Indication
Maraviroc and raltegravir are two new drugs in the treatment of human immunodeficiency virus (HIV) infection, which target other action sites than the existing antiretroviral drugs (Figure 1). Nucleoside reverse transcriptase inhibitors and protease inhibitors belong to the classic therapy against HIV infection.
Figure 1.
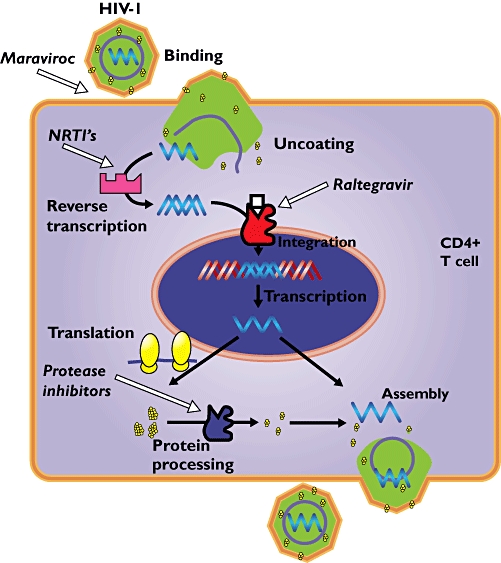
Replicative cycle of the HIV-1 virus in the CD4+ T cell with sites of drug interference indicated by the white arrows. NRTIs, nucleoside reverse transcriptase inhibitors
Mechanism
Maraviroc inhibits the formation of the binding complex at the surface of the host cell, preventing viral entry. The viral surface protein gp120 binds the CD4 receptor on the T cell. A second essential signal for viral entry is binding of gp120 to the coreceptor CCR5 of CXCR4. Completion of the binding complex makes it possible for protein gp41 to form a pore in the membrane of the T cell. Viral components can now penetrate the host cell. Maraviroc is a specific CCR5 antagonist that prevents CCR5-tropic HIV-1 entry (Figure 2). HIV-1 that uses CXCR4 as coreceptor is not affected by maraviroc.
Figure 2.
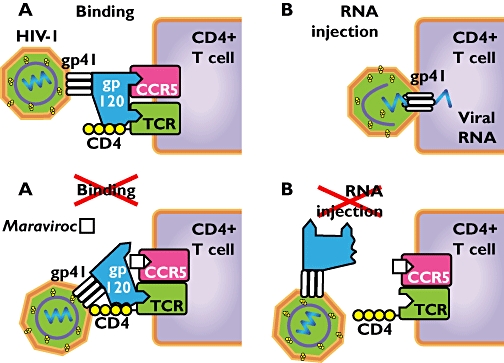
(A) The attachment process of HIV onto its host T cell depends on interaction between viral gp120 with T cell CD4 receptor (TCR) and coreceptor CCR5. (B) The viral protein gp41 is now able to form a pore into the host cell membrane, which allows the entry of viral DNA and enzymes into the T cell. (C) Maraviroc distorts the completion of the binding complex, because it occupies the coreceptor CCR5. (D) Without a complete binding complex the desired conformational change cannot occur and thus the gp41 protein cannot attach, prohibiting injection of viral RNA into the host cell
Raltegravir inhibits the catalytic activity of the integrase enzyme, thereby preventing the insertion of viral DNA into the host cell genome. In this case, HIV does penetrate the T cell, but will not replicate, because the viral DNA will not be transcribed. Without replication and synthesis of viral particles the propagation of HIV infection is prevented (Figure 1).
Adverse effects
The commonest adverse effects of maraviroc are diarrhoea, nausea and headache. Other common side-effects include dizziness, cough, insomnia, rash, weakness, muscle spasms and back pain.
A similar pattern of adverse reactions (gastrointestinal problems, asthenia and dizziness) is observed for raltegravir. Pruritus, lipodystrophy, hyperhidrosis and arthralgia are more specific adverse effects for raltegravir.
Literature
Kuritzkes D, Kar S, Kirkpatrick P. Fresh from the pipeline: Maraviroc. Nat Rev Drug Discov 2008; 7: 15–6.
Deeks SG, Kar S, Gubernick SI, Kirkpatrick P. Fresh from the pipeline: Raltegravir. Nat Rev Drug Discov 2008; 7: 117–8.
Opar A. New HIV drug classes on the horizon. Nat Rev Drug Discov 2007; 6: 258–9.
Available at http://www.emea.europa.eu/humandocs/Humans/EPAR/celsentri/celsentri.htm
Available at http://www.emea.europa.eu/humandocs/Humans/EPAR/isentress/isentress.htm


