Abstract
Modular tissue engineering is a novel microscale approach that aims to assemble tissue constructs with inherent vascularization. We transplanted endothelialized modules (sub-millimeter-sized collagen gel cylinders covered with human umbilical vein endothelial cell [HUVEC] on the outside surface) in the omental pouch of nude rats to characterize remodeling of the collagen gels and the fate of the transplanted HUVEC. Endothelialized modules randomly assembled in vivo to form channels among individual modules that persisted for at least 14 days. Transplanted HUVEC migrated and formed primitive vessels in these channels; however, host inflammation limited HUVEC survival beyond 3 days. Temporary depletion of peritoneal macrophages (by treatment with clodronate liposomes) prolonged the survival of HUVEC-derived vessels to 7 days, and some vessels appeared to be perfused with host erythrocytes and invested with host vascular cells (either rat von Willebrand factor or smooth muscle α-actin-positive cells). Despite treatment, HUVEC were presumed to be still subject to immune rejection. The presence of primitive HUVEC-derived vessels is encouraging in this first in vivo study of the modular approach, in a partially immune-compromised animal model. It suggests that with appropriate attention to the host response to transplanted endothelial cells and improved vessel survival, cells that would be embedded in modules could be adequately perfused.
Introduction
Acritical challenge for engineering large-scale tissue replacements is to ensure that the transplanted tissue grafts are well vascularized within the host. Without an internal vascular supply, tissue survival is limited by diffusion of essential nutrients and oxygen. Hence, only cells within a diffusion distance of 150–200 μm of the surrounding blood vessels are viable, and there can be significant cell loss at the core of transplanted tissues without vascularization.1,2 This phenomenon has been documented for several tissue engineering applications.3,4
New blood vessel formation in the adult occurs typically through angiogenesis, and there has been much effort to promote therapeutic angiogenesis through delivery of growth factors (e.g., VEGF and bFGF), but multiple growth factors are likely necessary to produce a mature vasculature.5 Several groups have shown that transplantation of primary endothelial cells (EC), particularly human umbilical vein endothelial cell (HUVEC) in immunocompromised rodents, can induce mature vessels. For example, HUVEC transfected with an antiapoptotic gene Bcl-2, suspended in a collagen-fibronectin gel, and transplanted into the abdominal wall of SCID mice developed into a complete microvascular bed capable of assuming arterial, venous, and capillary EC phenotype.6,7 Increased vessel density was shown to increase perfusion in an ischemic hindlimb model7 and also to effectively vascularize skin substitutes.8 Others have transplanted a mixture of HUVEC with mesenchymal precursor cells to form functional blood vessels that integrated with the host vasculature and were stable for 1 year.9 In another example, Levenberg et al. demonstrated that HUVEC-derived vascularization of 3D skeletal muscle tissue increases perfusion and survival of muscle constructs in vivo.10 Combined, these studies suggest that primary EC transplantation can drive vascularization in vivo.
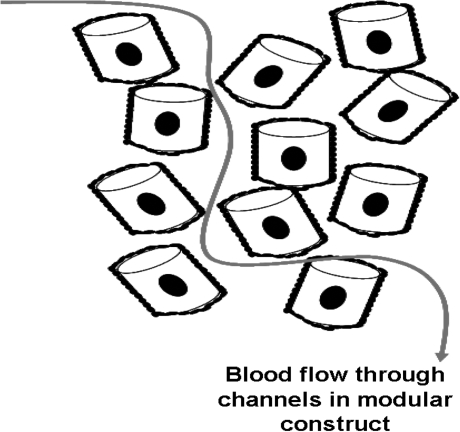
Modular tissue engineering is a means of assembling constructs with uniform cell density, scalability, mixed cell populations, and vascularization.11 Functional cells are embedded in sub-millimeter-sized collagen rods, and the outside surface is covered with EC. We refer to the collagen rod with embedded cells and EC composite unit as a module. Many modules are used to fill a tissue cavity, creating interconnected channels by virtue of the spaces among the randomly packed modules (Fig. 1). HUVEC grow to confluence on the surface of the collagen modules and remain nonthrombogenic when exposed to blood flow in vitro.11 By varying the type of embedded cells, it is expected that modular tissue engineering can be used for a number of applications; HepG2 cells11 and smooth muscle cells12 have been the subject of reports to date.
FIG. 1.
Schematic representation of a modular construct. Individual collagen gel cylinders (modules) normally with embedded cells are covered with endothelial cells (EC) on the outside surface. Modules randomly pack in situ and tissue spaces between individual modules form channels that allow for blood flow. In this manner, embedded cells are expected to be perfused with blood within the modular construct.
Here, we report on the fate of HUVEC-covered modules implanted in a surgically created omental pouch. No cells were embedded within the modules in the studies reported here. We chose the omentum pouch as it is well vascularized, permits a large number of implants, and can be easily retrieved for histological analysis.13 In pilot studies, we noted an early loss of transplanted HUVEC (in the nude rats) and significant inflammatory cell infiltration around the module implants. To prolong HUVEC survival, we explored the use of clodronate liposomes to temporarily deplete peritoneal macrophages. Clodronate (dichloromethylene-bisphosphonate) is encapsulated in liposomes, and when ingested by phagocytotic cells it makes them to be apoptotic.14 Repeated injections (2 or 3 days apart) of clodronate liposomes have shown to successfully deplete (temporarily) peritoneal and omental macrophages in rats.15 Our aim was to assess, for both untreated and treated rats, (i) remodeling of the collagen modules, (ii) HUVEC fate in vivo, and (iii) host inflammatory response to implanted modules.
Materials and Methods
Cells
Pooled HUVEC were purchased from Cambrex Corporation (East Rutherford, NJ) and maintained in EC growth medium with 2% FBS (EGM-2; Cambrex) at 37°C and 5% CO2. Cells were used between passages 3 and 5.
Module fabrication
Modules were prepared without embedded cells as before11,16 using bovine type 1 collagen (3.1 mg/mL; Cohesion Technologies, Palo Alto, CA), gelled (37°C, 60 min) in sterile 0.71 mm ID polyethylene tubing (PE60; Intramedic–BD, Oakville, Canada). Tubing was cut into small pieces (∼2 mm long × 0.6 mm diameter) using a custom automatic cutter and collected in HUVEC medium. Collagen modules were separated from the outer tubing with gentle vortexing. HUVEC (2.0 × 106) were seeded dynamically onto the surface of 1 mL of modules (produced using 3 m of tubing) for 45 min on a low-speed shaker and incubated for 7 days before implantation (HUVEC contracted the collagen modules to ∼1 mm long × 0.5 mm diameter). Collagen-only modules were prepared as above but without EC seeding.
Module transplants
Adult male (5 weeks old) nude (athymic) rats (200–250 g) were purchased from Charles River Laboratories (Wilmington, MA). They were individually housed in sterile cages and fed ad libitum. The study was approved by the University of Toronto animal care committee. Animals were divided into two groups: untreated and clodronate liposome treated. Treated animals received two injections of 1 mL of clodronate liposomes into the peritoneal cavities 4 days and 1 day before surgery, with a 23 G needle.15 Clodronate liposomes were prepared as previously described,15 and clodronate was a kind gift from Roche Diagnostics (Mannheim, Germany). Untreated animals received no injections. For module implants, animals were anesthetized and an upper midline incision exposed the abdomen. The greater omentum was spread out, and 7-0 silk suture was run along the edges and the top to create a pouch with a small opening. Modules (0.5 mL, with or without HUVEC) suspended in PBS were gently delivered by a sterilized micropipette (1 mL pipette tip; Axygen Scientific, Union City, CA) and allowed to settle. The pouch was folded over and sutured to secure the modules inside. The muscle layer was closed with 6-0 Vicryl sutures and the skin layer sutured with 4-0 nylon sutures. Animals were given a 0.3 mL injection of 0.25 mg/mL Buprenorphine solution for recovery. For both untreated and clodronate liposome–treated groups, animals were transplanted with endothelialized modules for 3 (n = 4), 7 (n = 5), and 14 (n = 5) days. Collagen-only modules were transplanted in clodronate liposome–treated animal (n = 1) and untreated rats (n = 3).
Histology and immunostaining
At explant, animals were sacrificed and the omental pouch was excised in 4% neutral buffered formalin (Sigma-Aldrich, Oakville, Canada) and fixed for 48 h. Tissue samples were embedded in paraffin wax, and 5 μm sections were cut at three levels (each level was 100 μm apart) within the block. Sections were processed and stained for hematoxylin and eosin (H&E; Fisher, Ottawa, Canada), Masson trichrome (Fisher), and various antibodies as outlined in Table 1. Apoptotic cells were identified by TUNEL staining. For immunostaining, endogenous peroxide and biotin activity was blocked with 3% hydrogen peroxide (Fisher) for 15 min and avidin/biotin blocking kit (SP-2001; Vector Laboratories, Burlingame, CA), and sections were developed with 30 min each of a biotinylated-linking reagent (ID Labs, London, Canada) and horseradish peroxidase–conjugated ultrastreptavidin labeling reagent (ID Labs). After washing well in PBS, color was developed with freshly prepared NovaRed solution (SK-4800; Vector). Finally, sections were counterstained lightly with Mayer's hematoxylin, dehydrated in alcohol, cleared in xylene, and mounted in Permount (Fisher). Fluorescent sections (smooth muscle α-actin [SMA] and von Willebrand factor [vWf ]) were followed by a streptavidin-FITC reagent (SA-5001; Vector) for 30 min and cover-slipped with Vectashield (H-1000; Vector) mounting medium for fluorescence. All sections were viewed with a Zeiss Axiovert light and fluorescent microscope equipped with a CCD camera.
Table 1.
List of Monoclonal Antibodies and Their Target Antigens Used for Immunostaining and Immunofluorescence
| Antibody | Supplier | Conditions | Target antigen |
|---|---|---|---|
| UEA-1 lectin | Vector Laboratories (Burlingame, CA) | Heat induced temperature retrieval, 1:300 dilution for 1 h | Human EC (does not stain rat EC) |
| ED1 (MCA341) and ED2 (MCA342) | AbD Serotec (Raleigh, NC) | 1% pepsin pretreatment, 1:600 for 1 h | Rat macrophages and monocytes (does not stain human cells) |
| vWf (CL20176A-R) | Cedarlane (Burlington, Canada) | After UEA-1 application, 1:5000 overnight | Rat EC and platelets (does not stain human cells) |
| SMA (AB5694) | Abcam (Cambridge, MA) | After UEA-1 application, 1:1000 overnight | Rat smooth muscle cells (does not stain rat EC) |
| TUNEL assay (DNA polymerase 1 large [Klenow] fragment, dATP, dCTP, dGTP, and Bio-11-dUTP) | Promega (Nepean, Canada) | 1% pepsin pretreatment, cocktail applied for 1 h at 37°C | Apoptotic cells (stains fragmented DNA of both rat and human cells) |
UEA-1, Ulex Europaeus Agglutinin I; vWF, von Willebrand factor; SMA, smooth muscle α-actin.
Histology quantification
Ulex Europaeus Agglutinin I (UEA-1) lectin–stained positive cells at day 3, 7, and 14 were counted using a Chalkley method17 adapted from the tumor angiogenesis literature. At a low magnification (100 ×), three hotspots were identified as areas containing the most positively stained cells in each section. At each hot spot, a 25-point Chalkley eyepiece graticule was applied (Chalkley grid area = 0.196 mm2) at 200 × magnification, and the eyepiece was rotated to align the maximum number of dots with UEA-1-positive structures (structures could be vessels or single cells). The dots that overlaid UEA-1-positive structures were counted, and the mean of the three counts was used for statistical analysis. The microvessel density (MVD) method was applied to count UEA-1-positive vessels (with a defined lumen). For each animal, the number of vessels in the three hot spots (same as identified in the Chalkley method) was counted at 400 × magnification. Average of the three MVD counts was used for statistical analysis.
Macrophages (ED1 or ED2 positive) were counted in five representative omentum sections at 400 × magnification, and the average number for each of four animals in each treatment group was recorded.
Statistical analysis
A one-way ANOVA with Tukey post hoc analysis was applied to compare means between multiple groups. Data were considered statistically significant at p < 0.05. All analysis was done with Statistica Version 5.1 software (Statsoft, Tulsa, OK).
Results
Remodeling of collagen gel modules
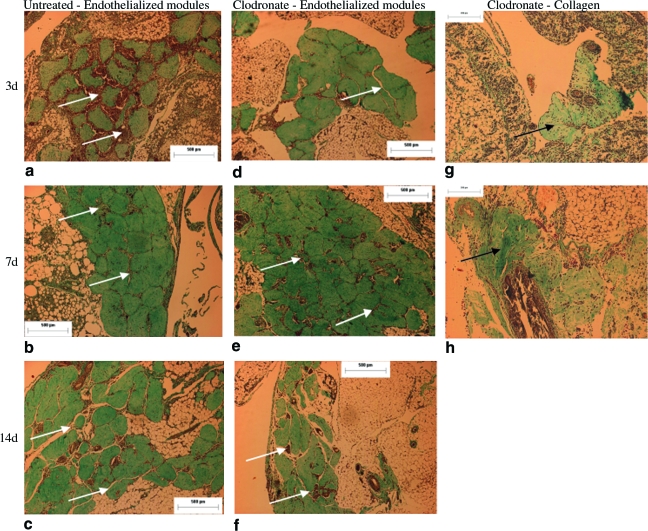
Implanted modules were identified with Masson trichrome stain (collagen stained green). Endothelialized modules retained their cylindrical shape and randomly assembled such that there were channels (albeit often containing cells) among individual modules that persisted for at least 14 days in both untreated (Fig. 2a–c) and clodronate liposome–treated (Fig. 2d–f) rats. Low-magnification pictures for both untreated and clodronate liposome–treated animals show that channels were formed among the majority of the implanted modules. In some implants a large number of modules assembled in one location (Fig. 2b), whereas in others, modules were more dispersed (Fig. 2c). While ∼400 modules were implanted, only 100–200 modules/animal could be found by histology; many modules were likely lost on filling the pouch or during tissue processing. Module assembly, distribution, and channel spacing were not affected by treatment with clodronate liposomes (Fig. 2d–f). Meanwhile, collagen-only (no EC) modules remodeled significantly and began to degrade as early as day 3 (Fig. 2g). Similar remodeling and degradation occurred with collagen-only module implants in 7-week-old nude rats (without clodronate treatment—results not shown) at days 3, 7, and 14, and in all cases, collagen modules lost their cylindrical shapes and did not form channels as seen with endothelialized modules.
FIG. 2.
Trichrome staining showed that in both untreated (a–c) and clodronate liposome–treated rats (d–f), endothelialized modules randomly assembled to form channels (indicated by white arrows) in situ. Channels were the spaces, often cell-filled, between individual modules and could be seen as long as 14 days in both groups. Scale bar = 500 μm. In comparison, collagen-only modules without EC (indicated by black arrows) in clodronate-treated rats degraded as early as 3 days (g) and continued to remodel until day 7 (h); channels were not apparent without EC. Scale bar = 200 μm. Color images available online at www.liebertonline.com/ten.
A mild inflammatory cell infiltration was observed with collagen-only implants. A similar response was observed in sham (no implant) operated animals (see below), suggesting that some of the observed inflammatory response is largely due to the trauma of suturing the omentum. With all endothelialized module implants, some level of cellular infiltration in the module-derived channels could be seen at all time points (Figs. 2 and 3). Many of the cells appeared to be erythrocytes (Fig. 3), but there were also several inflammatory cells. With untreated rats, there was a heavier cell infiltration at day 3 (Fig. 3a), which was reduced by day 7 (Fig. 3b), and few cells were detected by day 14 (Fig. 3c). Meanwhile, with clodronate liposome treatment, mild cellular infiltration was detected around the modules at day 3 (Fig. 3d), which was greater by day 7 (Fig. 3e) but reduced again by day 14 (Fig. 3f).
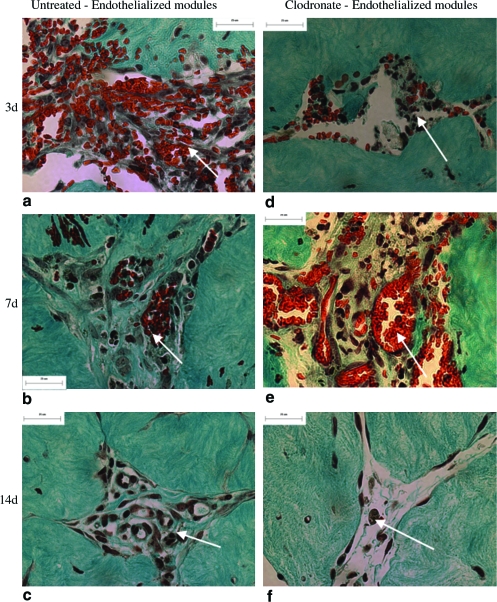
FIG. 3.
High-magnification trichrome sections show that in untreated rats, cells (mainly erythrocytes and some inflammatory cells, white arrows) infiltrated module-derived channels at day 3 (a). There was only moderate infiltration at day 7 (b) and less infiltration at day 14 (c). In clodronate liposome–treated rats, there was little cellular infiltration at day 3 (d), more at day 7 (e), and minimal infiltration at day 14 (f). Scale bar = 25 μm. Color images available online at www.liebertonline.com/ten.
Transplanted HUVEC
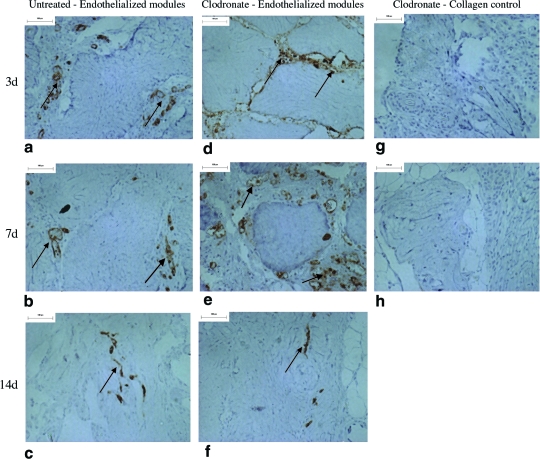
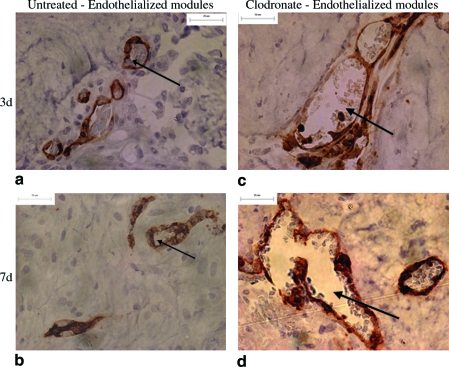
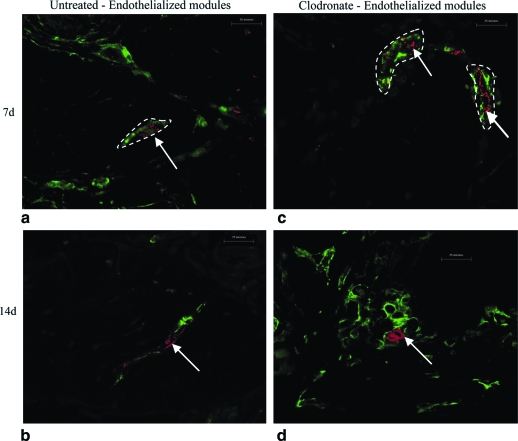
Transplanted HUVEC were identified with UEA-1 lectin (Figs. 4 and 5), a human EC-specific marker. Collagen-only implants did not stain with UEA-1, confirming that the lectin did not stain rodent cells (Fig. 4g–h). In untreated rats, HUVEC formed primitive microvessels (i.e., UEA-1-positive structures with a defined lumen) in module-derived channels at day 3 (Fig. 4a). A majority of these vessels disappeared after 7 days (Fig. 4b), and by day 14, there were few UEA-1-positive cells observed (Fig. 4c). These vessels are seen in higher magnification in Figure 5, where some of the vessels included erythrocytes in their lumens (Fig. 5a). In clodronate liposome–treated rats, HUVEC were apparent on the surface of the modules at day 3 (Fig. 4d), and by day 7, HUVEC migrated into module-derived channels to form primitive vessels (Fig. 4e). These UEA-1-positive vessels of varying sizes, a majority of which contained erythrocytes, are shown in Figure 5d. Similar to control rats, HUVEC-derived vessels receded by day 14 and few UEA-1-positive cells were observed (Fig. 4f). In all cases, HUVEC-derived vessels were located in close proximity to the modules, and there were no UEA-1-positive cells detected elsewhere in the omentum. There was no apparent effect of location within the implant on EC survival, at least for the small 0.5 mL implants used here.
FIG. 4.
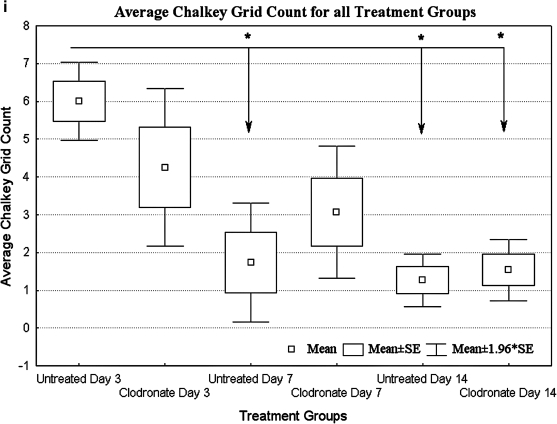
UEA-1-stained sections of endothelialized modules of untreated rats show some vessel formation at day 3 (a), loss of a majority of vessels by day 7 (b), and few UEA-1 cells at day 14 (c). Sections from clodronate-treated animals show UEA-1 cells on the surface of modules at day 3 (d), a number of HUVEC-derived vessels at day 7 (e), and few UEA-1-positive cells at day 14 (f). Collagen-only implants confirm that UEA-1 lectin was HUVEC specific (g, h). Arrows show examples of UEA-1-positive cells, some in the form of vessels. Scale bar = 100 μm. (i) Mean of Chalkley grid counts show that compared to untreated group at day 3, UEA-1 counts were significantly lower (p < 0.05) at days 7 and 14 for untreated animals and at day 14 in the clodronate liposome–treated group (relative to day 3, as marked with an asterisk). The y-axis is the Chalkley grid count, and data are represented as mean of each group (n = 4 for day 3, and n = 5 for days 7 and 14; ±SE). HUVEC, human umbilical vein endothelial cell. Color images available online at www.liebertonline.com/ten.
FIG. 5.
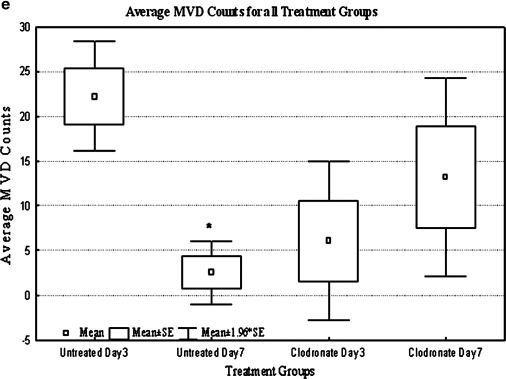
High-magnification (400 ×) UEA-1 sections show HUVEC-derived vessels in both untreated rats and clodronate liposome–treated rats at days 3 and 7. In untreated rats, numerous vessels were located at day 3 (a), while few vessels were found by day 7 (b). In clodronate liposome–treated rats, few vessels were detected at day 3 (c), while numerous vessels of varying sizes were detected at day 7 (d) with erythrocytes present in their lumen. Arrow indicates vessels with lumens. Scale bar = 25 μm. (e) Microvessel density (MVD) counts were significantly lower in the untreated group at day 7 relative to day 3 (p < 0.05). For clodronate liposome–treated animals, an opposite, although not statistically significant, trend was observed as MVD counts increased from day 3 to 7. The mean MVD count for clodronate liposome animals at day 7 was higher (but not significant, p = 0.17) than the corresponding untreated group. The y-axis is the MVD count, and data are represented as means of MVD count (n = 4 for day 3, and n = 5 for day 7; ±SE). Color images available online at www.liebertonline.com/ten.
The Chalkley method is an established method of quantifying angiogenesis and it was used to count UEA-1-positive structures (vessels and single cells). Consistent with the images, the UEA-1 cell population was high at day 3 and declined significantly (p < 0.05) after 14 days in both groups (Fig. 4i), presumably due to a macrophage response (see below). At day 7, there were generally more UEA-1-positive cells in clodronate liposome–treated animals; however, due to large variations within the group, none of the differences were statistically significant. On the other hand, the vessel structures at early time points were markedly different between the treated and control groups as discussed below.
The MVD (Fig. 5e) method was to quantify UEA-1-positive vessels (i.e., those structures that also contained lumens). There were significantly (p < 0.05) fewer vessels on day 7 than on day 3 in the untreated group. An opposite trend was observed for clodronate liposome–treated animals as the average MVD count increased (but not statistically significantly) from day 3 to 7.
Vessel maturity
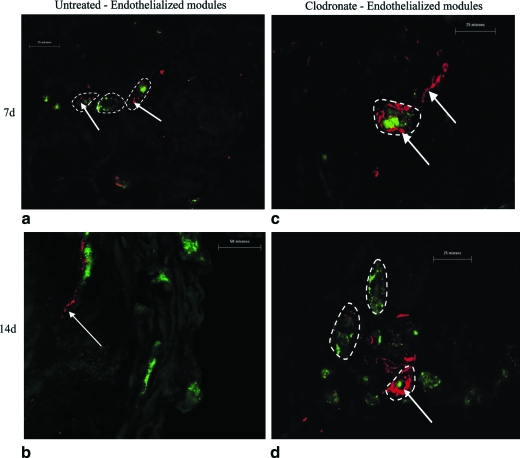
Sections were double stained with UEA-1 lectin (human EC) and either rat FVIII/vWf (rat EC—Fig. 6) or SMA (rat smooth muscle cells—Fig. 7). At day 3 in both untreated and clodronate liposome–treated rats, there were no host (rat) vWF-positive or SMA-positive cells associated with UEA-1 vessels and single cells. At day 7, while the majority of UEA-1-stained vessels were not associated with rat cells, some UEA-1 vessels (i.e., with human EC) also contained rat EC (Fig. 6a, c) and were surrounded by rat smooth muscle cells (Fig. 7a, c) in both untreated and treated animals. As noted earlier, there were generally more UEA-1 vessels with clodronate liposome treatment at day 7; however, the extent of association of host cells was not dependent on treatment. By day 14 in both groups, although only few UEA-1-positive cells and vessels were detected, these few UEA-1 cells were generally in proximity to rat EC (Fig. 6b, d). In a few instances, rat smooth muscle cells (Fig. 7b, d) were also seen around UEA-1-positive vessels. Also, a number of rat vWf- or SMA-positive vessels (without UEA-1 cells) were seen near endothelialized modules at day 14.
FIG. 6.
Double staining with UEA-1 lectin (red) and rat vWf (green) of endothelialized modules in untreated (a, b) and clodronate liposome–treated (c, d) animals at days 7 and 14. Images show UEA-1-positive structures (red) invested with rat vWF-positive cells (green) at day 7. At day 14, there were few isolated UEA-1-positive cells, but these were associated with vWf-positive rat cells. vWf-stained vessels (without UEA-1 staining) are particularly noticeable in panel (d), clodronate liposome treatment, day 14. Dashed lines show vessels, with and without UEA-1 staining. Scale bar = 25 μm. Color images available online at www.liebertonline.com/ten.
FIG. 7.
Double staining with UEA-1 lectin (red) and rat SMA (green) of endothelialized modules in untreated (a, b) and clodronate liposome–treated (c, d) animals at days 7 and 14. Images show UEA-1-positive structures (red) invested with SMA-positive cells (green). At day 7 in both untreated (a) and treated (c) animals some UEA-1 vessels were surrounded by SMA-positive cells (as outlined); these structures were larger in the clodronate liposome–treated animals. By day 14 (b, d) there was a proximity between the two cells, but the SMA-positive cells did not surround the UEA-1 cells (arrows) as at day 7. (d) Many SMA-positive cells, not associated with the UEA-1 cells. Dashed lines show vessels, with and without UEA-1 staining. Scale bar = 25 μm. Color images available online at www.liebertonline.com/ten.
Apoptotic cells
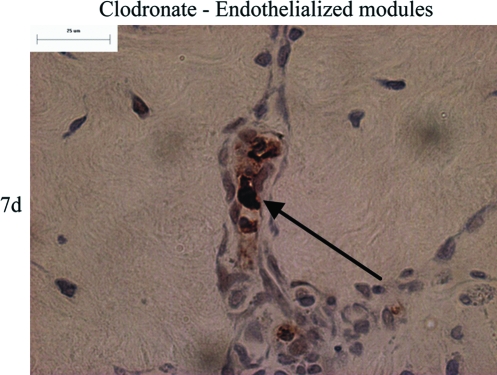
Apoptotic cells were identified with a TUNEL stain. There was no TUNEL staining detected around endothelialized modules in untreated rats at days 3 and 7. With clodronate liposome treatment, there were no apoptotic cells seen at day 3; however, there were a few apoptotic cells near endothelialized modules at day 7 (Fig. 8). TUNEL staining was not seen elsewhere in the omentum.
FIG. 8.
TUNEL staining (brown nuclei, black arrow) of animals treated with clodronate liposome shows occasional apoptotic cells around endothelialized modules at day 7. Scale bar = 25 μm. Color images available online at www.liebertonline.com/ten.
Inflammatory cells
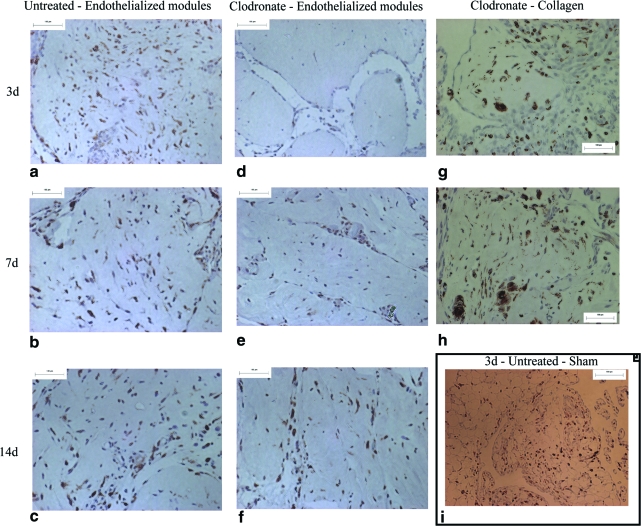
Inflammation around endothelialized module implants (Fig. 9) was characterized by rat macrophage markers, ED1 (expressed in nonactivated macrophages and dendritic cells) and ED2 (expressed in activated macrophages)18; images shown (Fig. 9) are with the ED2 marker, and similar trends were seen with ED1 staining. A large number of macrophages were detected in untreated rats at all time points (Fig. 9a–c). In clodronate liposome–treated rats (treated 4 days and 1 day before module implantation), there were few macrophages identified at day 3 with greater numbers at day 7 and finally full macrophage repopulation by day 14 (Fig. 9d–f, respectively). Similar repopulation by day 7 was seen with collagen only implants in clodronate liposome–treated rats (Fig. 9g, h). As expected, a few macrophages were seen in untreated sham-operated animals (Fig. 9i) at day 3, reflecting the impact of omental surgery. Five representative ED1 and ED2 sections for four animals in both untreated and clodronate liposome–treated groups (Table 2) were counted, and there was a significant (p < 0.01) reduction in macrophage numbers with clodronate liposome treatment at day 3. In both untreated and clodronate liposome–treated rats, the number of macrophages peaked at day 7, although the differences at day 7 were statistically significant (p < 0.05) only for clodronate-treated animals.
FIG. 9.
ED2-stained sections of endothelialized modules show high macrophage infiltration in untreated rats at days 3, 7, and 14 (a–c). For clodronate liposome–treated rats, macrophages were minimal at day 3 (d), but began to repopulate at day 7 (e), and tissue is fully repopulated by day 14 (f). Repopulation by day 7 is also seen in treated collagen-only implants (g, h). A few ED2-positive cells were seen in sham-operated, untreated animals (i) at day 3, reflecting the effect of omental surgery. Scale bar = 100 μm. Color images available online at www.liebertonline.com/ten.
Table 2.
Macrophage Accumulation Around Endothelialized Modules in Clodronate Liposome–Treated and Control Rats at Days 3, 7, and 14
| |
Clodronate liposome treated |
Untreated |
||
|---|---|---|---|---|
| Day | ED1 | ED2 | ED1 | ED2 |
| 3 | 65.9 ± 7.9a | 30.4 ± 5.9a | 107.4 ± 7.2 | 82.0 ± 11.0 |
| 7 | 81.5 ± 5.0 | 78.0 ± 10.8 | 102.3 ± 11.2 | 99.0 ± 18.1 |
| 14 | 68.0 ± 8.1 | 47.9 ± 10.1 | 85.8 ± 6.0 | 72.9 ± 9.3 |
The number of macrophages (ED1 or ED2 positive) around endothelialized modules in four animals per treatment group was counted in five sections at 400 ×, and average of the sections for each group is reported with the standard error. Macrophages were seen around modules at day 3, they peaked at day 7, and reduced by day 14 in both untreated and clodronate liposome–treated animals. When compared to untreated animals, there were fewer ED1- and ED2-positive macrophages with clodronate treatment at day 3 (ap < 0.01). At later time points, although there were generally fewer macrophages with clodronate treatment, these differences were not statistically significant (day 7, p = 0.07; day 14, p = 0.12).
Discussion
Fabrication of viable 3D tissue is dependent on sufficient vascularization in vivo. We have been exploring a modular tissue engineering strategy that incorporates intrinsically an internal vasculature and that can be used with a variety of functional cells. These studies were the first exploration of the strategy in vivo. There was substantial remodeling and agglomeration of the collagen modules without EC. This was not apparent with the endothelialized modules (Fig. 2). Collagen modules are larger and softer than those with EC, because the HUVEC contract the gel and this likely contributed to the observed remodeling. More importantly, there were channels separating the HUVEC-bearing modules, suggesting that if the channels were connected to the host vasculature, blood could reach all implanted modules. Transplanted HUVEC also appeared to have migrated from the surface of the modules to form primitive vessels within the module-derived channels (Fig. 5). This type of remodeling was encouraging because this suggests that individual modules (and eventually embedded cells) will be close to a vascular supply.
With UEA-1 staining, we were able to distinguish and enumerate transplanted HUVEC. At day 3, HUVEC-derived microvessels (i.e., UEA-1-positive structures with a lumen) appeared to consist completely of human EC in both untreated (Fig. 5a) and clodronate liposome–treated rats (Fig. 5c); UEA-1 does not stain rat EC, and vessels were not positive for rat FVIII or SMA. At day 7, some HUVEC-derived vessels were also associated with host (rat) smooth muscle cells (Fig. 7) or EC (Fig. 6), but by day 14 the majority of UEA-1 vessels had receded and very few UEA-1-positive cells were observed. Also, some vessels were invested with erythrocytes, suggesting that the vessels had anastomosed with host vasculature (Fig. 5d). Interestingly, in both groups at day 14, the few detectable UEA-1-positive cells were almost always seen in proximity to rat EC or smooth muscle cells, suggesting that transplanted cells may need to be supported by host cells for prolonged survival (Figs. 6d and 7d). Also at day 14, a number of host vessels (rat SMA and FVIII-positive cells, not necessarily with UEA-1-positive cells) were seen inside the module-derived channels, suggesting that vascularization of the modules with host cells was occurring (even though the majority of HUVEC did not persist).
HUVEC survival after transplantation
The poor survival of HUVEC vessels in our study was surprising because others have implanted human dermal microvascular EC in PLGA scaffolds in SCID mice and showed that the human cells begin to form vessels at day 3, anastomose (accumulate erythrocytes) with host vasculature at day 7, and were invested with supporting host cells that persisted until 28 days.19 On the other hand, others have shown that it is necessary to cotransplant HUVEC with supporting cells (mesenchymal precursors9 or embryonic fibroblasts10) or to transfect the cells with an antiapoptotic gene, Bcl-2,20 to form chimeric HUVEC/murine vessels in SCID mice for extended period of time. We checked for apoptosis with TUNEL staining in clodronate liposome–treated rats (Fig. 8) and noticed some TUNEL staining around the modules at day 7, suggesting that at least some of the HUVEC were undergoing apoptosis at that time point. This may account for the loss of HUVEC by day 14.
Host response
Omental transplants are more invasive than dermal implants so that there may have been increased surgery-related inflammation relative to other studies.19 In addition, SCID mice do not have B cells and have fewer macrophages than the T cell–deficient nude rats used here. We inferred from the beneficial (although temporary) effect of clodronate that macrophage responses directed at the xenogeneic EC were responsible for the low HUVEC survival.
Although nude rats are T cell deficient, they maintain normal or slightly higher levels of other leukocytes (macrophages, B cells, and natural killer cells) than euthymic rats and are known to reject xenografts.21–23 Thus, anti-HUVEC antibodies produced by the still-active B cells may have played a significant role in the rejection. Also, in xenogeneic-vascularized organ transplantation, host complement activation can directly cause donor endothelium damage.24 For example, complement depletion in nude rats receiving guinea pig cardiac transplants has shown to extend xenograft survival.24
Macrophages in the nude rat are known to produce cytokines that are directly toxic to donor EC.25 When nude rats treated with leflunomide and antiasialoGM-1 to deplete xenoreactive antibodies and natural killer cells received hamster hearts, infiltrating macrophages alone were capable of rejecting the vascularized xenografts.23 In our studies, macrophages clearly infiltrated endothelialized modules and it is likely that rejection mediated by these cells contributed to low HUVEC survival (Fig. 9). It is also conceivable that some isolated UEA-1 cells (those not in vascular structures) were actually a remnant of cells engulfed by macrophages; this possibility cannot be excluded because macrophages were generally present in the vicinity of modules.
To temporarily suppress the macrophage response, we treated the nude rats with clodronate liposomes. This initial macrophage depletion prolonged HUVEC viability until 7 days (Fig. 5e), which suggests that macrophage suppression could benefit HUVEC survival in the nude rat. However, macrophages were only completely depleted until day 3 and began to repopulate by day 7 (Fig. 9e), correlating to the HUVEC loss by day 14. Others have shown that with clodronate liposome treatment, macrophages are depleted for approximately 4 days, but with the addition of Freund's adjuvant (to elicit an inflammatory response), macrophage repopulation is accelerated.15 Similarly, the presence of a xenograft likely accelerated the inflammatory response in spite of clodronate liposome treatment. Also, the omental tissue contains a number of macrophage precursors26 that were not depleted with clodronate liposomes (precursors are not phagocytotic), and at the onset of inflammation, these cells likely matured and invaded host tissue to participate in xenograft rejection.27
Interestingly, with clodronate liposome treatment and subsequent macrophage depletion, vessel formation was delayed until 7 days (Fig. 5d). Macrophages secrete growth factors that induce HUVEC migration and vessel formation, as seen, for example, in a report of endothelial progenitor cell transplantation for wound healing.28 This also highlights the need to investigate such transplants in the presence of an inflammatory response. Ideally, we would like to induce HUVEC vessel formation without an adverse host response such that the donor-derived vessels stabilize and integrate with host vasculature. Some human cell transplants have been explored in nude rats. For example, intravenous injection of large numbers of human endothelial progenitor cells (EPC–CD34+) in nude rats improves vascularization in myocardial infarctions and ischemic limbs.29–32 These progenitor cells embed in various vessels and can be detected up to 1 month after transplant.26 Interestingly, only high doses of CD34+ cells are capable of inducing a therapeutic benefit and only in the presence of ischemic tissue.33 Not only are the models different, but also progenitor cells lack expression of mature human EC markers (unlike HUVEC) and hence may induce less of an xenogeneic response.34 Our observations hint at the possibility that HUVEC in nude rats are capable of driving vascularization; however, they presumably elicit a host (immune) response that limits this capacity. For this reason we are currently pursuing allogeneic and syngeneic endothelial transplant scenarios.
Conclusions
Transplantation of endothelialized modules (with HUVEC) in nude rats resulted in what appears to be primitive vessels in channels among modules at early times. With clodronate liposome–induced macrophage depletion, HUVEC-derived vessels persisted longer (until day 7) and some were invested with host vascular cells even at 14 days. However, inflammatory cell infiltration and a presumed immune response (in this xenogenic, partially immune-compromised animal model) lead to HUVEC cell loss and vessel regression by day 14 in both untreated and macrophage-depleted animals. Although the detection of primitive HUVEC vessels is encouraging, better means of modulating the host response are needed to preserve the long-term viability and stability of HUVEC-derived vessels.
Acknowledgments
The authors acknowledge the financial support from the U.S. National Institutes of Health (EB006903), and the technical expertise of Chuen Lo.
Disclosure Statement
No competing financial interests exist.
References
- 1.Nomi M. Atala A. Coppi P.D. Soker S. Principals of neovascularization for tissue engineering. Mol Aspects Med. 2002;23:463. doi: 10.1016/s0098-2997(02)00008-0. [DOI] [PubMed] [Google Scholar]
- 2.Griffith C.K. Miller C. Sainson R.C.A. Calvert J.W. Jeon N.L. Hughes C.C.W. George S.C. Diffusion limits of an in vitro thick prevascularized tissue. Tissue Eng. 2005;11:257. doi: 10.1089/ten.2005.11.257. [DOI] [PubMed] [Google Scholar]
- 3.Olsson R. Maxhuni A. Carlsson P.O. Revascularization of transplanted pancreatic islets following culture with stimulators of angiogenesis. Transplantation. 2006;82:340. doi: 10.1097/01.tp.0000229418.60236.87. [DOI] [PubMed] [Google Scholar]
- 4.Kelm J.M. Djonov V. Hoerstrup S.P. Guenter C.I. Ittner L.M. Greve F. Hierlemann A. Sanchez-Bustamante C.D. Perriard J.C. Ehler E. Fussenegger M. Tissue-transplant fusion and vascularization of myocardial microtissues and macrotissues implanted into chicken embryos and rats. Tissue Eng. 2006;12:2541. doi: 10.1089/ten.2006.12.2541. [DOI] [PubMed] [Google Scholar]
- 5.Epstein S.E. Fuchs S. Zhou Y.F. Baffour R. Kornowski R. Therapeutic interventions for enhancing collateral development by administration of growth factors: basic principles, early results and potential hazards. Cardiovasc Res. 2001;49:532. doi: 10.1016/s0008-6363(00)00217-0. [DOI] [PubMed] [Google Scholar]
- 6.Schechner J.S. Nath A.K. Zheng L. Kluger M.S. Hughes C.C.W. Sierra-Honigmannn M.R. Lorber M.I. Tellides G. Kashgarian M. Bothwell A.L.M. Pober J.S. In vivo formation of complex microvessels lined by human endothelial cells in an immunodeficient mouse. Proc Natl Acad Sci USA. 2000;97:9191. doi: 10.1073/pnas.150242297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enis D.R. Shepherd B.R. Wang Y.N. Qasim A. Shanahan C.M. Weissberg P.L. Kashgarian M. Pober J.S. Schechner J.S. Induction, differentiation, and remodeling of blood vessels after transplantation of Bcl-2-transduced endothelial cells. Proc Natl Acad Sci USA. 2005;102:425. doi: 10.1073/pnas.0408357102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shepherd B.R. Enis D.R. Wang F. Suarez Y. Pober J.S. Schechner J.S. Vascularization and engraftment of a human skin substitute using circulating progenitor cell-derived endothelial cells. FASEB J. 2006;20:1739. doi: 10.1096/fj.05-5682fje. [DOI] [PubMed] [Google Scholar]
- 9.Koike N. Fukumura D. Gralla O. Au P. Schechner J.S. Jain R.K. Creation of long-lasting blood vessels. Nature. 2004;428:138. doi: 10.1038/428138a. [DOI] [PubMed] [Google Scholar]
- 10.Levenberg S. Rouwkema J. Macdonald M. Garfein E.S. Kohane D.S. Darland D.C. Marini R. van Blitterswijk C.A. Mulligan R.C. D'Amore P.A. Langer R. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. 2005;23:879. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- 11.McGuigan A.P. Sefton M.V. Vascularized organoid engineered by modular assembly enables blood perfusion. Proc Natl Acad Sci USA. 2006;103:11461. doi: 10.1073/pnas.0602740103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leung B.M. Sefton M.V. A modular tissue engineering construct containing smooth muscle cells and endothelial cells. Ann Biomed Eng. 2007;35:2039. doi: 10.1007/s10439-007-9380-0. [DOI] [PubMed] [Google Scholar]
- 13.Suh S. Kim J. Shin J. Kil K. Kim K. Kim H. Kim J. Use of omentum as an in vivo cell culture system in tissue engineering. ASAIO J. 2004;50:464. doi: 10.1097/01.mat.0000138016.83837.8a. [DOI] [PubMed] [Google Scholar]
- 14.van Rooijen N. Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 15.Biewenga J. van der Ende M.B. Krist L.F. Borst A. Ghufron M. van Rooijen N. Macrophage depletion in the rat after intraperitoneal administration of liposome-encapsulated clodronate: depletion kinetics and accelerated repopulation of peritoneal and omental macrophages by administration of Freund's adjuvant. Cell Tissue Res. 1995;280:189. doi: 10.1007/BF00304524. [DOI] [PubMed] [Google Scholar]
- 16.McGuigan A.P. Sefton M.V. Design and fabrication of sub-mm-sized modules containing encapsulated cells for modular tissue engineering. Tissue Eng. 2007;13:1069. doi: 10.1089/ten.2006.0253. [DOI] [PubMed] [Google Scholar]
- 17.Hansen S. Sorensen F.B. Vach W. Grabau D.A. Bak M. Rose C. Microvessel density compared with the Chalkley count in a prognostic study of angiogenesis in breast cancer patients. Histopathology. 2004;44:428. doi: 10.1111/j.1365-2559.2004.01848.x. [DOI] [PubMed] [Google Scholar]
- 18.Takeya M. Hsiao L. Shimokawa Y. Takahashi K. Heterogeneity of rat macrophages recognized by monoclonal-antibodies—an immunohistochemical and immunoelectron microscopic study. J Histochem Cytochem. 1989;37:635. doi: 10.1177/37.5.2649558. [DOI] [PubMed] [Google Scholar]
- 19.Nor J.E. Peters M.C. Christensen J.B. Sutorik M.M. Linn S. Khan M.K. Addison C.L. Mooney D.J. Polverini P.J. Engineering and characterization of functional human microvessels in immunodeficient mice. Lab Investig. 2001;81:453. doi: 10.1038/labinvest.3780253. [DOI] [PubMed] [Google Scholar]
- 20.Zheng L. Gibson T.F. Schechner J.S. Pober J.S. Bothwell A.L.M. Bcl-2 transduction protects human endothelial cell synthetic microvessel grafts from allogeneic T cells in vivo. J Immunol. 2004;173:3020. doi: 10.4049/jimmunol.173.5.3020. [DOI] [PubMed] [Google Scholar]
- 21.Rolstad B. The athymic nude rat: an animal experimental model to reveal novel aspects of innate immune responses? Immunol Rev. 2001;184:136. doi: 10.1034/j.1600-065x.2001.1840113.x. [DOI] [PubMed] [Google Scholar]
- 22.Xia G.L. Ji P. Rutgeerts O. Waer M. Maintenance and reversibility of natural killer cell- and T cell-independent B lymphocyte xenotolerance in athymic nude rats. Transplantation. 1999;68:1181. doi: 10.1097/00007890-199910270-00019. [DOI] [PubMed] [Google Scholar]
- 23.Candinas D. Belliveau S. Koyamada N. Miyatake T. Hechenleitner P. Mark W. Bach F.H. Hancock W.W. T cell independence of macrophage and natural killer cell infiltration, cytokine production, and endothelial activation during delayed xenograft rejection. Transplantation. 1996;62:1920. doi: 10.1097/00007890-199612270-00042. [DOI] [PubMed] [Google Scholar]
- 24.Saadi S. Platt J.L. Immunology of xenotransplantation. Life Sci. 1998;62:365. doi: 10.1016/s0024-3205(97)00964-8. [DOI] [PubMed] [Google Scholar]
- 25.Lin Y. Vandeputte M. Waer M. Contribution of activated macrophages to the process of delayed xenograft rejection. Transplantation. 1997;64:1677. doi: 10.1097/00007890-199712270-00008. [DOI] [PubMed] [Google Scholar]
- 26.Krist L.F.G. Kerremans M. Broekhuis-Fluitsma D.M. Eestermans I.L. Meyer S. Beelen R.H.J. Milky spots in the greater omentum are predominant sites of local tumour cell proliferation and accumulation in the peritoneal cavity. Cancer Immunol Immunother. 1998;47:205. doi: 10.1007/s002620050522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu H. Naito M. Umezu H. Moriyama H. Takatsuka H. Takahashi K. Shultz L.D. Macrophage differentiation and expression of macrophage colony-stimulating factor in murine milky spots and omentum after macrophage elimination. J Leukoc Biol. 1997;61:436. doi: 10.1002/jlb.61.4.436. [DOI] [PubMed] [Google Scholar]
- 28.Suh W. Kim K.L. Kim J.M. Shin I.S. Lee Y.S. Lee J.Y. Jang H.S. Lee J.S. Byun J. Choi J.H. Jeon E.S. Kim D.K. Transplantation of endothelial progenitor cells accelerates dermal wound healing with increased recruitment of monocytes/macrophages and neovascularization. Stem Cells. 2005;23:1571. doi: 10.1634/stemcells.2004-0340. [DOI] [PubMed] [Google Scholar]
- 29.Iwasaki H. Kawamoto A. Ishikawa M. Oyamada A. Nakamori S. Nishimura H. Sadamoto K. Horii M. Matsumoto T. Murasawa S. Shibata T. Suehiro S. Asahara T. Dose-dependent contribution of CD34-positive cell transplantation to concurrent vasculogenesis and cardiomyogenesis for functional regenerative recovery after myocardial infarction. Circulation. 2006;113:1311. doi: 10.1161/CIRCULATIONAHA.105.541268. [DOI] [PubMed] [Google Scholar]
- 30.Matsumoto T. Kawamoto A. Kuroda R. Ishikawa M. Mifune Y. Iwasaki H. Miwa M. Horii M. Hayashi S. Oyamada A. Nishimura H. Murasawa S. Doita M. Kurosaka M. Asahara T. Therapeutic potential of vasculogenesis and osteogenesis promoted by peripheral blood CD34-positive cells for functional bone healing. Am J Pathol. 2006;169:1440. doi: 10.2353/ajpath.2006.060064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ott I. Keller U. Knoedler M. Gotze K.S. Doss K. Fischer P. Urlbauer K. Debus G. von Bubnoff N. Rudelius M. Schomig A. Peschel C. Oostendorp R.A.J. Endothelial-like cells expanded from CD34(+) blood cells improve left ventricular function after experimental myocardial infarction. FASEB J. 2005;19:992. doi: 10.1096/fj.04-3219fje. [DOI] [PubMed] [Google Scholar]
- 32.Sondergaard C.S. Bonde J. Dagnaes-Hansen F. Nielsen J.M. Zachar V. Holm M. Hokland P. Pedersen L. Minimal engraftment of human CD34+ cells mobilized from healthy donors in the infarcted heart of athymic nude rats. Stem Cells Dev. 2008 doi: 10.1089/scd.2008.0006. ahead of print. [DOI] [PubMed] [Google Scholar]
- 33.Kocher A.A. Schuster M.D. Szabolcs M.J. Takuma S. Burkhoff D. Wang J. Homma S. Edwards N.M. Itescu S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 34.Pober J.S. Orosz C.G. Rose M.L. Savage C.O.S. Can graft endothelial, cells initiate a host anti-graft immune response? Transplantation. 1996;61:343. doi: 10.1097/00007890-199602150-00001. [DOI] [PubMed] [Google Scholar]