Acquired severe aplastic anemia is a rare disease characterized by an immune-mediated functional impairment of hematopoietic stem cells. Transplantation of these cells from unrelated donors is a treatment option frequently offered to patients after failed immunosuppressive therapy. This systematic review indicates that unrelated donor hematopoietic stem cell transplantation in patients with acquired severe aplastic anemia after failure of immunosuppressive therapy is a valid treatment option.
Keywords: donor stem cell transplantation, aplastic anemia, systematic review
Abstract
Acquired severe aplastic anemia is a rare disease characterized by an immune-mediated functional impairment of hematopoietic stem cells. Transplantation of these cells from unrelated donors is a treatment option frequently offered to patients after failed immunosuppressive therapy. The aim was to investigate the outcome of these patients treated with unrelated donor transplants. Systematic literature searches were performed in MEDLINE, EMBASE, and The Cochrane Library. All databases were searched from inception to June 2009. Only full-text publications and studies including at least 10 patients were considered. The primary outcome was 5-year overall survival from the day of transplantation and the secondary outcomes were graft failure and graft-versus-host disease. A meta-analysis of survival estimates was conducted and heterogeneity was investigated. A total of 18 studies, one controlled trial and 17 case series were identified. The overall survival at five years and the corresponding confidence interval was stated in 8 studies and ranged from 28% to 94%. A meta-analysis revealed considerable heterogeneity between the studies that could not be explained and was also present in subgroups of the studies. The proportion of acute graft failure was 45% in one study using only umbilical cord blood, and it was reported to be 0–26% in 15 studies using mainly bone marrow as stem cell source after different follow-up periods. Acute GVHD grade II–IV was reported for 8–86% and extensive chronic GVHD for 0–38% of the evaluated patients in 16 studies. Recipient age, human leukocyte antigen match, performance status, year of transplantation, and conditioning with serotherapy were identified as significant factors for improved survival. Unrelated donor hematopoietic stem cell transplantation in patients with acquired severe aplastic anemia after failure to immunosuppressive therapy is a treatment option. A stable physical condition of the patients before receiving the transplant (for example, performance and age) may be associated with a better survival. Detailed HLA-matching facilitated by DNA-based typing, among other factors, may have contributed to recent improvements on survival after unrelated donor HSCT as a second-line treatment.
Introduction
Acquired severe aplastic anemia (SAA) is a rare1 and potentially fatal disease which is characterized by hypocellular bone marrow and pancytopenia, and mainly affects young adults. The incidence rate was estimated at less than 4 per million per year in the general population.2 The major signs and symptoms are severe infections, bleeding and exhaustion. The underlying pathophysiology is thought to be an aberrant immune response involving the T-cell mediated destruction of hematopoietic stem cells. In most cases, the cause is unknown, although various triggers such as drugs, toxins, and viruses have been reported.3,4
The treatment of SAA mainly includes immunosuppressive therapy (IST) with antithymocyte globulin and cyclosporine A, and allogeneic hematopoietic stem cell transplantation (HSCT).3–5 Allogeneic HSCT is seen as the treatment of choice for selected patients with an HLA-matched related donor.6,7 This donor type was documented for 66% (247 of 373) of the patients with an allogeneic HSCT who were registered in 2005 by the European Group for Blood and Marrow Transplantation (EBMT).8 More than 70% of patients with SAA are not expected to have a matched related donor3,9 and the question is whether or when to recommend allogeneic HSCT from an unrelated donor.
Clinical treatment algorithms have been suggested to find a decision that meets individual conditions, personal preferences and prognostic risk factors.7 Allogeneic HSCT is associated with a high treatment-related morbidity and mortality. Potentially life-threatening adverse events are sepsis, acute and chronic graft-versus-host disease, bleeding and organ toxicities. During the 1980s and 1990s, survival rates of patients with unrelated donor HSCT were about half of those seen in related donor HSCT, with age, time from diagnosis and HLA-matching as strong predictors of survival.3,4,10 Present data show that high-resolution molecular genetic HLA-matching may be an important factor for better survival in these patients.11 Unrelated donor HSCT has been applied conservatively and usually offered as a second-line treatment after one or more failed IST. The search for an unrelated donor seems advisable if patients with SAA have failed one course of IST.12,13
This review summarizes the evidence available on unrelated donor HSCT after failed IST in SAA patients to answer the clinical question of whether the outcome has improved sufficiently for it to be acknowledged as an alternative for IST and used in future randomized trials.
The primary objective was to investigate the overall survival of patients treated with unrelated donor HSCT in SAA patients. The secondary objective was to consider adverse events.
Design and Methods
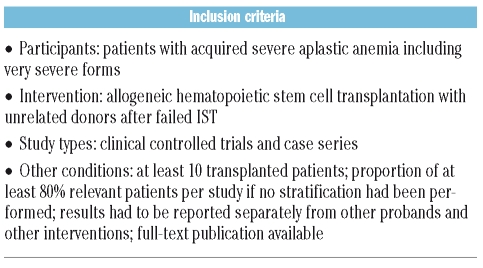
Study inclusion criteria
The study inclusion criteria are listed in Table 1. We included clinical controlled trials and case series that investigated patients with acquired severe aplastic anemia, including very severe forms, who received allogeneic HSCT from unrelated donors after failed IST. We arbitrarily set a minimum number of 10 transplanted patients per study to be considered, and required the proportion of relevant patients to be at least 80% per study if no stratification had been performed. Studies which did not present results separately from probands with other diseases and interventions were excluded from the studies. Only full-text publications were considered. All languages were included, as long as a title was available in English.
Table 1.
Inclusion criteria.
Search strategy
The sources used to search the studies are listed in Online Supplementary Table S1. MEDLINE (1950–2009), EMBASE (1980–2009) and The Cochrane Library (up to 2009) were searched without restrictions on study design, publication year, and language (final search 2nd June 2009). The terms and the syntax used for the search in MEDLINE/Ovid as shown in Online Supplementary Table S2 were tailored to the requirements of the other 2 databases. Additional steps were taken to complement electronic database searches: online trial registers and citations from experts submitted to the German Federal Joint Committee and to the IQWiG were screened. Reference lists of retrieved original articles and systematic reviews, as well as conference proceedings were handsearched. Moreover, in cases of assumed unpublished trials, we contacted the authors by e-mail and by post using the published contact details of the relevant institutions and asked for further information.
Study quality
Study quality was evaluated by description of the study characteristics (design, inclusion criteria, location, observation period), the patients’ characteristics (age, gender, number of failed IST, number of pre-transplantation IST courses, interval from diagnosis to transplant, cytomegalovirus and Epstein-Barr virus disease reactivation), the intervention (stem cell source, total body irradiation), the donor-recipient interaction (donor type, HLA-matching, HLA-typing, donor gender), and the outcome (method of survival analysis, follow-up, subgroup analysis, graft failure, acute and chronic GVHD, methods to investigate factors for improved survival).
Data extraction
Potentially relevant publications not in English were translated by medically trained native speakers. All steps of the literature screening and data extraction process were performed by two independent reviewers (FP, SL). Any disagreements were resolved by discussion.
Failed IST is a term used in this paper to describe patients who had failed pre-transplantation IST either as no response to IST (refractory patients), or as relapse after initial response
Data analysis
The primary outcome was survival from the day of transplantation based on Kaplan-Meier estimates as extracted directly from the text or deduced from the survival curve of the publication. The proportion of the number of survived patients at the end of observation was used to describe the outcome if Kaplan-Meier analyses were not reported.
The secondary outcomes were graft failure and graft-versus-host disease (GVHD), and they were documented according to the definition used in the individual publications. Graft failure included both primary and secondary types. Acute GVHD was considered if grade II–IV was stated and chronic GVHD was described in the present review if an extensive course was stated. Significant as well as non-significant factors for improved survival were searched in the identified studies provided that the statistical analysis method and the results of significance testing were reported.
Meta analysis
Survival estimates at five years and the corresponding 95% confidence intervals were extracted from the papers if present. If not explicitly stated, the survival estimates at five years were deduced from the survival curves if possible and the corresponding standard errors were estimated by using an approximate formula based on the survival probability and the number of patients at risk.14 In case of insufficient information about the number of patients at risk, this was estimated assuming uniformly distributed censoring times in the time period from beginning to the end of follow-up. Meta analyses based on the 5-year survival estimates and the corresponding standard errors were conducted using the generic variance approach15,16 and the random effects model.17 Calculations were conducted using SAS version 9.1.3 (SAS Institute Inc., Cary, North Carolina, United States).
The results of the meta-analyses were graphically displayed by means of a forest plot. Heterogeneity of the results was visually assessed and quantified using the I2 value.18 In case of very large heterogeneity a pooled estimate is not sensible and was not calculated.19 Heterogeneity was further explored according to the Cochrane Handbook guidelines by conducting several subgroup analyses.18
Results
Search results
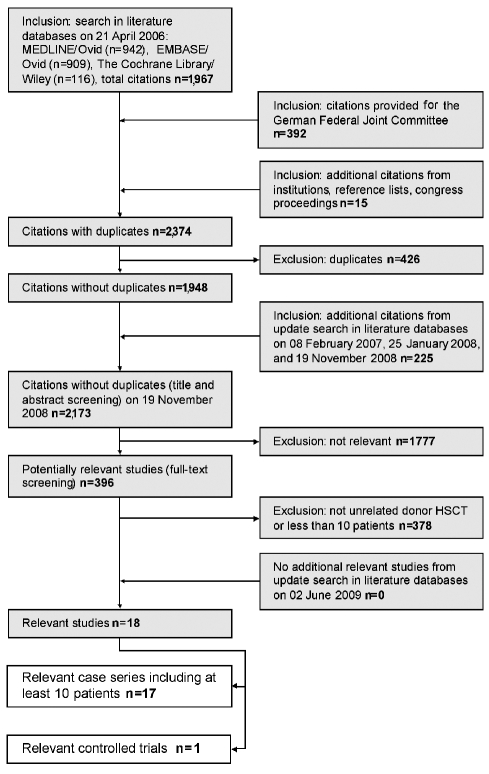
Of 2,173 retrieved publications, 396 full-text papers were obtained for further assessment (Figure 1). Eighteen studies were identified that met the inclusion criteria.10,11,15–17, 20–32 Seventeen case series which investigated at least 10 patients treated with unrelated donor HSCT after failed IS could be included.10,11,15–17,20–25,27–32 The transplantation arm of one controlled trial of unrelated donor HSCT versus IST in patients who had failed IST could also be included.26
Figure 1.
Results of the literature search.
Patients’ and design characteristics
An overview of the study characteristics is presented in Online Supplementary Table S3. The participating centers are located in the United States, Western Europe, Japan, and Korea. Five to 142 centers collaborated in 9 multicenter studies, the number of centers was not stated in 2 other multicenter studies, and 7 studies were conducted in single centers. The study design was reported as prospective in one controlled trial26 and in 4 case series.15,16,20,24 A consecutive enrolment was stated in 323,25,27 of 13 retrospective case series.10,11,17,21–23,25,27–32 Transplantations were performed between 1981 and 2006.
In 4 studies10,20,24,30 survival estimates were stated for subgroups only. In one study,20 patients younger than versus older than or equal to 20 years of age were investigated separately. Kim 200724 analyzed data in 2 groups with conditioning of 800 cGy versus 1000–1200 cGy total body irradiation. In one study,10 4 different donor types were investigated in subgroups according to the HLA status, although, only the 2 subgroups with HLA-matched unrelated donors versus HLA-mismatched unrelated donors were included in the present report. The year of transplant was investigated in subgroups in another study:30 before versus after and in 1998.
An overview of the patients’ characteristics is presented in Online Supplementary Table S4. A median of 32 patients (range 11–349, total 1,645) were investigated in the total study population of 14 studies11,15–17,21–23,25–29,31,32 and in 8 relevant subgroups of 4 other studies.10,20,24,30 The patients’ median age in the individual studies was between eight and 27 years and the ratio of males : females ranged from a male (30 : 10) to a female preponderance (11 : 20). Most of the 1,645 patients whose data were used for survival estimates had received a pre-transplantation IST to which they had not responded or after which they had relapsed. In 7 studies, a failed IST was not clearly stated.10,21,22,25,28,30,32 A few cases without SAA11,16,27,29 or without previous IST were included in some studies. The number of IST courses before HSCT was specified in 3 studies.20,24,26 The median interval from diagnosis to transplant was stated in 16 subgroups or studies with values per study spanning from six to 168 months.
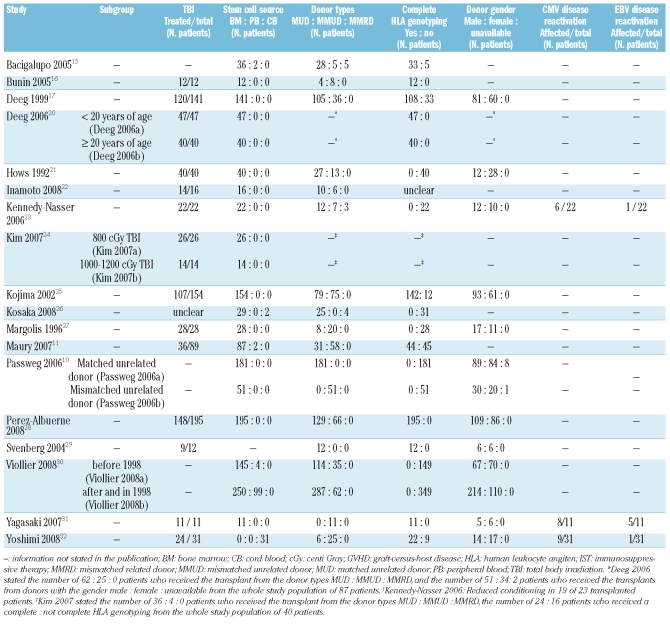
An overview of the treatment characteristics is presented in Table 2. In 12 studies,10,16,17,20–25,27,28,31 bone marrow was the exclusive stem cell source. In 2 studies,11,15 2 patients received peripheral blood stem cells, in one study,26 2 patients received umbilical cord blood stem cells, and the stem cell source was not stated in another study.29 Viollier et al. reported that, for the subgroup of patients treated after and in 1998, 99 of 349 patients received peripheral blood stem cells and 250 of 349 patients received bone marrow stem cells.30 Umbilical cord blood stem cells were the only stem cell source for all 31 patients in one study.32 In 14 studies,11,16,17,20–22,24,25,27–32 all patients received stem cell transplants from either HLA-matched or mismatched unrelated donors. Stem cell transplants from an HLA-mismatched related donor were included in 4 studies.10,15,23,26 DNA-based HLA-typing was performed for all participants in 5 published studies,16,20,28,29,31 and for the majority of participants in 5 other studies.15,17,24,25,32 In 8 studies,10,11,21–23,26,27,30 the HLA-typing was serology-based or unclear. Donor gender was reported in 12 studies.10,17,20,21,23,25,27–32 Reactivation of disease caused by cytomegalovirus and Epstein-Barr virus was observed in 3 studies.23,31,32
Table 2.
Treatment characteristics.
In the controlled trial, 205 children and young people under 18 years of age with SAA were enrolled,26 of whom 198 were treated with IST. Response rate was checked at least six months after the treatments were initiated. The 52 children who failed the first-line IST were assigned to 2 treatment groups: 31 were transplanted from alternative donors and 21 received a second-line IST. Of the 31 transplanted patients, 25 (81%) received a transplant from an HLA-matched unrelated donor, 2 (6%) received cord blood transplants from an unrelated donor, and 4 (13%) received a transplant from an HLA-mismatched related donor. This means a small fraction of the investigated transplanted patients did not have an unrelated transplantation.
Outcome
Primary outcome
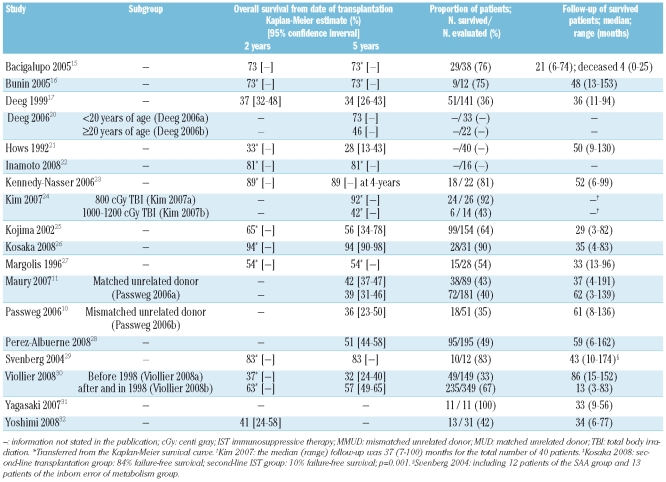
An overview of the survival is presented in Table 3. The median follow-up of surviving patients in 15 studies 10,11,15–17,21,23,25–32 ranged from 13 to 86 months. The 5-year overall survival and the 95% corresponding confidence intervals for 8 studies10,11,17,21,25,26,28,30 have been reported to be from 28% to 94% and could be estimated from the survival curve and follow-up data in 8 other studies15,16,20,22–24,27,29 from 42% to 92% (Table 3).
Table 3.
Survival.
Secondary outcomes
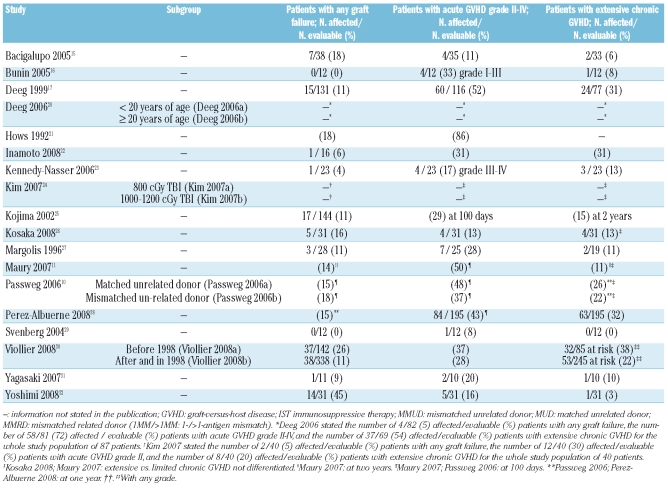
An overview of the adverse events is presented in Table 4. The proportion of graft failure was 45% in one study with all participants receiving stem cell transplants solely from umbilical cord blood32 (Table 4). This proportion was reported to be 0–26% in 15 of the other studies.10,11,15–17,21–23,25–31 The incidence of acute GVHD grade II–IV per study was 8–86% and the incidence of extensive chronic GVHD was 0–38% of the evaluated patients in 16 studies10,11,15–17,21–23,25–32 (Table 4).
Table 4.
Adverse events.
Factors for improved survival
One study reported the outcomes separately according to the different HLA donor status and the overall survival did not differ significantly.10 In another study, the data were presented separately for two different time periods and the overall survival was statistically significantly higher in the late cohort (from 1998) compared to the early cohort (before 1998).30
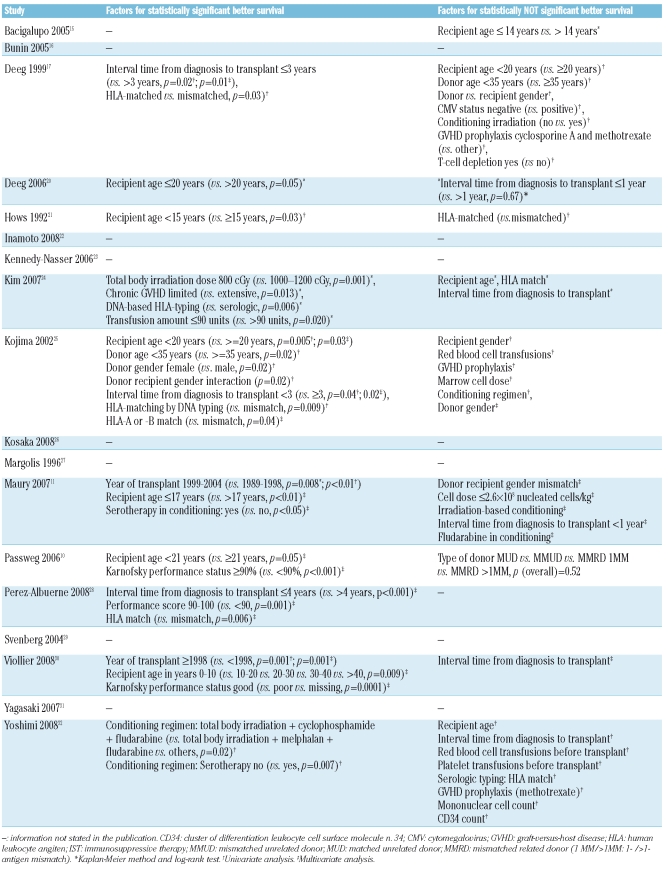
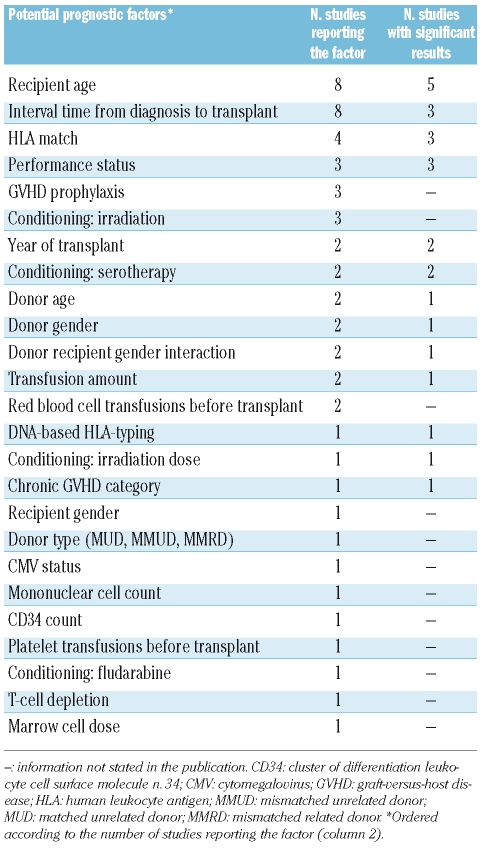
In 11 studies,10,11,15,17,20,21,24,25,28,30,32 factors for improved survival were stated (Table 5). Some factors, like irradiation dose, were reported in one study24 and other factors, like recipient age, were reported in up to 8 studies (Table 6). The following 5 factors were reported frequently (at least 2 times) and had more significant than non-significant results: recipient age, HLA match, performance status, year of transplant, and conditioning with serotherapy (Table 6). The factor irradiation was analyzed in 4 studies.11,17,24,32 Non-significant results were reported in 3 studies11,17,32 which analyzed the inclusion versus non-inclusion of irradiation in the conditioning regimen. Another study24 analyzed different doses and found that a lower dose (800 cGy) is a significant factor for improved survival when compared to a higher dose (1000–1200 cGy). DNA-based HLA-typing and chronic GVHD category (limited versus extensive) were also significant factors for improved survival; both factors were reported once.
Table 5.
Factors for improved survival
Table 6.
Comparison of potential prognostic factors for improved survival.
Comparative study
The estimated failure-free survival in the controlled trial26 at five years from the beginning of second-line therapy was about 85% in the allogeneic HSCT group compared to about 10% in the IST group (data not shown). There was no difference in the overall survival rate between the allogeneic HSCT and the IST group and was about 95% (data not shown).
Meta analysis
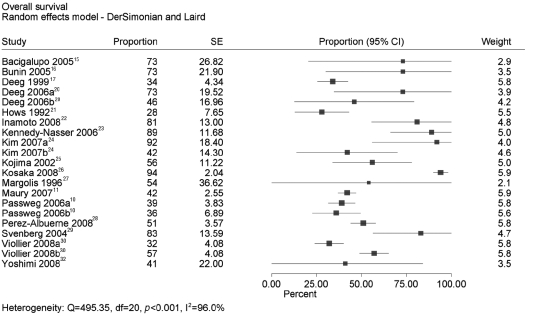
The 5-year survival probabilities and 95% confidence intervals have been stated in 8 studies.10,11,17,21,25,26,28,30 In 2 of these studies, estimates were reported only for the subgroups HLA matched (Passweg 2006a) versus mismatched (Passweg 2006b) unrelated donors in one study10 and for the subgroups date of transplantation before (Viollier 2008a) versus after or in (Viollier 2008b) the year 1998 in another study.30 Five-year survival probabilities and the corresponding standard errors were deduced for another 9 studies.15,16,20,22–24,27,29,32 In 2 of these studies, again estimates were reported only for the subgroups younger (Deeg 2006a) versus older or equal to (Deeg 2006b) 20 years of age in one study20 and for the subgroups total body irradiation dose 800 cGy (Kim 2007a) versus 1000–1200 cGy (Kim 2007b) in another study.24 Survival probabilities were not stated in one study.31
The meta analysis of the 5-year survival probabilities in 17 studies revealed a very high heterogeneity of I2=96% (Figure 2) and calculation of a pooled estimate was not justified. Weights were based on the random effects model. This level of heterogeneity did not change after removal of the 4 studies from the analysis which showed only subgroup results (13 studies analyzed; I2=96%; data not shown). Further, heterogeneity did not change after additional removal of the deduced estimates from 7 studies (6 studies analyzed; I2=98%; data not shown). The results of the following subgroup analyses did not explain the heterogeneity either (data not shown):
Figure 2.
Meta analysis of 5-year survival estimates.
Median age younger vs. older than or equal to 16;
Unicenter vs. multicenter;
Prospective vs. retrospective design;
HLA genotyping complete vs. partial or none;
Median interval from diagnosis to transplant shorter vs. longer than or equal to 14 months;
Study population less than vs. greater than or equal to 50 participants;
Transplantation was performed before vs. after or in 1998.
Data quality
Characteristics
The patients’ characteristics were described with varying details in the available publications. A clear and consistent definition of response criteria was lacking. In many studies, mixed populations of patients with a lack of response to IST (refractory SAA) and those with a recurrence of disease after initial treatment success (relapsed SAA) were investigated. Response was determined at various time points from three to six months after the beginning of the treatment where stated. The number of repeats of pre-transplantation IST varied considerably in one study and 87 patients received 1–11 IST repeats (median 3).20 This detailed information was not available for the other 17 studies, although it might be a considerable risk factor for long-term morbidity and mortality.
Comparative study
In the study by Kosaka et al.,26 the allocation of the patients to the transplantation group and the IST group was probably dependent on donor availability. This might be accepted as a qualified allocation, so called genetic randomization. However, the allocation was not described adequately in the paper. The patients’ characteristics of the transplantation group versus the IST group were different regarding the proportion of very severe aplastic anemia (32% versus 67%, p=0.03, statistically significant according to our calculation), and male gender (45% versus 67%, p=0.21, not statistically significant according to our calculation). Furthermore, the median follow-up was 35 (range 4–83) months versus 66 (9–80) months. These differences between the groups were not considered in the analysis and not discussed in the publication. In addition, the source of anti-thymocyte globulin was rabbit in the transplantation group and horse in the IST group. The median age (eight versus nine years) and the time from diagnosis to second-line therapy (eight versus seven months) were comparable. The response was evaluated at three and six months after the treatment. In the IST group, 6 patients had a response within 6–12 months and were not considered in the analysis.
Discussion
Outcomes
The outcome data varied considerably between the studies and the 5-year overall survival estimates ranged from 28% to 94%. This range is similar to data published in recent reviews, such as Young et al.4 (4-year or 5-year overall survival of 58–100% for alternative donor HSCT) and Marsh et al.7 (5-year overall survival of 36–73% for unrelated donor HSCT). The values in the present analysis of the cumulative incidence for treatment-associated complications, such as graft failure, acute and chronic GVHD, are also similar to the ranges stated in these articles provided that bone marrow was used as a stem cell source. Only one study relied solely on umbilical cord blood as the stem cell source.32 Merely 55% of 31 recipients achieved a sustained engraftment and 23% died because of graft failure. Chan et al.33 reported unrelated cord blood transplantation in 9 children of whom 89% (8 of 9 patients) engrafted, 2 after the second transplantation, and 78% were alive at a median follow-up of 34 months.
Factors for improved survival
Factors for improved survival were addressed in 11 studies and the results were inconsistent. Lower irradiation dose, DNA-based HLA-typing, and limited chronic GVHD were significant factors, but each factor was analyzed in merely one study. The following factors were reported to be significant in at least 2 studies and were significant in the majority of the studies reporting the respective factor: recipient age, HLA match, performance status, year of transplant, and conditioning with serotherapy. We gave the priority to the latter factors because of frequent reporting. However, this was not intended to detract from the importance of the previous factors reported.
Passweg et al.10 studied patients transplanted up to 1998 and did not find a significant effect of year of transplantation. Viollier et al.30 studied patients transplanted from 1990 to 1998 and from 1998 to 2005 and found significantly improved survival for patients transplanted after 1998, speculating that this result may be due to better donor matching.
Outcome assessment criteria
The considerable shortcomings of the available data mean that interpretation is limited. Although criteria for the quality of response after immunosuppressive treatment have been defined by the European Group for Blood and Marrow Transplantation (EBMT),34 it should be emphasized that authors use different definitions, not only in terms of response but also in terms of relapsed patients, refractory patients, and of the time interval from the beginning of treatment to ascertaining the response.35–37 For example, the more the IST is repeated in relapsed patients, the more the patient will be exposed to blood products. An association of the number of applied blood products with reduced survival estimates has been shown, and it was concluded that the number of IST repeats in turn can be a prognostic factor for survival.38 In addition, it was concluded that patients who fail 2 courses of treatment have almost always been heavily transfused and frequently have significant infections that make transplantation less likely to be successful.9 The number of IST courses was stated in merely 3 of 18 studies and the possible impact of this factor was not investigated.
Lack of controlled trials
According to recent recommendations,6 HLA-matched and HLA-mismatched unrelated donor HSCT, as well as HLA-mismatched related donor HSCT, are associated with significant morbidity but can be a clinical option when other therapies have failed. Unrelated donor HSCT is regarded by most clinicians as a sequential option which, by definition, could not be compared with a preceding treatment. On the other hand, it was stated that “a prospective comparison of unrelated donor transplantation versus a second trial of immunosuppression is needed to address this issue”,9 and it was also stated that “an ongoing IBMTR/EBMT study will compare the outcome after MUD BMT versus second course of IST in patients failing the first course of IST”.34
Evidence base for second-line unrelated donor HSCT after failed IST
In 2008, the results were published26 of the first prospective multicenter study to compare the efficacy of repeated IST with HSCT from an alternative donor in children with acquired SAA who failed to respond to an initial course of IST. There was no difference in the estimated overall survival after five years between the two treatment groups. The estimated failure-free survival after five years was significantly increased in the allogeneic HSCT group and may indicate that the transplanted patients may have an additional benefit.
The evidence for second-line unrelated donor HSCT after failed IST remains unclear. Well-performed controlled trials with stringent eligibility criteria need to be conducted to evaluate the true benefit. The documentation of data from all patients regardless of the type of intervention is feasible due to the very low prevalence of a disease like SAA. We believe that a disease-related rather than a procedure-related documentation in transplantation registers would provide data for additional comparative studies eligible for inclusion in future systematic reviews.
Second-line IST after failed first-line IST
Second-line IST after failed first-line IST may remain a treatment option. There are some uncontrolled studies providing results after second-line IST. The overall survival after a median follow-up of 30 months in 30 patients not responding to first IST was reported to be 93% by Di Bona et al.39 Scheinberg et al. reported an overall survival after 2.7 years of 70% for 22 refractory patients and of 83% for 21 relapsed patients.40 Tichelli et al. reported a long-term follow-up:41 The overall survival after ten years was 55% for 25 refractory patients and was 51% for 18 relapsed patients. However, the proportion of late clonal complications at 20 years was 53%.
Strengths and limitations of the present review
The strengths of this review are the broadness of the search strategy and the comprehensiveness of the published data included. Significant as well as non-significant factors that may influence the survival of the patients were considered in the present report. While the results of the present descriptive review may not be conclusive, they can provide useful summaries of the state of knowledge and be used for future design and data collections to obtain reliable comparative results.
This review has limitations. We described case series with heterogeneous clinical characteristics, apart from one controlled trial with methodological flaws, and we did not consider a systematic evaluation of case series on IST. While investigating factors that may influence survival, we considered results that were included in subgroup analyses, which increase the likelihood of false-positive results. Patients with disease other than SAA and patients not treated with pre-transplant IST were included. The time interval from diagnosis to transplant varied between one month and 28 years across the studies, and the number of IST courses were not stated clearly for any individual patient. We arbitrarily did not include studies with less than 10 participants and we did not consider asking the authors for individual patient data.
Heterogeneity was explored by conducting a meta analysis of the survival estimates at five years. The forest plot demonstrated a considerable difference between the studies and this heterogeneity was quantified by a very high I2 value. Therefore, a pooled estimate was not justified.
Conclusions
Unrelated donor hematopoietic stem cell transplantation in patients with acquired severe aplastic anemia after failure to immunosuppressive therapy is a treatment option. A stable physical condition of the patients before receiving the transplant (for example, performance and age) may be associated with a better survival. Detailed HLA-matching facilitated by DNA-based typing, among other factors, may have contributed to recent improvements in survival after unrelated donor HSCT as a second-line treatment. The results are based mainly on bone marrow as a stem cell source. Transplants from peripheral blood were used infrequently and transplants from umbilical cord blood were observed with an unusually large proportion of graft failure. The results of additional controlled trials comparing unrelated stem cell transplantation with further immunsuppressive therapy are needed for a meaningful analysis.
Footnotes
Funding: this work was supported by IQWiG (Institute for Quality and Efficiency in Health Care), an independent non-profit and non-government organization that evaluates the quality and efficiency of health care services in Germany. IQWiG receives its commissions (like the present work) from the Federal Joint Committee (the decision-making body of the self-administration of the German health care services) as well as from the Federal Ministry of Health, and also undertakes projects and research work on its own initiative.
The online version of this article contains a supplementary appendix.
Authorship and Disclosures
FP: principal investigator and takes primary responsibility for the paper; FP: coordinated the study, conducted the literature search, and also screened and analyzed the retrievals; SL: initiated the study; FP and SL: extracted the data; FP: drafted the manuscript; UG and FP: conducted the meta analyses; NK: provided a clinical perspective; MP: provided general advice; BZ: provided a patient-oriented perspective. All authors interpreted the data and made an intellectual contribution to the manuscript. All authors reviewed and approved the final version. The authors reported no potential conflicts of interest. Five authors (FP, UG, MP, BZ, SL) are IQWiG full-time employees, SL is deputy director of IQWiG; one author (NK) is deputy director of the Interdisciplinary Clinic for Stem Cell Transplantation, University Hospital Hamburg-Eppendorf.
References
- 1.Office of Rare Diseases: Rare Diseases Terms. Disease: Aplastic anemia [last reviewed 2006 July 19] Bethesda, Maryland, USA: National Institutes of Health; 2006. [Google Scholar]
- 2.Kaufman DW, Kelly JP, Issaragrisil S, Laporte JR, Anderson T, Levy M, et al. Relative incidence of agranulocytosis and aplastic anemia. Am J Hematol. 2006;81:65–7. doi: 10.1002/ajh.20489. [DOI] [PubMed] [Google Scholar]
- 3.Brodsky RA, Jones RJ. Aplastic anaemia. Lancet. 2005;365:1647–56. doi: 10.1016/S0140-6736(05)66515-4. [DOI] [PubMed] [Google Scholar]
- 4.Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108:2509–19. doi: 10.1182/blood-2006-03-010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Group for Blood and Marrow Transplantation (EBMT) Aplastic Anaemia Working Party (AAWP) Guidelines for treating of aplastic anemia. London, UK: 2000. [Google Scholar]
- 6.Ljungman P, Urbano-Ispizua A, Cavazzana-Calvo M, Demirer T, Dini G, Einsele H, et al. Allogeneic and autologous transplantation for haematological diseases, solid tumours and immune disorders: Definitions and current practice in Europe. Bone Marrow Transplant. 2006;37:439–49. doi: 10.1038/sj.bmt.1705265. [DOI] [PubMed] [Google Scholar]
- 7.Marsh J. Making therapeutic decisions in adults with aplastic anemia. Hematology Am Soc Hematol Educ Program. 2006:78–85. doi: 10.1182/asheducation-2006.1.78. [DOI] [PubMed] [Google Scholar]
- 8.Gratwohl A, Baldomero H, Frauendorfer K, Urbano-Ispizua A, Niederwieser D. Results of the EBMT activity survey 2005 on haematopoietic stem cell transplantation: focus on increasing use of unrelated donors. Bone Marrow Transplant. 2007;39:71–87. doi: 10.1038/sj.bmt.1705555. [DOI] [PubMed] [Google Scholar]
- 9.Horowitz MM. Current status of allogeneic bone marrow transplantation in acquired aplastic anemia. Semin Hematol. 2000;37:30–42. doi: 10.1016/s0037-1963(00)90028-3. [DOI] [PubMed] [Google Scholar]
- 10.Passweg JR, Perez WS, Eapen M, Camitta BM, Gluckman E, Hinterberger W, et al. Bone marrow transplants from mismatched related and unrelated donors for severe aplastic anemia. Bone Marrow Transplant. 2006;37:641–9. doi: 10.1038/sj.bmt.1705299. [DOI] [PubMed] [Google Scholar]
- 11.Maury S, Balere-Appert ML, Chir Z, Boiron JM, Galambrun C, Yakouben K, et al. Unrelated stem cell transplantation for severe acquired aplastic anemia: improved outcome in the era of high-resolution HLA matching between donor and recipient. Haematologica. 2007;92:589–96. doi: 10.3324/haematol.10899. [DOI] [PubMed] [Google Scholar]
- 12.Bacigalupo A. Treatment strategies for patients with severe aplastic anemia. Bone Marrow Transplant. 2008;42 (Suppl 1):S42–S44. doi: 10.1038/bmt.2008.113. [DOI] [PubMed] [Google Scholar]
- 13.Ballen KK, King RJ, Chitphakdithai P, Bolan CD, Jr, Agura E, Hartzman RJ, Kernan NA. The national marrow donor program 20 years of unrelated donor hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2008;14(9 Suppl):2–7. doi: 10.1016/j.bbmt.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 14.Altman D. Practical Statistics for Medical Research. London, UK: Chapman & Hall; 1990. [Google Scholar]
- 15.Bacigalupo A, Locatelli F, Lanino E, Marsh J, Socie G, Maury S, et al. Fludarabine, cyclophosphamide and anti-thymocyte globulin for alternative donor transplants in acquired severe aplastic anemia: a report from the EBMT-SAA Working Party. Bone Marrow Transplant. 2005;36:947–50. doi: 10.1038/sj.bmt.1705165. [DOI] [PubMed] [Google Scholar]
- 16.Bunin N, Aplenc R, Iannone R, Leahey A, Grupp S, Monos D, et al. Unrelated donor bone marrow transplantation for children with severe aplastic anemia: minimal GVHD and durable engraftment with partial T cell depletion. Bone Marrow Transplant. 2005;35:369–73. doi: 10.1038/sj.bmt.1704803. [DOI] [PubMed] [Google Scholar]
- 17.Deeg HJ, Seidel K, Casper J, Anasetti C, Davies S, Gajeweski JL, et al. Marrow transplantation from unrelated donors for patients with severe aplastic anemia who have failed immunosuppressive therapy. Biol Blood Marrow Transplant. 1999;5:243–52. doi: 10.1053/bbmt.1999.v5.pm10465104. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008] The Cochrane Collaboration; 2008. Section 9.5.2 Identifying and measuring heterogeneity. [Google Scholar]
- 19.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008] The Cochrane Collaboration; 2008. Section 13.6.2.4 When pooling is judged not to be appropriate. [Google Scholar]
- 20.Deeg HJ, O’Donnell M, Tolar J, Agarwal R, Harris RE, Feig S, et al. Optimization of conditioning for marrow transplantation from unrelated donors for patients with aplastic anemia after failure of immunosuppressive therapy. Blood. 2006;108:1485–91. doi: 10.1182/blood-2006-03-005041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hows J, Szydlo R, Anasetti C, Camitta B, Gajewski J, Gluckman E. Unrelated donor marrow transplants for severe acquired aplastic anemia. Bone Marrow Transplant. 1992;10 (Suppl 1):102–6. [PubMed] [Google Scholar]
- 22.Inamoto Y, Suzuki R, Kuwatsuka Y, Yasuda T, Takahashi T, Tsujimura A, et al. Long-Term outcome after bone marrow transplantation for aplastic anemia using cyclophosphamide and total lymphoid irradiation as conditioning regimen. Biol Blood Marrow Transplant. 2008:43–9. doi: 10.1016/j.bbmt.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy-Nasser AA, Leung KS, Mahajan A, Weiss HL, Arce JA, Gottschalk S, et al. Comparable outcomes of matched-related and alternative donor stem cell transplantation for pediatric severe aplastic anemia. Biol Blood Marrow Transplant. 2006;12:1277–84. doi: 10.1016/j.bbmt.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 24.Kim SY, Lee JW, Lim J, Cho BS, Eom KS, Kim YJ, et al. Unrelated donor bone marrow transplants for severe aplastic anemia with conditioning using total body irradiation and cyclophosphamide. Biol Blood Marrow Transplant. 2007;13:863–70. doi: 10.1016/j.bbmt.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 25.Kojima S, Matsuyama T, Kato S, Kigasawa H, Kobayashi R, Kikuta A, et al. Outcome of 154 patients with severe aplastic anemia who received transplants from unrelated donors: the Japan Marrow Donor Program. Blood. 2002;100:799–803. doi: 10.1182/blood.v100.3.799. [DOI] [PubMed] [Google Scholar]
- 26.Kosaka Y, Yagasaki H, Sano K, Kobayashi R, Ayukawa H, Kaneko T, et al. Prospective multicenter trial comparing repeated immunosuppressive therapy with stem cell transplantation from an alternative donor as second-line treatment for children with severe and very severe aplastic anemia. Blood. 2008;111:1054–9. doi: 10.1182/blood-2007-08-099168. [DOI] [PubMed] [Google Scholar]
- 27.Margolis D, Camitta B, Pietryga D, Keever-Taylor C, Baxter-Lowe LA, Pierce K, et al. Unrelated donor bone marrow transplantation to treat severe aplastic anaemia in children and young adults. Br J Haematol. 1996;94:65–72. doi: 10.1046/j.1365-2141.1996.d01-1772.x. [DOI] [PubMed] [Google Scholar]
- 28.Perez-Albuerne ED, Eapen M, Klein J, Gross TJ, Lipton JM, Baker KS, et al. Outcome of unrelated donor stem cell transplantation for children with severe aplastic anemia. Br J Haematol. 2008:216–23. doi: 10.1111/j.1365-2141.2008.07030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Svenberg P, Remberger M, Svennilson J, Mattsson J, Leblanc K, Gustafsson B, et al. Allogenic stem cell transplantation for nonmalignant disorders using matched unrelated donors. Biol Blood Marrow Transplant. 2004;10:877–82. doi: 10.1016/j.bbmt.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Viollier R, Socie G, Tichelli A, Bacigalupo A, Korthof ET, Marsh J, et al. Recent improvement in outcome of unrelated donor transplantation for aplastic anemia. Bone Marrow Transplantation. 2008:45–50. doi: 10.1038/sj.bmt.1705894. [DOI] [PubMed] [Google Scholar]
- 31.Yagasaki H, Takahashi Y, Kudo K, Ohashi H, Hama A, Yamamoto T, et al. Feasibility and results of bone marrow transplantation from an HLA-mismatched unrelated donor for children and young adults with acquired severe aplastic anemia. Int J Hematol. 2007;85:437–42. doi: 10.1532/IJH97.06229. [DOI] [PubMed] [Google Scholar]
- 32.Yoshimi A, Kojima S, Taniguchi S, Hara J, Matsui T, Takahashi Y, et al. Unrelated cord blood transplantation for severe aplastic anemia. Biol Blood Marrow Transplant. 2008;14:1057–63. doi: 10.1016/j.bbmt.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Chan KW, McDonald L, Lim D, Grimley MS, Grayson G, Wall DA. Unrelated cord blood transplantation in children with idiopathic severe aplastic anemia. Bone Marrow Transplant. 2008:589–95. doi: 10.1038/bmt.2008.227. [DOI] [PubMed] [Google Scholar]
- 34.Schrezenmeier H, Bacigalupo A, Aglietta M, Frickhofen N, Führer M, Gluckman E, et al. Consensus Document of a group of international experts [online] Maastricht, Netherlands: European Group for Blood and Marrow Transplantation (EBMT); 2000. Guidelines for Treatment of Aplastic Anemia. [Google Scholar]
- 35.Rosenfeld S, Follmann D, Nunez O, Young NS. Antithymocyte globulin and cyclosporine for severe aplastic anemia: association between hematologic response and long-term outcome. JAMA. 2003;289:1130–5. doi: 10.1001/jama.289.9.1130. [DOI] [PubMed] [Google Scholar]
- 36.Frickhofen N, Heimpel H, Kaltwasser JP, Schrezenmeier H. Antithymocyte globulin with or without cyclosporin A: 11-year follow-up of a randomized trial comparing treatments of aplastic anemia. German Aplastic Anemia Study Group. Blood. 2003;101:1236–42. doi: 10.1182/blood-2002-04-1134. [DOI] [PubMed] [Google Scholar]
- 37.Bacigalupo A, Bruno B, Saracco P, Di Bona E, Locasciulli A, Locatelli F, et al. Antilymphocyte globulin, cyclosporine, prednisolone, and granulocyte colony-stimulating factor for severe aplastic anemia: an update of the GITMO/EBMT study on 100 patients. European Group for Blood and Marrow Transplantation (EBMT) Working Party on Severe Aplastic Anemia and the Gruppo Italiano Trapianti di Midollo Osseo (GITMO) Blood. 2000;95:1931–4. [PubMed] [Google Scholar]
- 38.Marmont AM, Bacigalupo A, Van Lint MT, Frassoni F, Risso M, Cerri R, et al. Treatment of severe aplastic anemia with sequential immunosuppression. Exp Hematol. 1983;11:856–65. [PubMed] [Google Scholar]
- 39.Di Bona E, Rodeghiero F, Bruno B, Gabbas A, Foa P, Locasciulli A, et al. Rabbit antithymocyte globulin (r-ATG) plus cyclosporine and granulocyte colony stimulating factor is an effective treatment for aplastic anaemia patients unresponsive to a first course of intensive immunosuppressive therapy. Gruppo Italiano Trapianto di Midollo Osseo (GITMO) Br J Haematol. 1999;107:330–4. doi: 10.1046/j.1365-2141.1999.01693.x. [DOI] [PubMed] [Google Scholar]
- 40.Scheinberg P, Nunez O, Young NS. Retreatment with rabbit anti-thymocyte globulin and cyclosporin for patients with relapsed or refractory severe aplastic anaemia. Br J Haematol. 2006;133:622–7. doi: 10.1111/j.1365-2141.2006.06098.x. [DOI] [PubMed] [Google Scholar]
- 41.Tichelli A, Passweg J, Nissen C, Bargetzi M, Hoffmann T, Wodnar-Filipowicz A, et al. Repeated treatment with horse antilymphocyte globulin for severe aplastic anaemia. Br J Haematol. 1998;100:393–400. doi: 10.1046/j.1365-2141.1998.00578.x. [DOI] [PubMed] [Google Scholar]