Abstract
AIMS
Albendazole (ABZ) is used in several anthelminthic drug programmws. ABZ side-effects are generally mild, but ABZ-induced pancytopenia may be serious. In filariasis programmes, it may be necessary to administer ABZ to breastfeeding women. Few data are available on safety of ABZ for breastfed infants. In addition, the pharmacokinetics of ABZ and its metabolites in human milk is insufficiently investigated. The aim was to study pharmacokinetics of ABZ and its metabolites [ABZ sulphoxide (ABSX) and ABZ sulphone] in the breast milk lactating women after one single oral dose of ABZ.
METHODS
Thirty-three lactating women (age 18–40 years) participated in the study. They received a single oral 400-mg dose of ABZ. Five milk samples were taken at 0, 6, 12, 24 and 36 h. One serum sample was taken after 6 h. Samples were analysed using high-performance liquid chromatography and pharmacokinetic analysis was performed.
RESULTS
ABZ was detectable in milk samples 6 h after the oral dose. The mean concentration of serum ABZ was 63.7 ± 11.9 ng ml−1. The pharmacokinetic parameters for ABSX were calculated as follows: 351.9 ± 32.4 ng ml−1, 6.9 ± 0.5 h, 12.4 ± 2.2 h and 5190.3 ± 482.8 ng*h ml−1 for Cmax, Tmax, t½ and AUC0–36, respectively. The milk-to-serum ratios (range) for ABZ and ABSX were 0.9 (0.2–6.5) and 0.6 (0.1–1.5), respectively.
CONCLUSIONS
After an oral dose of 400 mg, ABZ and ABSX attain low concentrations in breast milk that are unlikely to be considered harmful for the breastfed infant.
Keywords: albendazole, albendazole sulphoxide, breast milk, Egypt, filariasis, humans
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Albendazole is used in several anthelminthic drug programs.
It may be necessary to include sectors of the community (such as breastfeeding women) not previously involved in community or clinical programmes (e.g. filariasis, hydatid disease) to achieve a reasonable level of elimination of the worm.
However, there are no studies in which the amount of the drug and/or its metabolites in the milk of lactating women was analysed, so as to assess the possible exposure of the breastfed infant.
WHAT THIS STUDY ADDS
In the present study, the concentrations of albendazole and its active metabolite (albendazole sulphoxide) were analysed in the milk of lactating women through 36 h after administration of a single (400 mg) oral dose of albendazole.
Albendazole was barely secreted in milk as such.
On the other hand, albendazole sulphoxide was analysed through the 36 h of the study, and it was concluded that albendazole and albendazole sulphoxide attain levels in breast milk that are unlikely to be considered harmful for the breastfed infant.
These findings would help in deciding whether to involve breastfeeding mothers in albendazole mass drug administration programmes.
Introduction
Albendazole (ABZ) is a long-established anthelminthic drug for both human and veterinary use having a broad spectrum of indications [1–3] including programmes to eliminate lymphatic filariasis [4]. In the latter case, the effectiveness of the elimination programmes necessitates high community treatment coverage as suggested by mathematical modelling [5]. The mass coverage should include 70–95% of the population, depending on the rate of endemicity, to achieve a 0.5% microfilarial prevalence within, optimally, a period of 5–6 years. This raises the question of the safety of involving sectors of the population not usually involved in mass drug treatments, such as breastfeeding women.
ABZ minor to moderate adverse effects (headache, fever, gastrointestinal upset, body aches, severe scrotal pain and shock) were reported in mass treatment of filariasis (when ABZ was used with diethylcarbamazine) [6]. Also, a case report of death due to ABZ-induced pancytopenia was reviewed [7] and suggested to be related to high and prolonged serum concentration of ABZ sulphoxide (ABSX). Almost 90% of breastfeeding women take a prescription drug during the first week post partum [8]. The rate of breastfeeding is considerably decreased if the woman requires a drug with uncertain impact on her breastfed child [9]. Although the use of ABZ for children as young as 12 months has been suggested [10], the safety of exposing younger children to ABZ is questioned [11]. Moreover, evidence as to whether ABZ is safe for breastfed babies is only suggestive [12].
ABZ is erratically absorbed from the intestine, being dependent on the type of meal and pH of the stomach, and is then rapidly metabolized to the active ABSX, which, in its turn, is further metabolized to the non-active sulphone (ABSO) [13]. Whereas detecting ABZ for pharmacokinetic analysis is difficult, ABSX is more likely to be detected, and is more informative than the inactive ABSO metabolite [14]. The amount of ABSX excreted in the milk of a lactating woman should reflect the potential impact of ABZ administered to the mother on her breastfed child.
Our purpose was to study the pharmacokinetics of ABZ and its metabolites (ABSX and ABSO) in the breast milk of lactating women after one single oral dose of ABZ and to estimate the ABZ dose that an infant might consume if breastfed.
Materials and methods
Subjects
Lactating women, who were breastfeeding a healthy full-term infant aged between 2 weeks and 6 months, postpartum, giving informed written consent to participate and able to provide milk samples for the duration of the study period, were included; all women were participating in the Egyptian Government ABZ/diethylcarbamazine (DEC) administration programme for lymphatic filariasis control and were not pregnant as determined by pregnancy test.
Women were excluded if:
Pregnant (determined by pregnancy test);
Using an investigational drug within 30 days or five half-lives (whichever was longer) preceding the first dose of study medication;
Presenting with clinical signs of hepatic or renal dysfunction;
Suffering from any significant clinical condition that would require treatment, or any intercurrent illness, e.g. diarrhoea or respiratory infection in either the mother or child;
Receiving treatment with a benzimidazole anthelminthic (ABZ, mebendazole, flubendazole, thiabendazole and triclabendazole) in the last 7 days; or treatment with praziquantel or cimetidine.
Thirty-three women with ages ranging from 18 to 40 years were included in the study. Every participant was administered 400 mg ABZ, orally at 08.00–09.00 h.
The ethical aspects of the study were approved by the Faculty of Medicine, Ain Shams University Research Ethics Committee (FMASU REC)*.
Collection of samples
Milk and blood samples were collected from the participants as follows.
Milk samples
M1 samples (first): collected immediately before drug administration. M2, M3, M4 and M5 samples: collected 6, 12, 24 and 36 h after drug administration, respectively.
In most cases, milk was collected by breast pump and milk was evacuated from the same breast each time. A few participants decided to evacuate their breasts manually. Participating women abstained from breastfeeding their infants during the study and a replacement infant formula was provided to all participants.
Blood samples
Blood samples were collected 6 h after drug administration, and centrifuged on-site. Serum was stored in 2 ml Eppendorf tubes.
Samples (milk and serum) were immediately transferred to a box filled with dry ice, before transportation for storage. Samples were stored at −70°C for 4–6 months.
Chemicals
Ethylacetate, dimethylsulphoxide (DMSO), acetonitrile [high-performance liquid chromatography (HPLC) grade], orthophosphoric acid (all from Honil Ltd, London UK); tetra-butyl-ammonium hydrogen sulphate (Sigma, Poole UK); sodium hydroxide (Merck, Darmstadt, Germany); ABZ, ABSX and ABSO; and proguanil (internal standard), all provided by GlaxoSmithKline (Brentford, UK).
Sample processing and extraction procedures
Milk samples were thawed at room temperature, and then sonicated for 10 min. One millilitre of each milk sample was pipetted, and then 5 µl of the 5 mg ml−1 of the internal standard was added. One hundred microlitres of 0.4 m NaOH was added to each tube and vortexed for 30 s. Ethylacetate (6 ml) was then added to each tube, followed by vortexing for 30 s. The mixture was centrifuged at 1565 g for 5 min, 5 ml of the supernatant was aspirated into another test tube, where 1 ml of HPLC grade water was added, and then vortexed for 30 s. The mixture was centrifuged at 880 g for 2 min. The top organic layer was aspirated (4 ml) into another test tube and then dried in a water bath (50°C) under a nitrogen stream for 20 min. The dried residue was reconstituted in 500 µl mobile phase, vortexed, and then 100 µl was injected into the HPLC system.
HPLC apparatus and conditions
The HPLC system used consisted of a Hewlett Packard 1050 series pump; Hewlett Packard 1050 series column oven; Hewlett Packard 1050 series UV-Vis detector and Hewlett Packard 1050 series online degasser (Hewlett Packard, Bracknell, UK). The system was equipped with a Rheodyne injector with a 100-µl loop.
The extraction and the chromatographic conditions were according to those described by Fletouris et al. [15]. An internal standard (proguanil) was selected according to Hoaksey et al. [16]. The separation of ABZ and its metabolites was carried out on Phenomenex® 250 × 4.6 mm, Nucleosil C18, 5 µm, 120A°. The mobile phase consisted of acetonitrile/0.01 m phosphoric acid solution (20 : 80, v/v) containing 5 mm tetra-butyl-ammonium hydrogen sulphate. The mobile phase was pumped at a flow rate of 1.5 ml min−1. The column temperature was adjusted to 50 ± 0.1°C. The analytes were detected at 292 nm.
Preparation of standards and calibration curves
ABSX, ABSO and the internal standard, proguanil, were prepared as 5-mg stocks, dissolved in 1 ml DMSO. Working standards were prepared as 10 ml of the stock solution added to 10 µl of the mobile phase (final concentration of 500 ng per 100 µl) and then further diluted as needed for calibration.
Concentrations of ABSX standards ranged from 0.625 to 199.6 ng per 100 µl. For ABZ, the range was 1.25–99.6 ng per 100 µl, and that for ABSO was 5–199.6 ng per 100 µl. For each concentration of the standards, 5 µg of the internal standard proguanil was added. Standard curves were plotted with graded concentrations of each standard against the ratio of the area of the respective standard to the area of the internal standard.
Pharmacokinetic analysis
From milk samples, the maximum concentration (Cmax), time at maximum concentration (Tmax) and half-life (t½) were determined using a noncompartmental model. To calculate the area under concentration–time curve (AUC), the trapezoidal sum of squares under the curve method was used. AUC reflects the total amount of drug to which the infant would be exposed over a 36-h period should it be breastfed. Pharmacokinetic calculations for ABSX were done for every individual participant, and then the mean ± SE of all participants' parameters were reported.
Statistical analysis
Descriptive statistics, linear regression and nonparametric Spearman correlation tests were done using Instat, version 3.05 (2002) (GraphPad Software Inc., La Jolla, CA, USA; http://www.graphpad.com). Pharmacokinetic parameters were calculated and graphed using Kinetica 2000, version 3.0 (InnaPhase Corp., Waltham, MA, USA) and Prism, version 3.0 (1999) (GraphPad Software Inc.).
Results
Method validation
The retention times for ABSX, internal standard, ABSO and ABZ were 2.6, 4.0, 5.5 and 8.5 min, respectively. The limit of quantification (LOQ) was 2.5, 12.5 and 5.0 ng ml−1 for ABSX, ABSO and ABZ, respectively. Linearity of the calibration curve was determined by plotting peak area ratio of the three analytes to internal standard against the analyte concentrations. A linear response was obtained for the three analytes with r2= 0.9945 for ABSX, 0.9993 for ABSO and 0.9972 for ABZ. The average recovery of ABZ and its metabolites in authentic milk samples ± SD was calculated to be 62.1 ± 17.6%. Within-day imprecision was <3% and between-day imprecision <6%. Inaccuracy was <15% of the nominal values of two spiked controls.
Albendazole and its metabolites in serum
Serum concentrations of ABZ and its two metabolites (ABSX and ABSO) were determined in the serum of all participants to ensure compliance and to have an idea of the systemic concentrations attained.
The mean (±SE) of the concentrations of ABZ, ABSX and ABSO were 63.7 ± 11.9, 608.0 ± 63.4 and 100.7 ± 10.7 ng ml−1, respectively.
Six hours after oral administration, the median ratio of serum concentrations of ABSX to ABZ was 11.4, with wide individual variation (2.2–100.0).
Albendazole and its metabolites in milk
In analyses of ABZ and ABSO, the concentrations detected in most samples after 24 h were below LOQ. There were insufficient points (three at least were required) for linear regression, and pharmacokinetic parameters could be calculated only for ABSX.
ABZ, ABSX and ABSO concentrations in the breast milk of participants are shown in (Table 1).
Table 1.
Mean ± SE in ng ml−1 of the concentrations of albendazole (ABZ), albendazole sulphoxide (ABSX) and albendazole metabolized to the non-active sulphone (ABSO) in the breast milk of lactating women who administered one single oral dose of 400 mg ABZ at 0 (M1), 6 (M2), 12 (M3), 24 (M4) and 36 (M5) hours after drug administration
| M1 | M2 | M3 | M4 | M5 | |
|---|---|---|---|---|---|
| ABZ mean ± SE | 0 | 31.9 ± 9.2 | 18.8 ± 6.7 | 7.5 ± 2.9 | ND* |
| n | 33 | 23 | 27 | 28 | 14 |
| ABSX mean ± SE | 0 | 312.8 ± 30.6 | 225.2 ± 27.7 | 94.1 ± 7.0 | 57.1 ± 11.1 |
| n | 33 | 23 | 25 | 23 | 15 |
| ABSO mean ± SE | 0 | 52.0 ± 8.1 | 56.0 ± 12.0 | 19.9 ± 5.8 | ND* |
| n | 33 | 24 | 25 | 28 | 14 |
ND, not-detected (below LOQ).
Pharmacokinetic parameters (Cmax, Tmax, t1/2 and AUC0–36) were calculated from the individual data from 20 participants who provided data for at least three time points for regression analysis. The mean ± SE of the selected parameters were: 351.9 ± 32.4 ng ml−1, 6.9 ± 0.5 h, 12.4 ± 2.2 h, 3932.0 ± 455.5 ng h−1 ml−1 and 5190.3 ± 482.8 ng*h ml−1 for Cmax, Tmax, t1/2, AUC0–24 and AUC0–36, respectively (Figure 1).
Figure 1.
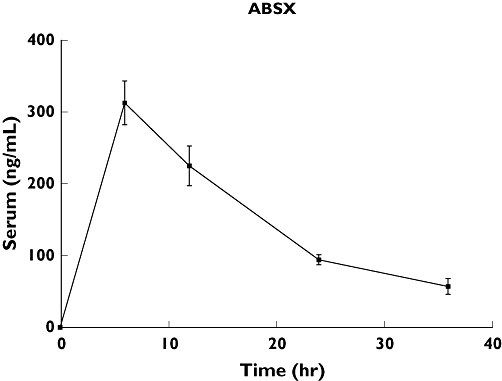
Concentrations–time curve of albendazole sulphoxide (ABSX) in the milk of lactating women after one single (400 mg) oral dose of ABZ. *Area under ABSX concentration–time curve (AUC)0–36 in milk = 5190.3 ng*h ml−1
Milk–serum ratios of albendazole and its metabolites
Ratios of milk–serum concentrations at 6 h after oral administration of ABZ were calculated and the medians (range) were: 0.9 (0.2–6.5) (n= 16); 0.6 (0.1–1.5) (n= 23) and 0.7 (0.2–1.9) (n= 18) for ABZ, ABSX and ABSO, respectively. In many instances, the concentrations were below LOQ. Thus, the number of calculated ratios varied.
Only for ABSX was there a significant (P= 0.0029, two-tailed) correlation (n= 23, Spearman r= 0.6, 95% confidence interval 0.2, 0.8) between milk and serum concentrations, 6 h after oral administration of ABZ (Figure 2). No significant correlation between milk and serum concentrations of ABZ (n= 16, Spearman r= 0.3, P= 0.2639) or ABSO (n= 18, Spearman r= 0.1, P= 0.6568) was found.
Figure 2.

Nonparametric correlation of the concentrations of serum albendazole sulphoxide (ABSX) in ng ml−1 to its concentrations in milk (n= 23; Spearman r= 0.6, 95% confidence interval 0.2, 0.8; P= 0.0029, two-tailed)
Discussion
In the present study, the pharmacokinetic parameters of ABZ and its major metabolites (ABSX and ABSO) in the milk of lactating women were determined after administration of a single oral (400 mg) dose of ABZ.
In the present study, the recovery of ABZ and its metabolites in milk and serum was similar to that in other studies using comparable methods. De Ruyck and colleagues [17] reported a recovery of 74 ± 1% for ABZ alone in cows' milk [compared with an estimated general (for ABZ and its metabolites) recovery of 62.1 ± 17.6% (mean ± SD) in the present study]. Moreover, Mirfazaelian and colleagues reported a recovery of 65% for ABSX in serum.
There are relatively few studies of ABZ pharmacokinetics in healthy subjects, probably due the difficulty of assay, and the high variability between individuals [15, 16].
In the present study, the milk–serum ratio of ABZ and ABSX were 0.9 and 0.6, respectively. While these figures reflected the median values, there was wide variability between the tested women. Besides the infant drug clearance, the milk–serum ratio is an important determinant of infant drug exposure [18]. In many instances, drugs are transferred into breast milk through passive diffusion and this is largely determined by the degree of ionization, lipophilicity and molecular weight [19]. Active, carrier-mediated mechanisms might be involved when the rate of transfer is greater than expected by passive diffusion [20]. Additionally, the breast cancer resistance protein ‘BCRP/ABCG2’ is involved in the transport of ABZ and ABSX into breast milk [21] and is reported to be regulated by several endogenous [22] and environmental [23] agents. So the implications of this regulation on the availability of ABZ and/or ABSX in the milk of breastfeeding mothers is not clear.
Drug clearance in infants develops at different rates depending on the system involved. Ito and Lee [18] reported that glomerular filtration rate takes 2–3 months to reach adult levels, starting from 25% at birth. Variable patterns are observed with CYP450 isozymes in infants.
ABZ is metabolized to the active sulphoxide (ABSX) through CYP3A4 [24]. Although CYP3A7 is expressed in fetal liver, it is largely replaced by the CYP3A4 isoform after birth [25]. CYP3A4 reaches 30–40% of adult activity 1 month after birth, while it is variable among neonates with different gestational age [26]. The intersubject variability in CYP3A4 activity is 5–10-fold [27] in adults, which probably could be responsible for the frequently observed wide variability of ABSX formation. Similar development and variability patterns apply to the intestinal CYP3A4 isoform [28].
ABSX (the main ABZ metabolite in milk, as shown in the present study) is expected to be less likely to be absorbed than the more lipophilic parent molecule.
The expected ‘dose’ of a drug that an infant would be exposed to after breastfeeding would be determined from the following equation [29]:
where Dinfant is the dose ingested by infant, Cmaternal is the maternal plasma concentration, M/PAUC is the AUC ratio in maternal milk and plasma and Vinfant is the volume of milk ingested by the infant daily (estimated as 0.15 l kg−1 day−1). If we collate the figures of ABSX from the different studies cited above and the findings of the present work, an estimated exposure of a breastfed infant to ABSX through 36 h (after a single maternal oral dose of 400 mg) would not exceed 0.1 mg kg−1 (of infant weight). Much lower exposure concentrations would be expected as regards ABZ, the parent drug, when secreted unchanged in milk. Interestingly, multiple dosing of ABZ could result in lower serum concentrations of ABSX (and hence in milk), due to the induction of its metabolism into the inactive sulphone metabolite [30].
Although the report of the Committee on Drugs of the American Academy of Pediatrics [31] did not include ABZ among the drugs that should be avoided by breastfeeding women, of special concern would be an effect of ABZ on growth. In a randomized clinical trial published in the Lancet, Forrester and colleagues [32] reported a decrease in selected markers of children's (between 2 and 10 years old) growth after administration of ABZ total doses up to 1200 mg (over 3 days) for the treatment of Trichuris trichiura (whipworm) infection. These findings, however, were in marked contrast to a number of other studies (e.g. Adams et al. in Kenya [33] and Hadju et al. in Indonesia [34]), where the growth of infants and young children was not affected and even increased following clearance of the helminth infection. Thus, the results of the Forrester study appear controversial, and the reduction in the selected growth markers could have resulted from the disease itself [35] rather than being an effect of ABZ treatment.
In conclusion, the results of the present study suggest that breastfed infants of breastfeeding mothers, who are administered a single 400-mg oral dose of ABZ, will be exposed to minimal concentrations of ABZ and to its active metabolite ABSX.
Footnotes
The Committee was not formally in existence at the time of formulating the study proposal and its conduct. On its establishment, it reviewed the protocols of all the studies that had been implemented before its formation and prior to the authors submitting this manuscript, which could not have been submitted without the Committee's approval.
Competing interests
M.B. is employed by GSK and has shares in the company. E.G. received a research grant from GSK for this research. J.H. worked for GSK at the time of implementing the research.
This work was funded by a grant from GlaxoSmithKline (GSK), UK. There was no involvement of the company in data acquisition or analysis.
REFERENCES
- 1.Reuter S, Jensen B, Buttenschoen K, Kratzer W, Kern P. Benzimidazoles in the treatment of alveolar echinococcosis: a comparative study and review of the literature. J Antimicrob Chemother. 2000;46:451–6. doi: 10.1093/jac/46.3.451. [DOI] [PubMed] [Google Scholar]
- 2.Del Brutto OH, Roos KL, Coffey CS, García HH. Meta-analysis: cysticidal drugs for neurocysticercosis: albendazole and praziquantel. Ann Intern Med. 2006;145:43–51. doi: 10.7326/0003-4819-145-1-200607040-00009. [DOI] [PubMed] [Google Scholar]
- 3.Horton J. Albendazole: a broad spectrum anthelminthic for treatment of individuals and populations. Curr Opin Infect Dis. 2002;15:599–608. doi: 10.1097/00001432-200212000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Ottesen EA, Ismail MM, Horton J. The role of albendazole in programmes to eliminate lymphatic filariasis. Parasitol Today. 1999;15:382–6. doi: 10.1016/s0169-4758(99)01486-6. [DOI] [PubMed] [Google Scholar]
- 5.Michael E, Malecela-Lazaro MN, Simonsen PE, Pedersen EM, Barker G, Kumar A, Kazura JW. Mathematical modelling and the control of lymphatic filariasis. Lancet Infect Dis. 2004;4:223–34. doi: 10.1016/S1473-3099(04)00973-9. [DOI] [PubMed] [Google Scholar]
- 6.McLaughlin SI, Radday J, Michel MC, Addiss DG, Beach MJ, Lammie PJ, Lammie J, Rheingans R, Lafontant J. Frequency, severity, and costs of adverse reactions following mass treatment for lymphatic filariasis using diethylcarbamazine and albendazole in Leogane, Haiti, 2000. Am J Trop Med Hyg. 2003;68:568–73. doi: 10.4269/ajtmh.2003.68.568. [DOI] [PubMed] [Google Scholar]
- 7.Opatrny L, Prichard R, Snell L, Maclean JD. Death related to albendazole-induced pancytopenia: case report and review. Am J Trop Med Hyg. 2005;72:291–4. [PubMed] [Google Scholar]
- 8.Anderson PO. Drug use during breast-feeding. Clin Pharm. 1991;10:594–624. [PubMed] [Google Scholar]
- 9.Ito S, Koren G, Einarson TR. Maternal non-compliance with antibiotics during breastfeeding. Ann Pharmacother. 1993;27:40–2. doi: 10.1177/106002809302700110. [DOI] [PubMed] [Google Scholar]
- 10.Montresor A, Awasthi S, Crompton DW. Use of benzimidazoles in children younger than 24 months for the treatment of soil-transmitted helminthiasis. Acta Trop. 2003;86:223–32. doi: 10.1016/s0001-706x(03)00042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allen HE, Crompton DW, de Silva N, LoVerde PT, Olds GR. New policies for using anthelmintics in high risk groups. Trends Parasitol. 2002;18:381–2. doi: 10.1016/s1471-4922(02)02386-3. [DOI] [PubMed] [Google Scholar]
- 12.Urbani C, Albonico M. Anthelminthic drug safety and drug administration in the control of soil-transmitted helminthiasis in community campaigns. Acta Trop. 2003;86:215–21. doi: 10.1016/s0001-706x(03)00036-6. [DOI] [PubMed] [Google Scholar]
- 13.Dayan AD. Albendazole, mebendazole and praziquantel. Review of non-clinical toxicity and pharmacokinetics. Acta Trop. 2003;86:141–59. doi: 10.1016/s0001-706x(03)00031-7. [DOI] [PubMed] [Google Scholar]
- 14.Marques MP, Takayanagui OM, Lanchote VL. Albendazole metabolism in patients with neurocysticercosis: antipyrine as a multifunctional marker drug of cytochrome P450. Braz J Med Biol Res. 2002;35:261–9. doi: 10.1590/s0100-879x2002000200016. [DOI] [PubMed] [Google Scholar]
- 15.Fletouris D, Bostoglou N, Psomas I, Mantis A. Rapid quantitative screening assay of trace benzimidazole residues in milk by liquid chromatography. J AOAC Int. 1996;79:1281–7. [PubMed] [Google Scholar]
- 16.Hoaksey P, Awadzi K, Ward SA, Coventry PA, Orme ML, Edwards G. Rapid and sensitive method for the determination of albendazole and albendazole sulphoxide in biological fluids. J Chromatogr. 1991;566:244–9. doi: 10.1016/0378-4347(91)80131-u. [DOI] [PubMed] [Google Scholar]
- 17.De Ruyck H, Van Renterghema R, De Riddera H, De Brabander D. Determination of anthelmintic residues in milk by high performance liquid chromatography. Food Control. 2000;11:165–73. [Google Scholar]
- 18.Ito S, Lee A. Drug excretion into breast milk—overview. Adv Drug Deliv Rev. 2003;55:617–27. doi: 10.1016/s0169-409x(03)00034-6. [DOI] [PubMed] [Google Scholar]
- 19.Atkinson HC, Begg EJ. Prediction of drug distribution into human milk from physicochemical characteristics. Clin Pharmacokinet. 1990;18:151–67. doi: 10.2165/00003088-199018020-00005. [DOI] [PubMed] [Google Scholar]
- 20.McNamara PJ, Meece JA, Paxton E. Active transport of cimetidine and ranitidine into the milk of Sprague Dawley rats. J Pharmacol Exp Ther. 1996;277:1615–21. [PubMed] [Google Scholar]
- 21.Merino G, Jonker JW, Wagenaar E, Pulido MM, Molina AJ, Alvarez AI, Schinkel AH. Transport of anthelmintic benzimidazole drugs by breast cancer resistance protein (BCRP/ABCG2) Drug Metab Dispos. 2005;33:614–8. doi: 10.1124/dmd.104.003319. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Zhou L, Gupta A, Vethanayagam RR, Zhang Y, Unadkat JD, Mao Q. Regulation of BCRP/ABCG2 expression by progesterone and 17beta-estradiol in human placental BeWo cells. Am J Physiol Endocrinol Metab. 2005;290:E798, 807. doi: 10.1152/ajpendo.00397.2005. [DOI] [PubMed] [Google Scholar]
- 23.Vlaming ML, Lagas JS, Schinkel AH. Physiological and pharmacological roles of ABCG2 (BCRP): recent findings in Abcg2 knockout mice. Adv Drug Deliv Rev. 2009;61:14–25. doi: 10.1016/j.addr.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Rawden HL, Kokwaro GO, Ward SA, Edwards G. Relative contribution of cytochromes P450 and flavin-containing monooxygenases to the metabolism of albendazole by human liver microsomes. Br J Clin Pharmacol. 2000;49:313–22. doi: 10.1046/j.1365-2125.2000.00170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lacroix D, Sonnier M, Moncion A, Cheron G, Cresteil T. Expression of CYP3A in the human liver—evidence that the shift between CYP3A7 and CYP3A4 occurs immediately after birth. Eur J Biochem. 1997;247:625–34. doi: 10.1111/j.1432-1033.1997.00625.x. [DOI] [PubMed] [Google Scholar]
- 26.Leeder JS. Ontogeny of drug-metabolizing enzymes and its influence on the pathogenesis of adverse drug reactions in children. Curr Ther Res. 2001;62:900–12. [Google Scholar]
- 27.Marques MP, Takayanagui OM, Lanchote VL. Albendazole metabolism in patients with neurocysticercosis: antipyrine as a multifunctional marker drug of cytochrome P450. Braz J Med Biol Res. 2002;35:261–9. doi: 10.1590/s0100-879x2002000200016. [DOI] [PubMed] [Google Scholar]
- 28.Merino G, Molina AJ, García JL, Pulido MM, Prieto JG, Alvarez AI. Intestinal elimination of albendazole sulfoxide: pharmacokinetic effects of inhibitors. Int J Pharm. 2003;263:123–32. doi: 10.1016/s0378-5173(03)00369-7. [DOI] [PubMed] [Google Scholar]
- 29.MEDSAFE. Information for health professionals. Available at http://www.medsafe.govt.nz/Profs/PUarticles/lactation.htm#Calculation (last accessed 15 May 2008.
- 30.Mirfazaelian A, Rouini MR, Dadashzadeh S. Time dependent pharmacokinetics of albendazole in human. Biopharm Drug Dispos. 2003;24:199–204. doi: 10.1002/bdd.355. [DOI] [PubMed] [Google Scholar]
- 31.American Academy of Pediatrics Committee on Drugs. Transfer of drugs and other chemicals into human milk. Pediatrics. 2001;108:776–89. [PubMed] [Google Scholar]
- 32.Forrester JE, Bailar JC, Esrey SA, José MV, Castillejos BT, Ocampo G. Randomised trial of albendazole and pyrantel in symptomless trichuriasis in children. Lancet. 1998;352:1103–08. doi: 10.1016/s0140-6736(97)08325-6. [DOI] [PubMed] [Google Scholar]
- 33.Adams EJ, Stephenson LS, Latham MC, Kinoti SN. Physical activity and growth of Kenyan school children with hookworm, Trichuris trichiura and Ascaris lumbricoides infections are improved after treatment with albendazole. J Nutr. 1994;124:1199–206. doi: 10.1093/jn/124.8.1199. [DOI] [PubMed] [Google Scholar]
- 34.Hadju V, Satriono AK, Stephenson LS. Relationships between soil-transmitted helminthiases and growth in urban slum school children in Ujung Pandang, Indonesia. Int J Food Sci Nutr. 1997;48:85–93. doi: 10.3109/09637489709006966. [DOI] [PubMed] [Google Scholar]
- 35.Winstanley P. Albendazole for mass treatment of asymptomatic trichuris infections. Lancet. 1998;352:1080–81. doi: 10.1016/S0140-6736(98)00027-0. [DOI] [PubMed] [Google Scholar]


