Abstract
Extramedullary myelopoiesis occurs in peripheral organs such as spleen and produces many types of myeloid cells with diverse functions in response to inflammation and infection. It is increased during immune responses and chronic inflammation and is a significant factor in regulating inflammatory diseases and immunity. Increased myeloid cells are found in FoxP3-deficient mice but the mechanism has been unclear. We investigated the mechanism by which FoxP3+ regulatory T cells regulate the extramedullary myelopoiesis. We found that antibody or genetic depletion of FoxP3+ regulatory T cells greatly increased the number of the myeloid progenitors in spleen during immune responses. Consistently, the splenic myelopoiesis was effectively suppressed by increased numbers of natural or induced FoxP3+ regulatory T cells. We demonstrated that myelopoiesis is positively regulated by splenic CD4+ T cells that produce myelopoietic cytokines (GM-CSF and IL-3), and these effector CD4+ T cells are induced from naïve CD4+ T cells in response to antigenic stimulation. FoxP3+ regulatory T cells were able to effectively suppress the differentiation of naïve T cells into myelopoietic cytokine-producing T cells. This suppression was found to be dependent on cell-contact but independent of TGF-β. Unlike splenic myelopoiesis, marrow myelopoiesis is not significantly affected by FoxP3+ regulatory T cells. We conclude that FoxP3+ T cells can negatively regulate splenic extramedullary myelopoiesis by suppressing the naïve T cell differentiation into myelopoietic cytokine-producing CD4+ T cells. Our results provide new insights into regulation of extramedullary myelopoiesis.
Introduction
Bone marrow is the primary site of hematopoiesis in adult mammals (1). During embryo development before formation of functional bone marrow, however, yolk sac, paraaortic splanchnopleural mesoderm, fetal liver and spleen serve as hematopoietic sites for survival of fetus (2). Spleen serves as a site of extramedullary hematopoiesis also in adult mammals (3, 4). Extramedullary hematopoiesis, and more specifically, extramedullary myelopoiesis (EM),2 is important for production of sufficient numbers of leukocytes such as phagocytes and antigen presenting cells during immune responses but excessive EM is often seen in autoimmunity and systemic inflammation. The important role of splenic EM in development of immunity is well evidenced by the marked reduction in phagocytosis and clearance of extracellular pathogens in asplenic patients (5, 6). Excessive splenic EM is a feature of many autoimmune diseases and chronic infection in humans and animals (7-9). Moreover, a heterogeneous group of myeloid cells called myeloid-derived suppressor cells with immune regulatory functions are increased in peripheral organs following infection and cancer formation and are likely to be a product of EM (10).
Myelopoiesis is both positively and negatively regulated by a number of cell types and cytokines in the body. For example, IL-3, stem cell factor (SCF), G-CSF, GM-CSF, and IL-6 are important promyelocytic cytokines (11-14). In contrast, some inflammatory cytokines and many chemokines negatively regulate myelopoiesis (15). While it is still unclear what cell types regulate extramedullary hematopoiesis, there is evidence that T cells have the potential to regulate the process (16). This is probably because T cells can produce GM-CSF, IL-3 and other hematopoietic cytokines upon activation. In line with this, it has been reported that reduced myelopoiesis occurs in T-cell deficient mice (17-21).
FoxP3+ regulatory T cells (Tregs)2 constitute a major subset of T cells with immunosuppressive functions (22-24). Tregs can suppress various cell types such as T cells, B cells, dendritic cells, macrophages, and NK cells to achieve immune tolerance (25-31). Deficiency of these T-cells due to congenital mutations in the FoxP3 gene or other genes important for induction or expansion of Tregs leads to autoimmune diseases in multiple organs (32-34). The important role of Tregs in regulation of myelopoiesis is well evidenced by the greatly increased numbers of Mac1 (CD11b/CD18)+ cells including neutrophils, monocytes and eosinophils in various tissues (33, 35-37). In this study, we investigated the roles of hematopoietic cytokine-producing T cells and Tregs in regulation of EM. We found that Tregs negatively regulate the splenic myelopoiesis but have a minimal effect on marrow myelopoiesis. Tregs regulate myelopoiesis through suppression of T cells that produce myelopoietic cytokines through a cell-contact-dependent but TGF-β-independent manner.
Methods
Mice
BALB/c mice were purchased from Harlan (Indianapolis, IN). FoxP3-deficient scurfy mice and dnTGFβRII mice were purchased from the Jackson laboratory (Bar Harbor, Maine). DO11.10 rag2(-/-) mice were purchased from Taconic (Germantown, NY). mOVA × DO11.10 rag2(-/-) transgenic mice were maintained at Purdue University. GM-CSF-deficient mice have been described previously (38). Mice were housed at Purdue University and used according to approved protocols and institutional guidelines. The FoxP3-deficient scurfy mice were used at 3 weeks of age, and other mice were used between 6 and 8 weeks of age.
Cell isolation and in vitro studies
CD4+ CD25+ T cells were isolated with a magnetic sorting method (Miltenyi Biotec, Auburn, CA) by isolation of splenic CD4+ T cells and then by positive selection of CD25+ cells (purity > 90% in FoxP3 expression). The CD25- fraction of CD4+ T cells was used as target T cells in many experiments. Naïve CD4+ T cells were further isolated by depleting the T cells expressing CD44 and CD69. For preparation of the culture supernatant of T cells, the two populations were cultured together or separately in the presence or absence of staphylococcal enterotoxin B (SEB2, 2 μg/ml) for 3 days in RPMI medium supplemented with 10% FBS. ELISA assays of GM-CSF and IL-3 were performed on the culture supernatant. Neutralizing anti-GM-CSF (clone MP1-22E9) and anti-IL-3 (clone MP2-8F8) antibodies were added to the culture at 10 μg/ml (Biolegend, San Diego, CA) when indicated. The culture supernatant was assayed also for its activity in supporting myelopoiesis with the hematopoietic colony forming assay as described below with splenic myeloid progenitor cells. Transwell membranes (Corning Life Sciences, Big Flats, NY) with 0.4 μm pores were used to physically separate the CD4+CD25- target cells from Tregs during culture in some experiments. For Treg suppression assays, 1 × 105 of CD4+CD25- T cells or naïve CD4+ T cells were co-cultured with Tregs at various ratios for 6 days in the presence of SEB (2 μg/ml).
Methylcellulose CFU-GM assay
The myeloid colony forming assay was performed as described before (39). Marrow cells and spleen cells were plated in 3 cm plates containing methylcellulose medium supplemented with cytokines or conditioned medium. Total marrow cells were plated at 5 × 104 cells per plate, and total splenocytes were plated at 5 × 105 cells per plate in 1.0% methylcellulose culture medium containing 30% vol/vol fetal bovine serum (Hyclone, Logan, UT). 1 U/ml recombinant human erythropoietin (Amgen Biologicals, Thousand Oaks, CA), 50 μM 2-mercaptoethanol, 10 ng/ml recombinant murine stem cell factor (Peprotech, Rocky Hill, NJ), 5% vol/vol pokeweed mitogen mouse spleen cell–conditioned medium or T cell-conditioned medium, and 0.1 mM hemin (Fluka) were added. Colonies of CFU-GM were scored after 8 days of incubation in a humidified environment at 5% CO2.
In vivo studies
For depletion studies, BALB/c mice were injected twice with 400 μg of anti-CD25 (PC61) at day 1 and 3. The mice were injected i.v. with 50 μg of SEB (Sigma-Aldrich) at day 5 and sacrificed at day 7, 10, or 20. As the result of the depletion of CD25+ cells, the frequency of FoxP3+ T cells was decreased from 10-12% to 3-4 % of CD4+ T cells in the spleen at day 10 (5 days after the SEB immunization). FoxP3-deficient scurfy mice and wild type mice were sacrificed at 3 weeks of age. Bone marrow and spleen cells were harvested and assayed to determine the number of CFU-GM. Spleen tissues were examined for immunohistochemical detection of FoxP3 and CD11b+ GR-1+ myelopoietic patches as described below.
For adoptive transfer of primary Tregs into scurfy mice, CD4+CD25+ cells (1 × 106 per pup) were transferred into 1-2 day-old new born pups. The mice were sacrificed 3 weeks later and the marrow and spleen were examined for CFU-GM by the colony assay described above, and for lineage (CD3/CD19/CD11b)-negative c-Kit+ progenitor cells, GM-CSF+ T cells, IL-3+ T cells, FoxP3+ T cells, and non-FoxP3+ T cells by flow cytometry.
For transfer of induced Tregs (iTregs) into DO11.10 rag2(-/-) mice, 1 × 107 iTregs or non-Tregs were injected into each mouse. The iTregs were prepared by culturing naïve CD4+ T cells isolated from the spleen of DO11.10 rag2(-/-) mice with concanavalin A (2.5 μg/ml), TGFβ1 (5 ng/ml) and IL-2 (100 U/ml) for 6 days. The non-Treg conventional cells were prepared in the same condition without TGFβ1. At day 2 post-injection of the cells, SEB (50 μg/mouse) was injected i.v. The mice were sacrificed 5 days later. The marrow and spleen tissues were examined for CFU-GM by the colony assay and for lineage (CD3/CD19/CD11b)-negative c-Kit+ progenitor cells, CD11b+GR-1bright and CD11b+GR-1dim myeloid cells by flow cytometry.
DO11.10 rag2(-/-) mice (tFoxP3+ cell-deficient) and mOVA × DO11.10 rag2(-/-) transgenic mice (tFoxP3+ cell-excessive) were immunized i.v. with SEB (50 μg/mouse) and the mice were sacrificed 5 days later. The marrow and spleen tissues were examined for CFU-GM by the colony assay and for FoxP3+ and FoxP3- CD4+ T cells by flow cytometry.
Immunohistochemistry
Spleens were harvested and frozen in the Tissue-Tek freezing medium (Sakura Finetek, Torrance, CA 90501). 6 μm tissue sections were fixed in cold acetone for 10 min and rehydrated in 1× PBS for surface staining. Sections were blocked with 10% rat serum for 10 min and stained with fluorescent anti-mouse GR-1 (clone RB6-8C5), anti-mouse CD11b (clone M1/70), and anti-mouse CD4 (clone GK1.5) for 40 min at 4°C. For nuclear FoxP3 staining, sections were stained with fluorescent anti-mouse FoxP3 (clone FJK-16s) for 40 min at 4°C according to the to the manufacturer's protocol (eBioscience, San Diego, CA). The slides were analyzed with a Leica microscope equipped with epifluorescence.
Flow cytometry
Fresh bone marrow cells and spleen cells, harvested from BALB/c mice or transgenic mice, were stained with antibodies to CD4 (clone RM4-5), CD11b, and GR-1 for 30 min on ice. The cells were further stained with anti-FoxP3 (clone FJK-16s) according to the manufacturer's protocol (eBioscience). For intracellular staining of GM-CSF and IL-3, cells were stained for surface antigens and activated for 4 hr in RPMI 1640 (10% FBS) with PMA (50 ng/ml) and ionomycin (1 μM) in the presence of monensin (2 mM). Cells were fixed, permeabilized and stained with anti-GM-CSF (clone MP1-22E9), anti-IL-3 (clone MP2-8F8) and anti-IFN-γ (clone XMG1.2) antibodies. The CD11b+Gr-1dim/bright cells were identified with antibodies to mouse CD11b (clone M1/70), GR-1 (clone RB6-8C5), c-Kit (clone 2B8), CD62L (MEL-14), and CD69 (H1.2F3). The antibodies were purchased from BD Bioscience or BioLegend (San Diego, CA). The stained cells were analyzed with a FACSCanto II (BD).
Statistical analyses
Averages with SEM are shown in most of the figures. Student's paired and unpaired t tests were used to determine the significance of the differences between two groups of data. P values < or = 0.05 were considered significant.
Results
FoxP3-deficient mice display excessive extramedullary, but normal marrow, myelopoiesis
Scurfy mice have a nonfunctional mutation in the FoxP3 gene and, thus, are completely deficient with FoxP3+ T-cells (32, 33). These mice have hematological abnormalities with increased CD11b+ cells in blood and lymphoid tissues with concomitantly increased production of GM-CSF (36, 37, 40). We found sites of myelopoiesis characterized by patches of CD11b+ GR-1+ cells in the spleen of the FoxP3-deficient mice but not in wild type mice (Fig. 1A). Also increased was the number of myeloid progenitors (CFU-GM) in the spleen of the FoxP3-deficient mice compared to wild type mice (Fig. 1B). In contrast, the numbers of CFU-GM in the bone marrow were not significantly increased in the FoxP3-deficient mice. Because effector T cells are increased in the FoxP3-deficient mice, we examined also the frequencies of GM-CSF+ and IL-3+ CD4+ T cells in the spleen and marrow. The spleen T cells and marrow T-cells were very different in expression of GM-CSF and IL-3 (Fig. 1C). Only some spleen, but not marrow, CD4+ T cells of the FoxP3-deficient mice were able to produce the two myelopoietic cytokines. These results show a positive correlation between EM and GM-CSF+ and IL-3+ CD4+ T cells; and a negative correlation between EM and FoxP3+ T cells. Also notable were the clear differences between spleen and marrow in myelopoietic activity and T cell cytokine phenotype.
Fig. 1.
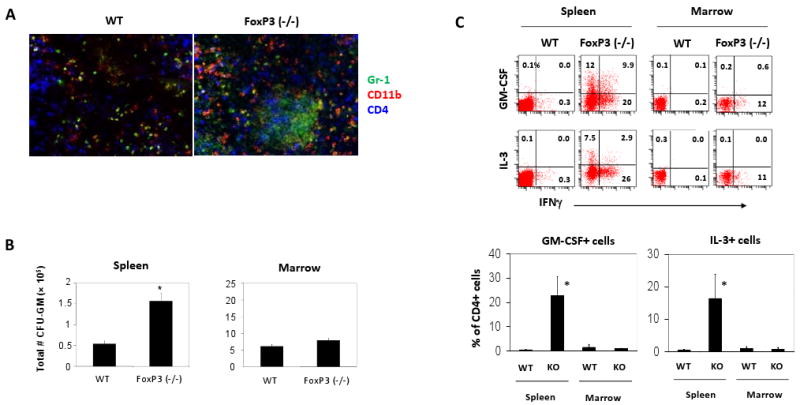
Increased myelopoietic activities and hematopoietic cytokine-producing cells in the spleen but not in the bone marrow of FoxP3 (-/-) mice. (A) Immunohistochemistry detection of the cells expressing GR-1 and CD11b in the spleen of FoxP3 (-/-) and wild type mice. (B) Total # of CFU-GM in the spleen and marrow (#/2.8 ×108 marrow cells). (C) Frequencies of GM-CSF+ CD4+ T cells and IL-3+ CD4+ T cells in spleen and marrow. Combined data of 3 independent experiments (averages and SEM; 3-5 mice) are shown. FoxP3 (-/-) mice were sacrificed at ∼3 weeks of age. The T cells were stimulated with PMA and ionomycin for 4 h to detect the cytokine-producing T cells. *Significant differences between the wild type and FoxP3 (-/-) mice (P < 0.05).
Depletion of Tregs increased the super antigen-induced splenic myelopoiesis
The excessive EM in the FoxP3-deficient mice may be due to the Treg deficiency and excessive development of T cells that produce myelopoietic cytokines but it is also likely that this is an indirect result of the chronic autoimmune disease in these mice. To clarify this, we established a mouse model of EM, induced with a superantigen staphylococcal enterotoxin B (SEB) (Fig. 2A), which polyclonally activates CD4+ T-cells by binding both to TCR of the T-cells and MHC class II molecules of antigen presenting cells (41). We found that SEB was effective in inducing EM in the spleen (Fig. 2A). Single injection (i.v.) of SEB increased the number of the myeloid progenitors (CFU-GM) in the spleen. This EM was transient following the injection of SEB, peaking at day 5. Interestingly, the bone marrow myelopoiesis did not change significantly upon the SEB challenge, a pattern similar to the unaltered numbers of marrow myeloid progenitors in the FoxP3-deficient mice.
Fig. 2.
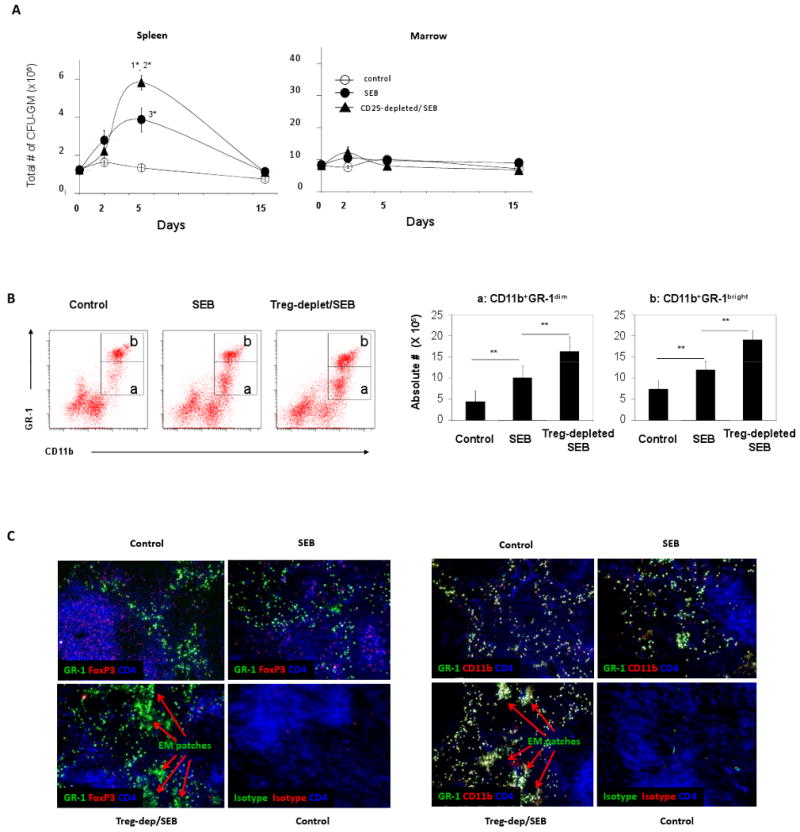
Depletion of Tregs increased a superantigen-induced myelopoiesis in BALB/c mice. (A) BALB/c mice were depleted of CD4+CD25+ cells with an anti-CD25 antibody (injected two times with a 2-day interval). Two days after the final injection of the antibody, SEB was injected i.v. into a tail vein of the mice. The mice were sacrificed at day 2, 5 and 15, and the change in CFU-GM numbers in bone marrow (# per 2.8 ×108 marrow cells) and spleen was determined by a myelopoietic colony forming assay (n=5 per group). P values for the comparisons: 1*=0.0001 between CD25-depleted/SEB and SEB groups; 2*=0.029 between CD25-depleted/SEB and control groups; 3*=0.053 between SEB and control groups. (B) Increased CD11b+GR-1dim and CD11b+GR-1bright myeloid cells in the spleen of Treg-depleted mice (n=4). CD3-CD19- splenic cells are shown. Total numbers of CD11b+ GR-1dim and CD11b+GR-1bright myeloid cells in the spleen are also shown. (C) Increased CD11b+ GR-1+ myelopoietic sites in the spleen of Treg-depleted mice. “EM patches” stands for “extramedullary myelopoietic patches”. Frozen tissue sections were stained with fluorescent antibodies in panel C. Error bars are SEM. The control mice were injected with control rat IgG. **Significant differences (P < 0.05).
To assess the role of FoxP3+ T-cells in vivo, we depleted the FoxP3+ T-cell-containing CD25+ cells utilizing a monoclonal anti-CD25 antibody before the SEB challenge. This treatment reproducibly depleted ∼95% of CD4+CD25+ cells in the spleen 2 days after the final antibody injection (not shown). The CD25+ cell-depleted group showed significantly enhanced spleen myelopoiesis compared to the non-depleted control group (Fig. 2A). However, the depletion had no effect on marrow myelopoiesis. This data suggests that FoxP3+ T-cells play a negative role in regulation of the EM but do not significantly affect marrow myelopoiesis. Along with the increased splenic CFU-GM, two populations of splenic myeloid cells (neutrophil-enriched CD11b+ GR-1bright and myeloblast-enriched CD11b+ GR-1dim cells) were increased following Treg-depletion (Fig. 2B). Consistently, we observed the emergence of CD11b+ GR-1+ hematopoietic sites (“EM patches”) in splenic red pulp areas surrounding the white pulp areas after the SEB challenge (Fig. 2C). The CD11b+ GR-1+ hematopoietic sites were greatly increased in both number and size in the Treg-depleted group (Fig. 2C). These results demonstrate that T cell activation in combination with Treg deficiency can cause excessive EM.
Myelopoietic activities of T cell-derived GM-CSF and IL-3
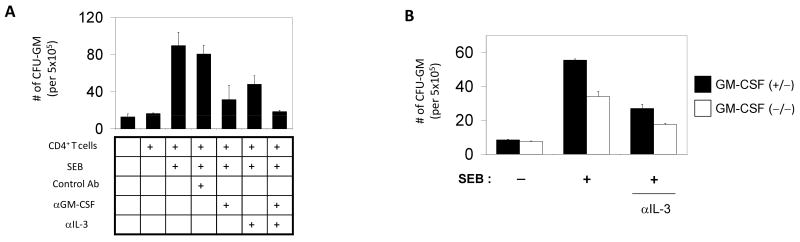
To determine the role of GM-CSF and IL-3 produced from T cells in regulation of myelopoiesis, we prepared conditioned media from the culture of CD4+ CD25- T cells and examined their activity in supporting myelopoiesis in a semi-solid methylcellulose assay. The T cell-conditioned medium was effective in supporting the splenic myeloid progenitors (Fig. 3A). Only the conditioned medium with SEB-activated T cells but not with resting T cells without antigen stimulation was effective in supporting the myelopoiesis of the splenic progenitors. Blocking of GM-CSF or IL-3 with neutralizing antibodies decreased the myelopoietic activity of the conditioned medium (Fig. 3A). Blocking of both GM-CSF and IL-3 was even more suppressive, suggesting the roles of the T cell-derived hematopoietic cytokines in supporting myelopoiesis. We tested also the T cells isolated from GM-CSF-deficient mice. We observed the lower activity of GM-CSF (-/-) T cells compared with GM-CSF (+/-) T cells in supporting myelopoiesis, which was further suppressed when IL-3 was blocked at the same time with a neutralizing antibody (Fig. 3B). These results demonstrate that the T cell-derived myelopoietic cytokines can positively regulate the myelopoiesis.
Fig. 3.
The roles of the GM-CSF and IL-3 produced by CD4+ T cells in regulation of myelopoiesis. (A) CD4+CD25- T cells were cultured in the presence of SEB, and the conditioned medium was examined for their activity to support myelopoiesis. Neutralizing antibodies to GM-CSF and IL-3 were used in the colony forming assay to determine the roles of the two cytokines in supporting the splenic myeloid progenitors. (B) CD4+CD25- T cells were isolated from GM-CSF-deficient mice or wild type mice, and the conditioned media of these T cells were examined for their activity in supporting the colony formation of splenic myeloid progenitors. An anti-IL-3 neutralizing antibody was added to the indicated cultures. Error bars are differences of duplicated measurements. Representative data of 4 independent experiments are shown.
Tregs directly suppress the T cells that produce myelopoietic cytokines
It is a question of interest how Tregs can regulate the EM. We hypothesized that Tregs would suppress the T cells that produce myelopoietic cytokines to suppress EM. To test the hypothesis, we cultured the two T-cell populations (Tregs and CD4+CD25- effector T cells) separately or together in the presence or absence of SEB and examined the production of GM-CSF/ IL-3 and myelopoietic activity of the culture supernatant (Fig. 4). Tregs suppressed the production of GM-CSF and IL-3 by the CD4+CD25- T cells in a dose-dependent manner (Fig. 4A). It was apparent that Tregs decreased the myelopoiesis-supporting activity of the CD4+CD25- effector T cells in a dose-dependent manner (Fig. 4B). Unlike the CD4+CD25- T cells, Tregs, themselves, were not good producers of the myelopoietic cytokines.
Fig. 4.
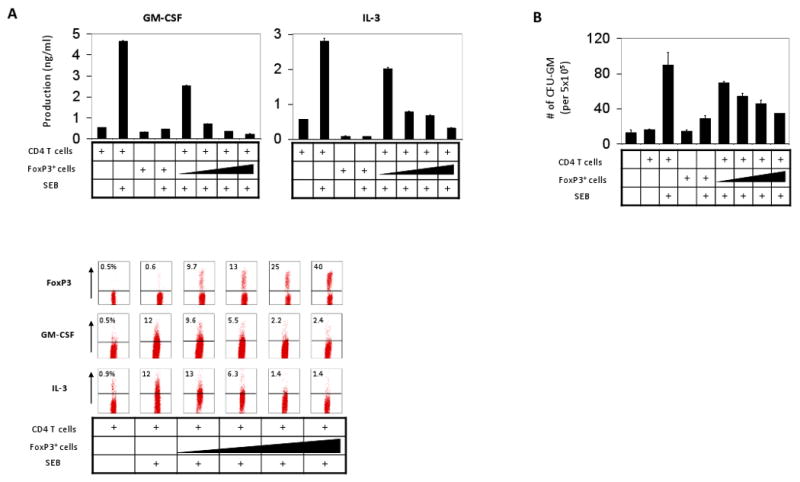
Tregs suppress the myelopoietic activity of hematopoietic cytokine-producing effector T cells. CD4+ CD25- T cells were cultured together with increasing numbers of Tregs (i.e. 0×, 0.25×, 0.5×, 1.0× and 2.0× of the number of CD4+CD25- T cells) for 5 days, and the conditioned media were examined for their activity in production of GM-CSF and IL-3 (A) and supporting the colony formation of splenic myeloid progenitors (B). The production of cytokines was examined by ELISA and intracellular staining (A). Error bars are differences of duplicated measurements. Representative data of 3 independent experiments are shown.
Tregs suppress the differentiation of naïve T-cells into myelopoietic cytokine-producing T cells
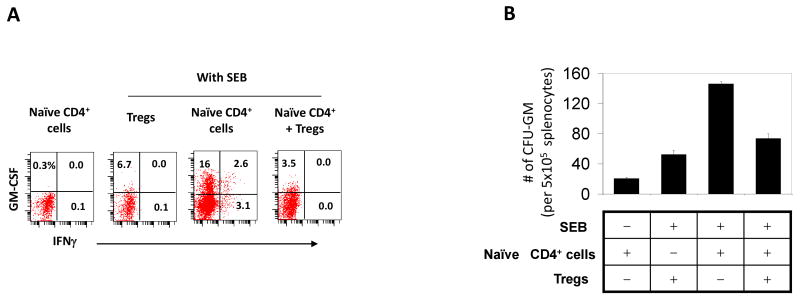
Naïve T cells do not produce GM-CSF and IL-3. Naïve T cells have to differentiate into effector T cells to gain the ability to produce the cytokines. Perhaps, Tregs would suppress the differentiation of naïve T-cells into hematopoietic cytokine-producing effector T-cells. To determine the possibility, naïve T cells, depleted of Tregs and memory/effector T cells, were isolated from spleen cells and cultured with irradiated splenocytes as antigen presenting cells in the presence of SEB. This condition was conducive for generation of GM-CSF+ CD4+ T cells from the naïve T-cells (Fig. 5A). In the absence of SEB, no GM-CSF+ T cells were made, suggesting the importance of the antigen signal in this process. Importantly, Tregs were effective in suppressing the differentiation of naïve T-cells into GM-CSF+ T cells (Fig. 5A). Tregs were effective also in suppressing the production of myelopoietic factors by the T cells differentiated from the naïve T cells (Fig. 5B).
Fig. 5.
Tregs suppress the differentiation of naïve T cells into GM-CSF+ T cells. (A) Naïve CD62L+ CD44- CD4+ T cells were cultured in the presence or absence of Tregs in the indicated conditions, and emergence of GM-CSF+ T cells was determined. (B) Conditioned media of the naïve CD4+ T cells cultured with or without Tregs were examined for their activity in supporting the colony formation of splenic myeloid progenitors. The cells were cultured for 5 days to obtain the cells and conditioned medium. Error bars are differences of duplicated measurements. Representative data of 3 independent experiments are shown.
The roles of cell-contact and TGF-β signaling in suppression of GM-CSF+ T cells
Cell-contact is typically required for the suppression of target cells by Tregs, and TGF-β is implicated in the process (42, 43). We investigated if cell-contact and TGF-β are important also for the suppression of myelopoietic cytokine-producing T cells by Tregs. We used Transwell membranes to separate the target cells in the lower chamber from the Tregs in the upper chamber. This separation was effective in abolition of the suppressive effect of Tregs on GM-CSF+ T cells (Fig. 6A).
Fig. 6.
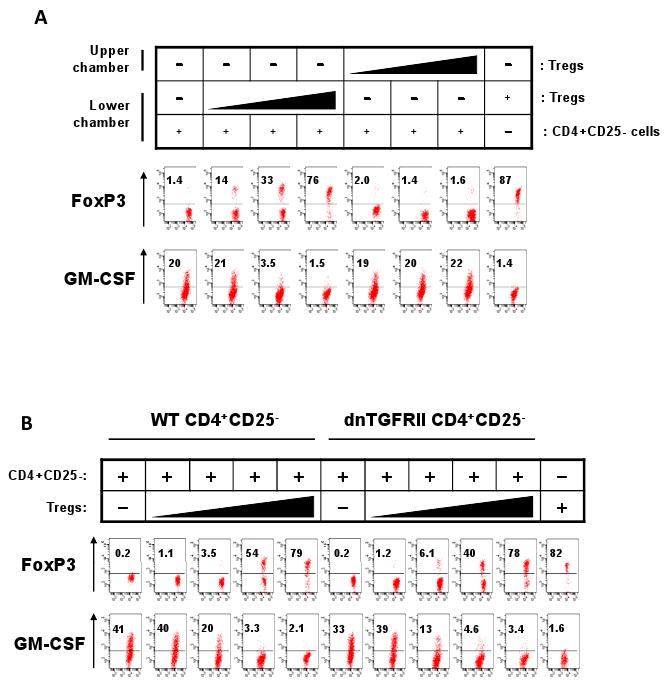
Treg-suppression of the myelopoietic activity of CD4+ T cells is cell-contact-dependent but TGF-β-independent. (A) CD4+ CD25- T-cells were cultured in the lower chambers of Transwells, and Tregs were cultured in either upper or lower chambers. The T cells were cultured for 6 days in the presence of SEB and the T cells in the lower chambers were examined for expression of GM-CSF. (B) CD4+ CD25- T-cells, isolated from wild type or dnTGFβRII mice, were cultured with or without Tregs for 6 days in the presence of SEB; and the CD4+ T cells were examined for expression of GM-CSF. Representative data of 3 independent experiments are shown.
To determine the role of TGF-β signaling in the cell-contact-dependent suppression of myelopoietic cytokine-producing T cells, we utilized the CD4+CD25- T cells isolated from the dominant negative (dn) TGF-βRII mice which are defective in transmitting TGF-β signaling (44). Tregs were able to suppress the CD4+CD25- T cells isolated from both wild type and dnTGF-βRII mice in production of GM-CSF (Fig. 6B), suggesting that the TGF-β signaling is not required for the process. These results demonstrate that Tregs need cell-contact but not the TGF-β signaling in suppression of the GM-CSF+ T cells.
Increased numbers of FoxP3+ T cells limit the numbers of myelopoietic cytokines and EM in the spleen
To confirm the positive function of Tregs in limiting splenic myelopoiesis, we transferred Tregs isolated from secondary lymphoid tissues of wild type mice into 1 or 2 day-old scurfy mice. As the result, the frequency of FoxP3+ T cells in the spleen was greatly increased in the scrufy mice with concomitant decreases in activated (CD69+) memory or effector (CD62L-) CD4+ T cells at 3 weeks of age (Fig.7A). Importantly, the levels of CD4+ T cells expressing GM-CSF or IL-3 were nearly normalized (i.e. decreased) following the Treg transfer (Fig.7B). Furthermore, the Treg transfer decreased the numbers of lineage-negative c-Kit+ progenitor cells and CFU-GM progenitor cells (Fig.7C and D).
Fig. 7.
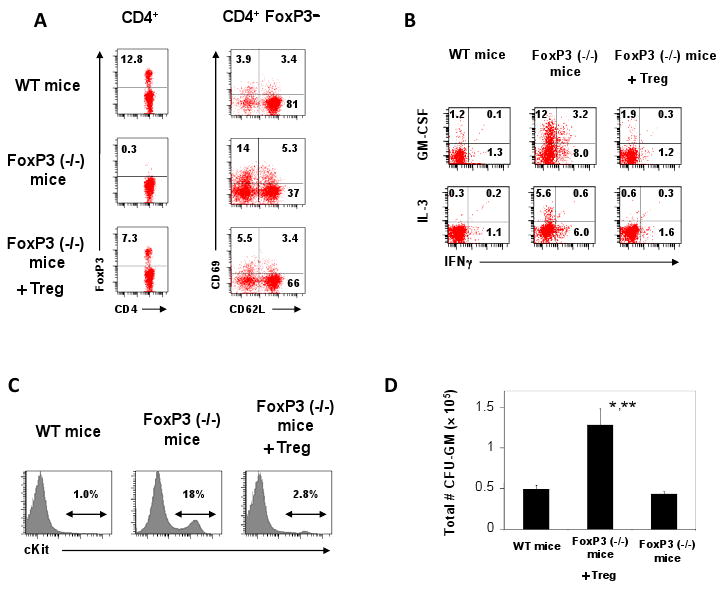
Tregs suppress the extramedullary myelopoiesis in the spleen of FoxP3(-/-) scurfy mice. (A) Frequencies of FoxP3+ CD4+ T cells and FoxP3- CD4+CD69+ /CD62L+ in the spleen of ∼3 week-old FoxP3(-/-) scurfy mice transferred with Tregs at day 1 or 2 following birth compared to age-matched wild type mice and control scurfy mice. (B) Frequencies of GM-CSF+ CD4+ T cells and IL-3+ CD4+ T cells in the spleen. (C) Frequencies of lineage (CD3/CD19/CD11b)-negative c-Kit+ progenitor cells in the spleen. (D) Frequencies of CFU-GM myeloid progenitor cells in the spleen. Averages and SEM of the data obtained from 4-6 mice are shown. *,**Significant differences from wild type mice (*, P < 0.05) or FoxP3(-/-) mice (**, P < 0.05).
The FoxP3+ T cells that are made in the periphery upon immunization are called induced FoxP3+ T cells (iFoxP3+ cells) while the FoxP3+ T cells that are made in the thymus are called thymus-generated FoxP3+ T cells (tFoxP3+ cells). We examined if iFoxP3+ cells can suppress the splenic myelopoiesis induced upon antigenic stimulation. We utilized DO11.10 rag2(-/-) mice which lack tFoxP3+ cells (45) (Fig. 8A). We transferred iFoxP3+ cells, generated in vitro from DO11.10 rag2(-/-) CD4+ T cells with TGFβ1, into DO11.10 rag2(-/-) mice and immunized the mice with SEB. For comparison, we transferred activated conventional non-Treg CD4+ T cells prepared without TGFβ1. The Treg transfer increased the frequency of FoxP3+ cells from 0.1 to ∼2.5% in the spleen at day 5 post-immunization (Fig. 8B). As the consequence, the numbers of lineage- c-Kit+ cells (Fig. 8C), CFU-GM (Fig. 8D) and CD11b+ GR-1dim (Fig. 8E) or CD11b+ GR-1bright cells (Fig. 8F) were significantly decreased following the immunization, while they were slightly increased in the mice transferred with non-Tregs. The incomplete suppression is probably due to the low (∼2.5%) frequency of transferred iTregs. Overall, the data suggest that iTregs have the ability to control the SEB-induced myelopoiesis in the spleen.
Fig. 8.
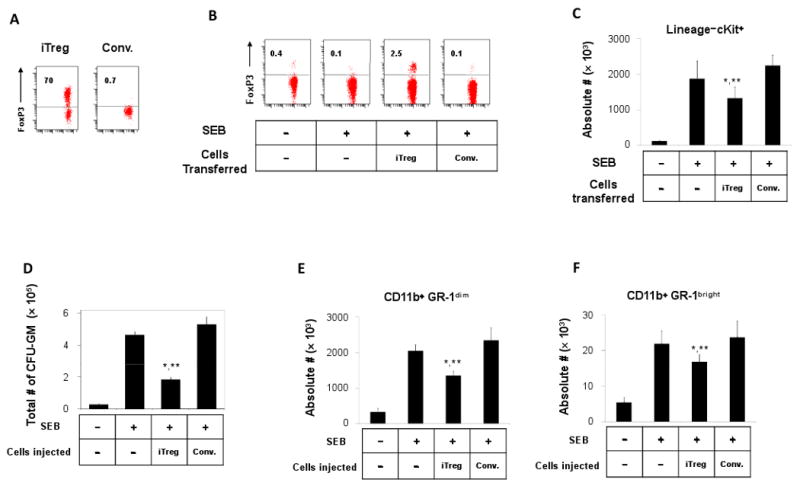
Induced Tregs suppress the extramedullary myelopoiesis in the spleen of DO11.10 rag2(-/-) mice. (A) Induced FoxP3+ T cells (iTreg) were prepared by activating DO11.10 rag2(-/-) CD4+ T cells with TGFβ1 for 6 days in vitro. Conventional CD4+ T cells (Conv) were prepared without using TGFβ1. (B) Frequencies of FoxP3+ T cells among splenic CD4+ T cells in the DO11.10 rag2(-/-) mice transferred with iTreg or conventional CD4+ T cells. Frequencies of lineage (CD3/CD19/CD11b)-negative c-Kit+ progenitor cells in the spleen (C), CFU-GM (D), CD11b+GR-1dim cells (E), and CD11b+GR-1bright cells (F) in the spleen of DO11.10 rag2(-/-) mice transferred with iTreg or conventional CD4+ T cells. Averages and SEM of the data obtained from 4-5 mice are shown. *,**Significant differences from the SEB group (*, P < 0.05) or the SEB/conventional T cell group (**, P < 0.05).
mOVA × DO11.10 rag2(-/-) mice have greatly increased tFoxP3+ T cells due to the transgenic expression of membrane ovalbumin and TCR-specific for the same antigen (Fig. 9A). SEB can induce or expand FoxP3+ T cells in DO11.10 rag2(-/-) mice but the absolute number of FoxP3+ T cells in these mice was still smaller than those in mOVA × DO11.10 rag2(-/-) mice after the immunization (Fig. 9B). We compared the myelopoietic response in the spleen of these mice with that of DO11.10 rag2(-/-) mice which are deficient with tFoxP3+ T cells. We observed that the SEB immunization in mOVA × DO11.10 rag2(-/-) mice induced relatively smaller increases of conventional CD69+ or CD62L-CD4+ T cells (Fig. 9C) and CFU-GM myeloid progenitors (Fig. 9D). Thus, these results additionally confirm that FoxP3+ T cells have a positive impact on limiting the splenic myelopoiesis induced upon antigenic stimulation.
Fig. 9.
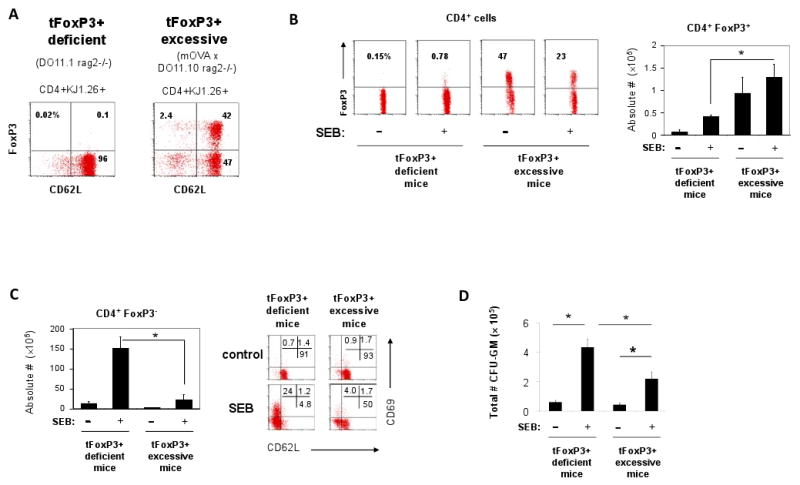
SEB-induced myelopoietic activity in the spleen of mOVA × DO11.10 rag2(-/-) mice compared to DO11.10 rag2(-/-) mice. (A) Frequencies of CD4+ FoxP3+ T cells in the spleen of mOVA × DO11.10 rag2(-/-) mice compared to DO11.10 rag2(-/-) mice prior to immunization. (B) CD4+ FoxP3+ T cells in the spleen of mOVA × DO11.10 rag2(-/-) mice compared to DO11.10 rag2(-/-) mice 5 days after immunization with SEB. Total numbers of CD4+ FoxP3+ T cells in the spleen are shown in the bar graph. (C) Frequencies of CD4+FoxP3- CD69+ /CD62L+ cells in the spleen of mOVA × DO11.10 rag2(-/-) mice compared to DO11.10 rag2(-/-) mice 5 days after immunization with SEB. (D) Frequencies of CFU-GM myeloid progenitor cells in the spleen 5 days after immunization with SEB. Averages and SEM of the data obtained from 4-6 mice are shown. *Significant differences between the groups (P < 0.05).
Discussion
Regulation of splenic EM in response to antigenic stimulation or inflammation has been largely unknown. Through the studies, we found that the splenic EM can be positively regulated by effector T cells that produce GM-CSF and IL-3, and FoxP3+ T-cells suppress the T cells that produce GM-CSF and IL-3 and negatively regulate splenic EM but do not significantly affect marrow myelopoiesis.
The T cells that produce hematopoietic cytokines such as GM-CSF and IL-3 are thought to be important for production of necessary phagocytes and antigen presenting cells during immune responses. However, excessive activities of these T cells and myelopoiesis would be harmful for the host because they could induce hyper-immune responses and inflammation. This view is supported by the fact that over-expression of GM-CSF induces chronic inflammation and autoimmune reactions (46, 47). In contrast, GM-CSF-deficient mice have no problem in basic myelopoiesis but are impaired in mounting immunity against bacterial pathogens (48-51). Appropriate regulation of myelopoietic cytokine-producing cells would be important to achieve both immunity and immune tolerance. Therefore, there is the need to negatively regulate EM. We found that Tregs provide the necessary negative signal to down-regulate EM induced upon antigenic stimulation or in systemic inflammation. Tregs themselves are not a good source of the myelopoietic cytokines. Rather, Tregs effectively suppress the production of the myelopoietic cytokines by the effector T cells. These findings are well-supported by the increased numbers of GM-CSF+ and IL-3+ T-cells and the high myelopoietic activity in the spleen of the FoxP3-deficient mice. Through the SEB-EM model, we found that the splenic EM occurs in the splenic red pulp areas surrounding white pulp areas following an immunological challenge. Depletion of FoxP3+ T-cells greatly expanded the size and number of the CD11b+ GR-1+ myelopoietic patches in spleen. On the other hand, transfer of Tregs decreased the myelopoietic activity in the spleen. These results demonstrate that Tregs play a negative role in the process.
In suppression of EM, FoxP3+ T-cells may suppress the effector T-cells in the white pulp areas or they may suppress the production of hematopoietic cytokines in the red pulp areas. We found almost no FoxP3+ T-cells within the CD11b+ GR-1+ myelopoietic patches in the red pulp areas during immune responses. Instead, almost all FoxP3+ T-cells were found in the white pulp areas and, more specifically, in the T cell area (Fig. 2C). This information supports the view that FoxP3+ Tregs function in white pulps where naïve T cells undergo activation and differentiation into effector T cells that produce myelopoietic cytokines. In this regard, our results demonstrated that Tregs are effective in blocking the differentiation of naive T cells into GM-CSF+ effector T cells (Fig. 5).
It is interesting that FoxP3+ Tregs, while they regulate the myelopoiesis in the spleen, do not affect that in the bone marrow. This is somewhat unexpected because the bone marrow is highly enriched with FoxP3+ T cells (which constitute ∼40% of the marrow CD4+ T cells) (45, 52). We have two explanations for this. First, the frequency of effector CD4+ T cells in the bone marrow (∼0.1 % of total marrow cells versus ∼15% of total spleen cells) is too low to affect the marrow hematopoiesis at significant levels. Second, the T cells in the bone marrow are intrinsically different from those of spleen in that they are unable to produce GM-CSF and IL-3. Thus, suppression of marrow effector T cells would not have any significant impact on production of the myelopoietic cytokines.
The CD11b+ GR-1+ myeloid cells with certain immune regulatory functions are getting a lot of attention these days. Many groups noted the emergence of these myeloid-derived suppressor cells in spleen and other organs in infection and cancer (10, 53). The myeloid cells that are induced in the absence of Tregs in our study could include these myeloid cells. However, one should note that the CD11b+ GR-1bright/dim myeloid cells induced in the spleen are highly heterogeneous and should not consider all of these cells are myeloid-derived suppressor cells. Indeed, it has been reported that CD11b+GR-1+ myeloid cells induced in the spleen of tumor-free animals often lack the regulatory function (54). The myeloid-derived suppressor cells themselves are highly heterogeneous and are considered normal constituents of myelopoiesis (10).
Overall, our results demonstrate that Tregs function to restrain the myelopoietic cytokine-producing T cells and, therefore, play an active role in prevention of extramedullary myelopoiesis induced upon antigenic stimulation in the spleen. EM can occur in diverse forms and tissues in addition to the spleen in diseased conditions such as chronic inflammation and cancer. It remains to be determined if Tregs have a similar function in regulation of other types of EM occurring in non-lymphoid tissues.
Acknowledgments
We thank S. Kang for helpful input (Purdue University).
Footnotes
This study was supported, in part, from grants from American Heart Association, NIH (R21AI063064, R01AI074745, R01DK076616), and Crohn's and Colitis Foundation of America to CHK.
Abbreviations: FoxP3, Forkhead box P3; Tregs, FoxP3+ regulatory T cells; CFU-GM, colony forming unit-granulocyte/macrophage; SEB, Staphylococcal Enterotoxin B; EM, Extramedullary Myelopoiesis; iTregs or iFoxP3+ cells, induced FoxP3+ regulatory T cells; tFoxP3+ T cells, thymus-generated FoxP3+ T cells.
References
- 1.Cumano A, Godin I. Ontogeny of the hematopoietic system. Annual review of immunology. 2007;25:745–785. doi: 10.1146/annurev.immunol.25.022106.141538. [DOI] [PubMed] [Google Scholar]
- 2.Heissig B, Ohki Y, Sato Y, Rafii S, Werb Z, Hattori K. A role for niches in hematopoietic cell development. Hematology. 2005;10:247–253. doi: 10.1080/10245330500067249. [DOI] [PubMed] [Google Scholar]
- 3.Spencer RP, Pearson HA. The spleen as a hematological organ. Semin Nucl Med. 1975;5:95–102. doi: 10.1016/s0001-2998(75)80007-9. [DOI] [PubMed] [Google Scholar]
- 4.Cumano A, Dieterlen-Lievre F, Godin I. Lymphoid potential, probed before circulation in mouse, is restricted to caudal intraembryonic splanchnopleura. Cell. 1996;86:907–916. doi: 10.1016/s0092-8674(00)80166-x. [DOI] [PubMed] [Google Scholar]
- 5.Brigden ML. Overwhelming postsplenectomy infection. West J Med. 1993;158:308–309. [PMC free article] [PubMed] [Google Scholar]
- 6.Sills RH. Splenic function: physiology and splenic hypofunction. Crit Rev Oncol Hematol. 1987;7:1–36. doi: 10.1016/s1040-8428(87)80012-4. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki H, Kundig TM, Furlonger C, Wakeham A, Timms E, Matsuyama T, Schmits R, Simard JJ, Ohashi PS, Griesser H, et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor beta. Science. 1995;268:1472–1476. doi: 10.1126/science.7770771. [DOI] [PubMed] [Google Scholar]
- 8.Murray PJ, Young RA, Daley GQ. Hematopoietic remodeling in interferon-gamma-deficient mice infected with mycobacteria. Blood. 1998;91:2914–2924. [PubMed] [Google Scholar]
- 9.Arellano-Rodrigo E, Esteve J, Gine E, Panes J, Cervantes F. Idiopathic myelofibrosis associated with ulcerative colitis. Leuk Lymphoma. 2002;43:1481–1483. doi: 10.1080/1042819022386590. [DOI] [PubMed] [Google Scholar]
- 10.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laiosa CV, Stadtfeld M, Graf T. Determinants of lymphoid-myeloid lineage diversification. Annual review of immunology. 2006;24:705–738. doi: 10.1146/annurev.immunol.24.021605.090742. [DOI] [PubMed] [Google Scholar]
- 12.Kaushansky K. Lineage-specific hematopoietic growth factors. The New England journal of medicine. 2006;354:2034–2045. doi: 10.1056/NEJMra052706. [DOI] [PubMed] [Google Scholar]
- 13.Nishimoto N, Kishimoto T. Interleukin 6: from bench to bedside. Nature clinical practice. 2006;2:619–626. doi: 10.1038/ncprheum0338. [DOI] [PubMed] [Google Scholar]
- 14.Barreda DR, Hanington PC, Belosevic M. Regulation of myeloid development and function by colony stimulating factors. Dev Comp Immunol. 2004;28:509–554. doi: 10.1016/j.dci.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Broxmeyer HE. Chemokines in hematopoiesis. Curr Opin Hematol. 2008;15:49–58. doi: 10.1097/MOH.0b013e3282f29012. [DOI] [PubMed] [Google Scholar]
- 16.Garland JM. Lymphocytes, lymphokines, and hematopoiesis. Immunol Ser. 1990;49:297–328. [PubMed] [Google Scholar]
- 17.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 18.Kelso A, Owens T. Production of two hemopoietic growth factors is differentially regulated in single T lymphocytes activated with an anti-T cell receptor antibody. J Immunol. 1988;140:1159–1167. [PubMed] [Google Scholar]
- 19.Wierenga EA, Backx B, Snoek M, Koenderman L, Kapsenberg ML. Relative contributions of human types 1 and 2 T-helper cell-derived eosinophilotrophic cytokines to development of eosinophilia. Blood. 1993;82:1471–1479. [PubMed] [Google Scholar]
- 20.Broxmeyer HE, Sehra S, Cooper S, Toney LM, Kusam S, Aloor JJ, Marchal CC, Dinauer MC, Dent AL. Aberrant regulation of hematopoiesis by T cells in BAZF-deficient mice. Mol Cell Biol. 2007;27:5275–5285. doi: 10.1128/MCB.01967-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monteiro JP, Benjamin A, Costa ES, Barcinski MA, Bonomo A. Normal hematopoiesis is maintained by activated bone marrow CD4+ T cells. Blood. 2005;105:1484–1491. doi: 10.1182/blood-2004-07-2856. [DOI] [PubMed] [Google Scholar]
- 22.Shevach EM, DiPaolo RA, Andersson J, Zhao DM, Stephens GL, Thornton AM. The lifestyle of naturally occurring CD4+ CD25+ Foxp3+ regulatory T cells. Immunol Rev. 2006;212:60–73. doi: 10.1111/j.0105-2896.2006.00415.x. [DOI] [PubMed] [Google Scholar]
- 23.Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nat Immunol. 2007;8:457–462. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- 24.Sakaguchi S, Setoguchi R, Yagi H, Nomura T. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in self-tolerance and autoimmune disease. Curr Top Microbiol Immunol. 2006;305:51–66. doi: 10.1007/3-540-29714-6_3. [DOI] [PubMed] [Google Scholar]
- 25.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cederbom L, Hall H, Ivars F. CD4+CD25+ regulatory T cells down-regulate co-stimulatory molecules on antigen-presenting cells. Eur J Immunol. 2000;30:1538–1543. doi: 10.1002/1521-4141(200006)30:6<1538::AID-IMMU1538>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 27.Azuma T, Takahashi T, Kunisato A, Kitamura T, Hirai H. Human CD4+ CD25+ regulatory T cells suppress NKT cell functions. Cancer Res. 2003;63:4516–4520. [PubMed] [Google Scholar]
- 28.Taams LS, van Amelsfort JM, Tiemessen MM, Jacobs KM, de Jong EC, Akbar AN, Bijlsma JW, Lafeber FP. Modulation of monocyte/macrophage function by human CD4+CD25+ regulatory T cells. Hum Immunol. 2005;66:222–230. doi: 10.1016/j.humimm.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim HW, Hillsamer P, Banham AH, Kim CH. Cutting edge: direct suppression of B cells by CD4+ CD25+ regulatory T cells. J Immunol. 2005;175:4180–4183. doi: 10.4049/jimmunol.175.7.4180. [DOI] [PubMed] [Google Scholar]
- 30.Terme M, Chaput N, Combadiere B, Ma A, Ohteki T, Zitvogel L. Regulatory T cells control dendritic cell/NK cell cross-talk in lymph nodes at the steady state by inhibiting CD4+ self-reactive T cells. J Immunol. 2008;180:4679–4686. doi: 10.4049/jimmunol.180.7.4679. [DOI] [PubMed] [Google Scholar]
- 31.Onishi Y, Fehervari Z, Yamaguchi T, Sakaguchi S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:10113–10118. doi: 10.1073/pnas.0711106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, Bricarelli FD, Byrne G, McEuen M, Proll S, Appleby M, Brunkow ME. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 33.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 34.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 35.Lin W, Truong N, Grossman WJ, Haribhai D, Williams CB, Wang J, Martin MG, Chatila TA. Allergic dysregulation and hyperimmunoglobulinemia E in Foxp3 mutant mice. J Allergy Clin Immunol. 2005;116:1106–1115. doi: 10.1016/j.jaci.2005.08.046. [DOI] [PubMed] [Google Scholar]
- 36.Lyon MF, Peters J, Glenister PH, Ball S, Wright E. The scurfy mouse mutant has previously unrecognized hematological abnormalities and resembles Wiskott-Aldrich syndrome. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:2433–2437. doi: 10.1073/pnas.87.7.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clark LB, Appleby MW, Brunkow ME, Wilkinson JE, Ziegler SF, Ramsdell F. Cellular and molecular characterization of the scurfy mouse mutant. J Immunol. 1999;162:2546–2554. [PubMed] [Google Scholar]
- 38.Dranoff G, Crawford AD, Sadelain M, Ream B, Rashid A, Bronson RT, Dickersin GR, Bachurski CJ, Mark EL, Whitsett JA, et al. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science. 1994;264:713–716. doi: 10.1126/science.8171324. [DOI] [PubMed] [Google Scholar]
- 39.Kim CH, Qu CK, Hangoc G, Cooper S, Anzai N, Feng GS, Broxmeyer HE. Abnormal chemokine-induced responses of immature and mature hematopoietic cells from motheaten mice implicate the protein tyrosine phosphatase SHP-1 in chemokine responses. J Exp Med. 1999;190:681–690. doi: 10.1084/jem.190.5.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Godfrey VL, Wilkinson JE, Russell LB. X-linked lymphoreticular disease in the scurfy (sf) mutant mouse. Am J Pathol. 1991;138:1379–1387. [PMC free article] [PubMed] [Google Scholar]
- 41.Scherer MT, Ignatowicz L, Winslow GM, Kappler JW, Marrack P. Superantigens: bacterial and viral proteins that manipulate the immune system. Annu Rev Cell Biol. 1993;9:101–128. doi: 10.1146/annurev.cb.09.110193.000533. [DOI] [PubMed] [Google Scholar]
- 42.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. The Journal of experimental medicine. 2001;194:629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Piccirillo CA, Letterio JJ, Thornton AM, McHugh RS, Mamura M, Mizuhara H, Shevach EM. CD4(+)CD25(+) regulatory T cells can mediate suppressor function in the absence of transforming growth factor beta1 production and responsiveness. The Journal of experimental medicine. 2002;196:237–246. doi: 10.1084/jem.20020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gorelik L, Flavell RA. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–181. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 45.Lee JH, Kang SG, Kim CH. FoxP3+ T cells undergo conventional first switch to lymphoid tissue homing receptors in thymus but accelerated second switch to nonlymphoid tissue homing receptors in secondary lymphoid tissues. J Immunol. 2007;178:301–311. doi: 10.4049/jimmunol.178.1.301. [DOI] [PubMed] [Google Scholar]
- 46.Biondo M, Nasa Z, Marshall A, Toh BH, Alderuccio F. Local transgenic expression of granulocyte macrophage-colony stimulating factor initiates autoimmunity. J Immunol. 2001;166:2090–2099. doi: 10.4049/jimmunol.166.3.2090. [DOI] [PubMed] [Google Scholar]
- 47.Lang RA, Metcalf D, Cuthbertson RA, Lyons I, Stanley E, Kelso A, Kannourakis G, Williamson DJ, Klintworth GK, Gonda TJ, et al. Transgenic mice expressing a hemopoietic growth factor gene (GM-CSF) develop accumulations of macrophages, blindness, and a fatal syndrome of tissue damage. Cell. 1987;51:675–686. doi: 10.1016/0092-8674(87)90136-x. [DOI] [PubMed] [Google Scholar]
- 48.Stanley E, Lieschke GJ, Grail D, Metcalf D, Hodgson G, Gall JA, Maher DW, Cebon J, Sinickas V, Dunn AR. Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:5592–5596. doi: 10.1073/pnas.91.12.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spight D, Trapnell B, Zhao B, Berclaz P, Shanley TP. Granulocyte-macrophage-colony-stimulating factor-dependent peritoneal macrophage responses determine survival in experimentally induced peritonitis and sepsis in mice. Shock. 2008;30:434–442. doi: 10.1097/SHK.0b013e3181673543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seymour JF. Extra-pulmonary aspects of acquired pulmonary alveolar proteinosis as predicted by granulocyte-macrophage colony-stimulating factor-deficient mice. Respirology. 2006;11(Suppl):S16–22. doi: 10.1111/j.1440-1843.2006.00801.x. [DOI] [PubMed] [Google Scholar]
- 51.LeVine AM, Reed JA, Kurak KE, Cianciolo E, Whitsett JA. GM-CSF-deficient mice are susceptible to pulmonary group B streptococcal infection. J Clin Invest. 1999;103:563–569. doi: 10.1172/JCI5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zou L, Barnett B, Safah H, Larussa VF, Evdemon-Hogan M, Mottram P, Wei S, David O, Curiel TJ, Zou W. Bone marrow is a reservoir for CD4+CD25+ regulatory T cells that traffic through CXCL12/CXCR4 signals. Cancer Res. 2004;64:8451–8455. doi: 10.1158/0008-5472.CAN-04-1987. [DOI] [PubMed] [Google Scholar]
- 53.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nature reviews. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brys L, Beschin A, Raes G, Ghassabeh GH, Noel W, Brandt J, Brombacher F, De Baetselier P. Reactive oxygen species and 12/15-lipoxygenase contribute to the antiproliferative capacity of alternatively activated myeloid cells elicited during helminth infection. J Immunol. 2005;174:6095–6104. doi: 10.4049/jimmunol.174.10.6095. [DOI] [PubMed] [Google Scholar]