Abstract
Neurotensin, a tridecapeptide, is widely distributed in the brain and gastrointestinal tract. It possesses analgesic, hypothermic, and antipsychotic-like properties. Neurotensin’s effects are mediated mainly through two receptor subtypes, NTS1 and NTS2. Activation of NTS1 has been implicated in most of the pharmacological effects of neurotensin, but is associated with hypothermia and hypotension. We report on a novel neurotensin analog with higher selectivity to NTS2, namely, NT79 which exhibits selective behavioral effects.
NT79 was tested in animal models for pain (thermal - hot plate test; visceral – acetic acid-induced writhing test), and in animal models that are predictive of antipsychotic-like effects (apomorphine-induced climbing; d-amphetamine-induced hyperactivity; disruption of prepulse inhibition). Its effects on body temperature and on blood pressure were also determined. Neurochemical changes in extracellular neurotransmitters were measured using in vivo microdialysis while the rats were simultaneously evaluated for acetic acid-induced writhing with and without pretreatment with NT79.
Binding data at molecularly-cloned hNTS1 and hNTS2 suggest selectivity for hNTS2. NT79 blocked the acetic acid-induced writhing with an ED50 of 0.14μg/kg, while having no effect on thermal nociception. The writhing was paralleled by an increase in 5-HT which was attenuated by NT79. NT79 demonstrated antipsychotic-like effects by blocking apomorphine-induced climbing, d-amphetamine-induced hyperactivity, and reducing d-amphetamine- and DOI-induced disruption of prepulse inhibition. Uniquely, it caused no significant hypothermia and was without effect on blood pressure. NT79, with its higher selectivity to NTS2, may be potentially useful to treat visceral pain, and psychosis without concomitant side effects of hypothermia or hypotension.
Keywords: neurotensin analogs, neurotensin receptors, antipsychotic drugs, antinociception, rat
1. Introduction
Neurotensin (NT), a widely distributed neuropeptide in the central nervous system, and the gastrointestinal tract (Carraway and Leeman, 1976; Kitabgi, et al., 1976) is known to exert several effects at these sites, including analgesia (Clineschmidt, et al., 1979), hypothermia, and antipsychotic-like activity by activating two well defined NT receptors, the high affinity, levocabastine-insensitive NT receptor (NTS1) (Tanaka, et al., 1990; Vita, et al., 1993) and the low affinity, levocabastine-sensitive receptor (NTS2) (Chalon, et al., 1996; Mazella, et al., 1996). For NT to exert its effects it needs to be administered centrally, since it is readily digested by peptidases. For many years our laboratory has been developing NT analogs that exhibit central effects when administered systemically. NT69L is the most studied of these analogs. It has antinociceptive properties (Tyler-McMahon, et al., 2000) and antipsychotic-like effects (Boules, et al., 2001; Cusack, et al., 2000; Shilling, et al., 2003). NT69L has high affinity to both NTS1 and NTS2 receptors with no apparent selectivity to either receptor subtype. Compounds that activate NTS1 cause hypothermia and a profound, but transient, drop in blood pressure, as is seen with NT69L (Fantegrossi et al., 2005). To avoid these potential adverse effects, our laboratory has been developing new NT analogs with higher selectivity for NTS2 than NTS1 receptor subtype. We hypothesized that a NT analog with higher selectivity at the NTS2 receptor will maintain its antipsychotic-like and antinociceptive properties without causing hypothermia and hypotension. Here we report on one of these new compounds, namely NT79, which was tested in animal models that are predictive of antipsychotic-like activity (apomorphine-induced climbing, d-amphetamine-induced hyperactivity, and reduction of disrupted prepulse inhibition). Additionally, NT79 was tested for its ability to lower body temperature, lower blood pressure, and block thermal (hot plate) and visceral (writhing) pain in animals. The data show NT79 to be unique in its effects. It exhibits antipsychotic-like activity, selective antinociceptive effect with no significant hypothermia and no hypotension.
2. Results
2.1. Binding affinity
Table 1 shows the sequences of NT79, NT69L (for comparison), and NT. Radioligand binding results show that NT79 had higher affinity for hNTS2 as compared to that for hNTS1 receptor.
Table 1.
Sequence and binding data comparisons for NT, NT69L, and NT79
| Kd (nM) | |||
|---|---|---|---|
| Reference Name | Compound Sequence | hNTS1 Geometric Mean±SEM | hNTS2 Geomtetric Mean±SEM |
| NT | Neurotensin(1-13) pyroGlu-Leu-Tyr-Glu-Asn-Lys-Pro-Arg-Arg-Pro-Tyr-Ile-Leu-OH | 1.94 ± 0.07 | 6.5 ± 0.1 |
| NT69L | [N-methyl-Arg8,L-Lys9,L-neo-Trp11,tert-Leu12]NT(8-13) | 3.1 ± 0.4 | 2.1 ± 0.2 |
| NT79 | [N-methyl-Arg8,D-3,1-Nal11,tert-Leu12]NT(8-13) | 2100 ± 300 | 10 ± 2 |
The Kd values reflect the geometric mean ± SEM. Each value is of duplicate determinations made in at least three independent experiments.
2.2. Effect of NT79 on body temperature
Unlike the atypical antipsychotic drugs (APDs) (Goudie, et al., 2007; Goudie, et al., 1999) and non-selective NT agonists, such as NT69L (Tyler-McMahon, et al., 2000), which cause a large (−4.5°C) and long-lasting (>4 h) drop in body temperature, NT79 caused only a mild decrease (−1.5°C) in body temperature. The drop in body was transient and returned to baseline 1 h post-injection (Figure 1).
Figure 1. Effect of NT79 injection on body temperature.
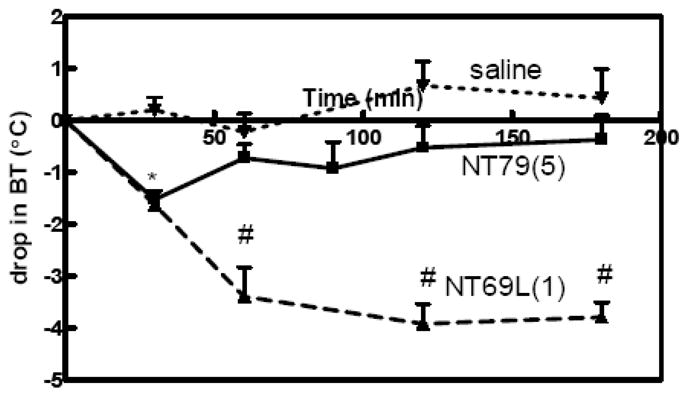
Sprague–Dawley rats (n=4) were given acute injection of NT79 (5mg/kg), NT69L (1mg/kg) i.p. or saline. Body temperature was recorded before (basal) and for 3 h after NT79, NT69L or saline injection. Rectal temperatures were recorded using a thermistor probe. Values are means ± S.E.M. ( ) dose administered in mg/kg. *Significantly different from saline; #significantly different from NT79 and saline (P<0.001).
2.3. Effect of NT79 on blood pressure
As with saline, NT79 resulted in a drop in blood pressure within 2 min post injection, after which it returned to baseline. Isoproterenol administration resulted in a drop in blood pressure 1 min post injection. The isoproterenol-induced drop in blood pressure persisted for 10 min after drug administration. There was no significant difference between the effect of saline and NT79 administration on blood pressure. Isoproterenol, which is confirmed to decrease blood pressure in animals, significantly (P<0.05) decreased blood pressure as compared to saline and NT79 (Figure 2).
Figure 2. Effect of NT79 on blood pressure.
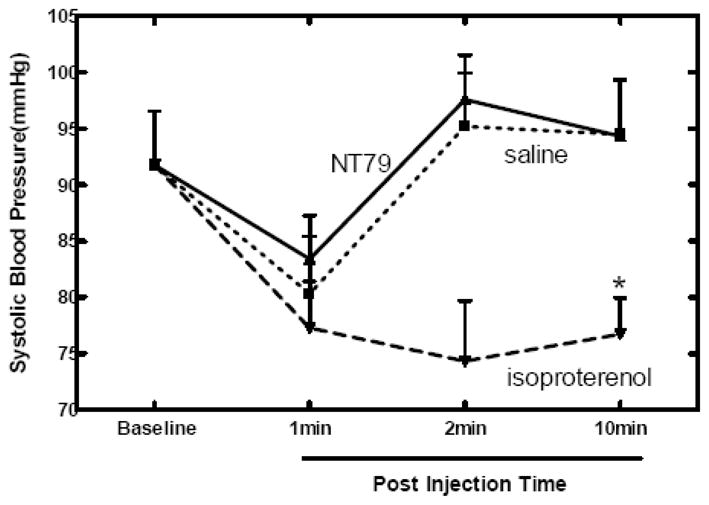
Animals (n=4) were maintained and blood pressure was measured as detailed in Materials and Methods. Injection of saline and NT79 did not alter blood pressure. Isoproteronol injection dropped blood pressure. *Significantly different from saline and NT79 (P<0.05).
2.4. Behavioral Testing
2.4.a. Antipsychotic-like activity
2.4.a.i. Blockade of Apomorphine-induced climbing
As expected, subcutaneous injection of apomorphine at 600 μg/kg caused distinctive climbing that lasted for about 60 min. Our laboratory (Cusack, et al., 2000) and others (Jolicoeur, et al., 1991) have found that NT when injected directly into brain or brain-penetrating NT receptor agonists, when injected systemically, reduce apomorphine-induced climbing behavior. Similarly, NT79 treatment caused a significant (P<0.001) blockade of the climbing (Figure 3).
Figure 3. Effects of NT79 on apomorphine-induced climbing behavior.
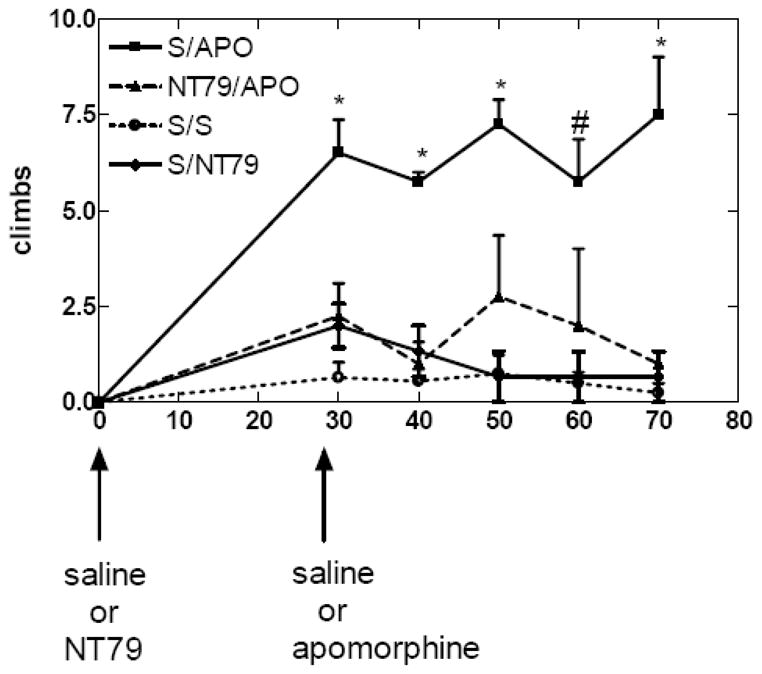
Rats (n=4–8) were maintained as described in the Methods section. Rats were acclimated in a clear plastic cage for 1h after which they were injected with either saline or NT79 (5 mg/kg i.p.). Thirty minutes later the rats were injected with saline or apomorphine (600 μg/kg s.c.). Climbing behavior was observed for 1 h after injection. The number of times the rat moved up and down on its hind legs counted as one climb. Values are means ± S.E.M. * Significantly different from all other treatments (P<0.002), #significantly different from S/S and S/NT79 (P=0.018). There were no significant differences in results between NT79/APO, S/S and S/NT79
S=saline APO=apomorphine
2.4.a.ii. Reduction in d-Amphetamine-induced hyperactivity
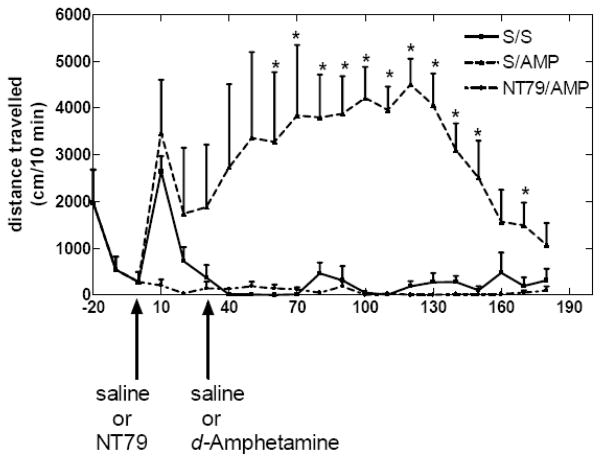
d-Amphetamine administration resulted in hyperactivity which was significantly (P<0.001) blocked by pretreatment with NT79. There was no significant difference in activity between saline and NT79 (Figure 4).
Figure 4. Effect of NT79 on blocking d-amphetamine-induced hyperactivity.
Each Sprague–Dawley rat (n=4) was placed in an Opto-Varimex Minor motility chamber (Columbus Instruments, Columbus, OH) activity chamber for 1 h for acclimation. After obtaining a 30-min baseline, each rat was removed for i.p. injection with NT79 (5 mg/kg) or saline, and then placed back in the activity chamber. Thirty minutes later the each rat was again removed from the chamber, injected with saline or d-amphetamine (5 mg/kg) i.p., and returned to the chamber. Activity was recorded for 3h. * Significantly different (P<0.001) from S/A. There was no significant difference between NT79/A and S/S.
S=saline A=amphetamine
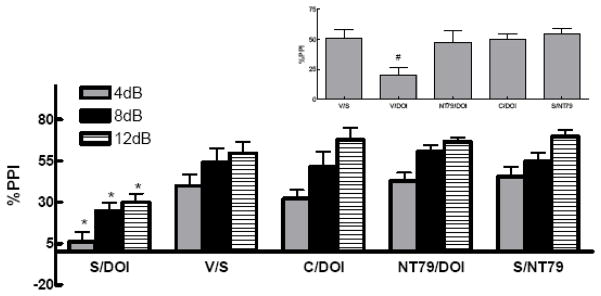
2.4.a.iii. Reduction of drug-induced disruption of prepulse inhibition (PPI)
The % PPI for the vehicle/saline group of rats was not significantly different from the saline/saline group (39±11 and 57±11, 60±9 and 42±6, 50±13 and 60±12 for vehicle and saline groups at 4, 8, and 12 dB pulse intensities, respectively). Therefore, the average of both groups was used as control. The saline/NT79 and vehicle/saline groups were used as controls for both studies. There was no significant difference (P=0.773) between all control groups tested (50±7, 48±3, 48±8, and 55±4 for vehicle/saline, saline/haloperidol, saline/clozapine, and saline/NT79, respectively).
2.4.a.iii.1) Effect of NT79 and haloperidol on d-amphetamine-induced disruption of PPI
Figure 5 shows a significant effect of treatment with NT79 or haloperidol in preventing the d-amphetamine-induced disruption of PPI (F 3,55 = 15.4; F 3,55 = 21.9; F 3,55 = 27.99, P<0.001) at 4, 8, and 12 dB prepulse intensities, respectively. Comparisons among the groups show that haloperidol and NT79 significantly (P=0.001) blocked d-amphetamine-induced disruption of PPI. Two-way ANOVA shows no significant interaction between treatment group and the pulse intensity (P=0.954).
Figure 5. Effect of NT79 and haloperidol on blocking d-amphetamine-induced disruption of PPI.
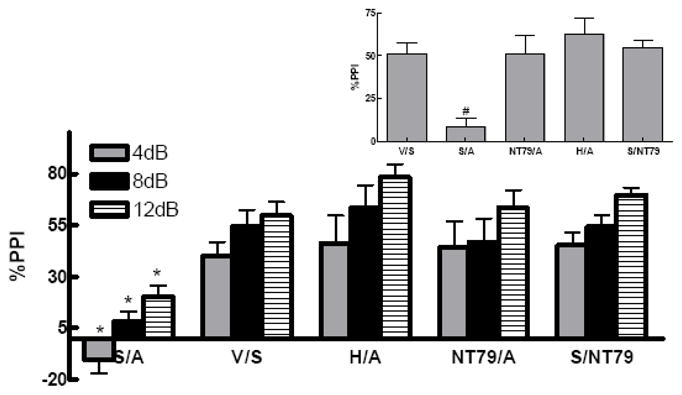
Sprague-Dawley rats (n=4–19) were administered s.c. injections of either NT79 (5 mg/kg), haloperidol (1 mg/kg) or saline. Thirty minutes later they were injected s.c. with either saline or d-amphetamine (5 mg/kg). Twenty minutes later the animals were placed in the startle chambers and PPI measured as previously described. Data is presented as the %PPI ± SEM at 4, 8 and 12 (dB above background) prepulse intensities. Inset shows the average %PPI. #Significantly different (P<0.001) from all other treatments. *Significantly different (P<0.05) from all other treatment at the corresponding pulse intensity. Values for the saline/d-amphetamine group and the haloperidol/d-amphetamine groups are from part of a larger PPI study from our laboratory (Briody et al., 2009) done in parallel with this NT79 work.
S=saline A=amphetamine V=vehicle H=haloperidol
2.4.a.iii.2) Effect of NT79 and clozapine on DOI-induced disruption of PPI
Figure 6 shows a significant effect of treatment with either NT79 or clozapine in preventing the disruption of PPI by the 5-HT2A receptor agonist DOI (F 3,34= 5.781; F 3,30 = 6.16; F 3,31 = 13.433, P<0.003) at 4, 8, and 12 dB pulse intensities, respectively. Comparisons among the groups show that clozapine and NT79 significantly (P=0.001) blocked DOI-induced disruption of PPI. Two-way ANOVA shows no significant interaction between treatment group and the pulse intensity (P=0.803).
Figure 6. Effect of NT79 and clozapine on prevention of DOI-induced disruption of PPI.
Rats (n=4–15) were treated as described in Figure legend 3. d-Amphetamine was replaced with DOI (0.5 mg/kg). Data is presented as the %PPI ± SEM at 4, 8 and 12 (dB above background) prepulse intensities. Inset shows the average %PPI. #Significantly (P<0.05) different from all other treatments. *Significantly (P<0.05) different from all other treatment at the corresponding pulse intensity. Values for the saline/DOI group the clozapine/DOI groups are from part of a larger PPI study from our laboratory (Briody et al., 2009) done in parallel with this NT79 work. V=vehicle C=clozapine S=saline
d-Amphetamine and DOI significantly increased the startle response (P<0.05 There was no significant difference in the startle response between the control group and the haloperidol/d-amphetamine or NT79/d-amphetamine group (300±60, 280±80, and 240±80 for the control, haloperidol/d-amphetamine, and NT79/d-amphetamine, respectively). Similarly, there was no significant difference in the startle response between the control group and the clozapine/DOI or NT79/DOI group (300±60, 150±30, and 140±60 for the control, clozapine/DOI, and NT79/DOI, respectively). There was no significant effect of NT79 on startle alone as compared to the control group (Tables 2).
Table 2.
Effects of treatment on pulse-alone startle responding
| Treatment (n) | Startle response (Mean ± SEM) |
|---|---|
| Saline/saline (10) | 300 ± 60 |
| Saline/NT79 (8) | 320 ± 70 |
| Saline/d-amphetamine (15)a | 600 ± 60* |
| NT79/d-amphetamine (4) | 240 ± 80 |
| Haloperoid/d-amphetamine (4)a | 280 ± 80 |
| Saline/DOI (19)a | 470 ± 50** |
| NT79/DOI (4) | 140 ± 60 |
| Clozapine/DOI (11)a | 150 ± 30 |
The table presents the effects of NT79 (5 mg/kg), haloperiod (1 mg/kg) and clozapine (5 mg/kg) on the startle-response amplitude in rats given s.c. injections of saline, the indirect dopamine agonist d-amphetamine (5 mg/kg), or the serotonin agonist DOI (0.5 mg/kg).
Significantly different from saline/saline, NT79/d-amphetamine, and haloperiod/d-amphetamine (P<0.05).
Significantly different from saline/saline, NT79/DOI and clozapine/DOI (P<0.05).
Values are from part of a larger PPI study from our laboratory (Briody et al., 2009) done in parallel with this NT79 work.
2.4.b. Antinociceptive activity
2.4.b.i. Effect of NT79 on thermal pain (hot plate test)
In contrast to centrally administered NT and systemically administered NT69L (1 mg/kg) (Tyler-McMahon et al., 2000), which causes a strong (~80% MPE) and long-lasting (4 h) antinociceptive effect in the hot plate test, NT79 (5 mg/kg) did not block thermal nociception (Figure 7).
Figure 7. Effect of NT79 on thermal pain (hot plate test).
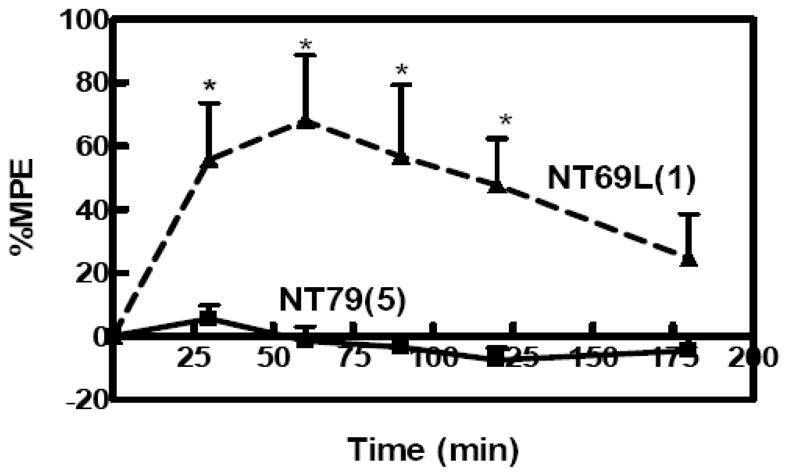
Antinociception was measured by hot plate test and presented as the percent of maximum possible effect (%MPE). %MPE = [(post-drug latency - pre-drug latency)/(cut-off - pre-drug latency)]×100. Baseline (pre-drug latency) was measured for each rat. The rats (n=4) were then injected with NT79 (5 mg/kg) i.p. and 30 min later placed on a metal plate (15×20 cm), maintained at a temperature of 52.5±0.15°C. The latency between the time the rat is placed on the surface and the time it licks either of its hindpaws was measured. Failure to respond in 30 s resulted in ending the trial and assignment of that latency. The effect of NT69L on thermal pain was added to the figure for comparison (Boules M., 2009). ( ) dose administered in mg/kg. *Significantly different from NT69L P<0.012.
2.4.b.ii. Effect of NT79 on visceral pain
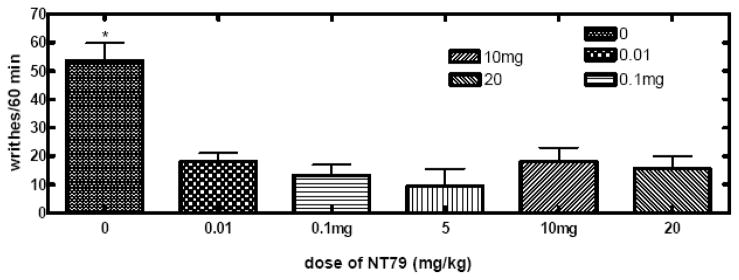
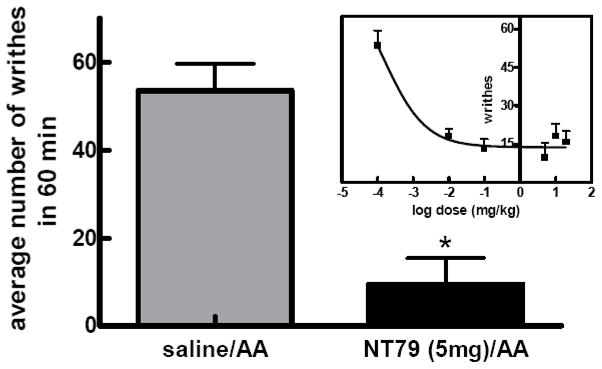
NT79 significantly (P<0.001) reduced the number of writhes caused by the injection of 2% acetic acid with an ED50 of 0.14 μg/kg (Figure 8a–b).
Figure 8. a–b. Effect of NT79 on visceral pain (writhing test).
Rats (n=4) were pretreated with NT79 (5 mg/kg) or saline, injected with 0.5 ml of a 2% aqueous solution of acetic acid and placed individually in clear plastic cages for observation. The number of writhes was counted during a 60 min observation period. A writhe is defined as stretching of the hind limbs accompanied by a contraction of abdominal muscles. The data is presented as average number of writhes in 60 min ±SEM. Figure 8a shows all doses tested. Figure 8b shows the average number of writhes for rats injected with NT79 at 5 mg/kg. Inset in Figure 8b shows the dose response curve for NT79 in blocking the acetic acid-induced writhing. Different groups of rats were injected with various doses of NT79 (0–20 mg/kg) and the writhing test was performed as described previously. The ED50 for NT79 was 0.14μg/kg (determined by nonlinear regression analysis using GraphPad Prism software). (Note: in the dose response curve, the dose 0.0001 mg/kg was used to calculate “log dose 0”).
2.5. In vivo microdialysis
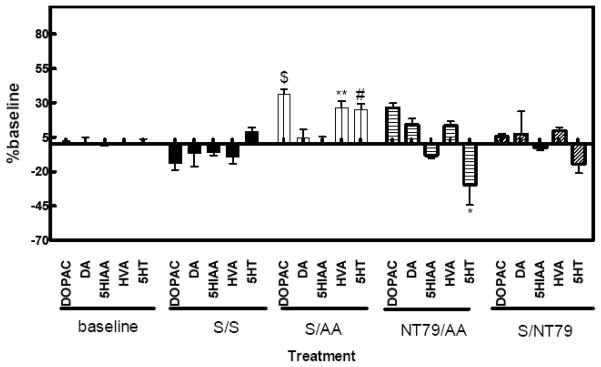
Writhing was paralleled by a significant (P<0.05) increase in 5-HT, and the DA metabolites DOPAC and HVA levels in the periaqueductal gray (PAG) as measured by in vivo microdialysis. The reduction in writhing after NT79 injection was associated with a significant decrease in 5-HT (P<0.05) (Figure 9). Table 3 shows the values of 5-HT, DA, and their metabolites in the PAG dialysate.
Figure 9. Effect of NT79 on monoamines in freely moving rats using microdialysis.
Rats (n=4) were injected with NT79 (5 mg/kg i.p.) or saline, after 6 fractions were collected using a probe inserted into the PAG to establish a baseline. Saline or 2% acetic acid was then injected i.p. and the fractions collected after the injections were compared to the baseline for each animal. The detection limits of DA, 5-HT and DOPAC are 22.6, 37.8 and 259.4 pM, respectively. The results represent mean ± SEM. *Significantly different from S and AA; #significantly different from S; **significantly different from S and NT/AA; $significantly different from S and NT.
S= saline NT= NT79 AA=acetic acid
Table 3.
In vivo microdialysis show effects of NT79 (5 mg/kg) and 2% acetic acid on the levels of 5-HT, DA, and their metabolites in PAG dialysate.
| (pg/20μl dialysate) | S/S | S/AA | S/NT79 | NT79/AA |
|---|---|---|---|---|
| 5-HT | 5±1* | 12±2 | 10±4 | 3±0.8* |
| 5-HIAA | 1600±100 | 1400±184 | 800±200 | 1300±10 |
| DA | 1.0±0.2 | 1.8±0.9 | 1.0±0.3 | 1.4±0.2 |
| HVA | 60±20 | 100±50 | 30±20 | 400±200 |
| DOPAC | 120±40 | 180±90 | 40±30 | 50±10 |
Rats were treated and samples collected as described in the legend to Figure 9. The monoamine levels were quantitated from measurements of the area under the curve (AUC) and the use of standard curves, which were generated by linear regression analyses (GraphPad Prism, GraphPad Software, Inc., La Jolla, CA, USA). The data represent the meant ± SEM of fractions collected after the second injection.
Significantly different from S/AA (P<0.05).
S=saline NT=NT79 AA=acetic acid
3. Discussion
The most studied NT agonist NT69L demonstrates antipsychotic-like activity and possesses antinociceptive properties, but causes hypothermia (Tyler-McMahon, et al., 2000), hypotension (Fantegrossi et al., 2005), and muscle relaxation (Osbahr, et al., 1979). Although these effects are temporary, they can affect the clinical testing of the compound. Here we report on a new NT analog, NT79, which similar to systemically administered NT69L and centrally administered NT, has antipsychotic-like and selective antinociceptive properties without causing hypothermia or hypotension. Unlike NT69L, which has about equal affinity for both NTS1 and NTS2, NT79 has a higher affinity for hNTS2 receptor subtype.
NTS1 has been implicated in many of the pharmacological effects of NT including analgesia against thermal stimuli, and antipsychotic-like behavior (Pettibone, et al., 2002; Tyler, et al., 1998). NTS1 receptor agonists are potent antinociceptive agents and hold great promise for treatment of pain (Tyler, et al., 1999). Additionally, they possess antipsychotic-like activity (Boules, et al., 2001; Cusack, et al., 2000; Shilling, et al., 2003). However, these compounds, as previously mentioned, are associated with potential adverse effects, such as hypotension and hypothermia. Reports on the presence of high levels of NTS2 mRNA and strong immunolabeling in the PAG and dorsal raphe nuclei, areas implicated in pain regulation, provide evidence for a potential role for NTS2 in antinociception (Sarret, et al., 2005). However, the role of NTS2 in antinociception remains controversial. The use of NTS2 antisense oligonucleotides (Dubuc, et al., 1999) or NTS2 deficient mice (Maeno, et al., 2004) markedly inhibited NT-induced antinociception. Some reports implicated NTS2 in the antinociceptive effect against visceral stimuli (Dubuc, et al., 1999), while other researchers propose that NTS2 is the main receptor implicated in analgesia of all types (thermal, chemical, or mechanical) (Bredeloux, et al., 2006). The present series of experiments evaluated a novel NT analog, NT79 with higher affinity to the hNTS2 receptor in thermal and visceral animal models for pain. As previously reported (Ghia, et al., 2004), acetic acid induced robust writhing in rats. The writhing was paralleled by elevated levels of 5-HT and the DA metabolites HVA and DOPAC in the PAG as measured by in vivo microdialysis suggesting that the effects observed may be related to altered metabolism of these monoamines. Although the presence of 5-HT receptors in areas of the brain involved in pain processes suggests a possible role for its receptors in pain modulation, researchers reported that the activation of 5-HT receptors can facilitate, inhibit, or fail to alter pain responses (Bardin, et al., 2001), depending on the nature of nociceptive stimuli applied (Millan, 2002). Such dual effects could also be related to the existence of multiple 5-HT receptors expressed both at the periphery and in the central nervous system.
The elevated levels of 5-HT after administration of acetic acid is consistent with several reports showing that in peripheral tissues, 5-HT application produces pain in humans (Ernberg, et al., 2000; Richardson, et al., 1985) and nociceptive behaviors (Hong and Abbott, 1994; Parada, et al., 2001; Sufka, et al., 1992; Wheeler-Aceto, et al., 1990) and hyperalgesia in rodents (Abbott, et al., 1996; Hong and Abbott, 1994; Okamoto, et al., 2002; Parada, et al., 2001). 5-HT was also reported to participate in the nociception induced by formalin, carrageenan and by complete Freund’s adjuvant (Doak, 1997; Taiwo and Levine, 1992). It has been postulated that the elevated 5-HT levels may activate and/or stimulate populations of 5-HT3 receptors that mediate inflammatory nociception since in previous reports 5-HT produced nociceptive writhing responses that were attenuated by pretreatment with tropisetron (Giordano and Gerstmann, 2004). Additionally, the 5-HT3 receptors located on sensory nerve terminals including the viscera have been shown to mediate the 5-HT-induced pain and hyperalgesia (Giordano and Dyche, 1989; Giordano and Rogers, 1989; Orwin and Fozard, 1986; Richardson, et al., 1985). In the present study pretreatment with NT79 significantly reduced the acetic acid-induced writhing and 5-HT levels simultaneously suggesting a modulatory effect on serotonergic transmission (Jolas and Aghajanian, 1997). Additionally, the change in the serotonergic system was accompanied by an increase in the DA metabolites HVA and DOPAC. The increase in DA metabolism might also contribute to the nociceptive effect of 5-HT as reported previously (Tambeli, et al., 2006). Although NT79 showed strong analgesic effect in the visceral pain model, the effect on visceral pain seems to be selective since it did not block the thermal pain in the hot plate test.
In addition to its antinociceptive effect, NT and NT receptor agonists produce behavioral and neurochemical effects similar to those of antipsychotic drugs (APD) (Boules, et al., 2003; Boules, et al., 2005; Boules, et al., 2006; Boules, et al., 2007; McMahon, et al., 2002; Tyler-McMahon, et al., 2000). When evaluated in animal models predictive of antipsychotic efficacy both NT and NT receptor agonists blocked apomorphine-induced climbing with no effect on stereotypic behavior (Cusack, et al., 2000; Jolicoeur, et al., 1983; Jolicoeur, et al., 1993), d-amphetamine-induced hyperactivity (Boules, et al., 2001; Ervin, et al., 1981; Ford and Marsden, 1990; Jolicoeur, et al., 1993; Kalivas, et al., 1983; Kalivas, et al., 1984; Nemeroff, et al., 1983; Sarhan, et al., 1997) and pharmacologically-induced disruption of prepulse inhibition (Feifel, et al., 1997; Shilling PD, 2004; Shilling, et al., 2003).
In all of the above mentioned animal models, NT79 behaved like an antipsychotic drug. Apomorphine is a non-selective dopamine D2/D3 receptor agonist that has been used for decades as one of many tests to screen for neuroleptic compounds (Niemegeers and Janssen, 1979). At low dosages apomorphine (e.g., 25–200 μg/kg) activates presynaptic receptors (Stahle and Ungerstedt, 1984), while at higher dosages (e.g., 600 μg/kg) it has effects on post-synaptic sites (Anden, et al., 1967) which results in behaviors that can discriminate between typical and atypical neuroleptics with respect to their selective potency to block these effects. Specifically, atypical neuroleptics are more potent at blocking the climbing behavior compared to their potency at blocking oro-facial stereotypies (sniffing and licking behaviors) induced by high-dose apomorphine in rats or mice (Gerhardt, et al., 1985; Puech, et al., 1978). Thus, NT79 behaved like an atypical antipsychotic drug by significantly blocking only the climbing behavior. Similarly, NT79 significantly blocked the hyperactivity caused by d-amphetamine, which models psychosis and positive symptoms of schizophrenia in a manner similar to that of centrally administered NT and systemically injected NT69L (Boules, et al., 2001; Nemeroff, et al., 1983). Additionally, NT79 prevented the disruption of PPI induced by both the DA agonist d-amphetamine and the 5-HT2A serotonin agonist DOI similar to both the typical APD haloperidol and the atypical APD clozapine, respectively. In the present study the effect of the drugs on the startle response did not seem to contribute significantly to its effects on PPI since d-amphetamine and DOI caused higher pulse intensities but still disrupted PPI. Additionally, although d-amphetamine has motor stimulatory effects, and caused an increase in the startle response, this is unlikely to have affected the PPI since PPI and locomotor activity have dissociable mesolimbic substrates (Feifel and Reza, 1999). Thus our results provide further support to previous reports on the lack of correlation between the effect of drugs on PPI and the startle response (Auclair, et al., 2006).
In contrast to NT and NT69L, while maintaining the antipsychotic-like and selective antinociceptive properties, NT79 did not cause hypothermia or hypotension, effects that are usually associated with NT administration (Bissette, et al., 1976; Boules, et al., 2001; Dubuc, et al., 1992; Tyler-McMahon, et al., 2000). Thus, NT79 may represent a novel class of antipsychotic drug.
The present data also suggest that NT79 can be potentially useful to treat visceral pain, possibly through modulating the serotonergic neurotransmission. Additionally, it demonstrates an antipsychotic-like activity for NT79 without the adverse side effects (hypothermia and hypotension) associated with the NTS1 receptor agonists. The studies also show that behavioral data can be used in conjunction with in vivo microdialysis to gain insight into ongoing pain-related processes.
4. Materials and Methods
4.1. Animals
Independent groups of 4–19 Sprague-Dawley rats (250–300g) (Harlan Sprague Dawley, Indianapolis IN, USA) were used for each test group. Animals were maintained in a temperature-controlled room with 12/12 h light/dark cycle with free access to food and water. All animal experiments were approved by Mayo Clinic Institutional Animal Care and Use Committee in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals.
4.2. Drugs
NT79 was synthesized in the Mayo Proteomics Research Center (RC, MN) as previously described (Fauq, et al., 1998). All drugs were administered intraperitoneally (i.p.) unless otherwise stated.
4.3. Receptor binding assay in CHO cells expressing human NTS1 and NTS2
NT receptor binding was done as described by Gendron et al (Gendron, et al., 2004), with few modifications. Briefly, cells were harvested, centrifuged, and the pellet was homogenized in 10 volumes of 50mM Tris-HCl buffer, pH 7.5 with a Polytron homogenizer at setting 6 for 15s. After centrifugation the pellet was re-suspended in cold Tris buffer containing 0.2% BSA (bovine serum albumin), and 0.8mM 1–10 phenanthroline. Competition binding assays with [3H]-NT and varying concentrations of unlabeled NT, and peptide analogs were carried out with membranal preparations from CHO-K1 cells expressing hNTS1 or hNTS2. The membrane preparations were incubated with a final concentration of 0.2nM of 3[H]-NT for 45 min at 26°C. At the end of the incubation period, the membranes were harvested onto GF/B glass fiber filters presoaked in 0.2% polyethylenimine in water. Radioactivity was determined by liquid scintillation spectrometry. Analysis of the data was performed using Sigma Plot software for one site analysis. Nonspecific binding was defined as that not displaced by 1μM NT. Protein concentration was determined by BCA assay (Pierce #23228, USA) using BSA as standard.
4.4. Animal Behavior
4.4.a. Body temperature
Body temperature was measured by means of a thermistor probe inserted approximately 2 cm into the rectum of the rat before (baseline) and at 30 min intervals for 3h after i.p. injection of NT79 (5 mg/kg), NT69L (1 mg/kg) or saline.
4.4.b. Blood Pressure
Rats (n=4) were anaesthetized with a rodent cocktail [ketamine (100 mg/ml) and xylazine (20 mg/ml)], administered I.M. (0.7 ml/kg). The carotid artery was cannulated with a PE-50 catheter (Clay Adams) for direct blood pressure determination. The catheter was exteriorized between the shoulder blades, filled with a heparin solution (10 U/ml; Elkins-Sinn, Inc.), and sealed with stylets. After a recovery period of 3h, direct blood pressure was recorded in free-moving, non-restrained animals with a pressure transducer coupled to a Digi-Medical blood pressure analyzer (Micro-Medical). After a 30 min equilibration period, 300ul of saline was injected and their blood pressure was measured. Fifteen minutes after the rats’ blood pressure returned to baseline, NT79 (5mg/kg) was administered i.p. The rats’ blood pressure was then recorded in response to NT79. Isoproterenol, which is confirmed to decrease blood pressure in animals, was given at dose of 150ug/kg by subcutaneous injection.
4.4.c. Behavioral testing for the antipsychotic efficacy of NT79
4.4.c.i. Apomorphine-induced climbing
Rats (n=4–8) were acclimated to a plastic cage (18″ × 9″3/8 × 7″5/8) for 1h after which the rats were pretreated with either saline or NT79 (5mg/kg) i.p. followed 30 min later by saline or apomorphine (600 μg/kg, s.c.) dissolved in oxygen-free hot 0.9% NaCl solution containing 0.1% (w/v) ascorbic acid to prevent oxidation. The volume of injection was 1 ml/kg under the loose skin of the back of the animal’s neck. Immediately following these injections, the rats were placed in empty plastic cages for observation. Climbing behavior was monitored and recorded for 1 h. Climbing episodes were quantitated by recording the number of times the rat moved up and down in a vertical position and recorded for every 5 min observation period (Jolicoeur et al., 1981).
4.4.c.ii. d-Amphetamine-induced locomotor activity
Locomotor activity was monitored in a Plexiglas Opto-Varimex Minor motility chamber (Columbus Instruments, Columbus, OH). Following an acclimation period of 1 h baseline activity was recorded for 30 min. The rats (n=4) were then injected with NT79 (5 mg/kg) or saline and placed back in the activity chamber for 30 min after which they were injected with saline or d-amphetamine at 5 mg/kg. Activity was recorded for 3 h. All injections were given i.p.
4.4.c.iii. Prepulse inhibition
4.4.c.iv. Equipment
Four identical startle chambers (San Diego Instruments, CA) were used. Each chamber consisted of a clear Plexiglas cylinder that rests on a Plexiglas platform inside a ventilated and illuminated enclosure housed in a sound attenuated room. A continuous background noise of 65 dB and the various acoustic stimuli were produced within each chamber by a high-frequency loudspeaker. The whole-body startle response of each animal resulted in vibrations that were transduced into analog signals by a piezoelectric unit mounted underneath the platform.
4.4.c.v. Test Sessions
Tests were performed as previously described (Shilling PD, 2004). The animals were subjected to a 5-min acclimation to a 65dB background noise, which continued throughout the test session. The test session consisted of 5 trial types within a 15 minute time period. Trials were: no stimulus, in which only background noise was presented, pulse alone (a 120dB startle pulse) and prepulse plus pulse (prepulses 4, 8 or 12 dB above background). Prepulses were presented 100ms (onset to onset) before the pulse. All trial types were presented in pseudo-random order separated by an average of 15 s (range 8–23s). In addition, four pulse alone trials were presented at the beginning and end of the session. These are not used in the calculation of prepulse inhibition. PPI was calculated as a percentage of the pulse alone startle stimulus using the following formula: [1 − (startle magnitude after prepulse-pulse pair/startle magnitude after pulse only] × 100.
The rats (n=4–19) were administered either NT79 (5mg/kg), haloperidol (1 mg/kg), clozapine (20mg/kg) or saline. Thirty minutes later they were injected s.c. with either saline, d-amphetamine at 5mg/kg or DOI at 0.5mg/kg. Twenty minutes later the animals were placed in the startle chambers and PPI measured. NT79, DOI, and d-amphetamine were dissolved in saline; haloperidol and clozapine were dissolved in ethanol and/or saline with 0.04% (v/v) acetic acid (vehicle).
4.4.d. Antinociception
The antinociceptive effect of NT79 was tested in thermal (hot plate) and visceral (writhing) pain tests.
4.4.d.i. Hot plate test
Rats (n=4) were placed on a metal plate (15 × 20 cm) surrounded by a transparent plastic cage and heated to 52.5°C (MOD 35D Hot Plate, Life Science Instruments, CA, USA). Baseline testing for the hot plate test was done for each rat immediately prior to the experiment. NT79 (5 mg/kg) was then injected i.p. and 30 min later, each rat was placed on the hot plate the temperature of which was maintained at 52.5±0.15°C. The latency between the time the rat is placed on the surface and the time it licks either of its hind paws or jumps was recorded. Failure to respond in 30 s resulted in ending the trial and assignment of that latency to prevent tissue damage. Hot plate results were scored as the percentage of maximum possible effect (%MPE) and were calculated according to the following equation: MPE=100 × (test latency-baseline latency)/(cutoff time [30 s]-baseline latency). For comparison data from a previous study (Boules et al., 2009) was added to show the effect of NT69L (1mg/kg i.p.) on %MPE in the hot plate.
4.4.d.ii. Writhing test
Rats (n=4) were pretreated with different doses of NT79 (5 mg/kg), or saline, and 30 min later injected with 0.5 ml of a 2% aqueous solution of acetic acid and placed individually in clear plastic cages for observation. The average number of writhes ± SEM was counted during a 60 min observation period. A writhe is defined as stretching of the hind limbs accompanied by a contraction of abdominal muscles. To determine the ED50 dose for NT79, a dose-response curve was generated for NT79L in the writhing test with the use of various doses (0 – 20 mg/kg) of NT79L injected into different groups of rats (n≥8).
4.5. Determination of brain monoamines by in vivo microdialysis
4.5.a. Surgery and drug administration
Rats, n=4–6, (initial weight 220–250 g) were housed in a temperature controlled room with 12 h light-dark cycle and free access to food and water. The rats were cannulated in the PAG (A-7.5. L0.5, V4.4) to determine the extracellular level of dopamine (DA) and serotonin (5-HT) in response to 2% acetic acid and NT79. Writhing was recorded simultaneously. The exact location of the microdialysis probe is shown in Figure (10). The surgeries were done as described previously by our group (Liang, et al., 2008). Briefly, on the day of surgery, the rat was anesthetized with gasiform isoflurane (1% isoflurane in a mixture of 20% oxygen and 80% nitrogen gas), and immobilized in a stereotaxic frame (KOPF Ins., Tujunga, CA, USA). The anesthesia was maintained during the entire surgery. Each guide cannula (CMA Microdialysis Inc., Acton, MA, USA) was stereotaxically implanted into the PAG with final coordinates (A7.5, L0.5, V6.4) relative to bregma (Paxinos and Watson, 1997), then secured to the skull by screws and dental cement. Following surgery, each rat was housed individually with food and water ad libitum for 3–5 days to recover from cannulation surgery. Pretreatment with NT79 (5 mg/kg, i.p.) or saline occurred 40 min before acetic acid injection during microdialysis. Two groups of animals were injected with saline and 40 min later with either saline or NT79 (5 mg/kg i.p.). Samples were collected for 1 h (3 samples) after the second injection. The position of the probe was verified by visual inspection at the end of each experiment.
Figure 10. Microdialysis probe placement in the PAG.
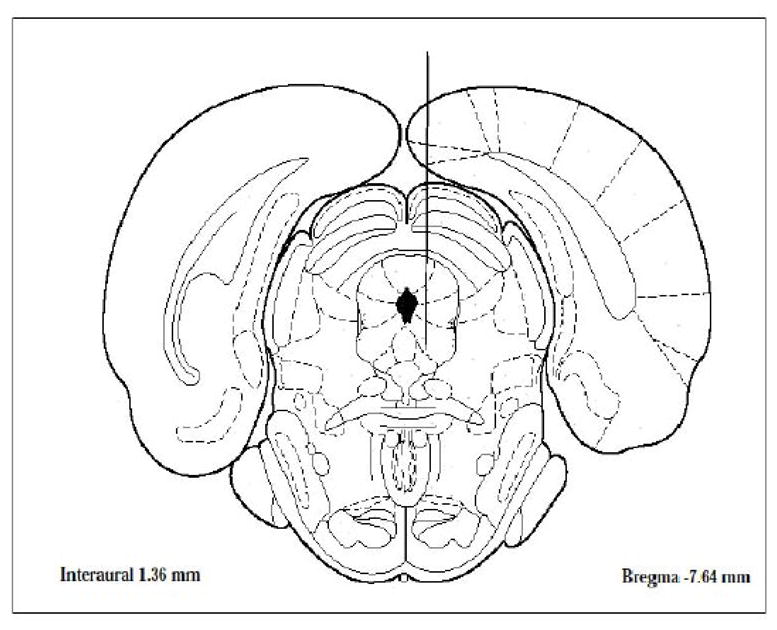
Numbers indicate distance (mm) from bregma. Sections are adapted from Paxinos and Watson (Paxinos and Watson, 1997).
4.5.b. Microdialysis procedure
Microdialysis experiments were carried out on conscious, freely moving Sprague Dawley rats. On the day of the experiment, the stylet in the guide cannula was replaced with the microdialysis probe (CMA/11 with 2 mm membrane, CMA Microdialysis Inc., Acton, MA, USA). The probe was perfused at 2 μl/min with artificial cerebrospinal fluid (146 mM NaCl, 1.2 mM CaCl2, 3 mM KCl, 1.0 mM MgCl2, 1.9 mM Na2HPO4, 0.1 mM NaH2PO4, pH 7.4). After at least 2 h equilibration, dialysate samples were automatically collected every 20 min into vials containing 2 μl perchloric acid (0.5 M) to delay the oxidation of monoamines. Three baseline fractions were collected before NT79 or saline injection. All samples were immediately applied to the HPLC-electrochemical detector for the determination of monoamines and their metabolites. Results were reported as % increase of baseline and the AUC after the second injection was given as the total percentage of increase above baseline.
4.5.c. Monoamine assay
Monoamines and their metabolites were measured on an ESA HPLC coupled with Coulochem II electrochemical detector system (ESA Inc., Chelmsford, MA, USA) with a 20-μl sample loop. They were separated on an MD-150 analytical column (3 × 150 mm, 3 μm C18, ESA Inc., Chelmsford, MA, USA) with MDTM mobile phase (ESA Inc., Chelmsford, MA, USA) at 0.6 ml/min. Potential settings for detection were E1 at −175 mV, E2 at 250 mV, GC at 350 mV. Peaks were displayed, integrated, and stored with ESA 501 Chromatography data system (ESA Inc., Chelmsford, MA, USA). The monoamine levels were quantitated from measurements of the area under the curve (AUC) and the use of standard curves, which were generated by linear regression analyses (GraphPad Prism, GraphPad Software, Inc., La Jolla, CA, USA). For the standard curves, the range for DA and 5-HT was 1–160 pg/20 μl; for DOPAC, HVA, and 5-HIAA it was 20–1600 pg/20 μl.
4.6. Statistical Analysis
The data was analyzed with One-way ANOVA followed by Tukey’s test for multiple comparisons using Sigma Stat software (SPSS, Inc., Chicago, IL) with P<0.05 being considered significant. Two-way ANOVA was used to determine the interaction between the treatment and the pulse intensities.
Acknowledgments
This work was funded by NIMH grant # MH71241 and by the Mayo Foundation for Medical Education and Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott FV, Hong Y, Blier P. Activation of 5-HT2A receptors potentiates pain produced by inflammatory mediators. Neuropharmacology. 1996;35:99–110. doi: 10.1016/0028-3908(95)00136-0. [DOI] [PubMed] [Google Scholar]
- Anden NE, Rubenson A, Fuxe K, Hokfelt T. Evidence for dopamine receptor stimulation by apomorphine. J Pharm Pharmacol. 1967;19:627–629. doi: 10.1111/j.2042-7158.1967.tb09604.x. [DOI] [PubMed] [Google Scholar]
- Auclair AL, Kleven MS, Besnard J, Depoortere R, Newman-Tancredi A. Actions of novel antipsychotic agents on apomorphine-induced PPI disruption: influence of combined serotonin 5-HT1A receptor activation and dopamine D2 receptor blockade. Neuropsychopharmacology. 2006;31:1900–1909. doi: 10.1038/sj.npp.1301015. [DOI] [PubMed] [Google Scholar]
- Bardin L, Tarayre JP, Koek W, Colpaert FC. In the formalin model of tonic nociceptive pain, 8-OH-DPAT produces 5-HT1A receptor-mediated, behaviorally specific analgesia. Eur J Pharmacol. 2001;421:109–114. doi: 10.1016/s0014-2999(01)01029-9. [DOI] [PubMed] [Google Scholar]
- Bissette G, Nemeroff CB, Loosen PT, Prange AJ, Lipton MA. Hypothermia and intolerance to cold induced by intracisternal administration of the hypothalamic peptide neurotensin. Nature. 1976;262:607–609. doi: 10.1038/262607a0. [DOI] [PubMed] [Google Scholar]
- Boules M, Fredrickson P, Richelson E. Current topics: brain penetrating neurotensin analog. Life Sci. 2003;73:2785–2792. doi: 10.1016/s0024-3205(03)00674-x. [DOI] [PubMed] [Google Scholar]
- Boules M, Fredrickson P, Richelson E. Neurotensin agonists as an alternative to antipsychotics. Expert Opin Investig Drugs. 2005;14:359–369. doi: 10.1517/13543784.14.4.359. [DOI] [PubMed] [Google Scholar]
- Boules M, Fredrickson P, Richelson E. Bioactive analogs of neurotensin. Peptides. 2006;27:2523–2533. doi: 10.1016/j.peptides.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Boules M, Shaw A, Fredrickson P, Richelson E. Neurotensin agonists: potential in the treatment of schizophrenia. CNS Drugs. 2007;21:13–23. doi: 10.2165/00023210-200721010-00002. [DOI] [PubMed] [Google Scholar]
- Boules M, Warrington L, Fauq A, McCormick D, Richelson E. A novel neurotensin analog blocks cocaine- and D-amphetamine-induced hyperactivity. Eur J Pharmacol. 2001;426:73–76. doi: 10.1016/s0014-2999(01)01197-9. [DOI] [PubMed] [Google Scholar]
- Boules M, Shaw A, Liang Y, Barbut D, Richelson E. NT69L, a Novel Analgesic, Shows Synergy with Morphine. Brain Res. 2009;1294:22–28. doi: 10.1016/j.brainres.2009.07.086. [DOI] [PubMed] [Google Scholar]
- Bredeloux P, Costentin J, Dubuc I. Interactions between NTS2 neurotensin and opioid receptors on two nociceptive responses assessed on the hot plate test in mice. Behav Brain Res. 2006;175:399–407. doi: 10.1016/j.bbr.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Briody S, Boules M, Oliveros A, Fauq I, Richelson E. Chronic NT69L potently prevents drug-induced disruption of prepulse inhibition without causing tolerance. Behav Brain Res. 2009 doi: 10.1016/j.bbr.2009.09.044. (available on line October 2, 2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraway R, Leeman SE. Characterization of radioimmunoassayable neurotensin in the rat. Its differential distribution in the central nervous system, small intestine, and stomach. J Biol Chem. 1976;251:7045–7052. [PubMed] [Google Scholar]
- Chalon P, Vita N, Kaghad M, Guillemot M, Bonnin J, Delpech B, Le Fur G, Ferrara P, Caput D. Molecular cloning of a levocabastine-sensitive neurotensin binding site. FEBS Lett. 1996;386:91–94. doi: 10.1016/0014-5793(96)00397-3. [DOI] [PubMed] [Google Scholar]
- Clineschmidt BV, McGuffin JC, Bunting PB. Neurotensin: antinocisponsive action in rodents. Eur J Pharmacol. 1979;54:129–139. doi: 10.1016/0014-2999(79)90415-1. [DOI] [PubMed] [Google Scholar]
- Cusack B, Boules M, Tyler BM, Fauq A, McCormick DJ, Richelson E. Effects of a novel neurotensin peptide analog given extracranially on CNS behaviors mediated by apomorphine and haloperidol. Brain Res. 2000;856:48–54. doi: 10.1016/s0006-8993(99)02363-x. [DOI] [PubMed] [Google Scholar]
- Cusack B, Jansen K, McCormick DJ, Chou T, Pang Y, Richelson E. A single amino acid of the human and rat neurotensin receptors (subtype 1) determining the pharmacological profile of a species-selective neurotensin agonist. Biochem Pharmacol. 2000;60:793–801. doi: 10.1016/s0006-2952(00)00409-3. [DOI] [PubMed] [Google Scholar]
- Doak GJ, Sawynok J. Formalin-induced nociceptive behavior and edema: Involvement of multiple peripheral 5-hydroxytryptamine receptor subtypes. Neuroscience. 1997;80:939–949. doi: 10.1016/s0306-4522(97)00066-3. [DOI] [PubMed] [Google Scholar]
- Dubuc I, Costentin J, Doulut S, Rodriguez M, Martinez J, Kitabgi P. JMV 449: a pseudopeptide analogue of neurotensin-(8–13) with highly potent and long-lasting hypothermic and analgesic effects in the mouse. Eur J Pharmacol. 1992;219:327–329. doi: 10.1016/0014-2999(92)90314-t. [DOI] [PubMed] [Google Scholar]
- Dubuc I, Sarret P, Labbe-Jullie C, Botto JM, Honore E, Bourdel E, Martinez J, Costentin J, Vincent JP, Kitabgi P, Mazella J. Identification of the receptor subtype involved in the analgesic effect of neurotensin. J Neurosci. 1999;19:503–510. doi: 10.1523/JNEUROSCI.19-01-00503.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernberg M, Lundeberg T, Kopp S. Pain and allodynia/hyperalgesia induced by intramuscular injection of serotonin in patients with fibromyalgia and healthy individuals. Pain. 2000;85:31–39. doi: 10.1016/s0304-3959(99)00233-x. [DOI] [PubMed] [Google Scholar]
- Ervin GN, Birkemo LS, Nemeroff CB, Prange AJ. Neurotensin blocks certain amphetamine-induced behaviours. Nature. 1981;291:73–76. doi: 10.1038/291073a0. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Ko MCH, Woods JH, Richelson E. Pharmacological effects of a novel neurotensin receptor agonist, NT69L, in rhesus monkeys. Pharm Biochem & Behavior. 2005;80:341–349. doi: 10.1016/j.pbb.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Fauq AH, Hong F, Cusack B, Tyler BM, Pang YP, Richelson E. Synthesis of (2S)-2-amino-3-(1H-4-indolyl) propanoic acid, a novel trryptophan analog for structural modification of bioactive peptides. Tetrahedron: Asymmetry. 1998;9:4127–4134. [Google Scholar]
- Feifel D, Minor KL, Dulawa S, Swerdlow NR. The effects of intra-accumbens neurotensin on sensorimotor gating. Brain Res. 1997;760:80–84. doi: 10.1016/s0006-8993(97)00306-5. [DOI] [PubMed] [Google Scholar]
- Feifel D, Reza TL. Effects of neurotensin administered into the ventral tegmental area on prepulse inhibition of startle. Behav Brain Res. 1999;106:189–193. doi: 10.1016/s0166-4328(99)00123-0. [DOI] [PubMed] [Google Scholar]
- Ford AP, Marsden CA. In vivo neurochemical and behavioural effects of intracerebrally administered neurotensin and D-Trp11-neurotensin on mesolimbic and nigrostriatal dopaminergic function in the rat. Brain Res. 1990;534:243–250. doi: 10.1016/0006-8993(90)90135-x. [DOI] [PubMed] [Google Scholar]
- Gendron L, Perron A, Payet MD, Gallo-Payet N, Sarret P, Beaudet A. Low-Affinity Neurotensin Receptor (NTS2) Signaling: Internalization-Dependent Activation of Extracellular Signal-Regulated Kinases 1/2. Mol Pharmacol. 2004;66:1421–1430. doi: 10.1124/mol.104.002303. [DOI] [PubMed] [Google Scholar]
- Gerhardt S, Gerber R, Liebman JM. SCH 23390 dissociated from conventional neuroleptics in apomorphine climbing and primate acute dyskinesia models. Life Sci. 1985;37:2355–2363. doi: 10.1016/0024-3205(85)90102-x. [DOI] [PubMed] [Google Scholar]
- Ghia JE, Crenner F, Metz-Boutigue MH, Aunis D, Angel F. The effect of a chromogranin A-derived peptide (CgA4-16) in the writhing nociceptive response induced by acetic acid in rats. Life Sci. 2004;75:1787–1799. doi: 10.1016/j.lfs.2004.02.035. [DOI] [PubMed] [Google Scholar]
- Giordano J, Dyche J. Differential analgesic actions of serotonin 5-HT3 receptor antagonists in the mouse. Neuropharmacology. 1989;28:423–427. doi: 10.1016/0028-3908(89)90040-3. [DOI] [PubMed] [Google Scholar]
- Giordano J, Gerstmann H. Patterns of serotonin- and 2-methylserotonin-induced pain may reflect 5-HT3 receptor sensitization. Eur J Pharmacol. 2004;483:267–269. doi: 10.1016/j.ejphar.2003.10.044. [DOI] [PubMed] [Google Scholar]
- Giordano J, Rogers LV. Peripherally administered serotonin 5-HT3 receptor antagonists reduce inflammatory pain in rats. Eur J Pharmacol. 1989;170:83–86. doi: 10.1016/0014-2999(89)90137-4. [DOI] [PubMed] [Google Scholar]
- Goudie AJ, Cole JC, Sumnall HR. Olanzapine withdrawal/discontinuation-induced hyperthermia in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1500–1503. doi: 10.1016/j.pnpbp.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Goudie AJ, Smith JA, Robertson A, Cavanagh C. Clozapine as a drug of dependence. Psychopharmacology (Berl) 1999;142:369–374. doi: 10.1007/s002130050901. [DOI] [PubMed] [Google Scholar]
- Hong Y, Abbott FV. Behavioural effects of intraplantar injection of inflammatory mediators in the rat. Neuroscience. 1994;63:827–836. doi: 10.1016/0306-4522(94)90527-4. [DOI] [PubMed] [Google Scholar]
- Jolas T, Aghajanian GK. Neurotensin and the serotonergic system. Prog Neurobiol. 1997;52:455–468. doi: 10.1016/s0301-0082(97)00025-7. [DOI] [PubMed] [Google Scholar]
- Jolicoeur FB, De Michele G, Barbeau A, St-Pierre S. Neurotensin affects hyperactivity but not stereotypy induced by pre and post synaptic dopaminergic stimulation. Neurosci Biobehav Rev. 1983;7:385–390. doi: 10.1016/0149-7634(83)90043-x. [DOI] [PubMed] [Google Scholar]
- Jolicoeur FB, Gagne MA, Rivest R, Drumheller A, St-Pierre S. Atypical neuroleptic-like behavioral effects of neurotensin. Brain Res Bull. 1993;32:487–491. doi: 10.1016/0361-9230(93)90295-m. [DOI] [PubMed] [Google Scholar]
- Jolicoeur FB, Rivest R, St-Pierre S, Drumheller A. Antiparkinson-like effects of neurotensin in 6-hydroxydopamine lesioned rats. Brain Res. 1991;538:187–192. doi: 10.1016/0006-8993(91)90428-x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Burgess SK, Nemeroff CB, Prange AJ. Behavioral and neurochemical effects of neurotensin microinjection into the ventral tegmental area of the rat. Neuroscience. 1983;8:495–505. doi: 10.1016/0306-4522(83)90195-1. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Nemeroff CB, Prange AJ. Neurotensin microinjection into the nucleus accumbens antagonizes dopamine-induced increase in locomotion and rearing. Neuroscience. 1984;11:919–930. doi: 10.1016/0306-4522(84)90203-3. [DOI] [PubMed] [Google Scholar]
- Kitabgi P, Carraway R, Leeman SE. Isolation of a tridecapeptide from bovine intestinal tissue and its partial characterization as neurotensin. J Biol Chem. 1976;251:7053–7058. [PubMed] [Google Scholar]
- Liang Y, Boules M, Shaw AM, Williams K, Fredrickson P, Richelson E. Effect of a novel neurotensin analog, NT69L, on nicotine-induced alterations in monoamine levels in rat brain. Brain Res. 2008;1231:6–15. doi: 10.1016/j.brainres.2008.07.037. [DOI] [PubMed] [Google Scholar]
- Maeno H, Yamada K, Santo-Yamada Y, Aoki K, Sun YJ, Sato E, Fukushima T, Ogura H, Araki T, Kamichi S, Kimura I, Yamano M, Maeno-Hikichi Y, Watase K, Aoki S, Kiyama H, Wada E, Wada K. Comparison of mice deficient in the high- or low-affinity neurotensin receptors, Ntsr1 or Ntsr2, reveals a novel function for Ntsr2 in thermal nociception. Brain Res. 2004;998:122–129. doi: 10.1016/j.brainres.2003.11.039. [DOI] [PubMed] [Google Scholar]
- Mazella J, Botto JM, Guillemare E, Coppola T, Sarret P, Vincent JP. Structure, functional expression, and cerebral localization of the levocabastine-sensitive neurotensin/neuromedin N receptor from mouse brain. J Neurosci. 1996;16:5613–5620. doi: 10.1523/JNEUROSCI.16-18-05613.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon BM, Boules M, Warrington L, Richelson E. Neurotensin analogs indications for use as potential antipsychotic compounds. Life Sci. 2002;70:1101–1119. doi: 10.1016/s0024-3205(01)01520-x. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Luttinger D, Hernandez DE, Mailman RB, Mason GA, Davis SD, Widerlov E, Frye GD, Kilts CA, Beaumont K, Breese GR, Prange AJ. Interactions of neurotensin with brain dopamine systems: biochemical and behavioral studies. J Pharmacol Exp Ther. 1983;225:337–345. [PubMed] [Google Scholar]
- Niemegeers CJ, Janssen PA. A systematic study of the pharmacological activities of dopamine antagonists. Life Sci. 1979;24:2201–2216. doi: 10.1016/0024-3205(79)90096-1. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Imbe H, Morikawa Y, Itoh M, Sekimoto M, Nemoto K, Senba E. 5-HT2A receptor subtype in the peripheral branch of sensory fibers is involved in the potentiation of inflammatory pain in rats. Pain. 2002;99:133–143. doi: 10.1016/s0304-3959(02)00070-2. [DOI] [PubMed] [Google Scholar]
- Orwin JM, Fozard JR. Blockade of the flare response to intradermal 5-hydroxytryptamine in man by MDL 72.222, a selective antagonist at neuronal 5-hydroxytryptamine receptors. Eur J Clin Pharmacol. 1986;30:209–212. doi: 10.1007/BF00614305. [DOI] [PubMed] [Google Scholar]
- Osbahr AJ, Nemeroff CB, Manberg PJ, Prange AJ. Centrally administered neurotensin: activity in the Julou-Courvoisier muscle relaxation test in mice. Eur J Pharmacol. 1979;54:299–302. doi: 10.1016/0014-2999(79)90090-6. [DOI] [PubMed] [Google Scholar]
- Parada CA, Tambeli CH, Cunha FQ, Ferreira SH. The major role of peripheral release of histamine and 5-hydroxytryptamine in formalin-induced nociception. Neuroscience. 2001;102:937–944. doi: 10.1016/s0306-4522(00)00523-6. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 3. Academic Press, Inc; San Francisco: 1997. [Google Scholar]
- Pettibone DJ, Hess JF, Hey PJ, Jacobson MA, Leviten M, Lis EV, Mallorga PJ, Pascarella DM, Snyder MA, Williams JB, Zeng Z. The effects of deleting the mouse neurotensin receptor NTR1 on central and peripheral responses to neurotensin. J Pharmacol Exp Ther. 2002;300:305–313. doi: 10.1124/jpet.300.1.305. [DOI] [PubMed] [Google Scholar]
- Puech AJ, Simon P, Boissier JR. Benzamides and classical neuroleptics: comparison of their actions using 6 apomorphine-induced effects. Eur J Pharmacol. 1978;50:291–300. doi: 10.1016/0014-2999(78)90134-6. [DOI] [PubMed] [Google Scholar]
- Richardson BP, Engel G, Donatsch P, Stadler PA. Identification of serotonin M-receptor subtypes and their specific blockade by a new class of drugs. Nature. 1985;316:126–131. doi: 10.1038/316126a0. [DOI] [PubMed] [Google Scholar]
- Sarhan S, Hitchcock JM, Grauffel CA, Wettstein JG. Comparative antipsychotic profiles of neurotensin and a related systemically active peptide agonist. Peptides. 1997;18:1223–1227. doi: 10.1016/s0196-9781(97)00145-9. [DOI] [PubMed] [Google Scholar]
- Sarret P, Esdaile MJ, Perron A, Martinez J, Stroh T, Beaudet A. Potent spinal analgesia elicited through stimulation of NTS2 neurotensin receptors. J Neurosci. 2005;25:8188–8196. doi: 10.1523/JNEUROSCI.0810-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilling PD MG, Priebe K, Richelson E, Feifel D. Neurotensin agonists block the prepulse inhibition deficits produced by a 5-HT(2A) and an alpha(1) agonist. Psychopharmacology (Berl) 2004;175:353–359. doi: 10.1007/s00213-004-1835-5. [DOI] [PubMed] [Google Scholar]
- Shilling PD, Richelson E, Feifel D. The effects of systemic NT69L, a neurotensin agonist, on baseline and drug-disrupted prepulse inhibition. Behav Brain Res. 2003;143:7–14. doi: 10.1016/s0166-4328(03)00037-8. [DOI] [PubMed] [Google Scholar]
- Stahle L, Ungerstedt U. Assessment of dopamine autoreceptor agonist properties of apomorphine, (+)-3-PPP and (−)-3-PPP by recording of yawning behaviour in rats. Eur J Pharmacol. 1984;98:307–310. doi: 10.1016/0014-2999(84)90608-3. [DOI] [PubMed] [Google Scholar]
- Sufka KJ, Schomburg FM, Giordano J. Receptor mediation of 5-HT-induced inflammation and nociception in rats. Pharmacol Biochem Behav. 1992;41:53–56. doi: 10.1016/0091-3057(92)90058-n. [DOI] [PubMed] [Google Scholar]
- Taiwo YO, Levine JD. Serotonin is a directly-acting hyperalgesic agent in the rat. Neuroscience. 1992;48:485–490. doi: 10.1016/0306-4522(92)90508-y. [DOI] [PubMed] [Google Scholar]
- Tambeli CH, Oliveira MC, Clemente JT, Pelegrini-da-Silva A, Parada CA. A novel mechanism involved in 5-hydroxytryptamine-induced nociception: the indirect activation of primary afferents. Neuroscience. 2006;141:1517–1524. doi: 10.1016/j.neuroscience.2006.04.030. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Masu M, Nakanishi S. Structure and functional expression of the cloned rat neurotensin receptor. Neuron. 1990;4:847–854. doi: 10.1016/0896-6273(90)90137-5. [DOI] [PubMed] [Google Scholar]
- Tyler-McMahon BM, Stewart JA, Farinas F, McCormick DJ, Richelson E. Highly potent neurotensin analog that causes hypothermia and antinociception. Eur J Pharmacol. 2000;390:107–111. doi: 10.1016/s0014-2999(99)00877-8. [DOI] [PubMed] [Google Scholar]
- Tyler BM, Cusack B, Douglas CL, Souder T, Richelson E. Evidence for additional neurotensin receptor subtypes: neurotensin analogs that distinguish between neurotensin-mediated hypothermia and antinociception. Brain Res. 1998;792:246–252. doi: 10.1016/s0006-8993(98)00150-4. [DOI] [PubMed] [Google Scholar]
- Tyler BM, Douglas CL, Fauq A, Pang YP, Stewart JA, Cusack B, McCormick DJ, Richelson E. In vitro binding and CNS effects of novel neurotensin agonists that cross the blood-brain barrier. Neuropharmacology. 1999;38:1027–1034. doi: 10.1016/s0028-3908(99)00011-8. [DOI] [PubMed] [Google Scholar]
- Vita N, Laurent P, Lefort S, Chalon P, Dumont X, Kaghad M, Gully D, Le Fur G, Ferrara P, Caput D. Cloning and expression of a complementary DNA encoding a high affinity human neurotensin receptor. FEBS Lett. 1993;317:139–142. doi: 10.1016/0014-5793(93)81509-x. [DOI] [PubMed] [Google Scholar]
- Wheeler-Aceto H, Porreca F, Cowan A. The rat paw formalin test: comparison of noxious agents. Pain. 1990;40:229–238. doi: 10.1016/0304-3959(90)90073-M. [DOI] [PubMed] [Google Scholar]