Summary
Multiple RNA polymerase II (RNAPII) molecules can transcribe a gene simultaneously, but what happens when such polymerases collide—for example due to polymerase pausing or DNA damage? Here, RNAPII collision was characterized using a reconstituted system for simultaneous transcription by two polymerases. When progression of leading polymerase is obstructed, rear-end collision entails a transient state in which the elongation complexes interact, followed by substantial backtracking of trailing polymerase. Elongation complexes remain stable on DNA, with their activity and the integrity of transcription bubbles remaining intact. Subsequent TFIIS-stimulated transcript cleavage allows resumed forward translocation, resulting in trailing polymerase oscillating at the obstruction. Conversely, if leading polymerase is merely stalled at a pause site, collision and TFIIS cooperate to drive it through. We propose that dynamic interactions between RNAPII elongation complexes help regulate polymerase traffic and that their conformational flexibility buffers the effect of collisions with objects on DNA, thereby maintaining stability in the face of obstacles to transcription.
Keywords: DNA
Introduction
RNA polymerase II (RNAPII) is responsible for transcription of all protein-encoding genes, as well as for the generation of numerous noncoding RNAs. The movement of RNAPII on DNA induces changes in the structure of chromatin and involves dynamic interaction with other objects on DNA, such as those relevant for replication, repair, and transcription itself.
Structural analysis, single-molecule approaches, and biochemical characterization of RNAPII activity in reconstituted transcription systems have shed light on processes such as transcriptional translocation, pausing, arrest, and RNAPII backtracking at the level of individual polymerases (Arndt and Kane, 2003; Herbert et al., 2008; Kornberg, 2007). On the other hand, techniques such as chromatin immunoprecipitation (ChIP) and live cell imaging have provided insight into the average behavior of large populations of polymerases on active genes in vivo (Saunders et al., 2006; Singer et al., 2005; Struhl, 2007). However, the gap of knowledge between these extremes, including how RNAPII elongation complexes dynamically interact, has not yet been filled.
Biochemical analyses of transcription support the idea that transcript elongation involves frequent pausing and even arrest, which has to be overcome in order for overall transcription to be efficient (Arndt and Kane, 2003; Conaway et al., 2000; Shilatifard et al., 2003). A defining response to all transcription obstacles that have been studied is retrograde motion of RNAPII, so-called backtracking, during which RNAPII loses contact with the 3′ end of the RNA and often needs the help of general elongation factor TFIIS (also called SII) to recover. TFIIS stimulates transcript cleavage by the RNAPII active site so that a new RNA end in correct register with the active site is generated (Izban and Luse, 1992; Reines, 1992).
Equally important for our insight into fundamental transcription principles has been the progress in understanding overall RNAPII traffic on active genes. ChIP has been used to generate extensive maps of RNAPII location across genes and genomes and recently provided compelling evidence for widespread promoter-proximal pausing (Margaritis and Holstege, 2008), but this technique provides little or no information on the dynamics of RNAPII movement. Recent developments in the analysis of overall RNAPII dynamics by cell imaging have uncovered evidence for transcription occurring in bursts with pulses of high polymerases density (Chubb et al., 2006) and support the idea that RNAPII frequently stops, also during processive elongation. Indeed, although it is generally accepted that transcript elongation occurs at an average rate of 20–30 nt/s, evidence for a rate of up to 70 nt/s, interrupted by periods of pausing, was reported (Darzacq et al., 2007).
In general, the speed of transcript elongation, combined with the high likelihood of transcriptional pausing/arrest, would be expected to result in frequent polymerase rear-end collisions. Interestingly, collision between bacteriophage T7 RNA polymerases results in the leading polymerase falling off the DNA (Zhou and Martin, 2006), while bacterial RNA polymerases seem to cooperate during elongation (Epshtein and Nudler, 2003). However, results of studies aimed at characterizing the consequences of transcriptional collision by eukaryotic RNAPII have not been reported, and the basis for the stability and dynamic interactions of colliding elongation complexes in general remains poorly understood.
In this study, we used an oligonucleotide-based RNAPII transcription system to study colliding RNAPII elongation complexes transcribing the same DNA strand. Our data suggest that dynamic interactions between conformationally elastic elongation complexes may make significant and fundamental contributions to transcript elongation.
Results
The Anatomy of an RNAPII Elongation Complex
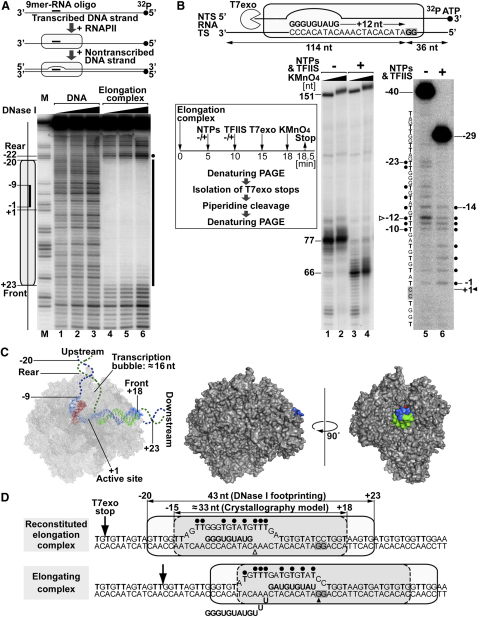
In order to understand RNAPII collision at the basic biochemical level, the characteristics of separate elongation complexes first needed to be understood in the context of the experimental system used here. Stepwise assembly of highly purified RNA and DNA oligonucleotides and yeast RNA polymerase II can reconstitute fully functional RNAPII elongation complexes (Kireeva et al., 2000) (Figure 1A, upper panel; see Figure S1A available online). The transcribed strand of such an elongation complex was radioactively labeled prior to assembly, and the footprint of the ternary complex was mapped with DNase I. RNAPII protected an ∼43 nt region extending approximately from position −20 to +23 relative to the polymerase active site (+1) (Figure 1A, lower panel), in good agreement with previous experiments using mammalian, promoter-driven RNAPII (see, for example, Linn and Luse, 1991). It also matched more precise estimates made from crystal structure models of yeast RNAPII (Wang et al., 2006, and references therein), though the enzymatic footprint, not surprisingly, extended beyond the protein-DNA interactions predicted by crystallography (Figures 1C and 1D).
Figure 1.
Elongation Complex Footprint and Mapping of the Transcription Bubble
(A) (Upper) Summary of elongation complex preparation, drawn to scale. (Lower) Elongation complexes with 5′ end-labeled, 131-mer transcribed DNA were digested with increasing amounts of DNase I, as indicated. The footprint region where DNase I cleavage is repressed (bold line) and a hypersensitive site (sphere) are indicated on the right. M, thymine-specific cleavage marker. Elongation complex (oval) and RNA (bold line) are indicated on the left. Position of the active site is taken as +1.
(B) (Upper) Schematic diagram of the locations of the RNA primer (bold text) and G stop (gray box) in the elongation complex. The 150-mer transcribed strand (TS), the corresponding end-labeled nontranscribed strand (NTS), and the direction of exonuclease digestion are indicated. (Lower) Elongation complexes incubated alone, or with ATP/UTP/GTP and TFIIS, were digested with T7 exonuclease to mark their position(s), modified with increasing amounts of KMnO4 (2 and 8 mM), and analyzed by denaturing PAGE (lanes 1–4). Bands corresponding to the major elongation complexes (2 mM KMnO4 experiment shown) were isolated, cleaved at modified thymines with piperidine, and resolved by denaturing PAGE (lanes 5 and 6). DNA sequence is shown on the left. Spheres, modified thymines. Arrowheads, positions of active sites.
(C) Crystal structure of the elongation complex with modeled DNA paths (dashed lines). Space-filling model shows the DNA (color) engulfed in protein bulk.
(D) Area protected by RNAPII (DNase I footprinting, solid line; crystallography model, dashed line) and their transcription bubbles. Only reconstituted elongation complex was mapped by DNase I footprinting. However, T7 exonuclease digests to the same distance from the edge of the two footprints (±1 base; positions indicated by vertical arrow) before and after transcription, showing that RNAPII moved forward upon NTP addition.
To map the precise position of the single-stranded RNAPII transcription bubble exposed upon the formation of the RNA:DNA hybrid, we took advantage of the ability to assemble an elongation complex at a defined position and of being able to transcribe it to another position. The transcribed strand was designed so that it lacked guanines for 12 nucleotides downstream from the 3′ end of the RNA, meaning that transcription to this position (the “G stop”) could be achieved by addition of ATP, GTP, and UTP (Figure 1B, upper panel; Figure S1B). The elongation factor TFIIS, which promotes transcript cleavage and thereby forward translocation of backtracked elongation complexes, was also added to ensure that the transcribed polymerase did not occupy backtracked positions. The 3′ end of the nontranscribed DNA strand was labeled prior to assembly and transcription. To map single-stranded DNA, the elongation complexes were treated with potassium permanganate (which modifies, but does not cleave at, thymines in single-stranded DNA). Moreover, to ensure that the single-stranded DNA region for a positionally defined elongation complex was mapped, elongation complexes were isolated based on their location marked by T7 exonuclease, which catalyzes the stepwise hydrolysis of nucleotides from the 5′ end of double-stranded DNA but is prohibited by the polymerase bulk from digesting beyond a point 8–9 nt upstream from the elongation complex delineated by DNase I footprinting (Figure 1B, lanes 1–4, 77 and 66 nt, respectively; Figure 1D). The isolated DNA was then treated with piperidine (which induces strand cleavage at the potassium permanganate-modified thymines), and the products were resolved by denaturing PAGE, uncovering the single-stranded bases in the respective elongation complexes (Figure 1B, lanes 5 and 6). The results are summarized in Figure 1D. Our data suggest that, all in all, the transcription bubbles extend for 15–20 nt, with only minor differences observed between the types of elongation complexes tested (see also Figure 5). This is in agreement with previous data using mammalian, promoter-driven RNAPII transcription (Fiedler and Timmers, 2001) and unpublished RNAPII structural information (R.D. Kornberg, personal communication).
Figure 5.
Transcription Bubbles Remain Intact and Separated upon Collision
(A) Experimental design.
(B) Elongation complexes (end-labeled NTS) were incubated with NTPs and TFIIS, digested with T7 exonuclease, and modified with increasing amounts of KMnO4 (12, 18, and 24 mM) as indicated and resolved by denaturing PAGE. Major T7 exonuclease-marked bands as seen in Figure 3, lane 17, line, were isolated, cleaved at modified thymines with piperidine, and analyzed by denaturing PAGE. The positions of isolated bands are indicated at the top. Thin lines between lanes on the autoradiograph indicate transcription bubbles. Naked NTS (6 mM KMnO4 footprinting) is shown in lane 16. Important distances and positions are indicated on the right. Arrowheads indicate the respective active sites.
(C) As in (B), but both in the absence and presence of TFIIS, modified with increasing amounts of permanganate (4, 8, and 18 mM).
(D) Summary of results obtained in the absence (upper) and presence (lower) of TFIIS, drawn to scale.
Collision between RNAPII Elongation Complexes
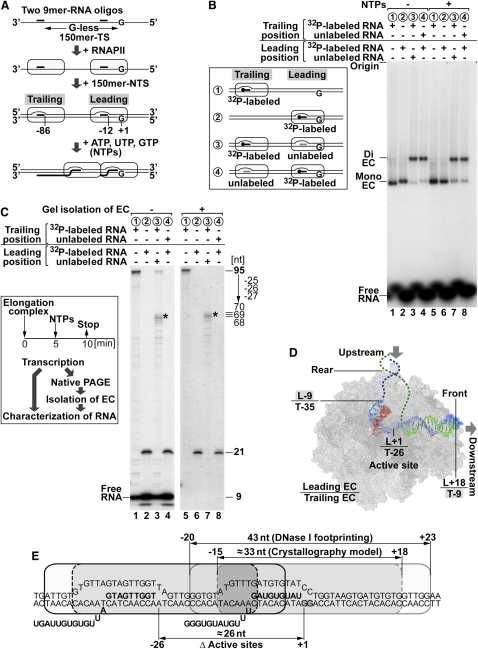
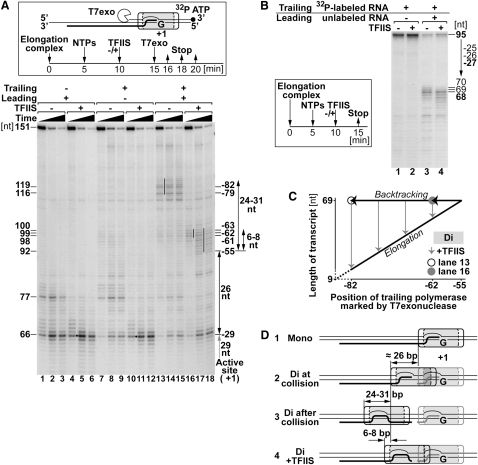
We now established a system in which the consequence of RNAPII collision could be studied (Figure 2A; Figure S1B). In this approach, two separate elongation complexes were assembled on 150-mer DNA oligonucleotides, with a labeled and unlabeled 9-mer RNA, respectively, where transcription up to a G stop was achieved by the addition of ATP/GTP/UTP (“NTPs”). The polymerase closest to the G stop (termed the “leading” polymerase) could transcribe 12 nt (producing a 21-mer product) before stopping due to the lack of CTP. The second polymerase (termed the “trailing” polymerase) could transcribe 86 nt in the absence of CTP (to produce a 95-mer product) if there was no leading polymerase on the DNA. The native agarose gel in Figure 2B shows that assembly of dielongation complexes was efficient, with only a small amount of contaminating monocomplex present (compare lanes 3 and 4 with lanes 1 and 2, respectively). Upon transcription, the integrity of dielongation complexes remained intact (Figure 2B, lanes 5–8), indicating that RNAPII elongation complexes can collide without substantial dissociation of either polymerase from the DNA.
Figure 2.
Collision between RNAPII Elongation Complexes
(A) Dielongation complex preparation on 150-mer oligonucleotides, drawn to scale.
(B) Native agarose gel electrophoresis of radioactive RNAPII elongation complexes. Schematic diagram of the elongation complexes reconstituted is shown in the box (numbers corresponding to each substrate also apply on the right and in C). Dielongation complexes were reconstituted with combinations of 32P-end-labeled and unlabeled RNA oligos that hybridize at the trailing or leading position, as indicated. The elongatin complexes alone, or incubated with NTPs, were analyzed by native agarose gel electrophoresis. Complexes containing one (Mono-) and two (Di-) RNAPII elongation complexes, and unincorporated 9-mer RNA (Free RNA), are indicated.
(C) (Box) Experimental procedure. (Right) The reconstituted elongation complexes incubated with NTPs in (B) were analyzed by denaturing PAGE (lanes 1–4). Alternatively, mono- and dielongation complexes were isolated after transcription by native agarose gel electrophoresis and analyzed by denaturing PAGE (lanes 5–8). The lengths of the transcripts are indicated. Asterisk indicates transcripts generated by the blocked, trailing elongation complex.
(D) Crystal structure model of the elongation complex. DNA is engulfed in protein bulk and not wrapped around RNAPII (see also Figure 8A). Numbers indicate the position of the indicated site of the leading or trailing polymerase (L/T) at collision, relative to that of the active site of the leading polymerase (+1).
(E) Summary model, indicating the theoretical overlap of footprints. Positions of the leading (gray line) and the trailing (black line) elongation complexes at collision were deduced from the lengths of the transcripts in (C). The size of the elongation complex and its transcription bubble are as in Figure 1.
Knowing that the integrity of the elongation complexes remained intact upon collision, we now resolved the RNA products by denaturing PAGE (Figure 2C, box). As expected, in monocomplexes, the trailing polymerase produced a 95-mer, while the leading polymerase produced a 21-mer RNA product (Figure 2C, lanes 1 and 2). With dielongation complexes, the length of the product produced by the leading polymerase did not change upon collision (Figure 2C, lane 4). More importantly, transcription by the trailing polymerase was significantly affected by the presence of a leading polymerase. Major transcripts with a length of 68–70 nt were produced (Figure 2C, lane 3, denoted by asterisk), although a weak 95 nt transcript was also visible. To investigate whether this 95 nt transcript originated from contaminating monocomplexes or from trailing polymerases transcribing past the leading polymerase, the reaction products from the transcription reaction were first resolved by native agarose gel electrophoresis (as in Figure 2B). The relevant elongation complexes were then excised and RNA was analyzed by denaturing PAGE. Using this procedure, the 9 nt RNA oligonucleotide was no longer visible, showing that all elongation complexes transcribed (Figure 2C, lanes 5–8). Moreover, the 95 nt product was also no longer observed (Figure 2C, lane 7), indicating that it originated from contaminating monocomplexes and that, as expected, the trailing polymerase cannot pass the leading polymerase.
With the knowledge of the anatomy of RNAPII elongation complexes obtained previously (see Wang et al., 2006, and references therein; Figure 1; R.D. Kornberg, personal communication), the consequences of collision could be assessed. The models predicted that the longest RNA product the trailing polymerase can produce before touching the leading polymerase should be approximately 95 minus 43 nt (the size of the footprint), i.e., 52 nt, or 95 minus 33 nt (the minimum DNA area protected by RNAPII in the elongation complex crystal structure), i.e., 62 nt, but instead 68–70 nt products were produced. The trailing polymerase thus continued transcription for at least 6–8 nt after first touching the leading polymerase (Figures 2C–2E). In contrast to nucleosomes, for example, where DNA is wrapped around a histone core, DNA is surrounded by protein mass in RNAPII (see Figures 1C and 8A), so “stripping of DNA” off the surface of RNAPII cannot account for the observations. We surmised that the conformation of elongation complexes might instead be flexible, buffering the effect of collision. If so, this might potentially result in retrograde movement (“recoil”) of the trailing elongation complex as normal conformation is regained. Alternatively, the polymerases might remain entangled, with potentially dramatic consequences for elongation complex integrity. In the following experiments, we therefore mapped the position of the trailing elongation complex and the transcription bubbles after collision and assessed the activity of the collided enzymes.
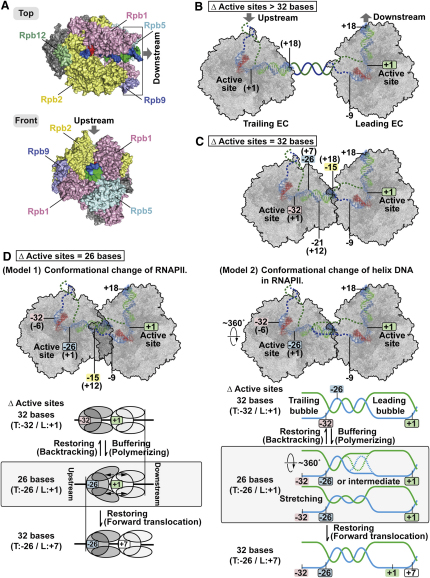
Figure 8.
Collision Models
(A) Crystal structure model of the RNAPII elongation complex (from Wang et al., 2006), viewed from the top, with RNAPII transcribing toward the right (upper panel), and from the front, with RNAPII transcribing toward the reader (lower panel). Boxed area, domains and subunits specific to eukaryotic RNAPII (see main text).
(B) Two RNAPII molecules en route to collision.
(C) Colliding polymerases at the point of first contact, with active sites 32 nt apart.
(D) The temporary collision state with only 26 nt between the active sites. Two possible explanations for this state are offered. Model 1 on the left invokes conformational changes in the protein component of elongation complexes. The upper panel shows the overlapped parts of the polymerases that have to be accommodated. The lower panel shows a schematic model for a proposed transient state (middle, boxed) that entails conformational changes in the structure of flexible RNAPII domains, helping to drive either backtracking (shown above) or forward translocation (below). Model 2 on the right invokes conformational change in the DNA component of elongation complex. The upper panel shows space between polymerases being created by ∼360° rotation of the trailing polymerase from the state in (C) and being filled by its forward translocation on DNA as a DNA helical turn between the transcription bubbles unwinds. The lower panel shows schematic models for proposal transient states (middle, boxed) that entail elastic conformational changes in helix DNA (twisting or/and stretching of DNA between the transcription bubbles), helping to drive either backtracking (shown above) or forward translocation (below). The models are not mutually exclusive.
Evidence for Backtracking of Trailing RNAPII upon Collision
To determine the position of the trailing polymerase after collision, T7 exonuclease mapping was performed, with and without TFIIS (Figure 3). As previously observed (Figure 1B), transcribing monocomplexes caused T7 exonuclease to make products 66 nt long (corresponding to position −29 relative to the RNAPII active site at +1) whether they started from the leading (Figure 3A, lanes 1–3), or the trailing position (lanes 7–9). This was confirmed by the addition of TFIIS (Figure 3A, lanes 4–6 and 10–12; Figure 3D, panel 1). Surprisingly, however, upon polymerase collision, the trailing polymerase was much further upstream than expected, with the products of exonuclease digestion 116 and 119 nt long (corresponding to position −79 and −82 relative to the active site of the leading polymerase) (Figure 3A, lanes 13–15, thin line; Figure 3D, panel 3). It is important to note that, in the previous experiment (Figure 2C, lane 3), no transcription products of a length (42–45 nts) that could explain these major exonuclease-mapped RNAPII positions were observed. The positions mapped by exonuclease digestion thus indicated that, upon collision, the active site of the trailing polymerase did not remain at the place of impact ∼26 nt upstream from the leading polymerase indicated by the length of its transcript (see Figures 2C and 2E; Figure 3D, panel 2). Rather, it moved ∼25 nt back from this position so that its active site ended up ∼50 nt from the active site of the leading polymerase.
Figure 3.
Exonuclease Mapping of Trailing Polymerase after Collision
(A) (Box) Experimental approach. (Lower) Mono- and dielongation complexes with 3′ end-labeled, nontranscribed strand DNA were incubated with NTPs (or with NTPs and TFIIS), digested with T7 exonuclease, and analyzed by denaturing PAGE. DNA fragment sizes are indicated to the left. The position of the elongation complexes relative to the active site of the leading polymerase (−82, −79, etc.) is indicated on the right. Grey arrow indicates distance between the active site of the elongation complex at the G stop and the position of that same elongation complex as marked by T7 exonuclease. Double arrows (black) mark distances between elongation complexes, and thin lines between lanes indicate major exonuclease stops (see text for details).
(B) (Box) Experimental design. (Right) Transcription products resolved by denaturing PAGE as in Figure 2C.
(C) Relationship between lengths of transcripts and positions of trailing elongation complex as marked by exonuclease. Angled line indicates forward translocation toward the point of collision. Horizontal lines with arrows indicate backtracking, and gray, vertical lines with arrows indicate transcript cleavage and the return of RNAPII to forward translocation.
(D) Summary of results, drawn to scale. Distances between elongation complexes are indicated.
The addition of TFIIS resulted in the products of exonuclease digestion being ∼20 nt shorter than this (position −63 to −61; Figure 3A, lane 16, short line; Figure 3D, panel 4). This suggests that the active sites of the polymerases were now 32–34 nt apart, but still not the ∼26 nt indicated by transcript length. It is important to note that although addition of TFIIS dramatically changed the position of the trailing polymerase after collision, the transcription products were similarly sized whether TFIIS was added or not (Figure 3B, compare lanes 3 and 4).
All in all, the only reasonable explanation for these results is that the trailing polymerase backtracks substantially after collision but can be reactivated by TFIIS (Figure 3C). This in turn indicates that it retains function after collision and backtracking. Moreover, even in the presence of TFIIS, the trailing elongation complex was located 6–8 nt upstream from the position expected from transcript length. This suggests that the trailing polymerase continues to transcribe forward against the opposing force of the leading polymerase, eventually quickly backtracking before rapid motion is repeated following TFIIS-mediated transcript cleavage (Figure 3C, and Movie S1). Interestingly, the exonuclease footprint appeared to move forward during the course of the experiment (Figure 3A, lanes 16–18, indicated by short and long lines, respectively). The ability of the T7 exonuclease to “catch” elongation complex positions further and further forward with time contrasted the lack of change over time in the case of monopolymerases (Figure 3A, lanes 10–12). It supports the above interpretation and indicates constant motion and dynamic interactions between colliding elongation complexes.
Substantial Backtracking of the Trailing Polymerase Is a Consequence of Collision
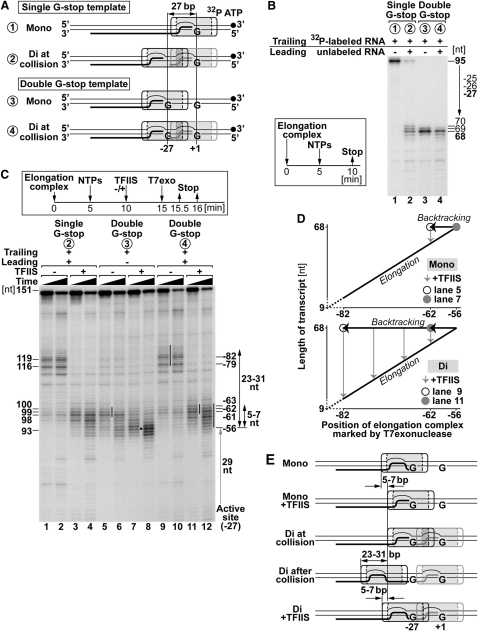
In theory, the backtracking of trailing polymerase observed above might simply be due to the underlying DNA sequence; i.e., the polymerase might rapidly backtrack from this position even if there were no collision. To investigate this point, we introduced a second G stop at position −27 relative to the active site of the leading polymerase, i.e., at the main position reached by the trailing polymerase during collision (Figure 4A, double G stop template; Figure S1B). Because the leading polymerase initiates transcription downstream from this point, we could now examine if the presence of the leading polymerase were the cause of the substantial backtracking observed. Figure 4B shows that, both in the absence and presence of a leading polymerase, the trailing polymerase stopped transcription at the same position on this template (lanes 3 and 4), producing transcripts of a length similar to that observed with a single G stop with a leading polymerase stalled at it (lane 2). Importantly, exonuclease footprinting showed that, in the absence of TFIIS and a leading polymerase, polymerase transcribing from the trailing position remained in the area immediately upstream of the newly introduced G stop (Figure 4C, lanes 5 and 6), rather than moving far back, as it did when there was a leading polymerase in front of it on the single G stop template (Figure 4C, lanes 1 and 2). In contrast, when the leading polymerase was present on the double G stop template, the trailing polymerase backtracked for more than 20 nt to the same positions it moved to on a single G stop template (Figure 4C, compare lanes 9 and 10 and lanes 1 and 2).
Figure 4.
Backtracking of Trailing Polymerase Caused by Collision with Leading Polymerase
(A) Experimental approach. A second G stop at position −27 relative to the first G stop was introduced. Drawn to scale. Numbers corresponding to each substrate also apply in (B) and (C).
(B) Transcription products resolved by denaturing PAGE as in Figure 2C.
(C–E) T7 exonuclease mapping of elongation complexes and models, as in Figure 3.
As observed earlier (Figure 3), TFIIS allowed the polymerase to move forward again after collision-induced backtracking (Figure 4C, lanes 3 and 4 and lanes 11 and 12). However, while the trailing polymerase was detected around position −56 in the absence of a leading polymerase in front of it (Figure 4C, lane 7, indicated by black sphere), it was primarily found around positions −61 to −63 (5–7 nt further back) in the presence of one (lane 11). This highlights the elastic nature of the elongation complex as well as its TFIIS-mediated oscillation and again indicates that the residence time of the trailing elongation complex in the furthest position it transcribes to (and where it is completely blocked by the leading polymerase) is so short that T7 exonuclease cannot catch it and mark the position. In contrast, a monoelongation complex facing the same G stop can be caught at the position indicated by the length of its transcript (Figure 4C, lane 8; Figure 4D).
The results are summarized in Figure 4E. Together, they indicate that substantial backtracking of the trailing polymerase is not a consequence of DNA sequence but is indeed the result of collision with the leading polymerase.
Transcription Bubbles Remain Intact upon Collision
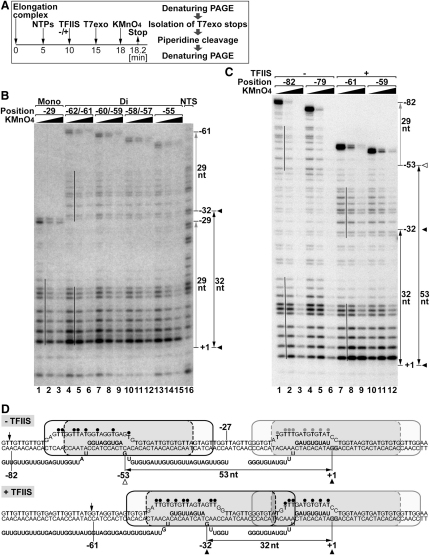
The exonuclease mapping presented above only provides information about the trailing polymerase. To obtain a view of both polymerases after collision and, moreover, to test if collision results in changes to the integrity of the transcription bubbles of the collided polymerases, potassium permanganate was used to detect thymines in single-stranded DNA regions (Figure 5A).
It was previously observed that upon polymerase collision, backtracking, and TFIIS-mediated forward motion, trailing polymerase stopped the exonuclease at positions ranging from approximately −55 to −62 relative to the G stop (see Figure 3, lane 17). If the overall organization of the colliding elongation complex were restored immediately after reaching the furthest position (−55), resulting in retrograde movement, the positions of the transcription bubbles of elongation complexes corresponding to the distinct exonuclease-generated bands should all be the same as that of the elongation complex at position −62. This was indeed the case: analysis of permanganate-treated DNA from these collided polymerases showed two well-separated transcription bubbles, which in all compared cases were similarly positioned (Figure 5B, lanes 4–6, 7–9, 10–12, and 13–15, respectively; position of the two bubbles is indicated by lines between lanes 4 and 5). Importantly, the transcription bubble also gave information about the position of the active site of the trailing polymerase, which was found to be ∼32 nt away from the active site of the leading one (with polymerases positioned in touching distance; see Figure 8C), rather than the ∼26 nt suggested by transcript length. Together, these data again point to a transient clash of stable, elastic elongation complexes and also further support the TFIIS-mediated oscillation indicated by the experiments in Figures 3 and 4.
We also note that the transcription bubble of the leading polymerase was almost indistinguishable from that in the (uncollided) monocomplex (Figure 5B, compare lanes 1–3 with lanes 4–6, for example) and that there was no evidence for single-stranded DNA between the transcription bubbles, arguing that little or no permanent loss of transcription bubble integrity occurred on collision.
Transcription bubbles were also mapped in the absence of TFIIS, using the same approach (Figure 5C, lanes 1–6; refer also to Figure 3A, lanes 13–15, which shows the main backtracked positions). In this particular experiment, somewhat more background cleavage at all thymines was evident, but comparison to Figure 5B (with lanes 4–9 of Figure 5B corresponding to lanes 7–12 of Figure 5C) and within the experiment made it straightforward to make conclusions. First, the position of the leading polymerase remained largely the same as in the presence of TFIIS, though evidence for some backtracking of leading elongation complexes could be seen (Figure 5C, compare lines at bottom of lanes 1 and 2 and lanes 7 and 8, respectively). More importantly, the transcription bubble of the substantially backtracked, trailing polymerase was observed at the position expected from exonuclease footprinting, approximately 51–70 nt upstream from the active site of the leading polymerase (Figure 5C, indicated by line near top between lanes 1 and 2). The distance between the RNAPII active sites was thus about 53 nt, showing that the elongation complexes were completely separated after collision.
These data, summarized in Figure 5D, complement and extend the exonuclease footprinting experiments. They indicate that transcription bubbles for both the leading and trailing polymerases remain intact after collision: the bubbles have the expected size and are completely separated.
Further Evidence for Dynamic Interactions between Colliding Elongation Complexes
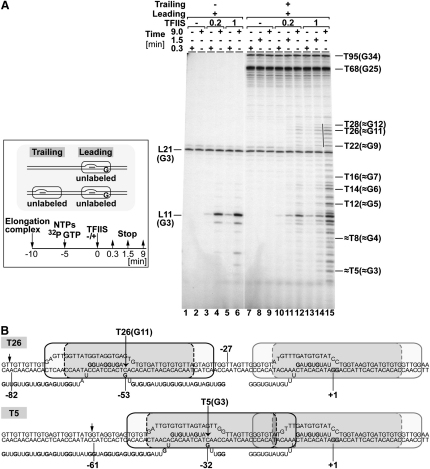
Besides defining the consequences of RNAPII collision, the footprint experiments also indicated that TFIIS allowed the trailing polymerase to “oscillate.” To more directly investigate this, experiments were performed in which the nascent transcripts were labeled by using radioactive GTP during transcription, enabling the RNA oligonucleotides cast off by transcript cleavage to be visualized (Figure 6). Interpretation of this experiment has to be done mindful of the fact that shorter RNA species have fewer radioactive labels incorporated and so are relatively underrepresented in the autoradiogram. As observed previously (Figure 2C), in the absence of TFIIS (Figure 6A, lanes 7–9), the trailing polymerase was blocked by the leading polymerase and predominantly produced 68–70 nt products (labeled T68) (and a 95 nt product [T95] originating from contaminating monocomplexes), while the leading polymerase concomitantly produced a 21 nt product (labeled L21), similar to what the monocomplex produced alone (lanes 1 and 2). No increase in the amount of these transcripts was observed over time, showing that, as expected, transcription to the end (or the obstacle) was completed already before TFIIS addition. TFIIS reactivates backtracked polymerase by cleaving the nascent RNA labeled by incorporation of radioactive GMP. Therefore, the length of the cleaved nascent RNA designates the distance of backtracking. T7 exonuclease mapping and permanganate probing previously showed that the majority of trailing complexes backtracked for 24–27 nt after collision in the absence of TFIIS (Figures 3 and 5). Indeed, cleaved transcripts corresponding to those lengths were observed when TFIIS was added (Figure 6A, lanes 11–15, thin line; Figure 6B, upper panel). Moreover, large amounts of shorter bands accumulated with time as a result of transcript cleavage, the majority of which were 5–15 nt, indicating that TFIIS captured backtracking polymerases at the corresponding positions.
Figure 6.
TFIIS Induces Oscillation of Trailing Polymerase upon Collision with Leading Polymerase
(A) (Box) Experimental design. (Right) Unlabeled elongation complexes were incubated with ATP/UTP/GTP and α-32P GTP. After 5 min, TFIIS was added (0.2 and 1 molar ratios to RNAPII). Products were analyzed by 20% denaturing PAGE. Length of transcripts generated by leading polymerase (L, on left side) and trailing polymerase (T, on the right), and the expected number of guanines in these transcripts (in brackets), are indicated.
(B) Depiction of the situation with a 26 nt (upper, T26), and with a 5 nt (lower, T5) long cleaved nascent RNA, an alternative view to that shown in Figure 5D. Numbers indicate positions relative to that of the leading polymerase's active site. Long arrows, position of cleavage induced by TFIIS. Short arrows, position of T7 exonuclease footprint.
These results support the observation made by footprinting in Figures 3–5 that the predominant “steady-state position” of the active site of trailing polymerase in the presence of TFIIS is ∼32 nt away from the active site of the leading polymerase (see lower panel in Figure 5B), rather that the 26–27 nt suggested by transcript length. Once TFIIS acts, the cleaved RNA intermediate is short lived (i.e., undetectable because subsequent forward translocation [chain elongation] is immediate), so that only the longer transcripts (which indicate a distance of ∼26 nt from the active site of the leading polymerase) are detected. The differences observed in the previous footprint experiments (Figures 3–5), 32 minus 26–27, i.e., 5–6 nt, are in this experiment appearing as the shortest of the oscillation range (5–15 nt). We note that, according to molecular modeling based on the polymerase crystal structure, the position of the trailing polymerases observed in the presence of TFIIS places the colliding polymerases in “touching distance,” or beyond (5–15 nt), rather than in a “compressed” state, as expected if the elastic/flexible elongation complexes are in effect driving each other back while regaining their conformation (see also Figures 8B–8D, Movie S1, and Discussion).
Rear-End Collision Can Drive RNAPII through a Pause Site
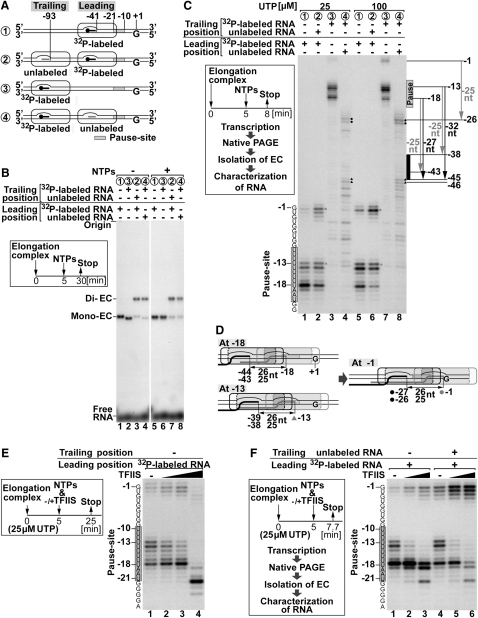
The situation characterized so far was one in which the leading polymerase was blocked from further forward translocation. But what happens if the leading polymerase is merely stalled at a pause site at collision? To address this question, a DNA template containing a 9 nt adenine tract upstream from the G stop to induce transcriptional pausing was used (Figure 7A). The native gel in Figure 7B shows that RNAPII elongation complexes also under these conditions can collide without substantial dissociation of either polymerase from the DNA, as observed previously (Figure 2B). Next, the RNA products isolated from elongation complexes were resolved by denaturing PAGE (Figure 7C, box). The vast majority of polymerases in monocomplexes paused in the A tract whether transcribing from the leading (Figure 7C, lanes 1 and 5) or the trailing position (lanes 3 and 7), both at high and low nucleotide concentration. The presence of a trailing polymerase dramatically increased transcription through the A tract by the leading polymerase (Figure 7C, compare lanes 1 and 2 and lanes 5 and 6, respectively), indicating that collision did not perturb the activity of the leading polymerase but rather drove it through the pause site to continue transcription. The transcript pattern produced by leading polymerases making it through the pause site to the G stop was not markedly altered by collision (Figure 7C, compare lanes 1 and 2 and lanes 5 and 6, respectively), suggesting that their escape from the stall upon collision was not due to base skipping.
Figure 7.
Stalled RNAPII Can Be Helped through a Potent Pause Site
(A) Schematic of the experiment. Numbers corresponding to each 140-mer oligonucleotide substrate apply also in (B) and (C).
(B) (Box) Experimental design. (Right) Stability of the elongation complexes upon cooperation assessed by native agarose gel electrophoresis.
(C) (Box) Experimental design. (Right) Transcription products resolved by denaturing PAGE. Sequence of the transcript and positions of major pause and stop positions of leading polymerase relative to the G stop are indicated to the left. The positions of the pause site and full-length transcript (−1, relative to the G stop), as well as the major pause and stop sites of trailing polymerase, are indicated on the right. Spheres indicate the leading polymerase stopped at the G stop (gray) and the corresponding trailing polymerases (black). Arrowheads indicate the foremost, pause-site-stalled, leading polymerase (gray) and the corresponding trailing polymerases (black).
(D) Dynamically interacting polymerases before and after the leading polymerase overcomes the pause site. Numbers and distances corresponding to those on the autoradiograph are shown.
(E) (Box) Experimental design. (Right) Monoelongation complexes incubated with NTPs and TFIIS (0.2, 1, and 5 molar ratios to RNAPII) analyzed by denaturing PAGE.
(F) (Box) Experimental design. (Right) Transcripts obtained with the stimulatory amounts of TFIIS in elongation reactions performed with mono- or dielongation complexes, respectively.
We also characterized the transcripts produced by the trailing polymerase upon collision on this template. This suggested the existence of two distinct trailing polymerase populations. The first was positioned only 25–26 nt upstream from the G stop (Figure 7C, lanes 4 and 8, black spheres) and must therefore represent trailing polymerases that had driven leading polymerases through the pause site and were blocked by this leading RNAPII at the G stop (indicated by gray spheres in lanes 2 and 6; see also Figure 7D, right). The second was positioned at several locations 32–33 nt, or more, upstream from the position of leading polymerases paused at the A tract. The furthest the trailing polymerases of this population transcribed was to position −45/−46 relative to the G stop (Figure 7C, lanes 4 and 8, black arrowheads). These transcripts therefore represent trailing polymerases that failed to interact sufficiently with the paused leading polymerase to push it (they only transcribed to position −13 relative to the G stop; Figure 7C, lanes 2 and 6; gray arrowheads) and which were therefore prohibited from progressing further. We note that the difference between polymerase positions (−13 versus −45/−46, i.e., 32–33 nt) again corresponds to their being in “touching distance” (Figure 8C). By contrast, no transcripts were observed in the region between −38 and −45 relative to the G stop (Figure 7C, bold line on right), indicating that all trailing polymerases that transcribed this close to a leading, stalled polymerase (25–-26 nt from its active site) drove it through the pause and so did not stop at these positions themselves (Figure 7D).
These results indicate that, as observed for the trailing polymerase previously, collision does not perturb the activity of the leading polymerase. Rather, collision greatly stimulates pause-site readthrough by this polymerase.
Polymerase Collision and TFIIS Action Cooperate to Allow Efficient Transcription through Pause Sites
TFIIS enhances transcription through pause sites (reviewed by Arndt and Kane, 2003). As expected, we found that at a high NTP concentration, TFIIS was capable of stimulating efficient pause-site readthrough. However, polymerase collision showed a significant additive effect, further promoting transcription (Figure S2). This was particularly clear at a limiting UTP concentration. Here, TFIIS enhanced transcription through the pause site when added at up to equimolar concentration relative to RNAPII (Figure 7E, compare lane 1 with lanes 2 and 3) but had an inhibitory effect when added at a higher concentration (lane 4). The stimulatory amounts of TFIIS were used in collision experiments. Notably, rear-end collision by the trailing polymerase stimulated pause-site readthrough by leading RNAPII to a higher degree than addition of the optimal TFIIS concentration (Figure 7F, compare lanes 3 and 4). Moreover, whereas less than 10% of polymerases transcribed through the pause site in the absence of both TFIIS and collision (Figure 7F, lane 1), the combination of TFIIS and RNAPII collision resulted in more than 60% of the polymerases transcribing through to the end (lane 6).
These results demonstrate that RNAPII collision and TFIIS action can cooperate to allow transcription through pause sites.
Discussion
Mechanism of RNAPII Collision: Complementing the Information Gained from RNAPII Crystallography
Considerable progress in our understanding of RNAPII transcription has been achieved through solving crystal structures of RNAPII at different stages of transcription (Kornberg, 2007). The enzymological evidence reported here complements and extends these data. Arguably, one of the most surprising findings of this study is the prediction of a temporary state during impact where the colliding elongation complexes are structurally “intermingled” (Figure 8). This state requires structural changes in the protein component or/and the DNA component of the RNAPII elongation complexes. In the former case, it seems likely that the elongation complex is “elastic,” with flexible domains of either (or both) polymerase(s) changing conformation (swivelling/rotating) to accommodate the presence of the bulk of the other polymerase and then regaining their original shape upon backtracking (Figure 8D, model 1). Interestingly, substantial movement of polymerase domains, not least of modules such as the jaw lobe and clamp, is indeed structurally feasible (Cramer et al., 2000, 2001; Darst et al., 2002; Gnatt et al., 2001). While the precise extent of freedom of jaw lobe movement remains unclear, the clamp might be free to move out by as much as 20 degrees (Darst et al., 2002).
In the case of structural changes in the DNA component of elongation complexes (Figure 8D, model 2), helix unwinding (twisting) between the transcription bubbles by polymerase rotation might gain space between the colliding polymerases, which enables the trailing polymerase to continue forward translocation on DNA while remaining in touching distance of the leading polymerase. Alternatively, or additionally, the DNA between the transcription bubbles may temporarily become stretched as the trailing polymerase keeps moving forward on DNA from the point where it has reached the point of contact with the leading polymerase. Reformation of a stable DNA double helix might thus also help drive backtracking. Finally, if some stripping of DNA contacts with RNAPII occurs, reformation of these might contribute as well.
In any case, the mechanism outlined here differs fundamentally from that proposed for transcription into a nucleosome. When RNAPII transcribes into the nucleosome, it appears to unwrap the DNA from the histone core. When it pauses and backtracks in doing so, the backtracked conformation is stabilized by restoration of DNA-histone contacts in front of it, causing transcriptional arrest (Kireeva et al., 2005). In agreement with this model, the prominent pause and arrest sites in the nucleosome are observed at intrinsic pause sites of DNA. In contrast, DNA is not “wrapped around” the elongation complex, and the transcripts generated by trailing polymerase in the furthest position it transcribes to and where it is completely blocked by the leading polymerase are not observed on naked DNA; their production is more consistent with polymerase hitting a flexible protein “wall.” Likewise, the backtracking resulting from this clash is initiated by the elastic nature of elongation complexes themselves (Figures 3–5).
Translocation by RNAPII is probably best explained by a Brownian ratchet model, according to which fluctuation between pre- and posttranslocated states is driven toward forward motion primarily by NTP binding and hydrolysis: binding of the incoming nucleotide increases dwell time in the posttranslocation position, so that chain elongation is favored (reviewed by Herbert et al., 2008). However, if NTP-mediated stabilization does not occur, backtracking is almost as likely as forward translocation because of Brownian motion. It thus seems reasonable to suggest that when collision with an immovable leading polymerase occurs, the transcription ratchet (the trailing polymerase) moves against greater and greater force so that when the next translocation step can no longer be secured (a state of low entropy, because the elongation complexes have fewer conformations available), rapid retrograde motion occurs, probably driven at least partly by the increase in entropy reforming the stable equilibrium states (high entropy) of both elongation complexes entails.
Oscillation in the Presence of TFIIS
The idea of conformational elasticity of elongation complexes was supported by exonuclease mapping of the trailing polymerase during collision in the presence of TFIIS. This polymerase's active site (as inferred from both T7 exonuclease mapping and permanganate footprinting; Figures 3–6) was positioned 5–8 nt upstream from the position predicted from the length of the transcript it was generating. The only reasonable explanation for these data is that the trailing polymerase constantly oscillates at impact, quickly transcribing forward after TFIIS-promoted transcript cleavage to the foremost position (to produce the transcripts we detect) and then immediately backtracking to upstream positions (the shortest of which were detected by footprinting techniques) before TFIIS acts again (Movie S1). Indeed, 5–15 nt RNA cleavage products accumulated with time, in a collision- and TFIIS-dependent manner (Figure 6). Moreover, the most forward position in which the polymerases spend the vast majority of time in the presence of TFIIS is also the position at which only minimal elongation complex-elongation complex incursion occurs (Figure 8C). RNAPII oscillation might help ensure that, facing obstacles, the polymerase can adapt dexterously to changes in circumstances by keeping itself in the active state.
Cooperation between RNAPII Molecules
Our results indicate that during collision at a pause site, structural elasticity and dynamic interactions between trailing and leading polymerase in effect generate bias in Brownian motion for the leading polymerase toward the forward direction, so that its active site is kept in register with the end of the RNA, even through the long A tract. Evidence for cooperation between bacterial RNA polymerase was previously obtained (Epshtein and Nudler, 2003; Epshtein et al., 2003). Our results on transcription by RNAPII confirm and extend the work on the bacterial enzyme by Epshtein and Nudler by pointing to the elasticity and dynamic interactions of elongation complexes as a driving factor and underlying mechanism, and by showing that polymerase collision and TFIIS-promoted transcript cleavage can be cooperative, helping the majority of leading polymerases through a potent pause site (Figure 7). It seems reasonable to suggest that TFIIS allows polymerases to oscillate against obstacles (Figures 3–6), while the resulting repeated collisions increase the time spent by the leading polymerase in the posttranslocation state, so that NTP can bind, and further progress is stimulated.
We note that the similarity between bacterial RNA polymerase and eukaryotic RNA polymerase II is confined to the protein core surrounding the active site (Cramer et al., 2000). In contrast, proteins and protein domains (such as Rpb9 and the Rpb1 jaw domain [on the jaw lobe side], as well as Rpb5 [on the clamp side]) that are likely to be touching the leading polymerase during collision are not conserved between prokaryotic and eukaryotic RNA polymerases (Figure 8A, upper panel, boxed). Therefore, the precise mechanism of RNA polymerase cooperation may have changed in the course of evolution. Indeed, the extension of the polymerase footprint downstream from the active site that has occurred during evolution (phage, prokaryote, eukaryote) might have served to improve the stability of elongation complexes during collision, enabling transcription of longer genes.
Biological Significance
During transcript elongation, RNAPII constantly encounters obstacles to its progression, such as DNA-binding proteins, nucleosomes, DNA damage, and other polymerases. Unfortunately, studies of RNAPII collision in vivo are hampered by the fact that conditions that lead to it occur randomly across the transcriptome, with recent evidence supporting the idea that longer genes are more likely to experience “traffic jams” than shorter ones (Swinburne et al., 2008).
Interestingly, results obtained in yeast using an artificial pause site (ARTAR) integrated into a GAL-driven lacZ gene (Kulish and Struhl, 2001) are potentially very informative. Expression of lacZ was dramatically reduced by insertion of the ARTAR sequence and almost undetectable if the gene encoding TFIIS was concomitantly deleted. However, if the gene was expressed at a very high level, the ARTAR had no, or little, negative effect, and TFIIS was no longer needed. Although other possibilities cannot be ruled out, it is tempting to speculate that during low-level expression, backtracking of not only ARTAR-paused RNAPII but also polymerases that collide with these would require TFIIS-stimulated transcript cleavage for reactivation. In contrast, in the highly expressed gene (where polymerases are transcribing “back to back”), the constant pushing by the polymerase behind might render such action unnecessary. We note that such polymerase “group action” could also underlie the transcriptional pulsing bursts with high polymerase density observed by others (Chubb et al., 2006).
Unfortunately, making firm conclusions about molecular events at pause sites (or even at complete blocks to transcription, such as DNA lesions) in vivo is greatly complicated by the fact that, in eukaryotes, numerous other elongation factors also affect transcription through obstacles (Arndt and Kane, 2003), and by the fact that elongating RNAPII can also be ubiquitylated and degraded in response to transcription problems (Somesh et al., 2005). Moreover, our recent results indicate that the (TFIIS-independent) intrinsic transcript cleavage activity of RNAPII itself, although typically virtually undetectable in vitro, may nevertheless be important for transcription and cell viability (S. Sigurdsson, A.B. Dirac-Svejstrup, and J.Q.S., unpublished data).
The data presented here suggest a mechanism by which the extraordinary robustness of transcription, with individual polymerases being required to traverse very long genes (in human cells up to a million nucleotides long) without dissociation, might be possible. In this model, the remarkable conformational flexibility of RNAPII elongation complexes buffers the effect of running into objects on DNA, so that when collisions occur, the polymerase in effect “bounces off” and retains full activity. The structural flexibility of RNAPII may be important to generally regulate the flow of polymerases on genes, and besides the help provided by numerous transcription elongation factors, actively transcribing polymerases can also assist each other.
Experimental Procedures
Oligonucleotides and Proteins
For the collision experiments, the sequence of the transcribed strand DNA (TS) was 5′-CCGCAACCACATTCCAACCACACATCACTTACCAGGATACACATCAAACATACACCCAACTAACCAACTACTAACACACAATCACACACTCACCTACCATAACCAACTCACAACAACAACACCTCTCCATACTCTACTCCTATACCACGC-3′. Full information about the sequences of the oligonucleotides can be found in Figure S1. RP-HPLC purified oligos were purchased from DNA Technology A/S (Aarhus, Denmark) and purified further by 5.2% denaturing PAGE. Radioactive 5′ end labeling was by T4 polynucleotide kinase (NEB), or, alternatively, 3′ ends were labeled with dideoxy-ATP (Amersham), or 3′ deoxy-ATP (NEN), using terminal transferase (Roche). Prior to T7 exonuclease assays, end-labeled oligos were repurified by 5.2% denaturing PAGE.
Recombinant yeast TFIIS and wild-type, 12 subunit RNAPII were purified from E. coli and yeast (BJ926), respectively (Christie et al., 1994; Edwards et al., 1990), and dialyzed against 100 mM KOAc, 20 mM HEPES-NaOH (pH 7.6, 26°C), 0.1 mM EDTA, 10% glycerol, 2 mM DTT, 0.1 mM PMSF. Unless specified, TFIIS was used in equimolar concentration to RNAPII.
Reconstitution and Characterization of RNAPII Ternary Complexes
Elongation complexes (ECs) were reconstituted essentially as described by Kashlev and colleagues (e.g., Kireeva et al., 2000). All experiments were done using nonstick microtubes and tips (Axygen). Oligos were prepared at the following concentrations in reconstitution buffer (as transcription buffer described below except 0.2 mM MgCl2). Typically, 4 μl 1 pmol/μl TS and 2 μl each of 4 pmol/μl RNA oligos were mixed and incubated at 65°C for 5 min, followed by gradual cooling to 25°C (−0.1°C/6 s for 40 min in thermal cycler). After addition of 2.6–3 μl 4 pmol/μl RNAPII (a 2.5–3 molar excess over TS), the reaction was incubated at 25°C for 25–30 min and at 37°C for 1 min. Then, 8 μl 1–1.25 pmol/μl NTS (pretreated by heating to 65°C for 5 min, then on ice for 2 min, and finally at 37°C for 2 min) was added and incubated for 10–15 min at 37°C. The final concentration of TS was 0.10–0.14 pmol/μl after adding supplement buffer to obtain transcription conditions (40 mM KCl, 20 mM Tris-Cl [pH 7.9], 7 mM MgCl2, 20 μM ZnCl2, 5 mM DTT, ±0.75 μg/μl BSA). Assembled ECs were kept on ice until use.
After addition of an equal volume of loading buffer (0.75 μg/μl BSA, 5 mM 2-mercaptoethanol, and with glycerol added to a final concentration of 3%), samples were resolved by 0.7% agarose gel electrophoresis in 0.25 × TB buffer (22.3 mM Trizmabase/SIGMA, 22.3 mM boric acid, 0.1 mM MgCl2, 0.01 mM ZnCl2, 5 mM 2-mercaptoethanol) at 4°C.
Transcription was initiated by adding nucleotides, typically 0.5 mM of each (no CTP) at 26°C. Transcription of A tract DNA was at 30°C and was initiated by 0.025 or 0.1 mM UTP, 1 mM GTP, and 0.5 mM ATP. For analysis of transcription products by 6% denaturing PAGE, the reaction was terminated by addition of an equal volume of urea analysis buffer (10 M urea, 80 mM EDTA, 0.05% [w/v] xylene cyanol, 0.05% bromphenol blue). Gels were dried on DEAE paper (DE81/Whatman) and subjected to autoradiography, as well as exposure for phosphor imaging. Where RNA was isolated from purified ECs, the ECs were resolved by agarose gel electrophoresis after transcription as described above, stained with SYBR Green I at 4°C, and visualized by fluorescence laser scanner (FLA-5000 FUJI). EDTA (to give a final concentration of 7.5 mM) and tRNA (to give a final concentration of 8 μg/ml) were added to the excised bands corresponding to ECs. These were then crushed by using a micropestle in a microtube and filtrated by Nanosep MF/0.45 μm (Pall). RNA was phenol/chloroform extracted and ethanol precipitated.
Footprinting Analyses of Elongation Complexes
DNase I footprinting: radioactively end-labeled ECs were incubated with 0.0056, 0.011, or 0.022 units/μl DNase I (Roche) at 24°C for 1 min. As control, naked DNA was digested with 0.0028, 0.0056, or 0.011 units/μl DNase I. Reactions were stopped by the addition of EDTA, and samples were resolved by agarose gel electrophoresis as described above, without MgCl2 and ZnCl2, and visualized with SYBR Green I as described above. DNA was then extracted and purified by QIAEX II (QIAGEN) and finally analyzed by 7% denaturing PAGE.
T7 exonuclease footprinting: radioactively end-labeled ECs were incubated with 0.4 units/μl T7 exonuclease (NEB) at 26°C. The reaction was terminated with an equal volume of urea analysis buffer as described above and analyzed by 5.2% denaturing PAGE. Where transcription bubbles were analyzed, samples were treated with freshly prepared KMnO4, and the reaction was stopped with an equal volume of stop buffer (2 M 2-mercaptoethanol, 40 mM EDTA, 100 μg/ml tRNA). This DNA was purified by phenol/chloroform extraction and ethanol precipitation, dissolved in TE (pH 8, 26°C) with an equal volume of urea isolation buffer (10 M urea, 20 mM EDTA, 5 mM Tris-Cl [pH 7.5, 26°C]) and resolved by 5.2% denaturing PAGE. After taking an autoradiograph of the wet gel, bands corresponding to the relevant T7 exonuclease (and permanganate-)- treated oligos were excised and crushed by micropestle. DNA was eluted in elution buffer (10 mM Tris-Cl [pH 7.5, 26°C], 1 mM EDTA, 0.1% SDS), filtered by Nanosep MF/0.2 μm (Pall) and purified by phenol/chloroform extraction and ethanol precipitation. Modified thymines were cleaved by piperidine, and the resulting DNA was analyzed by 6% denaturing PAGE.
Molecular Modeling
Modeling of elongation complex interaction was based on the crystal structure of the elongation complex (Wang et al., 2006) (PDB 2E2H) using PyMOL. TS (−9 to +18), NTS (+5 to +18), and RNA (−9 to −1) are shown, but TS (−10) and RNA (−10) were omitted. Numbers in brackets indicate positions relative to the active site (+1).
Acknowledgments
This work was supported by a grant from the European Community (Integrated Project DNA repair, grant number LSHG-CT-2005-512113) and by an in-house grant from Cancer Research UK (to J.Q.S.). We thank Stefan Sigurdsson for recombinant yeast TFIIS and helpful discussions and Caroline Kane for the TFIIS expression plasmid. Dave Bushnell and Roger Kornberg are thanked for sharing unpublished information and their insight into the RNAPII structure. Members of the Svejstrup lab, Dale Wigley, Craig Kaplan, Dave Bushnell, Dong Wang, Roger Kornberg, and Peter Verrijzer are thanked for comments on the manuscript.
Published: July 30, 2009
Footnotes
Supplemental Data include two figures and one movie and can be found with this article online at http://www.cell.com/molecular-cell/supplemental/S1097-2765(09)00400-6.
Supplemental Data
References
- Arndt K.M., Kane C.M. Running with RNA polymerase: eukaryotic transcript elongation. Trends Genet. 2003;19:543–550. doi: 10.1016/j.tig.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Christie K.R., Awrey D.E., Edwards A.M., Kane C.M. Purified yeast RNA polymerase II reads through intrinsic blocks to elongation in response to the yeast TFIIS analogue, P37. J. Biol. Chem. 1994;269:936–943. [PubMed] [Google Scholar]
- Chubb J.R., Trcek T., Shenoy S.M., Singer R.H. Transcriptional pulsing of a developmental gene. Curr. Biol. 2006;16:1018–1025. doi: 10.1016/j.cub.2006.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conaway J.W., Shilatifard A., Dvir A., Conaway R.C. Control of elongation by RNA polymerase II. Trends Biochem. Sci. 2000;25:375–380. doi: 10.1016/s0968-0004(00)01615-7. [DOI] [PubMed] [Google Scholar]
- Cramer P., Bushnell D.A., Fu J., Gnatt A.L., Maier-Davis B., Thompson N.E., Burgess R.R., Edwards A.M., David P.R., Kornberg R.D. Architecture of RNA polymerase II and implications for the transcription mechanism. Science. 2000;288:640–649. doi: 10.1126/science.288.5466.640. [DOI] [PubMed] [Google Scholar]
- Cramer P., Bushnell D.A., Kornberg R.D. Structural basis of transcription: RNA polymerase II at 2.8 angstrom resolution. Science. 2001;292:1863–1876. doi: 10.1126/science.1059493. [DOI] [PubMed] [Google Scholar]
- Darst S.A., Opalka N., Chacon P., Polyakov A., Richter C., Zhang G., Wriggers W. Conformational flexibility of bacterial RNA polymerase. Proc. Natl. Acad. Sci. USA. 2002;99:4296–4301. doi: 10.1073/pnas.052054099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzacq X., Shav-Tal Y., de Turris V., Brody Y., Shenoy S.M., Phair R.D., Singer R.H. In vivo dynamics of RNA polymerase II transcription. Nat. Struct. Mol. Biol. 2007;14:796–806. doi: 10.1038/nsmb1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A.M., Darst S.A., Feaver W.J., Thompson N.E., Burgess R.R., Kornberg R.D. Purification and lipid-layer crystallization of yeast RNA polymerase II. Proc. Natl. Acad. Sci. USA. 1990;87:2122–2126. doi: 10.1073/pnas.87.6.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epshtein V., Nudler E. Cooperation between RNA polymerase molecules in transcription elongation. Science. 2003;300:801–805. doi: 10.1126/science.1083219. [DOI] [PubMed] [Google Scholar]
- Epshtein V., Toulme F., Rahmouni A.R., Borukhov S., Nudler E. Transcription through the roadblocks: the role of RNA polymerase cooperation. EMBO J. 2003;22:4719–4727. doi: 10.1093/emboj/cdg452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler U., Timmers H.T. Analysis of the open region of RNA polymerase II transcription complexes in the early phase of elongation. Nucleic Acids Res. 2001;29:2706–2714. doi: 10.1093/nar/29.13.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnatt A.L., Cramer P., Fu J., Bushnell D.A., Kornberg R.D. Structural basis of transcription: an RNA polymerase II elongation complex at 3.3 Å resolution. Science. 2001;292:1876–1882. doi: 10.1126/science.1059495. [DOI] [PubMed] [Google Scholar]
- Herbert K.M., Greenleaf W.J., Block S.M. Single-molecule studies of RNA polymerase: motoring along. Annu. Rev. Biochem. 2008;77:149–176. doi: 10.1146/annurev.biochem.77.073106.100741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izban M.G., Luse D.S. The RNA polymerase II ternary complex cleaves the nascent transcript in a 3′→5′ direction in the presence of elongation factor SII. Genes Dev. 1992;6:1342–1356. doi: 10.1101/gad.6.7.1342. [DOI] [PubMed] [Google Scholar]
- Kireeva M.L., Komissarova N., Waugh D.S., Kashlev M. The 8-nucleotide-long RNA:DNA hybrid is a primary stability determinant of the RNA polymerase II elongation complex. J. Biol. Chem. 2000;275:6530–6536. doi: 10.1074/jbc.275.9.6530. [DOI] [PubMed] [Google Scholar]
- Kireeva M.L., Hancock B., Cremona G.H., Walter W., Studitsky V.M., Kashlev M. Nature of the nucleosomal barrier to RNA polymerase II. Mol. Cell. 2005;18:97–108. doi: 10.1016/j.molcel.2005.02.027. [DOI] [PubMed] [Google Scholar]
- Kornberg R.D. The molecular basis of eukaryotic transcription. Proc. Natl. Acad. Sci. USA. 2007;104:12955–12961. doi: 10.1073/pnas.0704138104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulish D., Struhl K. TFIIS enhances transcriptional elongation through an artificial arrest site in vivo. Mol. Cell. Biol. 2001;21:4162–4168. doi: 10.1128/MCB.21.13.4162-4168.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn S.C., Luse D.S. RNA polymerase II elongation complexes paused after the synthesis of 15- or 35-base transcripts have different structures. Mol. Cell. Biol. 1991;11:1508–1522. doi: 10.1128/mcb.11.3.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margaritis T., Holstege F.C. Poised RNA polymerase II gives pause for thought. Cell. 2008;133:581–584. doi: 10.1016/j.cell.2008.04.027. [DOI] [PubMed] [Google Scholar]
- Reines D. Elongation factor-dependent transcript shortening by template-engaged RNA polymerase II. J. Biol. Chem. 1992;267:3795–3800. [PMC free article] [PubMed] [Google Scholar]
- Saunders A., Core L.J., Lis J.T. Breaking barriers to transcription elongation. Nat. Rev. Mol. Cell Biol. 2006;7:557–567. doi: 10.1038/nrm1981. [DOI] [PubMed] [Google Scholar]
- Shilatifard A., Conaway R.C., Conaway J.W. The RNA polymerase II elongation complex. Annu. Rev. Biochem. 2003;72:693–715. doi: 10.1146/annurev.biochem.72.121801.161551. [DOI] [PubMed] [Google Scholar]
- Singer R.H., Lawrence D.S., Ovryn B., Condeelis J. Imaging of gene expression in living cells and tissues. J. Biomed. Opt. 2005;10:051406. doi: 10.1117/1.2103032. [DOI] [PubMed] [Google Scholar]
- Somesh B.P., Reid J., Liu W.F., Sogaard T.M., Erdjument-Bromage H., Tempst P., Svejstrup J.Q. Multiple mechanisms confining RNA polymerase II ubiquitylation to polymerases undergoing transcriptional arrest. Cell. 2005;121:913–923. doi: 10.1016/j.cell.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Struhl K. Transcriptional noise and the fidelity of initiation by RNA polymerase II. Nat. Struct. Mol. Biol. 2007;14:103–105. doi: 10.1038/nsmb0207-103. [DOI] [PubMed] [Google Scholar]
- Swinburne I.A., Miguez D.G., Landgraf D., Silver P.A. Intron length increases oscillatory periods of gene expression in animal cells. Genes Dev. 2008;22:2342–2346. doi: 10.1101/gad.1696108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Bushnell D.A., Westover K.D., Kaplan C.D., Kornberg R.D. Structural basis of transcription: role of the trigger loop in substrate specificity and catalysis. Cell. 2006;127:941–954. doi: 10.1016/j.cell.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Martin C.T. Observed instability of T7 RNA polymerase elongation complexes can be dominated by collision-induced “bumping”. J. Biol. Chem. 2006;281:24441–24448. doi: 10.1074/jbc.M604369200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.