Abstract
Objective
To review the relation in midlife and beyond between estrogen exposures and episodic memory in women.
Background
Episodic memory performance declines with usual aging, and impairments in episodic memory often portend the development of Alzheimer's disease. In the laboratory, estradiol influences hippocampal function and animal learning. However, it is controversial whether estrogens affect memory after a woman's reproductive years.
Method
Focused literature review, including a summary of a systematic search of clinical trials of estrogens in which outcomes included an objective measure of episodic memory.
Results
The natural menopause transition is not associated with objective changes in episodic memory. Strong clinical trial evidence indicates that initiating estrogen-containing hormone therapy after about age 60 years does not benefit episodic memory. Clinical trial findings in middle-age women before age 60 are limited by smaller sample sizes and shorter treatment durations, but these also do not indicate substantial memory effects. Limited short-term evidence, however, suggests that estrogens may improve verbal memory after surgical menopause. Although hormone therapy initiation in old age increases dementia risk, observational studies raise the question of an early critical window during which midlife estrogen therapy reduces late-life Alzheimer's disease. However, almost no data address whether midlife estrogen therapy affects episodic memory in old age.
Conclusions
Episodic memory is not substantially impacted by the natural menopause transition or improved by use of estrogen-containing hormone therapy after age 60. Further research is needed to determine whether outcomes differ after surgical menopause or whether episodic memory later in life is modified by midlife estrogenic exposures.
Keywords: Alzheimer's disease, estrogens, memory
Sex steroids are hormones produced primarily by the reproductive glands, either the ovaries or testes. In his eighth decade of life, the peripatetic neurologist and physiologist Charles-Édouard Brown-Séquard (1817-1894) reported self-observations of salubrious cognitive effects from injections with testicular extracts prepared from laboratory animals.1 During the 1920s, “monkey gland” testicular grafts were performed with surprising frequency in an attempt to rejuvenate aging men.2 Sex steroid therapy for women, however, achieved popularity only later, spurred in part by the 1966 publication of Robert Wilson's Feminine Forever, in which he promoted estrogenic hormone therapy for the “elimination of the menopause, woman's physical, mental, and final emancipation” (p. 5).3 Estrogen prescriptions increased throughout the 1970s, 1980s and 1990s, declining only after principle findings from the Women's Health Initiative trials of hormone therapy were first reported in 2002.4,5 Although Wilson mentions “loss of memory” (p. 42) as a menopausal symptom, it is only in recent years that memory function in relation to menopause and to estrogen-containing hormone therapy begun to be carefully examined.
SEX STEROIDS
Steroid hormones share a similar basic structure of three hexane rings and a pentane ring. Sex steroids include estrogens, androgens, and progestogens, and each has major effects on reproductive physiology. Although estrogens (e.g., 17β-estradiol) and progestogens (e.g., progesterone) are classified as female sex hormones and androgens (e.g., testosterone) as male sex hormones, this categorization is misleading. Estrogens, for example, arise in tissues other than the ovaries, are found in men as well as women, and have effects in both sexes.
With respect to human non-reproductive behaviors, estrogens are the best studied of the sex steroids. Although it was long believed that estrogens were not synthesized within the central nervous system, endogenous estradiol production can occur in hippocampus and other parts of the brain, where it presumably acts as a local mediator of synaptic transmission or plasticity.6,7 The two types of estrogen receptors — estrogen receptor alpha and estrogen receptor beta — are found in high concentrations in specific brain regions, particularly including the hippocampus and basal forebrain cholinergic neurons.8-11 Within the cell nucleus, estrogen-receptor dimers act as transcription factors, binding to estrogen response elements on the genome to control gene expression. Rapid effects of estrogens — such as those involving ion channel changes, kinase signaling, and neurotransmitter release — are mediated by other receptors located within cell membranes and do not involve genomic activation.12
Estradiol, the principal endogenous estrogen, is produced cyclically by the developing ovarian follicle. Estrone, whose affinities for the alpha and beta estrogen receptors are less than those of estradiol, circulates in somewhat lower levels during a woman's reproductive years. Estrone is derived both from estradiol and from the androgenic precursor androstenedione. After menopause, total estrogen levels are greatly reduced, but concentrations of estrone are relatively higher than those of estradiol.
EPISODIC MEMORY
Learning and memory are inextricably linked. Memory refers to a change in behavior as a result of prior experience or exposure due to a change in the brain. Learning is the process by which memories are created. Nothing is more fundamental to cognition and behavior than memory. Memory, however, encompasses disparate processes assessed with dissimilar tasks. Squire has argued for the distinction between declarative and nondeclarative forms of memory.13 Only the former is amenable to conscious access and description. One form of declarative memory — episodic memory — refers to recollection of discrete episodes, or events. A further distinction is between events consciously recalled for only a few seconds and events recalled after some longer period of time. In the psychology literature, the latter is usually referred to as long-term memory, and in the following discussion, reference to episodic memory will indicate the long-term variety. Clinicians often evaluate episodic memory with supraspan immediate recall tasks or delayed recall tasks. For the later, new information is presented for learning, followed some minutes later by a request for recall of this information.
Further distinction is sometimes made between episodic memory involving information that can be verbally encoded and retrieved (e.g., a list of unrelated or related words, a list of word pairs [paired associate learning], or a paragraph length story; verbal memory) and memory for information not easily described by words (e.g., a visual pattern; figural, visual, or nonverbal memory). Strictly speaking, of course, episodic memory cannot be assessed in isolation from other cognitive processes. For verbal forms of episodic memory, for example, a deficit in semantic memory would have an adverse impact on episodic memory test scores. Attention, motivation, processing speed and executive functioning would also influence performance on most episodic memory tasks.
Medial portions of the temporal lobes are essential to episodic memory encoding. In the 1950s, it was discovered that bilateral surgical resection of the hippocampus and nearby structures led to profound, persistent difficulty acquiring long-term episodic memories while sparing nondeclarative forms of memory and the retrieval of older episodic memories.14 In these surgical patients, most new information can be consciously retained and retrieved for only a few seconds. In the presence of severe medial temporal lobe damage, this long-term memory deficit persists unabated for decades.15
This article focuses on episodic memory, the most clinically relevant variety of memory. It is the cognitive domain most commonly assessed in clinical studies of estrogen. Episodic memory is adversely affected by normal, or usual, aging. Performance decline on episodic memory tasks begins in midlife and progresses gradually thereafter.16 It is the domain most severely affected in dementia due to Alzheimer's disease, particularly during early stages of the illness.17 Deficits may be detected more than a decade before the development of frank dementia18 and are thought to reflect early pathological alterations in the medial temporal lobes.19 The term mild cognitive impairment (or, more particularly, the amnestic variety of mild cognitive impairment) is often applied to relatively selective deficits in episodic memory. In older adults, this variety of mild cognitive impairment is often the harbinger of Alzheimer's disease.20
CIRCULATING ESTROGENS AND MEMORY
Menopause is associated with sharp declines in concentrations of circulating estradiol and estrone,21 and this change has the potential to affect central nervous system function.22 Depending on specific characteristics of the animal model system, estradiol replacement after ovariectomy can enhance aspects of learning and memory.23-26 Some forms of learning, for example, striatal-dependent motor learning, may be impeded by estradiol.26
Several estrogenic actions in the hippocampus appear particularly germane. Long-term potentiation provides a physiological basis for episodic memory formation, and in the CA1 region of the hippocampus estradiol enhances this process.27 Dendritic spine density within CA1 hippocampus fluctuates during the normal four-day rat estrous cycle, with greater density seen during proestrus when estradiol levels are higher.28 Spines represent points of excitatory synapses. In rats and primates, ovariectomy decreases and estradiol restores the density of dendritic spines.29,30 In the dentate gyrus of the hippocampus, estradiol promotes neurogenesis,31 although neurogenesis may be more pertinent to associative learning than to episodic memory.
After experimental brain lesions, estradiol helps protect against loss of acetylcholine,23,32 a neurotransmitter important in the formation of new episodic memories. Further, as previously reviewed,22 estrogens are neuroprotective and neurotrophic; reduce oxidative stress; reduce β-amyloid formation and tau protein hyperphosphorylation; modulate noradrenergic, serotonergic, and glutamatergic neurotransmission; and enhance cerebral blood flow and active glucose transport into the brain.
Sex steroids powerfully influence dimorphic sexual differentiation in the prenatal and neonatal brain33 and influence adult non-reproductive and reproductive behaviors. Cognitive effects are most often described in relation to tasks involving spatial skills. In rodents, for example, testosterone — produced by the fetal testes and aromatized in the developing brain to estradiol — affects adult spatial memory performance.34 In primates, prenatal androgen exposures may influence adult behaviors in spatial exploratory tasks.35
Prior to menarche, circulating estrogen levels are typically undetectable. During a woman's reproductive years, a number of studies have examined cognitive performance across the menstrual cycle. Sample sizes in most studies are small. Memory has been assessed in some studies but without consistent findings. A recent study found better verbal memory during the late follicular phase of the menstrual cycle when estradiol levels are elevated compared to the late luteal phase, and authors report an interesting association with relatively increased right anterior hippocampal volume on magnetic resonance imaging.36 In three reports, there was no relation between verbal memory and menstrual phase,37-39 but one of these described better nonverbal memory performance during the luteal phase, when estradiol and progesterone levels are increased relative to the menstrual phase of the cycle.37 Other investigators report no differences related to object memory or memory of spatial location.40
During midlife, there is no clear association between various estrogenic exposures and episodic memory. This relation has been examined in middle-age cohorts from Melbourne Australia41 (Figure 1), Taiwan,42 the United Kingdom,43 Sweden44 and the United States,45 where there was no link between episodic memory performance and reproductive status around the time of the natural menopausal transition and early postmenopause (Table 1). In three of these cohorts, episodic memory was also unrelated to circulating estradiol levels.41,44,45
Figure 1. Reproductive stage and episodic memory in the Melbourne Women's Midlife Health Project.
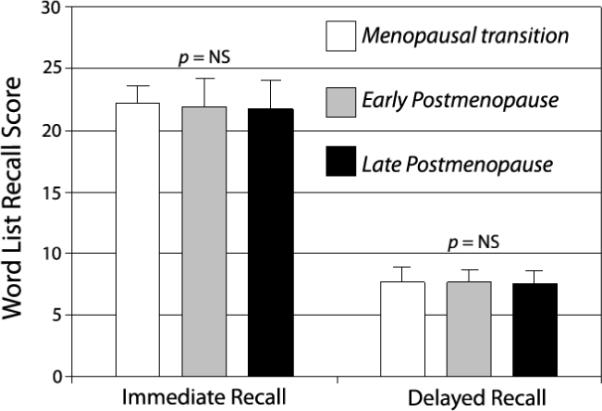
Based on 250 women aged 52-63 without surgical menopause and not using hormone therapy (see Henderson et al41). Early postmenopause, < 5 years after final menstrual period; late postmenopause, ≥ 5 years after natural menopause. Values represent total words from 3 immediate recall trials and 1 delayed recall trial. Error bars are standard deviations.
TABLE 1.
Natural Menopause Transition and Episodic Memory*
| First author, publication year | Location | Cohort description | Number of women | Episodic memory |
Estradiol levels | |
|---|---|---|---|---|---|---|
| Verbala | Nonverbalb | |||||
| Henderson, 200341 | Melbourne, Australia | Population-based, mean age 57 | 326 | NS | — | NS |
| Fuh, 200642 | Kinmen, Taiwan | Population-based, mean age about 48 | 495 | NS | NS | — |
| Kok, 200643 | United Kingdom | Population-based, age 53 (birth cohort) | 997 | NS | — | — |
| Herlitz, 200744 | Umea, Sweden | Population-based, mean age 49 | 242 | NS | NS | NS |
| Luetters, 200745 | United States (SWAN) | Community volunteers, mean age 50 | 1657 | NS | — | NS |
Midlife cohorts of naturally menopausal (Henderson, Fuh, Kok, Luetters) or predominately naturally menopausal (Herlitz) women that identified reproductive stage and included an objective measure of episodic memory.
NS = nonsignificant probability P>0.05; SWAN = Study of Women Across the Nation
Verbal episodic memory tasks were word-list learning or recall (Henderson, Fuh, Kok), paragraph recall (Luetters), or a composite based on free recall, cued recall and recognition of verbal material (Herlitz).
Nonverbal episodic memory tasks were nonsense figure recognition (Fuh) and face recognition (Herlitz).
Memory complaints are common in middle-age,46 and these cohort findings appear surprising. However, memory symptoms have multiple causes, and subjective complaints might have both cognitive and noncognitive determinants. For example, depression and stress are linked both to subjective and objective performance decrements. Further, trouble recalling an acquaintance's name (impaired semantic memory) or trouble multitasking (poor working memory or executive function) may be self-reported simply as bad memory. In the Melbourne Women's Midlife Health Project, episodic memory performance on a word-list learning task was similar for women reporting good memory and poor memory.
In older women, circulating estrogen levels are not clearly related to memory, but findings are inconsistent. In one study, investigators found significant positive relations between estradiol and bioavailable estradiol (but not estrone) concentrations and verbal episodic memory (word list recall), and inverse relations between estradiol and estrone and nonverbal memory (figural recall).47 German researchers reported an association between higher estradiol levels and better verbal memory (paired associates),48 but other investigators found an association with word list recall but not paired associates.49 In another study, older women with lower estradiol levels experienced more verbal memory decline over a two year interval.50 However, serum concentrations of estrone and estradiol were not closely related to several measures of memory among older community-dwelling women in the United States or Australia.51,52 In a Dutch population-based cohort, there was an inverse relation between levels of total estradiol and verbal episodic memory (word list recall).53
ESTROGENS AND MEMORY: FUNCTIONAL IMAGING
Estrogens increase cerebral blood flow,54,55 and several investigators have examined regional cerebral blood flow during performance of memory tasks. One study of women of reproductive age found a positive correlation between serum estradiol concentrations and the degree of left inferior frontal gyrus activation during successful encoding of verbal information.56 In this report, brain activation was based on blood oxygenation level-dependent functional magnetic resonance imaging. Investigators from the Baltimore Longitudinal Study of Aging used positron emission tomography to assess regional blood flow at rest and under conditions involving delayed recognition of verbal and figural information.57 Comparisons were between older postmenopausal women using hormone therapy and nonusers. During verbal memory processing, there were significant interactions based on hormone use in several brain regions, including right parahippocampal gyrus, right precuneus, right frontal regions, and left hypothalamus. During figural memory processing, significant interactions were observed for right parahippocampal and inferior parietal regions and for left visual association and anterior thalamic regions.57 In a small study of midlife women randomly assigned to transdermal estradiol or placebo, estradiol was associated with increased functional magnetic resonance imaging activation of the frontal lobe during a verbal recall task (forced recognition of previously learned words).58
Selective estrogen receptor modulators (SERMS) like raloxifene, where estrogenic effects are tissue specific, also affect brain activation. For example, on a visual encoding task (looking at pictures for later recall), postmenopausal women assigned to raloxifene compared to placebo showed decreased activation in the left parahippocampal gyrus and left lingual gyrus, and increased activation in the right superior frontal gyrus. No differences were observed during delayed recall (recognition) performance.59
ESTROGENS AND MEMORY: CLINICAL TRIAL EVIDENCE IN MIDDLE-AGE WOMEN
Compared to observational research on cognitive effects of estrogenic hormones, randomized placebo-controlled trials are expected to provide less biased evidence regarding a possible relation between hormone use and memory. A number of randomized clinical trials have now been completed, and these are described below. Because there is some suggestion that hormone effects might be modified by age when hormones are used, these studies are described separately for middle-age women and older women.
A recent systematic literature search identified 11 randomized controlled trials of estrogen-containing hormone therapy in midlife women. Search criteria included placebo-control, treatment duration of at least one month, and an objective outcome measure of verbal episodic memory or nonverbal episodic memory60 (Table 2). Most of these trials suggested no significant cognitive benefit or harm of hormonal treatment. Important caveats are that these studies were generally short-term, and samples sizes were generally small. The power to detect a clinically meaningful effect, if present, was therefore limited. The largest and longest study enrolled 180 women with cognitive complaints who had undergone natural menopause.61 Active treatment was with a combined hormonal preparation (conjugated estrogens plus medroxyprogesterone acetate), and treatment duration was four months. Conjugated estrogens, the most commonly prescribed form of estrogen in the United States, are composed of estrone sulfate and other estrogenic steroids. Outcome in this study was assessed with an extensive neuropsychological battery. There were no significant between-groups differences on any neuropsychological measure. There was a nonsignificant trend to worse performance in the estrogens group on a word-list learning task (California Verbal Auditory Learning Test, short and long delayed recall) but not on two other episodic memory tasks (paragraph recall and the Benton Visual Retention Test).
TABLE 2.
Estrogens and Episodic Memory: Randomized, Double-Blind, Placebo-Controlled Trials in Postmenopausal Women*
| First author, publication year |
Type of menopause |
Experimental design |
Mean age, years |
Number of women |
Mean duration |
Hormone treatment |
Episodic memory |
|
|---|---|---|---|---|---|---|---|---|
| Verbala | Nonverbalb | |||||||
| Younger postmenopausal women (mean age less than 60 years) | ||||||||
| Hackman, 197693 | Both | Parallel | Range 29 to 68 | 18 | 6 months | Estrone (O) | NSc | — |
| Vanhull, 197694 | Both | Parallel | 58 | 26 | 3 months | Estriol (O) | — | NS |
| Sherwin, 198862 | Surgical | Crossover | 45 | 40d | 3 months | E2 (IM); Other hormone treatments | E2 better | — |
| Phillips, 199263 | Surgical | Parallel | 48 | 19 | 2 months | E2(IM) | E2 better | NS |
| Polo-Kantola, 199895 | Surgicale | Crossover | 56 | 62 | 3 months | E2 ( TD) | — | NS |
| Linzmayer, 200196 | Not reported | Parallel | 57 | 49g | 2 months E2 + P(O)g | E2 (O); E2 + P, most NSh | E2, NS; | NS |
| Dunkin, 200597 | Both | Parallel | 57 | 17 | 10 weeks | E2 (TD) | NS | NS |
| Joffe, 200658 | Natural | Parallel | 51 | 50 | 12 weeks | E2 (TD) | NS | NS |
| Maki, 200798 | Natural | Parallel | 52 | 180 | 4 months | CE + P(O) | NS | NS |
| Older postmenopausal women (mean age greater than 60 years) | ||||||||
| Bender, 200199 | Both | Parallel | 81 | 52 | 9 months | CE + P(O) | NS | — |
| Grady, 2002100 | Both | Parallel | 67 | 1063 | 4 years | CE + P(O) | NS | — |
| Viscoli, 2005101 | Both | Parallel | 70 | 461 | 3 years | E2(O) | NS | NS |
| Wolf, 2005102 | Surgicale | Parallel | 64 | 35l | 24 weeks | E2 (O); E2 + P (O) | NS | NS |
| Almeida, 200670 | Surgicale | Parallel | 74 | 115 | 20 weeks | E2(O) | NS | NS |
| Resnick, 200672 | Natural | Parallel | 71 | 1416 | 4 years | CEE + P (O) | Placebo betterm | NSm |
| Yaffe, 2006103 | Natural | Parallel | 67 | 417 | 2 years | E2 (TD) | NS | NS |
Restricted to clinical trials reporting an objective measure of episodic memory and a mean treatment duration of at least one month. Table adapted with permission of the publisher from Henderson & Sherwin.60
CE = conjugated estrogens; E2= estradiol; IM = intramuscular; NS = nonsignificant probability P>0.05; O = oral; P = progestogen (medroxyprogesterone acetate daily in Maki, Grady, Resnick and trimonthly in Binder; dienogest in Linzmayer); TD = transdermal.
Verbal episodic memory tasks were word-list recall (Dunkin, Joffe, Grady, Almeida, Resnick, Yaffe); paragraph recall (Sherwin, Phillips, Dunkin, Joffe, Maki, Wolf, Yaffe); associate learning (Phillips, Linzmayer, Dunkin, Bender, Wolf); a composite score emphasizing verbal memory (Hackman); common, numerical and total verbal memory (Linzmayer); and incidental recall of naming task items (Viscoli).
Nonverbal episodic memory tasks were visual retention / visual reproduction (Vanhull, Phillips, Polo-Kantola, Linzmayer, Dunkin, Joffe, Maki, Resnick); manual labyrinth learning (Vanhull); a composite visuospatial memory score (Joffe); spatial location (Viscoli); face recognition (Almeida); associate learning (Wolf); and a visuospatial memory test (Yaffe).
Cognition was assessed with Guild Memory Test, with six subtests that included tasks of verbal episodic memory. Subtest results are not provided; differences are implied to be nonsignificant. For the composite score, group differences are reported as significant; reanalysis of published data indicates no significant differences.
Four treatment groups of 10 women each: estradiol, estradiol plus androgen, androgen, placebo.
Hysterectomy; oophorectomy status not reported.
Three treatment groups of 16 to 17 women each.
Estradiol plus progestogen was superior to placebo on associate learning, but comparisons with placebo were not significant on three other verbal memory tasks; estradiol alone and placebo were not significantly different on any comparison.
Three treatment groups of 10 to 13 women.
Statistical significance defined in this study as P<0.01. No significant between-groups differences after three years. After treatment for 1.4 more years, placebo was better for verbal memory (total word-list recall score, P<0.01); nonsignificant trends (0.01<P<0.05) favored placebo on two other verbal memory scores (short and long free delayed word-list recall) and hormone on a nonverbal memory task (visual retention).
Two small, short-term trials in midlife women reported significant cognitive benefits of estrogen treatment. These studies stand apart in that treatment was with high-dose intramuscular estradiol, mean age of participants was fairly young, and treatment was initiated immediately after surgical menopause (Table 2).62,63 In one of these two trials, outcomes included both nonverbal (visual reproduction) as well as verbal (associate learning and paragraph recall) episodic memory tasks; significant effects were seen only on verbal memory measures.63
In interpreting these research findings in middle-age women, the type of menopause may be relevant. Women who undergo surgical menopause (i.e., bilateral oophorectomy) differ from women who experience natural menopause in at least four important ways. First, there are demographic differences between these two groups of women. In the United States, for example, surgically menopausal women are more likely to be less well educated and socioeconomically disadvantaged.64 Second, by definition, such women have experienced menopause at an age earlier than would have occurred after natural menopause. Third, the natural menopause transition is characterized by irregular cycle length and a gradual decline in mean estradiol levels over a several year period beginning about two years before the final menstrual period.21 In contrast, loss of ovarian hormone production with surgical menopause is essentially instantaneous at he time of oophorectomy. Finally, after natural menopause testosterone is derived from androgen precursors produced by ovarian stroma or the adrenal cortex. Oophorectomy leads to loss of testosterone, as well as estradiol and progesterone. With natural menopause, testosterone levels are maintained.65
There is other evidence that cognitive consequences might be especially apparent when ovarian function is abruptly curtailed. Sherwin and Tulandi66 studied 19 reproductive-age women with uterine myomas, who received a gonadotropin-releasing hormone agonist for 12 weeks to suppress ovarian function. Women then were randomly assigned to receive either conjugated estrogens or placebo for an additional eight weeks while continuing to receive the gonadotropin-releasing hormone agonist. When assessed at the 12 week juncture, women's scores on a verbal episodic memory task (paragraph recall) had fallen from baseline performance levels. Eight weeks later, memory scores had returned to the pretreatment baseline in women receiving estrogens but not in those receiving placebo. Nonverbal memory performance in this trial was unaffected by hormonal interventions.
A recent case-control study also highlights the potential relevance of surgical menopause. Women in Olmsted County, Minnesota who had undergone oophorectomy at a relatively young age were at elevated risk of cognitive impairment or dementia later in life compared to women who had not experienced surgical menopause.67 Other randomized trials of surgically menopausal women have not shown cognitive effects of estrogen therapy.68-71 In these studies, however, surgical menopause was defined on the basis of hysterectomy rather than oophorectomy, and hormonal intervention did not occur immediately after surgery. Only one of these trials involved midlife women.68
ESTROGENS AND MEMORY: CLINICAL TRIAL EVIDENCE IN OLDER POSTMENOPAUSAL WOMEN
Among older women, nine randomized controlled trials of estrogen-containing hormone therapy have been reported of at least one month duration in which outcomes included an objective measure of episodic memory60 (Table 2). Unlike trials in younger postmenopausal women, several of these studies were quite large and extended over treatment periods of up to four years. However, findings in both age groups on tasks of episodic memory and other cognitive functions are similar in that most results indicate no significant effect of hormone treatment.
The largest trial of older women is from the Women's Health Initiative Study of Cognitive Aging and included over 1400 naturally-menopausal women aged 65 to 79 years.72 This ancillary study involved a subset of participants in the Women's Health Initiative.4 The first cognitive assessment occurred on average three years after treatment randomization, and women were followed for a mean of 1.4 years thereafter. At the first assessment, episodic memory was equivalent in the two treatment groups, suggesting no important memory effect of the hormonal intervention during this initial three year period. At follow-up, small differences favored the placebo group on several measures of episodic verbal memory (total immediate recall and short- and long-delay free recall on the California Verbal Learning Test), and there was a small advantage for estrogens on a nonverbal memory task (Benton Visual Retention Test). These clinical trial findings do suggest a cognitive effect of this estrogen-progestogen formulation, although mean between-groups differences were small (e.g., decline of 0.23 points per year on long-delay recall, where the mean score was about 9; improvement of 0.27 points per year on visual retention, where the mean error score was about 7).72
Memory findings are not yet available from the parallel ancillary study within the Women's Health Initiative involving women who had undergone hysterectomy. In this trial, allocation was to unopposed conjugated estrogens or placebo. In an earlier report from Women's Health Initiative investigators, the two treatment groups of hysterectomized women performed nearly the same on a global cognitive task (the 100-point modified Mini Mental State Examination), with women in the placebo group performing slightly, but significantly, better than women in the conjugated estrogens group after an average follow-up of five years (the mean difference was about one-fourth point).71
IS THERE A CRITICAL WINDOW DURING WHICH ESTROGENS CAN IMPROVE MEMORY?
Some clinically important effects of estrogens are modulated by age or timing. In the Women's Health Initiative, women who were randomly assigned to receive hormone therapy close to the age of menopause tended to develop less coronary heart disease than older postmenopausal women.73 Estrogens can accelerate atherosclerosis when administered in the presence of established vascular disease but can retard this process when the vascular endothelium is healthy.74,75 In a murine experimental stroke model, estradiol initiated at the time of ovariectomy, but not when initiated 10 weeks after ovariectomy, reduces infarct volume.76
Women aged 65 to 79 years in the Women's Health Initiative who received hormone therapy faced increased risk of dementia from any cause.77 In apparent contrast, observational research generally supports the inference that estrogen-containing hormone therapy reduces the risk of later Alzheimer's disease (e.g., reports from Leisure World,78 northern Manhattan,79 the Baltimore Longitudinal Study of Aging,80 and Cache County81). Because most hormone therapy is initiated during the menopausal transition or early postmenopause for alleviation of hot flashes and other vasomotor symptoms, exposures in observational studies tend to involve women considerably younger and more symptomatic than Women's Health Initiative participants. There is other observational evidence that past users of hormone therapy or younger hormone users face a lower risk of Alzheimer's disease than current hormone users or older users.81,82 Findings such as these have suggested a critical window close to the age of menopause during which estrogens may protect against coronary heart disease or clinical outcomes like Alzheimer's disease.83 Alternatively, observational studies of Alzheimer's disease risk may be biased by the so-called healthy user effect,83 as hormone users tend to be better educated and to practice healthier lifestyles than nonusers.
Some animal studies support the so-called critical window hypothesis in relation to cognitive outcomes. In one study of ovariectomized rats, animals that received parenteral estradiol immediately after surgery learned a delayed matching-to-position task better than vehicle-treated controls, as did animals whose treatment began three months after ovariectomy. When estradiol was delayed for 10 months, performance resembled that of untreated animals.84 In a similar manner, estradiol begun immediately after ovariectomy improves learning of a radial maze task but not estradiol initiated five months later.85 In older ovariectomized primates, cyclical low-dose estradiol improves performance on several memory tasks.86
Impaired memory is a risk factor for Alzheimer's disease,20 but effects of hormone use on Alzheimer's disease are not necessarily predictive of effects on memory performance in healthy women. There are no comparable observational or experimental data regarding midlife estrogen therapy and late-life episodic memory abilities.
CONCLUDING PERSPECTIVES AND A LOOK AHEAD
Animal studies provide fairly convincing evidence that ovariectomy and estradiol treatment can affect cognition, including aspects that involve hippocampal-dependent forms of memory similar to episodic memory. Although the number of reports is small and interpretation is not straight-forward, functional brain imaging reinforces the view that estrogens affect human cerebral function during performance of memory tasks. However, the natural menopause transition is not associated with objective change in midlife memory performance (Table 1, Figure 1), and it has been difficult to demonstrate performance differences on episodic memory tasks in human clinical trials, especially among older postmenopausal women where the quality of evidence is now quite solid (Table 2). These findings on memory are consistent with a recent Cochrane meta-analysis, which concluded that estrogens alone or estrogens plus a progestogen do not prevent cognitive decline in postmenopausal women.87 Among various interpretations is the possibility that endogenous estradiol production within the central nervous system after menopause is adequate to maintain neural processes involved in episodic memory.6,7
Another possibility concerns the type of hormonal preparation used in clinical trials. In the laboratory, different estrogens,88 different progestogens,89 and different routes of administration vary in their effects on neural function and other physiological processes. Is it possible that a hormonal formulation different from that used in large clinical trials like the Women's Health Initiative would have had robust positive effects on memory? Because it is the most abundant circulating estrogen during a woman's reproductive years and because it is the estrogen most often used in animal behavioral studies, it is argued that 17β-estradiol is a more appropriate therapeutic agent than, for example, conjugated estrogens, esterified estrogens, or estriol. For similar reasons, one might select progesterone over other progestogens or recommend cyclic hormone therapy over continuous combined estrogen-progestogen therapy. In some model systems, an estrogen alone might be preferred to an estrogen plus a progestogen.90 The Women's Health Initiative formulation can be reasonably justified, however,91 and clinically meaningful distinctions between particular estrogens or progestogens are not easily resolved by existing clinical data. With respect to human memory, no strong findings thus far indicate that estradiol would lead to different outcomes than conjugated estrogens (Table 2).
Selective estrogen receptor modulators would be expected to have profiles of nervous system action distinct from those of estrogens. In a large multicenter trial of older women, raloxifene compared to placebo did not improve verbal episodic memory (word list recall), but raloxifene users were less likely to show memory decline over a three year interval.92 As newer selective estrogen receptor modulators are developed and come to market, cognitive effects will need to be carefully evaluated.
Women who have undergone surgical menopause represent an important, inadequately studied subgroup. It is among these women that clinical trial results, albeit short-term, seem most promising62,63 (Table 2). Surgically menopausal women have experienced an abrupt loss of ovarian function in addition to an early loss and, conjecturally, estrogen effects on episodic memory may be clinically evident only in the acute setting when treatment has not been preceded by a prolonged interval of estrogen deprivation. Studies in surgically menopausal women also suggest that verbal memory, but not nonverbal memory, benefits from estradiol.63 However, any conclusions on modality specificity must be made cautiously, because studies have not been replicated and because psychometric properties of these memory tasks are different.
A related consideration is that treatment effects might differ for younger postmenopausal women compared to older postmenopausal women. Findings from surgically menopausal women are consistent with this conjecture. As noted, there is emerging experimental evidence that age or timing of hormone use in relation to menopause may modify treatment effects on clinically important cardiovascular disease outcomes,73 and there speculation based on observational research that the same may true for Alzheimer's disease risk.83 Whether this interaction with age or timing will hold for episodic memory is equally speculative.
Clinical trial data do not confirm that hormonal effects on episodic memory differ by age or proximity to menopause, but neither are findings definitive, since trials among younger women have involved fewer women treated for shorter periods of time. Two ongoing randomized clinical trials will have the opportunity to examine this critical window hypothesis more carefully in younger postmenopausal women. These are the Kronos Early Estrogen Prevention Study (KEEPS) and the Early versus Late Intervention Trial with Estradiol (ELITE) (ClinicalTrials.gov identifiers NCT00154180 and NCT00114517). Active therapy in these placebo-controlled trials is with oral conjugated estrogens or transdermal estradiol in the former and with oral estradiol in the later. Both trials are adequately powered to detect moderate differences on episodic memory tests between hormone and placebo groups.
Acknowledgments
Supported in part by National Institutes of Health R01-AG023038, R01-AG17160.
Footnotes
Off-label use: There is no approved indication for estrogens for the prevention or treatment of memory loss.
Conflicts of interest: None.
References
- 1.Brown-Séquard CE. The effects produced on man by subcutaneous injections of a liquid obtained from the testicles of animals. Lancet. 1889;2:105–7. [Google Scholar]
- 2.Sengoopta C. The Most Secret Quintessence of Life. Sex, Glands and Hormones, 1850-1950. University of Chicago Press; Chicago: 2006. [Google Scholar]
- 3.Wilson RA. Feminine Forever. M. Evans and Company; New York: 1966. [Google Scholar]
- 4.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 5.Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291:47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- 6.Kretz O, Fester L, Wehrenberg U, et al. Hippocampal synapses depend on hippocampal estrogen synthesis. J Neurosci. 2004;24:5913–21. doi: 10.1523/JNEUROSCI.5186-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hojo Y, Murakami G, Mukai H, et al. Estrogen synthesis in the brain--role in synaptic plasticity and memory. Mol Cell Endocrinol. 2008;290:31–43. doi: 10.1016/j.mce.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 8.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–25. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 9.Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J Comp Neurol. 2001;429:355–71. [PubMed] [Google Scholar]
- 10.Milner TA, Ayoola K, Drake CT, et al. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J Comp Neurol. 2005;491:81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- 11.Shughrue PJ, Scrimo PJ, Merchenthaler I. Estrogen binding and estrogen receptor characterization (ERα and ERβ) in the cholinergic neurons of the rat basal forebrain. Neuroscience. 2000;96:41–9. doi: 10.1016/s0306-4522(99)00520-5. [DOI] [PubMed] [Google Scholar]
- 12.Edwards DP. Regulation of signal transduction pathways by estrogen and progesterone. Annu Rev Physiol. 2005;67:335–76. doi: 10.1146/annurev.physiol.67.040403.120151. [DOI] [PubMed] [Google Scholar]
- 13.Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 14.Scoville WB. The limbic lobe in man. J Neurosurg. 1954;11:64–6. doi: 10.3171/jns.1954.11.1.0064. [DOI] [PubMed] [Google Scholar]
- 15.Corkin S. Lasting consequences of bilateral medial temporal lobectomy: clinical course and experimental findings in H.M. Semin Neurol. 1984;4:249–59. [Google Scholar]
- 16.Storandt M. Longitudinal studies of aging and age-associated dementias. In: Boller F, Grafman J, editors. Handbook of Neuropsychology. vol 4. Elsevier; New York: 1991. [Google Scholar]
- 17.Welsh K, Butters N, Hughes JP, Mohs RC, Heyman A. Detection and staging of dementia in Alzheimer's disease — use of the neuropsychological measures developed for the Consortium to Establish a Registry for Alzheimer's Disease. Arch Neurol. 1992;49:448–52. doi: 10.1001/archneur.1992.00530290030008. [DOI] [PubMed] [Google Scholar]
- 18.Elias MF, Beiser A, Wolf PA, Au R, White RF, D'Agostino RB. The preclinical phase of Alzheimer's disease: a 22-year prospective study of the Framingham cohort. Arch Neurol. 2000;57:808–13. doi: 10.1001/archneur.57.6.808. [DOI] [PubMed] [Google Scholar]
- 19.Hyman BT, van Hoesen GW, Damasio AR, Barnes CL. Alzheimer's disease: cell-specific pathology isolates the hippocampal formation. Science. 1984;225:1168–70. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- 20.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–8. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 21.Trévoux R, De Brux J, Castanier M, Nahoul K, Soule J-P, Scholler R. Endometrium and plasma hormone profile in the peri-menopause and post-menopause. Maturitas. 1986;8:309–26. doi: 10.1016/0378-5122(86)90039-3. [DOI] [PubMed] [Google Scholar]
- 22.Henderson VW. Hormone Therapy and the Brain: A Clinical Perspective on the Role of Estrogen. Parthenon Publishing; New York: 2000. [DOI] [PubMed] [Google Scholar]
- 23.Singh M, Meyer EM, Millard WJ, Simpkins JW. Ovarian steroid deprivation results in a reversible learning impairment and compromised cholinergic function in female Sprague-Dawley rats. Brain Res. 1994;644:305–12. doi: 10.1016/0006-8993(94)91694-2. [DOI] [PubMed] [Google Scholar]
- 24.Fader AJ, Hendricson AW, Dohanich GP. Estrogen improves performance of reinforced T-maze alternation and prevents the amnestic effects of scopolamine administered systemically or intrahippocampally. Neurobiol Learn Mem. 1998;69:225–40. doi: 10.1006/nlme.1998.3820. [DOI] [PubMed] [Google Scholar]
- 25.Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol Learn Mem. 2007;88:208–16. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zurkovsky L, Brown SL, Boyd SE, Fell JA, Korol DL. Estrogen modulates learning in female rats by acting directly at distinct memory systems. Neuroscience. 2007;144:26–37. doi: 10.1016/j.neuroscience.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foy MR, Henderson VW, Berger TW, Thompson RF. Estrogen and neural plasticity. Current Directions in Psychological Science. 2000;9:148–52. [Google Scholar]
- 28.Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–9. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–91. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leranth C, Shanabrough M, Redmond DE., Jr. Gonadal hormones are responsible for maintaining the integrity of spine synapses in the CA1 hippocampal subfield of female nonhuman primates. J Comp Neurol. 2002;447:34–42. doi: 10.1002/cne.10230. [DOI] [PubMed] [Google Scholar]
- 31.Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19:5792–801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luine V. Estradiol increases choline acetyltransferase activity in specific basal forebrain nuclei and projection areas of female rats. Exp Neurol. 1985;89:484–90. doi: 10.1016/0014-4886(85)90108-6. [DOI] [PubMed] [Google Scholar]
- 33.Schwarz JM, McCarthy MM. Cellular mechanisms of estradiol-mediated masculinization of the brain. J Steroid Biochem Mol Biol. 2008;109:300–6. doi: 10.1016/j.jsbmb.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roof RL. Neonatal exogenous testosterone modifies sex difference in radial arm and Morris water maze performance in prepubescent and adult rats. Behav Brain Res. 1993;53:1–10. doi: 10.1016/s0166-4328(05)80261-x. [DOI] [PubMed] [Google Scholar]
- 35.Herman RA, Wallen K. Cognitive performance in rhesus monkeys varies by sex and prenatal androgen exposure. Horm Behav. 2007;51:139–42. doi: 10.1016/j.yhbeh.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Protopopescu X, Butler T, Pan H, et al. Hippocampal structural changes across the menstrual cycle. Hippocampus. 2008 doi: 10.1002/hipo.20468. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 37.Phillips SM, Sherwin BB. Variations in memory function and sex steroid hormones across the menstrual cycle. Psychoneuroendocrinology. 1992;17:497–506. doi: 10.1016/0306-4530(92)90008-u. [DOI] [PubMed] [Google Scholar]
- 38.Mordecai K, Rubin L, Maki P. Effects of menstrual cycle phase and oral contraceptive use on verbal memory. Horm Behav. 2008;54:286–93. doi: 10.1016/j.yhbeh.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 39.Maki PM, Rich JB, Rosenbaum RS. Implicit memory varies across the menstrual cycle: estrogen effects in young women. Neuropsychologia. 2002;40:518–29. doi: 10.1016/s0028-3932(01)00126-9. [DOI] [PubMed] [Google Scholar]
- 40.Epting LK, Overman WH. Sex-sensitive tasks in men and women: a search for performance fluctuations across the menstrual cycle. Behav Neurosci. 1998;112:1304–1317. doi: 10.1037//0735-7044.112.6.1304. [DOI] [PubMed] [Google Scholar]
- 41.Henderson VW, Dudley EC, Guthrie JR, Burger HG, Dennerstein L. Estrogen exposures and memory at midlife: a population-based study of women. Neurology. 2003;60:1369–71. doi: 10.1212/01.wnl.0000059413.75888.be. [DOI] [PubMed] [Google Scholar]
- 42.Fuh J-L, Wang S-J, Lee S-J, Lu S-R, Juang K-D. A longitudinal study of cognition change during early menopausal transition in a rural community. Maturitas. 2006;53:447–53. doi: 10.1016/j.maturitas.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 43.Kok HS, Kuh D, Cooper R, et al. Cognitive function across the life course and the menopausal transition in a British birth cohort. Menopause. 2006;13:19–27. doi: 10.1097/01.gme.0000196592.36711.a0. [DOI] [PubMed] [Google Scholar]
- 44.Herlitz A, Thilers P, Habib R. Endogenous estrogen is not associated with cognitive performance before, during, or after menopause. Menopause. 2007;14:425–31. doi: 10.1097/01.gme.0000247019.86748.e3. [DOI] [PubMed] [Google Scholar]
- 45.Luetters C, Huang MH, Seeman T, et al. Menopause transition stage and endogenous estradiol and follicle-stimulating hormone levels are not related to cognitive performance: cross-sectional results from the study of women's health across the nation (SWAN) J Womens Health. 2007;16:331–44. doi: 10.1089/jwh.2006.0057. [DOI] [PubMed] [Google Scholar]
- 46.Mitchell ES, Woods NF. Midlife women's attributions about perceived memory changes: observations from the Seattle Midlife Women's Health Study. J Womens Health Gend Based Med. 2001;10:351–62. doi: 10.1089/152460901750269670. [DOI] [PubMed] [Google Scholar]
- 47.Drake EB, Henderson VW, Stanczyk FZ, et al. Associations between circulating sex steroid hormones and cognition in normal elderly women. Neurology. 2000;54:599–603. doi: 10.1212/wnl.54.3.599. [DOI] [PubMed] [Google Scholar]
- 48.Wolf OT, Kirschbaum C. Endogenous estradiol and testosterone levels are associated with cognitive performance in older women and men. Horm Behav. 2002;41:259–66. doi: 10.1006/hbeh.2002.1770. [DOI] [PubMed] [Google Scholar]
- 49.Hogervorst E, De Jager C, Budge M, Smith AD. Serum levels of estradiol and testosterone and performance in different cognitive domains in healthy elderly men and women. Psychoneuroendocrinology. 2004;29:405–21. doi: 10.1016/s0306-4530(03)00053-2. [DOI] [PubMed] [Google Scholar]
- 50.Yaffe K, Barnes D, Lindquist K, et al. Endogenous sex hormone levels and risk of cognitive decline in an older biracial cohort. Neurobiol Aging. 2007;28:171–8. doi: 10.1016/j.neurobiolaging.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 51.Barrett-Connor E, Goodman-Gruen D. Cognitive function and endogenous sex hormones in older women. J Am Geriatr Soc. 1999;47:1289–93. doi: 10.1111/j.1532-5415.1999.tb07427.x. [DOI] [PubMed] [Google Scholar]
- 52.Almeida OP, Lautenschlager N, Vasikaram S, Leedman P, Flicker L. Association between physiological serum concentration of estrogen and the mental health of community-dwelling postmenopausal women age 70 years and over. Am J Geriatr Psychiatry. 2005;13:142–9. doi: 10.1176/appi.ajgp.13.2.142. [DOI] [PubMed] [Google Scholar]
- 53.den Heijer T, Geerlings MI, Hofman A, et al. Higher estrogen levels are not associated with larger hippocampi and better memory performance. Arch Neurol. 2003;60:213–20. doi: 10.1001/archneur.60.2.213. [DOI] [PubMed] [Google Scholar]
- 54.Berman KF, Schmidt PJ, Rubinow DR, et al. Modulation of cognition-specific cortical activity by gonadal steroids: a positron-emission tomography study in women. Proceedings of the National Academy of Sciences, USA. 1997;94:8836–41. doi: 10.1073/pnas.94.16.8836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaya E, Sahin FK, Köken G, Köse M, Cevrioglu AS. Acute effect of intranasal estrogen on cerebral and cerebellar perfusion in postmenopausal women. Maturitas. 2008;59:72–82. doi: 10.1016/j.maturitas.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 56.Craig MC, Fletcher PC, Daly EM, et al. Physiological variation in estradiol and brain function: a functional magnetic resonance imaging study of verbal memory across the follicular phase of the menstrual cycle. Horm Behav. 2008;53:503–8. doi: 10.1016/j.yhbeh.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 57.Resnick SM, Maki PM, Golski S, Kraut MA, Zonderman AB. Effects of estrogen replacement therapy on PET cerebral blood flow and neuropsychological performance. Horm Behav. 1998;34:171–82. doi: 10.1006/hbeh.1998.1476. [DOI] [PubMed] [Google Scholar]
- 58.Joffe H, Hall JE, Gruber S, et al. Estrogen therapy selectively enhances prefrontal cognitive processes: a randomized, double-blind, placebo-controlled study with functional magnetic resonance imaging in perimenopausal and recently postmenopausal women. Menopause. 2006;13:411–22. doi: 10.1097/01.gme.0000189618.48774.7b. [DOI] [PubMed] [Google Scholar]
- 59.Neele SJ, Rombouts SA, Bierlaagh MA, Barkhof F, Scheltens P, Netelenbos JC. Raloxifene affects brain activation patterns in postmenopausal women during visual encoding. J Clin Endocrinol Metab. 2001;86:1422–4. doi: 10.1210/jcem.86.3.7454. [DOI] [PubMed] [Google Scholar]
- 60.Henderson VW, Sherwin BB. Surgical versus natural menopause: cognitive issues. Menopause. 2007;14:572–9. doi: 10.1097/gme.0b013e31803df49c. [DOI] [PubMed] [Google Scholar]
- 61.Maki PM, Gast MJ, Vieweg A, Burriss SW, Yaffe K. Hormone therapy in menopausal women with cognitive complaints: a randomized, double-blind trial. Neurology. 2007;69:1322–30. doi: 10.1212/01.wnl.0000277275.42504.93. [DOI] [PubMed] [Google Scholar]
- 62.Sherwin BB. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology. 1988;13:345–57. doi: 10.1016/0306-4530(88)90060-1. [DOI] [PubMed] [Google Scholar]
- 63.Phillips SM, Sherwin BB. Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology. 1992;17:485–95. doi: 10.1016/0306-4530(92)90007-t. [DOI] [PubMed] [Google Scholar]
- 64.Kjerulff K, Langenberg P, Guzinski G. The socioeconomic correlates of hysterectomies in the United States. Am J Public Health. 1994;83:106–8. doi: 10.2105/ajph.83.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Davison S, Bell R, Donath S, Montalto J, Davis SR. Androgen levels in adult females: changes with age, menopause and oophorectomy. J Clin Endocrinol Metab. 2005;90:3847–53. doi: 10.1210/jc.2005-0212. [DOI] [PubMed] [Google Scholar]
- 66.Sherwin BB, Tulandi T. “Add-back” estrogen reverses cognitive deficits induced by a gonadotropin-releasing hormone agonist in women with leiomyomata uteri. J Clin Endocrinol Metab. 1996;81:2545–9. doi: 10.1210/jcem.81.7.8675575. [DOI] [PubMed] [Google Scholar]
- 67.Rocca WA, Bower JH, Ahlskog JE, Grossardt BR, de Andrade M, Melton LJ., 3rd Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:1074–83. doi: 10.1212/01.wnl.0000276984.19542.e6. [DOI] [PubMed] [Google Scholar]
- 68.Polo-Kantola P, Erkkola R, Helenius H, Irjala K, Polo O. When does estrogen replacement therapy improve sleep quality? Am J Obstet Gynecol. 1998;178:1002–9. doi: 10.1016/s0002-9378(98)70539-3. [DOI] [PubMed] [Google Scholar]
- 69.Schiff R, Bulpitt CJ, Wesnes KA, Rajkumar C. Short-term transdermal estradiol therapy, cognition and depressive symptoms in healthy older women. A randomised placebo controlled pilot cross-over study. Psychoneuroendocrinology. 2005;30:309–15. doi: 10.1016/j.psyneuen.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 70.Almeida OP, Lautenschlager NT, Vasikaran S, Leedman P, Gelavis A, Flicker L. 20-week randomized controlled trial of estradiol replacement therapy for women aged 70 years and older: effect on mood, cognition and quality of life. Neurobiol Aging. 2006;27:141–9. doi: 10.1016/j.neurobiolaging.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 71.Espeland MA, Rapp SR, Shumaker SA, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291:2959–68. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- 72.Resnick SM, Maki PM, Rapp SR, et al. Effects of combination estrogen plus progestin hormone treatment on cognition and affect. J Clin Endocrinol Metab. 2006;91:1802–10. doi: 10.1210/jc.2005-2097. [DOI] [PubMed] [Google Scholar]
- 73.Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465–77. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 74.Clarkson TB. Estrogen effects on arteries vary with stage of reproductive life and extent of subclinical atherosclerosis progression. Menopause. 2007;14:373–84. doi: 10.1097/GME.0b013e31803c764d. [DOI] [PubMed] [Google Scholar]
- 75.Umetani M, Domoto H, Gormley AK, et al. 27-Hydroxycholesterol is an endogenous SERM that inhibits the cardiovascular effects of estrogen. Nat Med. 2007;13:1185–92. doi: 10.1038/nm1641. [DOI] [PubMed] [Google Scholar]
- 76.Suzuki S, Brown CM, Dela Cruz CD, Yang E, Bridwell DA, Wise PM. Timing of estrogen therapy after ovariectomy dictates the efficacy of its neuroprotective and antiinflammatory actions. Proc Natl Acad Sci U S A. 2007;104:6013–8. doi: 10.1073/pnas.0610394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291:2947–58. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 78.Paganini-Hill A, Henderson VW. Estrogen replacement therapy and risk of Alzheimer's disease. Arch Intern Med. 1996;156:2213–7. [PubMed] [Google Scholar]
- 79.Tang M-X, Jacobs D, Stern Y, et al. Effect of oestrogen during menopause on risk and age at onset of Alzheimer's disease. Lancet. 1996;348:429–32. doi: 10.1016/S0140-6736(96)03356-9. [DOI] [PubMed] [Google Scholar]
- 80.Kawas C, Resnick S, Morrison A, et al. A prospective study of estrogen replacement therapy and the risk of developing Alzheimer's disease: the Baltimore Longitudinal Study of Aging. Neurology. 1997;48:1517–21. doi: 10.1212/wnl.48.6.1517. [DOI] [PubMed] [Google Scholar]
- 81.Zandi PP, Carlson MC, Plassman BL, et al. Hormone replacement therapy and incidence of Alzheimer's disease on older women: the Cache County study. JAMA. 2002;288:2123–9. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]
- 82.Henderson VW, Benke KS, Green RC, Cupples LA, Farrer LA. Postmenopausal hormone therapy and Alzheimer's disease risk: interaction with age. J Neurol Neurosurg Psychiatry. 2005;76:103–5. doi: 10.1136/jnnp.2003.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Henderson VW. Estrogen-containing hormone therapy and Alzheimer's disease risk: understanding discrepant inferences from observational and experimental research. Neuroscience. 2006;138:1031–9. doi: 10.1016/j.neuroscience.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 84.Gibbs RB. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol Aging. 2000;21:107–16. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- 85.Daniel JM, Hulst JL, Berbling JL. Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology. 2006;147:607–14. doi: 10.1210/en.2005-0998. [DOI] [PubMed] [Google Scholar]
- 86.Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003;23:5708–14. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lethaby A, Hogervorst E, Richards M, Yesufu A, Yaffe K. Hormone replacement therapy for cognitive function in postmenopausal women. Cochrane Database of Systematic Reviews. 2008;(Issue 1Art) doi: 10.1002/14651858.CD003122.pub2. No.: CD003122. DOI: 10.1002/14651858.CD003122.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brinton RD, Proffitt P, Tran J, Luu R. Equilin, a principal component of the estrogen replacement therapy Premarin, increases the growth of cortical neurons via an NMDA receptor-dependent mechanism. Exp Neurol. 1997;147:211–20. doi: 10.1006/exnr.1997.6619. [DOI] [PubMed] [Google Scholar]
- 89.Nilsen J, Brinton RD. Divergent impact of progesterone and medroxyprogesterone acetate (Provera) on nuclear mitogen-activated protein kinase signaling. Proc Natl Acad Sci U S A. 2003;100:10506–11. doi: 10.1073/pnas.1334098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rosario ER, Ramsden M, Pike CJ. Progestins inhibit the neuroprotective effects of estrogen in rat hippocampus. Brain Res. 2006;1099:206–10. doi: 10.1016/j.brainres.2006.03.127. [DOI] [PubMed] [Google Scholar]
- 91.Women's Health Initiative Study Group Design of the Women's Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 92.Yaffe K, Krueger K, Sarkar S, et al. Cognitive function in postmenopausal women treated with raloxifene. N Engl J Med. 2001;344:1207–13. doi: 10.1056/NEJM200104193441604. [DOI] [PubMed] [Google Scholar]
- 93.Hackman BW, Galbraith D. Replacement therapy with piperazine oestrone sulphate ('Harmogen') and its effect on memory. Curr Med Res Opin. 1976;4:303–6. doi: 10.1185/03007997609109322. [DOI] [PubMed] [Google Scholar]
- 94.Vanhulle G, Demol R. Consensus on Menopause Research: A Summary of International Opinion. University Park Press; Baltimore: 1976. A double-blind study into the influence of estriol on a number of psychological tests in post-menopausal women; pp. 94–9. [Google Scholar]
- 95.Polo-Kantola P, Portin R, Polo O, Helenius H, Irjala K, Erkkola R. The effect of short-term estrogen replacement therapy on cognition: a randomized, double-blind, cross-over trial in postmenopausal women. Obstet Gynecol. 1998;91:459–66. doi: 10.1016/s0029-7844(97)00700-x. [DOI] [PubMed] [Google Scholar]
- 96.Linzmayer L, Semlitsch HV, Saletu B, et al. Double-blind, placebo-controlled psychometric studies on the effects of a combined estrogen-progestin regimen versus estrogen alone on performance, mood and personality of menopausal syndrome patients. Arzneimittelforschung. 2001;51:238–45. doi: 10.1055/s-0031-1300030. [DOI] [PubMed] [Google Scholar]
- 97.Dunkin J, Rasgon N, Wagner-Steh K, David S, Altshuler L, Rapkin A. Reproductive events modify the effects of estrogen replacement therapy on cognition in healthy postmenopausal women. Psychoneuroendocrinology. 2005;30:284–96. doi: 10.1016/j.psyneuen.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 98.Maki PM, Gast MJ, Vieweg A, Burriss SW, Yaffe K. Hormone therapy in recently menopausal women with cognitive complaints: a randomized double-blind trial. Neurology. doi: 10.1212/01.wnl.0000277275.42504.93. in press. [DOI] [PubMed] [Google Scholar]
- 99.Binder EF, Schechtman KB, Birge SJ, Williams DB, Kohrt WM. Effects of hormone replacement therapy on cognitive performance in elderly women. Maturitas. 2001;38:137–46. doi: 10.1016/s0378-5122(00)00214-0. [DOI] [PubMed] [Google Scholar]
- 100.Grady D, Yaffe K, Kristof M, Lin F, Richards C, Barrett-Connor E. Effect of postmenopausal hormone therapy on cognitive function: the Heart and Estrogen/progestin Replacement Study. Am J Med. 2002;113:543–8. doi: 10.1016/s0002-9343(02)01270-6. [DOI] [PubMed] [Google Scholar]
- 101.Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. Estrogen therapy and risk of cognitive decline: results from the Women's Estrogen for Stroke Trial (WEST) Am J Obstet Gynecol. 2005;192:387–93. doi: 10.1016/j.ajog.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 102.Wolf OT, Heinrich AB, Hanstein B, Kirschbaum C. Estradiol or estradiol/progesterone treatment in older women: no strong effects on cognition. Neurobiol Aging. 2005;26:1029–33. doi: 10.1016/j.neurobiolaging.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 103.Yaffe K, Vittinghoff E, Ensrud KE, et al. Effects of ultra-low-dose transdermal estradiol on cognition and health-related quality of life. Arch Neurol. 2006;63:945–50. doi: 10.1001/archneur.63.7.945. [DOI] [PubMed] [Google Scholar]


