Abstract
Klein, Dolph (Rutgers, The State University, New Brunswick, N. J.), and David Pramer. Bacterial dissimilation of streptomycin. J. Bacteriol. 82:505–510. 1961.—The influence of various nutritional and environmental factors on the dissimilation of streptomycin by a pseudomonad isolated from soil was investigated, and conditions most suitable for growth of the bacterium, in a medium that contained streptomycin as a sole source of energy, nitrogen, and organic carbon, were determined. Development of the bacterium as measured by plate counts was correlated with degradation of streptomycin as measured by both biological and spectrophotometric assay procedures.
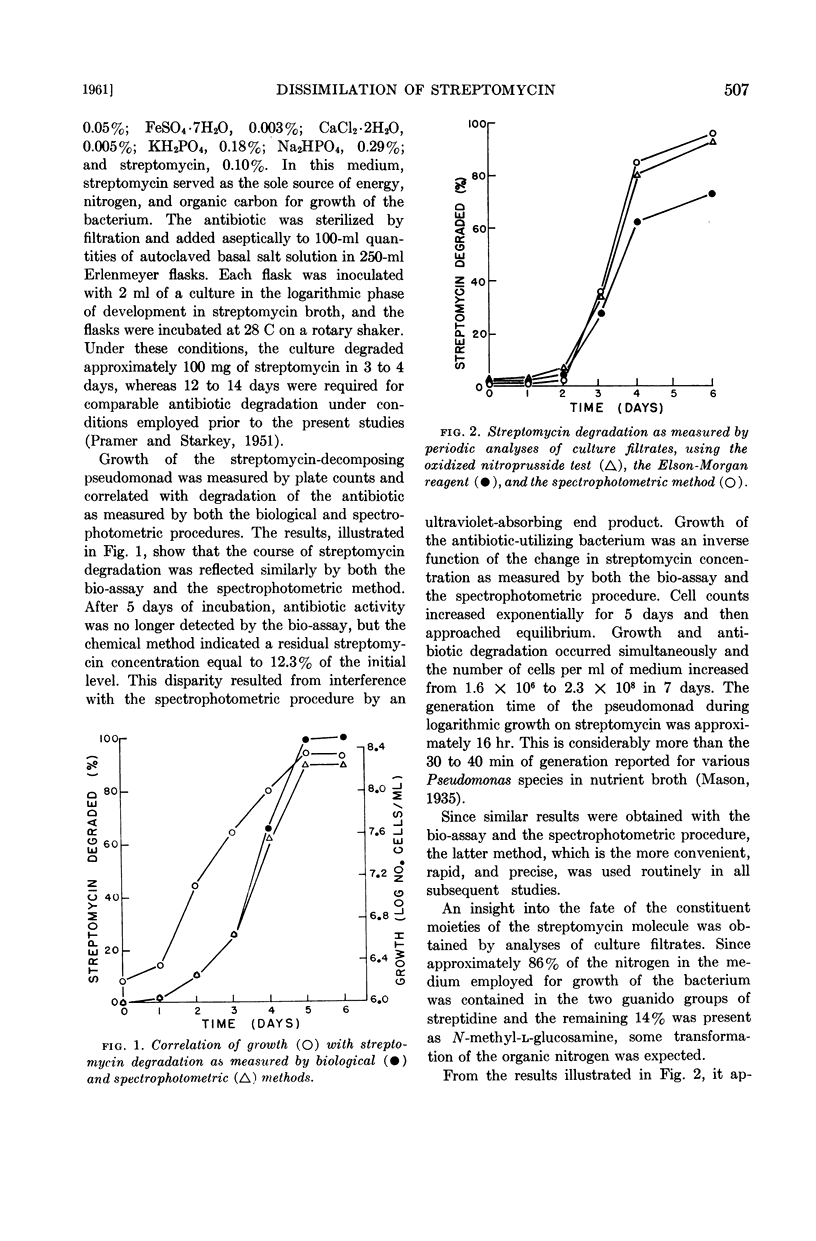
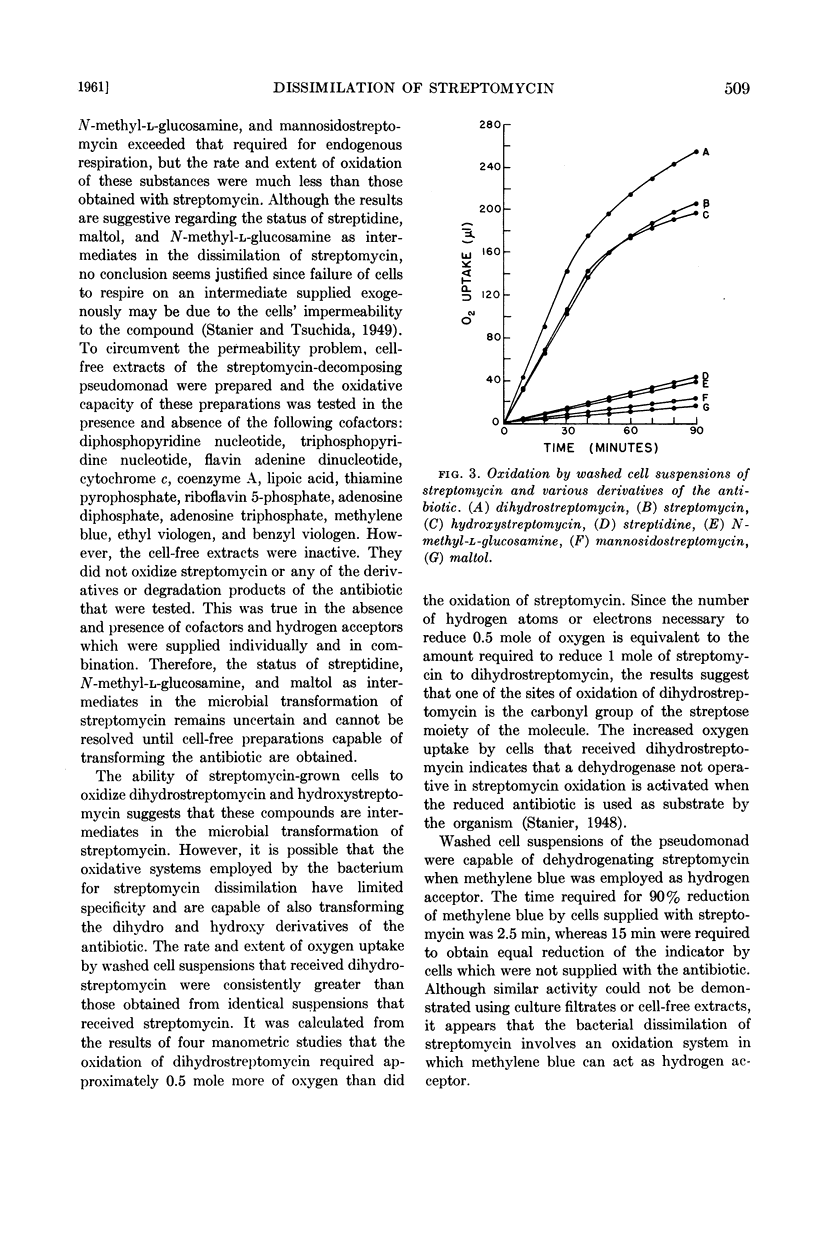
Periodic analyses of culture filtrates indicated that the three constituent moieties of the streptomycin molecule underwent simultaneous transformation when the antibiotic was degraded microbiologically. Washed cell suspensions were capable of immediate and rapid oxidation of streptomycin, dihydrostreptomycin, and hydroxystreptomycin, but cell-free sonic extracts, in the absence and presence of cofactors, did not oxidize streptomycin or any of a number of derivatives or degradation products of the antibiotic. Evidence was obtained that the bacterial dissimilation of streptomycin involves an oxidation system in which methylene blue can act as hydrogen acceptor.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANAND N., DAVIS B. D., ARMITAGE A. K. Uptake of streptomycin by Escherichia coli. Nature. 1960 Jan 2;185:23–24. doi: 10.1038/185023a0. [DOI] [PubMed] [Google Scholar]
- ESPERSEN E. Streptomycinase. Acta Pathol Microbiol Scand. 1951;29(3):277–282. [PubMed] [Google Scholar]
- Loo Y. H., Skell P. S., Thornberry H. H., Ehrlich J., McGuire J. M., Savage G. M., Sylvester J. C. Assay of Streptomycin by the Paper-Disc Plate Method. J Bacteriol. 1945 Dec;50(6):701–709. [PMC free article] [PubMed] [Google Scholar]
- Mason M. M. A Comparison of the Maximal Growth Rates of Various Bacteria under Optimal Conditions. J Bacteriol. 1935 Feb;29(2):103–110. doi: 10.1128/jb.29.2.103-110.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pramer D., Starkey R. L. Decomposition of Streptomycin. Science. 1951 Feb 2;113(2927):127–127. doi: 10.1126/science.113.2927.127. [DOI] [PubMed] [Google Scholar]
- SAKAKIBARA E. Studies on the streptomycin-resistant variants in bacteria. 5. On several properties of streptomycin-fastness. Acta Sch Med Univ Kioto. 1951;29(1):72–81. [PubMed] [Google Scholar]
- SAKAKIBARA E. Studies on the streptomycin-resistant variants in bacteria. 7. On streptomycin-decomposing enzyme (streptomycinase). Acta Sch Med Univ Kioto. 1951;29(2):116–124. [PubMed] [Google Scholar]
- Scudi J. V., Boxer G. E., Jelinek V. C. A Color Reaction Given by Streptomycin. Science. 1946 Nov 22;104(2708):486–487. doi: 10.1126/science.104.2708.486. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y. The Oxidation of Aromatic Compounds by Fluorescent Pseudomonads. J Bacteriol. 1948 Apr;55(4):477–494. doi: 10.1128/jb.55.4.477-494.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Tsuchida M. ADAPTIVE ENZYMATIC PATTERNS IN THE BACTERIAL OXIDATION OF TRYPTOPHAN. J Bacteriol. 1949 Jul;58(1):45–60. doi: 10.1128/jb.58.1.45-60.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]


