Although they have been used for over 40 years, the value of granulocyte transfusions is controversial. This paper reviews outcomes in patients with severe aplastic anemia given such transfusions at the NIH. See related perspective article on page 1644.
Keywords: granulocyte, transfusions, severe aplastic anemia
Abstract
Background
Infections, particularly those caused by invasive fungi, are a major cause of death in patients with severe aplastic anemia. The purpose of this study was to analyze our experience with granulocyte transfusions in such patients.
Design and Methods
A retrospective analysis was performed on all patients with severe aplastic anemia who had received granulocyte transfusions between 1997 and 2007 in our institute. Survival to hospital discharge was the primary outcome. Secondary outcomes included microbiological, radiographic and clinical responses of the infection at 7 and 30 days after initiating granulocyte therapy, and post-transfusion absolute neutrophil count, stratified by HLA alloimmunization status.
Results
Thirty-two patients with severe aplastic anemia underwent granulocyte transfusions; the majority had received horse antithymocyte globulin and cyclosporine A. One quarter of patients had demonstrable HLA alloimmunization prior to the initiation of granulocyte therapy. Infections were evenly divided between invasive bacterial and fungal infections unresponsive to maximal antibiotic and/or antifungal therapy. The median number of granulocyte components transfused was nine (range, 2–43). The overall survival to hospital discharge was 58%. Survival was strongly correlated with hematopoietic recovery. Among the 18 patients who had invasive fungal infections, 44% survived to hospital discharge. Response at 7 and 30 days correlated with survival. The mean post-transfusion absolute neutrophil count did not differ significantly between response groups (i.e. patients grouped according to whether they had complete or partial resolution of infection, stable disease or progressive infection). There was also no difference in mean post-transfusion absolute neutrophil count between the patients divided according to HLA alloimmunization status.
Conclusions
Granulocyte transfusions may have an adjunctive role in severe infections in patients with severe aplastic anemia. HLA alloimmunization is not an absolute contraindication to granulocyte therapy.
Introduction
Large studies of granulocyte transfusions in the era of granulocyte colony-stimulating factor (G-CSF) use1–6 have generally focused on neutropenic patients with acute leukemia and/or on patients undergoing hematopoietic stem cell transplantation (HSCT). Infections, especially those caused by invasive fungi, are a major cause of death in patients with severe aplastic anemia (SAA).7,8 Despite the introduction of new anti-fungal agents in the last few years, the response rate in a landmark trial comparing voriconazole and liposomal amphotericin was only about 30% for both arms.9 The purpose of this study was to analyze our experience with G-CSF and dexamethasone-mobilized granulocyte transfusions in patients with SAA.
Design and Methods
Selection of patients
Patients who had SAA and fulfilled various entry criteria were enrolled into four different treatment protocols from 1997 to 2007 at the Warren Grant Magnuson Clinical Center and the Mark O. Hatfield Clinical Research Center at the National Institutes of Health in Bethesda (MD, USA). SAA was diagnosed on the basis of a bone marrow cellularity of less than 30% and severe pancytopenia with at least two of the following peripheral blood count criteria: (i) absolute neutrophil count (ANC) less than 0.5×109/L; (ii) absolute reticulocyte count less than 60×109/L; and (iii) platelet count less than 20×109/L. During this period the following regimens were studied: horse anti-thymocyte globulin)/cyclosporine; cyclophosphamide/cyclosporine; horse anti-thymocyte globulin/cyclosporine/mycophenolate mofetil; rabbit anti-thymocyte globulin/cyclosporine; and alemtuzumab.10–12 Serum sickness prophylaxis with oral prednisone 1 mg/kg/day was given prior to the first dose of anti-thymocyte globulin, continued for 10 days, and then tapered off over the subsequent 7 days. All patients received pentamidine prophylaxis for Pneumocystis pneumonia; valacyclovir was given for Herpes simplex virus prophylaxis in alemtuzumab recipients. Neutropenic fever was treated initially with broad-spectrum antibiotics, followed by empiric amphotericin therapy within 24–48 h if fever persisted. Five patients underwent upfront HSCT because they had relapsed after SAA therapy several years previously. All adult patients and legal guardians of children (< 18 years of age) signed informed consent approved by the Institutional Review Board of the National Heart, Lung, and Blood Institute.
Patients were eligible to receive a granulocyte transfusion if they had the following: (i) proven or probable invasive fungal disease according to the European Organization for Research and Treatment of Cancer criteria,13 or (ii) a bacterial infection which, in the experience of our center, was associated with greater than 90% mortality, and (iii) an ANC of less than 0.2×109/L, and (iv) no response to appropriate antibiotic or antifungal therapy for 24–48 h.
Screening for alloimmunization to human leukocyte antigens
All patients were screened for the presence of antibodies to human leukocyte antigens (HLA) prior to beginning granulocyte therapy. The methods used for detecting these antibodies included a microlymphocytotoxicity assay, a flow cytometric panel reactive assay (flow-PRA®; OneLambda, Canoga Park, CA, USA), and an enzyme-linked immunosorbent assay (ELISA Lambda Antigen Tray; OneLambda, Canoga Park, CA, USA). An attempt was made to find (partially) HLA-matched granulocyte donors for patients who were alloimmunized. Subsequent testing for HLA antibody was performed only if there was a suspicion of de novo alloimmunization.
Donor selection and granulocytapheresis
After giving appropriate informed consent, volunteer apheresis donors received a single subcutaneous injection of filgrastim (Amgen, Thousand Oaks, CA, USA) 12–18 h prior to leukapheresis, and 8 mg of dexamethasone orally 12 h prior to leukapheresis. The dose of G-CSF was 5 μg/kg until July 2005, and 480 μg as a standard dose thereafter. Early in our series of granulocytapheresis, a few donors chose to receive either dexamethasone or G-CSF only; however, more than 95% of our granulocyte donors received both G-CSF and dexamethasone. Cytomegalovirus serostatus was not a selection criterion. ABO compatibility was preferred but not required. Less than 5% of donors were family members, generally HLA-matched siblings who had already donated peripheral blood CD34+ cells for HSCT. Granulocyte concentrates were collected with a blood cell separator (CS3000 Plus, Fenwal, Deerfield, IL, USA) processing seven liters of whole blood with trisodium citrate anticoagulant (Citra Anticoagulants, Braintree, MA, USA) and 6% hetastarch (Hespan, Braun Medical, Irvine, CA, USA).
Granulocyte concentrate processing and transfusion
Granulocyte concentrates were sedimented by gravity following collection if they were not ABO-compatible with the recipient. All granulocyte concentrates were irradiated, and transfused within 8–10 h of collection. Patients received pre-medication with antipyretics. Amphotericin therapy was avoided for 4–6 h before and 4–6 h after granulocyte transfusion. Once a course of granulocyte therapy had been initiated, the goal was to provide granulocyte concentrates daily or on alternate days.
Outcome measures
Survival to hospital discharge was the primary outcome of this study. Secondary outcomes included responses at 7 and 30 days after the initiation of granulocyte therapy, and post-transfusion ANC, stratified by HLA alloimmunization status. Accurate post-transfusion white blood cell (WBC) increments were reliably obtained for the first 10 patients; this information was calculated from a complete blood count immediately prior to the granulocyte transfusion, and a repeat count 1–4 h after the transfusion. Complete blood counts were performed in all patients 5–8 h post-transfusion, given that the granulocyte transfusions occurred in the late evening, and were followed by morning complete blood counts.
Response was categorized 7 and 30 days after starting the granulocyte transfusions by taking into account microbiological data (resolution of bacteremia), radiographic criteria (decrease in infiltrates or nodule size), and clinical criteria. The clinical criteria included defervescence or a temperature decrease of 1.5–2°C, hemodynamic stabilization, and improvement in symptoms such as dyspnea. A complete response was defined as improvement in all three criteria (microbiological, radiographic and clinical); a partial response was defined as improvement in one or two criteria; stable disease was defined as no improvement; progressive disease signified clinical deterioration or a breakthrough infection. ANC data over the course of granulocyte therapy were plotted using an “area under the curve” (AUC) approximation (by the linear trapezoidal method) in an effort to quantify the degree of “neutrophil protection” provided by granulocyte therapy. For instance, a patient who received granulocyte transfusions on two consecutive days would be expected to have a higher AUC than the same patient receiving granulocyte transfusions 3 days apart over a weekend.
Statistical analysis
Data were recorded and analyzed with a spreadsheet application (Microsoft Excel, Seattle, WA, USA), including the formula for ANC AUC analysis. Pearson’s correlation coefficient was calculated for the correlation analysis. Univariate analysis was conducted for the following factors potentially influencing survival: age, period of treatment (1997–2002 versus 2003–2007), number of granulocyte transfusions, presence of HLA alloimmunization prior to initiation of granulocyte therapy, presence of invasive fungal infection, site of infection (central nervous system or lung involvement versus no central nervous system/lung involvement). Logistic regression was performed using S-Plus (Insightful, Palo Alto, CA, USA).
Results
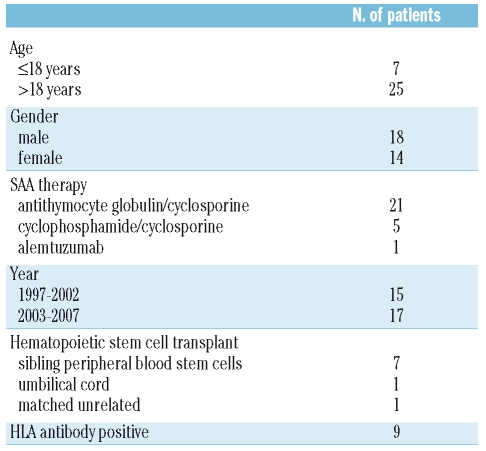
Thirty-two patients with SAA received granulocyte therapy during the 11-year period of the study. The patients’ characteristics are shown in Table 1. Their median age was 38 years (range, 7–67 years). Two-thirds (21/32) of the patients had received a horse anti-thymocyte globulin/cyclosporine-based regimen as therapy for SAA. The median time from diagnosis to entry into the study was 3 months (range, 1 month to 7 years). Three-quarters (23/32) of the patients had not received prior immunosuppressive therapy. One-quarter (8/32) of the patients had a poor performance status (Zubrod 3 or 4, in bed for 50% or more of the time); of these, four were receiving mechanical ventilation prior to the initiation of granulocyte therapy.
Table 1.
Characteristics of SAA patients who received granulocytes.
Twenty-eight percent (9/32) of patients had demonstrable HLA alloimmunization prior to the initiation of granulocyte therapy. The median number of granulocyte components transfused was nine (range, 2–43). All but seven of the 379 granulocyte concentrates transfused had been obtained following mobilization with G-CSF/dexamethasone or G-CSF. Six percent (22/379) of the components were split doses, generally between two pediatric recipients. The mean granulocyte dose transfused was 6.8 ± 2.3×1010 cells. The median interval between the initiation of SAA therapy and the initiation of granulocyte therapy was 21 days (range up to 111 days; five patients began receiving granulocyte transfusions from 2 to 32 days prior to immunosuppressive therapy).
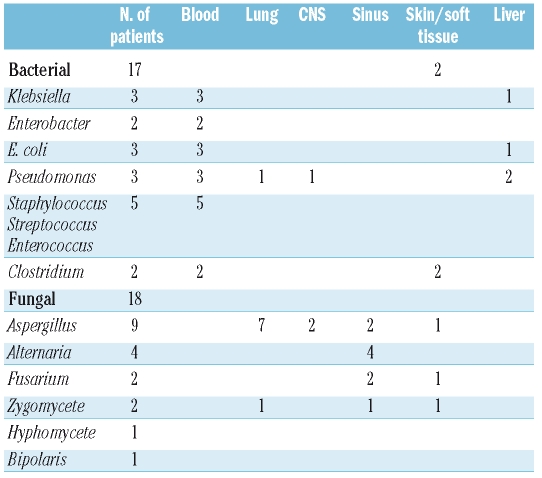
The types of infections are shown in Table 2. Some infections were polymicrobial, involving more than one bacterial strain, more than one mold, or combined bacterial and fungal infections. Of the 18 patients who had any invasive fungal infection, half were infected by Aspergillus species, predominantly in the lung; the sinuses were also frequently involved. Bacterial infections included bacteremia in all cases, including two patients who had Clostridium septicum myositis.
Table 2.
Characteristics of infections in SAA patients receiving granulocyte therapy.
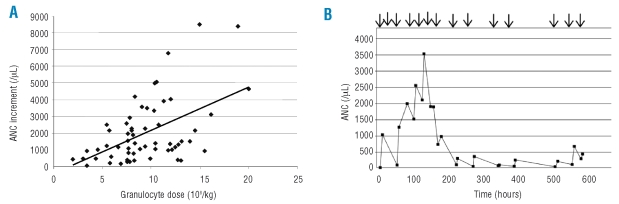
Figure 1A shows the relationship between the ANC increment and the granulocyte dose transfused for the first five non-alloimmunized patients for whom accurate pre-transfusion ANC were available. Figure 1B shows the ANC over time for one patient who developed de novo HLA alloimmunization during the course of granulocyte therapy. The mean post-transfusion ANC for the entire series of 374 transfusion episodes, was 1.433×109/L.
Figure 1.
(A) Relationship between ANC increment and granulocyte dose transfused for the first five non-alloimmunized patients who received 69 granulocyte concentrates. R2=0.26, p<0.01. (B) ANC over time for one patient who developed de novo HLA antibodies during the course of granulocyte therapy. Arrows denote granulocyte transfusions.
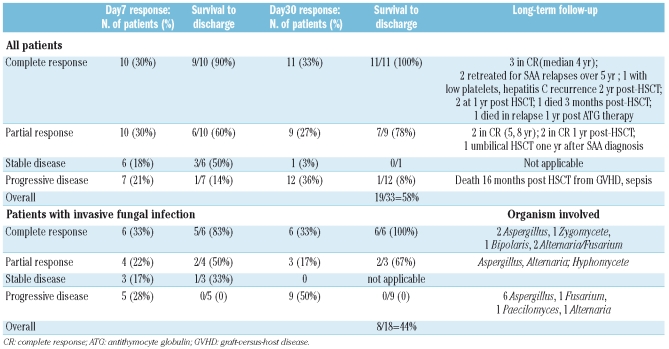
The patients’ outcomes on day 7 and day 30 are shown in Table 3a. For response classification, one patient had three distinct neutropenic infectious episodes over 5 months which were counted separately, and one patient who received only two granulocyte transfusions was excluded from analysis, yielding a total of 33 patient-episodes for analysis. The overall survival to hospital discharge was 58% (19/33). For the subset of 18 patients who had invasive fungal infections, the overall survival to hospital discharge was 44% (8/18). Survival appeared to be strongly correlated with hematopoietic recovery: of the 14 patients who recovered hematopoiesis, 13 (93%) survived; of the 18 patients who did not, 4 (22%) survived. Of note, seven patients received granulocyte therapy as a bridge between failed immunosuppressive therapy and HSCT; four of these patients survived to discharge, including two who had invasive fungal infection prior to HSCT. None of the patients who had a poor performance status at the time of initiating granulocytes therapy survived.
Table 3A.
Response at day 7 and day 30 versus survival to hospital discharge.
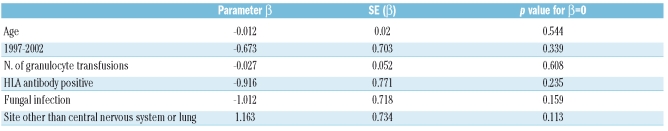
Logistic regression analysis of other variables that could affect survival did not show any statistical significance, although the trends were as expected: for instance, fungal infection and treatment before 2002 were negative determinants for survival, and site of involvement other than lung or central nervous system was a positive determinant for survival (Table 3b).
Table 3B.
Univariate logistic analysis for survival to discharge.
We explored the possibility of a dose-response effect by looking at the mean post-transfusion ANC versus response, the mean granulocyte dose versus response, and the ANC AUC versus response. There was no statistical difference in the mean post-transfusion ANC among the patients grouped according to response category (ANC of 1.397×109/L in the group with complete response versus 2.081×109/L in the group with progressive disease), or in the mean granulocyte dose by response category (9.15×108 cells/kg in the complete response group versus 11.41×108 cells/kg in the group with progressive disease, and 13.18×108 cells/kg in the partial response group). There was also no statistical difference in the ANC AUC among the different response groups (data not shown). The interval between granulocyte transfusions partially determined the dose-intensity of granulocyte therapy; it was not significantly different between response groups (1.59 days in the group with progressive disease, compared to 1.89 days in the group with complete response and 1.76 days in the group with partial response).
Thirteen patients had positive tests for HLA antibody at some point during granulocyte therapy. An attempt was made to provide at least partially-HLA matched granulocytes for the nine patients known to be alloimmunized prior to the initiation of granulocyte transfusions; two of these patients received granulocytes from HLA-matched siblings. The mean post-transfusion ANC among the remaining seven alloimmunized patients was 1.52×109/L. Of the patients who were not alloimmunized at baseline, 4/23 (17%) developed HLA antibodies after the initiation of granulocyte therapy; one of these patients experienced pulmonary symptoms as a reaction to granulocyte transfusion, prompting repeat HLA antibody testing. In the 19 non-alloimmunized patients, the mean post-transfusion ANC was 1.456×109/L, which was not significantly different from the mean post-transfusion ANC in the alloimmunized group.
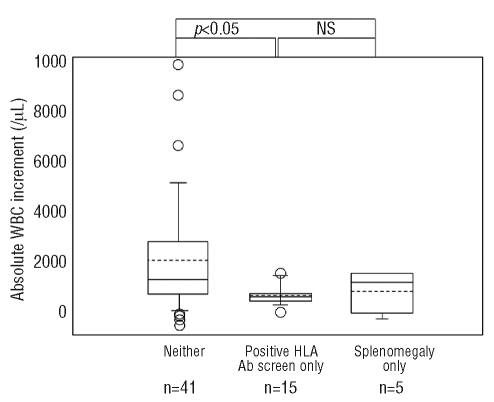
The effect of HLA alloimmunization or splenomegaly on the WBC increment for the first ten patients in our series, for whom accurate pre- and post-transfusion WBC counts were available, is shown in Figure 2. Among these ten patients, those who did not have splenomegaly or HLA alloimmunization had significantly higher WBC increments than those who had either. WBC increments were used in this analysis because the WBC differential (for ANC) was not typically repeated within a 8-hour period.
Figure 2.
Effect of splenomegaly and positive HLA antibody screen on absolute WBC increments 0–4 h post-transfusion. Ends of boxes represent 25% and 75% of data. End bars represent 5% and 95% of data. Dashed line inside box is the mean. Solid line inside the box is the median. Open circles represent outliers. Numbers on the x-axis refer to granulocyte transfusion episodes.
Early in the study we observed five cases of acute lung injury (defined as decreased oxygen saturation associated with radiographically detected pulmonary infiltrates) temporally related to granulocyte transfusion; all of these patients were receiving amphotericin-based therapy for fungal infections involving the lungs (n=2), sinuses (n=2) and skin (n=1). One reaction was attributable to evolving HLA alloimmunization. The other reactions were attributable to fluid overload. Of note, there was no pulmonary reaction to granulocyte transfusions in the latter 7 years of the study period. There were no other severe adverse events associated with granulocyte transfusions.
Discussion
This report details the largest clinical experience to date of granulocyte therapy as part of a multimodality approach to neutropenic infections in patients with SAA. More than half of our patients had invasive fungal infections (with or without concurrent bacterial infections); 44% of these patients survived to hospital discharge.
Invasive fungal infections, particularly those caused by Aspergillus species, have long been recognized as a major cause of death in SAA. In a series from our institution, in which all infections in 103 patients with aplastic anemia seen between 1978 and 1989 were examined, all 14 patients with aspergillosis died despite amphotericin B therapy and selected adjuvant surgical resection; all ten patients with systemic Candida infections also eventually died.14 In a more recent series of 42 patients with aplastic anemia followed between 1994 and 2000,7 despite the use of fluconazole prophylaxis, invasive fungal infections (yeasts and molds) accounted for 36% of neutropenic infections, and five of these nine episodes were fatal. The role of prophylactic antifungal therapy in patients expected to have prolonged neutropenia is controversial.
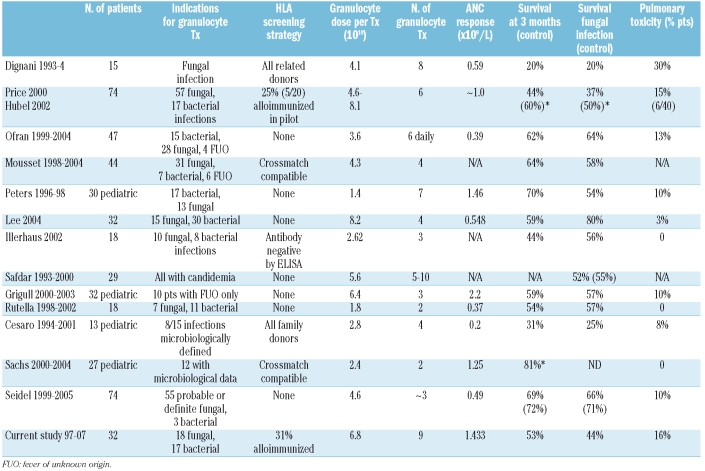
Before the introduction of G-CSF, granulocyte collections from normal donors (typical dose of 4×109 cells if hetastarch or corticosteroids were not used) produced inadequate cell doses except for neonates.15 A meta-analysis exploring the efficacy of granulocyte transfusions in the pre-G-CSF era confirmed the importance of cell dose.16 When granulocyte donors are stimulated with G-CSF with or without corticosteroids, granulocyte doses of 4–7×1010 are achievable. The majority of donors experience transient bone pain and myalgias; long-term follow-up of our granulocyte donors has not shown any adverse outcomes.17 Recent studies of therapeutic granulocyte transfusions for neutropenic infections in patients with leukemia and/or undergoing HSCT have shown survival rates of 31–81%; in patients with invasive fungal infections, the survival rates range from 20–80%. These studies, summarized in Table 4,1–6,18–25 vary in the definition of invasive fungal infections, the timing of initiating granulocyte therapy, the cell dose of granulocytes transfused, and the number of granulocyte doses given. One study was randomized, but only 60% of patients were actually neutropenic prior to receiving granulocytes, and 44% of patients randomized to the granulocyte arm received only one or two transfusions before neutrophil recovery.23
Table 4.
Selected studies of therapeutic granulocyte transfusion (Tx) in hematology/stem cell transplant patients in the G-CSF era (*1-month survival). Control group did not receive granulocytes.
Our study is unique in that all patients had SAA, a condition in which the response to immunosuppressive therapy can take up to 6 months to develop, leading to a neutropenic period that is typically longer than that seen after leukemia induction chemotherapy and HSCT. Fever in these severely neutropenic patients is aggressively managed with early use of broad-spectrum antibiotics and antifungal therapy. Granulocyte therapy is considered judiciously, but relatively early in the care of the patient rather than as a “last-ditch” or futile effort.2
Our response categorization correlated very well with survival to discharge, for all patients and for the subset of patients with fungal infections. This does not necessarily imply a cause-and-effect relationship between granulocyte transfusion and discharge, since “response” could have occurred with antimicrobials alone if given enough time. The strong correlation between survival to hospital discharge and hematopoietic recovery supports the role of granulocyte transfusion as a bridge to ANC recovery or HSCT. Absence of a dose response suggests a threshold phenomenon: we speculate that as long as the post-transfusion ANC exceeds a certain cutoff (such as 1×109/L), there is no correlation between post-transfusion ANC and clinical response. The interval between transfusions as a measure of dose intensity was similarly not significantly different among response groups; the mean interval was 1.7 days. This suggests that granulocyte therapy every other day may be as effective as daily granulocyte transfusions, which are logistically much more difficult to provide. Concurrent corticosteroid administration can potentially impair neutrophil function, but most of our patients had completed serum sickness prophylaxis by the time of initiating granulocyte therapy; in vitro, G-CSF can prevent corticosteroid-induced suppression of neutrophilic anti-hyphal function.26
Pre-existing alloimmunization did not preclude adequate post-transfusion ANC and did not cause severe pulmonary or other reactions, unlike the experience with granulocyte transfusion in chronic granulomatous disease.27 We had accurate pre-transfusion complete blood counts for only the first ten patients in the series: in this subset, the WBC increment was significantly lower for the patients with HLA antibodies than for those without. We achieved partial HLA matching within cross-reactive groups (CREG) or minimized triplet mismatches by HLA-Matchmaker28 for approximately half the granulocyte transfusions given to alloimmunized patients, perhaps accounting for the good post-transfusion ANC in this group of patients. In an earlier study2 using unrelated volunteer granulocyte donors, HLA alloimmunized patients did not have lower ANC increments or increased transfusion reactions. Another study demonstrated lower ANC increments in patients who were alloimmunized: in that study,29 each patient received four granulocyte concentrates from the same related donor (first-degree relative), prophylactically following HSCT with components that were all cross-match compatible by lymphocytotoxicity and leukoagglutination assays. There was no difference in ANC increments between alloimmunized and non-alloimmunized patients for the first two granulocyte transfusions; the difference became significant only with the third and fourth transfusions. The generalizability of these results is not clear.
De novo alloimmunization was associated with pulmonary toxicity in one of five of our patients; much more commonly, acute lung injury was attributable to fluid overload. Meticulous fluid management is crucial since these critically ill patients typically receive hydration because of the use of amphotericin (or derivatives) and antibiotics, fluid boluses for hypotension, multiple blood products and hyperalimentation. Our observed incidence of pulmonary toxicity was 16% (5/32), in keeping with the incidence reported in the literature (Table 4); of note, no pulmonary reaction has occurred in the latter 7 years of the study, reflecting the improved experience with fluid management in critically ill patients undergoing granulocyte therapy.
There are several limitations of this study. First, it is a retrospective, single institution, observational study. Our numbers were too small for us to generate a multivariate logistic regression model of factors that influence survival. Second, the study is limited to SAA patients; these results may not, therefore, be extendable to leukemia patients or HSCT recipients who routinely receive intensive cytotoxic chemotherapy. A large multicenter randomized controlled trial is underway to study the incremental benefits of granulocyte transfusion in infections post-HSCT. Finally, the availability of newer antibiotics and antifungal agents or cytokine regimens to treat neutropenic infections30 may deter the use of G-CSF and dexamethasone to stimulate normal donors, which can only be performed under the auspices of a clinical research protocol. Until a prospective randomized controlled trial is conducted, use of granulocyte transfusions in SAA patients will continue to be dictated by local institutional practice and clinical assessment of the patient.
Footnotes
Authorship and Disclosures
KQ was the principal investigator, and takes primary responsibility for the paper. EW, SFL, and TJW participated in the patients’ care, data collection and analysis. COW performed the logistic regression analysis. PS and NSY participated in the patients’ care, discussions and writing the manuscript. The authors reported no potential conflicts of interest.
References
- 1.Peters C, Minkov M, Matthes-Martin S, Potschger U, Witt V, Mann G, et al. Leucocyte transfusions from rhG-CSF or prednisolone stimulated donors for treatment of severe infections in immunocompromised neutropenic patients. Br J Haematol. 1999;106:689–96. doi: 10.1046/j.1365-2141.1999.01619.x. [DOI] [PubMed] [Google Scholar]
- 2.Price TH, Bowden RA, Boeckh M, Bux J, Nelson K, Liles WC, Dale DC. Phase I/II trial of neutrophil transfusions from donors stimulated with G-CSF and dexamethasone for treatment of patients with infections in hematopoietic stem cell transplantation. Blood. 2000;95:3302–9. [PubMed] [Google Scholar]
- 3.Hubel K, Carter RA, Liles WC, Dale DC, Price TH, Bowden RA, et al. Granulocyte transfusion therapy for infections in candidates and recipients of HPC transplantation: a comparative analysis of feasibility and outcome for community donors versus related donors. Transfusion. 2002;42:1414–21. doi: 10.1046/j.1537-2995.2002.00249.x. [DOI] [PubMed] [Google Scholar]
- 4.Safdar A, Hanna HA, Boktour M, Kontoyiannis DP, Hachem R, Lichtiger B, et al. Impact of high-dose granulocyte transfusions in patients with cancer with candidemia. Cancer. 2004;101:2859–65. doi: 10.1002/cncr.20710. [DOI] [PubMed] [Google Scholar]
- 5.Mousset S, Hermann S, Klein SA, Bialleck H, Duchscherer M, Bomke B, et al. Prophylactic and interventional granulocyte transfusions in patients with haematological malignancies and life-threatening infections during neutropenia. Ann Hematol. 2005;84:734–41. doi: 10.1007/s00277-005-1055-z. [DOI] [PubMed] [Google Scholar]
- 6.Grigull L, Pulver N, Goudeva L, Sykora KW, Linderkamp C, Beilken A, et al. G-CSF mobilized granulocyte transfusions in 32 paediatric patients with neutropenic sepsis. Support Care Cancer. 2006;14:910–6. doi: 10.1007/s00520-006-0041-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torres HA, Bodey GP, Rolston KVI, Kantarjian HM, Raad II, Kontoyiannis DP. Infections in patients with aplastic anemia. Cancer. 2003;98:86–93. doi: 10.1002/cncr.11478. [DOI] [PubMed] [Google Scholar]
- 8.Rosenfeld S, Follman D, Nunez O, Young NS. Antithymocyte globulin and cyclosporine for severe aplastic anemia: association between hematologic response and long-term outcome. JAMA. 2003;289:1130–5. doi: 10.1001/jama.289.9.1130. [DOI] [PubMed] [Google Scholar]
- 9.Walsh TJ, Pappas P, Winston DJ, Lazarus HM, Petersen F, Raffali J, et al. Voriconazole compared with liposomal amphotericin B for empirical antifungal therapy in patients with neutropenia and persistent fever. New Engl J Med. 2002;346:225–34. doi: 10.1056/NEJM200201243460403. [DOI] [PubMed] [Google Scholar]
- 10.Scheinberg P, Nunez O, Wu C, Young NS. Treatment of severe aplastic anaemia with combined immuno-suppression: anti-thymocyte globulin, ciclosporin and mycophenolate mofetil. Br J Haematol. 2006;133:606–11. doi: 10.1111/j.1365-2141.2006.06085.x. [DOI] [PubMed] [Google Scholar]
- 11.Scheinberg P, Nunez O, Young NS. Retreatment with rabbit anti-thymocyte globulin and ciclosporin for patients with relapsed or refractory severe aplastic anaemia. Br J Haematol. 2006;133:622–7. doi: 10.1111/j.1365-2141.2006.06098.x. [DOI] [PubMed] [Google Scholar]
- 12.Tisdale JF, Dunn DE, Geller N, Plante M, Nunez O, Dunbar CE, et al. High-dose cyclophosphamide in severe aplastic anaemia: a randomised trial. Lancet. 2000;356:1554–9. doi: 10.1016/S0140-6736(00)03126-3. [DOI] [PubMed] [Google Scholar]
- 13.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–21. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinberger M, Elattar I, Marshall D, Steinberg SM, Redner RL, Young NS, Pizzo PA. Patterns of infection in patients with aplastic anemia and the emergence of aspergillus as a major cause of death. Medicine. 1992;71:24–43. doi: 10.1097/00005792-199201000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Price TH. Granulocyte transfusion: current status. Semin Hematol. 2007;44:15–23. doi: 10.1053/j.seminhematol.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 16.Vamvakas EC, Pineda AA. Meta-analysis of clinical studies of the efficacy of granulocyte transfusions in the treatment of bacterial sepsis. J Clin Apher. 1996;11:1–9. doi: 10.1002/(SICI)1098-1101(1996)11:1<1::AID-JCA1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 17.Quillen K, Byrne P, Yau YY, Leitman SF. Ten-year followup of unrelated volunteer granulocyte donors who have received multiple cycles of granulocyte colony-stimulating factor and dexamethasone. Transfusion. 2009;49:513–8. doi: 10.1111/j.1537-2995.2008.01983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ofran Y, Avivi I, Oliven A, Oren I, Zuckerman T, Bonstein L, et al. Granulocyte transfusions for neutropenic patients with life-threatening infections: a single centre experience in 47 patients, who received 348 granulocyte transfusions. Vox Sang. 2007;93:363–9. doi: 10.1111/j.1423-0410.2007.00971.x. [DOI] [PubMed] [Google Scholar]
- 19.Lee JJ, Song HC, Chung IJ, Bom HS, Cho D, Kim HJ. Clinical efficacy and prediction of response to granulocyte transfusion therapy for patients with neutropenia-related infections. Haematologica. 2004;89:632–3. [PubMed] [Google Scholar]
- 20.Illerhaus G, Wirth K, Dwenger A, Waller CF, Garbe A, Brass V, et al. Treatment and prophylaxis of severe infections in neutropenic patients by granulocyte transfusions. Ann Hematol. 2002;81:273–81. doi: 10.1007/s00277-002-0439-6. [DOI] [PubMed] [Google Scholar]
- 21.Cesaro S, Chinello P, de Silvestro G, Marson P, Picco G, Varotto S, et al. Granulocyte transfusions from G-CSF-stimulated donors for the treatment of severe infections in neutropenic pediatric patients with onco-hematological diseases. Support Care Cancer. 2003;11:101–6. doi: 10.1007/s00520-002-0394-8. [DOI] [PubMed] [Google Scholar]
- 22.Sachs UJH, Reiter A, Walter T, Bein G, Woessmann W. Safety and efficacy of therapeutic early onset granulocyte transfusions in pediatric patients with neutropenia and severe infections. Transfusion. 2006;46:1909–14. doi: 10.1111/j.1537-2995.2006.00996.x. [DOI] [PubMed] [Google Scholar]
- 23.Seidel MG, Peters C, Wacker A, Northoff H, Moog R, Boehme A, et al. Randomized phase III study of granulocyte transfusions in neutropenic patients. Bone Marrow Transplant. 2008;42:679–84. doi: 10.1038/bmt.2008.237. [DOI] [PubMed] [Google Scholar]
- 24.Rutella S, Pierelli L, Piccirillo N, Sica S, Serafini R, Chiusolo P, et al. Efficacy of granulocyte transfusions for neutropenia-related infections: retrospective analysis of predictive factors. Cytotherapy. 2003;5:19–30. doi: 10.1080/14653240310000047. [DOI] [PubMed] [Google Scholar]
- 25.Dignani MC, Anaissie EJ, Hester JP, O’Brien S, Vartivarian SE, Rex JH, et al. Treatment of neutropenia-related fungal infections with granulocyte colony-stimulating factor-elicited white blood cell transfusions: a pilot study. Leukemia. 1997;11:1621–30. doi: 10.1038/sj.leu.2400811. [DOI] [PubMed] [Google Scholar]
- 26.Roilides E, Uhlig K, Venzon D, Pizzo PA, Walsh TJ. Prevention of corticosteroid-induced suppression of human polymorphonuclear leuko-cyte-induced damage of Aspergillus fumigatus hyphae by granulocyte colony-stimulating factor and gamma interferon. Infect Immun. 1993;61:4870–7. doi: 10.1128/iai.61.11.4870-4877.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stroncek DF, Leonard K, Eiber G, Malech HL, Gallin JI, Leitman SF. Alloimmunization after granulocyte transfusions. Transfusion. 1996;36:1009–15. doi: 10.1046/j.1537-2995.1996.36111297091747.x. [DOI] [PubMed] [Google Scholar]
- 28.Nambiar A, Duquesnoy RJ, Adams S, Zhao Y, Oblitas J, Leitman S, et al. HLAMatchmaker-driven analysis of responses to HLA-typed platelet transfusions in alloimmunized thrombocytopenic patients. Blood. 2006;107:1680–7. doi: 10.1182/blood-2004-10-4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adkins DR, Goodnough LT, Shenoy S, Brown R, Moellering J, Khoury H, et al. Effect of leukocyte compatibility on neutrophil increment after transfusion of granulocyte colony-stimulating factor-mobilized prophylactic granulocyte transfusions and on clinical outcomes after stem cell transplantation. Blood. 2000;95:3605–12. [PubMed] [Google Scholar]
- 30.Dignani MC, Rex JH, Chan KW, Dow G, deMagalhaes-Silverman M, Maddox A, et al. Immunomodulation with interferon-gamma and colony-stimulating factors for refractory fungal infections in patients with leukemia. Cancer. 2005;104:199–204. doi: 10.1002/cncr.21142. [DOI] [PubMed] [Google Scholar]