Abstract
Bioamines, such as norepinephrine and serotonin are key neurotransmitters implicated in multiple physiological and pathological brain mechanisms. Evolutionarily, the bioaminergic neuromodulatory system is widely distributed throughout the brain and is among the earliest neurotransmitters to arise within the hindbrain. In both vertebrates and invertebrates, monoamines play a critical role in the control of respiration. In mammals, both norepinephrine and serotonin are involved in the maturation of the respiratory network, as well as in the neuromodulation of intrinsic and synaptic properties, that not only differentially alters the activity of individual respiratory neurons but also the activity of the network during normoxic and hypoxic conditions. Here, we review the basic noradrenergic and serotonergic pathways and their impact on the activity of the pre-Bötzinger Complex inspiratory neurons and network activity.
1- Introduction
Bioamines, such as norepinephrine (NE) and serotonin (5-HT) are involved in the maturation of mammalian neural network as well as in the modulation of its intrinsic and synaptic properties. By changing the synaptic and intrinsic properties of a rhythmogenic network, these neuromodulators, in turn alter the frequency and phasing of the motor patterns produced by a given neuronal circuit (for review see: Marder and Bucher, 2007; Doi and Ramirez, 2008). The respiratory network is no exception and, as neuromodulators, NE and 5-HT in particular, have multiple functions in controlling respiratory rhythmic activity.
The respiratory network has to be continuously active throughout life to insure survival. During this time, the neural network controlling breathing is under influence of multiple neuromodulators, among which, bioamines are the earliest neurotransmitters to arise in the brainstem. During life, bioamines are released in a state-dependent manner from different nuclei that participate in the control of vital functions and arousal and their influence is an integral part of the neural network that generates breathing (Mason et al., 2007).
The respiratory rhythm is thought to be generated by neural networks located within the ventral respiratory column and the parafacial respiratory group (pFRG) (Alheid et al., 2002; Feldman and Del Negro, 2006). Within the ventral respiratory column is the Bötzinger Complex (BötC) which primarily contains expiratory neurons and the pre-Bötzinger Complex (pre-BötC) that is critical for generating inspiratory activity (Smith et al., 1991; Ramirez et al., 1998).
Over the past twenty years, the use of in vitro preparations, the “en bloc” developed by Suzue (1984) as well as slices (Smith et al., 1991), has improved the understanding of the basic principles of the generation and modulation of the inspiratory rhythm. After discussing how the respiratory rhythm may be generated, we will discuss the role of NE and 5-HT in the modulation of the respiratory rhythm generation with emphasis on own data collected from slices preparation containing the pre-BötC that generates inspiratory breathing activity that we will compare with others in vivo and in vitro data. Then, we will focus on the cellular mechanisms involved in this neuromodulation. Finally, we will review the role of bioamines in pathologies affecting the control of breathing. In this review article we will not consider membrane properties of motoneurons and discuss motoneuronal activities as the monitored rhythmic activity from the respiratory rhythm generator.
2- Generation of the inspiratory like rhythm
The neural network underlying inspiratory rhythm generation is proposed to be located in the ventrolateral medulla so called, the pre-BötC (Smith et al., 1991; Ramirez et al., 1998). When isolated in a transverse brain-slice preparation, the pre-BötC generates inspiratory rhythmic activities, that resemble eupnea, sighs, and during hypoxia, the network generates fictive gasps Lieske et al. (2000, Fig. 2). Recent studies described an additional network for respiratory rhythm generation: the pFRG which is thought to constitute a dual oscillator for the respiratory rhythm generation (Feldman and Del Negro, 2006). The respiratory rhythm is thought to be generated by the close interaction of the pre-BötC and the pFRG (Ballanyi et al., 1999; Janczewski et al., 2002; Onimaru and Homma, 2003; Mellen et al., 2003; Feldman and Del Negro, 2006), although the role of the pFRG has been questioned in adult rats (Fortuna et al., 2008). The pFRG is located rostral to the pre-BötC. The pre-BötC and pFRG have heterogeneous populations of respiratory neurons and some of these have pacemaker properties that have been proposed to be essential for rhythmogenesis (Ramirez et al., 2004; Peña et al., 2004; Tryba et al., 2006, Fig. 1). However, others have proposed a different hypothesis regarding how the rhythm is generated that relies on emergent network properties (Del Negro and Hayes, 2008; Feldman and Del Negro, 2006). Both synaptic coupling and intrinsic bursting properties likely play a critical role in respiratory rhythmogenesis. In the case of fictive gasping rhythmogenesis, it appears that synaptically released serotonin plays a critical synaptic role in expression of pacemaker properties (Tryba et al. 2006).
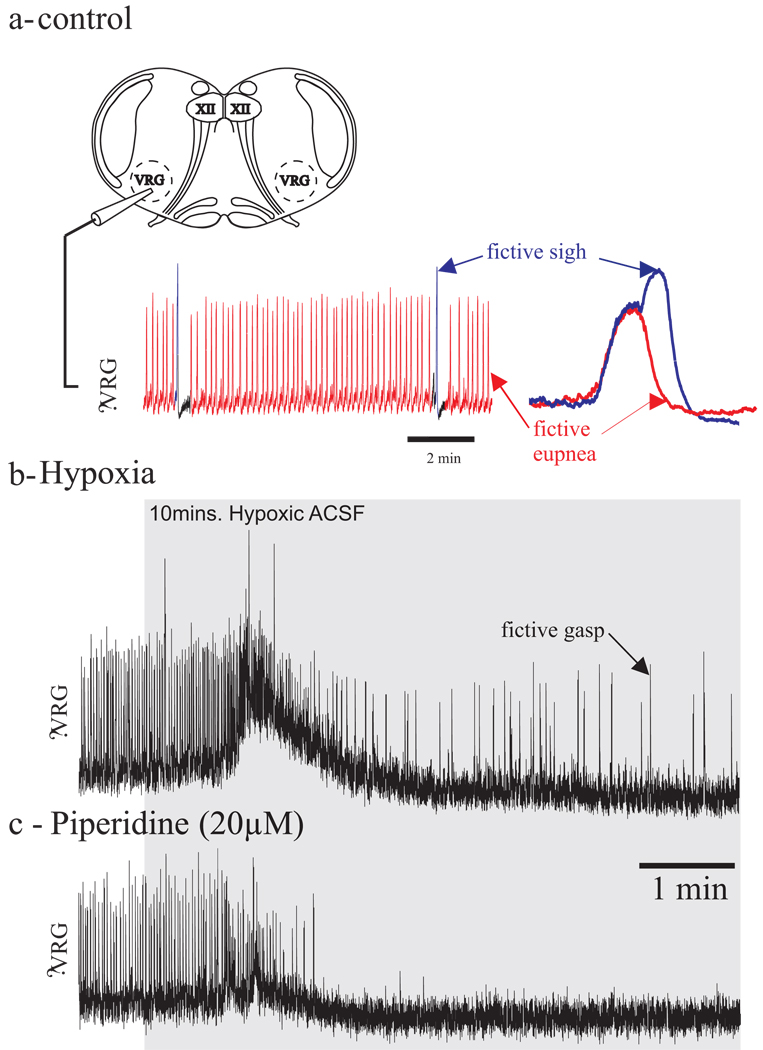
Figure 2. Gasping depends on activation of 5-HT2A serotonergic receptors.
1a- Schema of a transverse slice preparation containing the PreBötzinger Complex (650 µM thick slice, the potassium concentration raised at 8mM). Respiratory rhythmic activity is obtained with a suction electrode positioned on the surface of the slice in the area of the nucleus ambiguous (i.e., dorsal to the PBC).
The isolated respiratory network is capable of generating multiple rhythmic inspiratory-like population bursts under normoxic (slice bubbled with 95% O2-5% CO2) conditions that correspond to fictive eupnea (in red) and fictive sighs (in blue).
1b- Under hypoxic conditions (slice bubbled with 95% N2-5% CO2, in grey) the isolated respiratory network generates fictive gasps.
1c- Under hypoxic conditions (slice bubbled with 95% N2-5% CO2, in grey), blockade of 5-HT2A serotonergic receptors (piperidin) abolished gasping in vitro.
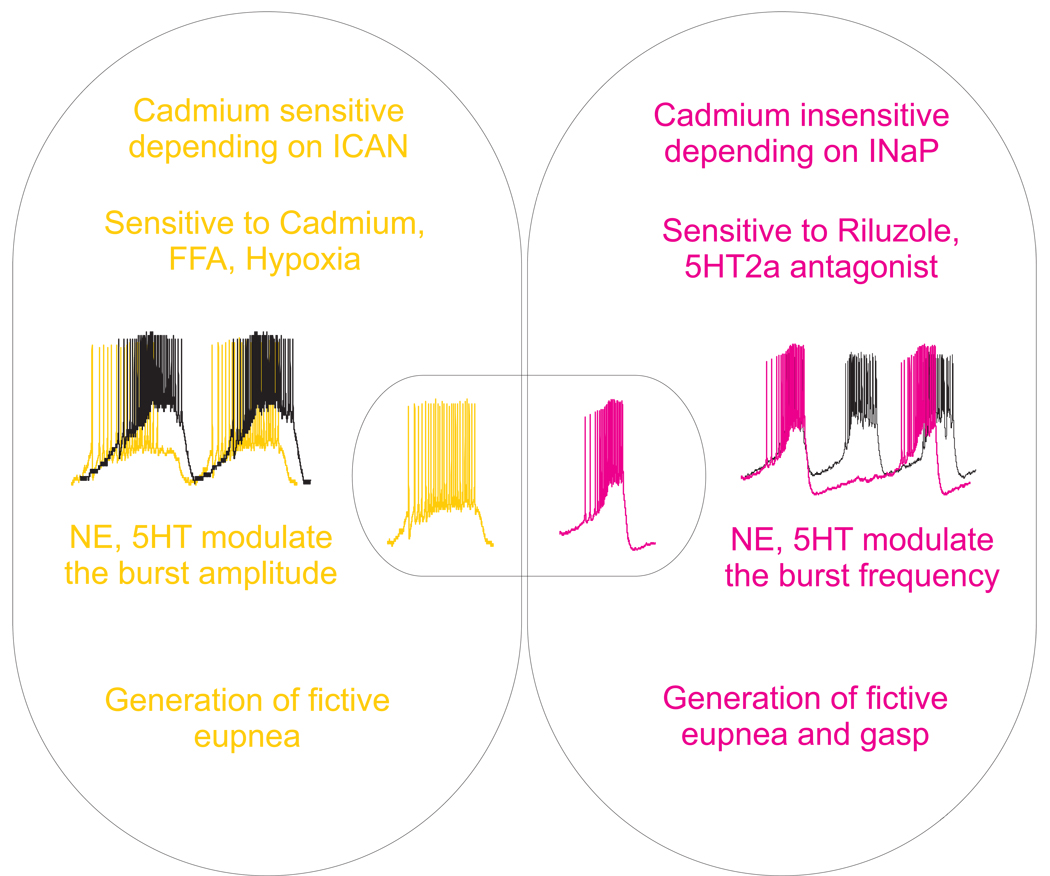
Figure 1. Modulation of pacemaker intrinsic properties.
In slices preparation containing the preBötC (650 µM thick slice, the potassium concentration raised at 8mM, bubbled with 95% O2-5% CO), two types of inspiratory neurons that express pacemaker properties have been identified. The bursting mechanism of one type depends on calcium-activated non-selective cation current and they are referred as “cadmium sensitive” (CS) pacemaker neurons (in yellow). The other type of pacemaker neurons have a bursting mechanism that depends on the persistent sodium current, these are so-called cadmium insensitive (CI) pacemaker neurons (in red). The two types of pacemaker neurons are proposed to play different roles in the modulation of the different respiratory like patterns. Bioamines such as, 5-HT and NE differentially modulate the different types of pacemaker neurons.
In slices preparation containing the pre-BötC, two types of inspiratory neurons that express pacemaker properties have been identified (Thoby-Brisson and Ramirez, 2001; Peña et al., 2004; Del Negro et al., 2005, Fig. 1). The bursting mechanism of one type depends on calcium-activated non-selective cation current (Peña et al., 2004; Del Negro et al., 2005; in yellow in Fig. 1) and they are referred as “cadmium sensitive” (CS) pacemaker neurons because bath applied cadmium (a non-specific calcium current blocker) abolishes their ability to burst. These pacemaker neurons are sensitive to the non-specific calcium current antagonist, flufenamic acid. The other type of pacemaker neurons have a bursting mechanism that depends on the persistent sodium current (Peña et al., 2004; Del Negro et al., 2005; in red in Fig. 1), these are so-called cadmium insensitive (CI) pacemaker neurons because they continue to burst after bath-application of cadmium. Their bursting mechanisms can be blocked by the persistent sodium current antagonist, riluzole. The inspiratory rhythm generator, as well as the different elements of the network such as pacemaker neurons, receive excitatory and inhibitory input from multiple sources containing NE and 5-HT.
3. Bioaminergic modulation of the respiratory network in vitro
The respiratory network is continuously modulated by endogenous bioamines that are required for its normal operation. Several studies suggest that endogenous NE and 5-HT are required for the maturation of the respiratory neuronal network (Bou-Flores et al., 2000; Viemari et al., 2004; Viemari, 2008). Nevertheless, Pet-1 null mice which lack ~ 70 % of all 5-HT neurons show normal gross anatomy of most brain structures (Hendricks et al., 2003). Similarly, Lmx1bf/f/p mice with near complete absence of central 5-HT neurons also reveals normal gross anatomy (Hodges et al., 2008) suggesting the presence of compensatory mechanisms. This may be due to the ability of other neurotransmitter systems (e.g. noradrenergic) to maintain normal respiratory activity in the absence of 5-HT (St-John and Leiter, 2007).
3.1 Noradrenergic modulation
In the brainstem of vertebrates there are 7 NE (A1–A7) and 3 adrenergic (C1–C3) groups. Of these, A6 (Locus coeruleus) is a well delineated cluster of NE neurons in the dorsal pons. It is estimated that 50 % of the NE projections in the CNS originate in the A6 which are directed toward the forebrain, cerebellum, brainstem and spinal cord (Aston-Jones et al., 1995). Anatomical studies have established reciprocal projections between the major groups of 5-HT and NE neurons in the brain (Aston-Jones et al., 1991). The physiological importance of such connections is evidenced by alterations in neuronal activity in lesion experiments. When 5-HT neurons are lesioned, the firing rate of A6 neurons is enhanced in a sustained fashion by about 70%, as is the case with 5-HT synthesis inhibition (Dremencov et al., 2007). When NE neurons are lesioned, dorsal raphe 5-HT neurons discharge erratically at a low rate (Svensson et al., 1975). Although it is difficult to investigate the respective role of both neuromodulators in vivo, the use of in vitro preparation has facilitated the understanding of the bioaminergic neuromodulation of the respiratory network. This experimental approach can be used to establish the net excitatory and/or inhibitory nature of a specific neurotransmitter at the post-synaptic level.
3.1.1. NE modulation of the respiratory rhythm generator
A5 and A6 pontine nuclei continuously modulate the respiratory rhythm generator in vitro (Hilaire et al., 2004) as well as in vivo (Guyenet et al., 1993). Recent in vitro experiments using slices and en bloc preparations also suggest that medullary catecholaminergic neurons (A1/C1) also contribute to respiratory rhythmogenesis. Local application of yohimbine (an alpha 2 noradrenergic receptors antagonist) and lesion experiments showed that A1/C1 groups are involved in the yohimbine depressing effect of the respiratory activity in vitro (Zanella et al., 2006). These results suggest that A1/C1 groups modulate the respiratory frequency in vitro but they do not rule out the possibility that A2/C2 neurons play a role in the stabilization of the rhythm, since they are affected in Mecp2 mutant mice that exhibit an irregular respiratory rhythm (Viemari et al., 2005b; Viemari, 2008). A5 and A6 neurons modulate respiration and contribute to its maturation before birth. A5 neurons depress the medullary respiratory rhythm generator which slows down the respiratory activity in vitro (Hilaire et al., 2004). In adult rats, A5 neurons are connected to the respiratory rhythm generator and display respiratory related activity. A6 neurons also display a respiratory related activity (Oyamada et al., 1998). In vitro experiments showed that A6 neurons are required for normal respiratory rhythm to emerge at birth (Viemari et al., 2004). Data from mutant mice (Viemari et al., 2004; 2005a) further suggest that A6 noradrenergic nuclei excite the respiratory rhythm generator. In Phox2a and Ret mutant mice, A6 neurons are missing and the respiratory activity is slower and more variable compared to wild-type (Viemari et al., 2004; 2005a). As summarized in recent reviews (Hilaire et al., 2004; Viemari, 2008), NE pontine groups exert opposite effects on the respiratory rhythm generator, A6 exerts a facilitation and A5 an inhibition. At the medullary level, A1/C1 modulates the frequency and A2/C2 is involved in the stabilization of the rhythm. In mice, exogenous NE application is typically excitatory, in in vitro brainstem–spinal cord and transverse slice preparations (Viemari et al., 2004; Viemari and Ramirez, 2006a) whereas in rats NE inhibits the respiratory rhythm generator via alpha-2 noradrenergic receptors possibly due to inter-species differences (Viemari and Hilaire, 2002).
In slices, exogenous application of NE induced a significant increase in the frequency of eupneic-like and sigh-like bursts previously characterized by Lieske et al. ( 2000). NE also increased burst duration and burst area of fictive eupneic-like events (Viemari and Ramirez, 2006). We showed that this excitatory effect is mediated through the activation of alpha-1 noradrenergic receptors since application of prazosin, an alpha-1 noradrenergic receptors antagonist, abolishes this excitation and application of the alpha-1 agonist, phenylephrine, mimics the NE excitatory effect (Viemari and Ramirez, 2006).
3.1.2. NE and inspiratory neurons
Activity of pre-inspiratory neurons could be directly regulated by excitation via alpha-1 noradrenergic receptors and inhibition via alpha-2 noradrenergic receptors (Arata et al., 1998). In this population, two subsets of neurons continued to burst after pharmacological isolation from the network and are identified as pacemaker neurons. In CS pacemaker neurons, NE increased the burst duration and the area encapsulated by the burst envelope without significantly affecting the burst frequency whereas in CI pacemaker neurons, NE increased the burst frequency without affecting either the burst duration or the burst area (Fig. 1). These results suggest that the two types of pacemaker neurons play different roles in respiratory rhythm generation. NE also modulates the activity of the two types of “non-pacemaker” inspiratory neurons (Viemari and Ramirez, 2006; Fig. 1). One type, so called “silent non-pacemaker” neurons, does not exhibit tonic spiking activity after application of a cocktail that blocks most fast synaptic transmission. A second type, so-called “active non-pacemaker”, exhibits irregular spiking activity after ionotropic glutamatergic receptor antagonists are applied. In synaptically isolated “non-pacemaker” neurons, bath-application of NE depolarized all silent “non-pacemaker” neurons. NE depolarized the active “non-pacemaker” neurons and induced cadmium sensitive bursting properties. We suggested that NE increases a calcium-activated non-selective cation current in the CS and conditional pacemaker neurons that is consistent with the fact that alpha-1 noradrenergic receptor activation opens non-specific cationic conductances (Carette, 1999). Alpha 1 noradrenergic receptors are also positively coupled to G protein q/11, Phospholipase C, inositol 1,4,5-triphosphate receptor, and protein kinase C. On CI pacemaker neurons, activation of the alpha-1 noradrenergic receptors exerts its effect by modulating a different cellular mechanism. One possibility is that NE increases the persistent sodium channel as occurs in the case of activation of the 5-HT2A receptors (Peña and Ramirez, 2002). Alpha-2 noradrenergic receptors are inhibitory and are coupled to G protein i/0 (Johnson et al., 1996), adenyl cyclase, cAMP and PKA.
3-2 serotonergic modulation
The 5-HT system also plays a fundamental role in the modulation of the respiratory activity. In the brainstem of vertebrates, two principal groups of 5-HT neurons have been described, B1–B3 and B4–B7 groups. The medullary raphé, which include the raphé magnus, pallidus and obscurus is considered to be involved in the respiratory control (Richerson, 2004). 5-HT is necessary for the development of the respiratory network and 5-HT excess, in MAO-A deficient mice or activation of 5-HT2A receptors in wild-type alter the wiring of the respiratory network (Bras et al, 2008).
3.2.1. 5-HT modulation of the respiratory rhythm generator
Serotonergic effects have been investigated by applying exogenous 5-HT, or by modifying the endogenous levels of 5-HT. Exogenous application of 5-HT exerts complex modulatory effects on the respiratory network. This can be attribute to the presence of multiple 5-HT receptor subtypes that cause diverse effects at the single neuron and network level.
In “en bloc” preparation, blockade of 5-HT uptake increased the frequency of the fictive respiratory activity recorded from the C4 ventral root (Morin et al., 1990 ; Di Pasquale et al., 1992), whereas application of 5-HT antagonist abolishes the respiratory activity (Di Pasquale et al, 1994). Activation of 5-HT1A receptors induces a facilitation of the rhythm (Monteau et al., 1994; Bou-flores et al, 2000). Recent in vivo studies also showed a facilitatory effect after applying 8-OH-DPAT (Stettner et al., 2008). However, these effects inducing a facilitation of the respiratory activity in the brainstem spinal cord preparation or in vivo cannot be attribute to a direct effect on the respiratory rhythm generator since several structures may contribute to the modulation of the respiratory rhythm generator.
Slice preparations have the ability to define the mechanisms of the effects of 5-HT on the respiratory rhythm generator. In this preparation, bath or pre-BötC specific application of 5-HT increases the frequency of the rhythmic activity (Al-Zubaidy et al., 1996; Schwarzacher et al., 2002). The effect of 5-HT on rhythmic activity recorded from the hypoglossal or the Pre-BötC can be mimicked by the 5-HT2 agonist, DOI, and blocked by the 5-HT2A,C antagonist ketanserine (Al-Zubaidy et al., 1996, Peña and Ramirez, 2002). The excitatory effect of DOI can be mimicked by blockade of endogenous 5HT uptake with alaproclate. A possible action on 5-HT2C receptors has been excluded by comparing the effects caused by 5-HT2A antagonists with those caused by a specific 5-HT2C receptors antagonist (Peña and Ramirez, 2002). However, Gunther et al. (2006) reported that blockade of 5-HT2B receptors, but not ketanserine, can abolish the hypoglossal activity and earlier work by Al-Zubaidy et al. (1996) showed that the respiratory rhythm persist after bath application of methylsergide. In slices, 5-HT endogenous activation is also required to stabilize the eupneic rhythm and the blockade of the 5-HT excitatory drive results in an irregular rhythmic activity in vitro (Peña and Ramirez, 2002). This excitatory effect obtained pharmacologically can be mimicked in transverse slices by stimulating raphé neurons the primary source of endogenous 5-HT. These results confirm previous results obtained in different in vitro preparations (Al-Zubaidy et al., 1996; Onimaru et al., 1998) as well as in anaesthetized cats (Lalley et al., 1995) and in conscious rats (Cayetanot et al., 2002).
3.2.2. 5-HT and inspiratory neurons
As we described for NE, 5-HT differentially affects the different types of pacemaker neurons that are involved in generating different respiratory network activities (Doi and Ramirez, 2008; Viemari, 2008; Viemari and Ramirez, 2006; Fig. 1). 5-HT increases the bursting of the CI pacemaker neurons upon pharmacological isolation (Peña and Ramirez, 2002; in red in Fig. 1). 5-HT, via activation of 5-HT2A receptors specifically increases the bursting frequency of CI pacemaker neurons that may result in an increased in frequency of the network activity. On the other hand, blockade of 5-HT2A receptors (with either ketanserine or piperidine) abolished the bursting properties in CI pacemaker neurons and increased the irregularity of the network (Peña and Ramirez, 2002; Tryba et al., 2006; Chevalier et al., 2008), whereas application of 5-HT uptake blockers restored the regularity after application of 5-HT2A antagonists.
As is the case with CI pacemaker neurons, CS pacemaker neurons are influenced by 5-HT (Fig. 1). Application of 5HT2A serotonergic receptors agonists induced an increase in amplitude of the depolarizing drive potential in CS pacemaker neurons (Peña and Ramirez, 2002; Fig. 2). As a possible consequence, activation of these receptors subtypes increased the amplitude of the integrated activity at the network level. This modulation of amplitude can be abolished after blockade of the calcium-activated non-selective cation current with flufenamic acid, suggesting the possibility that CS pacemaker neurons may play a role in this modulation. These data additionally suggest that synaptic neuromodulation plays an important role in the expression of intrinsic pacemaker properties.
5-HT1 receptors are linked to G protein i/o and inhibit adenylate cyclase and increase a K+ conductance. The synaptic localization of 5-HT1A and 5-HT1B is critical in determining the effects on excitability of respiratory neurons but also in interpreting the data collected when applying agonist and antagonist since they target the pre- and the post-synaptic elements that lead to opposite effects on respiratory output. Activation of 5-HT2A receptors generally leads to increased neuronal activity through different mechanisms (Doi and Ramirez, 2008; Hodges and Richerson, 2008). The 5-HT2A mediated effects on respiratory rhythm are thought to occur through PLC and PKC activation and modulation of transient and persistent sodium current (Peña and Ramirez, 2002). 5-HT4 and 5-HT7 are usually coupled to Gαs protein that increases the excitability and could contribute to reverse respiratory depression following fentanyl administration (Manzke et al., 2003).
Although 5-HT acts through different receptors and different cellular signaling mechanisms, depending on which 5-HT receptor is activated, 5-HT has a unique influence on the activity of different inspiratory cell types that, in turn, differentially alters fictive eupnea, sighs and gasping inspiratory activities (Peña and Ramirez, 2002; Ramirez et al., 2004; Tryba et al., 2006; 2008; Doi and Ramirez, 2008; Hodges and Richerson, 2008). 5-HT exerts its effects via a multitude of receptors within which a few of them are known to modulate the respiratory rhythm generator. Bath applied 5-HT increases and subsequently decreases bursting frequency in pre-inspiratory and inspiratory neurons. This complex modulation seems to be explained by the activation of 5-HT2A (excitatory), 5-HT2C (excitatory) and 5-HT1A (inhibitory) receptors (Onimaru et al., 1998). This modulation can gain in complexity since 5HT4A and 5HT7 receptors are expressed in the Pre-BötC (Manzke et al., 2008). For exemple, 5HT4A receptor activation reverses respiratory depression after fentanyl administration (Manzke et al., 2003) and 5-HT7 receptors have also received attention due to the moderate affinity for 8-OH-DPAT (Richter et al., 2003) which can explain the different results obtained after activation of 5-HT1A receptors (Morin et al., 1990; Onimaru et al., 1998). Finally, all these effects depend also on the experimental protocol as well as the concentration used in different laboratory.
3.2.3. Fictive gasping depend on activation of 5-HT2A serotonergic receptors
Under normoxic conditions (Fig. 2a, artificial cerebro-spinal fluid bubbled with carbogen, 95% O2-5% CO2) the isolated respiratory network is able to produce a second inspiratory like rhythm, the augmented inspiratory efforts, called fictive sigh (in blue in Fig 2a). A recent study by Tryba et al. (2008) postulated that CI pacemaker neurons are important for the generation of sighs confirming the study by Peña and Aguilera (2007) that suggested that riluzole blocked not only gasps but also sighs. Fictive sighs are also under influence of bioamines and 5-HT and NE both increased the fictive sigh frequency.
When the isolated respiratory network is exposed to lowered oxygen conditions, a third type of inspiratory rhythm called fictive gasps is generated (Fig. 2b). Gasping activity is characterized by a shorter duration and a more rapid inspiratory effort onset compare to the fictive eupneic activity. In contrast to fictive eupneic activity, fictive gasping is eliminated by riluzole alone (Peña et al., 2004). During hypoxic conditions, synaptically isolated CS-pacemaker neurons cease endogenous bursting, whereas CI pacemaker neurons, and the respiratory network, remains rhythmic (Thoby-Brisson and Ramirez, 2000; Peña et al., 2004). Thus, CI-pacemaker neurons have been previously shown to require endogenous serotoninergic 5-HT2A receptor activation to burst (Peña and Ramirez, 2002). Tryba et al. (2006) now suggested that they are involved in gasping generation in vitro since their blockage abolishes gasping (Fig. 2c). In contrast to CI pacemakers, CS pacemakers continue to burst following blockade of 5HT2A receptors (Pena and Ramirez, 2002) and CS pacemakers are not thought to participate in the gasping rhythm. Peña and Aguilera (2007) confirmed that pharmacological blockade of CI pacemaker neurons by application on riluzole drastically reduced gasping in vivo. Experiments in the in situ perfused preparation also suggest that both 5-HT2A and alpha-1 adrenergic receptors are important to sustain gasping after hypoxia-induced depression (St-John and Leiter, 2007). Taken together, these experiments performed in different preparations confirmed that neuromodulators and bioamines in particular play a crucial role in the generation of the different respiratory rhythms. Along these lines, while neuromodulation has been shown to be essential for rhythmogenesis in several invertebrate rhythmic networks, it should be noted that little evidence had previously identified its critical role in generating rhythmic central pattern generator and motor output, in vertebrate systems either at the cellular (Peña and Ramirez, 2002; Tryba et al., 2006) or network levels (Tryba et al., 2006). The fact that CI pacemaker bursting properties are conditionally dependent on serotonergic neuromodulation emphasizes the fact that respiratory rhythmogenesis is highly dependent on both synaptic and intrinsic bursting, or pacemaker, mechanisms.
4. Bioamines and respiratory diseases
Sudden Infant Death Syndrome has been associated with serotonin but also with disturbance in the noradrenergic systems (Hilaire, 2006). A study by Weese-Mayer et al. (2004) revealed that catecholaminergic neurons are abnormal in SIDS. But, abnormalities in the serotonergic modulation of respiratory nuclei are proposed to be a major risk factor for Sudden Infant Death Syndrome (Kinney, 2005; Paterson et al., 2006; Weese-Mayer et al., 2008). Sudden Infant Death Syndrome victims displayed a decreased 5-HT binding in an important area for the chemoreception of the medulla (Ozawa and Okado, 2002) reported reduced expression of 5-HT1A receptors in the medulla. Weese-Mayer et al., (2003) also reported that the promoter polymorphism in 5-HTT (serotonin gene transporter) may play an important role in Sudden Infant Death Syndrome risk. These studies indicate a possibly large involvement of the 5-HT system and strengthen the role of serotonin in the pathogenesis of Sudden Infant Death Syndrome but also in others respiratory disease such as Parder-Willi syndrome and sleep apneas (Wilken et al, 1997; Real et al, 2007; Zanella et al, 2008b; Stettner et al, 2008).
Sudden Infant Death Syndrome is hypothesized to occur, in part because of a disruption of autoresuscitation, or gasping activity during hypoxia (Kahn et al., 1988; Poets et al., 1999). In vitro data suggest that the central fictive gasping activity generated by the pre-BötC requires 5HT2A receptor neuromodulation (Tryba et al., 2006). Whether 5HT2A receptor activation is critical to gasping has been understandably difficult to verify in vivo, given in vivo application of serotonin agonists/antagonists will likely nonspecifically act on many receptors and sites that influence respiratory rhythm and pattern. That said, elucidating which serotonin receptor(s) may be involved in Sudden Infant Death Syndrome may be better clarified when more specific agonists/ antagonists and antibodies are developed.
Neuromodulation of the respiratory network by NE likely plays a role in Rett Syndrome (Viemari et al., 2005). Rett Syndrome results from either a mutation or gene duplication of the methyl-CpG binding protein 2 (MeCp2) gene encoding a transcription factor (Amir et al., 1999; Guy et al., 2001; Viemari et al., 2005). Rett Syndrome results in progressive mental regression, loss of the ability to walk, speak or purposefull hand use (Hagberg et al., 1983). Rett Syndrome victims eventually suffer from irregular breathing patterns (Elian and Rudolf, 1991). Mice with MeCp2 deficiency share similar phenotypes as children with Rett Syndrome. For example, MeCp2 mutant mice also have irregular breathing patterns and this irregular breathing activity in vivo (Viemari et al., 2005), parallels the irregular respiratory activity recorded from the brainstem slice preparations made from these mutants and containing the pre-BotC in vitro (Viemari et al., 2005; Pena and Tryba, 2007). These data suggested the possibility that the irregular breathing pattern of activity resulted from central deficits in respiratory rhythm modulation. In brainstem slice preparations made from mice with the MeCp2 mutation, bath-application of NE restored the regularity of fictive eupneic activity to control levels (Viemari et al., 2005; Pena and Tryba, 2007). There is also a developmental degeneration of noradrenergic neuromodulation of the respiratory central network in MeCp2 mutants and this is proposed to correlate with the developmental loss of regular respiratory activity (Viemari et al. 2005; Pena and Tryba, 2007). Thus, NE likely plays a critical role in stabilizing the central respiratory eupneic breathing activities in Rett Syndrome, whereas intervention, such as using NE-reuptake blockers may also provide a promising avenue to stabilize respiratory patterns in those who have Rett Syndrome (Zanella et al., 2008a; Roux et al., 2007; Pena and Tryba, 2007).
5. Conclusions
The mechanisms underlying respiratory rhythm generation in a neural network where the dynamic interactions between synaptic and intrinsic properties are highly modulated, is difficult and complex. Many very creative approaches to this issue have contributed to a better understanding, and we have only touched on a few of these approaches here, largely in vitro studies, which have the benefit of being able to dissect cellular and central network mechanisms, but may be limited regarding the entire in vivo system. That caveat noted, in vitro studies have suggested that the interplay between both intrinsic (pacemaker) bursting properties of neurons and synaptic neuromodulation (e.g., via 5-HT and NE) play a major role in respiratory rhythmogenesis. Understanding the important and in some cases, critical, role of these neuromodulators in stabilizing respiratory rhythmogenesis in a state-dependent fashion will likely elucidate the cellular mechanisms of diseases such as Sudden Infant Death Syndrome, Rett Syndrome, Prader-Willi syndrome and sleep apneas.
Acknowledgement
This work was supported by NIH Grant R01-HL 079294 to A.K.T.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alheid GF, Gray PA, Jiang MC, Feldman JL, McCrimmon DR. Parvalbumin in respiratory neurons of the ventrolateralmedulla of the adult rat. J.Neurocytol. 2002;31:693–717. doi: 10.1023/a:1025799830302. [DOI] [PubMed] [Google Scholar]
- Al-Zubaidy ZA, Erickson RL, Greer JJ. Serotonergic and noradrenergic effects on respiratory neural discharge in the medullary slice preparation of neonatal rats. Pflugers Arch. 1996;431:942–949. doi: 10.1007/s004240050089. [DOI] [PubMed] [Google Scholar]
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- Arata. A, Onimaru. H, Homma. I. The adrenergic modulation of firings of respiratory rhythm-generating neurons in medulla-spinal cord preparation from newborn rat. Exp Brain Res. 1998;119(4):399–408. doi: 10.1007/s002210050355. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Shipley MT, Grzanna R. The locus coeruleus, A5 and A7 noradrenergic cell groups. In: Paxinos G, editor. The Rat Nervous System. San Diego, CA: Academic Press; 1995. pp. 183–213. [Google Scholar]
- Aston-Jones G, Shipley MT, Chouvet G, Ennis M, van Bockstaele E, Pieribone V, Shiekhattar R, Akaoka H, Drolet G, Astier B, et al. Afferent regulation of locus coeruleus neurons: anatomy, physiology and pharmacology. Progress in Brain Research. 1991;88:47–75. doi: 10.1016/s0079-6123(08)63799-1. [DOI] [PubMed] [Google Scholar]
- Ballanyi K, Onimaru H, Homma I. Respiratory network function in the isolated brainstem-Spinal cord of newborn rats. Prog. Neurobiol. 1999;59:583–634. doi: 10.1016/s0301-0082(99)00009-x. [DOI] [PubMed] [Google Scholar]
- Bou-Flores C, Lajard AM, Monteau R, De ME, Seif I, Lanoir J, Hilaire G. Abnormal phrenic motoneuron activity and morphology in neonatal monoamine oxidase A-deficient transgenic mice: possible role of a serotonin excess. J. Neurosci. 2000;20:4646–4656. doi: 10.1523/JNEUROSCI.20-12-04646.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bras H, Gaytán SP, Portalier P, Zanella S, Pásaro R, Coulon P, Hilaire G. Prenatal activation of 5-HT2A receptor induces expression of 5-HT1B receptor in phrenic motoneurons and alters the organization of their premotor network in newborn mice. Eur J Neurosci. 2008;28(6):1097–1107. doi: 10.1111/j.1460-9568.2008.06407.x. [DOI] [PubMed] [Google Scholar]
- Carette B. Noradrenergic responses of neurones in the mediolateral part of the lateral septum: alpha1-adrenergic depolarization and rhythmic bursting activities, and alpha2-adrenergic hyperpolarization from guinea pig brain slices. Brain Res. Bull. 1999;48:263–276. doi: 10.1016/s0361-9230(98)00168-3. [DOI] [PubMed] [Google Scholar]
- Cayetanot F, Gros F, Larnicol N. Postnatal changes in the respiratory response of the conscious rat to serotonin 2A/2C receptor activation are reflected in the developmental pattern of fos expression in the brainstem. Brain Res. 2002;942(1–2):51–57. doi: 10.1016/s0006-8993(02)02690-2. [DOI] [PubMed] [Google Scholar]
- Chevalier M, Ben Mabrouk F, Tryba AK. Background Sodium Current Underlying Respiratory Rhythm Regularity. Eur J Neurosci. 2008;28(12):2423–2433. doi: 10.1111/j.1460-9568.2008.06537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Negro CA, Hayes JA. A 'group pacemaker' mechanism for respiratory rhythm generation. J Physiol. 2008;586(9):2245–2246. doi: 10.1113/jphysiol.2008.153627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Negro CA, Morgado-Valle C, Hayes JA, Mackay DD, Pace RW, Crowder EA, Feldman JL. Sodium and calcium current-mediated pacemaker neurons and respiratory rhythm generation. J. Neurosci. 2005;25:446–453. doi: 10.1523/JNEUROSCI.2237-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pasquale E, Monteau R, Hilaire G. Endogenous serotonin modulates the fetal respiratory rhythm: an in vitro study in the rat. Brain Res Dev Brain Res. 1994;80(1–2):222–232. doi: 10.1016/0165-3806(94)90107-4. [DOI] [PubMed] [Google Scholar]
- Di Pasquale E, Morin D, Monteau R, Hilaire G. Serotonergic modulation of the respiratory rhythm generator at birth: an in vitro study in the rat. Neurosci Lett. 1992;143:91–95. doi: 10.1016/0304-3940(92)90240-8. [DOI] [PubMed] [Google Scholar]
- Doi A, Ramirez JM. Neuromodulation and the orchestration of the respiratory rhythm. Respir. Physiol. Neurobiol. 2008;164:96–104. doi: 10.1016/j.resp.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dremencov E, El Mansari M, Blier P. Noradrenergic augmentation of escitalopram response by risperidone: electrophysiologic studies in the rat brain. Biological Psychiatry. 2007;61:671–678. doi: 10.1016/j.biopsych.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Elian M, Rudolf ND. EEG and respiration in Rett syndrome. Acta Neurol. Scand. 1991;83:123–128. doi: 10.1111/j.1600-0404.1991.tb04660.x. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat. Rev. Neurosci. 2006;7:232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortuna MG, West GH, Stornetta RL, Guyenet PG. Botzinger expiratory-augmenting neurons and the parafacial respiratory group. J Neurosci. 2008;28:2506–2515. doi: 10.1523/JNEUROSCI.5595-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günther S, Maroteaux L, Schwarzacher SW. Endogenous 5-HT2B receptor activation regulates neonatal respiratory activity in vitro. J Neurobiol. 2006;66(9):949–961. doi: 10.1002/neu.20253. [DOI] [PubMed] [Google Scholar]
- Guy J, Hendrich B, Holmes M, Martin JE, Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat Genet. 2001;27:322–326. doi: 10.1038/85899. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Koshiya N, Huangfu D, Verberne AJ, Riley TA. Central respiratory control of A5 and A6 pontine noradrenergic neurons. Am J Physiol. 1993;264:1035–1044. doi: 10.1152/ajpregu.1993.264.6.R1035. [DOI] [PubMed] [Google Scholar]
- Hagberg B, Aicardi J, Dias K, Ramos O. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett's syndrome: report of 35 cases. Ann Neurol. 1983;14:471–479. doi: 10.1002/ana.410140412. [DOI] [PubMed] [Google Scholar]
- Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, Yamamoto B, Silver J, Weeber EJ, Sweatt JD, Deneris ES. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–247. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- Hilaire G. Endogenous noradrenaline affects the maturation and function of the respiratory network: possible implication for SIDS, Auton. Neurosci. 2006:126–127. 320–331. doi: 10.1016/j.autneu.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Hilaire G, Viemari JC, Coulon P, Simonneau M, Bévengut M. Modulation of the respiratory rhythm generator by the pontine noradrenergic A5 and A6 groups in rodents. Respir Physiol Neurobiol. 2004;143(2–3):187–197. doi: 10.1016/j.resp.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Richerson GB. Interaction between defects in ventilatory and thermoregulatory control in mice lacking 5-HT neurons. Respir Physiol Neurobiol. 2008;164(3):350–357. doi: 10.1016/j.resp.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Tattersall G, Harris MB, McEvoy S, Richerson D, Deneris ES, Johnson RL, Chen ZF, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J. Neurosci. 2008;28:2495–2505. doi: 10.1523/JNEUROSCI.4729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janczewski WA, Onimaru H, Homma I, Feldman JL. Opioid-resistant respiratory pathway from the preinspiratory neurones to abdominal muscles: in vivo and in vitro study in the newborn rat. J. Physiol. (Lond.) 2002;545:1017–1026. doi: 10.1113/jphysiol.2002.023408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SM, Smith JC, Feldman JL. Modulation of respiratory rhythm in vitro: role of Gi/o protein-mediated mechanisms. J Appl Physiol. 1996;80:2120–2133. doi: 10.1152/jappl.1996.80.6.2120. [DOI] [PubMed] [Google Scholar]
- Kahn A, Blum D, Rebuffat E, Sottiaux M, Levitt J, Bochner A, Alexander M, Grosswasser J, Muller MF. Polysomnographic studies of infants who subsequently died of sudden infant death syndrome. Pediatrics. 1988;82:721–727. [PubMed] [Google Scholar]
- Kinney HC. Abnormalities of the brainstem serotonergic system in the sudden infant death syndrome: a review. Pediatr. Dev. Pathol. 2005;8:507–524. doi: 10.1007/s10024-005-0067-y. [DOI] [PubMed] [Google Scholar]
- Lalley PM, Bischoff AM, Schwarzacher SW, Richter DW. 5-HT2 receptor-controlled modulation of medullary respiratory neurones in the cat. J. Physiol. 1995;487:653–666. doi: 10.1113/jphysiol.1995.sp020907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieske SP, Thoby-Brisson M, Telgkamp P, Ramirez JM. Reconfiguration of the neural network controlling multiple breathing patterns: eupnea, sighs and gasps. Nat. Neurosci. 2000;3:600–607. doi: 10.1038/75776. [DOI] [PubMed] [Google Scholar]
- Manzke. T, Guenther. U, Ponimaskin. EG, Haller. M, Dutschmann. M, Schwarzacher. S, Richter. DW. 5-HT4(a) receptors avert opioid-induced breathing depression without loss of analgesia. Science. 2003;301(5630):226–229. doi: 10.1126/science.1084674. [DOI] [PubMed] [Google Scholar]
- Manzke T, Preusse S, Hülsmann S, Richter DW. Developmental changes of serotonin 4(a) receptor expression in the rat pre-Bötzinger complex. J Comp Neurol. 2008;506(5):775–790. doi: 10.1002/cne.21581. [DOI] [PubMed] [Google Scholar]
- Marder E, Bucher D. Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu. Rev. Physiol. 2007;69:291–316. doi: 10.1146/annurev.physiol.69.031905.161516. [DOI] [PubMed] [Google Scholar]
- Mason P, Gao K, Genzen JR. Serotonergic Raphe Magnus Cell Discharge Reflects Ongoing Autonomic and Respiratory Activities. J Neurophysiol. 2007;98:1919–1927. doi: 10.1152/jn.00813.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellen NM, Janczewski WA, Bocchiaro CM, Feldman JL. Opioid-Induced Quantal Slowing Reveals Dual Networks for Respiratory Rhythm Generation. Neuron. 2003;37:821–826. doi: 10.1016/s0896-6273(03)00092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteau R, Di Pasquale E, Hilaire G. Further evidence that various 5-HT receptor subtypes modulate central respiratory activity: in vitro studies with SR 46349B. Eur J Pharmacol. 1994;259(1):71–74. doi: 10.1016/0014-2999(94)90159-7. [DOI] [PubMed] [Google Scholar]
- Morin D, Hennequin S, Monteau R, Hilaire G. Serotonergic influences on central respiratory activity: an in vitro study in the newborn rat. Brain Res. 1990;535:281–287. doi: 10.1016/0006-8993(90)91611-j. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. CO2 dialysis in the medullary raphe of the rat increases ventilation in sleep. J. Appl. Physiol. 2001;90:1247–1257. doi: 10.1152/jappl.2001.90.4.1247. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Homma I. A Novel Functional Neuron Group for Respiratory Rhythm Generation in the Ventral Medulla. J Neurosci. 2003;23:1478–1486. doi: 10.1523/JNEUROSCI.23-04-01478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Shamoto A, Homma I. Modulation of respiratory rhythm by 5-HT in the brainstem-spinal cord preparation from newborn rat. Pflugers Arch. 1998;435:485–494. doi: 10.1007/s004240050543. [DOI] [PubMed] [Google Scholar]
- Oyamada Y, Ballantyne D, Muckenhoff K, Scheid P. Respiration-modulated membrane potential and chemosensitivity of locus coeruleus neurones in the in vitro brainstem-spinal cord of the neonatal rat. J Physiol. 1998;513:381–398. doi: 10.1111/j.1469-7793.1998.381bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa Y, Okado N. Alteration of serotonergic receptors in the brain stems of human patients with respiratory disorders. Neuropediatrics. 2002;33:142–149. doi: 10.1055/s-2002-33678. [DOI] [PubMed] [Google Scholar]
- Paterson DS, Trachtenberg FL, Thompson EG, Belliveau RA, Beggs AH, Darnall R, Chadwick AE, Krous HF, Kinney HC. Multiple serotonergic brainstem abnormalities in sudden infant death syndrome. JAMA. 2006;296:2124–2132. doi: 10.1001/jama.296.17.2124. [DOI] [PubMed] [Google Scholar]
- Peña F, Aguileta MA. Effects of riluzole and flufenamic acid on eupnea and gasping of neonatal mice in vivo. Neurosci. Lett. 2007;415:288–293. doi: 10.1016/j.neulet.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Peña F, Parkis MA, Tryba AK, Ramirez JM. Differential contribution of pacemaker properties to the generation of respiratory rhythms during normoxia and hypoxia. Neuron. 2004;43:105–117. doi: 10.1016/j.neuron.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Peña F, Ramirez JM. Endogenous activation of serotonin 2A receptors is required for normal respiratory rhythm generation in vitro. J. Neurosci. 2002;22:11055–11064. doi: 10.1523/JNEUROSCI.22-24-11055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña F, Tryba AK. Neural control and generation of respiratory rhythms:role of pacemaker neurons. Chpt. 7. In: Wiess Martin L., editor. Neural Network Research Horizons. New York: Nova Science Publishers, Inc.; 2007. pp. 207–225. ISBN 1-60021-485-1. [Google Scholar]
- Poets CF, Meny RG, Chobanian MR, Bonofiglo RE. Gasping and other cardiorespiratory patterns during sudden infant deaths. Pediatr. Res. 1999;45:350–354. doi: 10.1203/00006450-199903000-00010. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Schwarzacher SW, Pierrefiche O, Olivera BM, Richter DW. Selective lesioning of the cat pre-Botzinger complex in vivo eliminates breathing but not gasping. J. Physiol. (Lond.) 1998;507:895–907. doi: 10.1111/j.1469-7793.1998.895bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM, Tryba AK, Peña F. Pacemaker neurons and neuronal networks: an integrative view. Curr. Opin. Neurobiol. 2004;14:665–674. doi: 10.1016/j.conb.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Real C, Popa D, Seif I, Callebert J, Launay JM, Adrien J, Escourrou P. Sleep apneas are increased in mice lacking monoamine oxidase A. Sleep. 30(10):1295–1302. doi: 10.1093/sleep/30.10.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat. Rev. Neurosci. 2004;5:449–461. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- Richter DW, Manzke T, Wilken B, Ponimaskin E. Serotonin receptors: guardians of stable breathing. Trends Mol Med. 2003 Dec;9(12):542–548. doi: 10.1016/j.molmed.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Roux JC, Dura E, Moncla A, Mancini J, Villard L. Treatment with desipramine improves breathing and survival in a mouse model for Rett syndrome. Eur. J. Neurosci. 2007;25:1915–1922. doi: 10.1111/j.1460-9568.2007.05466.x. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stettner GM, Zanella S, Huppke P, Gärtner J, Hilaire G, Dutschmann M. Spontaneous central apneas occur in the C57BL/6J mouse strain. Respir Physiol Neurobiol. 2008;160(1):21–27. doi: 10.1016/j.resp.2007.07.011. [DOI] [PubMed] [Google Scholar]
- St-John WM, Leiter JC. Maintenance of gasping and restoration of eupnea after hypoxia is impaired following blockers of {alpha}-1 adrenergic receptors and serotonin 5HT2 receptors. J. Appl. Physiol. 2007 doi: 10.1152/japplphysiol.00599.2007. [DOI] [PubMed] [Google Scholar]
- Svensson TH, Bunney BS, Aghajanian GK. Inhibition of both noradrenergic and serotonergic neurons in brain by the alpha-adrenergic agonist clonidine. Brain Research. 1975;92:291–306. doi: 10.1016/0006-8993(75)90276-0. [DOI] [PubMed] [Google Scholar]
- Schwarzacher SW, Pestean A, Günther S, Ballanyi K. Serotonergic modulation of respiratory motoneurons and interneurons in brainstem slices of perinatal rats. Neuroscience. 2002;115(4):1247–1259. doi: 10.1016/s0306-4522(02)00540-7. [DOI] [PubMed] [Google Scholar]
- Suzue T. Respiratory rhythm generation in the in vitro brain stem-spinal cord preparation of the neonatal rat. J Physiol. 1984;354:173–183. doi: 10.1113/jphysiol.1984.sp015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoby-Brisson M, Ramirez JM. Role of inspiratory pacemaker neurons in mediating the hypoxic response of the respiratory network in vitro. J Neurosci. 2000;20:5858–5866. doi: 10.1523/JNEUROSCI.20-15-05858.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoby-Brisson M, Ramirez JM. Two types of inspiratory pacemaker neurons in the isolated respiratory network of mice. J. Neurophysiol. 2001;86:104–112. doi: 10.1152/jn.2001.86.1.104. [DOI] [PubMed] [Google Scholar]
- Tryba AK, Peña F, Lieske SP, Viemari JC, Thoby-Brisson M, Ramirez JM. Differential modulation of neural network and pacemaker activity underlying eupnea and sigh breathing activities. J. Neurophysiol. 2008;99:2114–2125. doi: 10.1152/jn.01192.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryba AK, Peña F, Ramirez JM. Gasping activity in vitro: a rhythm dependent on 5-HT2A receptors. J. Neurosci. 2006;26:2623–2634. doi: 10.1523/JNEUROSCI.4186-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viemari JC. Noradrenergic modulation of the respiratory neural network. Respir Physiol Neurobiol. 2008;164:123–130. doi: 10.1016/j.resp.2008.06.016. [DOI] [PubMed] [Google Scholar]
- Viemari J-C, Bévengut M, Burnet H, Coulon P, Pequignot JM, Tiveron MC, Hilaire G. Phox2a gene, A6 neurons and noradrenaline play a crucial role in the prenatal maturation of the respiratory rhythm generator in mice. J. Neurosci. 2004;24:928–937. doi: 10.1523/JNEUROSCI.3065-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viemari JC, Hilaire G. Noradrenergic receptors and in vitro respiratory rhythm: possible interspecies differences between mouse and rat neonates. Neurosci Lett. 2002;324(2):149–153. doi: 10.1016/s0304-3940(02)00191-x. [DOI] [PubMed] [Google Scholar]
- Viemari JC, Maussion G, Bevengut M, Burnet H, Pequignot JM, Nepote V, Pachnis V, Simonneau M, Hilaire G. Ret deficiency in mice impairs the development of A5 and A6 neurons and the functional maturation of the respiratory rhythm. Eur. J. Neurosci. 2005a;22:2403–2412. doi: 10.1111/j.1460-9568.2005.04441.x. [DOI] [PubMed] [Google Scholar]
- Viemari JC, Ramirez JM. Norepinephrine differentially modulates different types of respiratory pacemaker and nonpacemaker neurons. J. Neurophysiol. 2006;95:2070–2082. doi: 10.1152/jn.01308.2005. [DOI] [PubMed] [Google Scholar]
- Viemari JC, Roux JC, Tryba AK, Saywell V, Burnet H, Peña F, Zanella S, Bevengut M, Barthelemy-Requin M, Herzing LB, Moncla A, Mancini J, Ramirez JM, Villard L, Hilaire G. Mecp2 deficiency disrupts norepinephrine and respiratory systems in mice. J. Neurosci. 2005b;25:11521–11530. doi: 10.1523/JNEUROSCI.4373-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weese-Mayer DE, Berry-Kravis EM, Ceccherini I, Rand CM. Congenital central hypoventilation syndrome (CCHS) and sudden infant death syndrome (SIDS): Kindred disorders of autonomic regulation. Respir Physiol Neurobiol. 2008;164:38–48. doi: 10.1016/j.resp.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Berry-Kravis EM, Zhou L, Maher BS, Curran ME, Silvestri JM, Marazita ML. Sudden infant death syndrome: case-control frequency differences at genes pertinent to early autonomic nervous system embryologic development. Pediatr. Res. 2004;56:391–395. doi: 10.1203/01.PDR.0000136285.91048.4A. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Zhou L, Berry-Kravis EM, Maher BS, Silvestri JM, Marazita ML. Association of the serotonin transporter gene with sudden infant death syndrome: a haplotype analysis. Am. J. Med. Genet. A. 2003;122:238–245. doi: 10.1002/ajmg.a.20427. [DOI] [PubMed] [Google Scholar]
- Wilken B, Lalley P, Bischoff AM, Christen HJ, Behnke J, Hanefeld F, Richter DW. Treatment of apneustic respiratory disturbance with a serotonin-receptor agonist. J Pediatr. 1997;130(1):89–94. doi: 10.1016/s0022-3476(97)70315-9. [DOI] [PubMed] [Google Scholar]
- Zanella S, Mebarek S, Lajard AM, Picard N, Dutschmann M, Hilaire G. Oral treatment with desipramine improves breathing and life span in Rett syndrome mouse model. Respir Physiol Neurobiol. 2008a;160:116–121. doi: 10.1016/j.resp.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Zanella S, Roux JC, Viemari JC, Hilaire G. Possible modulation of the mouse respiratory rhythm generator by A1/C1 neurones. Respir. Physiol. Neurobiol. 2006;153:126–138. doi: 10.1016/j.resp.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Zanella S, Watrin F, Mebarek S, Marly F, Roussel M, Gire C, Diene G, Tauber M, Muscatelli F, Hilaire G. Necdin plays a role in the serotonergic modulation of the mouse respiratory network: implication for Prader-Willi syndrome. J. Neurosci. 2008b;28:1745–1755. doi: 10.1523/JNEUROSCI.4334-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]