Abstract
AIMS
A recent communication from the United States Food and Drug Administration highlighted a possible increased risk of stroke associated with use of the relatively new inhaled anticholinergic drug, tiotropium bromide. Using the United Kingdom General Practice Research Database, we set out to assess the risk of stroke in individuals exposed to inhaled tiotropium bromide and two other inhaled treatments for airways disease.
METHODS
We used the self-controlled case-series that reduces confounding and minimizes the potential for biases in the quantification of risk estimates.
RESULTS
Of 1043 people with a diagnosis of incident stroke who had had at least one prescription for tiotropium bromide, 980 satisfied inclusion criteria. The age-adjusted incidence rate ratio for all-cause stoke in individuals exposed to tiotropium bromide (n= 980), ipratropium bromide (n= 4181) and fluticasone propionate/salmeterol xinafoate (n= 1000) was 1.1 [95% confidence interval (CI) 0.9, 1.3], 0.8 (95% CI 0.7, 0.9) and 1.0 (95% CI 0.9, 1.2), respectively.
CONCLUSIONS
We found no evidence of an increased risk of all-cause stroke for individuals exposed to commonly prescribed inhaled treatments for chronic obstructive pulmonary disease.
Keywords: anti-cholinergics, drug safety, ipratropium, stroke, tiotroprium
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Conflicting studies have raised uncertainty over the vascular effects of the long-acting anticholinergic, tiotropium bromide.
WHAT THIS STUDY ADDS
Our results show no increased risk of stroke with tiotropium bromide, or with inhaled anticholinergics in general.
Introduction
Stroke is the second most common cause of death and major cause of disability worldwide [1]. Recently, the manufacturer of Spiriva, Boehringer Ingelheim (Ingelheim, Germany), informed the United States Food and Drug Administration that ongoing safety monitoring had identified a possible increase in risk of stroke in patients taking this inhaled medicine [2]. Spiriva contains tiotropium bromide, a long-acting quaternary ammonium anticholinergic used to treat bronchospasm associated with chronic obstructive pulmonary disease (COPD) [3]. Boehringer Ingelheim conducted a pooled analysis of the safety data from 29 placebo-controlled clinical studies, which included approximately 13 500 patients with COPD. Based on these studies, the estimate of the risk of stroke was eight patients per 1000 treated for 1 year with tiotropium bromide, and six patients per 1000 treated for 1 year with placebo [2]. This suggests that the estimated excess risk of all-cause stroke due to tiotropium bromide is two patients for each 1000 patients treated over a 1-year period. The Food and Drug Administration are thus in the process of analysing all available data [2]. In addition, a recent meta-analysis concluded that inhaled anticholinergics are associated with a significantly increased risk of the composite of cardiovascular death, myocardial infarction and stroke among patients with COPD [5]. By contrast, a single large long-term placebo controlled trial of tiotropium bromide recently reported no increase in the risk of stroke associated with tiotropium bromide use over 4 years of observation [4].
Due to the uncertainty surrounding the vascular effects of these agents, we studied the association between inhaled tiotropium bromide and stroke applying the self-controlled case-series method [6], which helps reduce confounding (by eliminating fixed-effect confounders such as comorbidities) in observational studies investigating drug exposure and adverse outcomes [7–9], on routinely collected information from the large UK General Practice Research Database (GPRD). As a control, analyses were repeated for the well-established anticholinergic, ipratropium bromide because a previous nested case–control study among patients with COPD, using the Manitoba health database, reported a slight increase in the risk of stroke [odds ratio 1.2, 95% confidence interval (CI) 1.0, 1.3] among those who had previously used inhaled ipratropium bromide [10]. Separate analyses were also undertaken for patients exposed to a different class of drug used in COPD [inhaled corticosteroid and β2 agonist combination (fluticasone propionate/salmeterol xinafoate)].
Methods
General practice research database
The GPRD is the world's largest computerized database of anonymized longitudinal medical records from primary care. Currently data are being collected on over 3.6 million patients from around 450 primary care practices throughout the UK [11]. Continuous information is recorded for each patient, including a record of each consultation, any diagnoses made, all prescribed medicine, and basic demographic data. Hospital referrals and diagnoses are also recorded. The geographical distribution and size of general practices represented in the database are largely representative of the population of England and Wales, and the individuals registered on the database are representative of the whole UK population in terms of age and sex [8].
Participants
All patients exposed to tiotropium bromide (Spiriva HandiHaler or Spiriva Respimat) between July 2002 (the date of the UK Marketing Authorization) and the end of December 2007 were included in the study. Eligible participants were those who had had a first-ever diagnosis of stroke (either ischaemic, haemorrhagic or unspecified) within a predefined study window. Episodes of transient ischaemic attack were not included, since the sensitivity of transient ischaemic attack recording in GPRD is likely to be lower than that for stroke. Medical diagnoses in the GPRD are recorded using Oxford Medical Information Systems and Read codes. Read codes became the standard for diagnostic classification in the GPRD during 1998, so both codes were utilized in this study. Study start dates were derived using the latter of the individual practice's up-to-standard date (GPRD-defined quality marker based on assessment of completeness, continuity and plausibility of data recording in key areas) or the patient's first registration date. Study end dates were derived using the earlier of the patient's transfer out date or the practice's last collection date. Patients must also have had a record of at least one incident stroke and at least a 12-month observation period prior to the first stroke and tiotropium bromide prescription. This was to ensure that stroke was truly incident, as diagnoses recorded close to patient registration may represent historic events.
Individuals were excluded if they had received dipyridamole or undergone a carotid endarterectomy more than 6 weeks prior to their event because this suggested that the stroke may not have been a new event. Cases were also excluded if their medical records indicated that the diagnosis of stroke was likely to have been retrospectively recorded. For example, if the patient's stroke was recorded along with other diagnoses on the day of a ‘new-patient’ or ‘well-person’ screen or if it were recorded at the same time as the first prescription. We also excluded people whose only diagnostic entry for their event appeared when the general practice received a post mortem report, because we were concerned that the date recorded would not accurately reflect the date of the cerebrovascular event.
Analyses
We used the self-controlled case-series method, which relies on intraperson comparisons in a population of individuals who have had the outcome of interest (cases). Age-adjusted incidence rate ratios (IRR) of the outcome of interest (incident stroke) were derived by comparing rates during defined intervals during exposure relative to all other observed time periods for each person [6, 12].
The start of the exposed period was defined as the date of first tiotropium bromide prescription. The end of the exposed period was defined as the date of the last prescription plus an estimate of exposure time according to the final prescription quantity and specified dosage. A 44-day wash-out period was then added to the end of the exposure date to account for delays in obtaining prescriptions and pharmacy supplies (14 days' allowance) and to ensure adequate tiotropium elimination (30 days). The terminal elimination half-life of tiotropium bromide is between 5 and 6 days following inhalation and 1 month was thus based on completion of five half-life's [3].
All other observation periods within the study window were taken as the baseline (unexposed) period. Participants included had a least one prescription (exposure) for tiotropium bromide and at least one recorded episode of stroke (event). Figure 1 illustrates a single individual who had a period of exposure to inhaled tiotropium bromide. The length of the exposed and baseline periods will vary for each participant.
Figure 1.
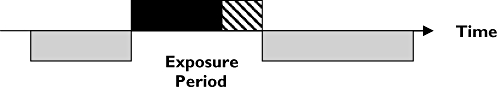
Pictorial representation of the self-controlled case-series method where age-adjusted incidence rate ratios of the outcome of interest are derived comparing defined intervals during the exposure period (drug administration and wash-out period) relative to all other observed time periods (baseline period) for each person. Baseline Period ( ); Drug Administration Period (
); Drug Administration Period ( ); Wash-out Period (
); Wash-out Period ( )
)
Using the self-controlled case-series method, confounding due to differences between participants cannot occur because all comparisons are within individual. However, confounding can occur with factors that change within an individual over time and are independently associated with both tiotropium bromide exposure and risk of stroke. Therefore, as the decision to prescribe tiotropium bromide may be associated with periods of COPD exacerbation, and such periods may be independently associated with an increased risk of stroke, we conducted sensitivity analyses in patients without known recent exacerbations and/or lower respiratory tract infections. This included patients who did not receive a prescription for antibiotics or oral corticosteroids within 6 weeks of tiotropium bromide initiation, both of which may act as markers of COPD exacerbation. Furthermore, analyses were repeated after adjustment to recorded stroke dates were made according to recent (within 6 weeks) symptoms, e.g. facial weakness. Additional sensitivity analyses were undertaken for patients who received more than 1 year of therapy and for patients without apparent breaks in their exposure. Finally, as mechanisms for stroke differ, a priori subgroup analyses for known haemorrhagic events were undertaken.
For comparison purposes identical analyses were repeated for individuals exposed to the shorter-acting inhaled anticholinergic, ipratropium bromide. Combination products containing ipratropium bromide such as Combivent and Duovent inhalers were included. Patients receiving chronic high-dose ipratropium bromide via nebulization were excluded. Additional control analyses were repeated in individuals exposed to Seretide (flixotide propionate and salmeterol xinafoate) inhalers.
We adjusted for age using ten 5-year age bands (45–49, 50–54, 55–59 years, etc.). IRR and 95% CIs were calculated for events occurring within each stratum of the exposed period compared with baseline periods using conditional Poisson regression. Data were analysed with Stata, version 9.0 (StataCorp, College Station, TX, USA).
Approval for our study was given by the Medicines and Healthcare products Regulatory Agency Independent Scientific Advisory Committee for Database Research and by the London School of Hygiene and Tropical Medicine Local Research and Ethics Committee.
Results
One thousand and forty-three patients were identified from the database that had had at least one prescription for tiotropium bromide and were known to have had at least one recorded episode of stroke (cases). Sixty-two patients were excluded as their history suggested either a previous stroke (n= 59) or that the diagnosis was retrospectively recorded (n= 3). The median age of eligible patients (n= 980) was 77 years [interquartile range (IQR) 70–82] and the median total observation and exposure periods were 14.0 years (IQR 9.1–17.3) and 14.1 months (IQR 3.7–30.7), respectively (see Table 1). The calculated IRR for all-cause stroke (see Table 2) was 1.1 (95% CI 0.9, 1.3).
Table 1.
Demographics for patients exposed to inhaled tiotropium bromide, ipratropium bromide and combination fluticasone propionate/salmeterol xinafoate
| Exposure | N | Median [IQR] age at first exposure (years) | Median [IQR] age at incident stroke (years) | Gender (%) | Median total [IQR] exposure (months) | Median [IQR] observation (years) |
|---|---|---|---|---|---|---|
| Tiotropium | 980 | 70 [62–76] | 72 [65–78] | M: 594 (61) F: 386 (39) | 14.1 [3.7–30.7] | 14.0 [9.1–17.3] |
| Ipratropium | 4181 | 66 [58–73] | 75 [68–81] | M: 2254 (54) F: 1927 (46) | 21.6 [1.7–55.5] | 10.9 [7.1–15.3] |
| Fluticasone & Salmeterol | 1000 | 67 [59–74] | 74 [66–80] | M: 544 (54) F: 456 (46) | 22.3 [4.7–54.1] | 9.0 [6.0–13.8] |
M, male; F, female.
Table 2.
Age-adjusted incidence rate ratios of all-cause incident stroke during exposure to inhaled tiotropium bromide, ipratropium bromide and combination fluticasone propionate/salmeterol xinafoate
| Exposure | N | n (baseline period) | n (exposed period) | IRR | 95% CI |
|---|---|---|---|---|---|
| Tiotropium | 980 | 773 | 207 | 1.1 | 0.9, 1.3 |
| Ipratropium | 4181 | 2839 | 1342 | 0.8 | 0.7, 0.9 |
| Fluticasone and salmeterol | 1000 | 609 | 391 | 1.0 | 0.9, 1.2 |
N, number of participants; n, number of events; IRR, age-adjusted incidence rate ratio; CI, confidence interval.
Over 70% of the strokes identified in this study were recorded with codes that did not distinguish the exact subtype of stroke. Nevertheless, we carried out a subgroup analysis (see Table 3) in the 61 patients with a clear diagnosis of haemorrhagic stroke (IRR = 1.5, 95% CI 0.7, 3.1). Sensitivity analysis showed no significant effect on altering the stroke date to recent symptoms, excluding patients with an apparent concurrent COPD exacerbation around the time of initiating therapy, or excluding patients with <1 year of exposure or with apparent breaks in their therapy (see Table 4).
Table 3.
Subgroup analyses for haemorrhagic stroke during exposure to inhaled tiotropium bromide, ipratropium bromide and combination fluticasone propionate/salmeterol xinafoate
| Exposure | N | n (baseline period) | n (exposed period) | IRR | 95% CI |
|---|---|---|---|---|---|
| Tiotropium | 61 | 42 | 19 | 1.5 | 0.7, 3.1 |
| Ipratropium | 204 | 140 | 64 | 0.6 | 0.4, 1.0 |
| Fluticasone and salmeterol | 49 | 28 | 21 | 1.3 | 0.5, 3.1 |
N, number of participants; n, number of haemorrhagic events; IRR, age-adjusted incidence rate ratio; CI, confidence interval.
Table 4.
Sensitivity analyses for tiotropium bromide cases
| N | n (baseline period) | n (exposed period) | IRR | 95% CI | |
|---|---|---|---|---|---|
| Adjusting stroke date to recent symptoms | 980* | 773 | 207 | 1.1 | 0.9, 1.3 |
| Excluding apparent COPD exacerbation | 931 | 745 | 186 | 1.0 | 0.8, 1.2 |
| Excluding apparent breaks in therapy† | 653 | 534 | 119 | 1.2 | 0.9, 1.5 |
| Patients with ≤1 year's exposure | 459 | 421 | 38 | 1.4 | 1.0, 2.0 |
| Patients with >1 year's exposure | 521 | 352 | 169 | 1.0 | 0.8, 1.3 |
N, number of eligible participants; n, number of events; IRR, age-adjusted incidence rate ratio; CI, confidence interval; COPD, chronic obstructive pulmonary disease.
57 stroke event date adjustments.
Breaks in therapy defined as a ≥3-month gap between prescription issues.
For comparison, the IRR for ipratropium bromide (n= 4181) and fluticasone propionate/salmeterol xinafoate (n= 1000) were 0.8 (95% CI 0.7, 0.9) and 1.0 (95% CI 0.9, 1.2), respectively.
Discussion
In a case-series analysis involving almost 1000 stroke events among exposed individuals we found no evidence of an increase in risk of all-cause stroke with tiotropium bromide, ipratropium bromide or combination fluticasone propionate/salmeterol xinafoate. However the calculated IRR for stroke with tiotropium bromide was highest at 1.1 (compared with fluticasone propionate/salmeterol xinafoate at 1.0 and ipratropium bromide at 0.8), albeit with overlapping CIs. Hence, our study should not be interpreted as providing definitive reassurance that the risk of stroke with tiotropium bromide is not truly higher than that of its comparators. However, our findings are consistent with the recently published 4-year Understanding the Potential Long-Term Impacts on Function with Tiotropium (UPLIFT) trial, which randomized 2986 patients to tiotropium bromide and 3006 patients to placebo, and also failed to demonstrate an increase in risk of stroke (relative risk 1.0, 95% CI 0.7, 1.3) [4]. However, it should be noted that the UPLIFT trial was not a pure placebo controlled trial and allowed use of concomitant medications as well as short-acting anticholinergics for the treatment of exacerbations, in contrast to previous tiotropium bromide trials, which showed increased risk.
Sensitivity analyses (see Table 4) yield a difference in the point estimate according to variance in the exposure time; however, this finding would require replication in additional studies.
Pooled analysis from 29 placebo-controlled clinical studies involving tiotropium bromide, which included approximately 13 500 patients with COPD, suggests a possible excess risk (rate ratio = 1.3) of stroke over a 1-year period [2]. However, with a baseline risk of 0.6% [2], such data are thus limited by statistical power. In addition, a more recent meta-analysis concluded that although inhaled anticholinergics are associated with a significantly increased risk of the composite of cardiovascular death, myocardial infarction and stroke among patients with COPD, no statistically significant excess risk was noted for the individual stroke end-point (rate ratio = 1.5, 95% CI 0.8, 2.6) [5]. However, this study combined analyses for both ipratropium and tiotropium bromide and was published prior to the large UPLIFT study. We therefore re-analysed the dataset including only trials involving tiotropium bromide and reporting stroke; and included the UPLIFT trial data. This analysis also fails to demonstrate a statistically significant effect for tiotropium bromide alone on stroke outcome (see Figure 2). It is important to note that only 60% of patients in our study were male compared with 80–90% in other trials.
Figure 2.
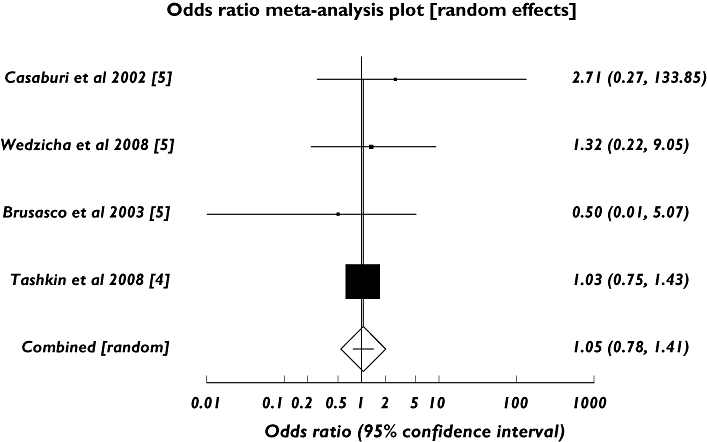
Meta-analysis of randomized trials of inhaled tiotropium bromide vs. control for the adverse outcome of stroke
The precise biological mechanism by which tiotropium bromide could increase the risk of stroke among patients with COPD is also uncertain. However, tiotropium did significantly increase sputum interleukin-8 (P= 0.04) compared with placebo in a year-long trial [13] and serum interleukin-8 may increase the risk of ischaemic stroke by destabilizing existing atherosclerotic plaques [14], although further investigation is needed [5].
Certain important study limitations need to be borne in mind. First, one major limitation of research using routinely collected clinical data is the robustness of the recording information. However, the validity of clinical data included in the GPRD has repeatedly been shown to be high, and data are rigorously checked and regularly audited and have been successfully used to conduct over 500 peer-reviewed published studies. Moreover, the GPRD has been widely used to study the epidemiology of stroke, with over 15 papers published to date. In addition, GPRD-recorded rates for stroke appear comparable to estimates obtained from other epidemiological studies [8]. Second, hospital prescriptions are unavailable from the sampling frame and this may have introduced a small degree of error in ascertaining the start of some exposure periods. However, particularly within the relatively recent time frame of our study, outpatient prescriptions issued by hospitals tend to be short (e.g. single inhaler supply), with all longer-term prescribing being undertaken by general practitioners. Under-ascertainment of exposure due to hospital prescriptions would therefore introduce only a very small error (bias towards the null if a true positive effect exists), likely to be nondifferential. In addition, we repeated the analyses presuming 1 month's exposure prior to the first general practice prescription and noted no difference in the derived risk estimate (IRR = 1.1, 95% CI 0.9, 1.3). Third, we assumed that all patients filled their prescriptions and inhaled their medication as prescribed, although this assumption is unlikely to hold true for all patients during their exposure and non-adherence to treatment is likely to affect any study examining drug efficacy or toxicity. Fourth, there may have been a delay between onset of stroke, clinical presentation, confirmation of diagnosis and recording in GPRD. This could possibly have produced a bias towards the null, if tiotropium bromide was discontinued between onset of stroke and diagnosis, but would be unlikely to have obscured entirely a clinically meaningful effect. Importantly, no effect on the point estimate was noted on adjusting the stroke date according to recent symptoms. Another potential source of bias is death due to stroke ending an observation earlier than it would have otherwise. Such bias is likely to be small, and could be in either direction.
There were also several strengths to the approach we used. Research using the GPRD has the great advantage of its large size, which means that we were able to include many more cases of stroke in exposed individuals than previous studies. This study included almost 1000 stroke cases in exposed individuals compared with just nine and 82 in the meta-analysis of randomized trials and UPLIFT study, respectively. Thus, although randomized controlled trials provide high-quality evidence, the power to detect an effect is low. Moreover, signals from multiple small studies in a meta-analysis could be inflated by small study bias. Although orthodox observational studies to investigate effects of drugs on adverse reactions can provide a large number of events, such studies are prone to confounding. The self-controlled case-series method we used reduces confounding by ensuring the comparisons are intraperson. Thus, our analysis removes the variation between individuals in risk factors for stroke and thus fixed confounders (e.g. comorbidities) are implicitly controlled for. Finally, when applied to datasets such as the GPRD, the risk information obtained relates to routine clinical use of a drug and therefore has good external validity.
Conclusion
We found no evidence of an increase in risk of all-cause stroke for individuals exposed to inhaled tiotropium bromide, ipratropium bromide or combination fluticasone propionate/salmeterol xinafoate. A prospective randomized study assessing outcomes such as stroke, myocardial infarction, cardiovascular arrhythmia and death would provide more definitive information.
Competing interests
I.D. holds stock and consults for GlaxoSmithKline. A.D.H. is a member of the editorial board of Drug and Therapeutics Bulletin. He has given non-remunerated advice to GlaxoSmithKline and London Genetics. He has received honoraria for speaking at educational meetings but donated these in whole or large part to medical charities.
We thank the GPRD research team for supplying the data (under the MRC licence scheme for academics) and providing advice and assistance. L.S. is supported by a Wellcome Trust Senior Research Fellowship in Clinical Science. A.H. is a British Heart Foundation Senior Research Fellow.
REFERENCES
- 1.Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371:1612–23. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- 2.US Food and Drug Administration. Early communication about an ongoing safety review of tiotropium (marketed as Spiriva HandiHaler) Available at http://www.fda.gov/cder/drug/early_comm/tiotropium.htm (last accessed 20 July 2008.
- 3.Spiriva HandiHaler (tiotropium bromide) Summary of Product Characteristics. Boehringer Ingelheim. Available at http://emc.medicines.org.uk/emc/industry/default.asp?page=displaydoc.asp&documentid=10039 (last accessed 20 August 2008.
- 4.Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, Decramer M UPLIFT Study Investigators. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543–54. [Google Scholar]
- 5.Singh S, Loke YK, Furberg CD. Inhaled anticholinergics and risk of major adverse cardiovascular events in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA. 2008;300:1439–50. doi: 10.1001/jama.300.12.1439. [DOI] [PubMed] [Google Scholar]
- 6.Whitaker H. The self controlled case series method. BMJ. 2008;337:a1069. doi: 10.1136/bmj.a1069. [DOI] [PubMed] [Google Scholar]
- 7.Grosso A, Douglas I, Hingorani A, MacAllister R, Smeeth L. Post-marketing assessment of the safety of strontium ranelate; a novel case-only approach to the early detection of adverse drug reactions. Br J Clin Pharmacol. 2008;66:689–94. doi: 10.1111/j.1365-2125.2008.03273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Douglas IJ, Smeeth L. Exposure to antipsychotics and risk of stroke: self controlled case series study. BMJ. 2008;337:a1227. doi: 10.1136/bmj.a1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hubbard R, Lewis S, West J, Smith C, Godfrey C, Smeeth L, Farrington P, Britton J. Bupropion and the risk of sudden death: a self-controlled case-series analysis using The Health Improvement Network. Thorax. 2005;60:848–50. doi: 10.1136/thx.2005.041798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macie C, Wooldrage K, Manfreda J, Anthonisen N. Cardiovascular morbidity and the use of inhaled bronchodilators. Int J Chron Obstruct Pulmon Dis. 2008;3:163–9. doi: 10.2147/copd.s1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.General practice research database. Available at http://www.gprd.com/home/ (last accessed 25 March 2008.
- 12.Farrington CP. Relative incidence estimation from case series for vaccine safety evaluation. Biometrics. 1995;51:228–35. [PubMed] [Google Scholar]
- 13.Powrie DJ, Wilkinson TM, Donaldson GC, Jones P, Scrine K, Viel K, Kesten S, Wedzicha JA. Effect of tiotropium on sputum and serum inflammatory markers and exacerbations in COPD. Eur Respir J. 2007;30:472–8. doi: 10.1183/09031936.00023907. [DOI] [PubMed] [Google Scholar]
- 14.Boekholdt SM, Peters RJ, Hack CE, Day NE, Luben R, Bingham SA, Wareham NJ, Reitsma PH, Khaw KT. IL-8 plasma concentrations and the risk of future coronary artery disease in apparently healthy men and women: the EPIC-Norfolk prospective population study. Arterioscler Thromb Vasc Biol. 2004;24:1503–8. doi: 10.1161/01.ATV.0000134294.54422.2e. [DOI] [PubMed] [Google Scholar]


