Mice and other animals have proved extremely useful testing grounds for hypotheses and for potential therapies. However, significant interspecies differences can confound correct interpretation and attempts to extrapolate results to man. In this study Fernandez and co-workers demonstrate that the human and murine protein C-protein S systems function in a similar way but do not interact efficiently with one another.
Keywords: anticoagulant, mitogenic activities, murine protein S
Abstract
Background
The protein C pathway down-regulates thrombin generation and promotes cytoprotection during inflammation and stress. In preclinical studies using models of murine injury (e.g., sepsis and ischemic stroke), murine protein S may be required because of restrictive species specificity.
Design and Methods
We prepared and characterized recombinant murine protein S using novel coagulation assays, immunoassays, and cell proliferation assays.
Results
Purified murine protein S had good anticoagulant co-factor activity for murine activated protein C, but not for human activated protein C, in mouse or rat plasma. In human plasma, murine protein S was a poor co-factor for murine activated protein C and had no anticoagulant effect with human activated protein C, suggesting protein S species specificity for factor V in addition to activated protein C. We estimated that mouse plasma contains 22±1 μg/mL protein S and developed assays to measure activated protein C co-factor activity of the protein S in murine plasma. Activated protein C-independent anticoagulant activity of murine protein S was demonstrable and quantifiable in mouse plasma, and this activity was enhanced by exogenous murine protein S. Murine protein S promoted the proliferation of mouse and human smooth muscle cells. The potency of murine protein S was higher for mouse cells than for human cells and similarly, human protein S was more potent for human cells than for mouse cells.
Conclusions
The spectrum of bioactivities of recombinant murine protein S with mouse plasma and smooth muscle cells is similar to that of human protein S. However, in vitro and in vivo studies of the protein C pathway in murine disease models are more appropriately performed using murine protein S. This study extends previous observations regarding the remarkable species specificity of protein S to the mouse.
Introduction
In translational research, investigators commonly use murine genetic manipulations and models of injury to generate preclinical information about pathogenic mechanisms and to assess potential drugs under development.1 Recombinant activated protein C (APC) is approved for the treatment of severe sepsis in humans and is a promising therapy for other indications, including ischemic stroke.2,3 Mice are used extensively for investigations of the antithrombotic and cytoprotective activities of APC.4 Most of the proteins involved in the protein C anticoagulant and cytoprotective pathways have been successfully targeted for genetic deletion in mice with the exception of protein S, an anticoagulant co-factor of APC. Homozygous protein C-deficient mice undergo fetal development, but they present a phenotype with brain abnormalities, secondary edema, and bleeding as major contributors to premature death, which often occurs in utero.5 Human APC has a half-life of 20–30 min in vivo in human blood; the half-life is determined by the protein’s irreversible inactivation by protease inhibitors such as protein C inhibitor and α1PI.6–8 These protease inhibitors irreversibly neutralize APC enzymatic activity by forming a covalent acyl enzyme complex with APC.
APC displays significant species specificity and murine APC is superior to human APC for translational research studies in mice.9–11 The anticoagulant species specificity of APC may be due primarily to protein S-APC interactions.12 Protein S in human or Rhesus monkey plasma serves well as a co-factor to human APC, and protein S in bovine, rabbit, or porcine plasmas serves optimally as a co-factor to bovine APC in anticoagulant activity assays.13–17 Purified rat protein S, however, is a notably inefficient co-factor for human APC,18 in contrast to purified rabbit protein S.19
Human protein S is present in plasma at a concentration of 25 μg/mL (or 330 nM)20 and functions as a non-enzymatic co-factor for APC in the proteolytic inactivation of activated factor V (FVa) and activated factor VIII (FVIIIa).21 The molecular mechanisms involved in the co-factor function of protein S are incompletely understood. Protein S increases the affinity of APC for negatively charged phospholipids by 10-fold and also alters the orientation of the active site of membrane-bound APC.22 About 60% of circulating human protein S is in a non-covalent complex with C4b-binding protein (C4bp), a complement regulatory factor. However, complex formation between protein S and C4bp does not occur in mouse plasma.23 Human protein S also has direct, APC-independent anticoagulant activity by virtue of direct binding and inhibition of activated factor X (FXa), FVa, and FVIIIa,24–27 and it may enhance the ability of tissue factor pathway inhibitor to inhibit the activated factor VII (FVIIa)/tissue factor complex.28 In a baboon thrombosis model, human protein S was antithrombotic independently of APC,29 but no information about protein S direct anticoagulant activity in other species is available.
In this study, we produced recombinant murine protein S and compared the co-factor activity of murine protein S with that of human protein S in plasma clotting assays using mouse, human, and bovine APC. In cell assays, we determined the potency of murine protein S for stimulating cell proliferation and the half-life of murine APC in plasma. We also developed an assay for APC co-factor activity of murine plasma protein S and a novel assay to investigate whether the protein S in murine plasma exerts direct anticoagulant activity. These new data and methods will help to define significant aspects of the components of the protein C pathway and show that recombinant murine protein S is a valuable instrument for future studies involving murine models of injury.
Design and Methods
Reagents
Mouse recombinant protein C and human protein C were prepared and activated as described elsewhere.9 Human FV was purified and activated, and goat anti-protein S was prepared and purified, as previously described.25 FXa, fibrinogen, human protein S and pro-thrombin were from Enzyme Research Laboratories, South Bend, IN, USA. Bovine APC was purchased from Haematologic Technologies (Essex Junction, VT, USA). p-APMSF was from Sigma, St. Louis, MO, USA. Synthetic phospholipid vesicles consisting of 80% 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine and 20% 1,2-dioleoyl-sn-glycero-3-phosphatidylserine (80%PC/20%PS) were prepared as described previously.9 Rat monoclonal antibodies, anti-mouse protein C TVM1 and 6B9 were prepared in house using purified recombinant mouse protein C as the antigen. Cell culture media and supplements were obtained from Invitrogen (Carlsbad, CA, USA). Rabbit anti-human Ki-67 was from DBS (Pleasanton, CA, USA) and rat monoclonal anti-mouse Ki-67 from DAKO (Carpinteria, CA, USA). Secondary antibodies for mitogenic studies were cyanine 3 (Cy3)-labeled anti-rabbit IgG and anti-rat IgG. For nuclear staining TO-PRO-3 was purchased from Invitrogen. [methyl-3H]thymidine was from Amersham/Pharmacia (Arlington Heights, IL, USA). Trichloracetic acid (TCA) was obtained from Sigma.
Plasma specimens
Mouse, rat and bovine plasma samples were obtained from Bioreclamation (Hicksville, NY, USA). Murine blood was also acquired from male CD-1/ICR mice or C57/BL6J mice by cardiac puncture. The blood was mixed with 3.8% sodium citrate and immediately centrifuged at 2,000 g for 20 min. The plasma was pooled and aliquots of 50–200 μL were frozen at −80°C until used. Normal pooled human plasma was obtained from George King Inc. (Overland Park, KS, USA). Rat or murine protein S-depleted plasma (PSdP) was prepared from 1.5 mL plasma by treatment with 20 μM p-amidinophenylmethylsulfonyl fluoride (pAPMSF), followed by adsorption on a 2 mL column of 4CNBr-Sepharose (GE Healthcare, Piscataway, NJ, USA) coupled with 4 mg of Dako rabbit anti-human protein S according to manufacturer’s instructions. The column was pre-equilibrated and the plasma was eluted in 0.2 mL fractions with Tris-buffered saline, pH 7.4 (TBS) containing 1 mM trisodium citrate. Fractions that were nearly undiluted were pooled and frozen in 50 μL aliquots. Dot immunobinding showed that more than 90% of the protein S was removed.
Smooth muscle cell cultures
Human vascular smooth muscle cells (VSMC) were isolated from human brain pial arteries, as described elsewhere.30 These cultures expressed smooth muscle α-actin, myosin heavy chain, calponin and SM22α and were negative for CD11b (microglia), glial fibrillar acidic protein (astrocytes), prolyl 4-hydroxylase (collagen-synthesizing fibroblasts) and endothelial cell von Willebrand factor. Mouse VSMC from aorta were obtained from the American Type Culture Collection (Manassas, VA, USA; ATCC code, CRL-2797). Cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) at 37°C, in a 5% CO2 humidified environment.
Construction and expression of murine protein S cDNA
Mouse liver Marathon-Ready cDNA was purchased from Clontech (Mountain View, CA, USA). Two primers MPSN (CGCGCTAGCGCAGGCACGGT) and MPS4 (CAGCTGGTGATAGGAATGTG) were designed to span the whole murine protein S cDNA from nucleotide (nt) 1 to nt 2158 according to Genbank sequence data by Chu et al.31 Polymerase chain reaction (PCR) was performed using primers MPSN and MPS4 and mouse liver cDNA as a template at an annealing temperature of 60°C for 30 cycles and 0.25 U of high fidelity PfuTurbo polymerase (Stratagene, La Jolla, CA, USA) was added to the reaction to limit the replication error. The approximately 2158-bp PCR product obtained was cloned into the Invitrogen expression vector pcDNA3.1 according to manufacturer’s instructions. The sequence of murine protein S cDNA was confirmed by sequencing. Stable transfection into a K293 cell line was performed using Effectene transfection reagent from Qiagen (German-town, MD, USA) according to the manufacturer’s instructions. The transfected cells were selected in DMEM/F-12 supplemented with 10% FBS containing 0.8 mg/mL of G418 antibiotic for about 3 weeks. The G418-resistant colonies were picked and grown in serum-free medium containing 10 μg/mL vitamin K1. Conditioned media were collected and tested for protein S antigen level by enzyme-linked immunoassay (ELISA) and western blotting. The positive colonies were chosen and used to produce conditioned media containing murine protein S.
Purification of recombinant wild-type murine protein S
Protein S was collected in serum-free DMEM/F12 medium, then 5 mM benzamidine and 5 mM EDTA were added and the pooled medium was loaded on a fast-flow Q-Sepharose column (10 cm x 2 cm) in 50 mM Tris, 150 mM NaCl, 1 mM EDTA pH 7.4. Protein S was eluted with the same buffer but containing 30 mM CaCl2 instead of EDTA. This protocol selected for properly γ-carboxylated protein S since under-carboxylated vitamin K-dependent proteins are not eluted by CaCl2.32 A second anion exchange chromatography step was performed using a mono Q column (Amersham/Pharmacia). In this step, a NaCl gradient (0–400 mM) was used to purify the murine protein S. After this step the fractions of interest were pooled and dialyzed against 50 mM Tris, 100 mM NaCl, pH 7.4. The protein S concentration was determined by monitoring absorbance at 280 nm, using an ɛ(1%, 1 cm) value of 9.5 and a molecular mass of 75,000 Da.
Enzyme-linked immunosorbent assay for protein S in mouse plasma
Costar half-well microplates (Corning, NY, USA) were coated with 5 μg/mL of IgG of rabbit anti-human protein S (Dako) in 0.1 M sodium carbonate, pH 9.0 (20 μL/well) for 1 h at 37°C and then blocked with 170 μL/well I-Block in TBS (Tropix, Bedford, MA, USA). Plasma (0.15 to 1.25 μL) or purified mouse protein S (0.25 to 2 μg/mL) was diluted in 20 μL Hepes-buffered saline (HBS), 0.5% bovine serum albumin (BSA), 0.005% Tween-20 and was incubated in the wells overnight at room temperature. Bound protein S was detected with 10 μg/mL goat anti-protein S IgG, followed by 1 μg/mL biotinylated mouse anti-goat IgG (Pierce, Rockford, IL, USA), 2 μg/mL streptavidin-horseradish peroxidase (Pierce) and o-phenylene-diamine substrate (Sigma). Between steps the wells were washed with TBS, 0.005% Tween-20, 2 mM CaCl2. The reaction was stopped upon appropriate color development (~10 min) with an equal volume of 1 M HCl and the product was measured at 490 nm.
Anticoagulant activity assays using purified protein S
Activated partial thromboplastin time (APTT) clotting assays were performed by incubating a mixture of 25 μL murine APC (180, 90, 45, 22.5, 11.2, 0 nM), 25 μL murine protein S (260 nM) or buffer, 10 μL mouse plasma, 25 μL of human fibrinogen (2 mg/mL), and 25 μL of Platelin LS (bioMerieux, Durham NC, USA) for 180 sec at 37°C. Reagents were diluted as needed in 50 mM Tris, 100 mM NaCl, pH 7.4 containing 0.1% BSA. The clotting times were then recorded after adding 25 μL of 30 mM CaCl2.
For FXa one-stage clotting assays, citrated mouse plasma (10 μL) was incubated for 180 sec at 37°C with 25 μL of 80%PC/20%PS vesicles (68 μM), 25 μL of human fibrinogen (2 mg/mL), 25 μL of FXa (34 nM), 25 μL of mouse or human APC (27 nM) and 25 μL of mouse or human protein S at varying concentrations. Clotting was initiated with 25 μL of 30 mM CaCl2.
Activated protein C co-factor activity of protein S in murine plasma
Plasma samples, as well as purified prothrombin, mouse APC, FVa, and fibrinogen at the concentrations to be used were frozen in multiple aliquots and thawed just before use. Plasma aliquots were thawed and treated for 20 min with pAPMSF just before use. Mouse test plasma (12 μL) was mixed with 2 μL of 3 μM prothrombin, 10 μL of 9 mg/mL fibrinogen, and 43 μL of 105 μM phospholipid vesicles (20%PS/80%PC) in HBS, 0.5% BSA in cuvettes of a coagulometer. Murine APC (25 μL of 2 μg/mL in HBS-BSA) or HBS-BSA was added, followed by 33 μL of 0.3 μg/mL FVa in HBS-BSA. After 2 min incubation at 37°C with mixing, 25 μL of 50 mM CaCl2 were added to initiate clotting, and the clotting time was measured. For standard curves in this assay, rat or mouse PSdP was mixed in various proportions with pooled mouse plasma so that the protein S in mouse plasma increased from 0–100% of normal, resulting in a linear increase in clotting time prolongation upon addition of APC.
Direct anticoagulant activity of murine plasma protein S
Mouse plasma smaples (15 μL) were pretreated for 15 min with 20 μM pAPMSF just before use and mixed with 5 μL of 3 μM prothrombin and 6 μL of 0.83 mg/mL neutralizing monoclonal antibody (TVM1) against murine and rat APC in HBS-BSA for 5 min. Phospholipids and CaCl2 in 15 μL of HBS-BSA were added to final concentrations of 15 μM and 9 mM, respectively, followed by 60 μL of fluorogenic substrate Z-GGR-aminomethyl coumarin (Bachem, Torrance, CA, USA) in HBS-BSA. To measure thrombin generation, fluorescence was read every 20 sec over a 45 min period at an excitation wavelength of 360 nm and an emission wavelength of 460 nm. The change in fluorescence/min was calculated to monitor thrombin generation. This assay is a modification of endogenous thrombin potential (ETP) assays. For standard curves, pooled normal mouse plasma and rat or mouse PSdP were mixed in various proportions as described above. In order to validate the assay, the effects of exogenous mouse protein S and rabbit anti-protein S were evaluated.
Cell proliferation assays
Immunofluorescence microscopy
To study the effect of murine protein S on proliferation of human- and mouse-VSMC, 6×103 cells were seeded into each well of 4-well chambered slides (Lab-Tek, Nunc, Rochester, NY, USA). After 24 h, cells were synchronized in G0 phase after transfer into DMEM containing 0.2% FBS for 18 h. Cells were then treated with various doses of protein S (2.5 to 250 nM) and cultured for 72 h. Next, cells were washed with phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde. The cells were immunostained for the mitotic marker Ki-67 using anti-Ki-67 antibodies (1:100) and these were detected by Cy3-labeled secondary antibodies, as explained in the Reagents section. Cells were then double stained with TO-PRO-3, a fluorescent nuclear marker. Fluorescence images were taken using a Zeiss 510 confocal microscope.
Cell counting
Human and mouse VSMC were cultured in 48-well culture plates and treated with protein S for 72 h as mentioned above. In order to quantify cell proliferation, cells were trypsinized and the total number of cells in each well was determined by counting trypan blue-excluding cells in a hemocytometer.
[3H]Thymidine incorporation assay
To determine cell proliferation by de novo DNA synthesis, cells were grown in 48-well culture plates in DMEM with 0.2% FBS, as above. Cells were next treated with protein S for 48 h and labeled with 1 μCi of [methyl-3H]thymidine for 24 h. Cells were then washed with PBS and incubated with cold 20% TCA for 30 min at 4°C. TCA-soluble material was removed and monolayers were washed with PBS and extracted with 0.5 N NaOH/0.5% SDS. TCA-precipitable counts were determined by scintillation counting using a Packard TRI-CARB 2100TR liquid scintillation analyzer.
Data analysis
Statistical comparisons by two-tailed t tests were conducted using Graph Pad Prism 5 software (San Diego, CA, USA). Differences with p values less than 0.05 were considered to be statistically significant. For the proliferation assays data were analyzed by one-way ANOVA followed by Tukey’s post hoc test. Again p values less than 0.05 were considered statistically significant.
Results
Recombinant murine protein S
Since the species specificity of protein S had been proposed to explain the major cause of reduction of human APC activity in animal plasma, we produced recombinant murine protein S. The nucleotide sequence of our murine cDNA for protein S (data not shown) was identical to the sequence reported by Chu et al.31 Comparing our murine cDNA protein S sequence to that of Lu et al.,33 we found Phe instead of Leu at residue 493. The mature murine protein S protein sequence has one amino acid less (634 residues) than the human protein S (635 residues). There are two potential glycosylation sites at residues 499 and 509 in murine protein S while in human protein S there is a third potential glycosylation site at residue 530. Recombinant murine protein S was expressed in stably transfected HEK293 cells and purified from conditioned serum-free medium via ion-exchange chromatography using a modification of a previously described protocol.34,35 Sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis (PAGE) demonstrated that the murine protein S was approximately 95% pure. In spite of having 80% sequence identity with human protein S, most antibodies available against human protein S did not recognize murine protein S. However two polyclonal antibodies recognized murine protein S sufficiently: goat anti-human protein S prepared in house, and Dako rabbit anti-human protein S. We were unsuccessful in producing high-affinity anti-mouse protein S antibodies despite attempts made in nine rats and two rabbits. The low-affinity antibodies raised did not perform as well as the anti-human protein S antibodies and did not neutralize mouse protein S activity.
Activity of activated protein C from various species
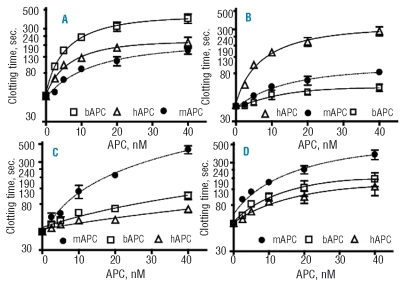
Protein C activity shows notable species specificity. We compared the anticoagulant activity of human, bovine and murine APC in plasma from several species (Figure 1). Using a modified APTT assay and bovine plasma (Figure 1A), we found that the anticoagulant response of bovine APC was 4.2-fold higher than that of murine APC and 1.8-fold higher than that of human APC, based on the initial slopes of dose-response curves. In human plasma (Figure 1B), the anticoagulant activity of human APC was 6-fold higher than that of murine APC and 17-fold higher than that of bovine APC. In rat plasma (Figure 1C), the anticoagulant activity of murine APC was 5-fold higher than that of bovine APC and 10-fold higher than that of human APC. Human APC anticoagulant activity was also remarkably low in rat plasma according to earlier reports.16 In mouse plasma (Figure 1D), the anticoagulant activity of murine APC was 3.5-fold higher than that of human APC and 1.8-fold higher than that of bovine APC.
Figure 1.
Anticoagulant activity of mouse, human and bovine APC in human, bovine, rat and mouse plasma. Increasing concentrations of mouse (●), human (▵), and bovine APC (□) were added to bovine (A), human (B), rat (C) and mouse (D) plasma specimens and APTT clotting assays were performed. Each experiment was performed in triplicate and mean values with standard deviation are presented. The indicated concentrations of APC refer to its concentration in the APC reagent solutions that were used for the protocol as detailed in the Design and Methods section.
Comparison of activated protein C co-factor activities of purified murine and human protein S
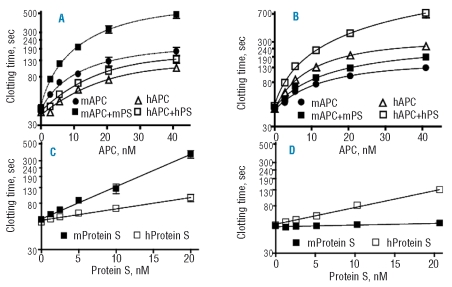
The activity of human or murine APC was assayed in the presence or absence of human and murine protein S in modified APTT assays using mouse plasma (Figure 2A). Murine protein S at 48 nM increased the anticoagulant activity of murine APC by 2-fold. However, the cofactor activity of human protein S for human APC in mouse plasma was very weak. For APTT assays using human plasma, 48 nM human protein S enhanced the activity of human APC by 1.7-fold, but murine protein S had almost no effect on murine APC in human plasma (Figure 2B). For murine APC, the co-factor activity of murine protein S in FXa one-stage clotting assays was at least four times greater than that of human protein S in mouse plasma whereas human protein S was a very weak co-factor for murine APC (Figure 2C). When assayed in human plasma under similar conditions, the co-factor activity of human protein S for human APC was six times greater than that of murine protein S (Figure 2D). Notably, murine protein S had essentially no anticoagulant co-factor activity for human APC at the concentrations tested.
Figure 2.
Anticoagulant co-factor activity of purified human and murine protein S. APTT clotting assays were performed using mouse (A) and human (B) plasma and increasing concentrations of human and murine APC in the absence or presence of recombinant human or murine protein S (48 nM) as indicated. For dose-response studies of protein S, FXa one-stage clotting assays were performed using 4 nM murine APC in mouse plasma (C) and 4 nM human APC in human plasma (D). The presence of human (□) or mouse (▪) protein S is indicated by the symbols in panels C and D. Each experiment was performed in triplicate and mean values with standard deviation are presented. The indicated concentrations of protein S represent the final concentrations in the clotting mixture.
Exogenous murine protein S had good co-factor activity for murine APC in mouse plasma, but not in human plasma, and exogenous human protein S had good co-factor activity for human APC in human plasma, but not in mouse plasma (Figure 2 A,B). It is suggested that other sources of species specificity must exist in addition to APC-protein S interactions.
Activated protein C co-factor activity of murine plasma protein S
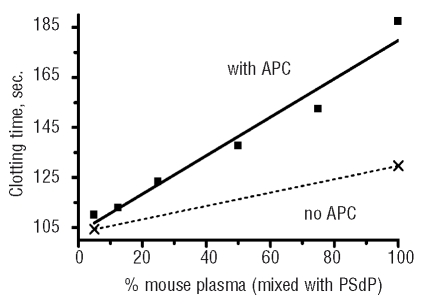
Addition of murine APC to mixtures of mouse plasma and rat PSdP resulted in a linear prolongation of clotting time as the proportion of mouse plasma in the mixture increased (Figure 3). APC had little effect in the PSdP, thus the dose response was due to the increasing amounts of protein S in increasing doses of murine plasma. The parent rat and mouse plasma samples used to prepare PSdP had similar clotting times in the absence of APC.
Figure 3.
APC co-factor activity of murine plasma protein S. The FVa-based assay is described in the Design and Methods sections. To vary plasma protein S levels, pooled normal mouse plasma as a source of protein S was mixed in various proportions with rat PSdP. 0% mouse plasma PS contains 100% rat PSdP. Clotting time was prolonged in a linear manner with increasing doses of normal mouse plasma containing protein S.
The basal clotting time of the frozen and thawed aliquots of rat PSdP was stable, but the basal clotting time of mouse PSdP increased markedly over time and could no longer be used. It is recognized that differences other than protein S in mouse and rat PSdP could have contributed to the dose-response curves observed, but when mouse PSdP was initially prepared, it behaved similarly to rat PSdP. Mouse and rat protein S share 93% sequence identity and 98% sequence similarity. In the absence of APC, the clotting time in PSdP was shorter than that in normal mouse plasma (Figure 3, dashed line), and this difference was not affected by inclusion of a neutralizing antibody (TVM-1) against mouse and rat APC (not shown). This suggests that mouse plasma protein S had direct anticoagulant activity, as was explored in the studies described below.
Direct anticoagulant activity of murine plasma protein S
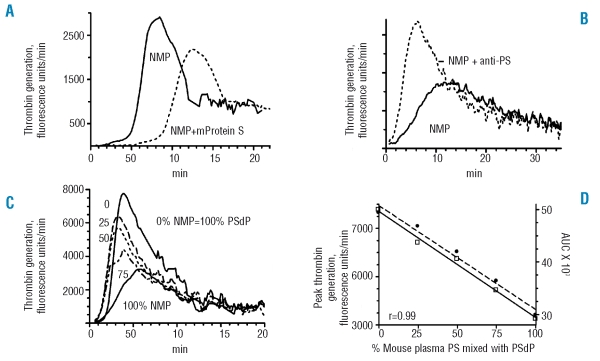
In a modified ETP assay, plasma samples were pretreated with neutralizing antibody against murine or rat protein C (TVM-1) to prevent any APC co-factor activity of protein S. As part of the validation of the assay, recalcified murine plasma with phospholipids was tested alone, or supplemented with partially purified recombinant mouse protein S (Figure 4A). Exogenous mouse protein S had an anticoagulant effect, causing a 2.3-fold prolongation of the lag time, a 47% increase in time to peak, and a 32% decrease in peak thrombin generation.
Figure 4.
Coagulation assays for the direct anticoagulant activity of murine plasma protein S. In all cases, plasma was treated with neutralizing antibody (TVM-1) against murine APC to exclude any contribution of protein S co-factor activity for APC. (A) Thrombin generation profile for mouse plasma. Following addition of calcium and phospholipids to mouse plasma, thrombin generation was monitored over time in a modified ETP assay as described in the Design and Methods section. Normal mouse plasma, solid line; mouse plasma with addition of partially purified recombinant murine protein S (0.1 μg), dashed line. (B) Thrombin generation in normal mouse plasma with and without addition of 2 mg/mL neutralizing rabbit anti-human protein S. (C) Thrombin generation in mixtures of normal mouse plasma and rat PSdP. See the legend to Figure 3 for mixtures. (D) Variation in ETP parameters with increasing dose of mouse normal plasma. In mixtures of pooled normal mouse plasma with rat PSdP, the area under the curve and peak thrombin generation in the ETP assay decreased in a linear manner with increasing doses of murine plasma containing protein S. The quantity of plasma used was 12 μL in the experiment represented in (B) and 15 μL in the experiments represented in the other panels.
Addition of Dako rabbit anti-protein S to murine plasma caused a decrease in the lag time and time to peak, and an increase in the area under the curve for thrombin generation (Figure 4B). When normal murine plasma was mixed in varying proportions with rat PSdP, the area under the curve for thrombin generation and peak thrombin generation decreased in a linear manner with increasing doses of murine plasma (Figure 4C,D). Other proteins in normal mouse plasma besides protein S could vary in the rat PSdP/mouse plasma mixtures and affect thrombin generation. However, freshly prepared mouse PSdP gave similar results as rat PSdP in the mixtures (data not shown), and the response to mouse protein S was anticoagulant (Figure 4A). These data are consistent with protein S in murine plasma having direct anticoagulant activity.
Physical characterization of mouse protein S and quantification of protein S in mouse plasma
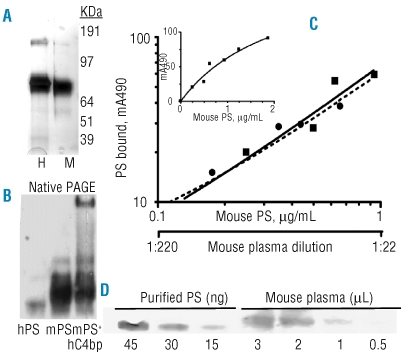
Murine and human protein S were subjected to 4–12% NuPAGE (Invitrogen) under reducing conditions and stained with Simple Blue (Invitrogen). The proteins appeared to be approximately 95% pure on SDS-PAGE (Figure 5A). Murine protein S migrated slightly fast than human protein S under both reducing and non-reducing conditions, consistent with a known difference in the number of glycosylation sites. Under native conditions, murine protein S migrated slightly slower than human protein S and a small proportion of multimers was observed (Figure 5B). When human C4bp was pre-incubated in a 1.4 molar excess with mouse protein S, protein S-C4bp complexes were formed, as shown near the top of the gel in Figure 5B.
Figure 5.
Protein and antigen analysis. (A) Denaturing SDS-PAGE electrophoresis of non-reduced (2 μg) purified protein S stained with Simple Blue. H= Human plasma-derived protein S. M= Murine recombinant protein S. The faster migration of mouse protein S is due to the lack of a carbohydrate side chain at residue 489. (B) Native PAGE immunoblot using 8% Tris-glycine gel of 0.5 ng human protein S (lane 1) and 30 ng mouse protein S in the absence and presence of 300 ng human C4bp (lanes 2, 3). (C) ELISA for murine protein S. Standard dilutions of purified murine protein S were used to calculate the concentration of murine protein S in dilutions of pooled murine plasma, using a log-log plot. The plots were superimposed at dilutions of mouse plasma that indicated an initial concentration of 22 μg/mL mouse protein S. The insert shows a linear plot of extended concentrations of purified mouse protein S. (D) Quantitative immunoblot for protein S in mouse plasma, using a 4–12% Bis-Tris gel. The first three lanes show purified murine protein S (45–15 ng), mixed with 10 μg BSA/lane; the next four lanes show pooled mouse plasma (3–0.5 μL). Blots in (B) and (D) were developed with 10 μg/mL goat anti-protein S, 1 μg/mL biotin-mouse anti-goat protein S (Pierce), 1 μg/mL streptavidin-horseradish peroxidase (Pierce) and chemiluminescent detection.
The ELISA for protein S was dose-responsive in the range of 0.25–2.0 μg/mL purified murine protein S (Figure 5C insert). Parallel slopes were obtained for purified murine protein S and mouse plasma when the data were plotted on a log-log scale in the range of 0.25–1.0 μg/mL protein S (Figure 5C). The curves were superimposable at dilutions of mouse plasma that indicated a plasma concentration of 22±1 μg/mL protein S. These ELISA assays will be useful for quantifying differences in protein S antigen among experimental mouse plasma samples in the dilution range of 0.25–1 μg/mL protein S. We used quantitative immunoblotting as another means to quantify protein S in mouse plasma (Figure 5D). Using this method, we found a value of 15±2 μg/mL protein S in pooled plasma of C57/BL6J mice, though other plasma components probably caused a slight shift in mobility and a decrease in sharpness of the protein S band. Thus, estimates for C57/BL6J mouse plasma range from 15–22 μg/mL protein S, with the ELISA calculation of 22 μg/mL probably being the more accurate value.
Protein S promotes proliferation of smooth muscle cells
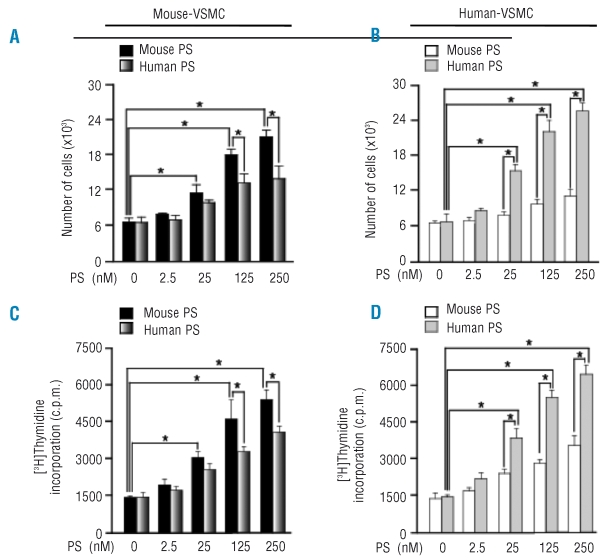
Protein S stimulates smooth muscle cell proliferation.36–38 Addition of exogenous murine or human protein S led to cell proliferation and [3H]-thymidine incorporation in mouse aortic smooth muscle cells and in human pial arterial smooth muscle cells (see Figure 6 for quantification and Online Supplementary Figure S1 for immunostaining). Murine protein S was more potent than human protein S on the mouse cells (Figure 6A,C). Conversely, when human VSMC were studied, human protein S was more potent than mouse protein S (Figure 6B,D). Thymidine incorporation showed the same pattern with protein S as cell proliferation.
Figure 6.
Protein S (PS) dose-dependent increase in the proliferation of VSMC. Mouse VSMC (left) and human VSMC (right) were treated with or without 25 nM of mouse protein S or human protein S for 72 h. The cells were then stained with Ki-67 antibodies to detect proliferation and TO-PRO-3 to visualize the nucleus. The number of cells at the end of 72 h of treatment with various concentrations of protein S was determined by counting the trypsinized mouse and human VSMC (A, B). Protein S-induced DNA synthesis in mouse and human VSMC was assessed by a [3H]thymidine incorporation assay (C, D). Data represent mean ± SD from a minimum of three independent cultures for each condition (*p<0.05).
Half-life of activated protein C in plasma
We previously characterized recombinant murine APC.9 In order to determine whether mouse plasma pro-tease inhibitors play important roles in the regulation of the protein C pathway, the in vitro half-lives of APC in pooled plasma samples from different species were measured (Online Supplementary Figure S1). APC in human plasma is inhibited by several members of the family of SERine Proteinase INhibitors (serpins) and, to a lesser extent, by α2-macroglobulin.6–8 We found that in human plasma, the rate of inactivation of human, bovine and murine APC was similar, with plasma half-lives of the proteins being approximately 20 min. In mouse plasma, the half-lives of mouse and human APC (~15 min) were significantly shorter than in human plasma.
The high concentration of α1PI in mouse plasma (2-fold higher than in human plasma) could contribute to inhibition of APC activity in mice.42 Indeed, we identified complexes of murine APC with α1PI in mouse plasma. However, bovine APC had a longer half-life, possibly because bovine APC has been shown to be resistant to inhibition by α1PI in vitro.43 It should be noted that in all cases, the half-life of APC in plasma is considerably longer than the half-lives of most coagulation proteases, such as thrombin and FXa.
Discussion
Murine models are ideal for in vivo studies of thrombosis because of the size of these animals, their breeding rate and the potential for generating genetically altered animals. However, the functional implications of differences between species have been an obstacle to both the design and confident interpretation of in vivo protein C pathway studies in mice. That differences between humans and mice constitute a particular problem was shown earlier by our group when murine APC was used in human plasma clotting assays.9 The impaired co-factor activity of human protein S for murine APC was proposed as the reason for this species incompatibility.
Studies using human APC in vivo in antithrombotic rat models are controversial, as Smirnov reported antithrombotic effects of low doses of human APC in the rat,39 whereas others failed to demonstrate antithrombotic effects of human APC in rat arterial thrombosis models.40,41 Human and rabbit APC can function with protein S from certain species and not others.15,18,19 Clearly there is a need to define the reactions of components of the murine protein C system better.
Since protein S modulates the anticoagulant activity of APC, we expressed and purified recombinant murine protein S for functional studies using in vitro clotting assays. The amino acid sequence of the mature form of murine protein S shows almost 80% identity with that of human protein S and differs by one residue in length. Despite the species specificity of the APC co-factor activity of murine protein S, murine protein S is structurally very similar to human protein S and is likely biologically equivalent with regard to its role in the protein C pathway. Recombinant murine protein S showed good anticoagulant co-factor activity for murine APC in APTT and FXa one-stage assays using mouse plasma. Residual murine protein S (1/5 of the total protein S) was present in the test plasma in these assays, so the effect of endogenous murine protein S cannot be completely overlooked. Murine protein S was not a co-factor for human APC in mouse plasma, but human protein S was partially active as a co-factor for murine APC. This suggests that murine protein S does not interact with human APC but that human protein S does interact with murine APC. In this regard it is puzzling that residues 36–39 of APC were found to be critical for interaction with protein S in mutagenesis studies,44 but these residues are identical in mouse and human APC. It is likely that other residues of APC are also critical for protein S interaction, and/or that complementary residues on mouse and human protein S differ.
Overall, our results suggest that the species specificity of APC is partially but not completely explained by the species specificity of protein S for APC. Explanations for differences between human and mouse plasma could involve C4bp, FV, or FVIII. Human plasma contains C4bp, which is capable of modulating the anticoagulant co-factor activity of protein S because about 40% of protein S in human plasma is bound to the β chain of this complement protein. However, mouse C4bp does not contain β chains,23 so one would expect all of murine protein S to be free in the plasma. The anticoagulant effect of APC and protein S is due to proteolytic inactivation of FVa and FVIIIa, with inactivation of FVa as the central anticoagulant function of the APC system. In one study, ten times more human APC was needed to inactivate purified rat FVa than that necessary to inactivate human FVa, indicating that specificity for the substrate, FVa, can also play a major role in the species specificity of APC.16 Moreover, FV itself may be an anticoagulant co-factor for APC with protein S,45 so some species specificity might be based on interactions of FV as a co-factor, not substrate, with APC or protein S. Protein S also binds to immobilized FVIII and inhibits the activation of factor X.46 FVIII activity is several fold higher in mice than in humans47 and it could be an important element for APC anticoagulant activity. Our studies suggest a species-specific effect of APC or protein S related to FV or FVIII, because murine protein S enhanced the activity of murine APC in mouse but not in human plasma.
The APC co-factor activity assay that we developed using murine APC and mouse plasma (containing murine protein S) was at least as sensitive as other published APC co-factor assays, in spite of using human FVa. In this case, human FVa may have simply been the initial stimulus, while subsequent clotting involved mouse FVa. Shorter baseline clotting times in PSdP in this assay in the absence of APC were suggestive of direct anticoagulant activity of protein S in mouse plasma. This concept was supported by our modified ETP assay, which included neutralizing antibody (TVM-1)48 against mouse APC to exclude any co-factor activity of protein S and was designed to reflect direct anticoagulant activity of endogenous and exogenous murine protein S. The effects of exogenous mouse protein S and Dako neutralizing antibodies against protein S in the ETP assay were analogous to the effects with human plasma, human protein S, and the Dako antibodies.49 It should be noted that only partially-purified mouse protein S, and not mouse protein S purified by the full method described here, had direct anticoagulant activity. This was most likely because of loss of a zinc ion from the MonoQ-purified mouse protein S, which affects direct anticoagulant activity but not APC co-factor activity of protein S.49 The assays for both APC co-factor activity and for direct anticoagulant activity of protein S are quantitative and will be useful when in vivo protein S activity levels might be affected by experimental conditions.
The concentration of 22 μg/mL protein S in mouse plasma, estimated by ELISA, compares to 10 μg/mL free protein S and 15 μg/mL of protein S in C4bp complexes in human plasma. As noted before, mouse plasma does not contain a form of C4bp capable of forming complexes with protein S, although we found that murine protein S is capable of forming complexes with human C4bp. Human protein S-C4bp complexes have only weak APC co-factor activity50 but do have direct anticoagulant activity.24
Free and C4bp-complexed forms of protein S bind to apoptotic cells, thus having the potential for local control of complement and coagulation systems.51 It has been suggested that protein S binding to apoptotic cells can stimulate phagocytosis by serving as a bridging molecule between the apoptotic cell and the phagocyte.52 Recently, it was shown that protein S binds through the Gla domain to apoptotic cells53 and forms dimers that preferentially bind to and activate the TAM receptor tyrosine kinase Mer on macrophages, promoting phagocytosis.54 Like Gas6,55 a protein S homolog, protein S emerges as a significant ligand for TAM receptors.56 It has been reported that protein S is a mitogen for rat VSMC36 and that there is a receptor for protein S on human VSMC.38 Here, we show that murine protein S has species-specific mitogenic properties on VSMC.
In summary, the murine recombinant protein S described in the present work is an important instrument for in vivo studies of the biology of the protein C pathway. The results show species incompatibility between murine protein S and human APC in mouse plasma, which highlights the requirement for murine APC in in vivo murine studies of the role of the protein C pathway in experimental pharmacology. Protein S is also a regulator of VSMC proliferation, which may have implications for the treatment of a variety of proliferative vascular diseases. Extrapolation of data from murine protein S to human protein S does, however, require careful evaluation of quantitative and qualitative interspecies differences.
Note added prior to publication
Two laboratories have now described protein S knockout mice (Burstyn-Cohen T, Heeb MJ, Lemke G. Lack of protein S in mice causes embryonic lethal coagulopathy and vascular dysgenesis. J Clin Invest 2009;119:2942-53, and Saller F, Brisset AC, Tchaikovski SN, Azevedo M, Chrast R, Fernández JA, et al. Generation and phenotypic analysis of protein S-deficient mice. Blood 2009;114:2307-14).
Acknowledgments
we thank Ms. Phoung M. Nguyen for excellent technical assistance, and Drs. Greg Lemke and Tal Burstyn-Cohen for pooled plasma from C57/BL6J mice.
Footnotes
Funding: this work was supported in part by National Institutes of Health grants HL31950 (to JHG), HL70002 and HL 088375 (to MJH) and HL081528 (to BVZ).
The online version of this article contains a supplementary appendix.
Authorship and Disclosures
JHG was the principal investigator and takes primary responsibility for the paper. JAF, MJH, and IS performed the laboratory work for this study. BVZ supervised the VSMC studies. JHG coordinated the research. JAF, MJH, and JHG wrote the paper.
The authors reported no potential conflicts of interest.
References
- 1.Traystman RJ. Animal models of focal and global cerebral ischemia. Ilar J. 2003;44:85–95. doi: 10.1093/ilar.44.2.85. [DOI] [PubMed] [Google Scholar]
- 2.Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A. Efficacy and safety of recombinant human APC for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 3.Cheng T, Petraglia AL, Li Z, Thiyagarajan M, Zhong A, Wu Z, et al. APC inhibits tissue plasminogen activator-induced brain hemorrhage. Nat Med. 2006;12:1278–85. doi: 10.1038/nm1498. [DOI] [PubMed] [Google Scholar]
- 4.Mosnier LO, Zlokovic BV, Griffin JH. The cytoprotective protein C pathway. Blood. 2007;109:3161–72. doi: 10.1182/blood-2006-09-003004. [DOI] [PubMed] [Google Scholar]
- 5.Jalbert LR, Rosen ED, Moons L, Chan JC, Carmeliet P, Collen D, et al. Inactivation of the gene for anticoagulant protein C causes lethal perinatal consumptive coagulopathy in mice. J Clin Invest. 1998;102:1481–8. doi: 10.1172/JCI3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heeb MJ, Gruber A, Griffin JH. Identification of divalent metal ion-dependent inhibition of APC by alpha 2-macroglobulin and alpha 2-antiplasmin in blood and comparisons to inhibition of FXa, thrombin, and plasmin. J Biol Chem. 1991;266:17606–12. [PubMed] [Google Scholar]
- 7.Heeb MJ, España F, Griffin JH. Inhibition and complexation of APC by two major inhibitors in plasma. Blood. 1989;73:446–54. [PubMed] [Google Scholar]
- 8.Heeb MJ, Schwarz HP, White T, Lammle B, Berrettini M, Griffin JH. Immunoblotting studies of the molecular forms of protein C in plasma. Thromb Res. 1988;52:33–43. doi: 10.1016/0049-3848(88)90038-2. [DOI] [PubMed] [Google Scholar]
- 9.Fernández JA, Xu X, Liu D, Zlokovic BV, Griffin JH. Recombinant murine APC is neuroprotective in a murine ischemic stroke model. Blood Cells Mol Dis. 2003;30:271–6. doi: 10.1016/s1079-9796(03)00034-2. [DOI] [PubMed] [Google Scholar]
- 10.Guo H, Liu D, Gelbard H, Cheng T, Insalaco R, Fern G, Griffin JH, et al. APC prevents neuronal apoptosis via protease activated receptors 1 and 3. Neuron. 2004;41:563–72. doi: 10.1016/s0896-6273(04)00019-4. [DOI] [PubMed] [Google Scholar]
- 11.Kerschen EJ, Fernández JA, Cooley BC, Yang XV, Sood R, Mosnier LO, et al. Endotoxemia and sepsis mortality reduction by non-anticoagulant APC. J Exp Med. 2007;204:2439–48. doi: 10.1084/jem.20070404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker FJ. Regulation of bovine APC by protein S: the role of the cofactor protein in species specificity. Thromb Res. 1981;22:321–7. doi: 10.1016/0049-3848(81)90125-0. [DOI] [PubMed] [Google Scholar]
- 13.Arnljots B, Dahlbäck B. Protein S as an in vivo cofactor to APC in prevention of microarterial thrombosis in rabbits. J Clin Invest. 1995;95:1987–93. doi: 10.1172/JCI117883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahlbäck B, Lundwall A, Stenflo J. Primary structure of bovine vitamin K-dependent protein S. Proc Natl Acad Sci USA. 1986;83:4199–203. doi: 10.1073/pnas.83.12.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greengard JS, Fernández JA, Radtke KP, Griffin JH. Identification of candidate residues for interaction of protein S with C4b binding protein and APC. Biochem J. 1995;305 (Pt 2):397–403. doi: 10.1042/bj3050397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katsuura Y, Mochizuki T, Tamura M, Hashide S, Kiyoki M, Nakagaki T, et al. Species specificity of anticoagulant activity of APC: involvement of FV as well as protein S. Thromb Res. 1996;82:147–57. doi: 10.1016/0049-3848(96)00061-8. [DOI] [PubMed] [Google Scholar]
- 17.Weinstein RE, Walker FJ. Enhancement of rabbit protein S anticoagulant cofactor activity in vivo by modulation of the protein S C4b binding protein interaction. J Clin Invest. 1990;86:1928–35. doi: 10.1172/JCI114926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yasuda F, Hayashi T, Tanitame K, Nishioka J, Suzuki K. Molecular cloning and functional characterization of rat plasma protein S. J Biochem (Tokyo) 1995;117:374–83. doi: 10.1093/jb/117.2.374. [DOI] [PubMed] [Google Scholar]
- 19.He X, Dahlbäck B. Molecular cloning, expression and functional characterization of rabbit anticoagulant vitamin-K-dependent protein S. Eur J Biochem. 1993;217:857–65. doi: 10.1111/j.1432-1033.1993.tb18314.x. [DOI] [PubMed] [Google Scholar]
- 20.Griffin JH, Gruber A, Fernández JA. Reevaluation of total, free, and bound protein S and C4b-binding protein levels in plasma anticoagulated with citrate or hirudin. Blood. 1992;79:3203–11. [PubMed] [Google Scholar]
- 21.Mosnier LO, Griffin JH. Protein C anticoagulant activity in relation to anti-inflammatory and anti-apoptotic activities. Front Biosci. 2006;11:2381–99. doi: 10.2741/1977. [DOI] [PubMed] [Google Scholar]
- 22.Yegneswaran S, Wood GM, Esmon CT, Johnson AE. Protein S alters the active site location of APC above the membrane surface. A fluorescence resonance energy transfer study of topography. J Biol Chem. 1997;272:25013–21. doi: 10.1074/jbc.272.40.25013. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez de Cordoba S, Perez-Blas M, Ramos-Ruiz R, Sanchez-Corral P, Pardo-Manuel de Villena F, Rey-Compos J. The gene coding for the beta-chain of C4b-binding protein (C4BPB) has become a pseudogene in the mouse. Genomics. 1994;21:501–9. doi: 10.1006/geno.1994.1308. [DOI] [PubMed] [Google Scholar]
- 24.Hackeng TM, van’t Veer C, Meijers JC, Bouma BN. Human protein S inhibits prothrombinase complex activity on endothelial cells and platelets via direct interactions with FVa and FXa. J Biol Chem. 1994;269:21051–8. [PubMed] [Google Scholar]
- 25.Heeb MJ, Mesters RM, Tans G, Rosing J, Griffin JH. Binding of protein S to FVa associated with inhibition of prothrombinase that is independent of APC. J Biol Chem. 1993;268:2872–7. [PubMed] [Google Scholar]
- 26.Heeb MJ, Rosing J, Bakker HM, Fernández JA, Tans G, Griffin JH. Protein S binds to and inhibits FXa. Proc Natl Acad Sci USA. 1994;91:2728–32. doi: 10.1073/pnas.91.7.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koppelman SJ, van’t Veer C, Sixma JJ, Bouma BN. Synergistic inhibition of the intrinsic FX activation by protein S and C4b-binding protein. Blood. 1995;86:2653–60. [PubMed] [Google Scholar]
- 28.Hackeng TM, Sere KM, Tans G, Rosing J. Protein S stimulates inhibition of the tissue factor pathway by tissue factor pathway inhibitor. Proc Natl Acad Sci USA. 2006;103:3106–11. doi: 10.1073/pnas.0504240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heeb MJ, Marzec UM, Gruber A, Hanson SR. Protein S is antithrombotic in vivo independent of APC. Blood. 2006;108:117A. [abstract] [Google Scholar]
- 30.Davis J, Wagner MR, Zhang W, Xu F, Van Nostrand WE. Amyloid beta-protein stimulates the expression of urokinase-type plasminogen activator (uPA) and its receptor (uPAR) in human cerebrovascular smooth muscle cells. J Biol Chem. 2003;278:19054–61. doi: 10.1074/jbc.M301398200. [DOI] [PubMed] [Google Scholar]
- 31.Chu MD, Sun J, Bird P. Cloning and sequencing of a cDNA encoding the murine vitamin K-dependent protein S. Biochim Biophys Acta. 1994;1217:325–8. doi: 10.1016/0167-4781(94)90294-1. [DOI] [PubMed] [Google Scholar]
- 32.Yan SC, Razzano P, Chao YB, Walls JD, Berg DT, McClure DB, et al. Characterization and novel purification of recombinant human protein C from three mammalian cell lines. Biotechnology (NY) 1990;8:655–61. doi: 10.1038/nbt0790-655. [DOI] [PubMed] [Google Scholar]
- 33.Lu D, Schmidel DK, Long GL. Structure of mouse protein S as determined by PCR amplification and DNA sequencing of cDNA. Thromb Res. 1994;74:135–42. doi: 10.1016/0049-3848(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 34.Fernández JA, Heeb MJ, Griffin JH. Identification of residues 413–433 of plasma protein S as essential for binding to C4b-binding protein. J Biol Chem. 1993;268:16788–94. [PubMed] [Google Scholar]
- 35.Fernández JA, Griffin JH, Chang GT, Stam J, Reitsma PH, Bertina RM, et al. Involvement of amino acid residues 423–429 of human protein S in binding to C4b-binding protein. Blood Cells Mol Dis. 1998;24:101–12. doi: 10.1006/bcmd.1998.0175. [DOI] [PubMed] [Google Scholar]
- 36.Gasic GP, Arenas CP, Gasic TB, Gasic GJ. Coagulation FX, FXa, and protein S as potent mitogens of cultured aortic smooth muscle cells. Proc Natl Acad Sci USA. 1992;89:2317–20. doi: 10.1073/pnas.89.6.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanthou C, Benzakour O. Cellular effects and signalling pathways activated by the anti-coagulant factor, protein S, in vascular cells protein S cellular effects. Adv Exp Med Biol. 2000;476:155–66. doi: 10.1007/978-1-4615-4221-6_13. [DOI] [PubMed] [Google Scholar]
- 38.Benzakour O, Formstone C, Rahman S, Kanthou C, Dennehy U, Scully MF, et al. Evidence for a protein S receptor(s) on human vascular smooth muscle cells. Analysis of the binding characteristics and mitogenic properties of protein S on human vascular smooth muscle cells. Biochem J. 1995;308:481–5. doi: 10.1042/bj3080481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smirnov MD, Pyzh MV, Borovikov DV, Atorozhilova AN, Dobrovolsky AB, Golubych VL, et al. Low doses of APC delay arterial thrombosis in rats. Thromb Res. 1990;57:645–50. doi: 10.1016/0049-3848(90)90082-n. [DOI] [PubMed] [Google Scholar]
- 40.Malm K, Dahlbäck B, Arnljots B. Prevention of thrombosis following deep arterial injury in rats by bovine APC requiring co-administration of bovine protein S. Thromb Haemost. 2003;90:227–34. doi: 10.1160/TH03-03-0174. [DOI] [PubMed] [Google Scholar]
- 41.Malm K, Arnljots B, Persson IM, Dahlbäck B. Antithrombotic and anticoagulant effects of wild type and Gla-domain mutated human APC in rats. Thromb Res. 2007;120:531–9. doi: 10.1016/j.thromres.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 42.Takahara H, Sinohara H. Inhibitory spectrum of mouse contrapsin and alpha-1-antitrypsin against mouse serine proteases. J Biochem (Tokyo) 1983;93:1411–9. doi: 10.1093/oxfordjournals.jbchem.a134276. [DOI] [PubMed] [Google Scholar]
- 43.Holly RD, Foster DC. Resistance to inhibition by alpha-1-anti-trypsin and species specificity of a chimeric human/bovine protein C. Biochemistry. 1994;33:1876–80. doi: 10.1021/bi00173a034. [DOI] [PubMed] [Google Scholar]
- 44.Preston RJ, Ajzner E, Razzari C, Karageorgi S, Dua S, Dahlbäck B, et al. Multifunctional specificity of the protein C/APC Gla domain. J Biol Chem. 2006;281:28850–7. doi: 10.1074/jbc.M604966200. [DOI] [PubMed] [Google Scholar]
- 45.Shen L, Dahlbäck B. FV and protein S as synergistic cofactors to APC in degradation of FVIIIa. J Biol Chem. 1994;269:18735–8. [PubMed] [Google Scholar]
- 46.Takeyama M, Nogami K, Saenko EL, Soeda T, Nishiya K, Ogiwara D, et al. Protein S down-regulates FXase activity independent of APC: specific binding of FVIII(a) to protein S inhibits interactions with FIXa. Br J Haematol. 2008;143:409–20. doi: 10.1111/j.1365-2141.2008.07366.x. [DOI] [PubMed] [Google Scholar]
- 47.Doering C, Parker ET, Healey JF, Craddock HN, Barrow RT, Lollar P. Expression and characterization of recombinant murine FVIII. Thromb Haemost. 2002;88:450–458. [PubMed] [Google Scholar]
- 48.Mosnier LO, Zampolli A, Kerschen EJ, Schuepbach RA, Banerjee Y, Fernández JA, et al. Hyper-anti-thrombotic, non-cytoprotective Glu149Ala-APC mutant. Blood. 2009;113:5970–8. doi: 10.1182/blood-2008-10-183327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heeb MJ, Prashun D, Griffin JH, Bouma BN. Plasma protein S contains zinc essential for efficient APC-independent anticoagulant activity and binding to FXa, but not for efficient binding to tissue factor pathway inhibitor. FASEB J. 2009 doi: 10.1096/fj.08-123174. prepublished online Feb. 24. PMID 19244162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maurissen LF, Thomassen MC, Nicolaes GA, Dahllback B, Tans G, Rosing J, et al. Re-evaluation of the role of the protein S-C4b binding protein complex in APC-catalyzed FVa-inactivation. Blood. 2008;111:3034–41. doi: 10.1182/blood-2007-06-089987. [DOI] [PubMed] [Google Scholar]
- 51.Webb JH, Blom AM, Dahlbäck B. Vitamin K-dependent protein S localizing complement regulator C4b-binding protein to the surface of apoptotic cells. J Immunol. 2002;169:2580–6. doi: 10.4049/jimmunol.169.5.2580. [DOI] [PubMed] [Google Scholar]
- 52.Anderson HA, Maylock CA, Williams JA, Paweletz CP, Shu H, Schacter E. Serum-derived protein S binds to phosphatidylserine and stimulates the phagocytosis of apoptotic cells. Nat Immunol. 2003;4:87–91. doi: 10.1038/ni871. [DOI] [PubMed] [Google Scholar]
- 53.Lemke G, Rothlin CV. Immunobiology of the TAM receptors. Nat Rev Immunol. 2008;8:327–36. doi: 10.1038/nri2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Uehara H, Shacter E. Auto-oxidation and oligomerization of protein S on the apoptotic cell surface is required for Mer tyrosine kinase-mediated phagocytosis of apoptotic cells. J Immunol. 2008;180:2522–30. doi: 10.4049/jimmunol.180.4.2522. [DOI] [PubMed] [Google Scholar]
- 55.Hafizi S, Dahlbäck B. Gas6 and protein S. Vitamin K-dependent ligands for the Axl receptor tyrosine kinase subfamily. FEBS J. 2006;273:5231–44. doi: 10.1111/j.1742-4658.2006.05529.x. [DOI] [PubMed] [Google Scholar]
- 56.Stitt TN, Conn G, Gore M, Lai C, Bruno J, Radziejewski C, et al. The anticoagulation factor protein S and its relative, Gas6, are ligands for the Tyro 3/Axl family of receptor tyro-sine kinases. Cell. 1995;80:661–70. doi: 10.1016/0092-8674(95)90520-0. [DOI] [PubMed] [Google Scholar]