Umbilical cord blood is an attractive source of stem cells for several cell-based therapies. In this paper, it is shown that umbilical cord blood-derived mesenchymal stroma cells, cultured in the presence of platelet lysate, have an increased proliferative potential but comparable immunomodulatory functions relative to their bone marrow-derived counterparts.
Keywords: mesenchymal stromal cells, umbilical cord blood, platelet lysate, immunomodulatory properties, cell therapy
Abstract
Background
Mesenchymal stromal cells are employed in various different clinical settings in order to modulate immune response. However, relatively little is known about the mechanisms responsible for their immunomodulatory effects, which could be influenced by both the cell source and culture conditions.
Design and Methods
We tested the ability of a 5% platelet lysate-supplemented medium to support isolation and ex vivo expansion of mesenchymal stromal cells from full-term umbilical-cord blood. We also investigated the biological/functional properties of umbilical cord blood mesenchymal stromal cells, in comparison with platelet lysate-expanded bone marrow mesenchymal stromal cells.
Results
The success rate of isolation of mesenchymal stromal cells from umbilical cord blood was in the order of 20%. These cells exhibited typical morphology, immunophenotype and differentiation capacity. Although they have a low clonogenic efficiency, umbilical cord blood mesenchymal stromal cells may possess high proliferative potential. The genetic stability of these cells from umbilical cord blood was demonstrated by a normal molecular karyotype; in addition, these cells do not express hTERT and telomerase activity, do express p16ink4a protein and do not show anchorage-independent cell growth. Concerning alloantigen-specific immune responses, umbilical cord blood mesenchymal stromal cells were able to: (i) suppress T- and NK-lymphocyte proliferation, (ii) decrease cytotoxic activity and (iii) only slightly increase interleukin-10, while decreasing interferon-γ secretion, in mixed lymphocyte culture supernatants. While an indoleamine 2,3-dioxygenase-specific inhibitor did not reverse mesenchymal stromal cell-induced suppressive effects, a prostaglandin E2-specific inhibitor hampered the suppressive effect of both umbilical cord blood- and bone marrow-mesenchymal stromal cells on alloantigen-induced cytotoxic activity. Mesenchymal stromal cells from both sources expressed HLA-G.
Conclusions
Umbilical cord blood- and bone marrow-mesenchymal stromal cells may differ in terms of clonogenic efficiency, proliferative capacity and immunomodulatory properties; these differences may be relevant for clinical applications.
Introduction
In addition to hematopoietic stem cells, the bone marrow (BM) also contains mesenchymal stromal cells (MSC).1,2 These latter cells exhibit multilineage differentiation potential and are endowed with immunomodulatory properties that have been demonstrated both in vitro and in vivo.3,4 Ex vivo-expanded MSC have been employed in the setting of hematopoietic stem cell transplantation, in view of both their immunomodulatory activity and their ability to support hematopoiesis. In particular, in a phase I/II multicenter study, MSC proved safe and effective when administered to 55 patients with severe, steroid-refractory acute graft-versus-host disease (GvHD).5 Moreover, in another phase I/II study, co-transplantation of MSC, together with T-cell depleted peripheral blood stem cells, overcame the problem of graft failure in 14 children receiving a hematopoietic stem cell transplant from an HLA-haploidentical family donor.6 Co-transplantation of MSC and umbilical cord blood (UCB) stem cells is also under investigation.7,8 Recently, in view of the immunosuppressive properties of MSC, as well as their role in tissue repair and trophism, infusion of these cells has also been proposed as a novel approach for reparative/regenerative medicine in the treatment of autoimmune disorders and chronic inflammatory diseases.9–11
Although BM represents the most commonly employed source of MSC for both experimental and clinical uses,3,12–16 MSC have been isolated from other sources, including adipose tissue, placenta, amniotic fluid, fetal tissues and UCB.17–22
Notably, it has not always been possible to grow MSC from UCB and in many cases the yield was low. In particular, the presence of mesenchymal progenitors in full-term UCB has been questioned in recent years by many groups, whose attempts to obtain MSC have either failed23,24 or yielded low numbers.21,25 In fact, the frequency of MSC in UCB is very low and, in fetal blood, it has been reported to decline with gestational age from about 1/106 mononuclear cells in first trimester fetal blood to 0.3/106 mononuclear cells in term cord blood.20 Despite this limitation, Bieback et al. have demonstrated that, when critical parameters for the selection of ‘good quality’ term UCB units are employed, MSC can be successfully isolated from more than 60% of processed cord blood units.22
MSC have been mainly expanded in vitro in the presence of fetal calf serum (FCS). Cells thus obtained, when infused into patients, carry the potential risk of both transmitting zoonoses and causing immune reactions directed against residual animal proteins. For these reasons, culture supplements devoid of animal components, such as platelet lysate (PL), have been tested in recent years for the isolation and expansion of MSC.26–28 In particular, our group previously demonstrated that a 5% PL-supplemented medium can support large-scale, ex vivo expansion of BM-derived MSC (BM-MSC) and that this medium is superior to 10% FCS in terms of both the clonogenic efficiency and proliferative capacity of the expanded MSC.27 Conversely, BM-MSC expanded in PL seem to be endowed with relatively low immunosuppressive activity, as compared with BM-MSC grown using FCS as the culture supplement.27
The aim of this study was to test the ability of a PL-supplemented medium to support the generation and ex vivo expansion of MSC from full-term UCB (UCB-MSC), as well as to characterize these latter cells for their biological and functional properties, in comparison with PL-expanded BM-MSC. In particular, we focused on an investigation of both the genetic stability and the immunoregulatory function, exerted on alloantigen-specific immune responses, of UCB-MSC. Moreover, we studied the possible mechanisms at the basis of the immunosuppressive effect of UCB-MSC.
Design and Methods
Collection and selection of umbilical cord blood units
UCB units were collected after full-term delivery and stored at the Cord Blood Bank of our Hospital after obtaining signed, written informed consent. The Institutional Review Board of the Fondazione IRCCS Policlinico San Matteo approved the study. Citrate phosphate dextrose-A, 20 mL, was employed as the anticoagulant in the collection bags. Whole UCB units were used to generate the MSC. Ten fresh UCB units (median volume 45 mL; range, 40–60 mL) were selected according to the following criteria: (i) total nucleated cell count ranging from 500 to 750×106; (ii) manipulation performed within 24 h of delivery; (iii) overall cell viability greater than 75%, investigated by 7-amino-actinomycin D and Aldefluor. Samples obtained from UCB units before and after mononuclear cell (MNC) separation were analyzed by a Becton Dickinson FACSCanto instrument (BD BioSciences, San Jose, CA, USA) using FACSDiva software 5.0, according to the European Working Group on Clinical Cell Analysis guidelines.29,30 Cell viability was determined using the 7-amino-actinomycin D dye test (Molecular Probes, Eugene, OR, USA) within the context of expression of surface markers identified by fluorochrome-labeled antibodies. The following monoclonal antibodies were used: anti-CD34 phycoerythrin, anti-CD45 peridinin chlorophyll protein, anti-CD133 allophycocyanin (all from BD BioSciences) and Aldefluor (StemCell Technologies, Vancouver, Canada). Aldefluor was detected using the green fluorescence channel following the manufacturer’s instructions.
Preparation of the platelet lysate
The PL was prepared as previously described.27 In brief, aliquots of 50 mL platelet-rich plasma, collected by apheresis, were obtained from ten healthy volunteers at the Transfusion Service of our hospital. All apheresis products contained a minimum of 5×1011 platelets. Written informed consent from donors was always obtained and all apheresis products were screened for infectious agents according to national regulations. Immediately after collection, platelet apheresis products were frozen at −80°C and subsequently thawed at 37°C to obtain the release of platelet-derived growth factors. Heparin (5000 UI) was added to the platelet bags to avoid gel formation. Apheresis products were centrifuged three times at 900 g for 30 min to eliminate platelet bodies. Finally, PL preparations obtained through this procedure were pooled in a single culture supplement to be used for the generation and expansion of UCB-MSC.
Isolation and culture of umbilical cord blood-derived mesenchymal stromal cells
MNC were isolated from the ten UCB units by density gradient centrifugation (Ficoll 1.077 g/mL; Lymphoprep, Nycomed Pharma, Zurich, Switzerland) after 1:1 dilution with Dulbecco’s phosphate-buffered saline (D-PBS; Euroclone, Celbio, Milan, Italy) and plated in non-coated 75–175 cm2 polystyrene culture flasks (Corning Costar, Celbio) at a density of 160,000/cm2 in complete culture medium: Mesencult (StemCell Technologies) supplemented with 2 mM L-glutamine, 50 μg/mL gentamycin (Gibco-BRL, Life Technologies, Paisely, UK) and 5% PL. This concentration of PL was chosen on the basis of results previously obtained with BM-MSC.27 Cultures were maintained at 37°C, in a 5% CO2 humidified atmosphere. After 48 h, non-adherent cells were discarded; culture medium was replaced twice a week. Upon the appearance of MSC-like clones, cells were harvested using trypsin (Sigma-Aldrich, Milan, Italy), re-plated for expansion at a density of 4,000 cells/cm2 and propagated in culture until reaching a senescence phase. Senescent cells were monitored for up to 8 weeks, in order to reveal any change in morphology and/or proliferation rate. Cell growth was analyzed by direct cell counts and cumulative population doublings were determined. The number of population doublings was calculated using the formula:
where N=cells harvested/cells seeded and results are expressed as cumulative population doublings.31
Multilineage differentiation potential of umbilical cord blood-derived mesenchymal stromal cells
The adipogenic and osteogenic differentiation capacity of UCB-MSC was determined at passage (P) 2, as previously described.27 To detect osteogenic differentiation, cells were stained for alkaline phosphatase activity using Fast Blue (Sigma-Aldrich) and for calcium deposition with Alzarin Red (Sigma-Aldrich). Adipogenic differentiation was evaluated through the morphological appearance of lipid droplets stained with Oil Red O (Sigma-Aldrich).
Immunophenotypic characterization of umbilical cord blood-derived mesenchymal stromal cells
Fluorescein isothiocyanate-, phycoerythrin-, peridinin chlorophyll protein-cyanin 5.5- labeled monoclonal antibodies specific for the following antigens were employed: (i) CD45 (clone HI30), CD14 (clone MΦP9), CD34 (clone 581), CD13 (clone L138), CD80 (clone L307.4), CD31 (clone L133.1), HLA A-B-C (clone G46-2.6), HLA-DR (clone G46-6[L243]), CD90 (clone 5E10), CD73 (clone AD2) (all from BD Biosciences), CD105 (clone SN6; Serotec, Kidlington, Oxford, UK), and HLA-G (clone MEM-G/9; Exbio, Prague, Czech Republic) for the assessment of the surface phenotype of the MSC; (ii) CD3 (clone SK7), CD4 (clone SK3), CD8 (clone SK1), CD56 (clone NCAM16.2), CD25 (clone 2A3), CD152 (CTLA4; clone BNI3), and Foxp3 (clone PCH101; eBioscience, San Diego, CA, USA) for evaluation of lymphocyte subsets. Appropriate isotype-matched controls (BD Bioscience, eBioscience) were included. Intracellular staining for CD152 (CTLA4), Foxp3 and HLA-G was performed as previously described.27,32 Two-color or three-color direct immune fluorescence cytometry was performed with a FACScalibur flow cytometer (BD Biosciences) and data calculated using CellQuest software (BD Biosciences).
Telomerase activity detection assay and reverse transcription polymerase chain reaction analysis of human telomerase reverse transcriptase
Telomerase activity was measured by the polymerase chain reaction (PCR)-based telomeric-repeat amplification protocol (TRAP) using the TRAPeze kit (Intergen Company, FL, USA) on samples containing 0.6 and 6.0 μg of protein. Protein extract from a telomerase-positive human cell line (JR8) was used as a positive control.33 A sample was scored as telomerase activity-positive when positive TRAP results were obtained from at least one protein concentration.
For assessment of human telomerase reverse transcriptase (hTERT), total cellular RNA was extracted from frozen samples with the RNeasy microkit (Qiagen GmbH). A 0.5 μg aliquot from each sample was reverse-transcribed using the reverse transcription (RT)-PCR Core kit (Applied Biosystems, Foster City, CA, USA) with random hexamers, and the resultant cDNA was then amplified with the same kit. hTERT cDNA was amplified as previously described.34
Western immunoblotting
MSC were lysed on ice in lysis buffer. Total cellular lysates were separated on 15% sodium dodecyl sulfate-polyacrylamide gel and were transferred onto Hybond ECL nitrocellulose membranes (GE Healthcare Europe GmbH, Cologno Monzese, Italy). Nitrocellulose membranes were blocked in PBS-Tween 20 with 5% skimmed milk, first incubated overnight with the primary antibody specific for p16ink4a (Abcam Inc., Cambridge, MA, USA) and then with the secondary peroxidase-linked whole antibody (GE Healthcare Europe). Bound antibody was detected using an enhanced chemiluminescence western blotting detection system (GE Healthcare Europe). DU145 human prostate cancer and U2OS human osteogenic sarcoma cell lines were used as positive and negative controls for p16ink4a expression, respectively.
Clonogenic assay
Single-cell suspensions of 100,000–1,000 cells/mL in complete medium and 0.3% (w/v) agarose were plated in triplicate in 35 mm culture dishes, over chilled 0.6% agarose feeder layers. Cultures were incubated at 37°C in a 5% CO2 humidified atmosphere and examined at 14 days after plating under an inverted microscope.
Molecular karyotyping
Molecular karyotyping of UCB-MSC at early and late passages (P3 and P8–9, respectively) was performed through array-comparative genomic hybridization (array-CGH) with the Agilent kit 44B (Human Genome CGH Microarray, Agilent Technologies, Santa Clara, CA, USA), as previously described.27,34 A pool of characterized genomic DNA (Human Genomic DNA Male, Promega, Madison, WI, USA) was used as control DNA for all experiments. Quality control parameters for every experiment were evaluated using the CGH Analytics Agilent software-QC tool.
Mixed lymphocyte cultures and cytotoxicity assays
Peripheral blood MNC were obtained by Ficoll-Hypaque density gradient from heparinized peripheral blood samples from healthy volunteers. Primary mixed lymphocyte cultures (MLC) were set up according to previously described methods.27,32,35 Briefly, non-irradiated, third-party UCB-MSC, allogeneic to both responder and irradiated stimulator peripheral blood MNC, were added at a responder cell to MSC ratio of 10:1. A control MLC was set-up in the absence of UCB-MSC. UCB-MSC employed in the MLC experiments had been harvested and cryopreserved at P3. The total number of peripheral blood MNC recovered after the 10-day MLC was counted with vitality assessed by trypan blue (Sigma-Aldrich). The recovered cells were then analyzed by flow cytometry and the percentages of lymphocyte subsets (CD3+CD4+ and CD3+CD8+ T cells, as well as CD3negCD56+ NK cells) were determined. The number of lymphocyte subsets per milliliter of culture was then calculated and compared with the initial number of cells (day 0). Differentiation of regulatory T cells (Treg) was evaluated by measuring the percentages of CD4+CD25+ and CD4+CD25bright T lymphocytes, together with the expression of Foxp3 and CTLA4 on CD4+CD25+ lymphocytes. Alloantigen-induced cell-mediated cytotoxic activity was tested in a 5-hour 51Cr-release assay, as previously described.27,32,35 Results are expressed as percent specific lysis of target cells. 51Cr-labeled target cells included phytohemagglutinin-activated stimulator peripheral blood MNC and the same lots of UCB-MSC that had been added to the MLC.
To evaluate the possible involvement of indoleamine 2,3-dioxygenase (IDO) and/or prostaglandin E2 (PGE2) in the immunosuppressive effect of PL-expanded UCB-MSC, we set up MLC in which, in some wells, specific inhibitors of IDO activity (1-methyl-tryptophan, Sigma-Aldrich), or of PGE2 (NS-398, Cayman Chemicals, Irvine, CA, USA) were added to the MSC/MLC co-culture at a concentration of 1 mM and 5 μM, respectively.36 In this set of experiments, BM-MSC, expanded in 5% PL-supplemented medium,27 were employed as a control for UCB-MSC. Cell counts per milliliter of culture recovered after 10 days of MLC, as compared to day 0, and alloantigen-induced cell-mediated cytotoxic activity (using 51Cr-labeled phytohemagglutinin-activated stimulator peripheral blood MNC as target cells) were evaluated for each culture condition. After 72 h of culture, supernatants were collected for the evaluation of IDO activity and PGE2 quantification.
Detection of indoleamine 2,3-dioxygenase activity
IDO activity was evaluated by quantifying tryptophan and kynurenine concentrations in 72-h culture supernatants with high-performance liquid chromatography (HPLC), using the HPLC pump, model SCL-10 VP (Shimadzu, Kyoto, Japan). For separation, pre-columns (cartridge holder and guard cartridge) from Phenomenex (Torrance, CA, USA) and reverse-phase C18 (octyl) columns (250 mm length, 460 mm internal diameter, 5 micron grain size) from Beckman-Coulter (Milan, Italy), were used. The incorporated UV/VIS detector model UV-SPD-M10 VP (Shimadzu) was employed for detection of both kynurenine and nitrotyrosine at a wavelength of 360 nm. Tryptophan was detected by a fluorescence detector (Shimadzu, Model RF-535) at 285-nm excitation wavelength and an emission wavelength of 365-nm. Samples were prepared as previously reported.37 L-tryptophan, L-kynurenine, 3-nitro-L-tyrosine, trichloroacetic acid, potassium phosphate and acetonitrile for the HPLC elution buffer were obtained from Sigma-Aldrich. All chemicals used were of analytical grade. Peak area counts were used to calculate concentrations (EZStart software, version 7.3). Tryptophan and kynurenine were referred to nitrotyrosine. The reproducibility of the system was controlled by nitrotyrosine counts and variations less than 5% were tolerated.
Measurement of cytokines and HLA-G by enzyme-linked immunsorbent assay
The concentrations of interferon-γ (IFN-γ), interleukin (IL)-10, IL-6, IL-12, IL-7, IL-2, IL-15 and transforming growth factor β (TGF-β) in MLC supernatants after 12, 24, and 48 h were quantified by enzyme-linked immunosorbent assay (ELISA) using monoclonal antibody pairs (Pierce Endogen, Rockford, IL, USA), as previously described.27 PGE2 levels were evaluated using a commercially available ELISA (R&D System, Minneapolis, MN, USA), according to the manufacturer’s instructions. The concentration of soluble HLA-G in culture supernatants was quantified by ELISA (sHLA-G ELISA, Exbio); clone MEM-G/9 was employed as the anti-HLA-G capture antibody.
Statistical analysis
The non-parametric Kolmogorov-Smirnov test for independent samples was performed for the comparison of cumulative cell counts at P0 and P5 of UCB- and BM-MSC. Due to the small size of the groups, the maximum significance value obtained was p=0.1.
Results
Characterization of umbilical cord blood-derived mes-enchymal stromal cells
Ten UCB units obtained at full-term delivery were selected according to the ‘quality’ criteria described in the Design and Methods section. MNC were separated, plated as P0 and cultured in medium supplemented with 5% PL. The cultures at P0 were monitored for up to 4 weeks to allow identification of MSC clones in the flasks. Two out of the ten UCB units (20%; UCB3 and UCB6) gave rise to three and four MSC-like clones after 15 and 14 days of culture, respectively. We did not obtain MSC from the remaining eight UCB units, despite the long culture period. MSC clones from UCB3 (UCB3-MSC) and UCB6 (UCB6-MSC) were expanded ex vivo and characterized for their morphology, differentiation potential, immunophenotype, proliferative capacity, biosafety profile and immunoregulatory properties. UCB-MSC displayed the typical spindle-shaped morphology, similar to that of BM-MSC expanded in the same culture medium (Online Supplementary Data, Figure S1A).27 As already observed for BM-MSC expanded in the presence of PL,27 UCB-MSC required only 2–3 min of incubation with trypsin at room temperature to become completely detached from the flasks, whereas 5–8 min at 37°C are usually necessary to harvest MSC grown in the presence of 10% FCS.
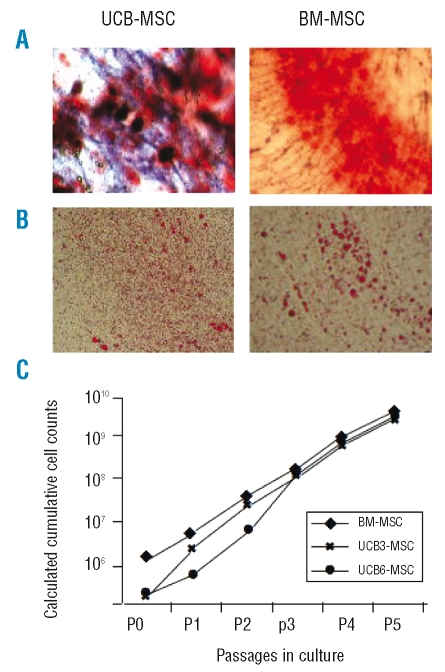
UCB-MSC were induced to differentiate into osteoblasts and adipocytes and examined for this capacity by histological staining. The cells were able to differentiate into osteoblasts, as demonstrated by the histological detection of alkaline phosphatase activity (purple reaction) and calcium deposition stained with Alzarin Red (Figure 1A), and into adipocytes, as revealed by the formation of lipid droplets, stained with Oil Red O (Figure 1B).
Figure 1.
A) Osteogenic differentiation capacity of UCB-MSC, as compared with BM-MSC (right panel). Representative photographs from UCB3-MSC at P3 and BM-MSC from donor 2.27 Magnification x 20. B) Adipogenic differentiation capacity of UCB-MSCs, as compared with BM-MSC (right panel). Representative photographs from UCB3-MSC at P3 and BM-MSC from donor 2.27 Magnification x 20. C) Calculated cumulative cell counts from P0 to P5 of UCB3-and UCB6-MSC, as compared with BM-MSC cultured in the presence of 5% PL-additioned medium (mean of eight BM-donors) and already reported.27
The surface phenotype of UCB-MSC was analyzed by flow cytometry every two passages (at P1, P3, P5 and so on) and showed the typical panel of MSC markers, in agreement with previous reports.2,3,20–24,27 In particular, by the second passage, contamination with hematopoietic cells was no longer detectable and more than 98% of the UCB-MSC were positive for CD90, CD73, CD105 and CD13 surface antigens and negative for CD34, CD45, CD14, CD80, CD31 molecules. The expression of HLA-DR was always less than 2%, whereas HLA-class I was uniformly present on UCB-MSC (more than 98% of positive cells). (Online Supplementary Data, Figure S1B).
Calculated cumulative cell counts from P0 to P5 for UCB3-MSC and UCB6-MSC, together with counts for BM-MSC cultured in the presence of 5% PL,27 for comparison, are shown in Figure 1C. UCB-MSC yielded similar numbers at P5 (1.62×109 MSC for UCB3 and 2.02×109 MSC for UCB6), as compared with BM-MSC (3.2±1.02×109 MSC as the mean±SD of eight BM donors; p>0.1),27 even when starting with low numbers of cells. In fact, the number of UCB-MSC collected after trypsinization at P0 was 0.2×106 for both units, whereas the mean number of MSC from eight BM donors at the same passage was 2.6±0.58×106 cells (p<0.1). UCB-MSC growth was also evaluated in terms of population doubling; cumulative population doublings from P1 to P5 were as follows: 12.9 for UCB3-MSC, 13.3 for UCB6-MSC, and 10.9 for BM-MSC (mean of eight BM donors). Because of the extremely low frequency of clones in the case of UCB-MSC, a comparison of the colony-forming unit-fibroblast (CFU-F) assay with BM-MSC could not be performed. The median time to reach 80% confluence for all passages from P1 to P5 was 6.5 days for both UCB3- and UCB6-MSC, as compared to 5.5 days in the case of BM-MSC expanded in the presence of 5% PL.27 Taken together, these results suggest that UCB-MSC, although displaying a rather low clonogenic efficiency, possess high proliferative potential.
UCB-MSC were cultured continuously in vitro until reaching senescence and monitored daily for up to 8 weeks, in order to investigate their propensity to undergo spontaneous transformation in vitro. The proliferative capacity of UCB3-MSC and UCB6-MSC decreased progressively until the cells reached a senescent phase after 73 and 81 days of culture at P9 and P10, respectively. The cells maintained their typical spindle-shaped morphology, differentiation capacity and surface markers throughout the culture period.
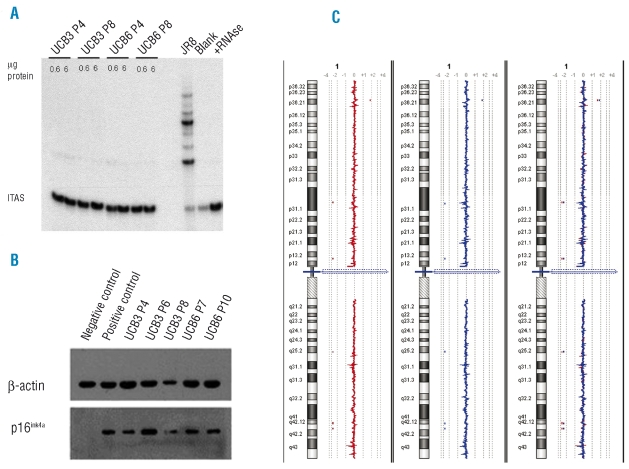
Biosafety profile of umbilical cord blood-derived mesenchymal stromal cells – lack of telomerase expression during long-term in vitro culture
MSC cultures from both UCB3 and UCB6 were tested at two different in vitro passages (P4 and P8) for the expression of telomerase catalytic activity by the TRAP assay. TRAP results failed to evidence the presence of enzyme catalytic activity in any of the samples tested (Figure 2A). To gain insights into the molecular mechanisms responsible for the repression of telomerase activity in UCB-MSC, we assessed the expression of the TERT gene, which codes for the catalytic component of human telomerase,38 in the same cultures screened for telomerase activity. RT-PCR failed to demonstrate evidence of expression of TERT mRNA (Online Supplementary Data, Figure S2), thus indicating that the absence of telomerase activity in cultured UCB-MSC was ascribable to a lack of TERT gene transcription.
Figure 2.
(A) Telomerase activity of UCB3- and UCB6-MSC cultures at P4 and P8. Telomerase activity was detected by the TRAP assay using two protein concentrations. The telomerase-positive cell line JR8 was used as a positive control. The blank represents a negative control to which no protein extract was added. The lane labeled +RNAse represents an additional negative control containing 0.6 μg cell extract of JR8 pretreated with RNAse. The location of the internal amplification standard (ITAS) is reported. (B) P16ink4a expression in UCB3- and UCB6-MSC cultures at different in vitro passages as evaluated by western immunoblotting. The p16ink4a-positive DU145 cell line and the p16ink4a-negative U2OS cell line were used as positive and negative controls, respectively. (C) Representative array-CGH profiles of chromosomes 1 of UCB6-MSC at P3 (left, red profile) and P9 (middle, blue profile). The array-CGH profiles are linear and perfectly overlapping (right), thus demonstrating that in vitro expanded UCB-MSC do not show unbalanced chromosomal rearrangements.
MSC cultures from both UCB3 and UCB6 were found to express p16ink4a protein, as detected by western immunoblotting, at all tested in vitro passages (Figure 2B). In addition, UCB3-MSC (P3 and P6) and UCB6-MSC (P4 and P11) did not show anchorage-independent cell growth, since they failed to generate colonies when plated in double-layer agarose (data not shown).
UCB-MSC were also tested for their genomic assets; in particular UCB3-MSC and UCB6-MSC were investigated at early passages (P3) and at later passages in culture (P8 and P9, respectively) by means of array-CGH (see Figure 2C for UCB6-MSC at P3 and P9). The results of array-CGH experiments revealed that UCB-MSC expanded in vitro do not show unbalanced chromosomal rearrangements; in fact, deletions or duplications of genomic material, excluding copy number variations constitutionally present, were absent in the UCB-MSC studied.
Immune regulatory properties of umbilical cord blood-derived mesenchymal stromal cells
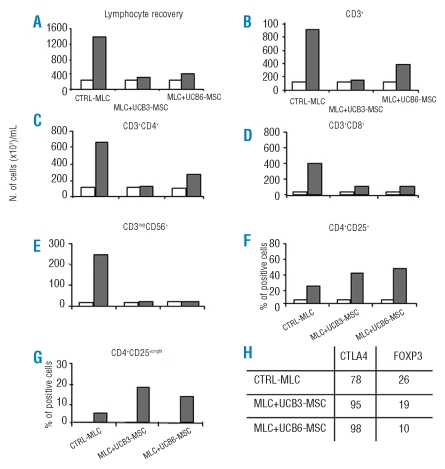
In a first set of experiments, the immune regulatory capacity of UCB-MSC was evaluated by assessing UCB-MSC interactions with alloantigen-specific immune responses, elicited in vitro in primary MLC. In agreement with previously reported studies,4 we observed that UCB-MSC were able to strongly inhibit alloantigen-induced lymphocyte proliferation (Figure 3A). A strong inhibitory effect was evident on whole T lymphocytes and their subsets (CD3+, Figure 3B; CD3+CD4+, Figure 3C; CD3+CD8+, Figure 3D), as well as on NK lymphocytes (CD3negCD56+, Figure 3E). The percentage of CD4+CD25+ T cells increased considerably, as compared with day 0, after 10-day primary MLC, both in the presence and absence of UCB-MSC, even though the percentage of this subset was higher in UCB-MSC MLC than in control MLC (Figure 3F). In an attempt to discriminate CD4+CD25+ Treg from conventional, recently activated CD4+CD25+ T lymphocytes, the degree of expression of CD25 (CD4+CD25bright T cells, Figure 3G), as well as of CTLA4 and FoxP3 molecules, was evaluated within the CD4+CD25+ T-cell subset (Figure 3H). We found a higher percentage of CD4+CD25bright and CTLA4+ cells in UCB-MSC MLC than in control MLC, while the percentage of FoxP3+ was lower in the presence than in the absence of UCB-MSC (Figure 3H).
Figure 3.
Immune modulatory effect of UCB-MSC on the expansion of T and NK-lymphocyte subsets, induced by allogeneic stimulus. Recovery of total number of lymphocytes (A), CD3+ (B), CD3+CD4+ (C), CD3+CD8+ (D), CD3neg sCD56+ NK cells (E), CD4+CD25+ (F), CD4+CD25bright ( G ) T-lymphocytes subsets and with respect to the initial number (white columns), was assessed after 10 days of primary culture (gray columns). Percentages of CTLA4+ and Foxp3+ cells were calculated on gated CD4+CD25+ T cells ( H ). MLC was performed in the absence (Ctrl-MLC) or presence of third-party MSC derived from UCB3 (MLC+UCB3-MSC) or UCB6 (MLC+UCB6-MSC). Results are expressed as the number of cells/mL in panels A–E and as percent of positive cells in panels F–H.
The kinetics of cytokine production induced in vitro by allogeneic stimulus documented that addition of UCB-MSC: (i) inhibits IFN-γ secretion; (ii) strongly increases IL-6 secretion in MLC supernatants (Online Supplementary Data, Table S1). Differently from what was found in a previous study, in which the addition of BM-MSC grown in 5% PL to the MLC substantially increased IL-10 secretion,27 UCB-MSC only slightly enhanced IL-10 secretion in 24-h MLC supernatants; (iii) IL-2, IL-7, IL-12, and IL-15 were undetectable in all experimental conditions.
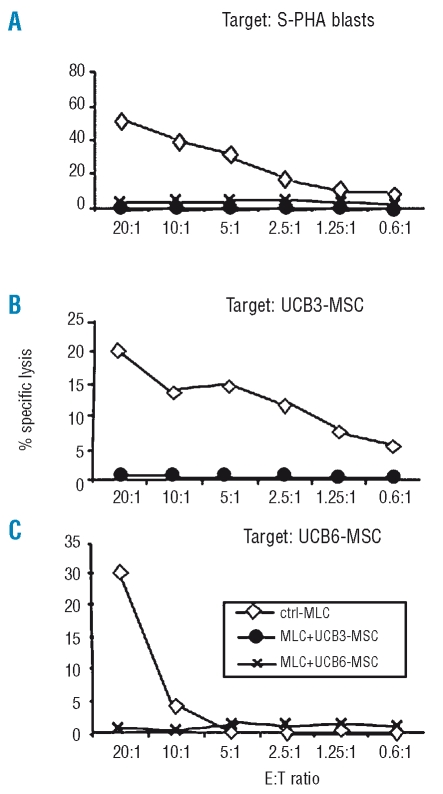
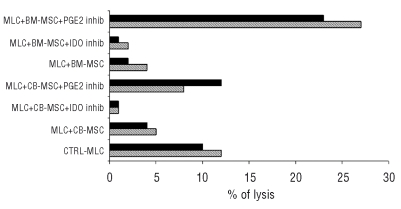
In order to assess the effect of UCB-MSC on alloantigen-induced cytotoxic activity, effector cells recovered after 10 days of MLC were tested in a cytotoxicity assay, employing as targets either MLC-stimulator phytohemogglutinin-activated blasts (Figure 4A) or third-party UCB-MSC from the same lots added to the MLC at day 0 (Figure 4B, and 4C). All the experiments showed a striking inhibitory effect mediated by both lots of UCB-MSC (UCB3-MSC and UCB6-MSC) on alloantigen-induced cytotoxic activity.
Figure 4.
Immune modulatory effect of third-party UCB-MSC on cell-mediated cytotoxic activity induced by an allogeneic stimulus (MLC). 51Cr-labeled target cells included phytohemagglutin-activated stimulator peripheral blood mononuclear cells (S-PHA) (A) and the same UCB3-MSC (B) and UCB6-MSC (C) added to MLC. Effector to target (E:T) ratios ranged between 20:1 and 0.6:1. Results are expressed as percent specific lysis of target cells.
With the aim of better understanding the biological mechanisms responsible for the immunosuppressive effect exerted by UCB-MSC on alloantigen-induced immune responses, in a second set of experiments, MLC were carried out in the presence of IDO- or PGE2-specific inhibitors. As controls, the same experimental conditions were also tested in either the presence or the absence of BM-MSC cultured in PL-supplemented medium.27 Moreover, the constitutive expression of intracellular, membrane and soluble HLA-G was evaluated in UCB-MSC, as well as in BM-MSC harvested at P3.
In terms of alloantigen-induced lymphocyte proliferation, neither the IDO-specific inhibitor nor the PGE2-specific inhibitor was able to reverse the MSC-induced suppressive effect (data not shown). By contrast, when alloantigen-induced cytotoxic activity was evaluated, a clear-cut effect of the PGE2-specific inhibitor was observed; indeed, addition of the PGE2-specific inhibitor to MLC was able to reverse the suppressive effect exerted by both UCB-MSC and BM-MSC on alloantigen-specific cytotoxic activity, even though this reagent was apparently more effective when BM-MSC were employed (Figure 5). In contrast, the IDO-specific inhibitor was not able to reverse the suppressive effect exerted by either UCB-MSC or BM-MSC on alloantigen-specific cytotoxic activity (Figure 5).
Figure 5.
Involvement of IDO and PGE2 in UCB- and BM-MSC mediated suppression of cell-mediated cytotoxic activity induced by an allogeneic stimulus (MLC). 51Cr-labeled target cells were phytohemagglutinin-activated stimulator peripheral blood mononuclear cells. Effector to target (E:T) ratios ranged between 10:1 and 1:1; results are expressed as percentage specific lysis of target cells. BM-derived MSC (BM1-MSC and BM2-MSC) expanded in PL-supplemented medium were employed as controls.27 IDO or PGE inhibitors (inhib) were added to the MLC as described in the Design and Methods section. Results obtained at the E:T of 1:1 for Exp. 1 (hatched bar) and 10:1 for Exp. 2 (full bar) are shown, UCB3-MSC and BM1-MSC were employed in Exp. 1 whereas UCB6-MSC and BM2-MSC were tested in Exp 2.
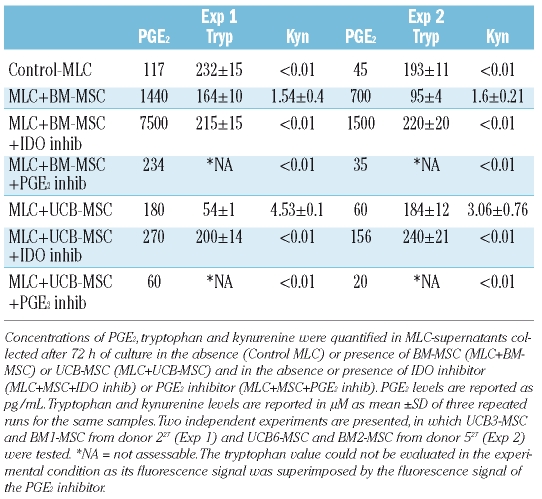
As shown in Table 1, PGE2 concentrations were considerably higher in supernatants of MLC carried out in the presence of BM-MSC than in the presence of UCB-MSC. These data might explain the striking effect of the PGE2-specific inhibitor observed in MLC performed using BM-MSC (Figure 5). The presence of the PGE2-specific inhibitor considerably decreased PGE2 secretion in MLC experiments carried out with either BM-MSC or UCB-MSC (Table 1). Interestingly, a striking increase in PGE2 secretion was observed in the presence of the IDO-specific inhibitor (Table 1). This observation may explain why the IDO-specific inhibitor was unable to reverse the suppressive effect exerted in vitro by MSC on alloantigen-specific cytotoxic activity (Figure 5). In fact, the striking increase in PGE2 secretion, induced by the presence of the IDO-specific inhibitor, could exert effective suppression on alloantigen-specific cytotoxic activity, thus masking the effect of the IDO-specific inhibitor on IDO-mediated suppressive activity.
Table 1.
Concentration of PGE2, tryptophan (Tryp) and kynurenine (Kyn) in MLC-supernatants.
IDO activity was evaluated in MLC supernatants, as indirect evidence of IDO-mediated tryptophan degradation. Detectable levels of kynurenine were found only in culture supernatants recovered from MLC carried out in the presence of either UCB-MSC or BM-MSC (Table 1). This finding indicates that IDO activity is dependent on the interaction between MSC and cells active in MLC. Kynurenine was more abundant in the presence of UCB-MSC than in the presence of BM-MSC. As expected, kynurenine was undetectable in culture supernatants collected from MSC MLC carried out in the presence of the IDO-specific inhibitor; however, kynurenine was also undetectable in culture supernatants collected from MSC MLC carried-out in the presence of PGE2-inibitor. The latter observation is in accordance with recently published data demonstrating that PGE2 is able to up-regulate IDO activity in dendritic cells.39,40 The inter-relationship between PGE2 secretion and IDO activity could also hypothetically explain why, even though PGE2 levels detected in the MLC+BM-MSC+PGE2 inhibitor culture condition of Experiment 1 were comparable to those detected in control MLC (Table 1), alloantigen–induced cytotoxic activity was strikingly higher in the former than in the latter culture condition (Figure 5).
Evaluation of constitutive HLA-G-expression in MSC showed that UCB-MSC displayed a higher percentage of membrane HLA-G+ cells as compared with BM-MSC, while more than 95% of both UCB-MSC and BM-MSC were positive for intracellular HLA-G. UCB-MSC and BM-MSC secreted similar amounts of soluble HLA-G in culture supernatants (Online Supplementary Data, Table S2).
Discussion
In this study, we have demonstrated that MSC generated from full-term UCB in a culture medium containing 5% PL are similar to BM-MSC in terms of morphology, differentiation potential, immunophenotype and proliferative ability. Their clonogenic efficiency does, however, differ. In spite of their high proliferative potential, UCB-MSC do not show any signs of in vitro transformation. Moreover, in view of our findings, although obtained in a limited number of UCB-MSC samples, UCB-MSC are apparently as efficient as BM-MSC in suppressing an alloantigen-specific immune response (Online Supplementary Figures S3 and S4).
Bieback et al. demonstrated that MSC could be isolated from full-term UCB with a success rate greater than 60%, when critical parameters for the selection of ‘good quality’ units were employed.22 These critical parameters included: a storage time shorter than 15 h, a net UCB volume greater than 33 mL; a MNC count greater than 1×108 and absence of clots or signs of hemolysis. The quality criteria adopted in our study were less stringent in terms of storage time and UCB cellularity, but included a viability test on the manipulated cells. Despite this, the 5% PL-supplemented culture medium, which we had documented to be superior to 10% FCS in terms of clonogenic efficiency and proliferative capacity when employed for BM-MSC,27 did not enable better isolation of UCB-MSC, as compared to a FCS-based medium.22 In fact, only 20% of the UCB units we tested gave rise to MSC, and MSC could not be obtained from the remaining UCB units, despite the extended culture period. We cannot exclude that either the relatively long storage time of the UCB units employed or their lower cellularity might have influenced our inferior isolation rate, despite the use of a PL-supplemented medium.
Once obtained, UCB-MSC expanded in the presence of 5% PL display the typical MSC morphology, immune phenotype and differentiation capacity (Online Supplementary Figure S1 and Figure 1A and 1B). UCB-MSC may possess high proliferative potential, yielding numbers comparable to BM-MSC cultured with the same medium27 at P5, although starting from lower cell counts at P0 (Figure 1C).
Given the high proliferative capacity and the published reports on in vitro transformation of MSC,41,42 we monitored UCB-MSC during their whole culture period and, particularly, during their senescent phase, which occurred after 9 and 10 passages for UCB3-MSC and UCB6-MSC, respectively. Neither phenotypic nor functional alterations of the cells were observed; the favorable bio-safety profile of UCB-MSC was further demonstrated by the absence of telomerase activity and hTERT expression, the expression of p16ink4a protein, the absence of anchorage-independent cell growth and by a normal molecular karyotype as proven by array-CGH analysis (Figure 2).
These data suggest that UCB-MSC, expanded in our culture system in the presence of 5% PL, do not have a tendency to undergo spontaneous transformation, in accordance with data published evaluating BM-MSC cultured with both PL- and FCS-supplemented media.27,33 To our knowledge, this is the first report to include a thorough investigation of the genetic stability of MSC derived from UCB. In our opinion, this type of evaluation, also in view of the high proliferative potential of UCB-MSC, is essential for any clinical application of these cells.
When tested for their capacity to influence the alloantigen-specific immune response, in comparison to BM-MSC grown in 5% PL (Online Supplementary Figures S3 and S4 for details on BM-MSC),27 UCB-MSC have similar suppressive effects on T- and NK-lymphocyte subset proliferation and on alloantigen-induced cytotoxic activity, while only slightly increasing IL-10 in MLC supernatants. The results obtained in our study suggest that UCB-MSC are able to exert an immunosuppressive effect on alloantigen-specific immune responses through several mechanisms, including IDO activation and production of kynurenine, PGE2 secretion and HLA-G expression.4,11,36,43–49 All these biological mechanisms have been previously described to be active in MSC derived from other sources, such as BM-MSC.4,11,35,43–49 In particular, it is noteworthy that our data confirm and extend previously reported results, underlining the inter-relationship between PGE2 secretion and IDO activation, two well-known mechanisms involved in the anti-inflammatory immune response.39,40 Indeed, the presence of a specific inhibitor of PGE2, besides reducing PGE2 levels in culture supernatants, was also able to inhibit IDO activity. Moreover, in the presence of an IDO-specific inhibitor, we observed a striking increase in PGE2 secretion.
A distinctive feature of UCB-MSC seems to be the constitutive surface expression of HLA-G on the majority of cells, while it has been reported that BM-MSC mainly express only the soluble isoform of HLA-G.45 However, it is worth considering that MEM-G/9 monoclonal antibody, which is specific for both membrane-bound (HLA-G1) and soluble (HLA-G5) HLA-G isoforms was employed to evaluate HLA-G expression in flow cytometry. We were, therefore, unable to formally prove that soluble HLA-G5 is the only HLA-G isoform expressed by UCB-MSC, as documented by Selmani et al.45 for BM-MSC. Further experiments are warranted to clarify this point.
Membrane HLA-G expression and soluble HLA-G iso-forms have been demonstrated to exert a strong suppressive effect on proliferation and activation of effector functions of both T and NK lymphocytes. For instance, it is well known that HLA-G expression at the fetomaternal interface is one of the most potent mechanisms protecting the fetus from maternal immune attack.48 Moreover, surface HLA-G expression is one of the systems employed by tumor cells to evade the cytotoxic activity of both tumor-specific T lymphocytes and NK cells, and it has been recently suggested that transfer of membrane patches containing HLA-G molecules from membrane HLA-G+ cells to activated T and NK lymphocytes (trogocytosis) might be a mechanism of immune suppression protecting HLA-G\ tumor cells.49 It may, thus, be speculated that UCB-MSC expressing membrane HLA-G may be more protected than BM-MSC from attack mediated by the host immune system. However, it has recently been demonstrated in a murine model that while local implantation of MSC results in ectopic bone formation in syngeneic recipients, it leads to transplant rejection in allogeneic mice.50 This is in line with previously published data that MSC can be lysed by cytotoxic T-lymphocytes, when infused into MHC-mismatched mice, resulting in their rejection.51 These observations support the use of MSC in hard tissue repair strategies, preferably in an autologous or tolerant host.50 Further studies specifically addressing this issue are underway.
In conclusion, while the ability of BM-MNC to generate MSC reaches 100% under appropriate culture conditions, the success rate of isolating MSC from UCB ranges, according to different reports, from 20 to 63%.21–24,52–54 In particular, Reinisch et al.54 have recently shown that MSC can be obtained from full-term UCB in the presence of human PL, yielding cell numbers suitable for clinical applications. The same authors reported an isolation efficiency of 46%, considering both FCS-expanded and PL-expanded MSC. However, their PL preparation procedure, percentage of PL employed in the culture medium (10%), as well as MSC plating density, differed from those used in our approach. These differences might explain the different results obtained in their study and ours, in particular in terms of isolation efficiency. Despite this, also in our experience, UCB-MSC have a high proliferative capacity, which allows the expansion of sufficient cell numbers for clinical applications, in a reasonable time-frame.
Given their high proliferative capacity, immunosuppressive properties and potential for avoiding attack by immune cells, as well as the ease of their collection, UCB-MSC could be considered for use in clinical practice for the prevention and treatment of alloreactive-related immune complications, namely severe GvHD and graft rejection, following hematopoietic stem cell transplantation. However, as a note of caution, recent data, obtained in a xenogenic model of NOD/SCID mice, showed that human UCB-MSC, when administered in multiple doses, are effective in the prevention, but not in the treatment of GvHD.55 This discrepancy with the clinical efficacy displayed by MSC on acute GvHD5 in human hematopoietic stem cell transplantation might be explained by the animal model employed and also by the unfavorable ratio between the number of UCB-MSC and the huge number of effector cells mediating the tissue damage at the time of onset of acute GvHD.
UCB-MSC could also serve in strategies of reparative/regenerative medicine, in which the combination of the immunosuppressive and tissue repair properties could improve the management of autoimmune and chronic inflammatory diseases.9–11
Our results, although obtained in a limited number of MSC samples tested, suggest that the differences between BM- and UCB-derived MSC and between cells expanded in the presence of PL and FCS may be relevant to the clinical application of MSC.
Supplementary Material
Acknowledgments
we are grateful to Prof. M. Galliano for precious advice on the HPLC system, and to Dra. M.S. Sbarra and E. Saino. We also thank Dr. C. Rognoni for advice on statistical analyses.
Footnotes
Funding: this work was partly supported by grants from the Istituto Superiore di Sanità (National Program on Stem Cells), European Union (FP6 program ALLOSTEM), MIUR (Ministero dell’Istruzione, dell’Università e della Ricerca, Progetti di Rilevante Interesse Nazionale, PRIN), Regione Lombardia (Research Project: ‘Trapianto di cellule staminali adulte per scopi di terapia cellulare sostitutiva, riparativa e rigenerativa’), Fondazione CARIP-LO, Fondazione IRCCS Policlinico San Matteo (Progetti di Ricerca Corrente) to FL; by grants from the Ministero della Salute (Progetti di Ricerca Finalizzata 2005), Fondazione IRCCS Policlinico San Matteo (Progetti di Ricerca Corrente), Istituto Superiore di Sanità (ISS per Alleanza contro il Cancro: Programma Straordinario di Ricerca Oncologica 2006) and European Union (FP6 program THERCORD) to RM; and by a grant from MURST “IDEE PROGETTUALI (Grandi Programmi Strategici, D.M. n° 24695, Prot. RBIPO6FH7J) and PRIN 2006 to LV.
The online version of this article contains a supplementary appendix.
Authorship and Disclosures
MAA and MEB contributed equally to this work, designed the study, performed research, analyzed data and wrote the paper; AMC, AM, CDF, and RV performed research and analyzed data; CP was responsible for preparing the platelet lysate and contributed to writing the paper; NZ, FN, and LV performed research, analyzed data and contributed to writing the paper; LMB contributed to data analysis and writing the paper; WEF advised on the design of the study and contributed to the final writing of the paper; RM advised on the design of the study, analyzed data and contributed to writing the paper; FL advised on the design of the study, analyzed data and contributed to the final writing of the paper. MAA and MEB contributed equally to this manuscript.
The authors reported no potential conflicts of interest.
References
- 1.Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230–47. [PubMed] [Google Scholar]
- 2.Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, et al. Clarification of the nomenclature for MSC: the International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393–5. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- 3.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 4.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 5.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–86. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 6.Ball LM, Bernardo ME, Roelofs H, Lankester A, Cometa A, Egeler RM, et al. Co-transplantation of ex-vivo expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem cell transplantation. Blood. 2007;110:2764–7. doi: 10.1182/blood-2007-04-087056. [DOI] [PubMed] [Google Scholar]
- 7.Bernardo ME, Ball LM, Cometa AM, Zecca M, Giorgiani G, Maccario R, et al. Co-transplantation of parental mesenchymal stem cells to improve the outcome of cord blood transplantation in children Bone Marrow Transplant 200739S117375128 [Google Scholar]
- 8.MacMillan ML, Blazar BR, DeFor TE, Wagner JE. Transplantation of ex-vivo culture-expanded parental haploidentical mesenchymal stem cells to promote engraftment in pediatric recipients of unrelated donor umbilical cord blood: results of a phase I–II clinical trial. Bone Marrow Transplant. 2009;43:447–54. doi: 10.1038/bmt.2008.348. [DOI] [PubMed] [Google Scholar]
- 9.Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341–7. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 10.García-Olmo D, García-Arranz M, Herreros D, Pascual I, Peiro C, Rodríguez-Montes JA. A phase I clinical trial of the treatment of Crohn’s fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum. 2005;48:1416–23. doi: 10.1007/s10350-005-0052-6. [DOI] [PubMed] [Google Scholar]
- 11.Locatelli F, Maccario R, Frassoni F. Mesenchymal stromal cells, from indifferent spectators to principal actors. Are we going to witness a revolution in the scenario of allograft and immune-mediated disorders? Haematologica. 2007;92:872–7. doi: 10.3324/haematol.11479. [DOI] [PubMed] [Google Scholar]
- 12.Bianco P, Gehron Robey P. Marrow stromal stem cells. J Clin Invest. 2000;105:1663–8. doi: 10.1172/JCI10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deans RJ, Moseley AB. Mesenchymal stem cells: biology and potential clinical use. Exp Hermat. 2000;28:875–84. doi: 10.1016/s0301-472x(00)00482-3. [DOI] [PubMed] [Google Scholar]
- 14.Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Bioch & Cell Biol. 2004;36:568–84. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–13. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 16.Koç ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI, et al. Rapid hematopoietic recovery after co-infusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000;18:307–16. doi: 10.1200/JCO.2000.18.2.307. [DOI] [PubMed] [Google Scholar]
- 17.Im G-I, Shin Y-W, Lee K-B. Do adipose tissue-derived mesenchymal stem cells have the same osteogenic and chondrogenic potential as bone marrow-derived cells? Osteoarthritis Cartilage. 2005;13:845–53. doi: 10.1016/j.joca.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 18.in’t Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22:1338–45. doi: 10.1634/stemcells.2004-0058. [DOI] [PubMed] [Google Scholar]
- 19.in ’t Anker PS, Noort WA, Scherjon SA, Kleijburg-van der Keur C, Kruisselbrink AB, van Bezooijen RL. Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica. 2003;88:845–52. [PubMed] [Google Scholar]
- 20.Campagnoli C, Roberts IA, Kumar S, Bennet PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98:2396–402. doi: 10.1182/blood.v98.8.2396. [DOI] [PubMed] [Google Scholar]
- 21.Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235–42. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- 22.Bieback K, Kern S, Kluter H, Eichler H. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells. 2004;22:625–34. doi: 10.1634/stemcells.22-4-625. [DOI] [PubMed] [Google Scholar]
- 23.Mareschi K, Biasin E, Piacibello W, Aglietta M, Madon E, Fagioli F. Isolation of human mesenchymal stem cells: bone marrow versus umbilical cord blood. Haematologica. 2001;86:1099–100. [PubMed] [Google Scholar]
- 24.Wexler SA, Donaldson C, Denning-Kendall P, Rice C, Bradley B, Hows JM. Adult bone marrow is a rich source of human mesenchymal “stem” cells but umbilical cord blood and mobilized adult blood are not. Br J Haematol. 2003;121:368–74. doi: 10.1046/j.1365-2141.2003.04284.x. [DOI] [PubMed] [Google Scholar]
- 25.Goodwin HS, Bicknese AR, Chien SN, Bogucki BD, Quinn CO, Wall DA. Multilineage differentiation activity by cells isolated from umbilical cord blood: expression of bone, fat and neural markers. Biol Blood Marrow Tranplant. 2001;7:581–8. doi: 10.1053/bbmt.2001.v7.pm11760145. [DOI] [PubMed] [Google Scholar]
- 26.Doucet C, Ernou I, Zhang Y, Llense JR, Begot L, Holy X, et al. Platelet lysates promote mesenchymal stem cell expansion: a safety substitute for animal serum in cell-based therapy applications. J Cell Physiol. 2005;205:228–36. doi: 10.1002/jcp.20391. [DOI] [PubMed] [Google Scholar]
- 27.Bernardo ME, Avanzini MA, Perotti C, Cometa AM, Moretta A, Lenta E, et al. Optimization of in vitro expansion of human multipotent mesenchymal stromal cells for cell-therapy approaches: further insights in the search for a fetal calf serum substitute. J Cell Physiol. 2007;211:121–30. doi: 10.1002/jcp.20911. [DOI] [PubMed] [Google Scholar]
- 28.Schallmoser K, Bartmann C, Rohde E, Reinisch A, Kashofer K, Stadelmeyer E, et al. Human platelet lysate can replace fetal bovine serum for clinical-scale expansion of functional mesenchymal stromal cells. Transfusion. 2007;47:1436–46. doi: 10.1111/j.1537-2995.2007.01220.x. [DOI] [PubMed] [Google Scholar]
- 29.Brando B, Barnett D, Janossy G, Mandy F, Autran B, Rothe G, et al. Cytofluorimetric methods for assessing absolute numbers of cell subsets in blood. Cytometry (Comm Clin Cytometry) 2000;42:327–46. doi: 10.1002/1097-0320(20001215)42:6<327::aid-cyto1000>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 30.Keeney M, Brown W, Gratama J, Papa S, Lanza F, Sutherland DR European Working Group on Clinical Cell Analysis. Single platform enumeration of viable CD34pos cells. J Biol Regul Homeost Agent. 2003;17:247–55. [PubMed] [Google Scholar]
- 31.Barachini S, Trombi L, Danti S, D’Alessandro D, Battolla B, Legitimo A, et al. Morpho-functional characterization of human mesenchymal stem cells from umbilical cord blood for potential uses in regenerative medicine. Stem Cell Dev. 2009;18:293–305. doi: 10.1089/scd.2008.0017. [DOI] [PubMed] [Google Scholar]
- 32.Maccario R, Podestà M, Moretta A, Cometa A, Comoli P, Montagna D, et al. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favours the differentiation of CD4+ T-cell subsets expressing regulatory/suppressive phenotype. Haematologica. 2005;90:516–25. [PubMed] [Google Scholar]
- 33.Bernardo ME, Zaffaroni N, Novara F, Cometa AM, Avanzini MA, Moretta A, et al. Human bone marrow-derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res. 2007;67:9142–9. doi: 10.1158/0008-5472.CAN-06-4690. [DOI] [PubMed] [Google Scholar]
- 34.Folini M, Brambilla C, Villa R, Gandellini P, Vignati S, Paduano F, et al. Antisense oligonucleotide-mediated inhibition of hTERT, but not hTERC, induces rapid cell growth decline and apoptosis in the absence of telomere shortening in human prostate cancer cells. Eur J Cancer. 2005;41:624–34. doi: 10.1016/j.ejca.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 35.Moretta A, Locatelli F, Mingrat G, Rondini G, Montagna D, Comoli P, et al. Characterization of CTL directed towards non-inherited maternal alloantigens in human cord blood. Bone Marrow Transplant. 1999;24:1161–6. doi: 10.1038/sj.bmt.1702054. [DOI] [PubMed] [Google Scholar]
- 36.Spaggiari MG, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer cell proliferation, cytotoxicity and cytokine production: role of indolamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111:1327–33. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- 37.Widner B, Werner ER, Schennach H, Wachter H, Fuchs D. Simultaneous measurement of serum tryptophan and kynurenine by HPLC. Clin Chem. 1997;43:2424–6. [PubMed] [Google Scholar]
- 38.Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Lingner J, et al. Telomerase catalytic subunit homologs from fission yeast and human. Science. 1997;277:955–9. doi: 10.1126/science.277.5328.955. [DOI] [PubMed] [Google Scholar]
- 39.Braun D, Longman RS, Albert ML. A two-step induction of indoleamine 2,3 dioxygenase (IDO) activity during dendritic-cell maturation. Blood. 2005;106:2375–81. doi: 10.1182/blood-2005-03-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.von Bergwelt-Baildon MS, Popov A, Saric T, Chemnitz J, Classen S, Stoffel MS, et al. CD25 and indoleamine 2,3-dioxygenase are up-regulated by prostaglandin E2 and expressed by tumor-associated dendritic cells in vivo: additional mechanisms of T-cell inhibition. Blood. 2006;108:228–37. doi: 10.1182/blood-2005-08-3507. [DOI] [PubMed] [Google Scholar]
- 41.Rubio D, Garcia-Castro J, Martín MC, de la Fuente R, Cigudosa JC, Lloyd AC, et al. Spontaneous human adult stem cell transformation. Cancer Res. 2005;65:3035–9. doi: 10.1158/0008-5472.CAN-04-4194. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, Huso DL, Harrington J, Kellner J, Jeong DK, Turney J, et al. Outgrowth of a transformed cell population derived from normal human BM mesenchymal stem cell culture. Cytotherapy. 2005;7:509–19. doi: 10.1080/14653240500363216. [DOI] [PubMed] [Google Scholar]
- 43.Meisel R, Zibert A, Laryea M, Gobel U, Daubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indolamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619–21. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 44.Nasef A, Mathieu N, Chapel A, Frick J, François S, Mazurier C, et al. Immunosuppressive effects of mesenchymal stem cells: involvement of HLA-G. Transplantation. 2007;84:231–7. doi: 10.1097/01.tp.0000267918.07906.08. [DOI] [PubMed] [Google Scholar]
- 45.Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26:212–22. doi: 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]
- 46.Morandi F, Raffaghello L, Bianchi G, Meloni F, Salis A, Millo E, et al. Immunogenicity of human mesenchymal stem cells in HLA-class I-restricted T-cell responses against viral or tumor-associated antigens. Stem Cells. 2008;26:1275–87. doi: 10.1634/stemcells.2007-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rizzo R, Campioni D, Stignani M, Melchiorri L, Bagnara GP, Bonsi L, et al. A functional role for soluble HLA-G antigens in immune modulation mediated by mesenchymal stromal cells. Cytotherapy. 2008;10:364–75. doi: 10.1080/14653240802105299. [DOI] [PubMed] [Google Scholar]
- 48.Carosella ED, Favier B, Rouas-Freiss N, Moreau P, Lemaoult J. Beyond the increasing complexity of the immunomodulatory HLA-G molecule. Blood. 2008;111:4862–70. doi: 10.1182/blood-2007-12-127662. [DOI] [PubMed] [Google Scholar]
- 49.Romani L, Zelante T, De Luca A, Fallarino F, Puccetti P. IL-17 and therapeutic kynurenines in pathogenic inflammation to fungi. J Immunol. 2008;180:5157–62. doi: 10.4049/jimmunol.180.8.5157. [DOI] [PubMed] [Google Scholar]
- 50.Prigozhina TB, Khitrin S, Elkin G, Eizik O, Morecki S, Slavin S. Mesenchymal stromal cells lose their immunosuppressive potential after allotransplantation. Exp Hematol. 2008;36:1370–6. doi: 10.1016/j.exphem.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 51.Nauta AJ, Westerhuis G, Kruisselbrink AB, Lurvink EG, Willemze R, Fibbe WE. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a non-myeloablative setting. Blood. 2006;108:2114–20. doi: 10.1182/blood-2005-11-011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 53.Perdikogianni C, Dimitriou H, Stiakaki E, Martimianaki G, Kalmanti M. Could cord blood be a source of mesenchymal stromal cells for clinical use? Cytotherapy. 2008;10:452–9. doi: 10.1080/14653240701883079. [DOI] [PubMed] [Google Scholar]
- 54.Reinisch A, Bartmann C, Rohde E, Schallmoser KM, Bjelic-Radisic V, Lanzer G, et al. Humanized system to propagate cord blood-derived multipotent mesenchymal stromal cells for clinical application. Regen Med. 2007;2:371–82. doi: 10.2217/17460751.2.4.371. [DOI] [PubMed] [Google Scholar]
- 55.Tisato V, Naresh K, Girdlestone J, Navarrete C, Dazzi F. Mesenchymal stem cells of cord blood origin are effective at preventing but not treating graft-versus-host disease. Leukemia. 2007;21:1992–9. doi: 10.1038/sj.leu.2404847. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.