Summary
An algal sulfated galactan has high anticoagulant and antithrombotic activities. Its serpin-dependent anticoagulant action is due to promoting thrombin and factor Xa inhibition by antithrombin and heparin cofactor II. Here, we evaluated the anticoagulant effect of the algal sulfated galactan using serpin-free plasma. In contrast to heparin, the sulfated galactan is still able to prolong coagulation time and delay thrombin and factor Xa generation in serpin-free plasma. We further investigated this effect using purified blood coagulation proteins, discovering that sulfated galactan inhibits the intrinsic tenase and prothrombinase complexes, which are critical for factor Xa and thrombin generation, respectively. We also investigated the mechanism by which sulfated galactan promotes factor Xa inhibition by antithrombin using specific recombinant mutants of the protease. We show that sulfated galactan interacts with the heparin-binding exosite of factor Xa and Arg-236 and Lys-240 of this site are critical residues for this interaction, as observed for heparin. Thus, sulfated galactan and heparin have similar high-affinity and specificity for interaction with factor Xa, though they have differences in their chemical structures. Similar to heparin, the ability of sulfated galactan to potentiate factor Xa inhibition by antithrombin is calcium-dependent. However, in contrast to heparin, this effect is not entirely dependent on the conformation of the γ-carboxyglutamic acid-rich domain of the protease. In conclusion, sulfated galactan and heparin have some similar effects on blood coagulation, but also differ significantly at the molecular level. This sulfated galactan opens new perspective for the development of antithrombotic drugs.
Keywords: antithrombotic drug, heparin, heparin-binding exosite, prothrombinase complex, sulfated galactan, tenase complex
Introduction
The leading cardiovascular diseases with high mortality/morbidity in humans involve the heart and blood vessels [1] and frequently result in thromboembolism. Once the diagnosis of venous thromboembolism is established, prompt anticoagulation treatment is necessary to prevent thrombus growth and reduce the risk of pulmonary embolism. Furthermore, any condition that predicts an increased risk of thrombosis requires the preventive use of anticoagulants. Heparin has been used for more than 50 years for the treatment and prevention of thrombosis [2]. This glycosaminoglycan is also required for extracorporeal circulation during cardiovascular surgeries and renal dialysis [3]. The current sources of heparin are animals, as it is obtained only from pig intestine or bovine lung. It is the second most frequently used natural drug. As a consequence of the increasing use of heparin there is now an urgent need for new anticoagulant drugs or alternative sources of heparin [4].
Fortunately, heparin is not the only polysaccharide capable of inhibiting blood coagulation proteases. A variety of sulfated galactans and sulfated fucans from marine algae and invertebrates also are anticoagulants [5-7]. The distinct structures of these polysaccharides when compared with heparin raise the following questions: How can these polysaccharides act as anticoagulant? Can they bind with high affinity to specific domain of proteins from the coagulation system?
One may speculate that sulfated galactans and sulfated fucans bind nonspecifically to basic patches on proteins of the coagulation system, and this binding leads to inhibition of the protease activity. However, this hypothesis is not supported by recent studies that describe the anticoagulant action of sulfated galactans and sulfated fucans. The structural requirements for interaction of sulfated polysaccharides with coagulation inhibitors and their target proteases are stereospecific and not merely a consequence of charge density or sulfate content [7-11]. In addition, upon systematic analysis of sulfated polysaccharides from several species of marine algae, we were able to identify a sulfated galactan with anticoagulant activity as potent as unfractionated heparin [5,12]. This potent anticoagulant sulfated galactan was extracted from the red alga Botryocladia occidentalis and is composed of the following repeating structure (-4-α-D-Gal-1→3-β-D-Gal-1→). One-third of the total α-units are 2,3-di-sulfated and one-third are 2-sulfated (Fig. 1). The potent anticoagulant activity of this algal galactan was attributed mostly to the 2,3-di-sulfated units [5].
Figure 1. Structure of sulfated galactan from the marine alga B. occidentalis.
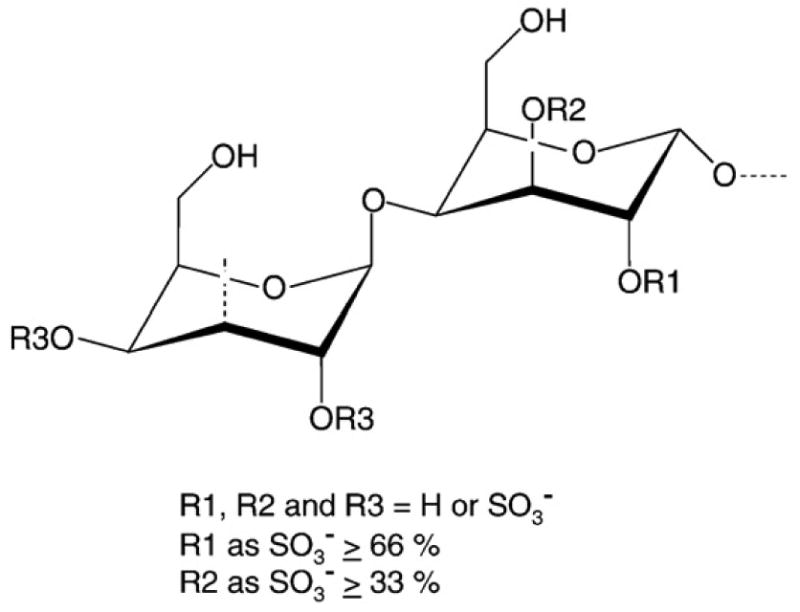
This polysaccharide has a repeating structure (-4-α-D-Galp-1→3-β-D-Galp-1→), with a variable sulfation pattern. Approximately one third of the total α-units are 2,3-di-sulfated, and another one-third are 2-sulfated (Farias et al. 2000).
This sulfated galactan has been studied as a catalyst of antithrombin-mediated inactivation of thrombin [9,13]. In some respects, the ways in which sulfated galactan and heparin assist in antithrombin-mediated thrombin inactivation are quite similar. Both polysaccharides potentiate inactivation of thrombin in similar molar concentrations, interact with the protease in the same region, around exosite II, and promote formation of a covalentlyly bound complex between thrombin and antithrombin. However, at the molecular level, the mechanisms by which these polysaccharides function are different. The first difference is the size of the glycan chain required for the inhibitory effect. The bulk of the sulfated galactan, and not a sequence-specific region, as in the case of heparin, determine its interaction with antithrombin [8,9]. Also, while both polysaccharides share the same binding region in thrombin (exosite II), the sulfated galactan and heparin differ in their specific binding site and/or in the conformational activation they induce in antithrombin [9,13].
In the present study, we expanded our understanding of the anticoagulant action of sulfated galactan on two different directions. First, we demonstrate that the sulfated galactan has a serpin-independent anticoagulant action that is preponderant in the plasma system. This polysaccharide inhibits the intrinsic tenase and prothrombinase complexes, which are critical for thrombin generation. Second, we show that this polysaccharide, similar to heparin, interacts with the heparin-binding exosite on the heavy chain of the factor Xa. These observations may facilitate the development of future anticoagulant drugs from the sulfated galactan.
Materials and Methods
Materials
Human plasmas immunodepleted of heparin cofactor II and/or antithrombin were obtained from Affinity Biologicals (Ancaster, ON, Canada). Activated partial thromboplastin time (APTT) reagents were from Wiener Lab. (Rosario, Argentina). Human heparin cofactor II, antithrombin, thrombin, factors Va, Xa and X were from Haematologic Technologies (Essex Junction, VT, U.S.A.). Human factor IXa was from Enzyme Research Laboratories (South Bend, IN, U.S.A.). Factor VIII was from Baxter (Deerfield, IL, U.S.A.). Factor VIII was activated with human thrombin, as previously described [14]. Prothrombin was purified as described [15]. PC (α-L-phosphatidylcholine) and PS (α-L-phosphatidylserine) were from Sigma Chemical Co. (St. Louis, MO, U.S.A.). Phospholipid vesicles, composed of 75% (w/w) PC and 25% (w/w) PS were prepared by sonication. Briefly, phospholipids in chloroform were dried with a stream of N2; resuspended in 50 mM Tris/HCl, 150 mM NaCl, pH 7.5; and sonicated for 2 min. The solution was adjusted further to 500 μM (final concentration). S-2238 (H-D-phenylalanyl-L-pipecolyl-L-arginine-p-nitroaniline-dihydrochloride) and S-2765 (N-α-benzyloxycarbonyl-D-Arg-Gly-Arg-p-nitroanilide) were from Chromogenix (Mölndal, Sweden). The snake venom enzyme Ancrod and dermatan sulfate from porcine mucosa were purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.). Heparin was the 5th International Standard from the National Institute for Biological Standards and Control (Potter Bar, United Kingdom). Recombinant GD-factor Xa (Gla-domainless factor Xa, i.e. factor Xa from which N-terminal residues 1-45 have been removed) forms (wild type and mutants R93A, K96A, R125A, R165A, K169A, K236A and R240A) were expressed and purified as described elsewhere [16]. Recombinant Ixolaris, a protein characterized from tick salivary gland, was purified as described [17].
Sulfated galactan
The sulfated galactan was extracted from the red alga B. occidentalis by protease digestion and purified by anion-exchange chromatography, as previously described [5]. Low-molecular-weight sulfated galactan was obtained by mild acid hydrolysis of the native polysaccharide and purified by gel filtration chromatography, as described [9]. The purity and structure of these polysaccharides were checked by agarose gel eletrophoresis and NMR spectroscopy.
Activated partial thromboplastin time assay
A mixture of 100 μL of normal or antithrombin/heparin cofactor II-free human plasma and various concentrations of sulfated polysaccharide were incubated with 100 μL of APTT reagent (kaolin bovine phospholipid reagent). After 2 min of incubation at 37°C, 100 μL of 25 mM CaCl2 was added to the mixtures, and the clotting time was recorded in a coagulometer (Amelung KC4A). The results were expressed as ratios of clotting time in the presence (T1) and in the absence (To) of the sulfated polysaccharide.
Thrombin- or factor Xa-generation test in plasma
For these experiments, we used defibrinated human plasmas, which were prepared as follows: normal and serpin-free plasmas were incubated with 0.1 unit/mL of Ancrod for 30 min at 37°C. Thereafter, the clot formed was removed, and the plasma was centrifuged (2700 × g, 10 min). The supernatant was used for thrombin and factor Xa generation tests. Formation of thrombin or factor Xa in plasma was followed after activation of the intrinsic pathway. Fifty μL of normal or serpin-free plasma was incubated with sulfated polysaccharides (0.2 μg/mL final concentration) in 50 μL of 50 mM Tris/HCl, 150 mM NaCl and 0.1% PEG-8000, pH 7.5 (TS/PEG buffer) and 10 μL of APTT reagent, for 2 min at room temperature. Thrombin- or factor Xa-generation tests were started by the addition of 100 μL of 12.5 mM CaCl2, and aliquots of 10 μL of the mixtures were removed every 15 sec into microplate wells, containing 40 μL of 50 mM Tris/HCl and 50 mM EDTA. The amount of thrombin or factor Xa formed was evaluated as described above, using chromogenic substrates S-2238 or S-2765, respectively. Hydrolysis of the substrate was detected using a Thermomax Microplate Reader (Molecular Devices, Menlo Park, CA, USA), equipped with a microplate mixer and heating system. Reactions were recorded continuously at 405 nm, for 5 min, at 37°C. The total volume of the reactions was 210 μL.
Effect of sulfated polyaccharides on the activity of intrinsic tenase complex
The effect of heparin or sulfated galactan on factor X activation by the intrinsic tenase complex was followed using a previously described discontinuous assay [18]. Different concentrations of sulfated polysaccharide were incubated with 200 pM factor IXa, 1 unit/mL factor VIIIa and 20 μM PC/PS vesicles, in TS/PEG buffer containing 10 mM CaCl2, final volume of 100 μL, for 2 min, at 37°C. Reaction was started by the addition of 50 nM factor X, and 6 min later, aliquots of 25 μL were removed into microplate wells, containing 25 μL of TS/PEG buffer containing 50 mM EDTA. The amount of factor Xa formed was determined after the addition of 50 μL of 100 μM chromogenic substrate S-2765 to the reaction mixtures, based on absorbance at 405 nm, recorded at 37°C for 15 min at 9 sec-intervals, using a Thermomax Microplate Reader (Molecular Devices, Menlo Park, CA, U.S.A.). Velocities (milli-absorbance units/min) obtained in the first few minutes of reaction were used to calculate the amount of factor Xa formed using a standard curve.
Effect of sulfated polyaccharides on the activity of prothrombinase complex
The effect of heparin or sulfated galactan on prothrombin activation by the prothrombinase complex was followed using a previously described discontinuous assay [19]. Different concentrations of sulfated polysaccharide were incubated with 10 pM factor Xa, 1 nM factor Va and 20 μM PC/PS vesicles, in TS/PEG buffer containing 10 mM CaCl2, final volume of 100 μL, for 2 min at 37°C. The reaction was started by the addition of 0.5 μM human prothrombin. After 4 min, aliquots of 10 μL were removed into microplate wells, containing 40 μL of TS/PEG buffer containing 50 mM EDTA. The amount of thrombin formed was determined after addition of 50 μL of 200 μM of the chromogenic substrate S-2238, as described above for factor Xa.
Prothrombin activation monitored by SDS/PAGE
Activation of prothrombin into thrombin by the prothrombinase complex was also monitored by SDS/PAGE. For this assay, incubation mixtures contained 1 nM FXa, 3 nM FVa and 20 μM PC/PS vesicles in TS/PEG buffer + 10 mM CaCl2. The incubations were performed for 15 min at room temperature in the absence or in presence of 10 μg/mL sulfated polysaccharides. The reaction was started by the addition of 1.4 μM human prothrombin, final volume of 500 μl. In order to decrease the formation of unspecific cleavage products by thrombin, the reactions were carried out in the presence of 6 μM DAPA [dansylarginine N-(3-ethyl-1,5- pentanediyl) amide]. Samples (50 μl) were taken at various times, the reaction was stopped with 15 mM EDTA and 5 μM D-Phe-Pro-Arg-chloromethyl ketone (20 μl), and the samples were subjected to SDS/PAGE (12% acrylamide) under non-reducing conditions and gels were stained with Coomassie Blue.
Effect of sulfated polysaccharides on inactivation of thrombin or factor Xa mediated by antithrombin
These experiments were based on assays of the amidolytic activity of thrombin or factor Xa measured by hydrolysis of chromogenic substrates. Increasing concentrations of sulfated polysaccharides in TS/PEG buffer, containing either 10 mM CaCl2 or 20 mM EDTA, were incubated for 5 min at 37°C, with 5 nM antithrombin and 0.5 nM factor Xa or thrombin. Residual factor Xa or thrombin activities were determined by the addition of 100 μM of the chromogenic substrate S-2765 or S-2238, respectively, in a final volume of 100 μL. Hydrolysis of the substrate was detected as described above. In some experiments, plasma-derived factor Xa was replaced by recombinant GD-factor Xa (wild-type or mutants, described above). Removal of antithrombin from the incubation mixture abolishes the effect of the polysaccharides on factor Xa or thrombin inhibition.
Effects of Ixolaris on the inactivation of factor Xa by antithrombin in the presence of sulfated polysaccharides
Increasing concentrations of sulfated polysaccharide in TS/PEG buffer, containing 10 mM CaCl2, were incubated for 5 min at 37°C, with 5 nM antithrombin and 0.5 nM of plasma-derived factor Xa, in the absence or presence of 10 nM Ixolaris. In same assays, incubations were performed with a fixed concentration of sulfated polysaccharide (1 μg/mL of heparin or 10 μg/mL of sulfated galactan) but in the presence of increasing concentrations of Ixolaris (0.5 pM to 50 nM). Residual factor Xa activity was determined using 100 μM of the chromogenic substrate S-2765, as described above.
Sulfated galactan-affinity chromatography
Binding of plasma-derived or recombinant GD-factor Xa was analyzed by affinity chromatography employing a sulfated galactan-Sepharose (1 mL), prepared as described (Melo et al. 2008). One mL of plasma-derived (100 nM) or GD-factor Xa (100nM) were applied onto the column that was pre-equilibrated in TS/PEG buffer containing 10 mM CaCl2., connected to an HPLC system (GE Healthcare) and washed with 5 mL of the same buffer at a flow rate of 0.5 mL/min. Then, the column was subjected to a NaCl gradient (0.15 to 3M) prepared in TS/PEG buffer containing 10 mM CaCl2. Fractions of 0.5 mL were collected and checked for factor Xa activity using S-2765 chromogenic substrate. Concentration of NaCl was estimated based on conductivity. In some cases, plasma-derived factor Xa was previously incubated with 200 nM Ixolaris, for 15 min at room temperature, before the chromatography procedure.
Statistical analysis
Data expressed as mean ± standard deviation were analysed by one-way ANOVA. Differences were considered statistically significant at p < 0.05.
Results
Sulfated galactan has a serpin-independent anticoagulant effect
Sulfated galactan from the red alga B. occidentalis is a potent anticoagulant, with an activity of 130 IU/mg when compared with heparin standard (229 IU/mg), by an APTT assay. We further investigated the anticoagulant effect of this polysaccharide using serpin-free plasmas.
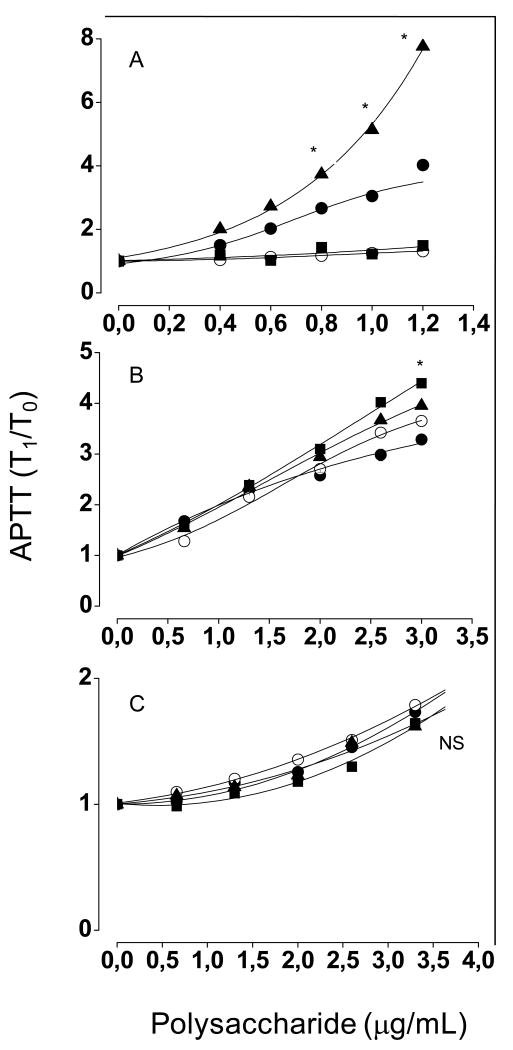
The addition of increasing concentrations of heparin to normal human plasma increased the clotting time, as expected (closed circles in Fig. 2A). In antithrombin-free and both antithrombin and heparin cofactor II-free plasmas this effect was abolished (closed squares and open circles in Fig. 2A). Surprinsingly, in heparin cofactor II-free plasma, the clotting times were even higher after the addition of heparin, when compared with normal control plasma (closed triangles in Fig. 2A). A possible explanation for this observation is that heparin, free from sequestration by heparin cofactor II, yields a more potent antithrombin-mediated anticoagulant effect. In contrast with heparin, sulfated galactan retained its ability to prolong clotting times even in the absence of serpins. Addition of this polysaccharide to either normal or serpin-free plasmas increased the clotting time with similar potency (Fig. 2B). Remarkably, the sulfated galactan at high concentrations (>2.5 μg/mL) was even more active on serpin-free plasmas. The anticoagulant activity of sulfated galactan in serpin-free and normal plasmas was estimated as ∼182 UI/mg and ∼130 UI/mg, respectively. For heparin, the anticoagulant activitiy in serpin-free plasma is <20 UI/mg. This observation suggests that the sulfated galactan, free from sequestration by the serpins, is even more active as an anticoagulant by an alternative pathway.
Figure 2. Anticoagulant activity of heparin (A), high- (B) and low- (C) molecular-weight sulfated galactans based on APTT, performed with normal human plasma (●), antithrombin- (■), heparin cofactor II- (▲) or both serpins-free plasmas (○).
A mixture of 100 μL of normal or serpin-free human plasma and increasing concentrations of sulfated polysaccharide were incubated with 100 μL of APTT reagent. After 2 min of incubation at 37°C, 100 μL of 25 mM CaCl2 was added to the mixtures, and the clotting time was recorded. The results are expressed as ratios of clotting time in the presence (T1) and in the absence (T0) of sulfated polysaccharide. The panels show mean±SD, n=3. (A) *p < 0,05 for ▲, ■ or ○ vs. ●; (B) *p < 0,05 for ■ vs. ●; (C) NS, not significant (p > 0,05).
Sulfated galactan has a significant higher molecular size than heparin (>100 vs ∼15 kDa). Thus, it is possible that sulfated galactan inhibits coagulation by a non-specific mechanism that happens to be also a serpin-independent effect. In order to rule out this possibility, we prepared a low-molecular-weigth derivative from the sulfated galactan with approximately the same molecular mass as heparin (∼15 kDa). This derivative had a lower anticoagulant activity than the native polysaccharide on normal plama (closed circles in Fig. 2C, compare it with panel B). But, more significantly, it showed similar activities in both serpin-free and normal plasmas, as already observed for the native polysaccharide.
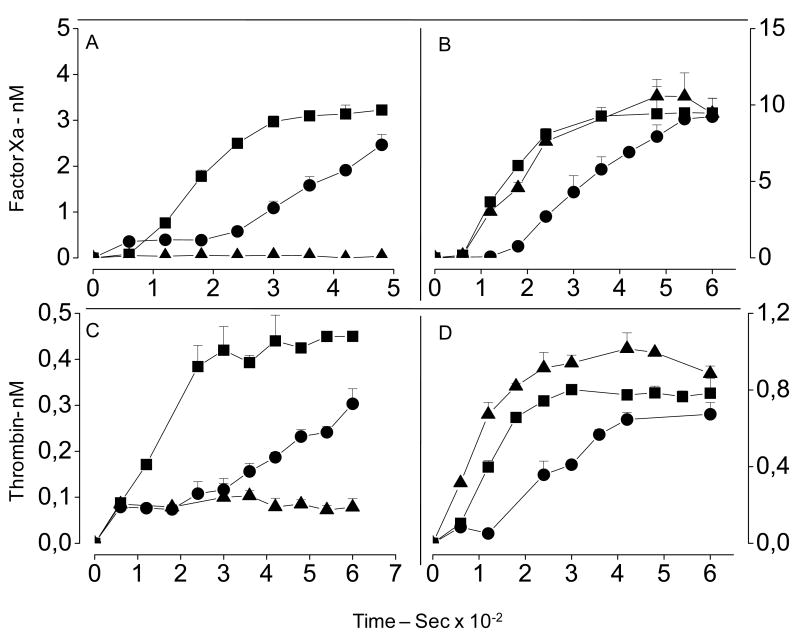
We looked for further evidence of a serpin-independent anticoagulant activity for sulfated galactan using thrombin and factor Xa generation tests. In these assays, normal or serpin-free defibrinated plasma was incubated in the presence or in the absence of sulfated polysaccharides, and then activated with cephalin (Fig. 3). In the absence of sulfated polysaccharides, the assay was characterized by an explosive generation of factor Xa or thrombin until a plateau was reached (closed squares in Fig. 3). When 0.2 μg/mL of heparin (closed triangles, Figs 3A and C) or sulfated galactan (closed circles) were added to the plasma, we observed a delay and a decrease in the generation of the proteases. When similar assays were performed with serpin-free plasmas, the effect of heparin was totally abolished (closed triangles in Figs 3B and D). In contrast, during the period of the experiment, sulfated galactan still retained most of its activity, thus delaying and decreasing generation of factor Xa and thrombin in both assays (closed circles in Figs 3B and D).
Figure 3. Factor Xa (A, and B) and thrombin (C and D) generation tests in normal (A and C) and serpin-free (B and D) plasmas.
Defibrinated plasmas (50 μL) were incubated in the absence (■) or in the presence of 0.2 μg/mL of heparin (▲) or sulfated galactan (●) with 50 μL of TS/PEG buffer and 10 μL of Cephalin reagent. After incubation for 2 min at room temperature, the protease generation reaction was started by addition of 100 μL 12.5 mM CaCl2, and aliquots of 10 μL were removed each 15 s into microplate wells containing 40 μL TS/PEG buffer + 50 mM EDTA. The amounts of factor Xa or thrombin generated were determined using the chromogenic substrates S-2765 or S-2238, respectively. Substrate hydrolysis was detected using a Thermomax Microplate Reader. Reactions were recorded continuously at 405 nm for 15 min at 37°C. The panels show mean±SD, n=3.
Overall, these experiments clearly demonstrated that sulfated galactan has a serpin-independent anticoagulant activity.
Sulfated galactan inhibits activation of prothrombin by the prothrombinase complex
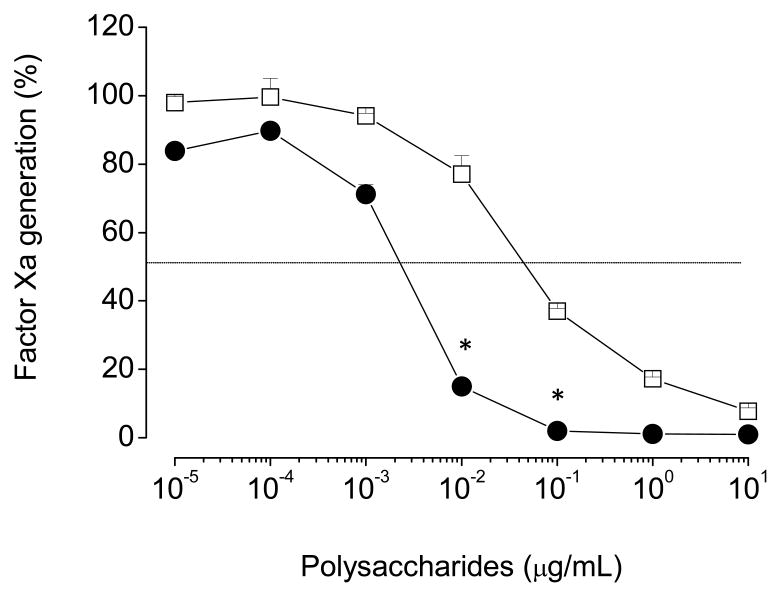
To determine possible sites for the serpin-independent action of sulfated galactan on the coagulation system, we tested it on the prothrombinase complex. For these assays, we incubated increasing concentrations of sulfated polysaccharides with factor Xa, factor Va, prothrombin, phospholipids and calcium. After a 4 min-incubation period, the amount of thrombin generated was determined. No inhibition of thrombin generation occurred with heparin up to 10 μg/mL (open squares, Fig. 4A). This result is consistent with those from Barrow et al. [20]. In contrast, sulfated galactan inhibited thrombin generation by the prothrombinase complex (closed circles, Fig. 4A).
Figure 4. Effect of sulfated polysaccharides on thrombin generation by the prothrombinase complex.
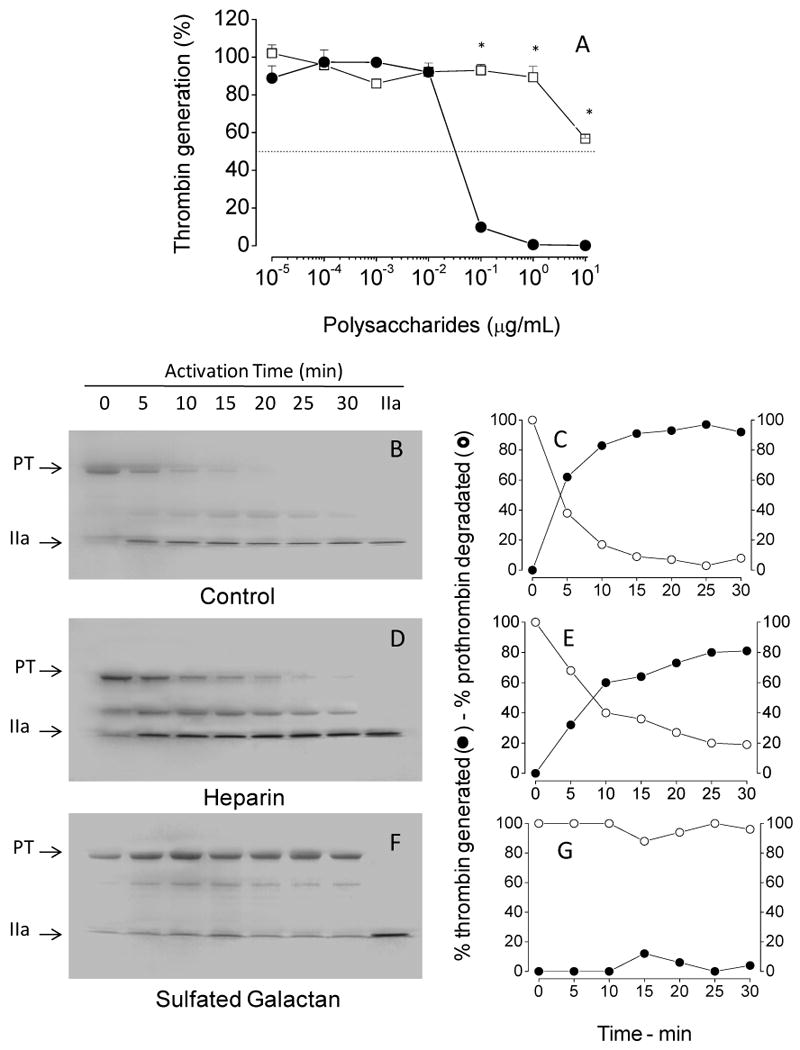
(A) Assays based on protease activity: Increasing concentrations of sulfated galactan (●) or heparin (□) were incubated with 10 pM plasma-derived factor Xa, 1 nM factor Va and 20 μM PC/PS vesicles in TS/PEG buffer containing 10 mM CaCl2, final volume of 100 μL, for 2 min at 37°C. The prothrombinase complex was then activated by the addition of 0.5 μM human prothrombin, and after 4 min, aliquots of 10 μL were removed for the assay of thrombin activity using the chromogenic substrate S-2238, as described in the legend of Fig. 3. The panel shows mean±SD, n=3. (B-G) Assays based on SDS/PAGE: The effect of sulfated polysaccharides on prothrombinase complex was analyzed on 12% SDS-PAGE. The incubation mixtures contain: 1 nM plasma-derived factor Xa, 3 nM factor Va, 20 μM phospholipids and 10 μg/mL sulfated polysaccharide in TS/PEG buffer containing 10 mM CaCl2, final volume 500 μL. After incubation for 10 min at 37°C, the activation reaction was started by addition of 0.5 μM prothrombin. Aliquots from each reaction mixture were removed at the time point indicated in the panels and immediately quenched in SDS-PAGE loading buffer. The gel was stained with Coomassie blue and band intensities for prothrombin and thrombin were monitored by densitometric analysis. PT, prothrombin and IIa, thrombin. *p < 0,05 for □ vs. ●.
The effects of sulfated galactan on thrombin generation were documented further by monitoring prothrombin activation using SDS/PAGE. As shown in Figs 4B and C, conversion of prothrombin into thrombin was almost complete after 15 min of zymogen incubation with the prothrombinase complex. Heparin did not impair conversion of prothrombin into thrombin through the prothrombinase assay (Figs 4D and E). In contrast, sulfated galactan abolishes the activation process. We observe only tenuous electrophoretic bands, corresponding to thrombin, in the time course assay (Figs 4F and G).
Thus, a serpin-independent action of the sulfated galactan on the coagulation system is due to, at least in part, by its ability to inhibit thrombin generation by the prothrombinase complex.
Sulfated galactan inhibits generation of factor Xa by the intrinsic tenase complex
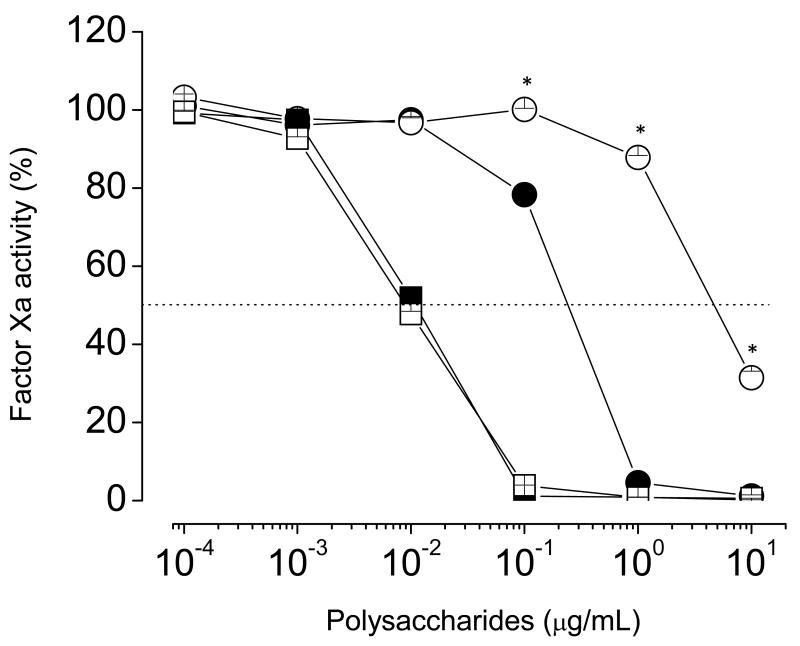
We tested the intrinsic tenase complex as another possible target for the serpin-independent effect of sulfated galactan on the coagulation system. In this assay, we incubated increasing concentrations of the polysaccharides with a mixture of factor IXa, factor VIIIa, factor X, phospholipids and calcium, followed by determination of the amount of factor Xa generated after a 6 min-incubation period (Fig. 5). Increasing concentrations of sulfated galactan or heparin resulted in essentially complete inhibition of factor X activation by the intrinsic tenase complex. However, the concentrations required for inhibition of the intrinsic tenase complex by these two sulfated polysaccharides were significantly different. Total inhibition was obtained with ∼0,002 μg/mL of sulfated galactan. Heparin required ∼20-fold higher concentration to achieve the same effect.
Figure 5. Effect of sulfated galactan (●) and heparin (□) on generation of factor Xa by the intrinsic tenase complex.
Increasing concentrations of sulfated polysaccharide were incubated with a mixture containing 200 pM factor IXa, 1 unit/mL factor VIIIa and 20 μM PC/PS vesicles, in TS/PEG buffer containing 10 mM CaCl2, final volume of 100 μL, for 2 min at 37°C. The tenase complex was activated by the addition of 50 nM factor X, and 6 min later, aliquots of 25 μL were removed into microplate wells containing 25 μl TS/PEG buffer + 50 mM EDTA. The amount of factor Xa formed was determined after the addition of 50 μL of 100 μM chromogenic substrate S-2765, as described in the legend of Fig. 3. The panels show mean±SD, n=3. *p < 0,05 for □ vs. ●.
The effect of sulfated galactan on the intrinsic tenase complex was achieved with a ∼10-fold-lower concentration than that required for prothrombin inhibition (compare curves in Fig. 4A and Fig. 5), suggesting that sulfated galactan is more potent inhibitor of factor Xa generation by tenase complex than of thrombin formation by the prothrombinase complex.
It is possible that the sites of action of sulfated galactan on the intrinsic tenase and on the prothrombinase complexes are similar, since several domains are preserved on the serine proteases of the coagulation system [21-23].
Despite the effect of heparin on the tenase complex, it did not seem to contribute to the anticoagulant action of this glycosaminoglycan in whole plasma, suggesting that sulfated galactan is more effective anticoagulant than heparin. In order to affect clotting assays and protease generation tests, heparin required the presence of serpin (Fig. 2A and Fig. 3). Our results are consistent with those reported by others [20].
Ca2+ affects antithrombin-mediated factor Xa inhibition by the sulfated galactan
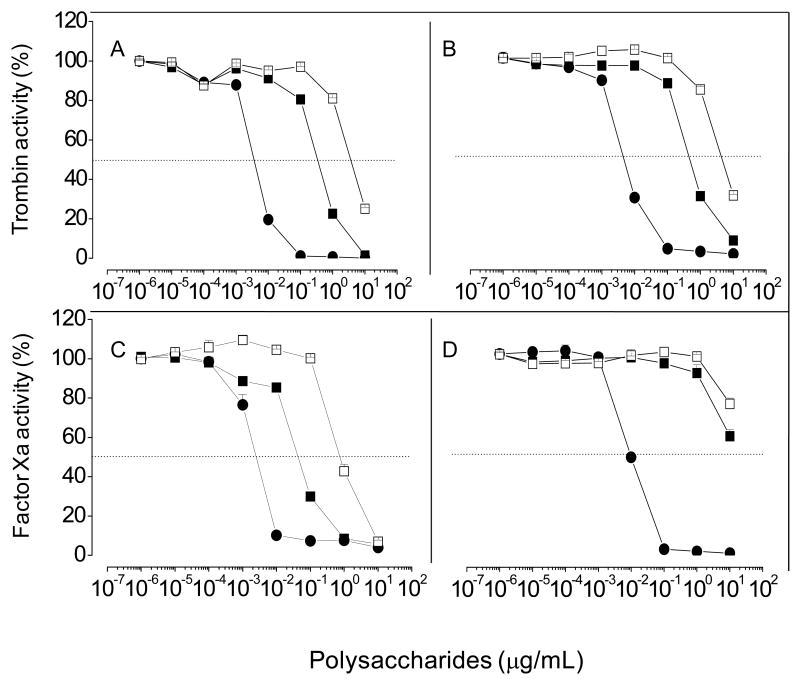
Heparin binds factor Xa with high affinity in the presence of physiological concentrations of Ca2+. This effect results in an additional contribution to promotion of the antithrombin-mediated inhibition of the protease, due to a template mechanism [24]. To understand the effect of Ca2+ on sulfated galactan-catalyzed antithrombin inhibition of coagulation proteases we initially compared the polysaccharide concentration-dependence of inactivation of thrombin (Figs 6A and B) or factor Xa (Figs C and D) by antithrombin in the presence of either 10 mM CaCl2 or 20 mM EDTA (Figs 6A/C and B/D, respectively). Similar inhibition curves were obtained for thrombin in the presence or absence of Ca2+ (Panel A vs B), as expected. In contrast, for factor Xa, the curves were shifted to the right in the absence of Ca2+ (Panels C vs D). The concentration of sulfated galactan necessary to achieve 50% of factor Xa inhibition is ∼200-fold higher in the presence of EDTA when compared with assays with CaCl2. The effect of Ca2+ was apparently more drastic for sulfated galactan than for heparin. Assays with the sulfated galactan with a reduced molecular weight showed a similar Ca2+-dependence (open squares in Fig. 6).
Figure 6. Effect of Ca2 on heparin- or sulfated galactan-catalyzed inactivation of thrombin (A, B) or factor Xa (C, D) by antithrombin.
Thrombin (0.5 nM) or factor Xa (0.5 nM) inactivation by antithrombin (5 nM) was monitored at increasing concentrations of heparin (●), high- (■) or low- (□) molecular-weigth sulfated galactan in TS/PEG buffer, containing either 10 mM CaCl2 (A and C) or 20 mM EDTA (B and D). After 60 s, the remaining thrombin or factor Xa activity was determined by the addition of S-2238 or S-2765, respectively, as described in the legend of Fig. 3. The panels show mean±SD, n=3.
It is postulated that in the absence of Ca2+, the highly anionic N-terminal Gla domain of factor Xa interferes with heparin binding to factor Xa to approximate the enzyme and the inhibitor for enhanced encounter complex formation [25]. In support of this hypothesis, the inhibitory curves of recombinant GD-factor Xa (Gla-domainless factor Xa) by heparin were identical, irrespective of the presence or absence of CaCl2 (open vs closed squares in Fig. 7), as expected. By contrast, a significant decrease in the sulfated galactan-catalyzed inhibition of GD-factor Xa in the absence of Ca2+ was observed (open vs closed circles, Fig. 7). This indicates that occupation of additional Ca2+-binding sites on the protease are required for optimum interaction with the polysaccharide.
Figure 7. Effect of Ca2 on heparin- or sulfated galactan-catalyzed inactivation of GD-factor Xa by antithrombin.
Inactivation of recombinant GD-factor Xa (0.5 nM) by antithrombin (5 nM) was monitored at increasing concentrations of heparin (squares) and sulfated galactan (circles) in TS/PEG buffer, containing either 10 mM CaCl2 (closed symbols) or 20 mM EDTA (open symbols). The total volume of reaction mixture was 100 μL. After 60 s, the remaining factor Xa activity was determined by the addition of S-2238 or S-2765 susbtrate (25 μL of a 200 μM solution), and substrate hydrolysis was monitored as described in the legend of Fig. 3. *p < 0,05 for ○ vs.●.
In conclusion, sulfated galactan and heparin show some similarity on their Ca2+-dependent inhibitory effect on the coagulation proteases, mediated by antithrombin. However, sulfated galactan has some particularity, since its effect is not exclusively dependent on the conformation of the Gla domain of factor Xa.
Mapping the sulfated galactan binding-site on factor Xa
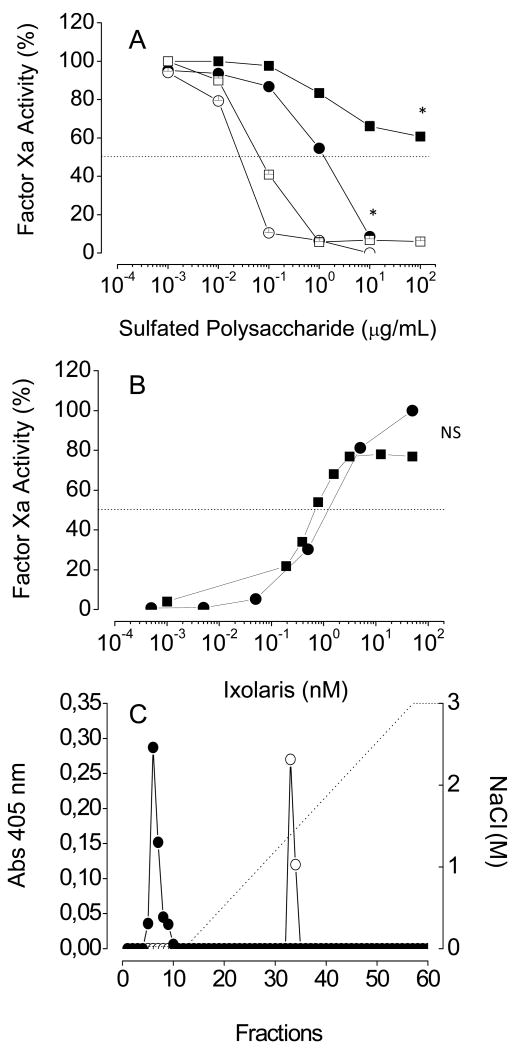
Factor Xa has a heparin-binding exosite located on the heavy chain of the protease [16]. To determine if sulfated galactan actually binds to the same exosite, we performed assays of protease inactivation by the sulfated polysaccharide in the presence of Ixolaris, a specific ligand of the heparin-binding exosite on factor Xa [25]. A significant decrease in the effect of sulfated galactan as catalyst of antithrombin inhibition of factor Xa in the presence of Ixolaris was observed (closed vs open symbols in Fig. 8A). A similar effect was observed for heparin. Thus, the inhibitory reaction was reversed at 50 nM Ixolaris, (Fig. 8B). These results indicate that sulfated galactan (as heparin) and Ixolaris compete for the same binding-site on factor Xa and this binding is crucial for the catalysis of antithrombin-mediated inactivation of the enzyme.
Figure 8. Effect of Ixolaris on antithrombin-mediated factor Xa inactivation by heparin or sulfated galactan.
(A) Dependence on the concentration of sulfated polysaccharide: Increasing concentrations of heparin (squares) or sulfated galactan (circles) were incubated with 0.5 nM plasma-derived factor Xa and 5 nM antithrombin, in the absence (open symbols) or in the presence (closed symbols) of 10 nM Ixolaris, in TS/PEG buffer containing 10 mM CaCl2. The total volume of reaction mixtures was 100 μL. After 60 s, the remaining factor Xa activity activity was determined using S-2765 substrate. (B) Dependence on the concentration of Ixolaris: The assays were as described for Panel A, except that increasing concentration of Ixolaris and fixed concentrations of heparin (1 μg/ml) or sulfated galactan (10 μg/ml) were used. (C) Sulfated galactan-affinity chromatography: One mL of plasma-derived factor Xa (100 nM), before (○) or after (●) incubation with 200 nM Ixolaris in TS/PEG buffer, containing 50 mM CaCl2 was applied to a sulfated galactan-Sepharose column (1 mL), connected to a HPLC system. The column was washed with 5 mL of the TS/PEG buffer and eluted with a linear gradient of 0.15 - 3 M NaCl, at a flow rate of 0.5 mL/min. Fractions of 0.5 mL were collected and checked by factor Xa activity using S-2765 substrate. *p < 0,05 for ■ vs. □ or ○ vs. ●; NS, not significant (p > 0,05).
Further evidence for sulfated galactan and Ixolaris having overlapping binding sites on factor Xa was obtained using sulfated galactan-affinity chromatography. The protease was retained on the column and eluted at ∼1.5 M NaCl (open circles in Fig. 8C). However, previous incubation of the protease with Ixolaris prevented the binding of the protease to sulfated galactan-Sepharose (closed circles in Fig. 8C).
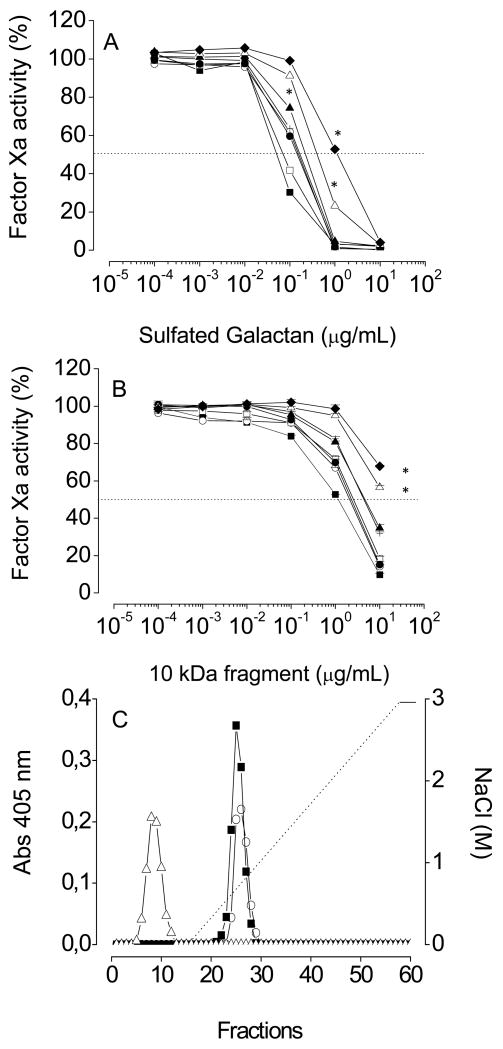
Studies using site-directed mutagenesis showed that seven basic residues in the heavy chain of factor Xa constitute the heparin-binding exosite of the enzyme [16]. We further used GD-factor Xa mutants to map the critical residues for binding of sulfated galactan to the protease (Fig. 9). The catalytic effect of sulfated galactan on GD-factor Xa inactivation by antithrombin was preserved in most mutants as compared to wild-type (Fig. 9A). However, the inhibitory curves for K236A and R240A mutants showed a slight shift to the right (opened triangles and closed diamonds in Fig. 9A), suggesting participation of these residues for binding to sulfated galactan. It is possible that the high molecular weight of the sulfated galactan masks a more defective interaction of the sulfated galactan with the mutant proteases. Therefore, we employed the low-molecular-weight derivative of sulfated galactan to investigate this question. The effect of this compound was also highly preserved on the mutants (Fig. 9B), but again the curves with K236A and R240A mutants were shifted to the right when compared to wild-type GD-factor Xa.
Figure 9. Interaction of GD-factor Xa with sulfated galactan. Concentration-dependence of high- (A) or low- (B) molecular-weight sulfated galactan to catalyze antithrombin-mediated inactivation of GD-factor Xa.
Increasing concentrations of the sulfated galactan were incubated with 5 nM antithrombin and 0.5 nM of wild or mutant Gla domainless factor Xa in TS/PEG buffer, final volume 100 μL, for 5 min at 37°C. Residual protease activity was determined by the addition of 100 μM of the chromogenic substrate S-2765, as described in the legend of Fig. 3. The symbols are as follows: wild-type (■), R125A (□), R93A (○), K96A (+), R165A (▲), K236A (△), K169A (●) and R240A (◆). (C) Sulfated galactan-affinity chromatography: Wild (■), R93A (○) or K236A (△) mutants of Gla domainless factor Xa (100 nM of each) were applied on a 1 mL sulfated galactan-Sepharose column, pre-equilibrated in TS/PEG buffer. The column was washed with 5 mL of the same solution at a flow rate of 0.5 mL/min, and then eluted with a 0.15 - 3 M NaCl gradiente. Fractions of 0.5 mL were collected and checked for factor Xa activity, as described in the legend of Fig. 3. Mutant R240A was not retained on the column, while mutants R96A, R125A and R169A were eluted from the column at 0.75 M NaCl, as the wild protease and the R93A mutant. (A) *p < 0,05 for ◆, △, ▲ vs ■ (B) *p < 0,05 for ◆, △ vs ■.
The direct interaction of the sulfated galactan with these mutants was further investigated using affinity chromatography. Displacement of the protease from sulfated galactan-Sepharose with increasing concentrations of NaCl (Fig. 9C) showed that wild-type, as well as mutants R93A, R96A, R125A and R169A eluted from the column with 0.75 M NaCl (shown only for wild-type and R93A). In contrast, K236A and R240A mutants were not retained on the affinity column (shown only for K236A). These direct-binding results are consistent with the inhibitory curves of the protease activities shown in Figs 9A and B and further suggest that, similar to heparin, both Lys-236 and Arg-240 are higly critical for interaction with sulfated galactan.
Discussion
Sulfated galactan has a serpin-independent effect on coagulation
We have demonstrated in this study that sulfated galactan has a serpin-independent anticoagulant effect. This is derived from the observation that sulfated polysaccharide is capable of prolonging coagulation time and delay thrombin and factor Xa generation in serpin-free plasmas. This effect is due to the inhibition of the intrinsic tenase and prothrombinase complexes, respectively. In a previous study we suggested that the sulfated galactan lost its anticoagulant activity when tested in serpin-free plasma [9]. However, we employed thrombin time (TT) instead of APTT to assay the anticoagulant activity of the polysaccharide. Comparison between the assays using normal and serpin-free plasmas clearly demonstrates that the serpin-independent anticoagulant effect of the sulfated galactan predominates over the serpin-dependent action in an in vitro plasma system. By contrast, an opposite effect is seen for heparin as expected.
There are other sulfated polysaccharides that can prolong clotting time independent of serpins. One of these polysaccharides is the pentosan polysulfate, obtained by chemical oversulfation of naturally occurring pentosan [26]. Another sulfated polysaccharide with similar effect on coagulation is a fucosylated chondroitin sulfate obtained from sea cucumber [27]. This compound is similar to a “depolymerized holothurian glycosaminoglycan” [28]. These sulfated polysaccharides have also a serpin-dependent anticoagulant activity, mostly due to potentiation of thrombin inhibition by heparin cofactor II.
The observation that the antithrombotic action of heparin may be partly antithrombin-independent [29-32], suggested that the removal of all antithrombin-dependent action of heparin could further reduce its side-effects (such as bleeding risk) and increase the therapeutic action [20]. Based on this proposition, several researchers attempted to prepare derivatives of heparin with serpin-independent action and, perhaps, antithrombotic effect. One of the approaches involved periodate oxidation of low-molecular-weigth heparin, followed by oversulfation of the saccharide chain which reduces its affinity for antithrombin. This chemically modified heparin became a potent inhibitor of the intrinsic tenase and prothrombinase complexes [33]. The serpin-independent anticoagulant effect of this modified heparin was predominant in the plasma system. Nevertheless, sulfated galactan from the marine alga B. occidentalis has clear advantage over these previously known anticoagulants in that: 1) it does not require any chemical modification or laborious chemical synthesis; 2) it is not obtained from mammals, reducing the possibility of contamination with prions; and 3) it occurs at high concentrations in an abundant marine plant.
Sulfated galactan has antithrombotic effect when tested in experimental models of venous and arterial thrombosis [12, 13]. A critical challenge in the future studies of this sulfated galactan as an antithrombotic polysaccharide will be determining the magnitude of the serpin-independent and serpin-dependent effects of its antithrombotic action in in vivo settings. In order to clarify this aspect it is necessary to properly follow these two effects of the sulfated galactan in the course of experimental thrombosis in appropriate models.
Recent observations from our group demonstrate that sulfated galactan with high molecular size activates factor XII and this action was associated with a prothrombotic effect of the polysaccharide at high doses. Similar effect was observed for oversulfated chondroitin sulfate, found as contaminant of heparin preparations [34]. This contaminant activates the kinin-kallikrein pathway and induces hypothension when administered by intravenous infusion. The low-molecular-weight derivative of sulfated galactan was devoid of this effect on factor XII, thus this fragment is more promising as a new antithrombotic drug.
Sulfated galactan interacts with the heparin binding-exosite of factor Xa
The large acidic sulfate substituents dominate the physical characteristics of sulfated polysaccharides that can equally dominate their effects in biological systems. There is no doubt that these molecules will be attracted to, and affect the activity of any basic protein. Thus, one might speculate that these interactions can lead to potent but non-specific biological activities, raising the skepticism that their anticoagulant activity is dependent on specific structures found on these sulfated polysaccharides. There is, however, ever increasing evidence suggesting that distinct sulfation patterns and structural motifs of the saccharide chains are involved in making specific interactions with proteins of the coagulation system [7-11]. Sulfated galactan has high affinity for thrombin and antithrombin. It interacts predominantly with exosite II on thrombin and, similar to heparin, promotes the formation of a covalent complex between antithrombin and the protease. The sulfated galactan and heparin differ in their specific binding site as well as in the conformational activation they induce in antithrombin [13]. Further evidence for the specificity of sulfated galactan is provided by the observation that it interacts with the heparin-binding exosite of factor Xa involving residues Lys-236 and Arg-240, as observed for heparin [22]. Nevertheless, it was interesting to note that, unlike the accelerating effect of heparin, deletion of the Gla domain of factor Xa did not abolish the effect of Ca2+ ions on sulfated galactan-catalyzed inactivation of the protease by antithrombin, possibly suggesting the existence of additional Ca2+-dependent galactan binding-sites on factor Xa. It is also possible that sulfated galactan modulates the catalytic pocket of Ca2+-stabilized conformer of factor X.
Conclusion and perspective
How can sulfated galactan and heparin, two sulfated polysaccharides with marked differences in their chemical structures, bind with high affinity to similar patches of proteins from the coagulation system? This question may be clarified based on conformational analysis of the polysaccharides. In spite of their diferences in chemical structures, sulfated galactan and heparin may fulfill the sulfate group spacing requirements for the interactive basic amino acid residues in target proteins. Conformational analysis, including molecular dynamics, may help to correlate structure and the biological effect of these compounds. However, sulfated polysaccharides are flexible, thus resulting in incomplete constraints to define their relative orientation.
Obviously, it is desirable that a sulfated polysaccharide with therapeutic action on thrombosis binds to target proteins of the coagulation system with high affininity and specificity. The ability of sulfated galactan to inhibit both the intrinsic tenase and prothrombinase complexes has clear advantages over heparin. However, further studies will be required to ensure that sulfated galactan has not acquired a broader specificity profile for interaction with many other plasma proteins.
Acknowledgments
This work was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) and HL-62565 (ARR). We would like to thank Adriana A. Piquet for technical assistance.
References
- 1.Fareed J, Hoppensteadt DA. Heparins in the new millennium: Will unfractionated heparin survive? Semin Thromb Haemost. 2000;26:87–88. [PubMed] [Google Scholar]
- 2.Béguin S, Lindhout T, Hemker HC. The mode of action of heparin in plasma. Thromb Haemost. 1988;60:457–62. [PubMed] [Google Scholar]
- 3.Blossom DB, et al. Outbreak of Adverse Reactions Associated with Contaminated Heparin. N Engl J Med. 2008;359:2674–84. doi: 10.1056/NEJMoa0806450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mourão PAS, Pereira MS. Searching for alternatives to heparin. Trends Cardiovasc Med. 1999;9:225–32. doi: 10.1016/s1050-1738(00)00032-3. [DOI] [PubMed] [Google Scholar]
- 5.Farias WRL, Valente AP, Pereira MS, Mourão PAS. Structure and anticoagulant activity of sulfated galactans - Isolation of a unique sulfated galactan from the red algae Botryocladia occidentalis and comparison of its anticoagulant action with that of sulfated galactans from invertebrates. J Biol Chem. 2000;275:29299–307. doi: 10.1074/jbc.M002422200. [DOI] [PubMed] [Google Scholar]
- 6.Pereira MS, Mulloy B, Mourão PAS. Structure and anticoagulant activity of sulfated fucans – Comparison between the regular, repetitive, and linear fucans from echinoderms with the more heterogeneous and branched polymers from brown algae. J Biol Chem. 1999;274:7656–7667. doi: 10.1074/jbc.274.12.7656. [DOI] [PubMed] [Google Scholar]
- 7.Pereira MS, Melo FR, Mourão PAS. Is there a correlation between structure and anticoagulant action of sulfated galactans and sulfated fucans? Glycobiology. 2002;12:573–80. doi: 10.1093/glycob/cwf077. [DOI] [PubMed] [Google Scholar]
- 8.Pereira MS, Vilela-Silva ACES, Valente AP, Mourão PAS. A 2-sulfated, 3-linked alpha-L-galactan is an anticoagulant polysaccharide. Carbohydr Res. 2002;337:2231–2238. doi: 10.1016/s0008-6215(02)00215-x. [DOI] [PubMed] [Google Scholar]
- 9.Melo FR, Pereira MS, Foguel D, Mourão PAS. Antithrombin-mediated anticoagulant activity of sulfated polysaccharides — different mechanisms for heparin and sulfated galactans. J Biol Chem. 2004;279:20824–20835. doi: 10.1074/jbc.M308688200. [DOI] [PubMed] [Google Scholar]
- 10.Shipp EL, Hsieh-Wilson LC. Profiling the sulfation specificities of glycosaminoglycans interactions with growth factor and chemotactic proteins using microarrays. Chem Biol. 2007;14:195–208. doi: 10.1016/j.chembiol.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Gama CI, Tully SE, Sotogaku N, Clark PM, Rawat M, Vaidehi N, Goddard WA, Nishi A, Hsieh-Wilson LC. Sulfation patterns of glycosaminoglycans encode molecular recognition and activity. Nat Chem Biol. 2006;2:467–473. doi: 10.1038/nchembio810. [DOI] [PubMed] [Google Scholar]
- 12.Farias WRL, Nazareth RA, Mourão PAS. Dual effects of sulfated D-galactans from the red algae Botryocladia occidentalis preventing thrombosis and inducing platelet aggregation. Thromb Haemostasis. 2001;86:1540–1546. [PubMed] [Google Scholar]
- 13.Melo FR, Pereira MS, Monteiro RQ, Foguel D, Mourão PAS. Sulfated galactan is a catalyst of antithrombin-mediated inactivation of α-thrombin. Biochimica et Biophysica Acta. 2008;1780:1047–1053. doi: 10.1016/j.bbagen.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Astermark J, Hogg PJ, Bjork I, Stenflo J. Effects of gamma-carboxyglutamic acid and epidermal growth factor-likemodules of factor IX on factor X activation. Studies using proteolytic fragments of bovine factor IX. J Biol Chem. 1992;267:3249–56. [PubMed] [Google Scholar]
- 15.Ngai PK, Chang JY. A novel one-step purification of human alpha-thrombin after direct activation of crude prothrombin enriched from plasma. Biochemistry Journal. 1991;280:805–806. doi: 10.1042/bj2800805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rezaie AR. Identification of Basic Residues in the Heparin-binding Exosite of Factor Xa Critical for Heparin and Factor Va Binding. J Biol Chem. 2000;275:3320–3327. doi: 10.1074/jbc.275.5.3320. [DOI] [PubMed] [Google Scholar]
- 17.Nazareth RA, Tomaz LS, Ortiz-Costa S, Atella GC, Ribeiro JMC, Francischetti IMB, Monteiro RQ. Antithrombotic properties of Ixolaris, a factor VIIa/tissue factor complex inhibitor. Thromb Haemost. 2006;96:7–13. doi: 10.1160/TH06-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monteiro RQ, Rezaie AR, Bae JS, Calvo E, Andersen JF, Francischetti IM. Ixolaris binding to factor X reveals a precursor state of factor Xa heparin-binding exosite. Protein Sci. 2008;17:146–53. doi: 10.1110/ps.073016308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monteiro RQ, Zingali RB. Bothrojaracin, a proexosite I ligand, inhibits factor Va-accelerated prothrombin activation. Thromb Haemostasis. 2002;87:288–293. [PubMed] [Google Scholar]
- 20.Barrow RT, Parker ET, Krishnaswamy S, Lollar P. Inhibition by heparin of the human blood coagulation intrinsic pathway factor X activator. J Biol Chem. 1994;269:26796–800. [PubMed] [Google Scholar]
- 21.Stenflo J. Structure-function relationships of epidermal growth factor modules in vitamin K-dependent clotting factors. Blood. 1991;78:1637–51. [PubMed] [Google Scholar]
- 22.Rezaie AR. Heparin-binding exosite of factor Xa. Trends Cardiovasc Med. 2000;10:333–8. doi: 10.1016/s1050-1738(01)00070-6. [DOI] [PubMed] [Google Scholar]
- 23.Sheehan JP, Sadler JE. Molecular Mapping of the Heparin-Binding Exosite of Thrombin. Proc Natl Acad Sci (U S A) 1994;91:5518–5522. doi: 10.1073/pnas.91.12.5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rezaie AR. Calcium enhances heparin catalysis of the antithrombin-factor Xa reaction by a template mechanism. Evidence that calcium alleviates Gla domain antagonism of heparin binding to factor Xa. J Biol Chem. 1998;273:16824–7. doi: 10.1074/jbc.273.27.16824. [DOI] [PubMed] [Google Scholar]
- 25.Monteiro RQ, Rezaie AR, Ribeiro JMC, Francischetti IMB. Ixolaris: a Factor Xa heparin-binding exosite inhibitor. Biochem J. 2005;387:871–877. doi: 10.1042/BJ20041738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sie P, Ofosu F, Fernandez F, Buchanan MR, Petitou M, Boneu B. Respective role of antithrombin III and heparin cofactor II in the in vitro anticoagulant effect of heparin and of various sulfated polysaccharides. Br J Haematol. 1986;64:707. doi: 10.1111/j.1365-2141.1986.tb02232.x. [DOI] [PubMed] [Google Scholar]
- 27.Glauser BF, Pereira MS, Monteiro RQ, Mourão PA. Serpin-independent anticoagulant activity of a fucosylated chondroitin sulfate. Thromb Haemost. 2008;100:420–8. [PubMed] [Google Scholar]
- 28.Nagase H, Enjyoji K, Minamiguchi K, Kitazato KT, Kitazato K, Saito H, Kato H. Depolymerized holothurian glycosaminoglycan with novel anticoagulant actions: antithrombin III- and heparin cofactor II-independent inhibition of factor X activation by factor IXa-factor VIIIa complex and heparin cofactor II-dependent inhibition of thrombin. Blood. 1995;85:1527–34. [PubMed] [Google Scholar]
- 29.Kishimoto TK, Viswanathan K, Ganguly T, Elankumaran S, Smith S, Pelzer K, Lansing JC, Sriranganathan N, Zhao G, Galcheva-Gargova Z, Al-Hakim A, Bailey GS, Fraser B, Roy S, Rogers-Cotrone T, Buhse L, Whary M, Fox J, Nasr M, Dal Pan GJ, Shriver Z, Langer RS, Venkataraman G, Austen KF, Woodcock J, Sasisekharan R. Contaminated heparin associated with adverse clinical events and activation of the contact system. N Engl J Med. 2008;358:2457–67. doi: 10.1056/NEJMoa0803200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holmer E. In: Heparin Chemical and Biological Properties, Clinical Applications. Lane DA, Lindahl U, editors. 1989. pp. 575–595. [Google Scholar]
- 31.Leizorovicz A, Haugh MC, Chapuis FR, Samama MM, Boissel JP. Low molecular weight heparin in prevention of perioperative thrombosis. BMJ. 1992;305:913–20. doi: 10.1136/bmj.305.6859.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nurmohamed MT, Rosendaal FR, Büller HR, Dekker E, Hommes DW, Vandenbroucke JP, Briët E. Low-molecular-weight heparin versus standard heparin in general and orthopaedic surgery: a meta-analysis. Lancet. 1992;340:152–6. doi: 10.1016/0140-6736(92)93223-a. [DOI] [PubMed] [Google Scholar]
- 33.Anderson JA, Fredenburgh JC, Stafford AR, Guo YS, Hirsh J, Ghazarossian V, Weitz JI. Hypersulfated low molecular weight heparin with reduced affinity for antithrombin acts as an anticoagulant by inhibiting intrinsic tenase and prothrombinase. J Biol Chem. 2001;276:9755–61. doi: 10.1074/jbc.M010048200. [DOI] [PubMed] [Google Scholar]
- 34.Melo FR, Mourão PAS. An algal sulfated galactan has an unusual dual effect on venous thrombosis due to activation of factor XII and inhibition of the coagulation proteases. Thromb Haemost. 2008;99:531–8. doi: 10.1160/TH07-10-0649. [DOI] [PubMed] [Google Scholar]