There are few published data from real-world clinical experience with miglustat, an oral inhibitor of glucosylceramide synthase, in type 1 Gaucher disease. This study suggests that miglustat is an effective therapy for the long-term maintenance of patients with type 1 Gaucher disease previously stabilized with enzyme replacement therapy.
Keywords: type 1 Gaucher disease, substrate reduction therapy, efficacy and safety, maintenance, real clinical setting
Abstract
There are few published data from real-world clinical experience with miglustat (Zavesca®), an oral inhibitor of glucosylceramide synthase, in type 1 Gaucher disease. We report data from a prospective, open-label investigational study that evaluated substrate reduction therapy with miglustat 100 mg t.i.d. as a maintenance therapy in patients with Type 1 Gaucher disease who had been switched from previous enzyme replacement therapy. Long-term data on changes in organ size, blood counts, disease severity bio-markers, bone marrow infiltration, overall clinical status and safety/tolerability were analyzed from 28 patients with Type 1 Gaucher disease who were attending routine clinic visits. Assessments were performed at six, 12, 24, 36 and 48 months of therapy. Disease severity biomarkers improved up to 48 months after initiation of miglustat, while other disease parameters remained stable. Miglustat showed an acceptable profile of safety and tolerability throughout treatment. In conclusion, miglustat is an effective therapy for the long-term maintenance of patients with Type 1 Gaucher disease previously stabilized with enzyme replacement therapy.
Introduction
Type 1 Gaucher disease (GD1) is caused by deficient activity of the enzyme, glucocerebrosidase, and presents with a considerable degree of heterogeneity in terms of clinical manifestations, severity and clinical outcome.1–3 Enzyme replacement therapy (ERT; Cerezyme®) has demonstrated efficacy in treating the core symptoms of GD1, and is generally well tolerated. The achievement of therapeutic goals4 is considered a useful way of monitoring clinical efficacy of treatment in patients with GD1.5
Because GD1 is a chronic and non-curable disease, maintenance of therapeutic goals, quality of life and treatment cost-effectiveness in stabilized patients are key objectives during long-term treatment.6–8 Maintenance therapy using SRT with miglustat (Zavesca®), an oral inhibitor of the ceramide-specific enzyme, glucosyltransferase,9 may represent a valuable alternative treatment option in GD1 patients who have achieved therapeutic goals on ERT. There is limited published information on real-world clinical experience with miglustat in such patients. However, clinical trial findings10–13 and data from everyday clinical practice14,15 have indicated improvements across a range of efficacy endpoints in GD1, both in treatment-naïve patients and those previously or currently treated with ERT. Moreover, miglustat was well tolerated during trial extension studies.12,13
Following regulatory approval of miglustat in Spain, the prospective ZAGAL study was designed by the Spanish Gaucher Disease (GD) Foundation according to European Working Group on GD Advisory Council recommendations, and was subsequently approved by the Ethics Committee for Clinical Investigation of Aragon (CEICA). The aim of this study, which draws on data from the National Spanish GD Registry (SGDR), was to evaluate the efficacy, safety and tolerability of miglustat in patients with mild-to-moderate GD1 being treated in the real-world clinical practice setting. We have previously reported data on the short-term efficacy of miglustat in treatment-naïve patients and those switched from previous ERT.15 Here we present long-term efficacy and safety/tolerability findings recorded during up to 48 months of miglustat treatment in patients switched from previous ERT.
Design and Methods
The ZAGAL study protocol aim was designed to optimize and standardize miglustat use in GD patients in the real-world setting, and set out recommendations for gathering efficacy, safety and quality of life (QoL) data from miglustat-treated patients in a structured, longitudinal manner. Patients with mild-to-moderate GD1 who were previously stabilized on ERT were enrolled between May 2004 and April 2008. Patients received miglustat orally at a dose of 100 mg t.i.d. Recommendations were provided on the correct administration of miglustat and how to follow a low-carbohydrate diet during the first weeks of treatment.
The efficacy of miglustat as a maintenance therapy was evaluated based on organ size (liver size by abdominal clinical examination and spleen length by ultrasound), hemoglobin concentration, platelet count, and plasma biomarkers (chitotriosidase [CT] and CCL18/PARC). Changes in bone marrow infiltration were quantified using the Spanish MRI Scoring system.16 Assessments were performed at six, 12, 24, 36 and 48 months.
A neurological and neurophysiological study with superficial electroneurogram in sural and peroneal nerves, as well as a Memory Impairment Screen (MIS), was applied to all patients by the same specialist. Quality of life was evaluated using the SF-36 questionnaire, with assessments performed prior to commencement of miglustat therapy and two years after.
Statistical analyses were performed using an SPSS database; comparisons between means were conducted using the non-parametric Mann–Whitney U test.
Results and Discussion
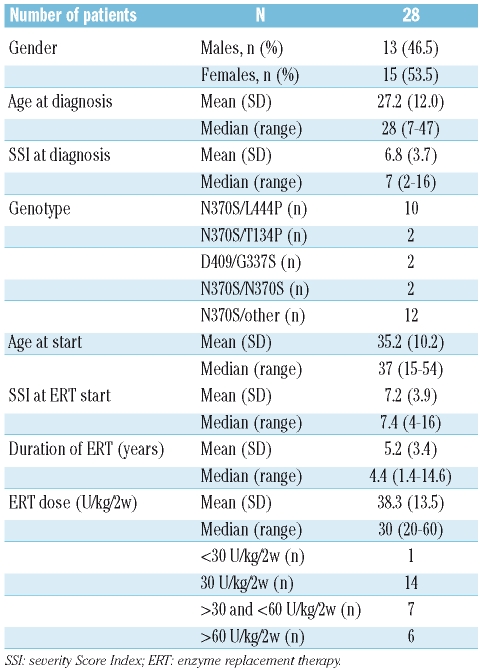
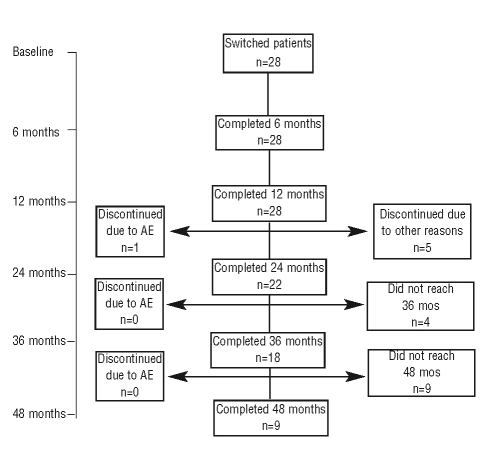
Twenty-eight patients were included in the study, with a mean time on ERT of 5.2 years (range: 1.4–14.6) (Table 1, Figure 1). Most patients had reached therapeutic goals during imiglucerase therapy4: hemoglobin >11 g/dL (27/28); platelet counts >120×109/L (17/28); liver volume decreased 1.5-fold (26/28); 8-fold reduction in spleen size (25/28). In December 2008, all patients had completed at least 12 months on miglustat therapy and were evaluated: 22 patients had completed 24 months, 18 had completed 36 months and 9 patients had completed 48 months on miglustat therapy and were continuing with treatment. Mean percentage changes from baseline in standard disease parameters at 12, 24, 36 and 48 months are detailed in the Online Supplementary Appendix.
Table 1.
General patients’ characteristics.
Figure 1.
Algorithm with patients’ distribution.
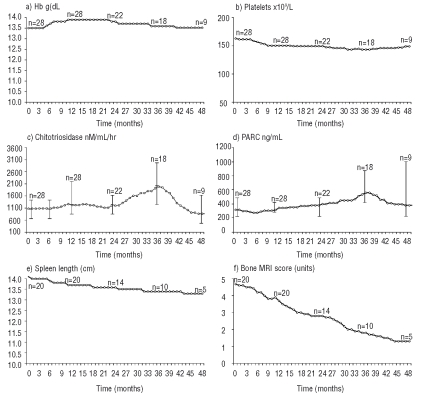
Most patients showed a satisfactory response to therapy (Figure 2); hemoglobin values and platelet counts were maintained throughout the study. Spleen and liver size did not increase over time. Mean plasma CT and CCL18/PARC values were increased at 36 months, but the differences versus baseline were not significant (p=0.333 and p=0.114, respectively). Plasma CT activity increased more than two-fold in 5 patients, decreased by over 20% in another 5, and was unchanged in the remaining patients. CCL18/PARC concentration showed a similar percentage increase at 36 months in 6 patients, a moderate decrease (approximately 20%) in 4 patients, and remained stable in 2 patients.
Figure 2.
Changes from baseline in standard disease parameters at 48 months.
The changes in the serum markers, CT and CCL18/PARC, did not correlate with changes in any of the therapeutic goal parameters (hemoglobin, platelet counts, liver and spleen volume, and bone infiltration) (Figure 2). The lack of reductions in plasma CT may be due to the fact that many patients in this cohort had previously undergone prolonged ERT and, as a result, miglustat did not reduce this marker any further. The fact that improvements were observed in other clinical parameters (platelets, spleen size, bone infiltration) indicates that plasma CT is not useful as a surrogate marker of long-term treatment response in patients who have undergone prolonged ERT, and/or that the mechanism of further improvement in these patients does not involve further reduction in the number of Gaucher cells. It is also possible that differences in patient compliance or inter-individual variations affected the plasma CT data. Overall, however, our data suggest that plasma CT and CCL18/PARC might not fully reflect systemic disease burden in GD1, and cast doubt on the strict validity of these biomarkers in patient monitoring.
Our data support findings from previous clinical trials with miglustat10–14 indicating that clinical and analytical responses obtained with ERT therapy are maintained in patients switched to miglustat. Our findings should also be considered alongside previously reported data comparing miglustat with ERT in the largest clinical trial in GD1 patients to date.14 Most patients were stabilized on miglustat therapy, given alone or in combination with ERT, on all key disease parameters assessed.14 In addition, data from post-marketing clinical experience with miglustat have so far confirmed that clinically relevant therapeutic benefits can be achieved with long-term miglustat therapy, with an acceptable safety/tolerability profile.17,18
Skeletal abnormalities are a serious manifestation of disease in GD1.19 Due to physico-chemical properties that enable a wide distribution throughout body tissues, miglustat has the potential to reach effector cells within bone. A pooled analysis of the effect of miglustat on bone manifestations and on bone mineral density (BMD), using data collected prospectively over two years from GD1 patients in three multinational, open-label clinical trials with miglustat, was published in 2007. Early and sustained increases in lumbar spine and femoral neck BMD were seen after starting miglustat monotherapy, with significant increases from baseline evident at six, 12, and 24 months. No bone crises, avascular necrosis or bone fractures were reported during two years of follow-up.20
No patients in our study reported bone pain or bone crisis during 48 months of therapy. Bone marrow infiltration in the spine was reduced by a mean of 2.9 points on the S-MRI scale (range, 1–4 points) during 36 months of miglustat therapy (Table 1). While the mechanism of action of miglustat on bone manifestations has not been fully clarified, it is known to affect osteoclastogenesis,21 and to penetrate bone sufficiently to reach deep-lying effector cells.22,23 For these reasons, miglustat might be a valuable treatment option for the improvement of bone disease in patients with GD1. However, further, dedicated studies may be required to fully evaluate this.
Analysis of data from the SF-36 assessment at 24 months revealed that improvements from baseline across all items were similar in miglustat-treated patients and those receiving ERT. The quality of life scores in this patient cohort were also similar to those recorded in the general Spanish population (data not shown).
With regards to safety and tolerability, 6 out of 28 patients (21%) complained of mild gastrointestinal disturbances which resolved after changing to a low-carbohydrate diet, in line with data from long-term extension trials.12,13 Patients receiving miglustat can be susceptible to gastrointestinal disturbances due to inhibitory effects on gut disaccharidases. In our cohort, low-dietary carbohydrate intake was recommended during the first weeks of miglustat therapy, with adjustments made thereafter according to individual tolerance. We consider that the occurrence of gastrointestinal adverse events in our patient cohort was lower than expected, likely due to the low-carbohydrate diet recommendation during the first weeks of therapy.
Five patients had moderate weight loss (mean [SD] 3.8 [2.38] kg; range 1–8 kg; representing a weight loss of 1.5 to 8.3% of total body weight). Conversely, 9 patients had moderate body weight gain (mean [SD] 1.3 [1.00] kg; range 1–4 kg). Body weight was stable in the remaining patients. Eight patients experienced a mild hand tremor during the first weeks after commencing treatment, but this had no effect on manual dexterity. No peripheral neuropathy or cognitive impairment was reported. The mean (SD) MIS score was 6.3 (1.66) points among the 22 patients evaluated at baseline, 6.4 (1.49) points in the 20 patients evaluated at 12 months, 6.2 (1.52) points in the 18 patients evaluated at 24 months, and 6.0 (1.50) in the 11 patients evaluated at 36 months. The range of MIS scores across all intervals was 4–8.
Of the patients who discontinued treatment, 3 patients discontinued due to poor compliance and one discontinued due to gastrointestinal discomfort related to the study drug (patient decided to stop therapy). Three patients died of unrelated diseases; one patient died after nine months on therapy because of melanoma complications, another died after seven months on therapy because of myocardial infarction, and one died after 18 months on therapy because of complications related to genital cancer. In summary, the results of this study confirm that miglustat is effective and well tolerated in patients with mild-to-moderate GD1 treated over the long term in everyday clinical practice. Miglustat is effective in the long-term maintenance of disease stability achieved on previous ERT, and has an acceptable safety and tolerability profile, which features preventable gastrointestinal effects.
Acknowledgments
the authors are also extremely grateful to the patients and their families whose participation made this work possible.
Footnotes
Funding: this study was partially supported by the following grants: FIS 04/2476, FIS 05/0695, FIS 06/1253, EC07/90737, FEETEG and CIBERER U-752.
All authors are members of the SGDG, which is supported by the Spanish Gaucher Disease Foundation (FEETEG). The corresponding author and some of the other authors are researchers at the Aragon Institute of Public Health (I+CS). This study was funded by FEETEG. PA, KA, MAFG, AB, RF, DA, AM, PL report no conflicts of interest. PG and MP have received consultancy fees from Actelion Pharmaceuticals Ltd. for participation in clinical trial programs and other projects, and speaker fees for participation in scientific congresses and sponsored events. PG and MP donate all fees to the National Gaucher Foundation, which supports research in the field of lysosomal storage disorders.
The authors would like to thank all the members of the Spanish Gaucher Disease Group (SGDG) who provided clinical data and samples. The complete list of physicians of the SGDG who have contributed is available at: www.feeteg.org.
The online version of this article contains a supplementary appendix.
Authorship and Disclosures
PG: responsible for the Spanish Registry of Gaucher Disease, conducted the study, analyzed and interpreted the data, and drafted the manuscript; PL: contributed to the statistical analysis and to the manuscript; PA: contributed to the enzymatic, genetic, serum biomarker, and statistical analyses; KA, AB, RF, DA, AM and MAF-G: collected clinical, analytical and follow-up data and contributed to analyses; MP: helped conduct the study, interpreted genetic and biochemical data, and edited the manuscript.
References
- 1.Giraldo P, Pocoví M, Pérez-Calvo J, Rubio-Félix D, Giralt M. Report of the Spanish Gaucher’s disease registry: clinical and genetic characteristics. Haematologica. 2000;85:792–9. [PubMed] [Google Scholar]
- 2.Sidransky E. Gaucher disease: complexity in a “simple” disorder. Mol Genet Metab. 2004;83:6–15. doi: 10.1016/j.ymgme.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Beutler E, Demina A, Laubscher K, Garver P, Gelbart T, Balicki D, et al. The clinical course of treated and untreated Gaucher disease. A study of 45 patients. Blood Cells Mol Dis. 1995;21:86–108. doi: 10.1006/bcmd.1995.0012. [DOI] [PubMed] [Google Scholar]
- 4.Pastores GM, Weinreb NJ, Aerts H, Andria G, Cox TM, Giralt M, et al. Therapeutic goals in the treatment of Gaucher disease. Semin Hematol. 2004;41:4–14. doi: 10.1053/j.seminhematol.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Weinreb N, Taylor J, Cox T, Yee J, vom Dahl S. A benchmark analysis of the achievement of therapeutic goals for type 1 Gaucher disease patients treated with imiglucerase. Am J Hematol. 2008;83:890–5. doi: 10.1002/ajh.21280. [DOI] [PubMed] [Google Scholar]
- 6.Pastores G. Management of patients with Gaucher’s disease: Clinical perspectives. Eur J Intern Med. 2006;17:S9–12. doi: 10.1016/j.ejim.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Weinreb NJ, Charrow J, Andersson HC, Kaplan P, Kolodny EH, Mistry P, et al. Effectiveness of enzyme replacement therapy in 1028 patients with type 1 Gaucher disease after 2 to 5 years of treatment: A report from the Gaucher Registry. Am J Med. 2002;113:112–9. doi: 10.1016/s0002-9343(02)01150-6. [DOI] [PubMed] [Google Scholar]
- 8.Connock M, Burls A, Frew E, Fry-Smith A, Juarez-Garcia A, McCabe C, et al. The clinical effectiveness and cost-effectiveness of enzyme replacement therapy for Gaucher’s disease: a systematic review. Health Technol Assess. 2006;10(iii–iv):ix-136. doi: 10.3310/hta10240. [DOI] [PubMed] [Google Scholar]
- 9.Platt FM, Jeyakumar M, Andersson U, Priestman DA, Dwek RA, Butters TD, et al. Inhibition of substrate synthesis as a strategy for glycolipid lysosomal storage disease therapy. J Inherit Metab Dis. 2001;24:275–90. doi: 10.1023/a:1010335505357. [DOI] [PubMed] [Google Scholar]
- 10.Cox T, Lachmann R, Hollak C, Aerts J, van Weely S, Hrebícek M, et al. Novel oral treatment of Gaucher disease with N-butyldeoxynojirimycin (OGT 918) to decrease substrate biosynthesis. Lancet. 2000;355:1481–5. doi: 10.1016/S0140-6736(00)02161-9. [DOI] [PubMed] [Google Scholar]
- 11.Heitner R, Elstein D, Aerts J, Weely S, Zimran A. Low-dose N-butyldeoxynojirimycin (OGT 918) for type I Gaucher disease. Blood Cells Mol Dis. 2002;28:127–33. doi: 10.1006/bcmd.2002.0497. [DOI] [PubMed] [Google Scholar]
- 12.Elstein D, Hollak C, Aerts JM, van Weely S, Maas M, Cox TM, et al. Sustained therapeutic effects of oral miglustat (Zavesca, N-butyldeoxynojirimycin, OGT 918) in type I Gaucher disease. J Inherit Metab Dis. 2004;27:757–66. doi: 10.1023/B:BOLI.0000045756.54006.17. [DOI] [PubMed] [Google Scholar]
- 13.Pastores GM, Barnett NL, Kolodny EH. An open-label, non comparative study of miglustat in type 1 Gaucher disease: efficacy and tolerability over 24 months of treatment. Clin Ther. 2005;27:1215–27. doi: 10.1016/j.clinthera.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Elstein D, Dweck A, Attias D, Hadas-Halpern I, Zevin S, Altarescu G, et al. Oral maintenance clinical trial with miglustat for type 1 Gaucher disease: switch from or combination with intravenous enzyme replacement. Blood. 2007;110:2296–301. doi: 10.1182/blood-2007-02-075960. [DOI] [PubMed] [Google Scholar]
- 15.Giraldo P, Pocovi M. Short-term effect of miglustat in every day clinical use in treatment-naïve or previously treated patients with type 1 Gaucher’s disease. Haematologica. 2006;91:703–6. [PubMed] [Google Scholar]
- 16.Roca M, Mota J, Alfonso P, Pocoví M, Giraldo P. S-MRI score: a simple method for assessing bone marrow involvement in Gaucher disease. Eur J Radiol. 2007;62:132–7. doi: 10.1016/j.ejrad.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 17.Mehta A. Clinical experience with substrate reduction therapy. Eur J Intern Med. 2006;17:S13–5. doi: 10.1016/j.ejim.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Hughes DA, et al. Post-marketing surveillance of miglustat in type 1 Gaucher disease. J Inherit Metab Dis. 2007;30(Suppl 1) [abstract 477-P] [Google Scholar]
- 19.Wenstrup RJ, Roca-Espiau M, Weinreb NJ, Bembi B. Skeletal aspects of Gaucher disease: a review. Br J Radiol. 2002;75 (Suppl 1):A2–12. doi: 10.1259/bjr.75.suppl_1.750002. [DOI] [PubMed] [Google Scholar]
- 20.Pastores GM, Elstein D, Hrebicek M, Zimran A. Effect of miglustat on bone disease in adults with type 1 Gaucher disease: a pooled analysis of three multinational, open-label studies. Clin Ther. 2007;29:1645–54. doi: 10.1016/j.clinthera.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Fukumoto S, Iwamoto T, Sakai E, Fukumoto S, Iwamoto T, Sakai E, et al. Current topics in pharmacological research on bone metabolism: osteoclast differentiation regulated by glycosphingolipids. J Pharmacol Sci. 2006;100:195–200. doi: 10.1254/jphs.fmj05004x3. [DOI] [PubMed] [Google Scholar]
- 22.Treiber A, Morand O, Clozel M. The pharmacokinetics and tissue distribution of the glucosylceramide synthase inhibitor miglustat in the rat. Xenobiotica. 2007;37:298–314. doi: 10.1080/00498250601094543. [DOI] [PubMed] [Google Scholar]
- 23.Aerts JM, Hollak CE, Boot RG, Groener JE, Maas M. Substrate reduction therapy of glycosphingolipid storage disorders. J Inherit Metab Dis. 2006;29:449–56. doi: 10.1007/s10545-006-0272-5. [DOI] [PubMed] [Google Scholar]