Abstract
Collagen content and tensile properties of engineered articular cartilage have remained inferior to glycosaminoglycan (GAG) content and compressive properties. Based on a cartilage explant study showing greater tensile properties after chondroitinase ABC (C-ABC) treatment, C-ABC as a strategy for cartilage tissue engineering was investigated. A scaffold-less approach was employed, wherein chondrocytes were seeded into non-adherent agarose molds. C-ABC was used to deplete GAG from constructs 2 weeks after initiating culture, followed by 2 weeks culture post-treatment. Staining for GAG and type I, II, and VI collagen and transmission electron microscopy were performed. Additionally, quantitative total collagen, type I and II collagen, and sulfated GAG content were measured, and compressive and tensile mechanical properties were evaluated. At 4 wks, C-ABC treated construct ultimate tensile strength and tensile modulus increased 121% and 80% compared to untreated controls, reaching 0.5 and 1.3 MPa, respectively. These increases were accompanied by increased type II collagen concentration, without type I collagen. As GAG returned, compressive stiffness of C-ABC treated constructs recovered to be greater than 2 wk controls. C-ABC represents a novel method for engineering functional articular cartilage by departing from conventional anabolic approaches. These results may be applicable to other GAG-producing tissues functioning in a tensile capacity, such as the musculoskeletal fibrocartilages.
Introduction
Tissue engineering replacement therapies depend on the regeneration of functional tissue.1 Although the classical paradigm of a scaffold, cells, and positive biochemical or biomechanical stimulation has led to advancements in tissue engineering, significant limitations exist. One important limitation is engineering tissue that has mechanical properties on par with native values, such that an implanted construct could function under in vivo loads. With respect to articular cartilage, several groups have been able to achieve compressive properties and sulfated glycosaminoglycan (sGAG) content that span native values using scaffold-based tissue engineering approaches.2–4 However, a challenge in all current articular cartilage tissue engineering strategies is obtaining collagen content and tensile properties that approach native values.3
Another limitation inherent in the classical approach may be the use of scaffolds, because scaffolds pose potential problems such as stress-shielding, toxicity of degradation products, altering cellular phenotype, and initiation of inflammatory or foreign body responses.5–9 Another complication may be the interpretation of the biomechanical properties of tissue-engineered constructs, because the remaining biomaterial may contribute to the measured properties. Our laboratory has recently developed a scaffold-less self-assembly approach to articular cartilage tissue engineering,6 which has been enhanced through serum-free and confinement methods.10 Using this system, we have also engineered constructs with sGAG content and compressive properties in the range of native values.
Work with cartilage explants has shown that in vitro growth of immature cartilage results in an imbalance between sGAG and collagen compared with the in vivo situation, resulting in poorer tensile properties.11,12 Furthermore, recent work in a cartilage explant model demonstrated that sGAG removal with chondroitinase ABC (C-ABC), followed by 2 weeks of subsequent culture, resulted in sGAG repletion and significant increases in tissue tensile modulus and ultimate tensile strength.13 The authors suggested that a sGAG imbalance causes pre-stress in the collagen network that prevents optimal function, a concept supported theoretically.14 Because the collagen network is the main contributor to tensile properties, its proper control and balance within the proteoglycan gel are paramount for tensile function.2,15
C-ABC is a well-studied bacteria-derived enzyme that cleaves the sGAG side chains chondroitin and dermatan sulfate, as well as hyaluronic acid.16,17 It has been used extensively to study GAG biology,18–20 the contribution of extracellular matrix (ECM) components to tissue mechanical properties,21–24 and cartilage integration into defects.25–27 As such, we chose to apply C-ABC to developing engineered constructs to cause an initial depletion of sGAG, with the hypothesis that tensile material properties would increase without permanently compromising compressive properties, ultimately leading to a more mature neo-tissue.
Materials and Methods
Chondrocyte isolation, self-assembly, and culture
Bovine chondrocytes were isolated and self-assembled as previously described.6,10 Briefly, tissue isolated from the distal femur and patellofemoral groove of three 1-week-old male calves (Research 87, Boston, MA) was digested in collagenase type II (Worthington Biochemical Corp., Lakewood, NJ) for 24 h. Cells were collected, counted using a hemocytometer, and frozen at −80°C. After thawing, cells were again counted and assessed for viability, before being seeded in 5-mm-diameter nonadherent, cylindrical wells made of 2% agarose. The chondrocytes were not embedded in agarose; agarose only served as the mold. Into each well, 5.5 million live cells were seeded in 150 μL of medium, followed by an additional 350 μL of culture medium 4 h later. Each construct was allowed to sit undisturbed for the next 20 h, during which time the cells coalesced into free-floating constructs. A full medium change was then performed. Chemically defined medium consisting of Dulbecco's modified Eagle medium with 4.5 mg/mL of glucose and L-glutamine (Biowhittaker/Cambrex, Walkersville, MD); 100 nM of dexamethasone (Sigma, St. Louis, MO); 1% Fungizone; 1% penicillin/streptomycin; 1% insulin, transferrin, selenium plus linoleic acid and bovine serum albumin (BD Scientific, Franklin Lakes, NJ); 50 μg/mL of ascorbate-2-phosphate; 40 μg/mL of L-proline; and 100 μg/mL of sodium pyruvate (Fisher Scientific, Pittsburgh, PA) was used throughout the study. The 500 μL of media, in each well were changed daily throughout the experiment, and all culture took place at 37°C, 10% carbon dioxide.
At 2 weeks, all constructs were unconfined10 and transferred to tissue culture plates having only the bottom of the wells coated with a thin layer of agarose. Half of the constructs were randomly assigned to be treated with protease-free C-ABC (Sigma) at an activity of 2 U/mL of medium for 4 h at 37°C.13 After treatment, constructs were thoroughly washed five times with 400 μL of fresh medium. Half of the treated constructs were returned to culture for 2 additional weeks, and the other half, along with untreated controls, were immediately processed for histology and transmission electron microscopy (TEM), quantitative biochemistry, and mechanical testing. From culture, constructs were photographed, weighed wet, and portioned for analysis.A 3-mm-diameter punch was taken from the construct's center for creep indentation. The remaining outer ring was halved for biochemistry and tensile testing. The small amount of material needed for TEM was also taken from the outer ring of two samples per group. Histology and immunohistochemistry (IHC) samples were prepared from additional constructs.
Gross morphology, histology, and IHC
Construct diameter was measured using ImageJ (National Institutes of Health, Bethesda, MD). For histology, constructs were cryoembedded and sectioned at 14 μm. Samples were fixed in 10% phosphate buffered formalin and stained with Safranin O/fast green to examine GAG distribution and picrosirius red to examine collagen distribution. For IHC, after fixing with chilled acetone, slides were rinsed with IHC buffer, quenched of peroxidase activity with hydrogen peroxide and methanol, and blocked with horse serum (Vectastain ABC kit, Vector Labs, Burlingame, CA). Samples were not pretreated to expose the collagen epitopes, because we have not found antigen retrieval or epitope enhancement to be necessary with our current type I, II, or VI collagen IHC systems. Sections were then incubated with mouse anti-collagen type I (Accurate Chemicals, Westbury, NY), rabbit anti-collagen type II (Cedarlane Labs, Burlington, NC), or rabbit anti-collagen type VI (US Biological, Swampscott, MA). The secondary antibody (anti-mouse or anti-rabbit immunoglobulin G, Vectastain ABC kit) was then applied, and color was developed using the Vectastain ABC reagent and DAB (Vectastain ABC kit). In addition to IHC staining of the experimental groups, bovine articular cartilage was used as a positive control for type II and VI collagens and as a negative control for type I collagen. Native bovine tendon was used as a positive control for type I collagen. As additional negative controls, tissue was stained as described above but without application of the primary antibodies. Slides were examined using a light microscope.
Transmission electron microscopy
TEM samples were prepared as previously described.28 Briefly, samples were sectioned and examined in longitudinal and cross-sectional orientations. Fixed samples were stained overnight with 1% cupromeronic blue (US Biological) in 0.2 M of acetate buffer (pH 5.6) containing 0.3 M of magnesium chloride. After being rinsed in the acetate buffer, specimens were placed in 0.5% sodium tungstate overnight. Samples were dehydrated in increasing concentrations of ethanol and then infiltrated and embedded in LX-112 medium. The samples were then polymerized in a 70°C oven for 2 days. Ultrathin sections were cut in a Leica Ultracut microtome (Leica, Deerfield, IL), stained with uranyl acetate and lead citrate in a Leica EM Stainer, and examined in a JEM 1010 transmission electron microscope (JEOL, USA, Inc., Peabody, MA) at an accelerating voltage of 80 kV. Digital images were obtained using AMT Imaging System (Advanced Microscopy Techniques Corp, Danvers, MA).
Biochemical analysis
Samples were frozen overnight and lyophilized for 48 h, after which dry weights were obtained. Samples were re-suspended in 0.8 mL of 0.05 M acetic acid containing 0.5 M sodium chloride. To this suspension, 0.1 mL of a 10-mg/mL pepsin (Sigma) solution in 0.05 M acetic acid was added, and the suspension was mixed at 4°C for 96 h. Next, 0.1 mL of 10x Tris-buffered saline (TBS) buffer was added along with 0.1 mL of pancreatic elastase (1 mg/mL dissolved in 1x TBS buffer). This suspension was mixed at 4°C overnight. This digestion protocol allows collagen epitopes to be preserved for enzyme-lined immunosorbent assay (ELISA). After this protocol, no residual construct remained. From the digest, total cell number was determined using the Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen, Carlsbad, CA) assuming 7.8 pg of DNA per cell.29 sGAG content was tested using the Blyscan Sulfated GAG Assay kit, a 1,9-dimethyl-methylene blue colorimetric assay (Accurate Chemical and Scientific Corp., Westbury, NY). Additionally, after hydrolyzation of the digest by 2 N sodium hydroxide for 20 min at 110°C, samples were assayed for total collagen content using a chloramine-T hydroxyproline assay.30 SIRCOL collagen assay standard (Accurate Chemical and Scientific Corp.) was used such that the standard curve was reflective of collagen amount, eliminating the need to convert from hydroxyproline to collagen. ELISA for type I and type II collagens was performed per the manufacturer's protocol (Chondrex, Redmond, WA). Measured sGAG, total collagen, and type II collagen values for all groups were normalized to wet weight based on prior work with C-ABC by Asanbaeva and colleagues,13 upon which this present study was modeled. Additionally, total collagen and type II collagen were normalized to dry weight for the control and C-ABC treated groups at 4 weeks.
Creep indentation testing
Compressive mechanical properties were determined using creep indentation testing assuming a linear biphasic model,31 which determines the specimen's aggregate modulus, Poisson's ratio, and permeability. A creep indentation apparatus was used to determine the compressive creep and recovery behavior of the constructs.32 Each sample was attached to a flat stainless steel surface with a thin layer of cyanoacrylate glue and equilibrated for 20 min in phosphate buffered saline. The sample was then placed into the creep indentation apparatus, which automatically loaded and unloaded the specimen while recording the tissue's creep and recovery behavior. A tare load of 0.2 g, followed by a test load of 0.7 g, was applied to all but the 2-week C-ABC-treated samples with a 1-mm-diameter, flat-ended, porous, rigid tip. The 2-week C-ABC-treated samples were loaded with a tare of 0.05 g and test of 0.27 g because excessive deformation resulted when these specimens were tested with the other loads. All loads were applied until equilibrium was reached. Specimen thickness was measured using a micrometer. To calculate the specimen's material properties, a semi-analytical, semi-numeric, linear biphasic model was used.33
Tensile testing
Samples were cut into a dog-bone shape and affixed to paper tabs for gripping.34 A micrometer was used to obtain gauge length, thickness, and width measurements for each sample. Tensile tests were performed to failure at a strain rate of 0.01/s of gauge length on an electromechanical materials testing system (Instron Model 5565, Canton, MA). The apparent Young's modulus (EY) was determined using least squares fitting of the linear region of the stress–strain curve. The ultimate tensile strength (UTS) reported was the maximum stress reached during a test. All specimens failed inside the gauge length.
Statistical analysis
Five to seven samples were used for each of the four experimental groups, with the groups consisting of 2- and 4-week control and C-ABC treated constructs. A one-way analysis of variance was run for all assays, and a Student-Newman-Keuls post hoc test was performed if significance (p < 0.05) was found. As mentioned above, separate constructs were used for histology and IHC. All data are presented as means ± standard deviations.
Results
Gross characteristics
All constructs appeared hyaline-like (Fig. 1A, F, K, P). Table 1 shows growth characteristics of the constructs. For each metric (thickness, diameter, and total wet weight), each group was significantly different from all others. C-ABC treatment resulted in an immediate and significant decrease in all of these parameters, but these effects did not negatively affect subsequent growth. The control and C-ABC-treated groups had substantial increases in thickness, diameter, and total wet weight between 2 and 4 weeks.
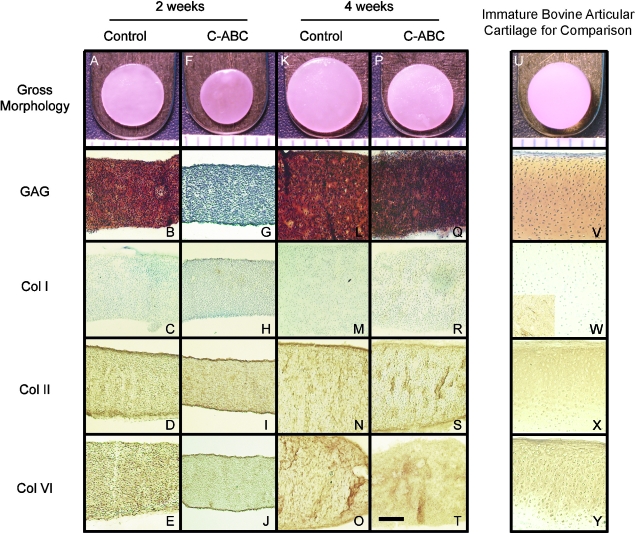
FIG. 1.
Representative gross and histological pictures of self-assembled tissue constructs for all groups. Two-week control (A–E), 2-week chondroitinase C-ABC treated (F–J), 4-week control (K–O), 4-week C-ABC treated (P–T). Ruler markings = 1 mm in A, F, K, P, and the scale bar = 200 μm in T applies to all histological images. Histological images were taken at 100X, and safranin-O/fast green was used to stain for glycosaminoglycan (GAG). Note the loss of GAG after C-ABC treatment (G), the return of GAG staining at 4 weeks (Q), and the absence of type I collagen staining in all treatment groups (C, H, M, R). A picture of an immature native articular cartilage explant and staining of immature native articular cartilage tissue are provided for comparison. The inset in the lower left of W is bovine tendon stained for collagen type I. Color images available online at www.liebertonline.com/ten.
Table 1.
Construct Thickness, Diameter, and Total Wet Weight
| Time | Treatment | Thickness (mm) | Diameter (mm) | Wet weight (mg) |
|---|---|---|---|---|
| 2 wks | Control | 0.38 ± 0.04A | 5.24 ± 0.09A | 8.7 ± 0.94A |
| C-ABC | 0.28 ± 0.01B | 4.59 ± 0.20B | 4.8 ± 0.6B | |
| 4 wks | Control | 0.68 ± 0.06C | 6.26 ± 0.25C | 21.31 ± 3.42C |
| C-ABC | 0.44 ± 0.02D | 5.49 ± 0.15D | 11.56 ± 1.49D |
Data are given as mean ± S.D. Within a column, groups not sharing a similar letter are significantly different from one another (p < 0.05). For each metric, each group was significantly different from all others.
Histology, IHC, and TEM
After treatment with C-ABC, GAG staining was lost from the construct, but staining returned at 4 weeks (Fig. 1B, G, L, Q). Collagen staining using picrosirius red showed diffuse collagen presence throughout the constructs in all groups. Examination of these slides with polarized light microscopy showed no preferential collagen orientation. There was no type I collagen staining found in any group with IHC (Fig. 1C, H, M, R). In contrast, type II and VI collagen staining was diffuse throughout the constructs for all groups (Fig. 1D, I, N, S and E, J, O, T). For comparison, staining of immature bovine articular cartilage for GAG and types I, II, and VI collagen is also provided (Fig. 1V, W, X, Y). The bovine tendon tissue control stained positive for collagen type I (Fig. 1W inset).
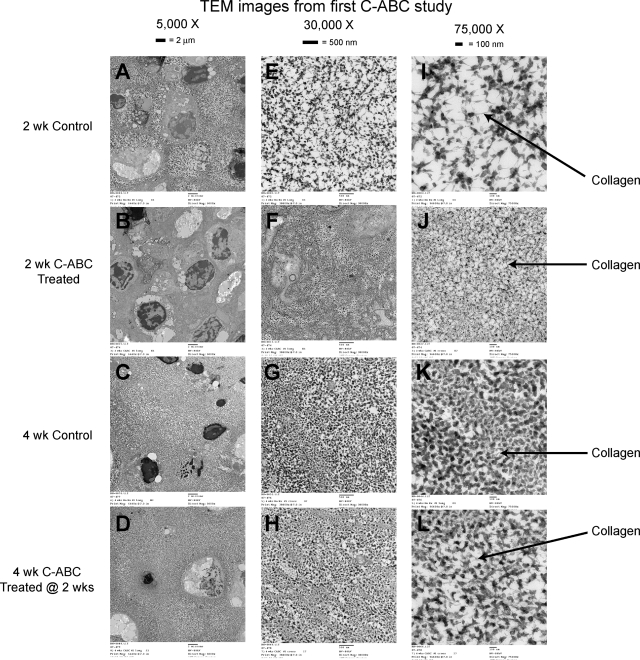
Figure 2 shows TEM images of the constructs. At 2 weeks, C-ABC-treated specimens show GAG loss and a denser collagenous matrix. Between 2 and 4 weeks, the constructs deposited ECM, resulting in greater spacing between cells (Fig. 2A, B vs Fig. 2C, D). Additionally, GAG staining returned to the matrix in the C-ABC-treated group over 2 weeks of culture (Fig. 2F, J vs Fig. 2H, L). Collagen molecules are evident in each group (grayish members indicated by arrows).
FIG. 2.
Representative transmission electron microscopy images: 2-week control (A, E, I), 2-week C-ABC treatment (B, F, J), 4-week control (C, G, K), 4-week C-ABC treatment (D, H, L). (A–D) Magnification 5,000X, (E–H) magnification 30,000X, (I–L) magnification 75,000X. Cupromeronic blue stains the GAGs black.
sGAG content, cell number, and percentage water
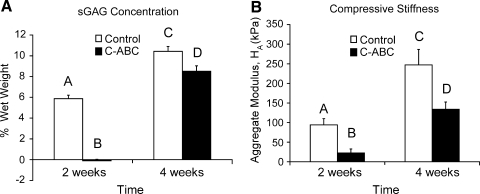
C-ABC treatment removed all of the sGAG from the constructs, but there was robust sGAG repletion between 2 and 4 weeks. Controls also experienced an increase in sGAG from 2 to 4 weeks (Fig. 3A). In Figure 3, total sGAG content was normalized to the constructs' wet weights. Each group was significantly different from all others. Immediately after C-ABC treatment, constructs had significantly more DNA per construct than the other groups (Table 2). Controls at 2 weeks and control and C-ABC-treated constructs at 4 weeks had similar DNA content, reflective of the number of cells initially seeded. The 4-week C-ABC-treated group contained 80 ± 1% water, which was significantly less than the 4-week (83 ± 1%) and 2-week (84 ± 3%) control groups. Percentage water for the 2-week C-ABC-treated group was unobtainable because dry weights of these constructs were below the lower limit of our balance.
FIG. 3.
(A) Construct sulfated GAG (sGAG) concentration and (B) compressive stiffness. Total sGAG normalized to wet weight of each group was significantly different from all others. The same was seen for the aggregate modulus. In both panels, groups not connected by the same letter are significantly different from one another (p < 0.05).
Table 2.
Construct Cell Number, Total Collagen, and Permeability
| Time | Treatment | Cell number (×106) | Total collagen (% WW) | Permeability (× 10−15 m4/N·s) |
|---|---|---|---|---|
| 2 wks | Control | 5.5 ± 0.3A | 12.0 ± 1.0A | 24 ± 14A |
| C-ABC | 6.9 ± 0.8B | 38.0 ± 8.5B | 16 ± 0.7A | |
| 4 wks | Control | 5.8 ± 0.6A | 8.1 ± 0.9A | 84 ± 64B |
| C-ABC | 5.0 ± 0.4A | 11.0 ± 2.2A | 29 ± 0.7A |
Data are given as mean ± S.D. Within a column, groups not sharing a similar letter are significantly different from one another (p < 0.05).
Total collagen and type I and type II collagen ELISA
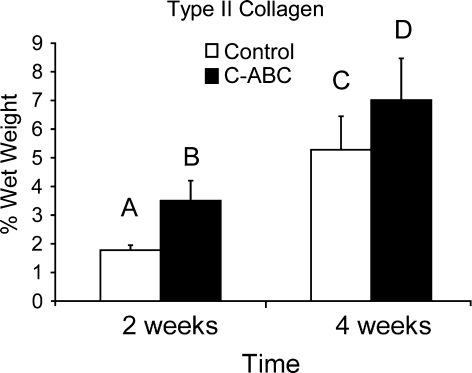
Table 2 shows total collagen per wet weight. At 2 weeks, total collagen per wet weight as measured using hydroxyproline was significantly greater in the C-ABC-treated group than in any other group; there was no significant difference between the controls and C-ABC-treated constructs at 4 weeks. The large value immediately after treatment can be attributed to the significant decrease in wet weight of this group due to sGAG removal and loss of its associated water. At 4 weeks, total collagen normalized to dry weight was 69 ± 8% and 67 ± 6% for the control and C-ABC-treated groups, respectively. With respect to type II collagen, each group was significantly different from all others (Fig. 4), with the 4-week C-ABC-treated group having the most type II collagen per wet weight. Type II collagen was 65% and 61% of the total collagen for the 4-week control and C-ABC-treated groups, respectively, which is similar to other articular cartilage tissue engineering efforts.35,36 In terms of dry weight, type II collagen measured 32 ± 7% and 34 ± 6% for the 4-week control and C-ABC-treated groups, respectively. There was no type I collagen detected using the ELISA.
FIG. 4.
Type II collagen normalized to wet weight in the 4-week C-ABC-treated group was significantly greater than in 4-week controls. Groups not connected by the same letter are significantly different from one another (p < 0.05).
Biomechanics
Figure 3B shows the aggregate modulus, a measure of compressive stiffness, obtained from creep indentation testing. At 2 weeks, the aggregate modulus of control constructs was significantly greater than that of the C-ABC-treated constructs, reflecting the removal of sGAGs. The remaining stiffness (22 kPa) reflects the unique contribution of collagen to compressive stiffness. Stiffness increased over 2 weeks of subsequent culture in both groups. Permeability was significantly greater in the 4-week control group than in any other group (Table 2). The Poisson ratio was significantly lower in the 2-week C-ABC-treated group (0.02 ± 0.02) than in any other group. The other groups' Poisson ratios were 0.22 ± 0.12 for the 2-week control, 0.17 ± 0.09 for the 4-week control, and 0.12 ± 0.09 for the 4-week C-ABC-treated group.
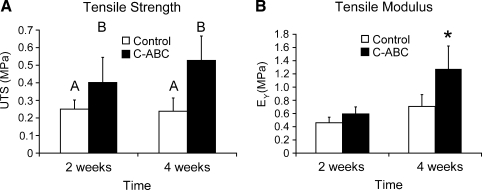
Figure 5 shows results from construct tensile testing. At 2 and 4 weeks, UTS was significantly greater in the C-ABC treated groups. The apparent Young's modulus (EY) of the 4-week C-ABC-treated group was significantly greater than that of any of the other groups. At 4 weeks, the UTS of the C-ABC-treated group was 121% greater than that of the control and EY was 80% greater.
FIG. 5.
(A) Ultimate tensile strength and (B) tensile modulus. The ultimate tensile strength (UTS) and apparent Young's modulus (EY) were significantly greater at 4 weeks in the C-ABC-treated group (121% and 80%, respectively). (A) Groups not connected by the same letter are significantly different from one another. (B) *indicates significantly different from all other groups (p < 0.05).
Discussion
The results of this study show that, with 2 weeks of culture after a one-time C-ABC treatment of developing tissue-engineered articular cartilage constructs, tensile properties were 80% greater for the apparent Young's modulus and 121% greater for ultimate tensile strength. The greater tensile properties could be due to greater collagen concentration, greater fibril diameter, greater cross-linking in the collagen network, or some combination of these factors, although only the first was assessed in this initial study. Low levels of collagen are associated with poor mechanical properties in most cell-seeded grafts for cartilage repair.37,38 In the present study, type II collagen concentration was greater at 4 weeks in the C-ABC-treated group than in untreated controls, although total collagen concentration was not significantly different. Furthermore, although depleted at 2 weeks, sGAGs returned, and the compressive stiffness of treated constructs recovered to where it was greater than that of 2-week controls. These results mirror the findings of Asanbaeva and colleagues,13 which showed that sGAGs returned and tensile properties increased after C-ABC treatment in a serum-based culture of cartilage explants. In that work, it was suggested that C-ABC treatment causes maturational growth of the tissue that parallels the in vivo situation and contrasts with expansive growth characteristic of in vitro culture. This study extends that work to tissue engineering in a scaffold-less, serum-free system. Furthermore, our results add compressive testing and collagen profiling and suggest that maturational and expansive growth phases could be a framework for discussing articular cartilage tissue engineering studies.
Several changes occurred in control and treated constructs between 2 and 4 weeks. First, total collagen concentration decreased. This decrease occurred concomitantly with an increase in sGAG concentration. Higher sGAG concentration led to greater water content, which resulted in constructs with greater total wet weights. Hence, the drop in total collagen concentration (per wet weight) in constructs from 2 to 4 weeks was due to more substantial sGAG production than total collagen production over that same time. Articular cartilage tissue engineering efforts suffer from greater production of GAG than of collagen.38,39 However, this same phenomenon was not observed for type II collagen concentration in this study, because type II collagen production kept better pace with sGAG between 2 and 4 weeks. For example, in controls, the total sGAG per construct (WW% of GAG times total wet weight) at 4 weeks was approximately 4.2 times that at 2 weeks, whereas total collagen per construct (WW% of total collagen times total wet weight) and type II collagen per construct (WW% of collagen type II times total wet weight) were approximately 1.6 and 7.6 times their 2-week values, respectively. It may be that the correct balance of matrix components is important for engineering functional articular cartilage. Another change was the increase in DNA of C-ABC constructs after treatment. Prior work in articular cartilage explants has shown that enzymatic treatment of the matrix can induce DNA synthesis, occurring over a few days.40 C-ABC may have also synchronized the cells in S-phase. Either of these reasons could account for the transient increase in DNA after C-ABC treatment. The increase in cell number resolved by 4 weeks, probably due to loss of cellular component from a looser matrix in the first few days after treatment. These changes in matrix biochemistry per construct between 2 and 4 weeks were not of the same magnitude on a concentration (per wet weight) basis, due to the intricate interplay between matrix components and hydration.
The biomechanical properties are the macroscopic functional representation of tissue's underlying structure and biochemical content.14,15,21,24 The immediate effects of C-ABC on compressive properties and tensile strength measured in this study reflect this fact. Removal of the sGAGs resulted in an immediate decrease in compressive stiffness, since the significant decrease in fixed charge density reduces the construct's ability to support compressive load.23,41 The return of sGAGs at 4 weeks reflects recovery of the compressive stiffness. At 4 weeks, sGAG concentration of the C-ABC-treated constructs was 8.5 ± 0.5%, which is slightly greater than the 4% to 7% range for native tissue.42 It has been demonstrated that the interaction between growth factors and proteoglycans can influence matrix development.43 The fact that chondroitin sulfate is also known to bind growth factors may explain the robust sGAG repletion.44 Thus, once chondroitin sulfate is removed, any endogenously produced growth factor would be available for binding its cellular receptor, as opposed to becoming bound in the developing matrix. The low Poisson's ratio seen in the 2-week C-ABC-treated group may be associated with the high total collagen concentration, because Kiviranta and colleagues45 have shown that greater collagen content correlates with a lower Poisson's ratio in bovine articular cartilage explants. Furthermore, greater tensile strength upon sGAG depletion reflects the fact that the collagen network could become more organized upon removal of prestress.14,24
The apparent Young's modulus at 4 weeks was significantly greater in the C-ABC-treated group than in the control group, and the increase in tensile strength observed immediately after treatment with C-ABC remained at 4 weeks, despite the sGAGs returning. If equal amounts of sGAG generate similar prestress, the fact that more sGAGs and greater tensile properties were seen at 4 weeks in the C-ABC-treated group than in the 2-week controls indicates that the removal of prestress is not the only contribution to the increase in tensile properties. Hence, the increase in tensile properties at 4 weeks is not purely a mechanical phenomenon. An interesting biological possibility comes from the recent work of Kafienah and colleagues37 that showed that lumican protein levels were greater in engineered cartilage and that knocking down lumican expression led to greater type II collagen accumulation and larger collagen fibril diameter. Larger diameter fibers could lead to greater cartilage stiffness. It may be that C-ABC causes a temporary decrease in lumican by freeing it from the matrix post-treatment. At 4 weeks, type II collagen concentration of the C-ABC-treated constructs was 7 ± 1.5%. This value is half the lower limit of native tissue, which ranges from 15% to 22%.42 However, although the present study saw significant greater type II collagen per wet weight in the C-ABC-treated group (∼1.3 times greater than in controls at 4 weeks), the fact that there is a disparity between the magnitude of change for type II collagen and tensile properties suggests that the relationship between these construct properties may not be linear and highlights the potential role for other factors related to the collagen network (see below) contributing to the greater tensile properties. The change in type II collagen was similar in magnitude to the increase in total collagen per wet weight (∼1.4 times). It makes sense that the material properties (parameters normalized to material size) would follow biochemical properties also normalized to a measure of material size (wet weight in this case). The positive functional outcome and absence of type I collagen in the present study (as collagen I would suggest a shift in chondrocyte phenotype) suggest that C-ABC is an option for enhancing the collagen network in tissue engineered articular cartilage.
Although greater type II collagen concentration is one possible biological explanation, other types of collagen or greater collagen cross-linking likely contribute to the increase in tensile properties. If we consider for a moment the collagen network as a polymer, then based on the theory of rubber elasticity, a greater modulus reflects a greater number of cross-links in the polymer.46 Thus, when the collagen network is collapsed after C-ABC treatment, an increase in inter-collagen type II pyridinium cross-linking may occur, which has been shown to correlate to tensile properties.2,12,47 Additionally, type IX collagen may be playing a larger role in cross-linking the type II collagen present.36 Supporting the idea of other collagens playing a role is the difference between total and type II collagen observed in this experiment. Finally, the order of magnitude difference between the compressive and tensile stiffness measured in our neo-tissue reflects the difference observed in native cartilage.48
The present study represents a novel method of functional tissue engineering of articular cartilage. Conventional methods used for tissue engineering articular cartilage focus on biochemical2,3,49 and biomechanical4,50–53 stimulation with anabolic intent. In contrast, the use of C-ABC's ECM-degrading properties, although perhaps counterintuitive at first glance, demonstrate how catabolic modulation of a particular ECM constituent can positively affect another. In vivo and in vitro studies have shown that C-ABC has minimal effects on rabbit54 and equine55 chondrocytes and has been successfully used in investigations of articular cartilage repair25–27 and central nervous system recovery after injury.56 Although there may be unknown, nonspecific C-ABC activities, there is no evidence in the literature to suggest that brief in vitro exposure to C-ABC causes unwanted effects. In this study, C-ABC treatment did not affect the ability of treated constructs to produce sGAG, nor did it induce type I collagen production. It is conceivable that C-ABC could be applied in tissue engineering strategies for other sGAG-containing tissues, such as heart valves, ligaments, tendons, the knee meniscus, the intervertebral disc, and the temporomandibular joint disc. Because it is not yet known for certain what characteristics a tissue-engineered construct will need to function in vivo, C-ABC represents another tool for tissue engineers that may prove useful in the long run. Future studies should aim to elucidate the biological or mechanical mechanism(s) by which C-ABC treatment affects functional properties and investigate whether C-ABC can be incorporated into conventional tissue engineering strategies through combination with other stimuli. Moreover, longer time points should be investigated to see whether the compressive stiffness of C-ABC-treated constructs continues to increase. Ultimately, an appropriate balance of ECM components and, perhaps more importantly, tensile and compressive properties, will be needed for successful implantation and integration of tissue-engineered constructs in in vivo models.
Acknowledgments
The authors gratefully acknowledge funding support from National Institute of Arthritis and Musculoskeletal and Skin Diseases R01AR053286 and Dr. Gwen Hoben for her assistance with IHC. We also acknowledge the Institutional Core Grant #CA16672 High Resolution Electron Microscopy Facility, University of Texas M. D. Anderson Cancer Center, for the TEM work.
Disclosure Statement
K.A. Athanasiou has contributed to OsteoBiologics (now part of Smith & Nephew), Diabetica Solutions, and VidaCare. He is now working with Rice University to develop technologies related to cartilage repair. Other authors have no competing interests to declare.
References
- 1.Butler D.L. Goldstein S.A. Guilak F. Functional tissue engineering: the role of biomechanics. J Biomech Eng. 2000;122:570. doi: 10.1115/1.1318906. [DOI] [PubMed] [Google Scholar]
- 2.Bastiaansen-Jenniskens Y.M. Koevoet W. de Bart A.C. van der Linden J.C. Zuurmond A.M. Weinans H. Verhaar J.A. van Osch G.J. Degroot J. Contribution of collagen network features to functional properties of engineered cartilage. Osteoarthritis Cartilage. 2008;16:359. doi: 10.1016/j.joca.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Ng K.W. Saliman J.D. Lin E.Y. Statman L.Y. Kugler L.E. Lo S.B. Ateshian G.A. Hung C.T. Culture duration modulates collagen hydrolysate-induced tissue remodeling in chondrocyte-seeded agarose hydrogels. Ann Biomed Eng. 2007;35:1914. doi: 10.1007/s10439-007-9373-z. [DOI] [PubMed] [Google Scholar]
- 4.Waldman S.D. Couto D.C. Grynpas M.D. Pilliar R.M. Kandel R.A. A single application of cyclic loading can accelerate matrix deposition and enhance the properties of tissue-engineered cartilage. Osteoarthritis Cartilage. 2006;14:323. doi: 10.1016/j.joca.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Athanasiou K.A. Niederauer G.G. Agrawal C.M. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials. 1996;17:93. doi: 10.1016/0142-9612(96)85754-1. [DOI] [PubMed] [Google Scholar]
- 6.Hu J.C. Athanasiou K.A. A self-assembling process in articular cartilage tissue engineering. Tissue Eng. 2006;12:969. doi: 10.1089/ten.2006.12.969. [DOI] [PubMed] [Google Scholar]
- 7.Anderson J.M. Biological responses to materials. Ann Rev Mat Res. 2001;31:81. [Google Scholar]
- 8.Yoon D.M. Fisher J.P. Chondrocyte signaling and artificial matrices for articular cartilage engineering. Adv Exp Med Biol. 2006;585:67. doi: 10.1007/978-0-387-34133-0_5. [DOI] [PubMed] [Google Scholar]
- 9.Frenkel S.R. Di Cesare P.E. Scaffolds for articular cartilage repair. Ann Biomed Eng. 2004;32:26. doi: 10.1023/b:abme.0000007788.41804.0d. [DOI] [PubMed] [Google Scholar]
- 10.Elder B.D. Athanasiou K.A. Effects of confinement on the mechanical properties of self-assembled articular cartilage constructs in the direction orthogonal to the confinement surface. J Orthop Res. 2008;26:238. doi: 10.1002/jor.20480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williamson A.K. Chen A.C. Masuda K. Thonar E.J. Sah R.L. Tensile mechanical properties of bovine articular cartilage: variations with growth and relationships to collagen network components. J Orthop Res. 2003;21:872. doi: 10.1016/S0736-0266(03)00030-5. [DOI] [PubMed] [Google Scholar]
- 12.Williamson A.K. Masuda K. Thonar E.J. Sah R.L. Growth of immature articular cartilage in vitro: correlated variation in tensile biomechanical and collagen network properties. Tissue Eng. 2003;9:625. doi: 10.1089/107632703768247322. [DOI] [PubMed] [Google Scholar]
- 13.Asanbaeva A. Masuda K. Thonar E.J. Klisch S.M. Sah R.L. Mechanisms of cartilage growth: modulation of balance between proteoglycan and collagen in vitro using chondroitinase ABC. Arthritis Rheum. 2007;56:188. doi: 10.1002/art.22298. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz M.H. Leo P.H. Lewis J.L. A microstructural model for the elastic response of articular cartilage. J Biomech. 1994;27:865. doi: 10.1016/0021-9290(94)90259-3. [DOI] [PubMed] [Google Scholar]
- 15.Quinn T.M. Morel V. Microstructural modeling of collagen network mechanics and interactions with the proteoglycan gel in articular cartilage. Biomech Model Mechanobiol. 2007;6:73. doi: 10.1007/s10237-006-0036-z. [DOI] [PubMed] [Google Scholar]
- 16.Derby M.A. Pintar J.E. The histochemical specificity of Streptomyces hyaluronidase and chondroitinase ABC. Histochem J. 1978;10:529. doi: 10.1007/BF01003135. [DOI] [PubMed] [Google Scholar]
- 17.Prabhakar V. Raman R. Capila I. Bosques C.J. Pojasek K. Sasisekharan R. Biochemical characterization of the chondroitinase ABC I active site. Biochem J. 2005;390:395. doi: 10.1042/BJ20050532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Front P. Aprile F. Mitrovic D.R. Swann D.A. Age-related changes in the synthesis of matrix macromolecules by bovine articular cartilage. Connect Tissue Res. 1989;19:121. doi: 10.3109/03008208909043893. [DOI] [PubMed] [Google Scholar]
- 19.Malemud C.J. Papay R.S. Hering T.M. Holderbaum D. Goldberg V.M. Haqqi T.M. Phenotypic modulation of newly synthesized proteoglycans in human cartilage and chondrocytes. Osteoarthritis Cartilage. 1995;3:227. doi: 10.1016/s1063-4584(05)80014-7. [DOI] [PubMed] [Google Scholar]
- 20.Sandy J.D. Brown H.L. Lowther D.A. Control of proteoglycan synthesis. Studies on the activation of synthesis observed during culture of articular cartilages. Biochem J. 1980;188:119. doi: 10.1042/bj1880119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korhonen R.K. Laasanen M.S. Toyras J. Lappalainen R. Helminen H.J. Jurvelin J.S. Fibril reinforced poroelastic model predicts specifically mechanical behavior of normal, proteoglycan depleted and collagen degraded articular cartilage. J Biomech. 2003;36:1373. doi: 10.1016/s0021-9290(03)00069-1. [DOI] [PubMed] [Google Scholar]
- 22.Laasanen M.S. Toyras J. Hirvonen J. Saarakkala S. Korhonen R.K. Nieminen M.T. Kiviranta I. Jurvelin J.S. Novel mechano-acoustic technique and instrument for diagnosis of cartilage degeneration. Physiol Meas. 2002;23:491. doi: 10.1088/0967-3334/23/3/302. [DOI] [PubMed] [Google Scholar]
- 23.Rieppo J. Toyras J. Nieminen M.T. Kovanen V. Hyttinen M.M. Korhonen R.K. Jurvelin J.S. Helminen H.J. Structure-function relationships in enzymatically modified articular cartilage. Cells Tissues Organs. 2003;175:121. doi: 10.1159/000074628. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt M.B. Mow V.C. Chun L.E. Eyre D.R. Effects of proteoglycan extraction on the tensile behavior of articular cartilage. J Orthop Res. 1990;8:353. doi: 10.1002/jor.1100080307. [DOI] [PubMed] [Google Scholar]
- 25.Hunziker E.B. Kapfinger E. Removal of proteoglycans from the surface of defects in articular cartilage transiently enhances coverage by repair cells. J Bone Joint Surg Br. 1998;80:144. doi: 10.1302/0301-620x.80b1.7531. [DOI] [PubMed] [Google Scholar]
- 26.Jo C.H. Kim E.M. Ahn H.J. Kim H.J. Seong S.C. Lee M.C. Degree of degeneration and chondroitinase ABC treatment of human articular cartilage affect adhesion of chondrocytes. Tissue Eng. 2006;12:167. doi: 10.1089/ten.2006.12.167. [DOI] [PubMed] [Google Scholar]
- 27.Lee M.C. Sung K.L. Kurtis M.S. Akeson W.H. Sah R.L. Adhesive force of chondrocytes to cartilage. Effects of chondroitinase ABC. Clin Orthop Relat Res. 2000:286. doi: 10.1097/00003086-200001000-00029. [DOI] [PubMed] [Google Scholar]
- 28.Allison D.D. Wight T.N. Ripp N.J. Braun K.R. Grande-Allen K.J. Endogenous overexpression of hyaluronan synthases within dynamically cultured collagen gels: Implications for vascular and valvular disease. Biomaterials. 2008;29:2969. doi: 10.1016/j.biomaterials.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Kim Y.J. Sah R.L. Doong J.Y. Grodzinsky A.J. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal Biochem. 1988;174:168. doi: 10.1016/0003-2697(88)90532-5. [DOI] [PubMed] [Google Scholar]
- 30.Woessner J.F., Jr. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 31.Mow V.C. Kuei S.C. Lai W.M. Armstrong C.G. Biphasic creep and stress relaxation of articular cartilage in compression? Theory and experiments. J Biomech Eng. 1980;102:73. doi: 10.1115/1.3138202. [DOI] [PubMed] [Google Scholar]
- 32.Athanasiou K.A. Agarwal A. Muffoletto A. Dzida F.J. Constantinides G. Clem M. Biomechanical properties of hip cartilage in experimental animal models. Clin Orthop. 1995;316:254. [PubMed] [Google Scholar]
- 33.Mow V.C. Gibbs M.C. Lai W.M. Zhu W.B. Athanasiou K.A. Biphasic indentation of articular cartilage–II. A numerical algorithm and an experimental study. J Biomech. 1989;22:853. doi: 10.1016/0021-9290(89)90069-9. [DOI] [PubMed] [Google Scholar]
- 34.Aufderheide A.C. Athanasiou K.A. Assessment of a bovine co-culture, scaffold-free method for growing meniscus-shaped constructs. Tissue Eng. 2007;13:2195. doi: 10.1089/ten.2006.0291. [DOI] [PubMed] [Google Scholar]
- 35.Kelly T.A. Ng K.W. Wang C.C. Ateshian G.A. Hung C.T. Spatial and temporal development of chondrocyte-seeded agarose constructs in free-swelling and dynamically loaded cultures. J Biomech. 2006;39:1489. doi: 10.1016/j.jbiomech.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 36.Riesle J. Hollander A.P. Langer R. Freed L.E. Vunjak-Novakovic G. Collagen in tissue-engineered cartilage: types, structure, and crosslinks. J Cell Biochem. 1998;71:313. doi: 10.1002/(sici)1097-4644(19981201)71:3<313::aid-jcb1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 37.Kafienah W. Cheung F.L. Sims T. Martin I. Miot S. Ruhland C.V. Roughley P.J. Hollander A.P. Lumican inhibits collagen deposition in tissue engineered cartilage. Matrix Biol. 2008;27:526. doi: 10.1016/j.matbio.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 38.Wong M. Siegrist M. Gaschen V. Park Y. Graber W. Studer D. Collagen fibrillogenesis by chondrocytes in alginate. Tissue Eng. 2002;8:979. doi: 10.1089/107632702320934074. [DOI] [PubMed] [Google Scholar]
- 39.Mauck R.L. Nicoll S.B. Seyhan S.L. Ateshian G.A. Hung C.T. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng. 2003;9:597. doi: 10.1089/107632703768247304. [DOI] [PubMed] [Google Scholar]
- 40.Lee D.A. Bentley G. Archer C.W. The control of cell division in articular chondrocytes. Osteoarthritis Cartilage. 1993;1:137. doi: 10.1016/s1063-4584(05)80029-9. [DOI] [PubMed] [Google Scholar]
- 41.Gu W.Y. Yao H. Effects of hydration and fixed charge density on fluid transport in charged hydrated soft tissues. Ann Biomed Eng. 2003;31:1162. doi: 10.1114/1.1615576. [DOI] [PubMed] [Google Scholar]
- 42.Mow V.C. Ratcliffe A. Poole A.R. Cartilage and diarthrodial joints as paradigms for hierarchical materials and structures. Biomaterials. 1992;13:67. doi: 10.1016/0142-9612(92)90001-5. [DOI] [PubMed] [Google Scholar]
- 43.Ferdous Z. Wei V.M. Iozzo R. Hook M. Grande-Allen K.J. Decorin-transforming growth factor interaction regulates matrix organization and mechanical characteristics of three-dimensional collagen matrices. J Biol Chem. 2007;282:35887. doi: 10.1074/jbc.M705180200. [DOI] [PubMed] [Google Scholar]
- 44.Nandini C.D. Sugahara K. Role of the sulfation pattern of chondroitin sulfate in its biological activities and in the binding of growth factors. Adv Pharmacol. 2006;53:253. doi: 10.1016/S1054-3589(05)53012-6. [DOI] [PubMed] [Google Scholar]
- 45.Kiviranta P. Rieppo J. Korhonen R.K. Julkunen P. Toyras J. Jurvelin J.S. Collagen network primarily controls Poisson's ratio of bovine articular cartilage in compression. J Orthop Res. 2006;24:690. doi: 10.1002/jor.20107. [DOI] [PubMed] [Google Scholar]
- 46.Rodriguez F. Philadelphia: Taylor & Francis; 1996. Principles of Polymer Systems. [Google Scholar]
- 47.Asanbaeva A. Masuda K. Thonar E.J. Klisch S.M. Sah R.L. Cartilage growth and remodeling: modulation of balance between proteoglycan and collagen network in vitro with beta-aminopropionitrile. Osteoarthritis Cartilage. 2008;16:1. doi: 10.1016/j.joca.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 48.Chahine N.O. Wang C.C. Hung C.T. Ateshian G.A. Anisotropic strain-dependent material properties of bovine articular cartilage in the transitional range from tension to compression. J Biomech. 2004;37:1251. doi: 10.1016/j.jbiomech.2003.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waldman S.D. Usmani Y. Tse M.Y. Pang S.C. Differential effects of natriuretic peptide stimulation on tissue-engineered cartilage. Tissue Eng Part A. 2008;14:441. doi: 10.1089/tea.2007.0035. [DOI] [PubMed] [Google Scholar]
- 50.Mauck R.L. Soltz M.A. Wang C.C. Wong D.D. Chao P.H. Valhmu W.B. Hung C.T. Ateshian G.A. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122:252. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 51.Ng K.W. Mauck R.L. Statman L.Y. Lin E.Y. Ateshian G.A. Hung C.T. Dynamic deformational loading results in selective application of mechanical stimulation in a layered, tissue-engineered cartilage construct. Biorheology. 2006;43:497. [PubMed] [Google Scholar]
- 52.Waldman S.D. Spiteri C.G. Grynpas M.D. Pilliar R.M. Kandel R.A. Long-term intermittent shear deformation improves the quality of cartilaginous tissue formed in vitro. J Orthop Res. 2003;21:590. doi: 10.1016/S0736-0266(03)00009-3. [DOI] [PubMed] [Google Scholar]
- 53.Waldman S.D. Spiteri C.G. Grynpas M.D. Pilliar R.M. Kandel R.A. Long-term intermittent compressive stimulation improves the composition and mechanical properties of tissue-engineered cartilage. Tissue Eng. 2004;10:1323. doi: 10.1089/ten.2004.10.1633. [DOI] [PubMed] [Google Scholar]
- 54.Nahir A.M. Shomrat D. Awad M. Chondroitinase ABC affects the activity of intracellular enzymes in rabbit articular cartilage chondrocytes. J Rheumatol. 1995;22:702. [PubMed] [Google Scholar]
- 55.Iqbal J. Bird J.L. Hollander A.P. Bayliss M.T. Effect of matrix depleting agents on the expression of chondrocyte metabolism by equine chondrocytes. Res Vet Sci. 2004;77:249. doi: 10.1016/j.rvsc.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 56.Crespo D. Asher R.A. Lin R. Rhodes K.E. Fawcett J.W. How does chondroitinase promote functional recovery in the damaged CNS? Exp Neurol. 2007;206:159. doi: 10.1016/j.expneurol.2007.05.001. [DOI] [PubMed] [Google Scholar]