Abstract
Treatment of xerostomia would benefit from development of a functional implantable artificial salivary gland. Salivary gland tissue from surgical patients was assessed by histology and immunohistochemistry to establish the phenotype of normal salivary gland cells including the native basement membranes. Ductal and acinar cells were identified in tissue and cultured cells from dispersed tissue. High levels of laminin and perlecan/HSPG2 (heparan sulfate proteoglycan 2) were noted in basement membranes, and perlecan also was secreted and organized by cultured acinar populations, which formed lobular structures that mimicked intact glands when cultured on Matrigel™ or a bioactive peptide derived from domain IV of perlecan. On either matrix, large acini-like lobular structures grew and formed connections between the lobes. α-Amylase secretion was confirmed by staining and activity assay. Biomarkers, including tight junction protein E-cadherin and water channel protein aquaporin 5 found in tissue, were expressed in cultured acinar cells. Cells cultured on Matrigel or domain IV of perlecan peptide organized stress fibers and activated focal adhesion kinase. We report a novel technique to isolate acinar cells from human salivary gland and identify a human peptide sequence in perlecan that triggers differentiation of salivary gland cells into self-assembling acini-like structures that express essential biomarkers and which secrete α-amylase.
Introduction
Xerostomia is a permanent and devastating sequela of head and neck radiation that affects approximately 40,000 patients annually in the United States.1 Direct radiation damage of the acinar cells that secrete fluid and protein results in salivary gland hypofunction. Histopathologic and immunohistochemical studies of chemoradiated salivary glands have shown profound acinar cell loss that can be attributed to lethal DNA damage under conditions in which ductal cells are preserved.2 Selective loss of acinar cells compromises the quantity and quality of saliva and produces conditions such as xerostomia, dysphagia, dental caries, mucositis, and other oropharyngeal infections. Patients suffer considerable morbidity, and their quality of life deteriorates significantly.1 Present treatments are unsatisfactory. We envision the creation of an implantable artificial salivary gland that can aid these patients to regain salivary functions.
Other groups have reported the isolation and culture of human salivary gland cells for tissue engineering purposes. Isolation of primary cells from primate and human salivary glands permitted the growth of ductal cells.3 While these cells are epithelial and can form tight junctions, they do not secrete fluid or the full array of salivary proteins produced by acinar cells.3,4 Recent studies have reported the successful isolation of human salivary acinar cells that express many essential markers.5,6 Joraku et al. reported reconstitution of salivary units that expressed α-amylase and an array of tight junction markers in a culture system consisting of collagen and Matrigel™.5 Although promising, this system cannot be used for human tissue engineering because Matrigel, being mouse derived, is not compatible with human systems. Recently, it was reported that a similar isolation of acinar cells required the use of animal serum, which promotes the growth of fibroblasts that often overtake epithelial cell cultures.6 Although their cultures formed acinotubular-type structures when grown on basement membrane extract (BME), the murine-derived BME cannot be used for tissue engineering in humans.6
Our study delineates a human-compatible system for the differentiation of human salivary gland cells into functional salivary units. To differentiate, cells require cues from several factors, including their extracellular matrix (ECM), growth factors, and integrin-mediated cell–cell interactions.7 In the past, basement membrane proteins used as substratum were found to be vital to the growth and differentiation of secretory cells, including mammary gland epithelial cells.8 The basement membrane is typically composed of collagen type IV, perlecan, laminin, and nidogen/entactin.9,10 Perlecan/heparan sulfate proteoglycan 2 (HSPG2), one of the critical components of the basement membrane, is a multidomain proteoglycan that forms functional attachments to multiple ECM components. Domain IV of perlecan (PlnDIV) contains a novel peptide sequence, which supports adhesion, spreading, and focal adhesion kinase (FAK) activation.11 Additionally, PlnDIV contains immunoglobulin (Ig) repeats that are similar to those found in Ig superfamily members such as the neural cell adhesion molecule or the platelet endothelial cell adhesion molecule.11
We tested the hypothesis that salivary gland cells cultured on PlnDIV peptide will receive the appropriate cues that allow them to differentiate and mimic their glandular phenotype. We used PlnDIV peptide to promote attachment and subsequent differentiation of cultured human acinar cells into salivary units, a useful first step toward the culture of acinar cells free of animal products that can be implanted into patients. These cells have the potential to polarize and differentiate into salivary units that express essential salivary biomarkers and can be used to engineer a functional artificial salivary gland.
Materials and Methods
Tissue samples
Normal tissue specimens of the human parotid and submandibular glands were obtained from patients undergoing head and neck surgery. A consent and protocol approved by the Institutional Review Board (IRBs) of both the Christiana Care Health System (CCHS) and the University of Delaware was utilized for tissue collection. Specimens were placed on ice immediately after excision and kept cold at all times until processing in the laboratory.
Hematoxylin and eosin staining
Tissues were frozen and cryosectioned at a thickness of 8 μm. Sections were fixed using a 1:1 mixture of acetone and methanol, stained with hematoxylin and eosin, and dehydrated by three dips each in 70%, 80%, 95%, and 100% (v/v) ethanol. The slides were mounted with Cytoseal mounting medium (Electron Microscopy Sciences, Fort Washington, PA) and coverslipped.
Periodic acid Schiff staining
Tissue cryosections were fixed using an acetone and methanol mixture, and hydrated using water. The slide then was oxidized with 0.5% (v/v) periodic acid solution, rinsed with distilled water, and then treated with the Schiff reagent. Slides were rinsed, counterstained with Mayer's hematoxylin, and then rinsed again. Lastly, they were dehydrated and coverslipped using Cytoseal mounting medium.
Cell culture
The ductal cell isolation protocol previously reported by Tran et al. was modified to select for acinar cells.4 Freshly obtained salivary gland tissue was washed with cold F-12 medium (Invitrogen, Carlsbad, CA) supplemented with 1% (v/v) penicillin–streptomycin, 1% (v/v) amphotericin B (Invitrogen), and 1% (v/v) Betadine solution. This wash was followed by a series of F-12 medium washes without the Betadine solution. The tissue specimen then was minced into small pieces and was subjected to an enzymatic tissue digestion step. The minced tissue slurry was added to a dissociation buffer with the Liberase RI enzyme (0.2 U/mL from Roche Diagnostics, Indianapolis, IN) and 0.1% (w/v) trypsin (Fisher Scientific, Pittsburgh, PA) in F-12 medium and incubated for ∼1.5 h at 37°C. Smaller pieces of minced tissue that dissociated faster yielded acini sooner than the larger tissue chunks. These dissociated acini were centrifuged earlier (incubation time ∼1 h) at a low speed (180 g for 5 min) and pelleted sooner to avoid over-digestion and possible destruction during the ongoing enzymatic action. The larger clumps were resuspended in the dissociation buffer until they dissociated into individual acini and in some cases into individual acinar cells. These gentler centrifugation steps and sequential digestion steps allowed for the isolation of viable acinar cells. After incubation, the buffer was centrifuged (180 g for 5 min) to obtain a cell pellet, which then was resuspended in serum-free Hepato-STIM™ medium (BD Biosciences Discovery Labware, Bedford, MA) or a medium substituted with human serum albumin, supplemented with 1% penicillin–streptomycin and 1% amphotericin B. The cell suspension was filtered through a 70 μm cell strainer (BD Biosciences Discovery Labware). The isolated cells then were seeded into a six-well culture plate and maintained at 37°C in a humidified atmospheric chamber containing 5% (v/v) CO2. Cells were passaged with 0.05% trypsin (w/v) (Invitrogen) ethylenediaminetetraacetic acid upon confluency. The enzymatic action of trypsin was stopped by addition of trypsin soybean inhibitor (Sigma, St. Louis, MO). The cell suspension was pelleted and resuspended in medium, and cells were counted using a hemacytometer. Cells then were replated at a dilution of 1:10.
For all studies involving ECM proteins, laminin (100μg/mL) (BD Biosciences Discovery Labware), Matrigel (1:3 dilution) (BD Biosciences Discovery Labware), or PlnDIV peptide (10 μg/mL) was added as a thin coating on chamber slides (eight well). PlnDIV peptide was kindly synthesized by Lisa Haines-Butterick (Chemistry and Biochemistry, University of Delaware). Laminin or PlnDIV peptide was allowed to air-dry overnight, while Matrigel was added to the chambers an hour before cell seeding. All residual Matrigel was removed until a thin film remained. Cells were seeded at equal density on each matrix. Cells in their third or fourth passage were used for all experimental analyses. Cells in these passages behaved the same way in all experiments.
Immunohistochemistry
Antibodies used in this study included polyclonal anti-human α-amylase (Sigma), Zonula Occludens-1 (ZO-1) (Zymed Laboratories, South San Francisco, CA), laminin (Collaborative Research in Bedford, MA), aquaporin 5 (AQP5) (Alpha Diagnostics International, San Antonio, TX), cytokeratin (CK) 14 (Gift from Dennis Roop, Baylor College of Medicine, Houston, TX), and monoclonal antibodies CK 19 (Amersham, Little Chalfont, United Kingdom), perlecan A76 (as previously described12), anti-FAK phospho Y397 (Millipore, Bedford, MA), anti-mouse fluorescein isothiocyanate–conjugated E-cadherin (BD Pharmingen, San Diego, CA), and Alexa 488–conjugated Phalloidin (Invitrogen). Alexa 488– and Alexa 568–conjugated secondary antibodies against mouse and rabbit IgG (Invitrogen) were used.
Tissue cryosections (8 μm thick) of human salivary gland were fixed with cold methanol for 10 min, and rehydrated with 1 × phosphate-buffered solution (PBS) for 5 min. Subsequently, tissue sections were blocked with 3% (w/v) bovine serum albumin in PBS for 16 h at 4°C. Primary antibodies were prepared in blocking solution and incubated for 45 min at 37°C in a humidified chamber. After washing with 1 × PBS, secondary antibodies prepared in blocking buffer were added and incubated for 40 min at 37°C. Draq5 (Biostatus, Leicestershire, United Kingdom) was subsequently added as a nuclear stain. Slides were washed, mounted, and coverslipped.
Staining with cultured human salivary gland cells grown in eight-well chamber slides (Lab-tek® Products; Nalge Nunc International, Naperville, IL) was performed as described above with minor changes. Cells were seeded on eight-well chamber slides coated with laminin (100 μg/mL), Matrigel (1:3 dilution), or PlnDIV (10 μg/mL) peptide. In brief, cells fixed with methanol were rehydrated with 1 × PBS and permeabilized with 0.1% (v/v) Triton X-100. Cells were blocked overnight in 3% bovine serum albumin in PBS (w/v). Primary antibody was applied for 1 h at 37°C in a humidified chamber. Secondary antibody was applied for 40 min at 37°C followed by Draq5 addition as a nuclear stain. Negative controls were performed for both the tissue sections and cultured cells without addition of the primary antibody. Primary antibodies specific for antigens expressed by other cell types present in the same tissues or cells also served as negative controls for nonspecific binding to tissues. Cells were mounted with Gel Mount (Biomeda Corporation, Foster City, CA) and stored in the dark at 4°C before viewing with the confocal microscope.
Western blotting
Salivary gland cells were grown on plastic-coated, Matrigel-coated, or PlnDIV peptide–coated dishes as above. Cells were lysed with RIPA buffer (150 mM NaCl, 1% NP-40, 0.5% deoxycholic acid, 0.1% sodium dodecyl sulphate, and 50 mM Tris pH 8.0) containing a protease inhibitor cocktail as well as phosphatase inhibitors. Lysate was centrifuged for 10 min at 13,000 rpm (Eppendorf 5402 centrifuge) at 4°C, and the pellet was discarded. A BCA (bicinchoninic acid) protein estimation assay was performed to measure the concentration of protein in the supernatant. Aliquots from each sample (50 μg) were separated on sodium dodecyl sulfate polyacrylamide gel electrophoresis gradient gels. Proteins then were transferred onto a nitrocellulose membrane. The membrane was incubated in 1:1000 dilution of the primary antibody, pFAK Y397 (BD Biosciences, Bedford, MA) or Anti-FAK (Millipore), followed by 1:200,000 dilution of horseradish peroxidase linked to mouse IgG. Negative controls were performed using preimmune serum and secondary antibodies. Protein bands were detected by using a chemiluminescent substrate (Thermo-Fisher, Rockford, IL) and were quantified using the Scion Imaging Software (Scion Corporation, Frederick, MD).
α-Amylase assay
Culture medium from human parotid gland cells grown on plastic, Matrigel, or PlnDIV peptide was collected every 2, 4, and 6 days. Culture medium was changed every 48 h. An α-amylase activity kit (Salimetrics, State College, PA) used a chromogenic substrate, 2-chloro-p-nitrophenol linked with maltose. Culture medium (50 μL) was mixed with 200 μL of prewarmed α-amylase substrate and color development measured at 405 nm. Absorbance was measured every 5 min for 1 h. The change in absorbance was used to calculate amylase activity.
Viability assay
Human parotid gland cells (10,000/well) were cultured on plastic, laminin, Matrigel, or PlnDIV peptide. A water-soluble tetrazolium (WST) salt (Roche Molecular Biochemicals, Mannheim, Germany) was added in the cell culture plates and incubated for 1 h at 37°C. The conversion of the WST salt to formazan, a colorimetric change, was measured using a plate reader at 470 nm. The proliferation of cells was measured every 2, 4, and 6 days in culture.
Results
Establishment of glandular morphology and isolation of glandular cells from salivary gland specimens
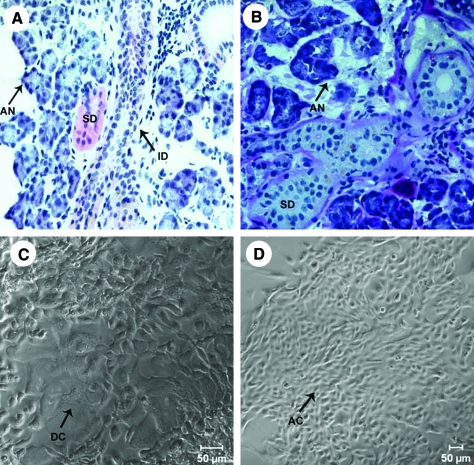
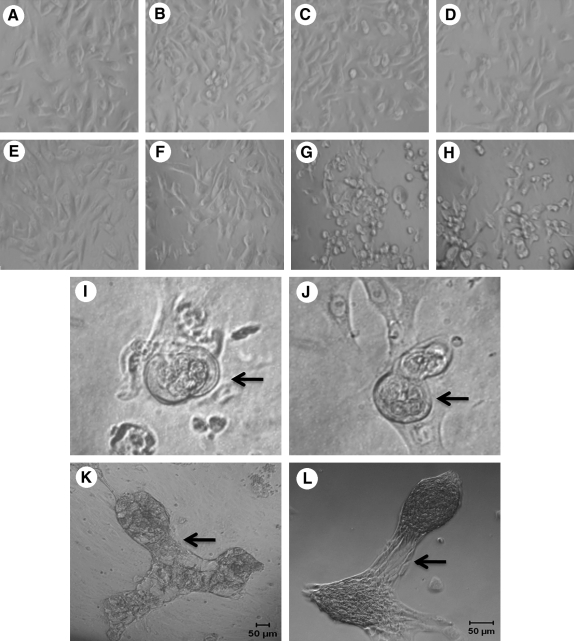
Our goal in this study was to recreate the structure of the native salivary gland in culture and ensure that it has all the essential proteins that will aid in secretion of salivary fluid. To recreate functional salivary units, the native structure of the excised salivary glands was evaluated by hematoxylin and eosin staining. Purple-stained acini surrounded large intercalated and striated ducts (Fig. 1A). Acinar and ductal structures were identified using periodic acid Schiff staining (Fig. 1B). Stained acinar cells easily were distinguished from the unstained ductal cells. Cells were isolated from the salivary gland tissue as described above. Initially, a mixed culture of cells was obtained (Fig. 1C). The mixed culture of cells contained a majority of small epithelial cells, which had acinar-like morphology and a minority of larger cells that appeared to be of ductal origin. Cultured cells maintained through multiple passages under these conditions lost the minority of ductal cells, indicating that the culture conditions favored the growth of acinar cells. Because acinar cells are mainly responsible for secreting salivary fluid, acinar-enriched cultures (Fig. 1D) were used for further study.
FIG. 1.
Glandular morphology and isolation of acinar and ductal cells from tissue. Hematoxylin and eosin staining of human salivary gland tissue (A). Periodic acid Schiff staining of the salivary gland tissue (B). Mixed cultures of salivary gland cells isolated from tissue (C). Cultures enriched for acinar cells (D). Arrows point to AN, acini; AC, acinar cells; ID, intercalated duct; SD, striated ducts; and DC, ductal cells. Color images available online at www.liebertonline.com/ten.
Biomarkers found in cultured salivary gland cells parallel those identified in glandular tissue structures
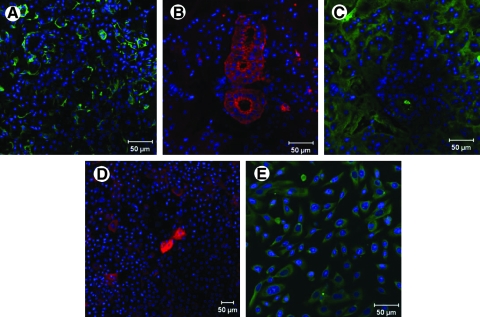
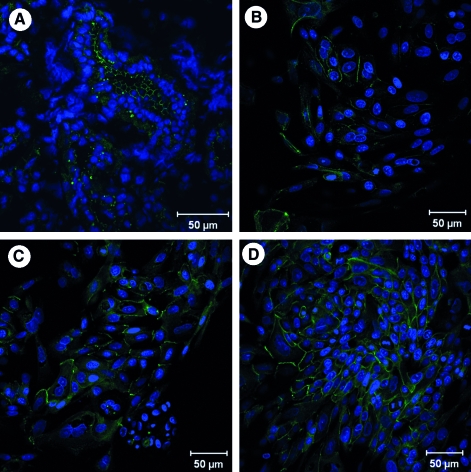
Salivary gland cells were characterized by their expression of cell-type-specific biomarkers. Myoepithelial cells present in the salivary gland tissue were identified using the marker CK 14. Myoepithelial cells stained strongly and were found wrapped around the acini in the tissue (Fig. 2A). To parallel the findings in the tissue with the isolated cells, the mixed culture of isolated cells was stained with CK 14. None of the cells in the mixed culture were positive for CK 14, suggesting that no myoepithelial cells were present in our mixed culture (not shown). Further, tissue staining with the ductal cell marker, CK 19, revealed positive staining of large ductal structures (Fig. 2B). Interestingly, the large cells from mixed cell cultures also stained positively for CK 19, indicating that they were derived from the ductal structures in the gland (Fig. 2D).
FIG. 2.
Myoepithelial, ductal, and acinar cells identified in tissue and cultured cells. Confocal microscopy images show expression of cytokeratin (CK) 14, a myoepithelial cell marker (green) (A); expression of CK 19, a ductal cell marker (red) (B, D); and expression of α-amylase, a marker for acinar cells (C, E). Panels (A–C) show staining in tissue sections of the salivary gland, while panels (D) and (E) show staining of cultured cells. Note that no myoepithelial cells were detected in the cultures.
α-Amylase, the predominant enzyme found in saliva that is abundantly produced and secreted by the acinar cells, was used as a marker to identify the acinar cells in the gland. Staining of salivary gland tissue sections displayed abundant expression of α-amylase in the acini structures (Fig. 2C). To parallel studies with cultured cells, the mixed population of cells was subcultured and subjected to conditions that favored the growth of acinar cells, yielding an acinar-enriched culture. α-Amylase staining for these acinar-enriched cultures was strongly positive (Fig. 2E). Because our tissue engineering approach is designed for the direct use of cultured acinar cells, rather than the re-engineering of ductal ones, we used the acinar-enriched cultures for all future analyses.
Salivary gland basement membrane and acinar-enriched cultures reveal robust expression of perlecan and laminin
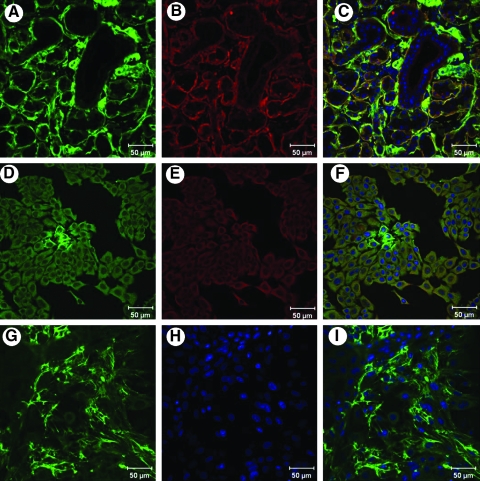
Because some self-assembly was observed in the acinar-enriched cell cultures, expression of certain candidate basement membrane components was investigated in tissue sections as well as in the cultured cells. Tissue cryosections of the salivary glands were stained with antibodies to the common ECM proteins. Immunohistochemical analyses revealed the presence of significant levels of perlecan and laminin in the basement membrane of the salivary gland tissue (Fig. 3A–C). Among the acinar-enriched cell cultures, 8 days of culturing led to robust expression of perlecan and laminin in their secretory route (Fig. 3D–F). At 12 days in culture, perlecan was secreted and organized by the acinar cell cultures (Fig. 3G, I).
FIG. 3.
Perlecan and laminin are highly expressed in salivary gland tissue and in cultured cells. Panels (A–C) represent tissue sections of the human salivary gland, while panels (D–I) show cultured salivary gland cells. Panel (G) shows cultured salivary acinar cells secreting and organizing perlecan in culture. Panels (C), (F), and (I) show merged images. Perlecan is seen in green, and laminin is seen in red. Nuclei stain blue.
Acinar cultures on PlnDIV peptide, laminin, or Matrigel did not show differences in cell viability
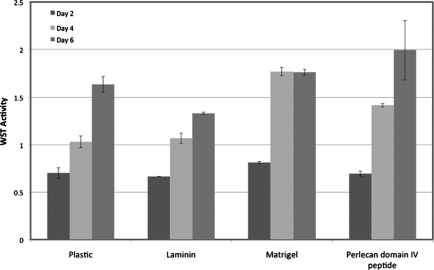
Cell viability of acinar-enriched cultures grown on plastic, laminin, Matrigel, or PlnDIV peptide was evaluated using the WST assay. Cell viability and growth remained similar for cells plated on each of the matrices. No significant differences were noted in the rate of proliferation of the cells among the different matrices (Fig. 4).
FIG. 4.
Acinar-enriched cells cultured on different extracellular matrices do not show differences in growth rates. Cells were cultured in 96-well plates coated with laminin (100 μg/mL), Matrigel™ (1:3 dilution), or domain IV of perlecan (PlnDIV) peptide (10 μg/mL).
Self-assembly of acinar-enriched cell cultures on Matrigel or PlnDIV peptide
The morphology of the acinar cells grown on plastic, laminin, Matrigel, or PlnDIV peptide was assessed. Cell morphology remained similar on all four of these matrices at day 1 (Fig. 5A–D). At day 6, the cultured cells showed noticeable differences. While the morphology of cells on plastic or laminin remained the same (Fig. 5E, F), the cells growing on Matrigel or PlnDIV peptide began to differentiate and self-assemble into clusters (Fig. 5G, H). At day 8, small lobules developed inside the self-assembled structures on Matrigel as well as on PlnDIV peptide (Fig. 5I, J). Large lobular structures with interconnections developed by day 15 on Matrigel (Fig. 5K) and on PlnDIV peptide (Fig. 5L).
FIG. 5.
Acinar-enriched cell cultures grown on Matrigel or PlnDIV peptide self-assemble. Cells cultured on plastic (A, E), laminin (B, F), Matrigel (C, G), or PlnDIV peptide (D, H). Phase contrast images show no differences when cultured for 24 h (A–D), but after 6 days (E–H) show differentiated structures forming on Matrigel (G) or PlnDIV peptide (H). Development of acini-like structures on Matrigel (I) or PlnDIV peptide (J) after 8 days in culture. Arrows in (I) and (J) point to lobules forming within the spherical acini-like structures. Development of salivary units is seen on Matrigel (K) or PlnDIV peptide (L) after 15 days in culture. Arrows in (K) and (L) point to connections formed between the lobular structures.
Acinar-enriched cell cultures express tight junction and water channel proteins
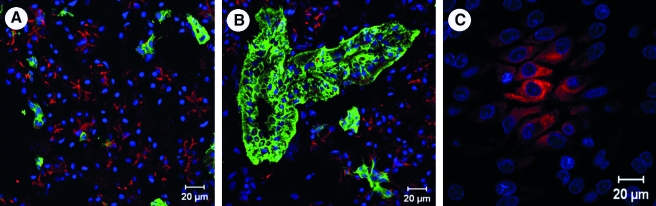
Tissue sections and cultured cells were analyzed for the presence of tight junctional components. Both tissue sections (Fig. 6A) and the acinar-enriched cultures grown on plastic, Matrigel, or PlnDIV peptide (Fig. 6B–D) all were found to express E-cadherin at their tight junctions. Tissue sections and acinar-enriched cultures grown on Matrigel or PlnDIV peptide were stained for AQP5 to determine the presence of this critical water channel protein. The acini in the salivary gland tissue sections were observed to highly express AQP5 at their cell membranes (Fig. 7A). The ductal structures, however, did not show the presence of this water channel protein (Fig. 7B). Among the acinar cells cultured on Matrigel or PlnDIV peptide, population clusters undergoing self-assembly were positive for AQP5 (Fig. 7C).
FIG. 6.
Tight junctions form in the salivary gland tissue and cell cultures. Confocal microscopy images show tight junctional marker E-cadherin (green) in the human salivary gland tissue (A), and in cultured cells plated on plastic (B), Matrigel (C), or PlnDIV peptide (D). Nuclei stain blue. Color images available online at www.liebertonline.com/ten.
FIG. 7.
Aquaporin 5 (AQP5) is expressed in salivary gland tissue and acinar cells cultured on PlnDIV peptide. AQP5, water channel protein, expression (red) and ductal cell marker, CK 19 (green) expression in the human salivary gland tissue (A, B) and cultured cells grown on PlnDIV peptide (C). Note that similar results (not shown) were observed with cells plated on Matrigel. Nuclei stain blue.
Stress fiber formation and FAK activation observed in acinar-enriched cell cultures grown on Matrigel or PlnDIV peptide
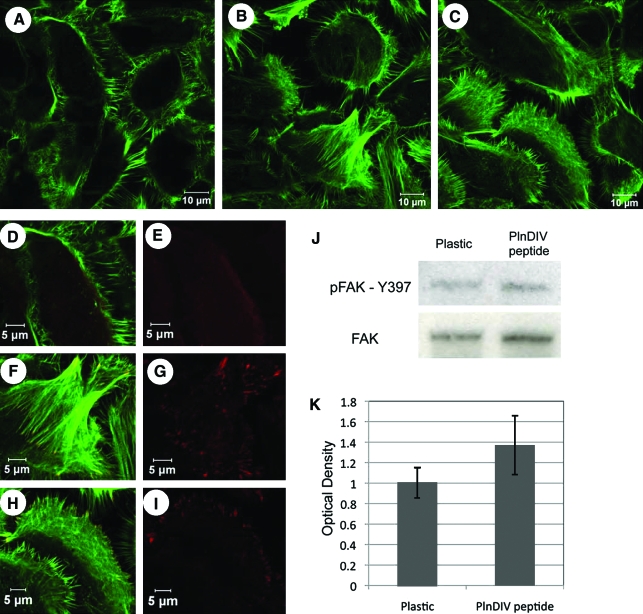
Acinar-enriched cell cultures grown on Matrigel or PlnDIV peptide assembled stress fibers and complex filopodial structures (Fig. 8B, C) as shown at higher magnification in Figure 8F and H. In contrast, cells grown on plastic displayed extensive actin filaments along the periphery of the cells (Fig. 8A, D) consistent with the more stationary, attached behavior seen in Figure 5A and E. Z-stacks performed on the phalloidin stainings confirm that our observations were consistent on all focal planes (data not shown). Phalloidin staining (green) of cells grown on Matrigel or PlnDIV peptide revealed a dense organized network of actin stress fibers (Fig. 8F, H), indicating that cells cultured on either of these matrices are able to migrate and form higher-order lobular acinar structures such as those shown in Figure 5I and J.
FIG. 8.
PlnDIV peptide supports stress fiber formation, formation of filopodia, and focal adhesion kinase (FAK) activation. Stress fiber formation seen with phalloidin (green) in cells growing on plastic (A), Matrigel (B), or PlnDIV peptide (C). High-magnification images reveal phosphorylated FAK throughout the cytoplasm on plastic (D, E) and at sites of focal adhesions (red) on Matrigel (F, G), or PlnDIV peptide (H, I). Western blot comparison of total and phospho-FAK levels from cells grown on plastic and PlnDIV peptide are shown (J) and are quantified (K). No differences between phospho-FAK levels in cells grown on plastic or PlnDIV peptide were observed. Color images available online at www.liebertonline.com/ten.
Experiments with salivary gland cells plated on either Matrigel or PlnDIV peptide showed clear FAK phosphorylation on tyrosine 397 at discrete cellular sites that correspond to sites of focal adhesions (red staining, Fig. 8F–I). Cells coated on plastic displayed expression of phosphorylated FAK throughout the cytoplasm and occasionally localized it to punctate adhesion sites (Fig. 8D, E). Western blot analysis of phosphorylation at this site, however, showed no clear differences in levels of FAK activation in cells plated on PlnDIV peptide when compared to cells on plastic (Fig. 8J, K). As expected, cells cultured on Matrigel expressed robust total FAK and FAK phosphorylation, higher than either on plastic or PlnDIV peptide (not shown). Thus, although cells growing on plastic or PlnDIV peptide express similar levels of activated and total FAK, they localize it differentially with cells growing on PlnDIV peptide able to localize it to focal adhesions at sites of rapid cytoskeletal reorganization.
PlnDIV peptide or Matrigel support the formation of three-dimensional acini-like salivary units that express large amounts of α-amylase
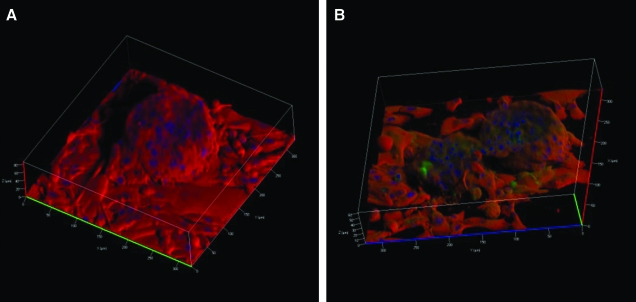
To better view α-amylase expression within the well-differentiated structures, acinar-enriched cultures were grown on Matrigel or PlnDIV peptide for 20 days. Z-stacks of the acini-like structures were obtained to view expression of α-amylase in optical slices through the structures. At day 20, a representative structure on Matrigel grew to approximately 86 μm above the surface of the culture plate, while structures on PlnDIV peptide grew approximately 61 μm. Three-dimensional reconstructions from the optical slices showed acini-like structures on Matrigel (Fig. 9A) and on PlnDIV peptide (Fig. 9B) rising from a monolayer of cells. Structures on both matrices formed tight junctions and demonstrated robust expression of α-amylase.
FIG. 9.
PlnDIV peptide or Matrigel trigger differentiation of salivary gland cells into three-dimensional acini-like structures expressing α-amylase. Structure on Matrigel (A) and PlnDIV peptide (B). α-Amylase expression is seen in red, and E-cadherin expression is seen in green. Color images available online at www.liebertonline.com/ten.
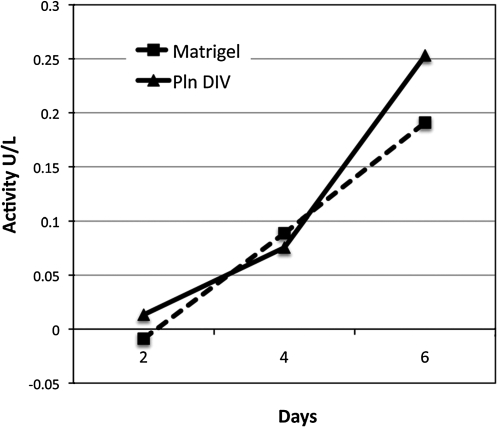
α-Amylase activity of acinar-enriched cultures grown on PlnDIV peptide or Matrigel increases over time
To demonstrate α-amylase secretion by the acinar-enriched cultures growing on PlnDIV peptide or Matrigel, α-amylase activity in their culture media was measured over a 6-day period. Activity measurements from cell cultures of both Matrigel and PlnDIV peptide gave similar values and were found to increase over time (Fig. 10).
FIG. 10.
Differentiated cells cultured on PlnDIV peptide or Matrigel secrete α-amylase over time. Amylase activity detected from culture medium of cells coated on Matrigel or PlnDIV peptide increased in a time-dependent manner.
Discussion
Tissue engineering of a salivary gland requires functional cells that express the vital proteins needed to restore secretion. Here, we report a technique for isolation and maintenance of human salivary gland cells and their differentiation via a human sequence in perlecan that allows for self-assembly of these cells into functional acini-like units, suitable for tissue engineering. We envision that the salivary acinar cells will grow and differentiate, initially, in an artificial scaffold and eventually fully integrate into the patients' subcutaneous buccal mucosa or native salivary gland tissue.
To recreate the native structure of the salivary gland in vitro, biomarkers from the salivary gland tissue were compared in parallel with cells isolated from specimens of the glandular tissue. Tissue cryosections from the human parotid and submandibular glands were obtained for initial histological and immunohistochemical studies. Parallel biomarker studies with CK 19 and α-amylase revealed the presence of ductal and acinar cells, respectively, in both salivary gland tissue and primary cell cultures. Myoepithelial cells were identified in the tissue; however, negative staining for CK 14 revealed their absence from primary cell cultures. Although mixed cultures of acinar and ductal cells were isolated initially, novel culturing techniques were used to obtain acinar-enriched cultures.
The basement membrane, one of the most important components of the ECM of polarized epithelial cells, directs the growth and differentiation of human epithelia.13 In addition to separating the parenchymal cells and connective tissues, the basement membrane also is involved in encasing, organizing, and guiding the behavior of the parenchymal cells that reside on it.14 In the case of the salivary epithelium, a major function of the basement membrane is maintenance of directional secretion. Abundant expression of perlecan and laminin in the tissue reveals the importance of these proteins for maintaining the phenotype of salivary gland cells. The functionality of the cultured salivary acinar cells in vitro is confirmed by their secretion and organization of their own basement membrane including the critical component perlecan that can support further growth and differentiation by virtue of its ability to interact with cell adhesion molecules that can influence integrin-mediated events.11,15 Uniquely, no non-human-derived material was needed for isolation or culture of the acinar cells.
To aid further differentiation of salivary gland cells into organized glandular structures that express essential biomarkers, acinar cells were cultured on ECM proteins such as perlecan, laminin, and the commonly used BME, Matrigel. Because full-length perlecan cannot be produced in recombinant form because of its large size, and because we wished to avoid animal proteins, human-compatible PlnDIV peptide sequence was used in studies involving perlecan. Evaluation of the behavior of acinar cells grown on Matrigel, laminin, or PlnDIV peptide showed no differences in growth and proliferative capabilities of cells. Morphological analysis revealed that Matrigel and PlnDIV peptide supported gradual differentiation of the cells. Lobe formation with interconnections were observed in cells grown on Matrigel as well as on PlnDIV peptide. Differentiated structures self-assembled into acini-like structures that are reminiscent of salivary units.
α-Amylase, one of the major proteins in saliva that helps the breakdown of starches and initiates digestion, is highly produced and secreted by acinar cells. Differentiated acini-like structures mimicked salivary units and produced and secreted α-amylase. Additionally, α-amylase secretion from differentiated structures increased over time, as seen by amylase activity measurements, suggesting development of functional salivary units.
Tight junctions, vital components in polarized secretory epithelia, hold cells together and form a barrier to maintain transcellular transport. E-cadherin expression confirmed formation of tight junctions in acinar cell cultures grown on plastic, Matrigel, as well as PlnDIV peptide. Additional markers of polarization include AQP, which are important indicators of fluid secretion and play an essential role in saliva production. Lack of AQP5 decreased rates of salivary secretion in mice.16,17 AQP5 expression in apical membrane of acini in salivary gland tissue revealed their secretory abilities. Because epithelial cells must be polarized to secrete fluid unidirectionally, the expression of AQP5 at sites of self-assembly in cultured cells revealed their polarization and secretory potential. Additionally, recent reports have shown that activation of muscarinic acetylcholine receptors is important for translocation of AQP5 to the apical plasma membrane of salivary gland cells.18,19 Self-assembled and differentiated acinar cells expressing AQP5 might receive appropriate signals from stimulatory receptors for AQP5 translocation.
For cells to differentiate and self-assemble, they must receive appropriate signals from the ECM. Stress fiber formation and signals generated from cytoskeletal tension provide essential cues for dynamic cellular organization into higher-order structures.20 Formation of ordered F-actin stress fibers and activation of FAK also are crucial for cell migration.21 The organization of dense stress fiber networks and filopodia in acinar cells cultured on Matrigel or PlnDIV peptide demonstrated their differentiation and migratory potential. Focal adhesions are dynamic protein complexes that form connections between the cell cytoskeleton and the ECM.22 Salivary gland cells grown on Matrigel or PlnDIV peptide supported FAK activation and correctly localized it to adhesion sites, reflecting signal transduction events between the ECM proteins and the cytoskeleton. FAK staining was observed in the cytoplasm of cells grown on plastic, but not many punctate focal adhesions were seen. Thus, although these cells cultured on plastic are expressing some basement membrane proteins on their own, they evidently lack contextual information required to consistently localize FAK to sites of integrin clustering. In contrast, the redistribution of phospho-FAK seen on Matrigel and PlnDIV peptide to the ends of stress fiber assemblies on filopodia can support the organized differentiation of salivary acinar cells.
Information provided by the PlnDIV peptide accelerated the self-assembly of salivary gland cells into lobular structures better than did the intact perlecan produced by the cells themselves. The intact perlecan molecule that is produced by cultured acinar cells is expected to be modified by heparan sulfate chains that in previous studies were found to conceal the adhesive domain IV motifs.11 Fibroblasts cultured on intact perlecan failed to make stable focal adhesion contacts because of the antiadhesive activity of gagosylated perlecan domain I.23 Consistent with this, our studies showed less FAK activation and, more strikingly, a failure to cluster phosphorylated FAK to sites of focal adhesions when cells were cultured on plastic. The mechanism by which PlnDIV peptide triggers self-assembly of salivary gland cells remains unknown. Our earlier studies suggest that adhesion to PlnDIV peptide is mediated through nonintegrin receptors as inhibition of β1 integrins only partially affected adhesion to surfaces modified with the peptide.11 The peptide's origin from an Ig loop on PlnDIV suggests that signaling could be induced via interactions with Ig superfamily members on the surfaces of the acinar cells through homophilic or heterophilic binding events. These interactions can initiate signaling events that alter cytoskeletal dynamics and organization leading to cell migration, differentiation, and ultimately acinar assembly.24
Prior reports of cell transplantation into animal models resulted in regeneration of ductal cells but lacked acinar cell differentiation.25 The cells characterized in this study have the potential to be functional in an artificial scaffold that provides them the appropriate conditions to differentiate. Efforts to devise a three-dimensional culture system with a biomaterial scaffold consisting of PlnDIV peptide are underway. The culture system consisting of PlnDIV peptide reported here will aid the development of an artificial salivary gland that will foster formation of functional salivary units capable of secreting salivary fluid and that can be implanted into patients to relieve xerostomia.
Acknowledgments
This work was supported by private philanthropic contribution and NIH/NCI P01-CA098912 and COBRE P20-RR016458 (to M.C.F.-C). The authors thank Dr. Randall Duncan and Dr. Ken van Golen for providing their expert advice on cytoskeletal studies.
Disclosure Statement
No competing financial interests exist.
References
- 1.Nagler R.M. Baum B.J. Prophylactic treatment reduces the severity of xerostomia following radiation therapy for oral cavity cancer. Arch Otolaryngol Head Neck Surg. 2003;129:247. doi: 10.1001/archotol.129.2.247. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan C.A. Haddad R.I. Tishler R.B. Mahadevan A. Krane J.F. Chemoradiation-induced cell loss in human submandibular glands. Laryngoscope. 2005;115:958. doi: 10.1097/01.MLG.0000163340.90211.87. [DOI] [PubMed] [Google Scholar]
- 3.Tran S.D. Sugito T. Dipasquale G. Cotrim A.P. Bandyopadhyay B.C. Riddle K. Mooney D. Kok M.R. Chiorini J.A. Baum B.J. Re-engineering primary epithelial cells from rhesus monkey parotid glands for use in developing an artificial salivary gland. Tissue Eng. 2006;12:2939. doi: 10.1089/ten.2006.12.2939. [DOI] [PubMed] [Google Scholar]
- 4.Tran S.D. Wang J. Bandyopadhyay B.C. Redman R.S. Dutra A. Pak E. Swaim W.D. Gerstenhaber J.A. Bryant J.M. Zheng C. Goldsmith C.M. Kok M.R. Wellner R.B. Baum B.J. Primary culture of polarized human salivary epithelial cells for use in developing an artificial salivary gland. Tissue Eng. 2005;11:172. doi: 10.1089/ten.2005.11.172. [DOI] [PubMed] [Google Scholar]
- 5.Joraku A. Sullivan C.A. Yoo J. Atala A. In-vitro reconstitution of three-dimensional human salivary gland tissue structures. Differentiation. 2007;75:318. doi: 10.1111/j.1432-0436.2006.00138.x. [DOI] [PubMed] [Google Scholar]
- 6.Szlavik V. Szabo B. Vicsek T. Barabas J. Bogdan S. Gresz V. Varga G. O'Connell B. Vag J. Differentiation of primary human submandibular gland cells cultured on basement membrane extract. Tissue Eng Part A. 2008;14:1915. doi: 10.1089/ten.tea.2007.0208. [DOI] [PubMed] [Google Scholar]
- 7.Flaim C.J. Chien S. Bhatia S.N. An extracellular matrix microarray for probing cellular differentiation. Nat Methods. 2005;2:119. doi: 10.1038/nmeth736. [DOI] [PubMed] [Google Scholar]
- 8.Wicha M.S. Lowrie G. Kohn E. Bagavandoss P. Mahn T. Extracellular matrix promotes mammary epithelial growth and differentiation in vitro. Proc Natl Acad Sci USA. 1982;79:3213. doi: 10.1073/pnas.79.10.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Defilippi P. Bozzo C. Geuna M. Rossino P. Silengo L. Tarone G. Modulation of extracellular matrix receptors (integrins) on human endothelial cells by cytokines. EXS. 1992;61:193. doi: 10.1007/978-3-0348-7001-6_29. [DOI] [PubMed] [Google Scholar]
- 10.Hassell J. Yamada Y. Arikawa-Hirasawa E. Role of perlecan in skeletal development and diseases. Glycoconj J. 2002;19:263. doi: 10.1023/A:1025340215261. [DOI] [PubMed] [Google Scholar]
- 11.Farach-Carson M.C. Brown A.J. Lynam M. Safran J.B. Carson D.D. A novel peptide sequence in perlecan domain IV supports cell adhesion, spreading and FAK activation. Matrix Biol. 2008;27:150. doi: 10.1016/j.matbio.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whitelock J.M. Murdoch A.D. Iozzo R.V. Underwood P.A. The degradation of human endothelial cell-derived perlecan and release of bound basic fibroblast growth factor by stromelysin, collagenase, plasmin, and heparanases. J Biol Chem. 1996;271:10079. doi: 10.1074/jbc.271.17.10079. [DOI] [PubMed] [Google Scholar]
- 13.Schittny J.C. Timpl R. Engel J. High resolution immunoelectron microscopic localization of functional domains of laminin, nidogen, and heparan sulfate proteoglycan in epithelial basement membrane of mouse cornea reveals different topological orientations. J Cell Biol. 1988;107:1599. doi: 10.1083/jcb.107.4.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merker H.J. Morphology of the basement membrane. Microsc Res Tech. 1994;28:95. doi: 10.1002/jemt.1070280203. [DOI] [PubMed] [Google Scholar]
- 15.Zell T. Kivens W.J. Kellermann S.A. Shimizu Y. Regulation of integrin function by T cell activation: points of convergence and divergence. Immunol Res. 1999;20:127. doi: 10.1007/BF02786469. [DOI] [PubMed] [Google Scholar]
- 16.Ma T. Song Y. Gillespie A. Carlson E.J. Epstein C.J. Verkman A.S. Defective secretion of saliva in transgenic mice lacking aquaporin-5 water channels. J Biol Chem. 1999;274:20071. doi: 10.1074/jbc.274.29.20071. [DOI] [PubMed] [Google Scholar]
- 17.Krane C.M. Fortner C.N. Hand A.R. McGraw D.W. Lorenz J.N. Wert S.E. Towne J.E. Paul R.J. Whitsett J.A. Menon A.G. Aquaporin 5-deficient mouse lungs are hyperresponsive to cholinergic stimulation. Proc Natl Acad Sci USA. 2001;98:14114. doi: 10.1073/pnas.231273398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishikawa Y. Yuan Z. Inoue N. Skowronski M.T. Nakae Y. Shono M. Cho G. Yasui M. Agre P. Nielsen S. Identification of AQP5 in lipid rafts and its translocation to apical membranes by activation of M3 mAChRs in interlobular ducts of rat parotid gland. Am J Physiol Cell Physiol. 2005;289:C1303. doi: 10.1152/ajpcell.00211.2005. [DOI] [PubMed] [Google Scholar]
- 19.Ishikawa Y. Eguchi T. Skowronski M.T. Ishida H. Acetylcholine acts on M3 muscarinic receptors and induces the translocation of aquaporin5 water channel via cytosolic Ca2+ elevation in rat parotid glands. Biochem Biophys Res Commun. 1998;245:835. doi: 10.1006/bbrc.1998.8395. [DOI] [PubMed] [Google Scholar]
- 20.Weber G.F. Menko A.S. Actin filament organization regulates the induction of lens cell differentiation and survival. Dev Biol. 2006;295:714. doi: 10.1016/j.ydbio.2006.03.056. [DOI] [PubMed] [Google Scholar]
- 21.Small J.V. Rottner K. Kaverina I. Anderson K.I. Assembling an actin cytoskeleton for cell attachment and movement. Biochim Biophys Acta. 1998;1404:271. doi: 10.1016/s0167-4889(98)00080-9. [DOI] [PubMed] [Google Scholar]
- 22.Zamir E. Geiger B. Molecular complexity and dynamics of cell-matrix adhesions. J Cell Sci. 2001;114:3583. doi: 10.1242/jcs.114.20.3583. [DOI] [PubMed] [Google Scholar]
- 23.French M.M. Gomes R.R., Jr. Timpl R. Hook M. Czymmek K. Farach-Carson M.C. Carson D.D. Chondrogenic activity of the heparan sulfate proteoglycan perlecan maps to the N-terminal domain I. J Bone Miner Res. 2002;17:48. doi: 10.1359/jbmr.2002.17.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nielsen J. Kulahin N. Walmod P.S. Extracellular protein interactions mediated by the neural cell adhesion molecule, NCAM: heterophilic interactions between NCAM and cell adhesion molecules, extracellular matrix proteins, and viruses. Neurochem Res. 2008 doi: 10.1007/978-1-4419-1170-4_2. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 25.Sugito T. Kagami H. Hata K. Nishiguchi H. Ueda M. Transplantation of cultured salivary gland cells into an atrophic salivary gland. Cell Transplant. 2004;13:91. doi: 10.3727/000000004783983567. [DOI] [PubMed] [Google Scholar]