Abstract
Experimental and epidemiologic studies suggest that calcium and vitamin D supplements may lower blood pressure. We examined the effect of calcium plus vitamin D supplementation on blood pressure and the incidence of hypertension in post-menopausal women. The Women's Health Initiative calcium/vitamin D trial randomly assigned 36,282 post-menopausal women to receive 1000 mg of elemental calcium plus 400 IU of vitamin D3 daily, or placebo, in a double-blind fashion. Change in blood pressure and the incidence of hypertension were ascertained. Over a median follow-up time of 7 years, there was no significant difference in the mean change over time in systolic blood pressure (0.22 mm Hg, 95% CI −0.05 – 0.49 mm Hg) and diastolic blood pressure (0.11 mm Hg, 95% CI −0.04 – 0.27 mm Hg) between the active and placebo treatment groups. This null result was robust in analyses accounting for non-adherence to study pills and in baseline subgroups of interest, including Blacks, women with hypertension or high levels of blood pressure, with low intakes of calcium and vitamin D, or low serum levels of vitamin D. In 17,122 non-hypertensive participants at baseline, the hazard ratio for incident hypertension associated with calcium/vitamin D treatment was 1.01 (95% CI 0.96 – 1.06.) In postmenopausal women, calcium plus vitamin D3 supplementation did not reduce either blood pressure or the risk of developing hypertension over seven years of follow-up.
Keywords: Calcium supplementation, 25-hydroxyvitamin D, 1, 25-dihydroxyvitamin D, blood pressure, hypertension, clinical trials
Introduction
The role of calcium in the prevention and treatment of hypertension is controversial, despite decades of study. An overall healthy dietary pattern that is rich in calcium from low-fat dairy products, fruits and vegetables has been shown to lower blood pressure substantially compared to a typical diet higher in fat and sodium and lower in calcium, magnesium, potassium and fiber.1-4 However, meta-analyses5-12 and systematic reviews13 of the epidemiologic and clinical trial evidence regarding dietary intake of calcium as a single nutrient have generally concluded that the effect on systolic blood pressure (BP) lowering is small, on the order of 2 mm Hg. The effect on diastolic BP, if any, may be even smaller. Nevertheless, at a population level, sustained BP lowering of this degree by calcium supplementation could have important benefits on cardiovascular disease.
Although the relation between vitamin D and BP has been less studied, two small, short-term intervention studies suggest that vitamin D, either as ultraviolet light exposure or as an oral supplement, may lower BP.14, 15 In addition, the risk of incident hypertension was lower in a 4-year prospective study among men and women with higher plasma levels of 25(OH) vitamin D.16 Animal studies have also shown that oral supplementation with vitamin D lowered blood pressure in hypertensive rats; in this model vitamin D inhibited renin expression in the juxtaglomerular apparatus and inhibited smooth muscle proliferation.17-20
Most individual trials of calcium supplementation (with or without vitamin D) have been relatively small, short in duration or both. Meta-analyses and reviews have called for high-quality, long-term studies including subgroups that might have greater BP lowering from calcium supplementation, such as individuals with elevated BP or hypertension (especially low renin or “salt-sensitive” hypertension), Blacks, or those with habitual low intake of calcium or vitamin D.21-24 The Women's Health Initiative included a methodologically rigorous randomized, double-blind, placebo-controlled trial of dietary supplementation with calcium plus vitamin D supplementation and long-term follow-up. We examined the effect on BP and the incidence of hypertension in this study of 36,282 post-menopausal women. Because of the large size of the trial we were able to examine subgroups that might have differing degrees of benefit.
Methods
Study population and intervention
Between 1993 and 1998, postmenopausal women aged 50-79 were recruited at 40 U.S. clinical centers into the Women's Health Initiative randomized trials assessing the risks and benefits of hormone therapy (HT) and dietary modification (DM).25 Participants enrolled in one or both trials were further invited to join the calcium plus vitamin D (CaD) trial at their first (n=33,070) or second (n=3,212) annual follow-up visit.26 Women with less than 3 years predicted survival, a history of kidney stone or hypercalcemia, current oral corticosteroid use, or current calcitriol use were excluded. The primary outcome for the CaD intervention trial was incident hip fracture and the secondary outcome was colorectal cancer. Results of these analyses have been published.27, 28 The study protocol was approved by the Institutional Review Board at each participating institution, and written informed consent was granted by each participant prior to the randomization into the CaD trial.
Participants were randomly assigned in a double-blind fashion to receive 1000 mg of elemental calcium plus 400 IU of vitamin D3 daily, or placebo.26 Each active tablet provided 500 mg calcium (as calcium carbonate) and 200 IU of vitamin D3 (provided by GlaxoSmithKline). Participants were instructed to take two tablets daily, preferably in divided doses with meals. Women in both the active supplement and placebo groups were allowed to continue their own open-label use of calcium and vitamin D supplements as long as non-study use of vitamin D did not exceed 600 IU daily. The upper limit of non-study vitamin D intake was raised to 1000 IU after the Institute of Medicine released its report on the tolerable upper limits of vitamin D intake.29 Adherence to study medication was assessed by weighing returned bottles.
Blood pressure measurement and ascertainment of hypertension
Blood pressure was measured by certified staff using standardized procedures and instruments, in the right arm, with a conventional mercury sphygmomanometer and an appropriately sized cuff, after the participant was seated and resting for 5 minutes.30 Two measurements, obtained at least 30 seconds apart, were performed at the WHI enrollment visit and at each subsequent annual visit, including the CaD enrollment visit. The average of the two measurements was used for analyses. At enrollment, participants were asked whether they had been diagnosed by a physician with high blood pressure or hypertension and if they were taking medications for hypertension. Then, at each semi-annual contact, participants were asked, “Since the date given on the front of this form, has a doctor prescribed any of the following pills or treatments?” The choices included “pills for hypertension.” Medication inventories were conducted at WHI enrollment, and at the first, 3rd, 6th and 9th annual visits. The product or generic name of the medications on the label was entered into the study database and matched to the corresponding item in a pharmacy database: (Master Drug Data Base [MDDB: Medi-Span, Indianapolis, IN]). Drugs from the following classes were considered to be antihypertensive agents: angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, β-blockers, calcium channel blockers, diuretics, centrally acting antihypertensive agents, vasodilators, and combinations of these medications.
At enrollment in the WHI study, 94% of women with self-reported hypertension treatment had an antihypertensive agent in the baseline drug inventory, and 79% with incident self-reported hypertension treatment during the first year of the trial brought an antihypertensive medication to the year 1 drug inventory.
Covariates
Demographic and health history data were self-reported at WHI baseline. Dietary data were collected using a validated food frequency questionnaire.31 Total calcium and vitamin D intakes included both dietary and supplement sources determined from the medication and supplement inventory. Metabolic equivalent task (MET) scores were calculated from the frequency and duration of recreational physical activity.32 Baseline 25-hydroxyvitamin D levels were measured using the DiaSorin Liaison chemiluminescent immunoassay system (Stillwater, MN) in a subset of CaD trial participants as part of nested case-control studies examining fractures, breast cancer and colorectal cancer.27, 28 Only participants selected as controls were included in these biomarker analyses (n=2029).
Statistical methods
Of 36,282 post-menopausal women enrolled in the CaD trial, 36,189 had either a systolic or diastolic BP measurement at randomization. The primary outcome measure was blood pressure change (annual blood pressure measurements collected through 7 years of follow-up minus the blood pressure at the CaD randomization visit. All participants with at least one blood pressure change measurement were included in the intent-to-treat analysis using linear repeated measures regression modeling with an unstructured covariance matrix (using SAS PROC MIXED ver 9.1; SAS Institute, Cary, NC). Repeated measures regression allows for correlations among responses within an individual and allows for missing response data. This procedure does not impute missing data or carry the last observation forward, but allows the inclusion of available data from individuals with missing responses by using a maximum likelihood approach that gives valid results under missing at random assumptions. Plots of longitudinal data are based on fitted means from these models where both treatment assignment and time are modeled as class variables and treatment effect is allowed to vary over time (saturated model).
The effect of treatment on incident hypertension was examined using Cox proportional hazards models stratified by age and randomization status for other WHI trials. Incident hypertension was defined as self-report of medication prescribed for hypertension or any BP ≥ 140/90 mm Hg during 7 years of follow-up among 17,122 women who did not have hypertension at CaD enrollment (no self-report of hypertension treatment, no antihypertensive medications in inventory, and BP at all visits < 140/90 mm Hg prior to randomization.) We also examined the effect of treatment on incident pre-hypertension (at least one visit BP 120-139/80-89 mm Hg with no self-report of medication prescribed for hypertension) or hypertension during follow-up among 9416 normotensive women at CaD enrollment (no self-report of hypertension treatment, no antihypertensive medications in inventory, and BP < 120/80 mm Hg at all pre-randomization visits). For all analyses of incident hypertension, women were considered at risk from entry into the Calcium/Vitamin D trial until the date at which hypertension was determined or until the last date during the follow-up period that outcome data were available. Women who were normotensive at CaD enrollment were considered at risk for pre-hypertension or hypertension from date of entry into the Calcium/Vitamin D trial until the first annual visit with BP greater than or equal to120/80 mm Hg, a self-report of medication prescribed for hypertension, or the last date that outcome data were available.
To examine the effect of non-adherence to the active CaD supplements or placebo, sensitivity analyses were conducted in which participants were censored after their first visit at which non-adherence was detected, defined as use of less than 80 percent of the study pills.
To assess whether the effect of CaD supplementation on blood pressure and incident hypertension varied according to baseline risk factors, the same models were extended and formal tests of interactions were performed. These factors included demographic characteristics, other risk factors for hypertension (baseline blood pressure, presence of hypertension, body mass index, physical activity), baseline intake of sodium, calcium and vitamin D, and randomization status in the HT and DM trials. Because 16 interactions with baseline characteristics were investigated, chance alone would be expected to produce approximately one statistically significant interaction test at the 0.05 level of significance.
Results
Baseline characteristics, retention and adherence
Between 1995 and 2000, 36,282 women were randomized into the CaD trial: 18,176 were assigned to the active calcium + vitamin D supplementation, and 18,106 to placebo. Mean (SD) follow-up time was 7.0 (1.4) years. Participant characteristics, described in detail in Table 1, did not differ by CaD treatment assignment. The mean age at enrollment in WHI of women included in this study was 62±7 years; 83% described themselves as non-Hispanic white, 9% Black, 4% Hispanic, <1% American Indian, and 2% Asian/Pacific Islander. Mean BP was 126/75 mm Hg, and 46% of the participants had evidence of hypertension (28% with self-reported antihypertensive treatment and 18% with at least one visit BP ≥140/90 mm Hg).
Table 1.
Characteristics of the participants in the Calcium and Vitamin D Trial (n=36282) at time of WHI screening, according to randomly assigned group
| No. (%) |
|||
|---|---|---|---|
| Characteristic | Calcium/Vitamin D (n=18176) |
Placebo (n=18106) |
P-value* |
| Age at screening, years | |||
| 50-59 | 6726 (37.00) | 6696 (36.98) | 0.99 |
| 60-69 | 8276 (45.53) | 8243 (45.53) | |
| 70-79 | 3174 (17.46) | 3167 (17.49) | |
| Race/ethnicity | |||
| White | 15047 (82.78) | 15106 (83.43) | 0.35 |
| Black | 1682 (9.25) | 1635 (9.03) | |
| Hispanic | 789 (4.34) | 718 (3.97) | |
| Asian or Pacific Islander | 369 (2.03) | 353 (1.95) | |
| Other/Unknown | 289 (1.59) | 294 (1.62) | |
| Education | |||
| High school diploma or less | 4286 (23.74) | 4289 (23.84) | 0.94 |
| Some school after high school | 7216 (39.96) | 7156 (39.78) | |
| College degree or higher | 6555 (36.30) | 6543 (36.37) | |
| Blood pressure, mm Hg† | |||
| <120/80 | 6283 (34.70) | 6271 (34.74) | 0.41 |
| 120-139/80-89 | 7987 (44.11) | 7851 (43.50) | |
| 140-159/90-99 | 3105 (17.15) | 3209 (17.78) | |
| ≥160/100 | 730 (4.03) | 718 (3.98) | |
| Hypertensive status† | |||
| Not hypertensive | 9845 (54.16) | 9768 (53.95) | 0.47 |
| Treated with medications for hypertension (self-report) | 5137 (28.26) | 5068 (27.99) | |
| Blood pressure ≥140/90, not being treated | 3194 (17.57) | 3270 (18.06) | |
| Body mass index (BMI), kg/m2 † | |||
| <25 | 4974 (27.57) | 5117 (28.47) | 0.16 |
| 25 - <30 | 6409 (35.52) | 6327 (35.20) | |
| ≥30 | 6658 (36.90) | 6531 (36.33) | |
| Physical activity, MET hrs/week | |||
| <3.00 | 5517 (33.34) | 5478 (33.30) | 0.84 |
| 3.00 - <11.75 | 5463 (33.02) | 5477 (33.30) | |
| ≥11.75 | 5566 (33.64) | 5493 (33.40) | |
| Smoking | |||
| Never smoked | 9325 (51.85) | 9428 (52.62) | 0.31 |
| Past smoker | 7255 (40.34) | 7133 (39.81) | |
| Current Smoker | 1405 (7.81) | 1356 (7.57) | |
| Alcohol intake, drinks/wk | |||
| None or < 1 | 11447 (63.45) | 11378 (63.27) | 0.90 |
| 1 - 6 | 4683 (25.96) | 4706 (26.17) | |
| 7 or more | 1910 (10.59) | 1900 (10.56) | |
| Total calcium intake, mg/day‡ | |||
| <600 | 3554 (19.94) | 3447 (19.42) | 0.47 |
| 600 - 799 | 2550 (14.31) | 2556 (14.40) | |
| 800 - 1199 | 4715 (26.46) | 4655 (26.22) | |
| ≥ 1200 | 7002 (39.29) | 7095 (39.97) | |
| Dietary calcium intake, mg/day | |||
| <400 | 2406 (13.50) | 2378 (13.39) | 0.84 |
| 400 - 599 | 3847 (21.59) | 3776 (21.27) | |
| 600 - 1199 | 8556 (48.01) | 8558 (48.21) | |
| ≥ 1200 | 3012 (16.90) | 3041 (17.13) | |
| Total vitamin D intake, IU/day‡ | |||
| < 200 | 6827 (38.31) | 6671 (37.58) | 0.35 |
| 200 - 399 | 3379 (18.96) | 3423 (19.28) | |
| ≥ 400 | 7615 (42.73) | 7659 (43.14) | |
| Dietary vitamin D, IU/day | |||
| < 200 | 12327 (69.17) | 12243 (68.96) | 0.67 |
| ≥ 200 | 5494 (30.83) | 5510 (31.04) | |
| Dietary sodium intake, mg/day | |||
| < 2040 | 4456 (25.00) | 4438 (25.00) | 0.60 |
| 2040 - 2697 | 4507 (25.29) | 4386 (24.71) | |
| 2698 - 3250 | 4433 (24.88) | 4461 (25.13) | |
| ≥ 3521 | 4425 (24.83) | 4468 (25.17) | |
| 25 Hydroxyvitamin D level, nmol/liter§ |
|||
| < 34.4 | 254 (25.17) | 253 (24.80) | 0.80 |
| 34.4 – 47.6 | 250 (24.78) | 262 (25.69) | |
| 47.7 - 64.6 | 246 (24.38) | 260 (25.49) | |
| ≥ 64.7 | 259 (25.67) | 245 (24.02) | |
| Diet modification trial assignment | |||
| Not randomized | 5582 (30.71) | 5490 (30.32) | 0.30 |
| Intervention | 4767 (26.23) | 4878 (26.94) | |
| Comparison | 7827 (43.06) | 7738 (42.74) | |
| Hormone therapy trial assignment | |||
| Not randomized | 10122 (55.69) | 10071 (55.62) | 0.80 |
| Estrogen+Progestin active | 2508 (13.80) | 2535 (14.00) | |
| Estrogen+Progestin placebo | 2475 (13.62) | 2395 (13.23) | |
| Estrogen-alone active | 1531 (8.42) | 1543 (8.52) | |
| Estrogen-alone placebo | 1540 (8.47) | 1562 (8.63) | |
Abbreviations: BMI, body mass index (weight in kilograms divided by the square of height in meters); MET, metabolic equivalent.
From χ2 test of association.
At randomization into the Calcium/Vitamin D trial.
From diet and supplements.
Limited to Calcium/Vitamin D trial participants with available measurements, who were controls in nested case-control studies examining fractures, breast cancer and colorectal cancer.
Mean (SD) dietary calcium intake was 825 (438) mg/day, total calcium intake was 1150 (656) mg/day, dietary vitamin D intake was 175 (117) IU/day and mean total vitamin D intake was 367 (266) IU/day. The mean intake of dairy products was 1.5 servings/day and 52% of the participants reported use of calcium supplements. At CaD enrollment 40% of women met the current recommendation for 1200 mg /day of calcium intake from supplements and diet combined.
At the termination of the trial, 1551 participants (4.3%) had died and 2.7% percent had withdrawn or been lost to follow-up. In Year 1, the proportion taking 80% or more of study medication was 60% overall and remained stable through year 7, ranging between 56% and 63%, with small differences between treatment groups. The mean dose of open-label supplemental calcium increased by less than 100 mg/day during the trial from 325 mg/day at enrollment and was similar across both treatment groups.
Blood pressure change
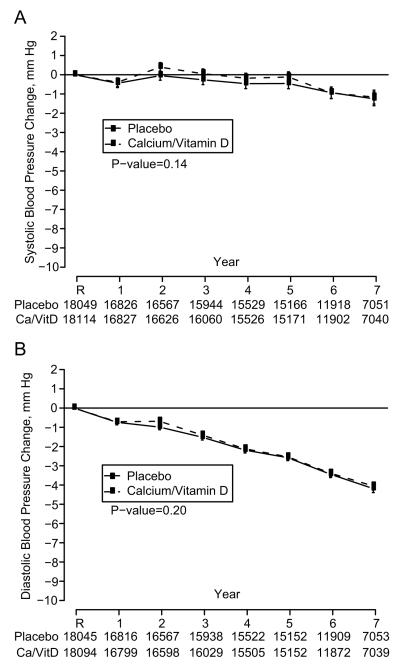
From baseline to the end of follow-up, systolic BP declined by about 1 mm Hg during follow-up, while diastolic BP declined by about 4 mm Hg. There was no difference in the change in either systolic BP (Figure 1a) or diastolic BP (Figure 1b) between women randomized to the active vs. placebo CaD supplements. In sensitivity analyses accounting for non-adherence, the results were similar (data not shown). The overall mean difference in change in systolic BP was 0.22 mm Hg (95% CI −0.05 – 0.49 mm Hg) and in diastolic BP was 0.11 mm Hg (95% CI −0.04 – 0.27 mm Hg), with a positive mean difference indicating that BP was lowered less in the active group than the placebo group, although differences were not significant (Table 2).
Figure 1.
Systolic blood pressure change (A) and diastolic blood pressure change (B) by calcium/vitamin D treatment assignment in 36,189 participants with measured blood pressure at randomization. R indicates randomization into the trial. P-values are for main effect of randomization assignment.
Table 2.
Effect of Calcium plus Vitamin D on Mean Difference (95% CI) in blood pressure change over the course of the trial overall and in subgroups for 36,189 participants with measured blood pressures at randomization.
| Mean Difference in Blood Pressure Change over Follow-up: Overall and by Baseline Subgroups* | ||||||||
|---|---|---|---|---|---|---|---|---|
| Systolic Blood Pressure | Diastolic Blood Pressure | |||||||
| Overall | Mean Difference |
Lower | Upper | P-value† | Mean Difference |
Lower | Upper | P-value† |
| Overall effect of Calcium/Vitamin D | 0.22 | −0.05 | 0.49 | 0.11‡ | 0.11 | −0.04 | 0.27 | 0.14‡ |
| Subgroup | ||||||||
| Age at screening, years | 0.95 | 0.58 | ||||||
| 50-59 | 0.22 | −0.22 | 0.66 | 0.10 | −0.15 | 0.36 | ||
| 60-69 | 0.18 | −0.21 | 0.58 | 0.06 | −0.17 | 0.28 | ||
| 70-79 | 0.31 | −0.33 | 0.95 | 0.29 | −0.08 | 0.66 | ||
| Ethnicity | 0.79 | 0.67 | ||||||
| White | 0.17 | −0.12 | 0.46 | 0.10 | −0.06 | 0.27 | ||
| Black | 0.46 | −0.44 | 1.35 | 0.07 | −0.45 | 0.58 | ||
| Hispanic | 0.97 | −0.37 | 2.30 | 0.22 | −0.55 | 0.98 | ||
| Asian or Pacific Islander | 0.20 | −1.68 | 2.09 | 0.85 | −0.23 | 1.94 | ||
| Other/Unknown | −0.13 | −2.25 | 2.00 | −0.30 | −1.52 | 0.93 | ||
| Education | 0.48 | 0.18 | ||||||
| High school diploma or less | 0.50 | −0.05 | 1.05 | 0.33 | 0.01 | 0.64 | ||
| Some school after high school | 0.20 | −0.23 | 0.62 | 0.14 | −0.11 | 0.39 | ||
| College degree or higher | 0.07 | −0.37 | 0.51 | −0.05 | −0.31 | 0.20 | ||
| Blood pressure, mm Hg§ | 0.21 | 0.28 | ||||||
| <120/80 | −0.02 | −0.40 | 0.36 | 0.13 | −0.11 | 0.37 | ||
| 120-139/80-89 | 0.08 | −0.26 | 0.42 | −0.03 | −0.25 | 0.18 | ||
| ≥ 140/90 | 0.52 | 0.03 | 1.01 | 0.26 | −0.05 | 0.57 | ||
| Hypertensive at enrollment | 0.09 | 0.18 | ||||||
| No | −0.01 | −0.37 | 0.34 | 0.01 | −0.20 | 0.21 | ||
| Yes∥ | 0.45 | 0.06 | 0.83 | 0.22 | −0.01 | 0.44 | ||
| BMI, kg/m2§ | 0.73 | 0.91 | ||||||
| <25 | 0.31 | −0.20 | 0.81 | 0.06 | −0.23 | 0.35 | ||
| 25 - <30 | 0.07 | −0.38 | 0.52 | 0.15 | −0.11 | 0.40 | ||
| ≥ 30 | 0.29 | −0.15 | 0.74 | 0.12 | −0.14 | 0.37 | ||
| Physical activity, MET hours/week | 0.26 | 0.68 | ||||||
| < 3.00 | 0.61 | 0.12 | 1.10 | 0.26 | −0.02 | 0.55 | ||
| 3.00 - < 11.75 | 0.03 | −0.46 | 0.52 | 0.09 | −0.19 | 0.37 | ||
| ≥ 11.75 | 0.30 | −0.18 | 0.79 | 0.14 | −0.14 | 0.42 | ||
| Total calcium intake, mg/day¶ | 0.62 | 0.80 | ||||||
| < 600 | −0.11 | −0.73 | 0.50 | −0.01 | −0.37 | 0.34 | ||
| 600-799 | 0.24 | −0.47 | 0.95 | 0.26 | −0.15 | 0.67 | ||
| 800-1199 | 0.23 | −0.29 | 0.76 | 0.12 | −0.18 | 0.42 | ||
| ≥ 1200 | 0.39 | −0.03 | 0.82 | 0.09 | −0.16 | 0.33 | ||
| Dietary calcium, mg/day | 0.53 | 0.96 | ||||||
| < 400 | −0.03 | −0.77 | 0.71 | 0.08 | −0.35 | 0.50 | ||
| 400-599 | 0.53 | −0.05 | 1.12 | 0.07 | −0.27 | 0.40 | ||
| 600-1199 | 0.11 | −0.28 | 0.49 | 0.09 | −0.13 | 0.31 | ||
| ≥ 1200 | 0.41 | −0.24 | 1.07 | 0.19 | −0.19 | 0.56 | ||
| Total vitamin D intake, IU/day¶ | 0.15 | 0.65 | ||||||
| <200 | 0.21 | −0.23 | 0.65 | 0.05 | −0.21 | 0.30 | ||
| 200 - <400 | 0.75 | 0.13 | 1.37 | 0.25 | −0.11 | 0.60 | ||
| ≥400 | 0.02 | −0.39 | 0.43 | 0.08 | −0.15 | 0.32 | ||
| Dietary vitamin D, IU/day | 0.10 | 0.11 | ||||||
| <200 | 0.08 | −0.25 | 0.40 | 0.02 | −0.17 | 0.20 | ||
| ≥200 | 0.57 | 0.09 | 1.06 | 0.29 | 0.01 | 0.57 | ||
| Dietary sodium intake, mg/day | 0.46 | 0.98 | ||||||
| < 2040 | 0.02 | −0.52 | 0.56 | 0.10 | −0.21 | 0.42 | ||
| 2040 - < 2698 | 0.44 | −0.10 | 0.98 | 0.16 | −0.15 | 0.47 | ||
| 2698 - < 3521 | 0.00 | −0.54 | 0.54 | 0.08 | −0.23 | 0.39 | ||
| ≥ 3521 | 0.46 | −0.08 | 1.00 | 0.05 | −0.26 | 0.36 | ||
| 25 Hydroxyvitamin D level, nmol/liter# | 0.82 | 0.26 | ||||||
| < 34.4 | −0.29 | −2.64 | 2.05 | 0.28 | −1.02 | 1.57 | ||
| 34.4 - 47.6 | −1.57 | −3.89 | 0.74 | −1.43 | −2.71 | −0.16 | ||
| 47.7 - 64.6 | −0.22 | −2.56 | 2.12 | −0.04 | −1.33 | 1.25 | ||
| ≥ 64.7 | −1.10 | −3.43 | 1.23 | −0.14 | −1.42 | 1.14 | ||
| Diet modification trial assignment | 0.13 | 0.32 | ||||||
| Intervention | 0.58 | 0.07 | 1.10 | 0.30 | −0.00 | 0.59 | ||
| Comparison | −0.08 | −0.48 | 0.33 | 0.00 | −0.23 | 0.24 | ||
| Hormone therapy trial assignment | 0.16 | 0.05 | ||||||
| Estrogen + Progestin active | 1.07 | 0.34 | 1.80 | 0.34 | −0.07 | 0.76 | ||
| Estrogen + Progestin placebo | 0.14 | −0.60 | 0.88 | 0.44 | 0.02 | 0.86 | ||
| Estrogen-alone active | −0.66 | −1.60 | 0.28 | −0.21 | −0.75 | 0.32 | ||
| Estrogen-alone placebo | 0.33 | −0.61 | 1.26 | 0.32 | −0.21 | 0.86 | ||
Abbreviations: BMI, body mass index (weight in kilograms divided by the square of height
Positive mean differences between treatment groups indicate that BP was lowered less in the Calcium/Vitamin D group than in the placebo group during follow-up.
Test of interaction between Calcium/Vitamin D assignment and variable of interest, based on linear repeated measures model with unstructured correlation matrix.
Test of main effect of Calcium/Vitamin D assignment, based on linear repeated measures model with unstructured correlation matrix.
At randomization into the Calcium/Vitamin D trial.
Includes women who self-reported treatment for hypertension or who had at least one BP ≥ 140/90 mm Hg up to the time of Calcium/Vitamin D randomization.
From diet and supplements
Limited to Calcium/Vitamin D trial participants with available measurements, who were controls in nested case-control studies examining fractures, breast cancer, and colorectal cancer.
There were no subgroups based on demographic characteristics, hypertension risk factors, calcium/vitamin D intake, or measured serum 25-hydroxy vitamin D who appeared to derive BP benefits from the supplements (Table 2). In addition to our a priori subgroups of approximate tertiles of baseline calcium intake (data not shown), we further subdivided the lowest tertile of the level of intake to attempt to identify a group with extremely low intake (<400 mg/day of dietary calcium [14th percentile] and <600 mg/day of total calcium [20th percentile]) who might benefit from the supplements. In each subgroup the mean BP differences were very close to zero, and none of the tertile or post hoc subgroups showed statistically significant treatment group differences either individually or overall. There were also no differences in BP between treatment groups in the subgroup of women with joint intake of dietary calcium <400 IU/day and total Vitamin D <200 IU/day (data not shown).
Incident hypertension and pre-hypertension
Over a mean follow-up time of 7 years, of 17,122 initially non-hypertensive women, 4429 (2131 assigned to active CaD, 2098 assigned to placebo) reported being prescribed medication for hypertension. Including participants who developed BP ≥ 140/90 mm Hg in the definition, 6692 women (3377 assigned to active CaD, 3315 assigned to placebo) developed incident hypertension. The risk of hypertension did not differ by calcium/vitamin D treatment assignment (Figure 2). The hazard ratio (HR) for incident hypertension, defined as newly prescribed antihypertensive medication or elevated measured blood pressure, was 1.01 (95% CI 0.96 – 1.06.) In the subsample of participants with measured 25-hydroxy vitamin D, those with lower levels had a higher risk of incident hypertension. No other subgroup interactions were observed.
Figure 2.
Effect of Calcium and Vitamin D assignment on risk of developing hypertension in 17,122 participants without hypertension during an average of 7 years follow-up, overall and by baseline subgroups. Hypertension defined as first self-report of medication prescribed for hypertension or BP of ≥ 140/90. (see attached PDF)
Among 9416 normotensive women, pre-hypertension or hypertension developed during follow-up in 6636. Treatment with calcium/vitamin D did not lower the risk of incident pre-hypertension or hypertension either overall or in any subgroup (HR 1.01, 95% CI 0.97 – 1.06.), (data not shown).
Discussion
Calcium plus vitamin D3 supplementation (1000 mg plus 400 IU daily, respectively) did not reduce BP over seven years of follow-up or the risk of developing pre-hypertension or hypertension in the WHI CaD randomized, placebo-controlled trial. This null result was robust in intention-to-treat analyses, analyses among adherent participants, and subgroup analyses. With the hazard ratio for incident hypertension of 1.01, comparing supplementation to placebo, and a narrow 95% confidence interval (0.96 –1.06), a clinically significant benefit for this calcium supplementation with regard to lowering BP or preventing hypertension is unlikely among generally healthy postmenopausal women.
A recent meta-analysis by van Mierlo, et al of randomized controlled trials of calcium supplementation or dietary intervention with a duration of 2 weeks or more included 40 trials and 2492 non-pregnant adults.10 The duration ranged from 3 to 208 weeks (median of 9.5 weeks) and daily calcium dose from 335 to 2000 mg (median 1055 mg.) The weighted estimate of the effect of calcium supplementation on BP was −2 mm Hg for systolic BP and −1 mm Hg for diastolic BP. The effect on systolic BP (−3 mm Hg) was slightly larger in populations with calcium intake ≤ 800 mg/d. The BP lowering effect was substantially larger (−10/5 mm Hg) in four trials in Asian populations, comprising 154 subjects, mostly with habitual calcium intakes <600 mg/day. A second recent meta-analysis included only randomized controlled trials of duration 8 weeks or longer in non-pregnant adults with BP ≥ 140/85 mm Hg. In this study of 13 trials that enrolled 484 subjects, BP lowering effect of calcium supplements was similar to that observed in the meta-analysis by van Mierlo, et al: −2.5 mm Hg for systolic BP and −0.8 mm Hg for diastolic BP.12
Several authors have argued that previous clinical trials and meta-analyses masked considerable heterogeneity of effect of blood pressure response to calcium supplementation.22, 33 The present study did not detect any heterogeneity of effect by age, ethnicity, baseline BP, sodium intake or calcium/vitamin D intake. The WHI CaD trial enrolled more than ten-fold the number of participants included in previous meta-analyses of randomized trials of calcium supplementation, and included large numbers of women in these potentially more responsive subgroups. The lower bound of the 95% CI overall and in each of these subgroups excludes a BP lowering effect of calcium supplementation of clinical or public health importance.
The WHI CaD trial intervention included supplementation with 400 IU vitamin D3. While data from large prospective studies has not shown that higher vitamin D intake lowered the incidence of hypertension, participants in these studies with plasma levels of 25-hydroxy vitamin D in the deficient range had an increase in the risk of incident hypertension (6-fold in men and 3-fold in women.)16 In the present study, we did not find evidence of lower BP in women who received the active vitamin D3-containing supplement in the lowest quartile of measured 25-hydroxy vitamin D, which fell into the range generally considered deficient. In fact, there was a trend toward increased risk of hypertension with supplementation in women with lower vitamin D levels. This was not statistically significant for hypertension defined only by antihypertensive treatment prescription. Due to the number of subgroup interactions we examined, this finding may be due to chance.
There are a number of potential limitations to this study. First, only postmenopausal women were included, so our results may not apply to men or younger women, although there is little basis to believe that these populations would respond differently to calcium supplementation.10 Second, non-adherence may have biased results toward the null; however, sensitivity analyses accounting for this suggested that the study result was robust. Third, the baseline level of dietary calcium intake was higher in the WHI CaD Trial than average for older women in the U.S (825 mg/day vs. 660 mg/day), and many women took supplemental calcium and vitamin D at baseline and as open-label supplements during the trial. However, because of the large size of the study, subgroup analyses included substantial numbers of participants with low intake of these nutrients, and a clinically important BP effect was also excluded in these subgroups. Fourth, different results might have been observed with a different formulation or dose of calcium, or a higher dose of vitamin D3. The dose of 400 IU of vitamin D in particular has been questioned as being inadequate to reduce the incidence of hip fracture and colorectal cancer, the main endpoints of the trial.27,28 The lack of any degree of BP lowering even in subgroups with low intakes of calcium or vitamin D, or in women with low vitamin D levels argues against this possibility, however. In addition, meta-analyses have not shown differences in BP lowering with higher (>1000-1200mg/day) vs. lower doses of calcium supplements.34,35 Finally, it is possible that combining calcium with other nutrients or other minerals or simultaneous dietary intervention focused on calcium and vitamin D rich foods might have led to different results. However, a meta-analysis of 3 trials of combinations of calcium, magnesium and potassium supplementation in 277 participants did not find a significant effect on BP.36 While one meta-analysis suggested the possibility of a trend toward greater BP lowering effect of dietary vs. non-dietary provision of calcium,9 unequivocally effective dietary interventions to lower BP have generally included sodium restriction, and manipulation of multiple foods groups, macronutrients and minerals.11
This study also has important strengths. The large size of the study allowed us to examine subgroups that had been hypothesized to have potential for greater benefit: Blacks, Asians, hypertensives, women with low habitual intake of calcium, and women with low serum levels of vitamin D. The study methodology was double-blind, and included adequate concealment of treatment assignment. The study was longer than any trial previously reported and there was little loss to follow-up.
In conclusion, in postmenopausal women over seven years of follow-up calcium plus vitamin D3 supplementation did not reduce either blood pressure or the risk of developing hypertension. There was no subgroup that appeared to benefit from calcium/vitamin D supplementation, and the precision of this study excludes a BP lowering effect of calcium supplementation of clinical or public health importance.
Perspectives
Calcium supplementation, and to a lesser extent, Vitamin D supplementation have long been hypothesized to lower blood pressure and delay the onset of hypertension. The results of previous calcium supplementation trials have been mixed, but in general have shown a small blood pressure lowering benefit. The WHI Calcium-Vitamin D supplementation trial provides the most definitive answer to the question: no benefit was seen overall or in any subgroup of postmenopausal women. Although the dose of both supplements was modest, particularly the vitamin D, the lack of benefit in women with low intake of these nutrients or low levels of vitamin D argues against a different result had dosages been higher. Because the WHI trial did not include younger women or men, no firm conclusions can be drawn about the effect of calcium or vitamin D on blood pressure in these groups. This study also did not address the question of blood pressure lowering by dietary calcium intake or dairy foods, but it suggests that short-cuts with dietary supplements can not be substituted for encouraging people to adopt dietary patterns that have been shown to lower blood pressure and decrease the risk of hypertension.
Acknowledgments
Source of Funding
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221.
Please see http://hyper.ahajournals.org for a short list of the Women's Health Initiative Investigators.
The following is a short list of the Women's Health Initiative Investigators:
Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Elizabeth Nabel, Jacques Rossouw, Shari Ludlam, Linda Pottern, Joan McGowan, Leslie Ford, and Nancy Geller.
Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Ross Prentice, Garnet Anderson, Andrea LaCroix, Charles L. Kooperberg, Ruth E. Patterson, Anne McTiernan; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker; (Medical Research Labs, Highland Heights, KY) Evan Stein; (University of California at San Francisco, San Francisco, CA) Steven Cummings.
Clinical Centers: (Albert Einstein College of Medicine, Bronx, NY) Sylvia Wassertheil-Smoller; (Baylor College of Medicine, Houston, TX) Aleksandar Rajkovic; (Brigham and Women's Hospital, Harvard Medical School, Boston, MA) JoAnn Manson; Brown University, Providence, RI) Annlouise R. Assaf; (Emory University, Atlanta, GA) Lawrence Phillips; (Fred Hutchinson Cancer Research Center, Seattle, WA) Shirley Beresford; (George Washington University Medical Center, Washington, DC) Judith Hsia; (Los Angeles Biomedical Research Institute at Harbor- UCLA Medical Center, Torrance, CA) Rowan Chlebowski; (Kaiser Permanente Center for Health Research, Portland, OR) Evelyn Whitlock; (Kaiser Permanente Division of Research, Oakland, CA) Bette Caan; (Medical College of Wisconsin, Milwaukee, WI) Jane Morley Kotchen; (MedStar Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Northwestern University, Chicago/Evanston, IL) Linda Van Horn; (Rush Medical Center, Chicago, IL) Henry Black; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (State University of New York at Stony Brook, Stony Brook, NY) Dorothy Lane; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Alabama at Birmingham, Birmingham, AL) Cora E. Lewis; (University of Arizona, Tucson/Phoenix, AZ) Tamsen Bassford; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of California at Davis, Sacramento, CA) John Robbins; (University of California at Irvine, CA) F. Allan Hubbell; (University at California at Los Angeles, Los Angeles, CA) Howard Judd; (University of California at San Diego, LaJolla/Chula Vista, CA) Robert D. Langer; (University of Cincinnati, Cincinnati, OH) Margery Gass; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Hawaii, Honolulu, HI) David Curb; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Massachusetts/Fallon Clinic, Worcester, MA) Judith Ockene; (University of Medicine and Dentistry of New Jersey, Newark, NJ) Norman Lasser; (University of Miami, Miami, FL) Mary Jo O'Sullivan; (University of Minnesota, Minneapolis, MN) Karen Margolis; (University of Nevada, Reno, NV) Robert Brunner; (University of North Carolina, Chapel Hill, NC) Gerardo Heiss; (University of Pittsburg, Pittsburgh, PA) Lewis Kuller; (University of Tennessee, Memphis, TN) Karen C. Johnson; (University of Texas Health Science Center, San Antonio, TX) Robert Brzyski; (University of Wisconsin, Madison, WI) Gloria E. Sarto; (Wake Forest University School of Medicine, Winston-Salem, NC) Denise Bonds; (Wayne State University School of Medicine/Hutzel Hospital, Detroit, MI) Susan Hendrix.
Footnotes
Disclosures:
Karen L. Margolis None
Roberta M. Ray None
Linda Van Horn None
JoAnn E. Manson None
Matthew A. Allison None
Henry R. Black None
Shirley A.A. Beresford None
Stephanie A. Connelly None
J. David Curb None
Richard H. Grimm None
Theodore A. Kotchen None
Lewis H. Kuller None
Sylvia Wassertheil-Smoller None
Cynthia A. Thomson None
James C. Torner None
Contributor Information
Karen L. Margolis, HealthPartners Research Foundation, Minneapolis, MN.
Roberta M. Ray, Fred Hutchinson Cancer Research Center, Seattle, WA.
Linda Van Horn, Northwestern University, Chicago, IL.
JoAnn E. Manson, Brigham and Women's Hospital, Harvard Medical School, Boston, MA.
Matthew A. Allison, University of California San Diego, San Diego CA.
Henry R. Black, Rush Medical College, Chicago, IL.
Shirley A.A. Beresford, University of Washington, Seattle, WA.
Stephanie A. Connelly, University of Tennessee, Memphis, TN.
J. David Curb, University of Hawaii, Honolulu, HI.
Richard H. Grimm, Jr., Berman Center for Outcomes of Clinical Research, Minneapolis, MN.
Theodore A. Kotchen, Medical College of Wisconsin, Milwaukee, WI.
Lewis H. Kuller, University of Pittsburgh, Pittsburg, PA.
Sylvia Wassertheil-Smoller, Albert Einstein College of Medicine, New York, NY.
Cynthia A. Thomson, University of Arizona, Tuscon, AZ.
James C. Torner, University of Iowa, Iowa City, IA.
References
- 1.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutlet JA, Windhauser MM, Lin PH, Karania N, DASH Collaborative Research Group A clinical trial of the effects of dietary patterns on blood pressure. New England Journal of Medicine. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. see comment. [DOI] [PubMed] [Google Scholar]
- 2.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, Simons-Morton DG, Karania N, Lin PH, DASH-Sodium Collaborative Research Group Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. New England Journal of Medicine. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 3.Dauchet L, Kesse-Guyot E, Czernichow S, Bertrais S, Estaquio C, Peneau S, Vergnaud AC, Chat-Yung S, Castetbon K, Deschamps V, Brindel P, Hercberg S. Dietary patterns and blood pressure change over 5-y follow-up in the SU.VI.MAX cohort. Am J Clin Nutr. 2007;85:1650–1656. doi: 10.1093/ajcn/85.6.1650. [DOI] [PubMed] [Google Scholar]
- 4.Wang L, Manson JE, Buring JE, Lee IM, Sesso HD. Dietary Intake of Dairy Products, Calcium, and Vitamin D and the Risk of Hypertension in Middle-Aged and Older Women. Hypertension. 2008;51:1073–1079. doi: 10.1161/HYPERTENSIONAHA.107.107821. [DOI] [PubMed] [Google Scholar]
- 5.Cappuccio FP, Elliott P, Allender PS, Pryer J, Follman DA, Cutler JA. Epidemiologic association between dietary calcium intake and blood pressure: a meta-analysis of published data. Am J Epidemiol. 1995;142:935–945. doi: 10.1093/oxfordjournals.aje.a117741. [DOI] [PubMed] [Google Scholar]
- 6.Allender PS, Cutler JA, Follmann D, Cappuccio FP, Pryer J, Elliott P. Dietary calcium and blood pressure: a meta-analysis of randomized clinical trials. Ann Intern Med. 1996;124:825–831. doi: 10.7326/0003-4819-124-9-199605010-00007. [DOI] [PubMed] [Google Scholar]
- 7.Birkett NJ. Comments on a meta-analysis of the relation between dietary calcium intake and blood pressure. Am J Epidemiol. 1998;148:223–228. doi: 10.1093/oxfordjournals.aje.a009627. discussion 32-3. [DOI] [PubMed] [Google Scholar]
- 8.Bucher HC, Cook RJ, Guyatt GH, Lang JD, Cook DJ, Hatala R, Hunt DL. Effects of dietary calcium supplementation on blood pressure. A meta-analysis of randomized controlled trials. JAMA. 1996;275:1016–1022. doi: 10.1001/jama.1996.03530370054031. [DOI] [PubMed] [Google Scholar]
- 9.Griffith LE, Guyatt GH, Cook RJ, Bucher HC, Cook DJ. The influence of dietary and nondietary calcium supplementation on blood pressure: an updated metaanalysis of randomized controlled trials. Am J Hypertens. 1999;12:84–92. doi: 10.1016/s0895-7061(98)00224-6. [DOI] [PubMed] [Google Scholar]
- 10.van Mierlo LA, Arends LR, Streppel MT, Zeegers MP, Kok FJ, Grobbee DE, Geleijnse JM. Blood pressure response to calcium supplementation: a meta-analysis of randomized controlled trials. J Hum Hypertens. 2006;20:571–580. doi: 10.1038/sj.jhh.1002038. [DOI] [PubMed] [Google Scholar]
- 11.Dickinson HO, Mason JM, Nicolson DJ, Campbell F, Beyer FR, Cook JV, Williams B, Ford GA. Lifestyle interventions to reduce raised blood pressure: a systematic review of randomized controlled trials. J Hypertens. 2006;24:215–233. doi: 10.1097/01.hjh.0000199800.72563.26. [DOI] [PubMed] [Google Scholar]
- 12.Dickinson HO, Nicolson DJ, Cook JV, Campbell F, Beyer FR, Ford GA, Mason J. Calcium supplementation for the management of primary hypertension in adults. Cochrane Database Syst Rev. 2006:CD004639. doi: 10.1002/14651858.CD004639.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Trumbo PR, Ellwood KC. Supplemental calcium and risk reduction of hypertension, pregnancy-induced hypertension, and preeclampsia: an evidence-based review by the US Food and Drug Administration. Nutr Rev. 2007;65:78–87. doi: 10.1111/j.1753-4887.2007.tb00284.x. [DOI] [PubMed] [Google Scholar]
- 14.Krause R, Buhring M, Hopfenmuller W, Holick MF, Sharma AM. Ultraviolet B and blood pressure. Lancet. 1998;352:709–710. doi: 10.1016/S0140-6736(05)60827-6. [DOI] [PubMed] [Google Scholar]
- 15.Pfeifer M, Begerow B, Minne HW, Nachtigall D, Hansen C. Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab. 2001;86:1633–1637. doi: 10.1210/jcem.86.4.7393. [DOI] [PubMed] [Google Scholar]
- 16.Forman JP, Giovannucci E, Holmes MD, Bischoff-Ferrari HA, Tworoger SS, Willett WC, Curhan GC. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49:1063–1069. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 17.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carthy EP, Yamashita W, Hsu A, Ooi BS. 1,25-Dihydroxyvitamin D3 and rat vascular smooth muscle cell growth. Hypertension. 1989;13:954–959. doi: 10.1161/01.hyp.13.6.954. [DOI] [PubMed] [Google Scholar]
- 19.Borges AC, Feres T, Vianna LM, Paiva TB. Cholecalciferol treatment restores the relaxant responses of spontaneously hypertensive rat arteries to bradykinin. Pathophysiology. 2002;8:263–268. doi: 10.1016/s0928-4680(02)00036-6. [DOI] [PubMed] [Google Scholar]
- 20.Feres T, Vianna LM, Paiva AC, Paiva TB. Effect of treatment with vitamin D3 on the responses of the duodenum of spontaneously hypertensive rats to bradykinin and to potassium. Br J Pharmacol. 1992;105:881–884. doi: 10.1111/j.1476-5381.1992.tb09072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCarron DA. Role of adequate dietary calcium intake in the prevention and management of salt-sensitive hypertension. Am J Clin Nutr. 1997;65:712S–716S. doi: 10.1093/ajcn/65.2.712S. [DOI] [PubMed] [Google Scholar]
- 22.Resnick LM. The role of dietary calcium in hypertension: a hierarchical overview. Am J Hypertens. 1999;12:99–112. doi: 10.1016/s0895-7061(98)00275-1. [DOI] [PubMed] [Google Scholar]
- 23.Geleijnse JM, Grobbee DE. Calcium intake and blood pressure: an update. J Cardiovasc Risk. 2000;7:23–29. doi: 10.1177/204748730000700105. [DOI] [PubMed] [Google Scholar]
- 24.Suter PM, Sierro C, Vetter W. Nutritional factors in the control of blood pressure and hypertension. Nutr Clin Care. 2002;5:9–19. doi: 10.1046/j.1523-5408.2002.00513.x. [DOI] [PubMed] [Google Scholar]
- 25.Hays J, Hunt J, Hubbell F, Anderson GL, Limacher M, Allen C, Rossouw JE. The Women's Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13:S18–S77. doi: 10.1016/s1047-2797(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 26.Jackson RD, Lacroix AZ, Cauley JA, Mcgowan J. The Women's Health Initiative Calcium-Vitamin D trial: Overview and Baseline characteristics of participants. Ann Epidemiol. 2003;13:S98–S106. doi: 10.1016/s1047-2797(03)00046-2. [DOI] [PubMed] [Google Scholar]
- 27.Jackson RD, LaCroix AZ, Gass M, Wallace B, Robbins J, Lewis CE, Bassford T, Beresford SA, Black HR, Blanchette P, Bonds DE, Brunner RL, Brzyski RG, Caan B, Cauley JA, Chlebowski RT, Cummings SR, Granek I, Hays J, Heiss G, Hendrix SL, Howard BV, Hsia J, Hubbell FA, Johnson KC, Judd H, Kotchen JM, Kuller LH, Langer RD, Lasser NL, Limacher MC, Ludlam S, Manson JE, Margolis KL, McGowan J, Ockene JK, O'Sullivan MJ, Phillips L, Prentice RL, Sarto GE, Stefanick ML, Van Horn L, Wactawski-Wende J, Whitlock E, Anderson GL, Assaf AR, Barad D. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669–683. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 28.Wactawski-Wende J, Kotchen JM, Anderson GL, Assaf AR, Brunner RL, O'Sullivan MJ, Margolis KL, Ockene JK, Phillips L, Pottern L, Prentice RL, Robbins J, Rohan TE, Sarto GE, Sharma S, Stefanick ML, Van Horn L, Wallace RB, Whitlock E, Bassford T, Beresford SA, Black HR, Bonds DE, Brzyski RG, Caan B, Chlebowski RT, Cochrane B, Garland C, Gass M, Hays J, Heiss G, Hendrix SL, Howard BV, Hsia J, Hubbell FA, Jackson RD, Johnson KC, Judd H, Kooperberg CL, Kuller LH, LaCroix AZ, Lane DS, Langer RD, Lasser NL, Lewis CE, Limacher MC, Manson JE. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354:684–696. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- 29.Dietary reference intakes for calcium, phosphorous, magnesium, vitamin D, and fluoride. National Academy Press; Washington, DC: 1997. Institute of Medicine Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. [Google Scholar]
- 30.Anderson GL, Manson J, Wallace R, Lund B, Hall D, Davis S, Shumaker S, Wang CY, Stein E, Prentice RL. Implementation of the Women's Health Initiative study design. Ann Epidemiol. 2003;13:S5–17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 31.Patterson R, Kristal A, Tinker L, Carter R, Bolton M, Agurs-Collins T. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–187. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 32.Ainsworth B, Haskell W, Leon A, Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Jr., Montoye HJ, Sallis JF, Jr., Paffenbarger RS., Jr. Compendium of physical activities: Classification of energy costs of human physical activities. Med Sci Sports and Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Zemel MB. Calcium modulation of hypertension and obesity: mechanisms and implications. J Am Coll Nutr. 2001;20:428S–435S. doi: 10.1080/07315724.2001.10719180. discussion 40S-42S. [DOI] [PubMed] [Google Scholar]
- 34.Dickinson HO, Nicolson DJ, Cook JV, et al. Calcium supplementation for the management of primary hypertension in adults. Cochrane Database Syst Rev. 2006;(2):CD004639. doi: 10.1002/14651858.CD004639.pub2. [DOI] [PubMed] [Google Scholar]
- 35.van Mierol LA, Arends LR, Streppel MT, et al. Blood Pressure response to calcium supplementation: a meta-analysis of randomized controlled trials. J Hum Hypertens. 2006 Aug;20(8):571–580. doi: 10.1038/sj.jhh.1002038. [DOI] [PubMed] [Google Scholar]
- 36.Beyer FR, Dickinson HO, Nicolson DJ, Ford GA, Mason J. Combined calcium, magnesium and potassium supplementation for the management of primary hypertension in adults. Cochrane Database Syst Rev. 2006;3:CD004805. doi: 10.1002/14651858.CD004805.pub2. [DOI] [PubMed] [Google Scholar]