vitreal surface oxygen tension increased 15%.18 Haemodilution in cats caused a non-significant decrease in choroidal oxygen delivery79 and an increase in pre-retinal oxygen tension.71 However, simultaneously decreasing haematocrit to 17% and mean arterial pressure to 50–55 mm Hg profoundly decreased calculated O2 delivery to the entire optic nerve in adult pigs.58 These results are disconcerting and suggest the need for caution with extreme haemodilution and hypotension in patients, but the study did not assess function in the optic nerve or retina, did not measure regional optic nerve blood flow, and did not describe anatomical findings. The pig might not be an optimal model because the blood supply to the optic nerve is from the external carotid artery.
There are no clinical studies examining the optic nerve, but in healthy volunteers very deep levels of haemodilution (haemoglobin, 50 g litre−1) were tolerated without any disturbance in systemic O2 delivery,100 and in a multicentre prospective study, allowing a lower haematocrit in critically ill patients did not worsen outcomes.45 It is not known whether any of these results can be extended to patients undergoing surgery such as spine surgery.
Fluid replacement
ION has been reported in the setting of massive fluid replacement, and many reports include patients operated upon in the prone position, raising the possibility that positioning contributes to altered venous haemodynamics within the optic nerve. Trauma patients with visual loss after massive blood replacement have been reported.24 Analysis is complicated because of numerous systemic alterations. Four burn patients with ≥25% body surface area burns and massive fluid resuscitation had IOP >30 mm Hg 48 h after admission, all of whom received >27 litre of i.v. fluid. Eye findings and vision diagnoses were not described.94 Large fluid replacement is generally seen in many case reports of ION.80 Patients in the ASA Postoperative Visual Loss Registry received an average of 9.7 litre of crystalloid intraoperatively,61 and increased postoperative weight gain was identified in a case–control study of visual loss after heart surgery,88 suggesting, although not proving, that fluid replacement might play a role.
Fluid resuscitation is necessary during lengthy, complex spine surgery where there can be substantial blood and fluid requirements from operative site losses.1,26 Because the central retinal vein exits out of the optic nerve, an internal ‘compartment syndrome’ may occur in the optic nerve. Alternatively, fluid accumulation in the vicinity of the lamina cribrosa might compress axons. Further support that increases in venous pressure within the optic nerve are potentially deleterious can be seen in reports of ION after head and neck surgery in which the internal jugular veins were ligated.56,65,87 However, head and neck venous drainage bypass pathways are available when internal jugular veins are ligated. Some have speculated that facial oedema indicates ION risk after spine surgery.27 However, facial oedema is often seen after spinal surgery in many patients who do not develop ION, and therefore, its relevance remains unclear, and the notion that facial oedema is a risk factor is unproven.
Cerebrospinal fluid (CSF) was sampled from the optic nerve subarachnoid space and the lumbar region in patients with idiopathic intracranial hypertension undergoing optic nerve sheath fenestration.54 Authors performed MRI and CT-cisternography and compared albumin, IgG, β-trace protein, and brain-derived protein lipocalin-like prostaglandin D-synthase content. From differences in ratios of CSF proteins in optic nerve vs the lumbar region, and cisternography, they concluded that CSF flow compartmentalized in the optic nerve under these pathological conditions. The implication is that relatively high CSF pressures in the intra-orbital optic nerve could result in compression. However, this theory has not been tested in patients undergoing fluid resuscitation.
Although these theories concerning the role of intraoperative fluids in perioperative ION have been postulated, none has been examined in either animal or human studies. Orbital MRI in ION patients has not provided support for any of these theories.78 No study has shown a relationship between periorbital oedema, IOP, and ION. Fluid administration could be a pathogenic factor in ION, especially in patients positioned prone or undergoing cardiac surgery, but the mechanisms involved, and also amounts and nature of fluid required, remain undefined.
Some types of ‘anatomic variation’ in the circulation of the optic nerve might predispose to ION. Potential watershed zones in the anterior and posterior circulation and the presence of disturbed autoregulation in normal patients76 cannot be predicted clinically. Human studies generally show preserved optic nerve blood flow to even lower ranges of perfusion pressure, but these studies are primarily of the anterior optic nerve.68,76 In animal studies, blood flow was preserved in various layers of the optic nerve, including the retrolaminar area, at a mean arterial pressure as low as 40 mm Hg.99
A small optic disc (low ‘cup-to-disc ratio’) might increase AION, but not PION, susceptibility8 because axons in the optic nerve pass through a narrower opening. Mechanisms of injury with a crowded disc include axoplasmic flow mechanical obstruction or stasis, stiff cribriform plate, and decreased neurotrophic factor delivery to retinal ganglion cells after ischaemia.5
Vasopressors
Shapira and colleagues88 showed an association between prolonged epinephrine infusions or long bypass time and ION in patients undergoing open-heart surgery. Lee and Lam59 reported ION after lumbar spine fusion during which phenylephrine infusion maintained arterial pressure. ION was reported in four critically ill patients with significant systemic illness on prolonged vasopressor and inotropic agent infusions to maintain arterial pressure and cardiac output.60 α-Adrenergic receptors are not known to be located in the optic nerve, and the blood–brain barrier prevents entry of systemically administered agents, except possibly in the prelaminar zone. Therefore, a role of vasopressors remains unclear.
High arterial pressure, diabetes mellitus, and coronary artery or cerebrovascular disease
High arterial pressure, diabetes mellitus, and coronary artery or cerebrovascular disease have been cited in case reports but are not present in all patients in whom ION has developed. Hypertension and coronary artery disease are nearly always found in patients undergoing coronary artery bypass grafting, but ION develops in few. Case–control data did not show association of these factors with ION in spine surgery patients and in a larger surgical population. However, Patil and colleagues74 reported an increased odds ratio of ION in spine surgery patients with a discharge code in NIS of peripheral vascular disease or diabetes mellitus. A case series showed that many perioperative PION patients have few vascular disease risk factors.86 Holy and colleagues50 did not find association of perioperative ION and hypertension, coronary artery disease, diabetes, or stroke. In a prospective study of non-surgical patients, ION was not associated with carotid artery disease.34 Although a basis for the notion that perioperative ION is related to atherosclerosis is that the optic nerve vasculature autoregulates abnormally, this association has not been studied in humans, and animal data are sparse and inconclusive.43 Because of these unknowns, it appears advisable to approach patients undergoing high-risk procedures cautiously.
Erectile dysfunction drugs
AION has been described in patients who used these agents, and cause and effect have been debated.25 Because of possible increased risk, it seems sensible to discontinue the use of these agents a day or two before surgery.
Surgery on the nose and paranasal sinuses
Surgery on the nose and paranasal sinuses has a risk of visual loss. Blindness was reported after endoscopic sinus surgery92 due to direct surgical damage to the optic nerve, or by compression from retrobulbar haematoma and ION. An ocular compartment syndrome may be present, necessitating immediate surgical decompression to preserve vision.64
Prognosis, treatment, and prevention of ION
Buono and Foroozan14 summarized the lack of proof that treatment altered the course of PION. In a few case reports, increasing arterial pressure or haemoglobin, or applying hyperbaric oxygen, improved visual outcome.80 Neuroprotective agents or drugs that lower IOP, valuable in theory, have never been shown to result in improvement.6 Apparent improvement of vision occurred in two patients with ION after spine surgery with correction of anaemia and hypotension. One had partial improvement which subsequently regressed, and the other had more clear improvement.93 However, it is difficult to ascertain if improvement came from treatment because some patients recover vision spontaneously after PION.14
Acetazolamide lowers IOP and may improve flow to the optic nerve head and retina.41 Diuretics such as mannitol or furosemide reduce oedema. Because steroids are of unproven benefit, their use must be carefully weighed. Increasing ocular perfusion pressure or haemoglobin concentration may be appropriate when ION is in conjunction with significant decreases in arterial pressure and haemoglobin (Table 1). Maintaining head-up position if increased ocular venous pressure is suspected may be advantageous. But its use has to be balanced against decreased arterial supply with the head up.
Table 1.
Recommendations of the ASA Task Force on Perioperative Blindness
| The consensus of the Task Force is that a high-risk patient's vision should be assessed when the patient becomes alert (e.g. in the recovery room, intensive care unit, or nursing floor). If there is concern regarding potential visual loss, an urgent ophthalmologic consultation should be obtained to determine its cause. Additional management may include optimizing haemoglobin or haematocrit levels, haemodynamic status, and arterial oxygenation. To rule out intracranial causes of visual loss, consider magnetic resonance imaging. The Task Force believes that there is no role for antiplatelet agents, steroids, or intraocular pressure-lowering agents in the treatment of perioperative ischaemic optic neuropathy |
| There is a subset of patients who undergo spine procedures while they are positioned prone and receiving general anaesthesia who have an increased risk for the development of perioperative visual loss. This subset includes patients who are anticipated before operation to undergo procedures that are prolonged, have substantial blood loss, or both (high-risk patients) |
| Consider informing high-risk patients that there is a small, unpredictable risk of perioperative visual loss |
| The use of deliberate hypotensive techniques during spine surgery has not been shown to be associated with the development of perioperative visual loss |
| Colloids should be used along with crystalloids to maintain intravascular volume in patients who have substantial blood loss |
| At this time, there is no apparent transfusion threshold that would eliminate the risk of perioperative visual loss related to anaemia |
| High-risk patients should be positioned with the head level with or higher than the heart when possible. In addition, the head should be maintained in a neutral forward position (e.g. without significant neck flexion, extension, lateral flexion, or rotation) when possible |
| Consideration should be given to the use of staged spine procedures in high-risk patients |
| Available from http://www.asahq.org/publicationsAndServices/BlindnessAdvisoryFinal.pdf |
Some general recommendations could be made for spine surgery, where risk of ION is higher, but whether any of these can prevent it remains unknown. The head should be in neutral position relative to the back; head down is discouraged. In patients with poorly controlled hypertension or significant or suspected atherosclerosis, maintaining systemic arterial pressure seems sensible. Where to keep arterial pressure should be weighed against possible surgical needs for deliberate hypotension to decrease blood loss and maintain adequate operative exposure. In cardiac surgery, many factors are involved in arriving at the optimal systemic perfusion pressures during cardiopulmonary bypass. Arterial pressure management is only one component in care of anaesthetized patients, where the focus is on the entire patient and not only the optic nerve. Hypertensive patients treated with angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, often together with β-blockers or calcium channel blockers, are commonly encountered. These patients frequently become hypotensive intraoperatively and might require vasopressors.23 Systemic risks of increasing arterial pressure with vasoactive agents or by infusion of i.v. fluids include serious complications of decreased renal, liver, and intestinal perfusion, congestive heart failure, and myocardial ischaemia. They are important considerations in determining the appropriate range for arterial pressure and fluid resuscitation.36
Although scientific evidence to guide practice with respect to level of haematocrit intraoperatively is incomplete, and bearing in mind that haematocrit determinations intraoperatively may not be exact, anaesthesiologists are advised to be cautious during simultaneous deliberate hypotension and haemodilution to haematocrit <25%. The effect of haemodilution will often be a large fluid resuscitation. Particularly in re-operation for spinal fusion, the expectation is that more fluid will be required. A relatively ‘dry’ fluid strategy has only been tested in abdominal surgery.38 Using the same practice in spine surgery might be hazardous. More reliance upon colloids might decrease the volume of crystalloid, although the impact upon occurrence of visual loss is not known. Earlier blood transfusion can decrease the use of i.v. fluid, but of course carries its own set of considerations and risks.
Staging complex spine procedures is another potential strategy. In some instances, the anaesthesiologist and surgeon might agree to follow a less ambitious plan. To decide if justified, it would be necessary to assess associated risks of multiple surgeries (such as infection and unstable spine). A summary of recommendations of the ASA Task Force on Perioperative Blindness that is applicable to patients undergoing spine fusion surgery appears in Table 1.4
Whether patients should be informed of ION, especially those undergoing higher-risk cardiac surgery and complex instrumented spinal fusion surgery, is controversial. The Task Force recommendation is to consider informing those undergoing prolonged spine fusion surgery with large anticipated blood loss.4 Because most patients are first seen by anaesthesiologists soon before surgery, consider requesting that the surgeon discuss the possible complication at an earlier, more relaxed, preoperative visit.
In summary, much is unknown about the pathogenesis of perioperative ION. Possible strategies the anaesthesiologist might consider using in the perioperative period have been outlined, although there is no scientific evidence at the present time that any of these interventions can prevent or treat perioperative ION. RVO, particularly when it occurs in a prone-positioned patient, is a preventable complication in most instances related to head positioning and external compression of the eyes during surgery.
Funding
S.R.'s research on visual loss has been supported by National Institutes of Health (Rockville, MD, USA) grant EY10343, and by grants from the Brain Research Foundation (Chicago, IL, USA) and the Illinois Society for the Prevention of Blindness (Chicago, IL, USA).
References
- 1.Alexandrakis G, Lam BL. Bilateral posterior ischaemic optic neuropathy after spinal surgery. Am J Ophthalmol. 1999;127:354–5. doi: 10.1016/s0002-9394(98)00343-2. [DOI] [PubMed] [Google Scholar]
- 2.Alm A, Bill A. Ocular circulation. In: Moses A, Hart C, editors. Adler's Physiology of the Eye. St Louis: CV Mosby; 1987. pp. 183–203. [Google Scholar]
- 3.Anonymous. Practice Guidelines for blood component therapy: a report by the American Society of Anaesthesiogists Task Force on Blood Component Therapy. Anesthesiology. 1996;84:732–47. [PubMed] [Google Scholar]
- 4.Arens JF, Connis RT, Domino KB, et al. Practice advisory for perioperative visual loss associated with spine surgery. A report by the American Society of Anaesthesiologists Task Force on Perioperative Blindness. Anesthesiology. 2006;104:1319–28. doi: 10.1097/00000542-200606000-00027. [DOI] [PubMed] [Google Scholar]
- 5.Arnold AC. Pathogenesis of nonarteritic anterior ischaemic optic neuropathy. J Neuroophthalmol. 2003;23:157–63. doi: 10.1097/00041327-200306000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Arnold AC, Levin LA. Treatment of ischaemic optic neuropathy. Semin Ophthalmol. 2002;17:39–46. doi: 10.1076/soph.17.1.39.10292. [DOI] [PubMed] [Google Scholar]
- 7.Awad H, Santilli S, Ohr M, et al. The effects of steep trendelenburg positioning on intraocular pressure during robotic radical prostatectomy. Anesth Analg. 2009;109:473–8. doi: 10.1213/ane.0b013e3181a9098f. [DOI] [PubMed] [Google Scholar]
- 8.Beck RW, Servais GE, Hayreh SS. Anterior ischaemic optic neuropathy. IX. Cup-to-disc ratio and its role in pathogenesis. Ophthalmology. 1987;94:1503–8. [PubMed] [Google Scholar]
- 9.Bernstein SL, Guo Y, Kelman SE, Flower RW, Johnson MA. Functional and cellular responses in a novel rodent model of anterior ischaemic optic neuropathy. Invest Ophthalmol Vis Sci. 2003;44:4153–62. doi: 10.1167/iovs.03-0274. [DOI] [PubMed] [Google Scholar]
- 10.Blauth CI, Arnold JV, Schulenberg WE, McCartney AC, Taylor KM. Cerebral microembolism during cardiopulmonary bypass. Retinal microvascular studies in vivo with fluorescein angiography. J Thorac Cardiovasc Surg. 1988;95:668–76. [PubMed] [Google Scholar]
- 11.Blauth CI, Smith PL, Arnold JV, Jagoe JR, Wootton R, Taylor KM. Influence of oxygenator type on the prevalence and extent of microembolic retinal ischaemia during cardiopulmonary bypass. Assessment by digital image analysis. J Thorac Cardiovasc Surg. 1990;99:61–9. [PubMed] [Google Scholar]
- 12.Brown RH, Schauble JF, Miller NR. Anaemia and hypotension as contributors to perioperative loss of vision. Anesthesiology. 1994;80:222–6. doi: 10.1097/00000542-199401000-00033. [DOI] [PubMed] [Google Scholar]
- 13.Bui BV, Edmunds B, Cioffi GA, Fortune B. The gradient of retinal functional changes during acute intraocular pressure elevation. Invest Ophthalmol Vis Sci. 2005;46:202–13. doi: 10.1167/iovs.04-0421. [DOI] [PubMed] [Google Scholar]
- 14.Buono LM, Foroozan R. Perioperative posterior ischaemic optic neuropathy: review of the literature. Surv Ophthalmol. 2005;50:15–26. doi: 10.1016/j.survophthal.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Buus DR, Tse DT, Farris BK. Ophthalmic complications of sinus surgery. Ophthalmology. 1990;97:612–9. doi: 10.1016/s0161-6420(90)32535-6. [DOI] [PubMed] [Google Scholar]
- 16.Byers B. Blindness secondary to steroid injections into the nasal turbinates. Arch Ophthalmol. 1979;97:79–80. doi: 10.1001/archopht.1979.01020010019004. [DOI] [PubMed] [Google Scholar]
- 17.Carr RE, Siegel IM. Unilateral retinitis pigmentosa. Arch Ophthalmol. 1973;90:21–6. doi: 10.1001/archopht.1973.01000050023005. [DOI] [PubMed] [Google Scholar]
- 18.Chamot SR, Petrig BL, Pournaras CJ, Riva CE. Effect of isovolumic haemodilution on oxygen delivery to the optic nerve head. Klin Monatsbl Augenheilkd. 2002;219:292–5. doi: 10.1055/s-2002-30656. [DOI] [PubMed] [Google Scholar]
- 19.Chang SH, Miller NR. The incidence of vision loss due to perioperative ischaemic optic neuropathy associated with spine surgery—The Johns Hopkins hospital experience. Spine. 2005;30:1299–302. doi: 10.1097/01.brs.0000163884.11476.25. [DOI] [PubMed] [Google Scholar]
- 20.Cheng MA, Sigurdson W, Tempelhoff R, Lauryssen C. Visual loss after spine surgery: a survey. Neurosurgery. 2000;46:625–31. doi: 10.1097/00006123-200003000-00020. [DOI] [PubMed] [Google Scholar]
- 21.Cheng MA, Todorov A, Tempelhoff R, McHugh T, Crowder CM, Lauryssen C. The effect of prone positioning on intraocular pressure in anesthetized patients. Anesthesiology. 2001;95:1351–5. doi: 10.1097/00000542-200112000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Chun DB, Levin DK. Ischaemic optic neuropathy after haemorrhage from a cornual ectopic gestation. Am J Obstet Gynecol. 1997;177:1550–2. doi: 10.1016/s0002-9378(97)70111-x. [DOI] [PubMed] [Google Scholar]
- 23.Colson P, Ryckwaert F, Coriat P. Renin angiotensin system antagonists and anaesthesia. Anesth Analg. 1999;89:1143–55. [PubMed] [Google Scholar]
- 24.Cullinane DC, Jenkins JM, Reddy S, et al. Anterior ischaemic optic neuropathy: a complication after systemic inflammatory response syndrome. J Trauma. 2000;48:381–7. doi: 10.1097/00005373-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Danesh-Meyer HV, Levin LA. Erectile dysfunction drugs and risk of anterior ischaemic optic neuropathy: casual or causal association? Br J Ophthalmol. 2007;91:1551–5. doi: 10.1136/bjo.2007.125880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dilger JA, Tetzlaff JE, Bell GR, Kosmorsky GS, Agnor RC, O'Hara JF. Ischaemic optic neuropathy after spinal fusion. Can J Anaesth. 1998;45:63–6. doi: 10.1007/BF03011996. [DOI] [PubMed] [Google Scholar]
- 27.Dunker S, Hsu HY, Sebag J, Sadun AA. Perioperative risk factors for ischaemic optic neuropathy. J Am Coll Surg. 2002;194:705–10. doi: 10.1016/s1072-7515(02)01210-3. [DOI] [PubMed] [Google Scholar]
- 28.Ettaiche M, Heurteaux C, Blondeau N, Borsotto M, Tinel N, Lazdunski M. ATP-sensitive potassium channels (K(ATP)) in retina: a key role for delayed ischaemic tolerance. Brain Res. 2001;890:118–29. doi: 10.1016/s0006-8993(00)03152-8. [DOI] [PubMed] [Google Scholar]
- 29.Faberowski N, Stefansson E, Davidson RC. Local hypothermia protects the retina from ischaemia. A quantitative study in the rat. Invest Ophthalmol Vis Sci. 1989;30:2309–13. [PubMed] [Google Scholar]
- 30.Feltgen N, Neubauer A, Jurklies B, et al. Multicenter study of the European Assessment Group for Lysis in the Eye (EAGLE) for the treatment of central retinal artery occlusion: design issues and implications. EAGLE Study report no. 1. Graefes Arch Clin Exp Ophthalmol. 2006;244:950–6. doi: 10.1007/s00417-005-0140-2. [DOI] [PubMed] [Google Scholar]
- 31.Fishman GA, Sokol S. Electrophysiological Testing in Disorders of the Retina, Optic Nerve, and Visual Pathway. San Franciso, CA: American Academy of Ophthalmology; 1990. [Google Scholar]
- 32.Ferraris VA, Ferraris SP, et al. Force SoTSBCGT (Society of Thoracic Surgeons Blood Conservation Task Force) Perioperative blood transfusion and blood conservation in cardiac surgery: the Society of Thoracic Surgeons and The Society of Cardiovascular Anesthesiologists clinical practice guideline. Ann Thorac Surg. 2007;83:S27–86. doi: 10.1016/j.athoracsur.2007.02.099. [DOI] [PubMed] [Google Scholar]
- 33.Foroozan R, Buono LM, Savino PJ. Optic disc structure and shock-induced anterior ischaemic optic neuropathy. Ophthalmology. 2003;110:327–31. doi: 10.1016/S0161-6420(02)01736-0. [DOI] [PubMed] [Google Scholar]
- 34.Fry CL, Carter JE, Kanter MC, Tegeler CH, Tuley MR. Anterior ischaemic optic neuropathy is not associated with carotid artery atherosclerosis. Stroke. 1993;24:539–42. doi: 10.1161/01.str.24.4.539. [DOI] [PubMed] [Google Scholar]
- 35.Geijer C, Bill A. Effects of raised intraocular pressure on retinal, prelaminar, laminar, and retrolaminar optic nerve blood flow in monkeys. Invest Ophthalmol Vis Sci. 1979;18:1030–42. [PubMed] [Google Scholar]
- 36.Gelman S, Mushlin PS. Catecholamine-induced changes in the splanchnic circulation affecting systemic hemodynamics. Anesthesiology. 2004;100:434–9. doi: 10.1097/00000542-200402000-00036. [DOI] [PubMed] [Google Scholar]
- 37.Glueck CJ, Wang PG, Bell H, Rangaraj V, Goldenberg N. Nonarteritic anterior ischaemic optic neuropathy: associations with homozygosity for the C677T methylenetetrahydrofolate reductase mutation. J Lab Clin Med. 2004;143:184–92. doi: 10.1016/j.lab.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 38.Grocott MP, Mythen MG, Gan TJ. Perioperative fluid management and clinical outcomes in adults. Anesth Analg. 2005;100:1093–106. doi: 10.1213/01.ANE.0000148691.33690.AC. [DOI] [PubMed] [Google Scholar]
- 39.Gupta M, Puri P, Rennie IG. Anterior ischaemic optic neuropathy after emergency caesarean section under epidural anaesthesia. Acta Anaesthesiol Scand. 2002;46:751–2. doi: 10.1034/j.1399-6576.2002.460621.x. [DOI] [PubMed] [Google Scholar]
- 40.Haller D, Gosepath J, Mann WJ. The management of acute visual loss after sinus surgery—two cases of rhinogenic optic neuropathy. Rhinology. 2006;44:216–8. [PubMed] [Google Scholar]
- 41.Hayreh SS. Anterior ischaemic optic neuropathy. III. Treatment, prophylaxis, and differential diagnosis. Br J Ophthalmol. 1974;58:981–9. doi: 10.1136/bjo.58.12.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hayreh SS. Anterior ischaemic optic neuropathy. VIII. Clinical features and pathogenesis of post-hemorrhagic amaurosis. Ophthalmology. 1987;94:1488–502. doi: 10.1016/s0161-6420(87)33273-7. [DOI] [PubMed] [Google Scholar]
- 43.Hayreh SS, Bill A, Sperber GO. Effects of high intraocular pressure on the glucose metabolism in the retina and the optic nerve in atheroschlerotic monkeys. Graefes Arch Clin Exp Ophthalmol. 1994;232:745–52. doi: 10.1007/BF00184278. [DOI] [PubMed] [Google Scholar]
- 44.Hayreh SS, Weingast TA. Experimental occlusion of the central artery of the retina. IV. Retinal tolerance time to acute ischaemia. Br J Ophthalmol. 1980;64:818–25. doi: 10.1136/bjo.64.11.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–17. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 46.Herren JI, Kunzelman KS, Vocelka C, Cochran RP, Spiess BD. Angiographic and histological evaluation of porcine retinal vascular damage and protection with perfluorocarbons after massive air embolism. Stroke. 1998;29:2396–403. doi: 10.1161/01.str.29.11.2396. [DOI] [PubMed] [Google Scholar]
- 47.Herzon GD, Zealear DL. Intraoperative monitoring of the visual evoked potential during endoscopic sinus surgery. Otolaryngol Head Neck Surg. 1994;111:575–9. doi: 10.1177/019459989411100507. [DOI] [PubMed] [Google Scholar]
- 48.Ho VTG, Newman NJ, Song S, Ksiazek S, Roth S. Ischaemic optic neuropathy following spine surgery. J Neurosurg Anesthesiol. 2005;17:38–44. [PMC free article] [PubMed] [Google Scholar]
- 49.Hollenhorst RW, Svien HJ, Benoit CF. Unilateral blindness occurring during anaesthesia for neurosurgical operations. Arch Ophthalmol. 1954;52:819–30. doi: 10.1001/archopht.1954.00920050825002. [DOI] [PubMed] [Google Scholar]
- 50.Holy SE, Tsai JH, McAllister RK, Smith KH. Perioperative ischaemic optic neuropathy: a case control analysis of 126,666 procedures at a single institution. Anesthesiology. 2009;110:246–53. doi: 10.1097/ALN.0b013e318194b238. [DOI] [PubMed] [Google Scholar]
- 51.Jampol LM, Goldbaum M, Rosenberg M, Bahr R. Ischaemia of ciliary arterial circulation from ocular compression. Arch Ophthalmol. 1975;93:1311–7. doi: 10.1001/archopht.1975.01010020945003. [DOI] [PubMed] [Google Scholar]
- 52.Kalyani SD, Miller NR, Dong LM, Baumgartner WA, Alejo DE, Gilbert TB. Incidence of and risk factors for perioperative optic neuropathy after cardiac surgery. Ann Thorac Surg. 2004;78:34–7. doi: 10.1016/j.athoracsur.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 53.Katz DM, Trobe JD, Cornblath WT, Kline LB. Ischaemic optic neuropathy after lumbar spine surgery. Arch Ophthalmol. 1994;112:925–31. doi: 10.1001/archopht.1994.01090190073024. [DOI] [PubMed] [Google Scholar]
- 54.Killer HE, Jaggi GP, Flammer J, Miller NR, Huber AR, Mironov A. Cerebrospinal fluid dynamics between the intracranial and the subarachnoid space of the optic nerve. Is it always bidirectional? Brain. 2007;130:514–20. doi: 10.1093/brain/awl324. [DOI] [PubMed] [Google Scholar]
- 55.Kim JW, Hills WL, Rizzo JF, Egan RA, Lessell S. Ischaemic optic neuropathy following spine surgery in a 16-year-old patient and a ten-year-old patient. J Neuroophthalmol. 2006;26:30–3. doi: 10.1097/01.wno.0000205980.32023.2d. [DOI] [PubMed] [Google Scholar]
- 56.Kirkali P, Kansu T. A case of unilateral posterior ischaemic optic neuropathy after radical neck dissection. Ann Ophthalmol. 1990;22:297–8. [PubMed] [Google Scholar]
- 57.Kumar N, Jivan S, Topping N, Morrell AJ. Blindness and rectus muscle damage following spine surgery. Am J Ophthalmol. 2004;138:889–91. doi: 10.1016/j.ajo.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 58.Lee LA, Deem S, Glenny RW, et al. Effects of anaemia and hypotension on porcine optic nerve blood flow and oxygen delivery. Anesthesiology. 2008;108:864–72. doi: 10.1097/ALN.0b013e31816c8a30. [DOI] [PubMed] [Google Scholar]
- 59.Lee LA, Lam AM. Unilateral blindness after prone lumbar surgery. Anesthesiology. 2001;95:793–5. doi: 10.1097/00000542-200109000-00036. [DOI] [PubMed] [Google Scholar]
- 60.Lee LA, Nathens AB, Sires BS, McMurray MK, Lam AM. Blindness in the intensive care unit: possible role for vasopressors? Anesth Analg. 2005;100:192–5. doi: 10.1213/01.ANE.0000139345.85653.AB. [DOI] [PubMed] [Google Scholar]
- 61.Lee LA, Roth S, Posner KL, et al. The American Society of Anaesthesiologists Postoperative Visual Loss Registry: analysis of 93 spine surgery cases with postoperative visual loss. Anesthesiology. 2006;105:652–9. doi: 10.1097/00000542-200610000-00007. [DOI] [PubMed] [Google Scholar]
- 62.Leibovitch I, Casson R, Laforest C, Selva D. Ischaemic orbital compartment syndrome as a complication of spinal surgery in the prone position. Ophthalmology. 2006;113:105–8. doi: 10.1016/j.ophtha.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 63.Little D. Induced hypotension during anaesthesia and surgery. Anesthesiology. 1955;16:320–32. doi: 10.1097/00000542-195505000-00004. [DOI] [PubMed] [Google Scholar]
- 64.Maniglia AJ. Fatal and major complications secondary to nasal and sinus surgery. Laryngoscope. 1989;99:276–83. doi: 10.1288/00005537-198903000-00008. [DOI] [PubMed] [Google Scholar]
- 65.Marks SC, Jaques DA, Hirata RM, Saunders JR., Jr Blindness following bilateral radical neck dissection. Head Neck. 1990;12:342–5. doi: 10.1002/hed.2880120412. [DOI] [PubMed] [Google Scholar]
- 66.Minagar A, Schatz NJ, Glaser JS. Liposuction and ischaemic optic neuropathy: case report and review of literature. J Neurol Sci. 2000;181:132–6. doi: 10.1016/s0022-510x(00)00409-3. [DOI] [PubMed] [Google Scholar]
- 67.Mojon DS, Hedges TR, 3rd, Ehrenberg B, et al. Association between sleep apnea syndrome and nonarteritic anterior ischaemic optic neuropathy. Arch Ophthalmol. 2002;120:601–5. doi: 10.1001/archopht.120.5.601. [DOI] [PubMed] [Google Scholar]
- 68.Movaffaghy A, Chamot SR, Petrig BL, Riva CE. Blood flow in the human optic nerve head during isometric exercise. Exp Eye Res. 1998;67:561–8. doi: 10.1006/exer.1998.0556. [DOI] [PubMed] [Google Scholar]
- 69.Murphy MA. Bilateral posterior ischaemic optic neuropathy after lumbar spine surgery. Ophthalmology. 2003;110:1454–7. doi: 10.1016/S0161-6420(03)00480-9. [DOI] [PubMed] [Google Scholar]
- 70.Myers MA, Hamilton SR, Bogosian AJ, Smith CH, Wagner TA. Visual loss as a complication of spine surgery. A review of 37 cases. Spine. 1997;22:1325–9. doi: 10.1097/00007632-199706150-00009. [DOI] [PubMed] [Google Scholar]
- 71.Neely KA, Ernest JT, Goldstick TK, Linsenmeier RA, Moss J. Isovolemic haemodilution increases retinal oxygen tension. Graefes Arch Clin Exp Ophthalmol. 1996;234:688–94. doi: 10.1007/BF00292355. [DOI] [PubMed] [Google Scholar]
- 72.Nuttall GA, Garrity JA, Dearani JA, Abel MD, Schroeder DR, Mullany CJ. Risk factors for ischaemic optic neuropathy after cardiopulmonary bypass: a matched case/control study. Anesth Analg. 2001;93:1410–6. doi: 10.1097/00000539-200112000-00012. [DOI] [PubMed] [Google Scholar]
- 73.Ozcan MS, Praetel C, Bhatti T, Gravenstein N, Mahla ME, Seubert CN. The effect of body inclination during prone positioning on intraocular pressure in awake volunteers: a comparison of two operating tables. Anesth Analg. 2004;99:1152–8. doi: 10.1213/01.ANE.0000130851.37039.50. [DOI] [PubMed] [Google Scholar]
- 74.Patil CG, Lad EM, Lad SP, Ho C, Boakye M. Visual loss after spine surgery: a population-based study. Spine. 2008;33:1491–6. doi: 10.1097/BRS.0b013e318175d1bf. [DOI] [PubMed] [Google Scholar]
- 75.Pazos GA, Leonard DW, Blice J, Thompson DH. Blindness after bilateral neck dissection: case report and review. Am J Otolaryngol. 1999;20:340–5. doi: 10.1016/s0196-0709(99)90039-x. [DOI] [PubMed] [Google Scholar]
- 76.Pillunat LE, Anderson DR, Knighton RW, Joos KM, Feuer WJ. Autoregulation of human optic nerve head circulation in response to increased intraocular pressure. Exp Eye Res. 1997;64:737–44. doi: 10.1006/exer.1996.0263. [DOI] [PubMed] [Google Scholar]
- 77.Pillanut LE, Harris A, Anderson DR, Greve EL. Current Concepts on Ocular Blood Flow in Glaucoma. The Hague, Netherlands: Kugler; 1999. [Google Scholar]
- 78.Rizzo JF, 3rd, Andreoli CM, Rabinov JD. Use of magnetic resonance imaging to differentiate optic neuritis and nonarteritic anterior ischaemic optic neuropathy. Ophthalmology. 2002;109:1679–84. doi: 10.1016/s0161-6420(02)01148-x. [DOI] [PubMed] [Google Scholar]
- 79.Roth S. The effects of isovolumic haemodilution on ocular blood flow. Exp Eye Res. 1992;55:59–63. doi: 10.1016/0014-4835(92)90092-7. [DOI] [PubMed] [Google Scholar]
- 80.Roth S. Postoperative visual loss. In: Miller RD, editor. Miller's Anesthesia. 7th Edn. New York: Elsevier; 2010. pp. 2821–41. [Google Scholar]
- 81.Roth S, Barach P. Post-operative visual loss: still no answers yet. Anesthesiology. 2001;95:575–6. doi: 10.1097/00000542-200109000-00006. [DOI] [PubMed] [Google Scholar]
- 82.Roth S, Nunez R, Schreider BD. Unexplained visual loss after lumbar spinal fusion. J Neurosurg Anesthesiol. 1997;9:346–8. doi: 10.1097/00008506-199710000-00010. [DOI] [PubMed] [Google Scholar]
- 83.Roth S, Pietrzyk Z. Blood flow after retinal ischaemia in cats. Invest Ophthalmol Vis Sci. 1994;35:3209–17. [PubMed] [Google Scholar]
- 84.Roth S, Thisted RA, Erickson JP, Black S, Schreider BD. Eye injuries after non ocular surgery: a study of 60,965 anesthetics from 1988 to 1992. Anesthesiology. 1996;85:1020–7. doi: 10.1097/00000542-199611000-00009. [DOI] [PubMed] [Google Scholar]
- 85.Roth S, Tung A, Ksiazek S. Visual loss in a prone-positioned spine surgery patient with the head on a foam headrest and goggles covering the eyes: an old complication with a new mechanism. Anesth Analg. 2007;104:1185–7. doi: 10.1213/01.ane.0000264319.57758.55. [DOI] [PubMed] [Google Scholar]
- 86.Sadda SR, Nee M, Miller NR, Biousse V, Newman NJ, Kouzis A. Clinical spectrum of posterior ischaemic optic neuropathy. Am J Ophthalmol. 2001;132:743–50. doi: 10.1016/s0002-9394(01)01199-0. [DOI] [PubMed] [Google Scholar]
- 87.Schobel GA, Schmidbauer M, Millesi W, Undt G. Posterior ischaemic optic neuropathy following bilateral radical neck dissection. Int J Oral Maxillofac Surg. 1995;24:283–7. doi: 10.1016/s0901-5027(95)80030-1. [DOI] [PubMed] [Google Scholar]
- 88.Shapira OM, Kimmel WA, Lindsey PS, Shahian DM. Anterior ischaemic optic neuropathy after open heart operations. Ann Thorac Surg. 1996;61:660–6. doi: 10.1016/0003-4975(95)01108-0. [DOI] [PubMed] [Google Scholar]
- 89.Shen Y, Drum M, Roth S. Anesth Analg. 2009. The prevalence of perioperative visual loss in the United States: a 10-year study from 1996 to 2005 of spinal, orthopedic, cardiac, and general surgery. August 27 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 90.Shen Y, Silverstein JC, Roth S. In-hospital complications and mortality after elective spinal fusion surgery in the United States: a study of the nationwide inpatient sample from 2001 to 2005. J Neurosurg Anesthesiol. 2009;21:21–30. doi: 10.1097/ANA.0b013e31818b47e9. [DOI] [PubMed] [Google Scholar]
- 91.Slavin ML. Ischaemic optic neuropathy after cardiac arrest. Am J Ophthalmol. 1987;104:435–6. doi: 10.1016/0002-9394(87)90247-9. [DOI] [PubMed] [Google Scholar]
- 92.Stankiewicz JA. Complications of endoscopic sinus surgery. Otolaryngol Clin North Am. 1989;22:749–58. [PubMed] [Google Scholar]
- 93.Stevens WR, Glazer PA, Kelley SD, Lietman TM, Bradford DS. Ophthalmic complications after spinal surgery. Spine. 1997;22:1319–24. doi: 10.1097/00007632-199706150-00008. [DOI] [PubMed] [Google Scholar]
- 94.Sullivan SR, Ahmadi AJ, Singh CN, et al. Elevated orbital pressure: another untoward effect of massive resuscitation after burn injury. J Trauma. 2006;60:72–6. doi: 10.1097/01.ta.0000197657.25382.b2. [DOI] [PubMed] [Google Scholar]
- 95.Tice DA. Ischaemic optic neuropathy and cardiac surgery. Ann Thorac Surg. 1987;44:677. doi: 10.1016/s0003-4975(10)62171-6. [DOI] [PubMed] [Google Scholar]
- 96.Vaphiades MS. Optic nerve enhancement in hypotensive ischaemic optic neuropathy. J Neuroophthalmol. 2004;24:235–6. doi: 10.1097/00041327-200409000-00011. [DOI] [PubMed] [Google Scholar]
- 97.Warner ME, Warner MA, Garrity JA, MacKenzie RA, Warner DO. The frequency of perioperative vision loss. Anesth Analg. 2001;93:1417–21. doi: 10.1097/00000539-200112000-00013. [DOI] [PubMed] [Google Scholar]
- 98.Weber E, Coyler M, Lesser R, Subramanian P. Posterior ischaemic optic neuropathy after minimally invasive prostatectomy. J Neuroophthalmol. 2007;27:285–7. doi: 10.1097/WNO.0b013e31815b9f67. [DOI] [PubMed] [Google Scholar]
- 99.Weinstein JM, Duckrow RB, Beard D, Brennan RW. Regional optic nerve blood flow and its autoregulation. Invest Ophthalmol Vis Sci. 1983;24:1559–65. [PubMed] [Google Scholar]
- 100.Weiskopf RB, Viele MV, Feiner J, et al. Human cardiovascular and metabolic response to acute, severe isovolemic anaemia. J Am Med Assoc. 1998;279:217–21. doi: 10.1001/jama.279.3.217. [DOI] [PubMed] [Google Scholar]
- 101.Williams EL, Hart WMJ, Tempelhoff R. Postoperative ischaemic optic neuropathy. Anesth Analg. 1995;80:1018–29. doi: 10.1097/00000539-199505000-00029. [DOI] [PubMed] [Google Scholar]
- 102.Williams GC, Lee AG, Adler HL, et al. Bilateral anterior ischaemic optic neuropathy and branch retinal artery occlusion after radical prostatectomy. J Urol. 1999;162:1384–5. [PubMed] [Google Scholar]
- 103.Wray SH. The management of acute visual failure. J Neurol Neurosurg Psychiatry. 1993;56:234–40. doi: 10.1136/jnnp.56.3.234. [DOI] [PMC free article] [PubMed] [Google Scholar]