Summary
Background
The rpoS, nlpD, pcm, and surE genes are among many whose expression is induced during the stationary phase of bacterial growth. rpoS codes for the stationary-phase RNA polymerase σ subunit, and nlpD codes for a lipoprotein. The pcm gene product repairs damaged proteins by converting the atypical isoaspartyl residues back to L-aspartyls. The physiological and biochemical functions of surE are unknown, but its importance in stress is supported by the duplication of the surE gene in E. coli subjected to high-temperature growth. The pcm and surE genes are highly conserved in bacteria, archaea, and plants.
Results
The structure of SurE from Thermotoga maritima was determined at 2.0 Å. The SurE monomer is composed of two domains; a conserved N-terminal domain, a Rossman fold, and a C-terminal oligomerization domain, a new fold. Monomers form a dimer that assembles into a tetramer. Biochemical analysis suggests that SurE is an acid phosphatase, with an optimum pH of 5.5–6.2. The active site was identified in the N-terminal domain through analysis of conserved residues. Structure-based site-directed point mutations abolished phosphatase activity. T. maritima SurE intra- and inter-subunit salt bridges were identified that may explain the SurE thermostability.
Conclusions
The structure of SurE provided information about the protein’s fold, oligomeric state, and active site. The protein possessed magnesium-dependent acid phosphatase activity, but the physiologically relevant substrate(s) remains to be identified. The importance of three of the assigned active site residues in catalysis was confirmed by site-directed mutagenesis.
Keywords: SurE, environmental stress, cell stationary phase, hyperthermophile, acid phosphatase
Introduction
Many genes are induced during the stationary phase of bacterial cell growth. These genes encode for proteins that are thought to increase the bacteria’s ability to survive under stress conditions, including environmental stress. The transcription of many of these genes is directed by the stationary-phase RNA polymerase subunit (σs), encoded by the rpoS gene [1]. In E. coli the rpoS gene is closely linked with three other genes, including one for a lipoprotein, nlpD [2], for an L-isoaspartate O-methyltransferase, pcm [3], and for a stationary-phase survival protein, surE [4]. The nlpD gene codes for an outer-membrane lipoprotein that has been associated with virulence and may function in cell wall formation or maintenance. Mutations in the nlpD gene decrease the survival of cells in the stationary phase [5]. The pcm and surE genes, which are found in most prokaryotes except for Gram-positive bacteria and myco-plasmas [6], are induced by declining cell growth rates during adverse environmental conditions or upon entry into the stationary phase [7]. In E. coli, the surE gene is found directly upstream of the pcm gene as part of a bicistronic operon, although pcm can also be transcribed from its own unique promoter [7]. In T. maritima, the pcm and surE genes do not form a contiguous operon, but rather the surE gene is found in an operon with the def gene, which codes the polypeptide de-formylase. In T. maritima, like many other bacterial species, there is an ORF downstream from surE that is homologous to the E. coli nlpD lipoprotein gene [8].
In aging cells, L-isoaspartyl residues accumulate by the spontaneous deamidation of asparagine or isomerization of aspartyl residues. The pcm gene product repairs these damaged proteins by converting isoaspartyl residues to L-aspartyl residues. The phenotype of a pcm mutant strain shows reduced viability in the stationary phase under environmental stresses, such as methanol or paraquat exposure [6].
The physiological role and biochemical function of the surE gene product is unknown. Genetic experiments [9] showed that a surE pcm double mutation suppresses the phenotype of stress-challenged pcm mutants. The double mutant also accrues higher levels of isoaspartyl residues than either the parent strain or either single mutant. The importance of surE in responding to stress is corroborated by the duplication of the gene in a strain of E. coli subjected to 2000 generations of high-temperature growth [10].
SurE protein and its sequence homologs are members of the cluster of orthologous genes (COG) 0496 [11]. This COG was identified with an all-against-all sequence comparison of the proteins encoded in completely sequenced genomes, and it comprises 23 proteins from bacteria (including cyanobacteria), archaea, and eukaryota (yeast and higher plants). The proteins that comprise each COG are assumed to have evolved from an ancestral protein and are therefore either orthologs or paralogs. BLAST analysis [12] of the NR database (E-value cutoff 0.002) revealed 44 sequence homologs and confirmed the presence of SurE-like proteins in plants.
Prior to this work, the biochemical and physiological roles of the SurE protein remained obscure, although a possible SurE homolog from Yarrowia lipolytica is able to complement a mutant Saccharomyces cerevisiae strain lacking two of its major acid phosphatases [13], suggesting that SurE might be an acid phosphatase as well. As a step toward understanding SurE function, we determined the crystal structure of SurE from T. maritima at 2.0 Å resolution and directly demonstrated that SurE displays acid phosphatase activity.
Results and Discussion
Crystal Structure of SurE
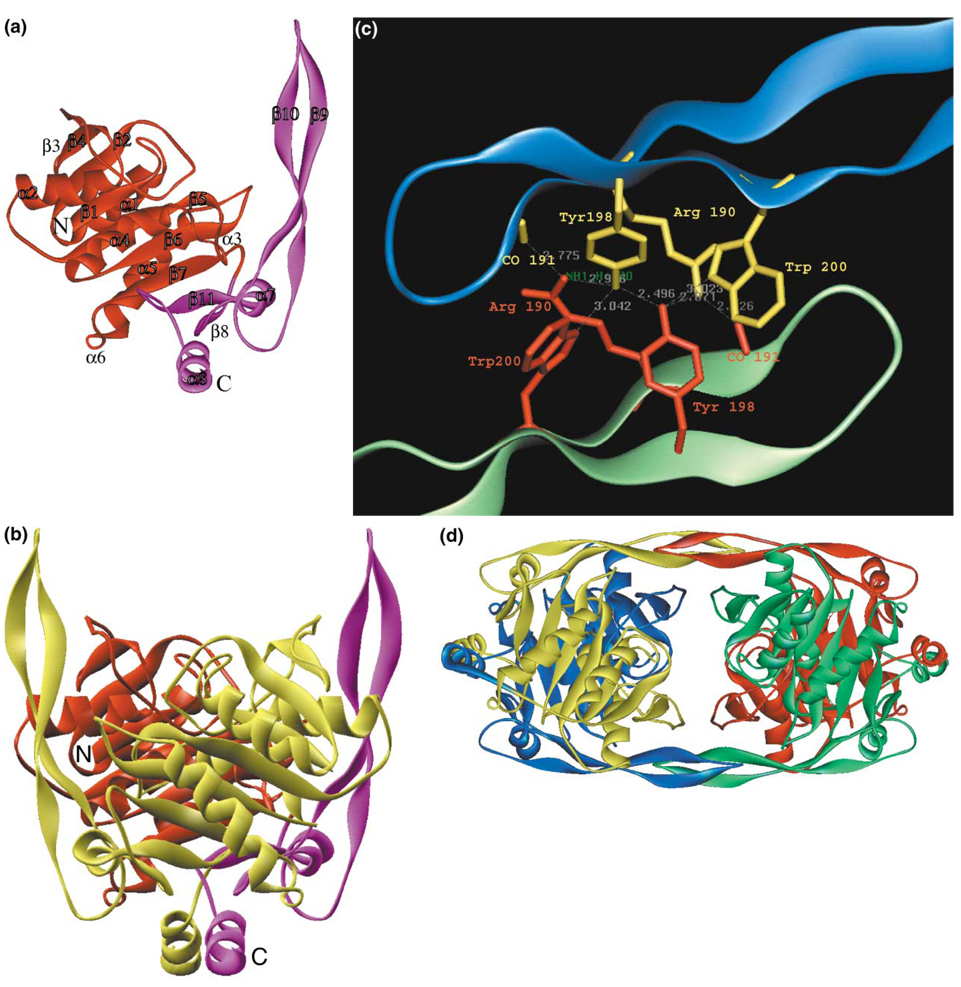
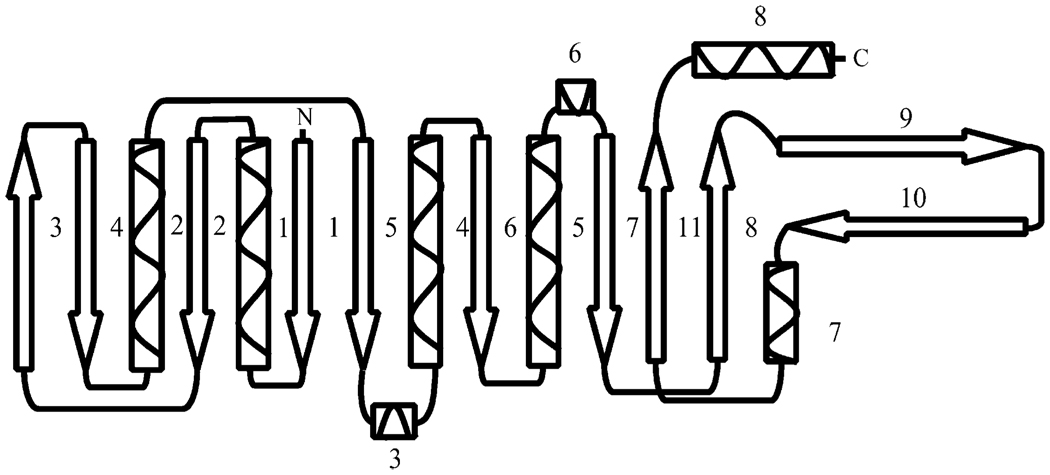
The SurE monomer structure shows two well-defined domains; a larger, globular N-terminal domain (1–168) and a smaller C-terminal domain (169–247; Figure 1). The N-terminal domain is a three-layer α/β/α sandwich that shows distal homology with the Rossmann fold (CATH class 3.40.50.170) of which the major feature is a long β sheet that is composed of nine mostly parallel β strands in the following order β3↑-β4↓-β2↓-β1↓-β5↓-β6↓-β7↓-β11↑-β8↑ (Figure 2). Strands β1–β7 are part of the N-terminal domain, and strands β8 and β11 are part of the C-terminal domain. Four long α helices, α1, α2, α4, and α5, and two short 310 helices, α3 and α6, complete the N-terminal domain sandwich.
Figure 1. Structure of SurE.
(a) Structure of the monomer with the N-terminal domain in red and C-terminal domain in maroon.
(b) Structure of the dimer with two-fold dyad in the plane of the figure. Color coding for one monomer is as in (a), and the second monomer is in yellow. N and C termini are labeled in the red/maroon monomer.
(c) Specific interactions responsible for the formation of the tetramer in β hairpin region of blue and green monomers.
(d) Structure of the SurE tetramer.
Figure 2. Diagram of the Secondary-Structural Elements in the SurE Monomer.
The C-terminal domain, in addition to the β8–β11 strands, has two long protrusions; one is formed by the C-terminal α helix (α8), and the second is formed by a 27 Å-long β hairpin (strands β9 and β10) (Figure 1d and Figure 2). Helices α7 and α8 are also a part of the C-terminal domain fold. The N- and C-terminal domains are connected through the β-pleated sheet. SurE shows no significant sequence homology with proteins deposited in the Protein Data Bank (PDB).
Analysis of the crystal packing shows that SurE forms a well-defined dimer (Figure 1b). The total area buried at the interface between two subunits is 6908 Å2. The two subunits interact by using several secondary-structure elements, including domain swapping of the C-terminal α helix (α8). The contacts between the subunits in the dimer include hydrophobic interactions, hydrogen bonds, and salt bridges. The dimer is formed through extensive interactions of the two C-terminal protrusions and two loops in the N-terminal domain (residues 39–45 and 101–103) with the α/β/α sandwich of the N-terminal domain (Figure 1b). The two dimers assemble into a tetramer (Figure 1c), whose existence was confirmed by gel exclusion chromatography. This showed an apparent molecular weight for SurE in solution of 123 kDa (data not shown), consistent with tetrameric structure.
The crystal structure showed that the SurE tetramer is assembled from dimers by way of the β-hairpin extensions. Highly networked and specific interactions between dimers involve several symmetry-related pairs of amino acids (Arg190, Tyr198, and Trp200) and a main chain. For example, Arg190 from the “blue” subunit (Figure 1d) makes two inter-subunit hydrogen bonds with the carbonyl of Val191 and Tyr198 from the “green” subunit; this Tyr198 in turn makes two additional hydrogen bonds with the symmetry-related Tyr198 and Trp200 from the “blue” subunit. In addition to hydrogen bonds, there are several hydrophobic interactions between the side chains of Val191, Tyr198, Trp200, and the hydrophobic chain of Arg190. The surface area buried at the interface between the two dimers is 2021 Å2, a sound oligomerization interface.
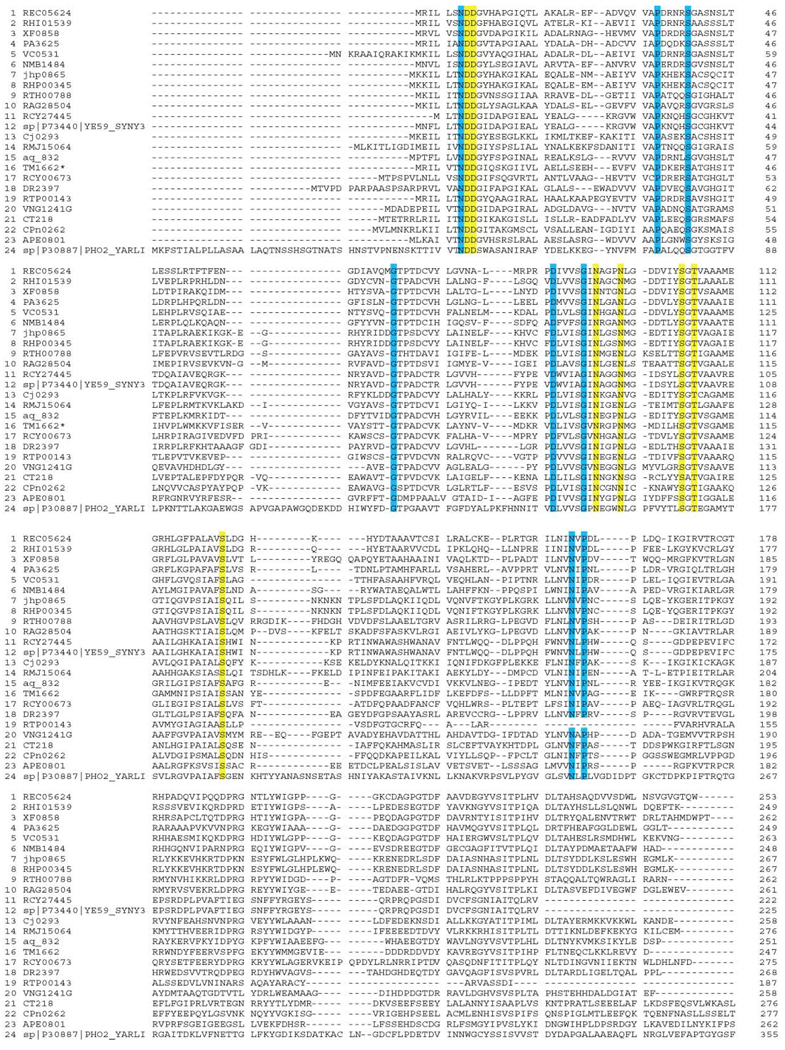
The T. maritima SurE must function at temperatures as high as 94°C. We sought to explain how the protein maintains structural stability at such high temperatures. One possible explanation for the high stability of SurE is the numerous salt bridges formed on the surface of the protein. There are five inter-subunit salt bridges (Asp156–Lys241, Asp185–Lys76/Glu115, Asp213–Arg243, Glu236–Arg177, and Glu244–Lys240) and ten intra-subunit salt bridges (Glu188–Arg190, Glu207–Arg181, Glu208–Arg182, Asp209–Arg180, Asp210–Arg180, Glu137–Arg141, Asp145–Lys148, Glu221–Arg220, Glu28–Arg2, and Glu115–Lys76). In general, residues involved in the formation of these salt bridges are not conserved or are only partly conserved across SurE orthologs (Figure 3). These salt bridges may provide an explanation for the exceptional stability of T. maritima SurE. Stabilizing effects of salt bridges have been observed in the past for a number of proteins, including engineered barnase [14], and have recently been theorized with electrostatic calculations [15]. In summary, it appears that the C-terminal domain is important mainly for maintaining the oligomeric state of SurE, whereas the N-terminal domain may be the functional domain.
Figure 3. Multiple Sequence Alignment (CLUSTAL W [1.81]) of Proteins Belonging to the SurE Family.
Completely conserved residues are highlighted with blue or yellow. Amino acids forming the presumed active site are highlighted with yellow.
SurE Is an Acid Phosphatase Activated by Magnesium
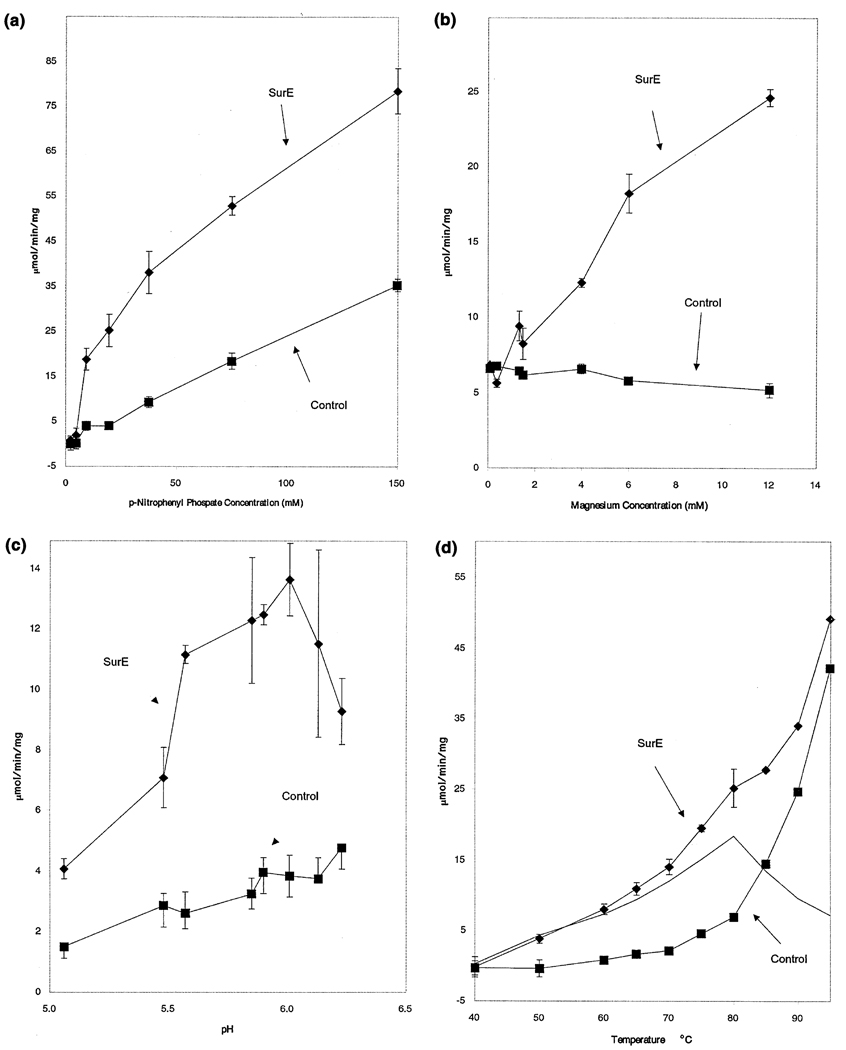
Gene complementation is a highly powerful tool for inferring protein function. In 1992, Treton and colleagues [13] reported that a genomic sequence containing the PHO2_Yarl1 ORF from the yeast Y. lipolytica complemented a double mutation in the two main acid phosphatases of S. cerevisiae. Based on this observation, the authors concluded that the PHO2_Yarl1 ORF encoded a new acid phosphatase. PHO2_Yarl1 shows 24% identity (over 200 residues) with T. maritima SurE. Using this information as a starting point, we examined SurE enzymatic activity. In standard assays SurE shows no detectable protease and nuclease activities. However, incubation of purified SurE protein with p-nitrophenyl phosphate (PNPP) resulted in enhanced hydrolysis when we compared it with buffer-only controls (Figure 4). The rate of hydrolysis was proportional to enzyme concentration and time (data not shown). Analysis of activity with various concentrations of PNPP revealed a Km of 31.5 mM and a Vmax of 51.6 µmol/min/mg protein at 76°C in the presence of 10 mM magnesium ion (Figure 4a and Figure 5). We found that the magnesium ion is a strong activator of the enzyme; little or no activity was seen in the absence of magnesium, and the activity continued to increase to at least 12 mM (Figure 6b). Calcium ion activated the enzyme but the specific activity of the enzyme was 10-fold lower (data not shown). The magnesium ion dependence seen here is similar to that found in the acid phosphatase from soybean root nodule[16], but is unlike that found in potato purple acid phosphatase [17] or seminal acid phosphatases [18]. Studies on the pH dependence of the activity revealed a maximum between pH 5.5 and 6.2 at 76°C (Figure 4c). Finally, we found that the phosphatase activity increased with temperature to at least 70 °C with significant activity still seen up to 95 °C with an apparent optimum of 80°C (Figure 6d). We considered the possibility that this activity might result from the contamination of the SurE protein with a phosphatase from the E. coli host. However, the properties of the enzyme described here are consistent with a novel type of phosphatase from thermophile T. maritima. The temperature dependence is consistent with that of a thermophilic organism, with little or no activity detected at 37 °C (data not shown) and significant activity detected beyond 85°C.
Figure 4. Enzymatic Analysis of SurE Phosphatase Activity on p-Nitrophenyl Phosphate (PNPP).
Assays were done as described in the Experimental Procedures, and each data point reflects the average of three trials, with the error bars reflecting ±1 standard deviation when they are larger than the plotted point.
(a) SurE catalyzes the hydrolysis of PNPP in a concentration-dependent manner. Data are shown from three experiments in which 0.1 µg of SurE protein (diamonds) or a buffer control (squares) was incubated in a 250 µl reaction with 100 mM MOPS (pH 6.0 at 75°C), 5% glycerol, and 10 mM MgSO4 at 75°C.
(b) SurE phosphatase is activated by magnesium ion. SurE (0.1 µg protein, diamonds) or a buffer control (squares) was incubated at 76°C with increasing concentrations of magnesium sulfate in a 250 µl reaction mixture with 100 mM sodium MOPS (pH 6.1 at 76°C), 5% glycerol, and 15 mM disodium PNPP.
(c) SurE is an acid phosphatase. SurE (0.1 µg protein, diamonds) or a buffer control (squares) was incubated at 76°C in a series of 100 mM sodium 2-[N-morpholino]ethanesulfonic acid (MES) buffers adjusted to different pH values at 76°C containing 5% glycerol, 6 mM MgSO4, and 15 mM disodium PNPP. Similar results were found when MOPS or phosphate buffers were used (data not shown).
(d) SurE is activated at elevated temperatures and has minimal activity at 40°C. SurE (0.1 µg, diamonds) or a buffer control (squares) was incubated at various temperatures in a 100 mM MOPS buffer (adjusted to pH 6 at 76°C) with 5% glycerol, 10 mM MgSO4, and 15 mM disodium PNPP. The solid line represents the enzyme specific activity and shows a peak at 80°C.
Figure 5. Enzymatic Activity of SurE phosphatase on PNPP, Adenosine-5′-Monophosphate, and α-Napthyl Phosphate.
Phosphate release was measured with the EnzChek system as described in the Experimental Procedures. The non-SurE-dependent spontaneous hydrolytic activity was subtracted from the SurE activity, and the resultant enzyme specific activity is shown in squares for α-napthyl phosphate (α-NP), in diamonds for adenosine-5′-monophosphate (AMP), and triangles for PNPP. The error bars for each symbol represent ± 1 standard deviation. For each substrate, the best-fit Km curve (as determined by a least-squares fit) is super-imposed through the points. The calculated Km and Vmax values for α-NP are 5.0 mM and 233 µmol/min/mg, respectively. For AMP, these values are 3.4 mM and 105 µmol/min/mg, while for PNPP the values are 31.5 mM and 51.6 µmol/min/mg. The kinetics for the PNPP substrate were determined with additional data points at higher concentrations up to 150 mM as shown in Figure 6a.
Figure 6. Presumed Active Site of SurE.
(a) A stereo view of conserved residues forming a well-defined cluster near the protein surface. Water molecules are represented as pink spheres.
(b) Location of the presumed active site in the SurE dimer and its solvent accessibility. Protein is shown as a space-filling model viewed down the two-fold dyad. Residues that are conserved and form the presumed active site are in space-filling representation (red and green in two different subunits, respectively). The conserved Asp88 in both subunits is labeled for reference.
SurE Acid Phosphatase Shows Substrate Selectively
We examined the phosphatase activity of SurE on a range of phosphate esters (Table 1). We found the highest activity with α-naphthyl phosphate, AMP, and GMP, but little or no activity with TMP, CMP, adenosine 2′-monophosphate, as well as a number of other small-molecule phosphate esters. The best substrate found for SurE is α-naphthyl phosphate. We calculated a turnover number of 4200/min for the α-naphtyl phosphate substrate, indicating that the activity is robust and is unlikely to be the result of a side activity an unrelated type of enzymatic reaction. The kinetic constants of the SurE phosphatase with PNPP, α-naphtyl phosphate, and AMP were calculated from the data shown in Figure 5. The Vmax values were comparable to those of purified plant and human acid phosphatases, but the Km values were generally larger than for these other enzymes. The differential affinity of SurE for substrates suggests that SurE is probably a specific phosphatase for which we have not discovered the primary endogenous substrate. We note that the soybean nodule enzyme has similar kinetic values for PNPP as the SurE enzyme, but a much lower Km (µM) value for its physiological substrate AMP [16].
Table 1.
Comparison of SurE Activity with Various Substrates with Other Acid Phosphatases
| SurE | Soybean Root Nodule1 |
Wheat Germ2 | Sweet Potato Purple3 |
Soybean Purple1 | Red Kidney Bean Purple3 |
Human Prostatic Type A |
Human Prostatic Type B |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substrate | Vmax, Km | Vmax, Km | Vmax, Km | Vmax, Km | Vmax | Vmax, Km | Vmax, Km | Vmax, Km | ||||||||
| p-Nitrophenyl phosphate | 51.6, 31.5 | 159, 13.25 | 605, 0.091 | 760, 0.095 | 213.8 | NR, 35.7 | NR, 0.59 | NR, 0.32 | ||||||||
| α-naphthyl phosphate | 233.3, 5.0 | - | 325, 0.29 | - | - | - | 598.4, 0.098 | 294.2, 0.167 | ||||||||
| Adenosine-5′-monophosphate | 104.6, 3.4 | 136, 0.08 | 49, 4.1 | 80.9, 0.36 | 68.5, 9 | - | - | - | ||||||||
| Vmax, 15 mM | % α-NP | Vmax, 15 mM | % α-NP | Vmax, 15 mM | % α-NP | Vmax, 15 mM | % α-NP | Vmax, 15 mM | % α-NP | Vmax, 15 mM | % α-NP | Vmax, 15 mM | % α-NP | Vmax, 15 mM | % α-NP | |
| α-naphthyl phosphate | 168 ± 15 | 100% | - | - | 318.8 | 190% | - | - | - | - | - | - | 594.5 | 354% | 290.96 | 173% |
| p-nitrophenyl phosphate | 14.9 ± 0.5 | 9% | 84.4 | 50% | 601.4 | 358% | 755.2 | 450% | 138.9 | 83% | - | - | - | - | - | - |
| Adenosine-5′-monophosphate | 83 ± 8 | 49% | 135.3 | 81% | 38.5 | 23% | 79.0 | 47% | 42.8 | 25% | - | - | - | - | - | - |
| Guanosine-5′-monophosphate | 113 ± 14 | 67% | 228.7 | 136% | - | - | - | - | - | - | - | - | - | - | - | - |
| Thymine-5′-monophosphate | 4 ± 6 | 2% | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Cytosine-5′-monophosphate | 11 ± 7 | 7% | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Adenosine-2′-monophosphate | ND | 0% | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| β-glycerol phosphate | ND | 0% | - | - | 301.7 | 180% | 1148.3 | 684% | 15.4 | 9% | - | - | - | - | - | - |
| Glucose-6-phosphate | ND | 0% | - | - | 126.8 | 75% | 401.2 | 239% | - | - | - | - | - | - | - | - |
| Phospho-enol-pyruvate | 3 ± 10 | 2% | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| O-phospho-serine | ND | 0% | - | - | 87.5 | 52% | - | - | - | - | - | - | - | - | - | - |
| O-phospho-threonine | ND | 0% | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
All Vmax data are listed in mmol/min/mg. All Km data are listed in mM. All % α-NP data are with reference to the SurE activity (in bold).
Data from Penheiter et al., 1997 [28]. 15 mM Vmax data are inferred from published Vmax and Km for the given substrate.
Data from Van Etten and Waymack, 1991 [29]. 15 mM Vmax data are inferred from published Vmax and Km for the given substrate.
Data from Schenk et al., 1999 [17]. 15 mM Vmax data are inferred from published Vmax and Km for the given substrate.
Sequence and Structure Homology Analysis
The Protein Data Bank (PDB) was searched [19] to identify proteins with structural similarity to SurE. The best match, with a Z score of 5.8 indicating rather low structural similarity, was to dihydropteridine reductase (PDB access code 1DHR [20], with positional root mean square deviation [rmsd] of superimposed Cα atoms equal to 3.5 Å for 125 equivalent positions). Other matches found by the DALI program showed even lower similarity. The CATH analysis revealed that the N-terminal domain of SurE shows reasonable structural homology to the phosphofructose kinase from E. coli (PDB code 1PFK; 3.9 Å rmsd over equivalent positions), but there is little sequence similarity between the enzymes, and the residues in the active site are not conserved. Phosphofructose kinase represents a variant of a Rossmann fold. The C-terminal oligomerization domain of SurE shows no meaningful structural or sequence homology with other proteins in the PDB. The C-terminal domain of SurE protein therefore represents a new protein fold.
SurE shows neither structural nor sequence homology to other acid phosphatases. Interestingly, the Y. lipolytica PHO2_Yarl1, the SurE functional and sequence homolog (24% identity with T. maritima SurE over 247 amino acids), also shows quite significant sequence identity with galactose oxidase (PDB access code 1GOH, 21.6% identity over 292 amino acids) and neural protease (PDB access code 1NPC, 25.4% identity over 252 amino acids). These homologies are misleading because SurE and galactose oxidase show no structural homology and in fact are very different folds (α/β/α and all-β, respectively). In addition, the presumed catalytic residues in SurE are not found in the galactose oxidase structure, and the catalytic residues of galactose oxidase are not conserved in the PHO2_Yarl1. Therefore, we conclude that SurE protein represents an acid phosphatase that has not been reported previously.
A Catalytic Site Suggested from the Structure
Enzymes performing the same function in different species should show strong amino acid sequence conservation in the active site. In SurE homologs there are 15 residues out of 247 that are completely conserved; all are in the N-terminal two-thirds of the protein. Seven of these amino residues (Asp8, Asp9, Asn95, Asn99, Ser107, Thr109, and Ser127 (highlighted in yellow on Figure 3) are clustered on the protein surface and form a well-defined pocket within the cleft between two subunits (Figure 6). These residues are located on β strands 5 and 6, α helix 4, a β turn near the N terminus and a loop between β strand 5 and 310 helix 3. The relatively small size of the binding pocket suggests that it binds a rather small ligand (Figure 6a). Three ordered water molecules were bound to this site (Figure 6a). Access to this site is quite well defined within the cleft running parallel to the interface between monomers. Because of the two-fold symmetry, there appear to be two independent sites in the dimer (four in the tetramer; the distance between Thr109 in the presumed active site of two monomers in the dimer is approximately 21 Å). The site is partly negatively charged (because of contribution from Asp8 and 9), but in close proximity there are positively (Lys36, partly conserved) and negatively (Asp88, fully conserved) charged patches that may contribute to substrate binding.
Site-Directed Mutagenesis of the Active Site
We carried out site-directed mutagenesis of the putative catalytic-site residues in SurE. Table 2 shows that the activity of SurE protein is dramatically affected for three point mutations in that site. Mutations of Asp8 or 9 to Asn caused almost complete loss of phosphatase activity (0.1% and 0.4%, respectively); mutation of Ser127 to Ala, with PNPP as a substrate, reduced the activity of SurE to 0.7% of wild-type. Mutant proteins appear to be structured and show CD spectra very similar to the wild-type. This result confirms the importance of these residues for substrate binding and/or catalysis and provides additional proof for identification of the catalytic site. The role of Asp8 and 9 in binding/catalysis is consistent with the magnesium-dependent activity of this acid phosphatase and the potential role of the metal ion in substrate binding. The active site of T. maritima SurE is unlike that of any known acid phosphatases.
Table 2.
Effects of Site-Directed Mutagenesis on SurE Phosphatase Activity1
| Mutation | Percent of Wild-Type Activity |
|---|---|
| Wild-type | 100 |
| D8N | 0.10 ± 0.07 |
| D9N | 0.43 ± 0.06 |
| S127A | 0.74 ± 0.36 |
Assays in which 0.1 µg of SurE protein (either wild-type, S127A, D9N, or D8N) or a buffer control was incubated in a 250 µL reaction with 100 mM MOPS (pH 6 at 75°C), 5% glycerol, 15 mM PNPP and 10 mM MgSO4 at 75°C were performed in triplicate as described in “Experimental Procedures” with the exception that the linear increase in absorbance was measured for 3–23 min for the mutants. The absorbance of the buffer-only controls was subtracted as a background. Data are shown ±1 standard deviation.
Functional Implications
In many bacteria, the rpoS RNA polymerase, which regulates transcription of genes in response to cell stationary phase, is clustered with three other genes, including genes for a lipoprotein, nlpD, an L-isoaspartate O-methyltransferase, pcm, and a novel acid phosphatase, surE, a stationary-phase survival protein. All these genes appear to be associated with environmental-stress response.
We have shown here that the product of the surE gene is a novel acid phosphatase. However, the physiological role of SurE during environmental stress is not clear. Acid phosphatases are expressed under a variety of conditions and in response to many stimuli. It is believed that acid phosphatases play an important role during cell starvation for phosphorous scavenging and remobilization. In fact, plants respond to phosphorous starvation by increasing the production and secretion of acid phosphatases. It is not clear whether, during stationary phase, bacterial cells also induce acid phosphatases to remobilize. In light of the fact that the SurE protein is not thought to be secreted [21], it is possible that it plays a role in the intracellular remobilization of phosphate from one or more specific compounds in the cell where nonspecific phosphatase activity would almost certainly be detrimental.
Experimental Procedures
Protein Expression and Purification
The ORF of surE was amplified by PCR from T. maritima genomic DNA (ATCC). The gene was cloned into the NdeI and BamHI sites of a modified pET15b cloning vector (Novagen) in which the TEV protease cleavage site replaced the Thrombin cleavage site, and a double stop codon was introduced downstream from the BamHI site. This construct provides for an N-terminal hexa-histidine tag separated from the gene by a TEV protease recognition site (ENLYFQ↓G). The fusion protein was overexpressed in E. coli BL21-Gold (DE3) (Stratagene) harboring an extra plasmid encoding three rare tRNAs (AGG and AGA for Arg, ATA for Ile). The cells were grown in LB at 37°C to an OD600 of approximately 0.6 and protein expression induced with 0.4 mM IPTG. After induction, the cells were incubated overnight with shaking at 15°C. The harvested cells were resuspended in binding buffer (500 mM NaCl, 5% glycerol, 50 mM HEPES [pH 7.5], 5 mM imidazole), flash-frozen in liquid N2 and stored at −70°C. The thawed cells were lysed by sonication after the addition of 0.5% NP-40 and 1 mM each of PMSF and benzamidine. The lysate was clarified by centrifugation (30 min at 27,000 g) and passed through a DE52 column preequilibrated in binding buffer. The flow-through fraction was then applied to a metal chelate affinity column charged with Ni2+. The hexa-histidine tag was eluted from the column in elution buffer (500 mM NaCl, 5% glycerol, 50 mM HEPES [pH 7.5], 500 mM imidazole), and the tag was then cleaved from the protein by treatment with recombinant His-tagged TEV protease. The cleaved protein was then resolved from the cleaved His-tag and the His-tagged protease by flowing the mixture through a second Ni2+-column.
The surE protein was dialyzed in 10 mM HEPES pH 7.5, 500 mM NaCl, and concentrated using a BioMax concentrator (Millipore). Before crystallization, any particulate matter was removed from the sample by passing through a 0.2 µm Ultrafree-MC centrifugal filter (Millipore). For the preparation of selenomethionine (SeMet) enriched protein, the T. maritima SurE was expressed in the methionine auxotroph strain B834(DE3) of E. coli (Novagen) and purified under the same conditions as the native protein in supplemented M9 media. The reducing reagent β-mercaptoethanol (5 mM) was added to all purification buffers.
p-Nitrophenyl Phosphate Phosphatase Assay
Phosphatase activity was measured by monitoring p-nitrophenol resulting from the hydrolysis of p-nitrophenyl phosphate. Reaction mixtures included the disodium salt of p-nitrophenyl phosphate (Sigma), the indicated buffer, 5% (v/v) glycerol, various concentrations of magnesium sulfate, and either purified SurE protein in 50 mM sodium HEPES with 500 mM NaCl and 5% glycerol (pH 7.5) or an equivalent volume of the buffer alone. Incubations were performed in a circulating water bath. At the specified time points, 100 µl of the reaction mixture was mixed with 900 µl of water, and the absorbance was measured at 410 nm. The activity was calculated from the linear increase in absorbance from 3 to 8 min with a molar extinction coefficient of 18,500 for p-nitrophenol. Buffers were adjusted to pH at the temperature used in the assay, except for the experiment in which temperature was varied. Here, the pH of the buffer was determined at 76°C.
General Phosphatase Assay
Phosphate release from other substrates was measured with the EnzChek system from Molecular Probes (E-6646) with a modified protocol. The EnzChek system determines free phosphate levels by measuring the absorbance at 360 nM of free 2-amino-6-mercapto-7-methylpurine that is released from ribose 1-phosphate via a phosphorylase reaction. In brief, 250 µl incubations of 100 mM sodium 3-[N-morpholino]propanesulfonic acid (MOPS) (pH 6.26; at 22°C), 10 mM magnesium sulfate, 5% glycerol, the specified substrate at the specified concentration and either 0.1 µg of SurE or an equivalent volume of the SurE storage buffer (as described above) were performed in a circulating water bath at 75°C. At the specified time points, 10 µl (for α-napthyl phosphate) or 30 µl (for all other substrates) of the reaction mixture was mixed with the EnzCheck components (5 µl of the 20× buffer, 20 µl of the MESG substrate, and 1 µl phosphorylase plus varying volumes of SurE solution and water to make a final volume of 100 µl). The EnzCheck reaction was allowed to proceed at room temperature for 7 min, at which time 900 µl of water was added to the reaction and the final solution absorbance was measured at 360 nm. A standard curve for phosphate was determined with a series of known concentrations of potassium phosphate. All reactions were performed in triplicate with a matched triplicate of the no-enzyme control.
Determination of the SurE Oligomeric State with Size Exclusion Chromatography
HPLC size exclusion chromatography was performed on a Superose-12 HR column (10 × 300 mm) (Amersham Pharmacia Biotech), preequilibrated with 10 mM HEPES pH 7.5, 0.5 M NaCl, with the system Gold (Beckman). The column was calibrated with cytochrome C (12.4 kDa), carbonic anhydrase (29 kDa), bovine serum albumin (66 kDa), alcohol dehydrogenase (150 kDa), β-amylase (200 kDa), and Blue Dextran (2,000 kDa). A 25 µl SurE protein sample either at a 2 mg/ml concentration or premixed with standard proteins was centrifuged at 14,000 rpm for 10 min before being injected into the column through a 20 µl injection loop. Filtration was carried out at 20°C at a flow rate of 1 ml/min. The eluted proteins were detected absorbance measurement at 280 nm.
Site-Directed Mutagenesis
Amino acid substitutions were introduced with the QuikChange Site-Directed Mutagenesis procedure (Stratagene). The primers’ design and the mutagenesis reaction were done according to the Quik-Change Site-Directed Mutagenesis instruction manual (Stratagene). To verify mutations, we sequenced plasmids with standard T7 promoter and T7 terminator primers.
Crystallization
We crystallized the protein by vapor diffusion in hanging drops by mixing 2 µl of the protein solution (4.5 mg/ml) with 2 µl of 0.1 M Tris/HCl (pH 8.5) and 1.75 M ammonium sulphate. We then equilibrated the protein at 20°C over 100 µl of this solution. Crystals, which appeared after 5 days, were flash frozen in liquid nitrogen with crystallization buffer plus 20% glycerol as cryoprotectant prior to data collection. The crystallization conditions reported here are different from the conditions described recently by Kwak et al. [22].
X-Ray Diffraction Data Collection and Reduction
Diffraction data were collected at 100 K at the 19BM beamline of the Structural Biology Center at the Advanced Photon Source, Argonne National Laboratory. Crystals of the SeMet derivative of SurE diffracted to 2.0 Å and belong to trigonal space group P3121 with the unit cell dimensions a = b = 115.52 Å, c = 78.67 Å, α = β = 90°, and γ = 120°. MAD data were collected to 2.0 Å resolution with an inverse beam strategy from a single crystal containing SeMet-labeled protein at three different X-ray wavelengths near the Se edge (Table 3). The absorption edge was determined by a fluorescent scan of the crystal as described previously [23]. The data were processed with the HKL2000 suite [24]. Crystal characteristics and data collection statistics are presented in Table 3.
Table 3.
Crystal, Data Collection, and Phasing Statistics
| Crystal | |||
|---|---|---|---|
| Unit cell | a = b = 115.52 Å, c = 78.67 Å, α = β = 90°, γ = 120° | ||
| Space group | P3121 | ||
| MW (247 residues) Da | 28075 | ||
| Mol (AU) | 2 | ||
| SeMet (AU) | 22 | ||
| MAD Data Collection Statistics | |||
| λ1 (peak) | λ (inflection) | λ (remote) | |
| λ (Å) | 0.9791 | 0.9793 | 0.9639 |
| Resolution (Å) | 2.0 | 2.0 | 2.0 |
| Reflections (measured) | 328,134 | 288,593 | 234,067 |
| Reflections (unique) | 79,278 | 78,802 | 75,790 |
| Completeness (%) (outer shell) | 100 | 99.4 | 95.6 |
| Rmerge1 (overall) | 0.126 | 0.098 | 0.124 |
| I/σ (overall) | 19.4 | 19.4 | 9.3 |
| Number of selenium sites | 22 | 22 | 22 |
| Phasing power2 | 1.19 | 1.206 | 0.6265 |
| FOMMAD3 | 0.246 | 0.246 | 0.157 |
| FOMMAD (overall) | 0.442 | ||
| FOM (sf)4 | 0.824 | ||
Rmerge = ΣΣ | Ii – Im |/ ΣΣ Ii, where Ii is the intensity of the measured reflection and Im is the mean intensity of all symmetry-related reflections.
Phasing power = FH/ERMS.
FP, FPH, and FH are the protein, derivative, and heavy-atom structure factors, respectively, and ERMS is the residual lack of closure.
Figure of merit from MAD phasing.
Figure of merit after solvent flipping.
Structure Determination and Refinement
All crystallographic procedures, including Patterson searches, MAD phasing, density modification, map calculation, and structure refinement, were carried out with the CNS suite [25]. Electron density maps were of high quality (data not shown) and allowed autotracing of the amino acid chain with the wARP program [26]. The procedure provided an initial model containing 221 out of 247 amino acid residues. The model was refined with 2.0 Å data with the CNS program from the CNS suite [25]. Manual adjustment and model building performed with the program QUANTA [27] allowed fitting of the addition of 26 amino acid residues. The final R factor and Rfree are 23.1% and 24.9%, respectively (Table 4). The final model included all 247 amino acid residues and 252 water molecules.
Table 4.
Structure Refinement Statistics
| Resolution range of data included (Å) | 50.0-2.0 |
| I/sigma cutoff | 0 |
| Number of reflections in working set (Rwork) | 74,997 |
| Number of reflections in test set (Rtest) | 3,681 |
| Number of protein atoms in the asymmetric unit | 3,934 |
| Number of water molecules in the asymmetric unit | 252 |
| Rwork (%) | 23.1 |
| Rfree (%) | 24.9 |
| Rmsd from ideal stereochemistry | |
| Bond lengths (Å) | 0.006 |
| Bond angles (°) | 1.5 |
| Dihedrals (°) | 24.1 |
| Impropers (°) | 0.84 |
| Mean B factor (Å2) | 26.7 |
| Ramachandran plot: | |
| Residues in most-favored region (%) | 95.3 |
| Residues in additionally allowed regions (%) | 4.7 |
Acknowledgments
We wish to thank all members of the Structural Biology Center at Argonne National Laboratory for their help in conducting experiments; Dr. Roman Laskowski for help with CATH analysis; and Lindy Keller for help in preparation of this manuscript. This work was supported by National Institutes of Health grant GM62414-01, the Ontario Research and Development Challenge Fund, and the U.S. Department of Energy, Office of Biological and Environmental Research, under contract W-31-109-Eng-38. J.E.K. was supported by United States Public Health Service training grant GM-07135. A.M.E. and C.H.A. are Canadian Institutes of Health Research investigators.
Footnotes
Accession numbers
Atomic coordinates have been deposited into the Protein Data Bank (PDB) as 1ILV.
References
- 1.Hengge-Aronis R. Survival of hunger and stress: the role of rpoS in early stationary phase gene regulation in Escherichia coli. Cell. 1993;72:165–168. doi: 10.1016/0092-8674(93)90655-a. [DOI] [PubMed] [Google Scholar]
- 2.Ichikawa JK, et al. A gene at 59 minutes on the Escherichia coli chromosome encodes a lipoprotein with unusual amino acid repeat sequences. J. Bacteriol. 1994;176:1630–1638. doi: 10.1128/jb.176.6.1630-1638.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fu JC, Ding L, Clarke S. Purification, gene cloning, and sequence analysis of an L-isoaspartyl protein carboxyl methyltransferase from Escherichia coli. J. Biol. Chem. 1991;266:14562–14572. [PubMed] [Google Scholar]
- 4.Li C, et al. A new gene involved in stationary-phase survival located at 59 minutes on the Escherichia coli chromosome. J. Bacteriol. 1994;176:6015–6022. doi: 10.1128/jb.176.19.6015-6022.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Streit WR, Hofmann K, Liebl W. Molecular characterization of the Sinorhizobium meliloti nlpD gene. Arch. Microbiol. 2000;174:292–295. doi: 10.1007/s002030000205. [DOI] [PubMed] [Google Scholar]
- 6.Visick JE, Cai H, Clarke S. The L-isoaspartyl protein repair methyltransferase enhances survival of aging Escherichia coli subjected to secondary environmental stresses. J. Bacteriol. 1998;180:2623–2629. doi: 10.1128/jb.180.10.2623-2629.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li C, Wu PY, Hsieh M. Growth-phase-dependent transcriptional regulation of the pcm and surE genes required for stationary-phase survival of Escherichia coli. Microbiology. 1997;143:3513–3520. doi: 10.1099/00221287-143-11-3513. [DOI] [PubMed] [Google Scholar]
- 8.Ichikawa JK, Clarke S. A highly active protein repair enzyme from an extreme thermophile: the L-isoaspartyl methyltransferase from Thermotoga maritima. Arch. Biochem. Biophys. 1998;358:222–231. doi: 10.1006/abbi.1998.0830. [DOI] [PubMed] [Google Scholar]
- 9.Visick JE, Ichikawa JK, Clarke S. Mutations in the Escherichia coli surE gene increase isoaspartyl accumulation in a strain lacking the pcm repair methyltransferase but suppress stress-survival phenotypes. FEMS Microbiol. Lett. 1998;167:19–25. doi: 10.1111/j.1574-6968.1998.tb13202.x. [DOI] [PubMed] [Google Scholar]
- 10.Riehle MM, Bennett AF, Long AD. Genetic architecture of thermal adaptation in Escherichia coli. Proc. Natl. Acad. Sci. USA. 2001;98:525–530. doi: 10.1073/pnas.021448998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tatusov RL, Koonin EV, et al. The COG database: new developments in phylogenetic classification of proteins from complete genomes. Nuc. Acids Res. 2001;29:22–28. doi: 10.1093/nar/29.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJJ. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 13.Treton BY, Le Dall MT, Gaillardin CM. Complementation of Saccharomyces cerevisiae acid phosphatase mutation by a genomic sequence from the yeast Yarrowia lipolytica identifies a new phosphatase. Curr. Genet. 1992;22:345–355. doi: 10.1007/BF00352435. [DOI] [PubMed] [Google Scholar]
- 14.Horovitz A, Serrano L, Avron B, Bycroft M, Fersht AR. Strength and co-operativity of contributions of surface salt bridges to protein stability. J. Mol. Biol. 1990;20:1031–1044. doi: 10.1016/S0022-2836(99)80018-7. [DOI] [PubMed] [Google Scholar]
- 15.Kumar S, Nussinov R. Salt bridge stability in monomeric proteins. J. Mol. Biol. 1999;293:1241–1255. doi: 10.1006/jmbi.1999.3218. [DOI] [PubMed] [Google Scholar]
- 16.Penheiter AR, Duff SM, Sarath G. Soybean root nodule acid phosphatase. Plant Physiol. 1997;114:597–604. doi: 10.1104/pp.114.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schenk G, de Jersey J, et al. Binuclear metal centers in plant purple acid phosphatses’ Fe-Mn in sweet potato and Fe-2n in soybean. Arch. Biochem. Biophys. 1999;370:183–189. doi: 10.1006/abbi.1999.1407. [DOI] [PubMed] [Google Scholar]
- 18.Lee H, Chu TM, Li SL, Lee CL. Homodimer and heterodimer subunits of human prostate acid phosphatase. Biochem. J. 1991;277:759–765. doi: 10.1042/bj2770759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holm L, Sander C. Protein structure comparison by alignment of distance matrices. J. Mol. Biol. 1993;233:123–138. doi: 10.1006/jmbi.1993.1489. [DOI] [PubMed] [Google Scholar]
- 20.Varughese KI, et al. Crystal structure of rat liver dihydropteridine reductase. Proc. Natl. Acad. Sci. USA. 1992;89:6080–6084. doi: 10.1073/pnas.89.13.6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakai K, Kanehisa M. Expert system for predicting protein localization sites in gram-negative bacteria. Proteins. 1991;11:95–110. doi: 10.1002/prot.340110203. [DOI] [PubMed] [Google Scholar]
- 22.Kwak JE, Suh SW, et al. Crystallization and preliminary X-ray crystallographic analysis of the SurE protein from Thermotoga maritima. Acta Crystallalogr. D. 2001;57:612–613. doi: 10.1107/s0907444901002141. [DOI] [PubMed] [Google Scholar]
- 23.Walsh MA, Dementieva I, Evans G, Sanishvili R, Joachimiak A. Taking MAD to the extreme: ultrafast protein structure determination. Acta Crystallogr. D. 1999;55:1168–1173. doi: 10.1107/s0907444999003698. [DOI] [PubMed] [Google Scholar]
- 24.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 25.Brunger AT, et al. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 26.Perrakis A, Morris R, Lamzin VS. Automated protein model building combined with iterative structure refinement. Nat. Struct. Biol. 1999;6:458–463. doi: 10.1038/8263. [DOI] [PubMed] [Google Scholar]
- 27.QUANTA. San Diego: Molecular Simulations Inc; 2000. [Google Scholar]
- 28.Penheiter AR, Duff SM, Sarath G. Soybean root nodule acid phosphatase. Plant Physiol. 1997;114:597–604. doi: 10.1104/pp.114.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Etten RL, Waymack PP. Substrate specificity and pH dependence of homogeneous wheat germ acid phosphatase. Arch. Biochem. Biophys. 1991;288:634–645. doi: 10.1016/0003-9861(91)90246-f. [DOI] [PubMed] [Google Scholar]