Abstract
The lack of easily isolated autologous endothelial cell (EC) sources is one of the major challenges with vascular tissue engineering interventions. This article examines the isolation and expansion of late-outgrowth endothelial progenitor cells (EPCs) from 50-mL samples of peripheral blood drawn from patients with significant coronary artery disease (CAD) and healthy young adult volunteers. In cases in which late-outgrowth EPCs were successfully isolated, the cells were assayed in vitro for their expression of EC markers, proliferation potential and ability to endothelialize synthetic materials, form new blood vessels, and produce nitric oxide. Late-outgrowth EPCs from patients with CAD and healthy volunteers exhibited critical EC markers and morphological characteristics that were analogous to a control population of human aortic ECs. To our knowledge, this is the first study to examine the suitability of late-outgrowth EPCs from patients with CAD for autologous endothelialization applications.
Introduction
A fully functional endothelial cell (EC) monolayer adherent to the lumen of synthetic or tissue-engineered vascular grafts has been shown to increase the patency of the replacement vessel.1 Graft endothelialization requires harvesting autologous ECs that are isolated with minimal patient morbidity, expandable to cell densities suitable for the application, minimally contaminated with other cell types, strongly and contiguously adherent to the vessel lumen, and functionally similar to intact endothelium.
The two most common sources of autologous ECs are cells harvested using trypsin and collagenase digestion from excised jugular or saphenous vein2 and microvascular ECs isolated from liposuctioned fat3—both of which require significant interventional surgical procedures. Cell contamination, although often not a problem with EC isolation from excised vessels, is a concern with adipose-derived cells, which are often heterogeneous and contaminated with other cell types (e.g., macrophages and fibroblasts) that are responsible for intimal hyperplasia and inflammation.4
Autologous ECs isolated from peripheral blood would represent an essentially noninterventional source for endothelialization therapies. Asahara et al.5 were first to report that peripheral blood contains a population of bone marrow–derived circulating cells that could differentiate into cells with some EC characteristics ex vivo, although the cells also displayed monocytic cell markers. These cells have been collectively termed endothelial progenitor cells (EPCs). Recent reports describe isolation of distinctly different EPC subpopulations from blood mononuclear cells (MNCs) dependent on the method of ex vivo culture.6–11 “Early outgrowth” EPCs (also known as colony forming unit ECs) form spindle-shaped clusters of cells only a few days after plating on fibronectin in angiogenic growth factor–enriched medium. Early-outgrowth EPCs express multiple EC markers and secrete high levels of angiogenic cytokines,12 although these cells have limited proliferation potential, express markers typical of macrophages, and show the ability to ingest bacteria, indicating hematopoietic origin.13 In contrast, “late outgrowth” EPCs (also called endothelial colony forming cells or endothelial outgrowth cells) are a much rarer subpopulation isolated from MNCs.14 Late-outgrowth EPCs are highly proliferative cells that express EC markers,8 exhibit no hematopoietic or monocytic character,15 and are capable of forming capillaries14 and endothelializing denuded vessels when injected in vivo.16 Late-outgrowth EPCs have been described as “true” EPCs because they are capable of generating ECs that express typical endothelial surface markers and exhibit typical EC function.17 It remains to be clarified whether these cells are derived from the bone marrow or dislodged from the vessel wall.6,18
Flow cytometry has been used to sort and enumerate the abundant early-outgrowth EPCs from MNC fractions through the use of a combination of surface markers, such as CD34+/CD133+/KDR+ that are predictive biomarkers of the extent of cardiovascular disease;19–23 in contrast, late-outgrowth EPCs are too scarce to enumerate using cell sorting directly from peripheral blood and are difficult to identify because of lack of unique cell markers to this cell subgroup.24 However, late-outgrowth EPCs can be cultured to yield large numbers of cells that express EC antigens,9 which suggests suitability for endothelialization therapies. There has been little work in demonstrating whether autologous late-outgrowth EPCs can be isolated and expanded in sufficient numbers from patients with significant coronary artery disease (CAD). It is also unclear whether late-outgrowth EPCs isolated from patients with CAD exhibit differences in proliferation, adhesion, and angiogenic potential from healthy individuals.
In this study, late-outgrowth EPCs were isolated and expanded from peripheral blood drawn from patients undergoing cardiac catheterization in the Duke University Medical Center who had documented advanced CAD according to angiography. In cases in which EPCs were successfully isolated, the cells were assayed in vitro for their expression of EC markers; their proliferation potential; and their ability to endothelialize synthetic materials, form new blood vessels, and produce nitric oxide (NO).
Materials and Methods
Donor subjects
The Duke University Institutional Review Board approved the protocol for collection and use of human blood employed in the study. Patients undergoing left heart catheterization were approached for consent at Duke University Medical Center (n = 13). All patients who have undergone cardiac catheterization, percutaneous coronary intervention, or cardiac surgery have had their demographic, clinical, angiographic, and procedural data entered into a standard database. Two operators systematically review all cardiac catheterization procedures in a standardized fashion, and the extent of CAD is documented. Enrolled patients with CAD had documented advanced CAD according to angiography. Clinical data were extracted from the patients' medical charts.
Young control individuals were normal healthy volunteers (n = 13) who had no history of chest pain and were surveyed for CAD risk factors including body mass index, smoking history, diabetes, hypertension, hyperlipidemia, and medication use.
Cell isolation and culture
Approximately 50 mL of peripheral blood was drawn after arterial sheath insertion for patients with CAD and stored in K2 ethylenediaminetetraacetic acid tubes (BD Labware, Franklin Lakes, NJ). For healthy individuals, approximately 50 mL of peripheral blood was drawn by vein puncture. Blood samples were processed the same day of collection. Late-outgrowth EPCs from patients with CAD and healthy individuals were isolated and grown according to a previously described protocol.25 Mononuclear cells were resuspended in 12 mL of complete endothelial basal medium (EBM)-2 plus endothelial growth medium (EGM)-2 SingleQuots (Cambrex, Walkersville, MD) with 1% antibiotic/antimycotic solution (Gibco, Carsbad, CA) and seeded into three separate wells of a six-well tissue culture plate coated with type 1 rat tail collagen (BD Biosciences) at 37°C, 5% carbon dioxide in a humidified incubator. An average of 34 × 106 MNCs were seeded per well. After 24 hours of culture, nonadherent cells were removed, and complete EGM-2 was added to each well. Medium was changed daily for 7 days and then every other day. Cells were used at passages 5 to 10 for all experiments.
Human aortic ECs (HAECs) (Cambrex) were grown to confluence in T-25 or T-75 polystyrene flasks with EBM-2 supplemented with EGM-2 SingleQuots, 10% fetal bovine serum (FBS), and 1% antibiotic/antimycotic solution. HAECs were used at passage 7 to 10 for all experiments.
Doubling time
A 12-well plate was coated with 3.33 μg/mL of fibronectin (Millipore, Billerica, MA). Cells were trypsinized and seeded at an initial density of 5 × 103 cells/cm2 in complete EGM-2. After 3 days, cells were fixed with 3.7% paraformaldehyde, and cell nuclei were stained with 4′,6-diamidino-2-phenylindole (Sigma, St. Louis, MO). Four pictures per well were taken, and the number of EPCs were counted using ImageJ computer software (version 1.37a, National Institutes of Health, Bethesda, MD).
Flow cytometry
Cells were detached using 0.025% trypsin (Cambrex). Detached cells were resuspended in 10% goat serum with antibodies against CD14 (BD Pharmingen, San Diego, CA), CD31 (Invitrogen, Camarillo, CA), CD45 (BD Pharmigen), CD105-PE (Invitrogen), and CD133 (Miltenyi Biotec, Auburn, CA). Alexa Fluor 488 goat anti-mouse immunoglobulin IgG heavy and light chain antibody (H + L) (Invitrogen) or phycoerythrin goat anti-mouse IgG (H+L) (Sigma) were used as secondary antibodies. Appropriate control isotype antibodies were used. Flow cytometric analysis was performed using a FACScalibur flow cytometer (BD Biosciences).
Substrate for measuring cell spreading and adhesion
To test cell behavior on a material with chemistry similar to that of expanded polytetrafluoroethylene (ePTFE) vascular grafts, Teflon-AF (DuPont, Wilmington, DE) films were spun-cast onto standard glass microscope slides (Gold Seal, Portsmouth, NH) as described previously.26 Before use, glass slides were cleaned using sonication with 2% PCC-54 detergent cleaning solution (Pierce, Rockford, IL) and a 1:1 mixture of methyl alcohol:hydrochloric acid.
Cell spreading
Teflon-AF-coated slides were incubated with 3.3 μg/mL of human fibronectin (Millipore) in Dulbecco's phosphate buffered saline (DPBS) for 1 h at 37°C. Cells were detached with 0.025% trypsin (Cambrex) for 5 min at 37°C and neutralized with trypsin neutralizing solution (Cambrex) (twice as much trypsin neutralizing solution s trypsin was useda), spun down, and stained with 5 μM of Cell Tracker Orange (Invitrogen). Cells were washed and plated onto the fibronectin-coated Teflon- AF slide. Ten random images were taken using fluorescence microscopy at 200 × magnification (Nikon TE2000U, Tokyo, Japan) and digital camera (DS-Qi1Mc, Nikon) at 1, 2, 3, 4, and 24 h after seeding. The projected cell area was measured using ImageJ software. On average, 50 to 100 cells were examined for each time point for each condition, and four experiments were performed for each condition.
Strength of adhesion
The strength of cell adhesion to Teflon-AF was measured as previously described.27 Briefly, Teflon-AF-coated slides were incubated with 3.3 μg/mL of fibronectin in DPBS for 1 h at 37°C. Cells were incubated with 0.025% trypsin for 5 min at 37°C, neutralized with trypsin neutralizing solution (Cambrex), spun down, and stained with Hoechst 33342 (Invitrogen). The cells were then seeded onto slides for 10 min at room temperature. The slide was placed in a variable-height flow chamber28 and five pre- and postflow images were taken at five different channel heights along the chamber. Steady laminar flow was applied for 2 min through using a programmable syringe pump (Harvard Apparatus, Holliston, MA). The total elapsed time from initial cell attachment to the onset of flow was typically 20 min. The flow medium consisted of DPBS with varying amounts of dextran (average molecular weight 500,000; Fischer Scientific, Pittsburg, PA) to increase the viscosity to 2 to 5 cP. Four experiments were performed per cell type.
Surface expression of α5β1 and αVβ3 integrins
The relative expression of α5β1 and αVβ3 integrins present on each cell type was measured after using 0.025% trypsin. Detached cells were resuspended in DPBS and incubated for 5 min. The cells were then incubated with 10 μg/mL of cycloheximide for 30 min to block protein synthesis.29 The cells were then rinsed, incubated with 10% goat serum (Sigma), incubated with 10 μg/mL mouse anti-α5β1 or 20 μg/mL mouse anti-αVβ3 antibodies (Chemicon), rinsed, incubated with Alexa fluor 488 goat anti-mouse secondary antibody (Invitrogen), rinsed, and fixed in 3.7% paraformaldehyde. The antibody concentration used was determined as the concentration to saturate the integrin binding sites (data not shown).
Fluorescence intensity per cell produced by the bound antibody was measured using a FACScalibur flow cytometer (BD Biosciences). Typically, 1 × 104 cells were collected and measured for fluorescence intensity. In addition, an isotype control was used for each sample condition, and the geometric mean fluorescent intensity found for the isotype control was subtracted from the geometric mean fluorescent intensity of the antibody bound cells to compensate for background fluorescence.
In vitro vasculogenesis assay
Endothelial tube formation was assessed in Matrigel (BD Biosciences);30 150 μL of Matrigel was pipetted into a 48-well plate and incubated at 37°C for 30 min to allow the polymerization of collagen. After solidification, 4 × 104 cells per well were resuspended in 150 μL of growth medium, added to the well, and incubated at 37°C. At 4, 6, and 24 h, three representative images were taken per well at 40 × magnification (Nikon TE2000U). The total tube length per field was measured using ImageJ. Four to five independent experiments were performed for each cell type.
Effect of shear stress on cell morphology and NO expression
Flow setup
Cells were seeded onto SlideFlasks (NUNC, Rochester, NY) at a density of 50 × 103 cells/cm2. The following day, the slides were placed in a parallel-plate flow chamber and connected to a circular flow setup consisting of a peristaltic pump (Cole Palmer, Vernon Hills, IL), pulse dampener (Cole Palmer), and flow chamber as described previously.28 The flow medium used consisted of EBM-2 + 10% FBS + 1% antibiotic/antimycotic. Cells were exposed to 15 dyn/cm2 for 48 h. Controls consisted of cells under identical culture conditions but not exposed to flow (static).
Staining
After exposure to flow, slides were removed from flow chambers, and the cells were washed with DPBS and fixed in 3.7% paraformaldehyde for 10 min. Cells were permeabilized with 0.1% triton X, rinsed with DPBS, and incubated with 10% goat serum (Sigma) for 30 min at 37°C to block nonspecific binding. Primary antibodies (platelet–EC adhesion molecule 1:100, F-actin 1:20; Invitrogen) were incubated with the cells for 1 h at 37°C in 10% goat serum. Cells were rinsed multiple times and then incubated with a goat anti-mouse Alexa488-conjugated secondary antibody (1:500) (Invitrogen). Nuclei were stained with 10 μg/mL of Hoechst 33342.
Quantitative real-time reverse transcriptase polymerase chain reaction
Late-outgrowth EPCs or HAECs were seeded onto polystyrene Slideflasks and placed parallel to a plate flow chamber flow loop, as described above. The cells were exposed to shear stresses of 15 dyn/cm2 for 48 h, after which the cellular RNA was isolated using the RNeasy Minikit (Qiagen, Germantown, MD). The quantity and purity of all RNA samples were measured using a NanoDrop Spectrophotometer (NanoDrop Technologies, Wilmington, DE). Reverse transcription of 50 ng of total RNA was performed using a complementary DNA (cDNA) kit (Bio-Rad, Hercules, CA) and a MyCycler (Bio-Rad). Reverse transcriptase polymerase chain reaction (RT-PCR) was performed using a SYBR-Green RT-PCR kit (Bio-Rad) and the MyIQ iCycler Optical Module (Bio-Rad). Whole-gene cDNA sequences of the target genes were obtained from PubMed, and primer sequences for Beta-2 microglobulin, Kruppel-like factor (KLF)2, and endothelial NO synthase (eNOS) were generated using the online design program Primer3.31 The melt curves of the primers were examined after reaction with reference RNA, and primers were selected that had uniform and single-product melt curves. After results were obtained, fold change from the reference RNA was calculated using the 2−ΔΔCT method. All samples were preformed in quadruplicate for all genes at each condition. Primer sequences are available upon request.
Nitric oxide assay
Media aliquots from 48-h shear stress experiments were frozen and lyophilized (Heto CT110, Appropriate Technical Resources, Laurel, MD). Nitric oxide concentration was determined by measuring breakdown products NO2− and NO32 using a commercial assay (Active Motif, Carlsbad, CA) according to the manufacturers' instructions. Media samples were filtered through a 10,000-Da micropore filter (Millipore) before assay.
Statistical analysis
Patient clinical characteristics are presented as means and standard deviations. All other results are presented as means and standard errors. Categorical differences between clinical characteristics were analyzed using the Fisher exact test. Differences between cell sources and between cultures from patients with CAD, cultures from healthy individuals, and HAECs were carried out using multivariate analysis of variance (ANOVA), with the significance of individual differences established using the post hoc Fisher protected least significant difference test, or t-test for comparing only two groups. For cell spreading and in vitro vasculogenesis assay, a repeated-measure ANOVA was performed. P values < 0.05 were considered to indicate a statistically significant difference.
Results
Characteristics of study subjects and EPC proliferation potential
Twenty-six individuals were enrolled, 13 patients with extensive CAD and 13 healthy volunteers. The characteristics of the two donor groups are shown in Table 1. Enrolled patients with CAD had a mean age of 61.5 ± 10.5, whereas healthy donors had an average age of 26.2 ± 2.2. The patients with CAD averaged 2.1 ± 0.3 vessels with significant vessel stenosis and had significantly higher frequency of tobacco use, diabetes, hypertension, hyperlipidemia, and medication use. The healthy volunteers had no symptomatic indicators or and did not self-report any disease.
Table 1.
Baseline Donor Characteristics of Young Healthy Donors Versus Patients with Coronary Artery Disease (CAD)
| Characteristic | Healthy Donors (n = 13) | Patients with CAD (n = 13) | P-Value |
|---|---|---|---|
| Male, n (%) | 9 (69) | 9 (69) | |
| Race, n (%) | |||
| White | 12 (92) | 10 (77) | |
| Other (2 black, 2 Asian) | 1 (8) | 3 (23) | |
| Age, mean ± SD (range) | 26 ± 3 (23–31) | 62 ± 11 (44–75) | <0.001 |
| Body mass index, mean ± SD | 24.3 ± 2.6 | 29.0 ± 4.1 | 0.01 |
| Smoking history, n (%) | 0 | 5 (39) | 0.04 |
| Diabetes, n (%) | 0 (0) | 7 (54) | 0.005 |
| Hypertension, n (%) | 2 (15) | 10 (77) | 0.005 |
| Hyperlipidemia | 1 (8) | 11 (85) | <0.001 |
| Number of vessels with significant CAD, mean ± SD | — | 2.1 ± 1.0 | |
| Percutaneous transluminal coronary angioplasty, n (%) | 0 (0) | 9 (69) | <0.001 |
| Coronary artery bypass graft, n (%) | 0 (0) | 7 (54) | 0.005 |
| Medication use, n (%) | |||
| Beta blockers | 0 (0) | 13 (100) | <0.001 |
| Statins | 0 (0) | 10 (77) | <0.001 |
| Angiotensin-converting enzyme inhibitors | 1 (8) | 9 (69) | 0.004 |
SD, standard deviation.
Frequency of isolation
Table 2 shows the success rate of late-outgrowth EPC isolation from patients with CAD. No single clinical characteristic precluded the isolation of late-outgrowth EPCs from patients with CAD. Colonies appeared after approximately 3 weeks in culture (Fig. 1A). Colonies were successfully isolated in seven of 13 patients with CAD (54% of total): five of nine male patients with CAD and two of four female patients with CAD. Isolation success was not dependent on any of the measured variables. Late-outgrowth EPC colonies were isolated in nine of 13 healthy donors (69% of total): six of 10 male donors and two of three female donors.
Table 2.
Characteristics of Donor Population with Coronary Artery Disease (CAD)
| |
|
Late-Outgrowth Endothelial Progenitor Cell Colonies Obtained |
|
|
|---|---|---|---|---|
| Characteristic | All Subjects with CAD | Yes | No | P-Value |
| Subjects, n | 13 | 7 | 6 | |
| Male, n (%) | 9 (69) | 5 | 4 | |
| Female, n (%) | 4 (31) | 2 | 2 | |
| Race, n (%) | ||||
| White | 10 (77) | 5 | 5 | |
| Other (2 black, 1 Asian) | 3 (23) | 2 | 1 | |
| Age, mean ± SD (range) | 62 ± 11 (44–75) | 61 ± 11 (44–75) | 62 ± 10 (45–75) | 0.80 |
| Body mass index, mean ± SD | 29.0 ± 4.1 | 27.9 ± 4.8 | 30.2 ± 3.0 | 0.25 |
| Smoking history, n (%) | 5 (39) | 2 | 3 | 0.59 |
| Diabetes | 7 (54) | 3 | 4 | 0.59 |
| Plasma glucose, mg/dL | 127 ± 45 | 126 ± 41 | 128 ± 52 | 0.94 |
| Hypertension | 10 (77) | 4 | 6 | 0.19 |
| Hyperlipidemia | 11 (85) | 5 | 6 | 0.46 |
| Familial hypercholesterolemia, n (%) | 4 (31) | 2 | 2 | >0.99 |
| Number of vessels with significant CAD, mean ± SD | 2.1 ± 1.0 | 1.7 ± 1.1 | 2.5 ± 0.8 | 0.18 |
| Percutaneous transluminal coronary angioplasty, n (%) | 9 (69) | 5 | 4 | >0.99 |
| Coronary artery bypass graft, n (%) | 7 (54) | 3 | 4 | 0.59 |
| Medication use, n (%) | ||||
| Beta-blockers | 13 (100) | 7 | 6 | — |
| Statins | 10 (77) | 4 | 6 | 0.19 |
| Angiotensin-converting enzyme inhibitors | 9 (69) | 4 | 5 | 0.56 |
SD, standard deviation.
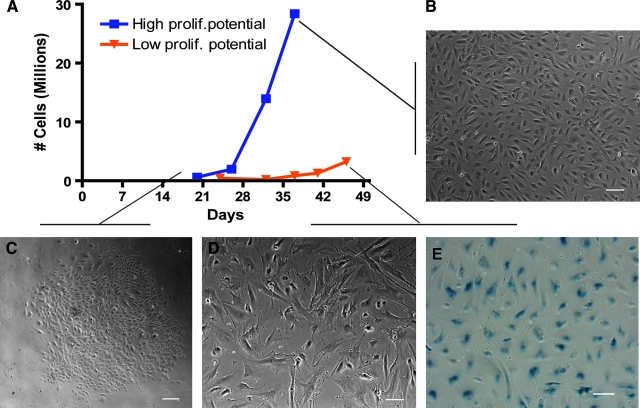
FIG. 1.
Representative growth and expansion of late-outgrowth endothelial progenitor cells (EPCs) isolated from peripheral blood of patients with coronary artery disease (CAD) and healthy donors. Cultures had high proliferation potential, low proliferation potential, or no colonies (A). Late-outgrowth EPC colonies appeared 2 to 3 weeks after plating peripheral blood mononuclear cells (C). Upon passaging, some cultures from patients with CAD and healthy donors showed exponential growth. Cells exhibited typical endothelial cell (EC) cobblestone morphology while growing to confluence and were defined as high proliferation potential (A, B). In four healthy donors, cultures were unable to be expanded to more than 107 cells, and the cultures were defined as low proliferation potential (A). Cells from low proliferation cultures had a larger area and more irregular shape (D). Low-proliferation cultures stained positive for beta-galactosidase, a marker of cell senescence (E). Scale bar 100 μm (B, D, E), 200 μm (C). Color images available online at www.liebertonline.com/ten.
Endothelial colonies grew in the original culture plates until 75% confluence and were passaged into T25 or T75 Flasks (Fig. 1C). Passaged cells that grew to a cell population of more than 107 cells over the course of three to six passages were defined as “highly proliferative” (Fig. 1A, B) (Table 3). All seven of the late-outgrowth EPC cultures successfully isolated from patients with CAD were highly proliferative. Five of 13 healthy donors yielded highly proliferative colonies (3 of 10 male donors and 2 of 3 from female donors), whereas the other four colonies isolated from healthy donors never attained more than 107 cells (Fig. 1A) (p = 0.10). These “limited proliferation” cultures displayed differing growth characteristics, with cells that became larger and more irregular and stained positively for β-galactosidase, a marker of senescence (Fig. 1D, E).32
Table 3.
Isolation Frequency of Late-Outgrowth Endothelial Progenitor Cells (EPCs)
| |
Young Healthy Donors |
Patients with Coronary Artery Disease |
|---|---|---|
| Late-Outgrowth EPC Isolation Frequency | n (%) | |
| Highly proliferative colonies (>107 EPCs) | 5 (38) | 7 (54) |
| Limited proliferation colonies (<107 EPCs) | 4 (30) | 0 (0) |
| No colonies (no EPCs) | 4 (30) | 6 (46) |
It took an average of 36 ± 5 days to reach 107 cells from donors with CAD, versus 40 ± 4 days for healthy donors (p = 0.64). There were no significant differences between donor groups in the initial number of MNCs counted (p = 0.54), nor were there significant differences between the number of MNCs counted and the ability to isolate late-outgrowth EPC colonies (high-proliferation, low -roliferation, or no colony cultures) (p = 0.69) (Table 4).
Table 4.
Number of Mononuclear Cells in Collected Peripheral Blood Samples
| |
Healthy Donors |
Patients with Coronary Artery Disease |
|---|---|---|
| Number of Mononuclear Cells (×106), Mean ± Standard Deviation (n) | ||
| All cultures | 98 ± 7 (13) | 107 ± 12 (13) |
| Highly proliferative colonies (>107 EPCs) | 113 ± 12 (5) | 105 ± 22 (7) |
| Limited proliferation colonies (<107 EPCs) | 83 ± 9 (4) | — (0) |
| No colonies (no EPCs) | 93 ± 12 (4) | 109 ± 9 (6) |
EPCs, endothelial progenitor cells.
Highly proliferative late-outgrowth EPC colonies from people with CAD and healthy subject groups were passaged four to six times and frozen. The doubling times of thawed late-outgrowth EPCs from patients with CAD and from healthy volunteers were 2.3 ± 0.7 days and 2.5 ± 0.6 days, respectively (p = 0.79). Doubling time for control HAECs was 1.2 ± 0.1 days (p = 0.15 versus outgrowth EPC from patients with CAD).
Culture phenotype
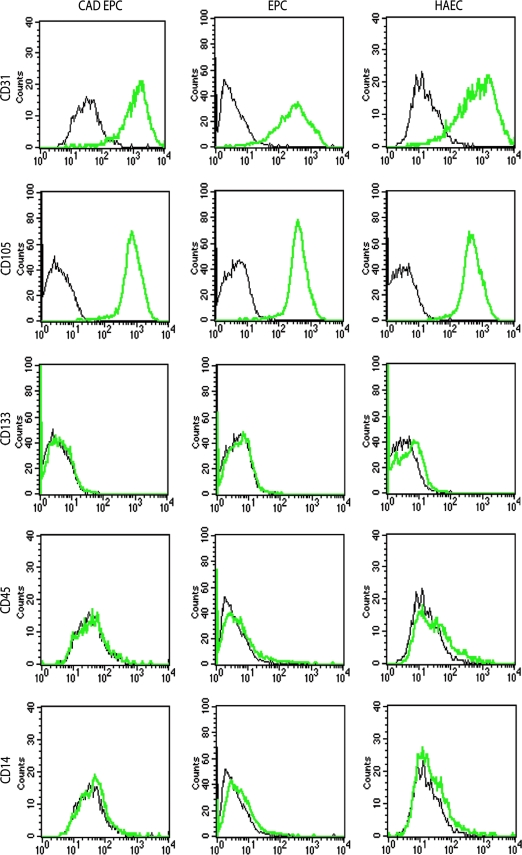
Flow cytometric studies were performed to assess the marker expression of expanded actively proliferating EPCs from several donors. Late-outgrowth EPCs derived from patients with CAD and healthy volunteers showed uniform expression of EC markers CD31 and CD105. Cells were negative for the stem cell marker CD133 and hematopoietic markers CD45 and CD14, indicating that the cultures were not contaminated with hematopoietic or monocytic cells (Fig. 2). HAEC histograms were similar to both EPC groups for all markers.
FIG. 2.
Immunotypic analysis of expanded late-outgrowth EPCs from patients with CAD (CAD EPCs), late-outgrowth EPCs from healthy donors (EPCs), and control human aortic ECs (HAECs) (black lines fluorescence signals of isotypic controls, bold green lines fluorescence signals of specific antigens). CAD EPCs, EPCs, and HAECs were positive for endothelial markers CD31 and CD105. CAD EPCs, EPCs, and HAECs showed no positive fraction for stem cell marker CD133 or hematopoietic markers CD45 and CD14. Shown are representative data from three independent experiments using different CAD EPCs and healthy EPCs with similar results. Color images available online at www.liebertonline.com/ten.
Cell attachment to Teflon-AF
Cell spreading
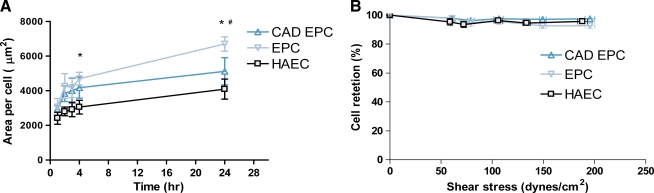
Teflon-AF was used as a surrogate for ePTFE vascular graft material.26 Spreading rates of late-outgrowth EPCs from patients with CAD were compared with those of cells isolated from healthy individuals on Teflon-AF surfaces that have the same hydrophobicity and nearly the same chemistry as ePTFE. Teflon-AF surfaces were incubated with 3.3 μg/mL fibronectin for 1 h to permit cell attachment. EPCs from healthy volunteers spread the fastest. Healthy EPCs were significantly larger in area than HAECs at 4 and 24 h and significantly larger than EPCs from CAD patients at 24 h (p < 0.05) (Fig. 3A).
FIG. 3.
Cell spreading and adhesion on fibronectin-coated Teflon-AF. Late-outgrowth EPCs from healthy donors spread at a higher rate than EPCs from patients with CAD and HAECs (A). EPCs from healthy donors had a significantly larger area at 4 and 24 h (* p < 0.05 EPC vs HAEC, # p < 0.05 EPC vs CAD EPC). ECs were seeded onto Teflon-AF coated with fibronectin for 20 min. All three cell types had greater than 95% adhesion after exposure up to 187 dyne/cm2 (B). Color images available online at www.liebertonline.com/ten.
Cell retention
The ability of late-outgrowth EPCs to remain adherent after exposure to fluid shear stress was tested by exposing the cells to short-term superphysiological flow. Late-outgrowth EPCs from patients with CAD, healthy donors, and HAECs adhered firmly to fibronectin-coated Teflon-AF after exposure to high shear stresses in a variable-height flow chamber. Both cell types showed greater than 95% retention after exposure to shear stress of 187 dyne/cm2 (Fig. 3B).
Integrin expression
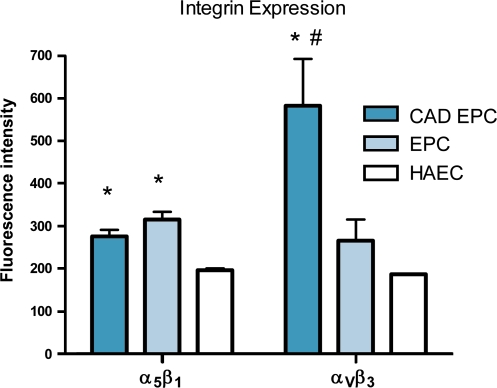
The primary integrins that mediate cell adhesion to extracellular matrix proteins such as fibronectin and collagen are αvβ3 and α5β1. Late-outgrowth EPCs from both donor groups expressed significantly higher levels of α5β1 than HAECs (p < 0.05). Late-outgrowth EPCs from patients with CAD expressed significantly higher levels of αvβ3 than cells from healthy donors and HAECs (p < 0.002) (Fig. 4).
FIG. 4.
Late-outgrowth EPCs from patients with CAD and healthy donors expressed significantly higher levels of α5β1 than HAECs. αvβ3 was expressed at significantly higher levels in CAD EPCs (* p < 0.05 vs HAECs, # p < 0.05 vs EPCs).
In vitro vasculogenesis assay
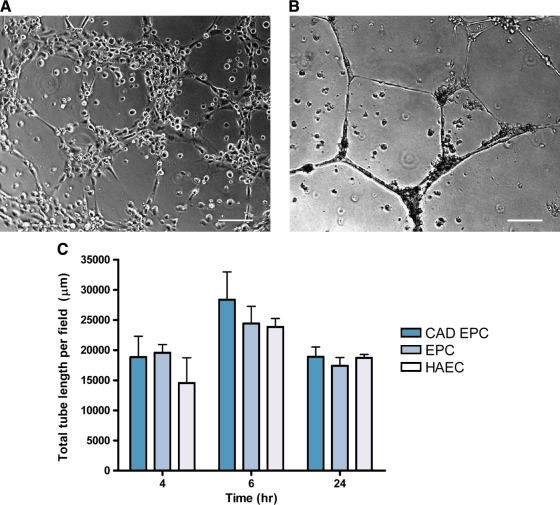
Late-outgrowth EPCs from patients with CAD and healthy individuals spontaneously formed capillaries when plated on three-dimensional Matrigel extracellular matrix. At 4 h, tube structures began to form (Fig. 5A). After 24 h, the cells coalesced and formed more-discrete tubes (Fig. 5B). Vessel length was significantly higher at 6 h than 4 or 24 h (p < 0.003), but no significant differences between CAD EPCs, EPCs, and HAECs were observed (p = 0.46), indicating that kinetics of the of the vasculogenesis properties of the cell types in Matrigel are similar (Fig. 5C).
FIG. 5.
In vitro vasculogenesis assay. Representative image of late-outgrowth EPCs from patients with CAD seeded onto Matrigel and viewed after 4 h using phase contrast microscopy (A). Tubes were more defined after 24 h (B). There were no significant differences in total vessel length versus cell type when measured at 4, 6, and 24 h (C). Scale bar 200 μm (A), 100 μm (B).
Effect of shear stress on cell morphology and NO expression
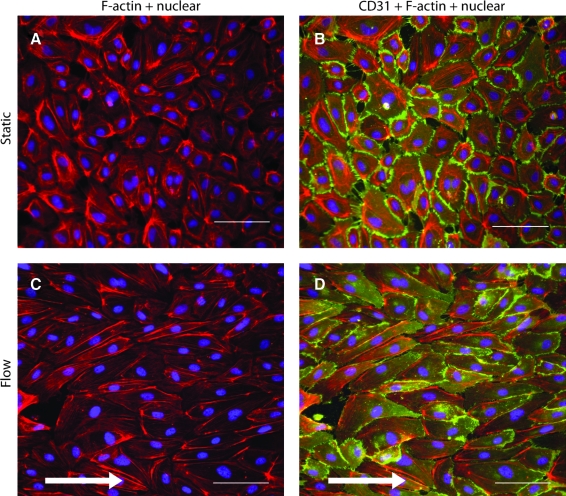
Late-outgrowth EPCs from both donor groups had typical EC cobblestone morphology under static conditions (Fig. 6A, B). CD31 staining was similar in both donor groups and was localized to the cell–cell borders. After exposure to 48-h laminar shear stress of 15 dyne/cm2, cells elongated, and f-actin oriented parallel to flow (Fig. 6C, D).
FIG. 6.
Effect of shear stress on cell morphology and nitric oxide (NO) expression. Representative immunofluorescent images of late-outgrowth EPCs from patients with CAD under static conditions stained for f-actin (A) and CD31 (B). Cultures were exposed to laminar shear stress (15 dyne/cm2) for 48 h. Late-outgrowth EPCs tended to elongate and align parallel to the direction of flow (C, D). Scale bar 100 μm. Color images available online at www.liebertonline.com/ten.
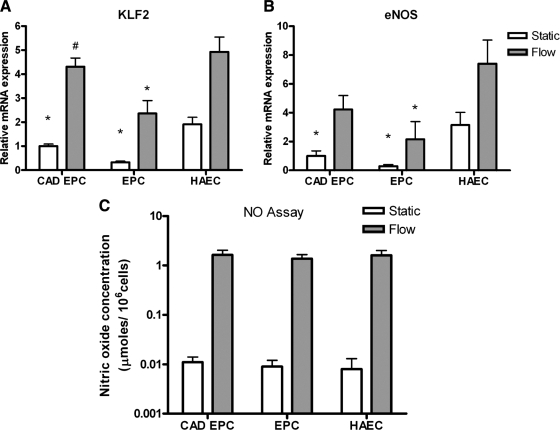
Late-outgrowth EPCs from healthy donors and donors with CAD cultured under static conditions expressed significantly lower levels of KLF2 and eNOS messenger RNA (mRNA) than HAECs (Fig. 7A, B) (p < 0.05). Upon exposure to laminar shear stress of 15 dyne/cm2 for 48 h, all cell groups increased mRNA levels for the flow-responsive transcription factor KLF2 and eNOS. EPCs from patients with CAD expressed significantly higher levels of KLF2 than late-outgrowth EPCs from healthy donors, although all cell types cultured under static conditions expressed similar low basal levels of NO products (Fig. 7C). Laminar shear stress of 15 dyne/cm2 for 48 h resulted in NO expression 150 times that under static conditions. No significant differences in NO production were observed between cell types (p = 0.88).
FIG. 7.
Effect of shear stress on flow responsive genes and nitric oxide expression. KLF2 and eNOS gene expression by patients with CAD and healthy donors were significantly lower than with HAECs (A, B). Upon exposure to 48 h of laminar shear, Kruppel-like factor-2 and endothelial NO synthase (eNOS) messenger RNA levels increased for all cell types, although expression was significantly lower for healthy donors than for HAECs (A, B) (* p < 0.05 vs HAECs, # p < 0.05 vs *EPCs). Late-outgrowth EPCs from patients with CAD, healthy individuals, and HAECs all increased their production of NO during exposure to flow (C). No significant differences were observed in NO release between the three cell groups.
Discussion
The lack of easily isolated autologous EC sources is one of the major challenges of vascular tissue engineering.2 The ability to isolate late-outgrowth EPCs noninvasively from peripheral blood and expand the colonies in long-term cultures is a major development for autologous cell therapies. Late-outgrowth EPCs have been pursued as an alternative cell source for endothelialization strategies.30,33–37 The 2- to 3-week time interval before EPC colonies are observed is a clinical drawback for emergency interventions, but the cells are highly proliferative, capable of undergoing more than 30 population doublings while maintaining a stable endothelial phenotype during long-term culture, justifying their use for elective procedures.9,30
If late-outgrowth EPCs are to be used as an autologous source of ECs for vascular conduits, it is likely that they will be procured from patients with extensive vascular disease. To our knowledge, the characteristics of late-outgrowth EPCs from these patients have not been fully characterized.
In the present study, we compared the ability to isolate late-outgrowth EPCs from peripheral blood donated by older patients with CAD and younger healthy volunteers. EPC isolation and expansion, EC marker expression, cell contamination, cell adhesion, and NO expression provided an essential set of characteristics needed to demonstrate the suitability of late-outgrowth EPCs from patients with CAD for autologous endothelialization applications.
The frequency that late-outgrowth EPC colonies were observed was similar for patients with CAD and young healthy donors, although no colonies with limited proliferation potential were recovered from patients with CAD. Although the observation was initially surprising, it was not at odds with recent reports finding that the number of late-outgrowth EPCs isolated in culture increases with the extent of patient coronary artery stenosis.38 Another study quantifying late-outgrowth EPCs from patients with age-related macular degeneration showed that only 11% of the young control subjects had cultures of more than 107 cells, whereas 38% of patients with neovascular age-related macular degeneration had highly proliferative colonies.39
Cell contamination is an important concern in EC harvesting, because cultures can be contaminated with fibroblasts and myofibroblasts that are capable of outgrowing EC cultures. Moreover, endothelialization methods using heterogeneous cell sources increased the extent of neointimal hyperplasia.4 Highly proliferative late-outgrowth EPC cultures from both donor types appeared homogeneous, with flow cytometry profiles closely resembling those of HAECs that were used as a positive EC control. Late-outgrowth EPCs from both groups were mature and fully differentiated, displaying antigenic characteristics of ECs, but negative for stem and hematopoietic markers. Additionally, late-outgrowth EPCs grown under static conditions had typical cobblestone morphology but were sensitive to shear stress, reorienting parallel to the direction of flow upon application of laminar flow at 15 dyne/cm2 for 48 h.
Because one of the causes of failure of endothelialized vascular grafts is cell detachment upon exposure to blood flow,40,41 seeded ECs must rapidly adhere, spread, and withstand physiological stresses. Previously reported low trypsin conditions during subculture were used to maximize the extent of cell adhesion to fibronectin-coated Teflon-AF.27 In the current study, late-outgrowth EPCs from patients with CAD and healthy donors rapidly attached to the substrate and assumed a larger area than HAECs. All EPCs maintained firm adhesion, with more than 95% of seeded cells remaining adherent after exposure to superphysiological shear stresses as high as 187 dyne/cm2. Although only the highly proliferative EPC cultures were used for all experiments, poorer proliferation potential could cause the greater cell area of late-outgrowth EPCs from healthy individuals than of late-outgrowth EPCs from patients with CAD and HAECs, as seen in other studies in which cell area increased with passage number and cell senescence.42
Initial adhesion strength depends on the number of α5β1 and αVβ3 interactions with the material.27 Late-outgrowth EPCs from patients with CAD and healthy individuals expressed higher levels of α5β1 and αVβ3 than HAECs, helping to explain the high levels of attachment to Teflon-AF. Late-outgrowth EPCs from patients with CAD had greater expression of αVβ3 than HAECs and EPCs from healthy individuals. These integrins are important not only for cell-biomaterial adhesion, but also fo adhesion to denuded arteries43 and angiogenesis,44 and thus the high levels of α5β1 and αVβ3 present may aid late-outgrowth EPC homing and vessel formation.
We speculated that, with greater expression of αVβ3 in late-outgrowth EPCs from patients with CAD, there may be differences in vessel forming ability between cell types. Capillary formation is not only important for revascularization of ischemic tissue, but also may improve performance of porous materials.45 The presence of CAD showed no effect on cell vessel forming ability, with all cell types able to form vessels spontaneously at comparable densities during in vitro vasculogenesis assays. Recent studies have shown that, with late-outgrowth EPCs, vessel formation decreases after cells are exposed to oxidative stress, although it remains to be seen whether there are differences from the patient disease state.46 A more-selective vessel-forming assay involving co-culture with fibroblasts may be more appropriate to evaluate the differences between cell types.47
Vascular ECs are known to release NO when challenged with fluid shear stress. Reduction in NO production is believed to lead to early development of atherosclerosis.48 The level of KLF2, a key transcription factor that regulates key factors in maintaining an antithrombotic EC surface, was significantly lower in late-outgrowth EPCs than HAECs. EPCs exposed to 48 h of shear increased their transcription of KLF2 and eNOS, in agreement with studies showing that KLF2 acts as a regulator of eNOS and can be induced by biomechanical stimulation.49,50 Late-outgrowth EPCs from patients with CAD and healthy individuals cultured under static culture conditions expressed low basal levels of NO end products, whereas late-outgrowth EPCs exposed to laminar shear stress over 48 h expressed approximately 150 times as much NO as under static conditions. No significant differences were observed between late-outgrowth EPCs from patients with CAD and healthy donors, indicating that EPCs from both groups are responsive to shear stress and have similar NO secretion rates in vitro. Levels of NO secreted by both donor groups were similar to those of HAECs, as found in recent studies demonstrating no differences in NO release between porcine late-outgrowth EPCs and porcine aortic ECs grown under static conditions.33
A drawback of using autologous late-outgrowth EPCs for endothelialization applications is their rare presence in peripheral blood MNC populations. Yoder et al. estimated the frequency of late-outgrowth EPCs in healthy volunteers as 0.017 colonies per 106 plated MNCs.14 With an average of 103 ± 7 × 106 MNCs per subject observed in our study, we would estimate that 1.75 endothelial colonies per 50-mL peripheral blood sample would be recovered. Others have similarly estimated 2.5 late-outgrowth EPC colonies per 50 mL of blood.38 Simply doubling the volume of blood collected should also increase the frequency of isolation. Alternatively, preselecting CD34+ CD45- MNCs before plating can enrich cell populations of late-outgrowth EPCs as much as 300 times over nonsorted cord blood cultures.51 Methods to specifically capture late-outgrowth EPCs using novel peptide ligands are also under development.52
The goal of the current study was to assess the feasibility of isolating therapeutic late-outgrowth EPCs from patients with known CAD, as indicated using angiography. Not surprisingly, this patient population differed in a range of clinical characteristics from our healthy donor population. The majority of the patients were also taking a range of medications at the time of catheterization. As such, we feel that this study reflects the pool of patients with CAD that would probably be candidates for autologous endothelialization therapies.
Because the sample was small and random, we were unable to systematically determine the effect of clinical factors or medication use on the isolation and characterization of late-outgrowth EPCs. Having said this, the effect of patient-specific factors merits consideration. It has previously been shown that acute myocardial infarction53,54 can cause mobilization of late-outgrowth EPCs and that statin treatment can maintain high levels of late-outgrowth EPCs within the vasculature.55 Patient age may also affect ability to isolate late-outgrowth EPCs.56 Our population with CAD included only patients with stable CAD; therefore, we do not believe that this represents a population in which EPCs were mobilized by acute injury. It may be of interest in future work to assess the effect of acute or chronic statin therapy before isolation of EPCs. Our data show that we were able to isolate functional late-outgrowth EPCs from patients with CAD taking and not taking statin therapy.
Finally, mononuclear cells derived from the bone marrow are multipotent, with the ability to generate vascular or muscular cells.57 As such, culture conditions strongly influence their phenotypic destination.58,59 Also, even mature ECs are known to change their surface marker expression during culture.60 Therefore, subjecting late-outgrowth EPCs from patients with CAD and healthy donors to the same long-term culture conditions may have influenced the cells acquiring the same EC phenotypic characteristics. Practically speaking, this concern is less important than the ability to noninvasively obtain healthy cells suitable for autologous endothelialization therapy from the peripheral blood of patients with significant CAD.
Conclusion
Late-outgrowth EPCs isolated from patients with CAD are a potentially clinically relevant cell source for autologous endothelialization applications. The isolation of EPCs from peripheral blood is less invasive than harvesting ECs from excised blood vessels and adipose tissue or late-outgrowth EPCs isolated from bone marrow. Late-outgrowth EPCs were capable of attaching, spreading, and maintaining firm adhesion to the underlying substrate and showed a functional response to flow by reorienting and increasing expression of NO. Once colonies were established and expanded from small volumes of peripheral blood, few differences were observed between late-outgrowth EPCs isolated from patients with CAD and those isolated from healthy individuals.
Acknowledgments
The authors would like to thank Beza Abebe and Enping Hong for helping with the vasculogenesis and flow experiments and Dr. Kam Leong for use of the lyophilizer. This work was supported by National Institutes of Health (NIH) Grant R01HL-44972 and a NIH biotechnology training grant (GM8555) fellowship to J.D.S.
Disclosure Statement
No competing financial interests exist.
References
- 1.Zilla P. Deutsch M. Meinhart J. Endothelial cell transplantation. Semin Vasc Surg. 1999;12:52. [PubMed] [Google Scholar]
- 2.Tiwari A. Salacinski H.J. Hamilton G. Seifalian A.M. Tissue engineering of vascular bypass grafts: role of endothelial cell extraction. Eur J Vasc Endovasc Surg. 2001;21:193. doi: 10.1053/ejvs.2001.1316. [DOI] [PubMed] [Google Scholar]
- 3.Jarrell B.E. Williams S.K. Stokes G. Hubbard F.A. Carabasi R.A. Koolpe E. Greener D. Pratt K. Moritz M.J. Radomski J. Speicher L. Use of freshly isolated capillary endothelial cells for the immediate establishment of a monolayer on a vascular graft at surgery. Surgery. 1986;100:392. [PubMed] [Google Scholar]
- 4.Arts C.H. Hedeman Joosten P.P. Blankensteijn J.D. Staal F.J. Ng P.Y. Heijnen-Snyder G.J. Sixma J.J. Verhagen H.J. de Groot P.G. Eikelboom B.C. Contaminants from the transplant contribute to intimal hyperplasia associated with microvascular endothelial cell seeding. Eur J Vasc Endovasc Surg. 2002;23:29. doi: 10.1053/ejvs.2001.1532. [DOI] [PubMed] [Google Scholar]
- 5.Asahara T. Murohara T. Sullivan A. Silver M. van der Zee R. Li T. Witzenbichler B. Schatteman G. Isner J.M. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 6.Lin Y. Weisdorf D.J. Solovey A. Hebbel R.P. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gulati R. Jevremovic D. Peterson T.E. Chatterjee S. Shah V. Vile R.G. Simari R.D. Diverse origin and function of cells with endothelial phenotype obtained from adult human blood. Circ Res. 2003;93:1023. doi: 10.1161/01.RES.0000105569.77539.21. [DOI] [PubMed] [Google Scholar]
- 8.Hur J. Yoon C.H. Kim H.S. Choi J.H. Kang H.J. Hwang K.K. Oh B.H. Lee M.M. Park Y.B. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24:288. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- 9.Ingram D.A. Mead L.E. Tanaka H. Meade V. Fenoglio A. Mortell K. Pollok K. Ferkowicz M.J. Gilley D. Yoder M.C. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 10.Shi Q. Rafii S. Wu M.H-D. Wijelath E.S. Yu C. Ishida A. Fujita Y. Kothari S. Mohle R. Sauvage L.R. Moore M.A.S. Storb R.F. Hammond W.P. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92:362. [PubMed] [Google Scholar]
- 11.Sieveking D.P. Buckle A. Celermajer D.S. Ng M.K.C. Strikingly different angiogenic properties of endothelial progenitor cell subpopulations: insights from a novel human angiogenesis assay. J Am Coll Cardiol. 2008;51:660. doi: 10.1016/j.jacc.2007.09.059. [DOI] [PubMed] [Google Scholar]
- 12.Urbich C. Aicher A. Heeschen C. Dernbach E. Hofmann W.K. Zeiher A.M. Dimmeler S. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Molec Cell Cardiol. 2005;39:733. doi: 10.1016/j.yjmcc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Rehman J. Li J. Orschell C.M. March K.L. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 14.Yoder M.C. Mead L.E. Prater D. Krier T.R. Mroueh K.N. Li F. Krasich R. Temm C.J. Prchal J.T. Ingram D.A. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Timmermans F. Van Hauwermeiren F. De Smedt M. Raedt R. Plasschaert F. De Buyzere M.L. Gillebert T.C. Plum J. Vandekerckhove B. Endothelial outgrowth cells are not derived from CD133+ cells or CD45+ hematopoietic precursors. Arterioscler Thromb Vasc Biol. 2007;27:1572. doi: 10.1161/ATVBAHA.107.144972. [DOI] [PubMed] [Google Scholar]
- 16.Gulati R. Jevremovic D. Witt T.A. Kleppe L.S. Vile R.G. Lerman A. Simari R.D. Modulation of the vascular response to injury by autologous blood-derived outgrowth endothelial cells. Am J Physiol Heart Circ Physiol 2004. 2004;287:H512. doi: 10.1152/ajpheart.00063.2004. [DOI] [PubMed] [Google Scholar]
- 17.Timmermans F. Plum J. Yoder M. Ingram D. Vandekerckhove B. Case J. Endothelial progenitor cells: identity defined? J Cell Molec Med. 2009;13:87. doi: 10.1111/j.1582-4934.2008.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ingram D.A. Mead L.E. Moore D.B. Woodard W. Fenoglio A. Yoder M.C. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood. 2005;105:2783. doi: 10.1182/blood-2004-08-3057. [DOI] [PubMed] [Google Scholar]
- 19.Scheubel R.J. Zorn H. Silber R.-E. Kuss O. Morawietz H. Holtz J. Simm A. Age-dependent depression in circulating endothelial progenitor cells inpatients undergoing coronary artery bypass grafting. J Am Coll Cardioly. 2003;42:2073. doi: 10.1016/j.jacc.2003.07.025. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt-Lucke C. Rossig L. Fichtlscherer S. Vasa M. Britten M. Kamper U. Dimmeler S. Zeiher A.M. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111:2981. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- 21.Werner N. Kosiol S. Schiegl T. Ahlers P. Walenta K. Link A. Bohm M. Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 22.Massa M. Rosti V. Ferrario M. Campanelli R. Ramajoli I. Rosso R. De Ferrari G.M. Ferlini M. Goffredo L. Bertoletti A. Klersy C. Pecci A. Moratti R. Tavazzi L. Increased circulating hematopoietic and endothelial progenitor cells in the early phase of acute myocardial infarction. Blood. 2005;105:199. doi: 10.1182/blood-2004-05-1831. [DOI] [PubMed] [Google Scholar]
- 23.Lambiase P.D. Edwards R.J. Anthopoulos P. Rahman S. Meng Y.G. Bucknall C.A. Redwood S.R. Pearson J.D. Marber M.S. Circulating humoral factors and endothelial progenitor cells in patients with differing coronary collateral support. Circulation. 2004;109:2986. doi: 10.1161/01.CIR.0000130639.97284.EC. [DOI] [PubMed] [Google Scholar]
- 24.Hirschi K.K. Ingram D.A. Yoder M.C. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28:1584. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Broxmeyer H.E. Srour E. Orschell C. Ingram D.A. Cooper S. Plett P.A. Mead L.E. Yoder M.C. Cord Blood Stem and Progenitor Cells. Methods Enzymol. 419:439–73. doi: 10.1016/S0076-6879(06)19018-7. [DOI] [PubMed] [Google Scholar]
- 26.Anamelechi C.C. Truskey G.A. Reichert W.M. Mylar(TM) and Teflon-AF(TM) as cell culture substrates for studying endothelial cell adhesion. Biomaterials. 2005;26:6887. doi: 10.1016/j.biomaterials.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 27.Brown M.A. Wallace C.S. Anamelechi C.C. Clermont E. Reichert W.M. Truskey G.A. The use of mild trypsinization conditions in the detachment of endothelial cells to promote subsequent endothelialization on synthetic surfaces. Biomaterials. 2007;28:3928. doi: 10.1016/j.biomaterials.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Truskey G.A. Proulx T.L. Relationship between 3T3 cell spreading and the strength of adhesion on glass and silane surfaces. Biomaterials. 1993;14:243. doi: 10.1016/0142-9612(93)90114-h. [DOI] [PubMed] [Google Scholar]
- 29.Lusska A. Wu L. Whitlock J.P., Jr. Superinduction of CYP1A1 transcription by cycloheximide. Role of the DNA binding site for the liganded Ah receptor. J Biol Chem. 1992;267:15146. [PubMed] [Google Scholar]
- 30.Fuchs S. Hermanns M.I. Kirkpatrick C.J. Retention of a differentiated endothelial phenotype by outgrowth endothelial cells isolated from human peripheral blood and expanded in long-term cultures. Cell Tissue Res. 2006;326:79. doi: 10.1007/s00441-006-0222-4. [DOI] [PubMed] [Google Scholar]
- 31.Rozen S. Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, editor; Misener S, editor. Bioinformatics Methods and Protocols: Methods in Molecular Biology. Totowa, NJ: Humana Press; 2000. pp. 365–386. [DOI] [PubMed] [Google Scholar]
- 32.van der Loo B. Fenton M.J. Erusalimsky J.D. cytochemical detection of a senescence-associated [beta]-galactosidase in endothelial and smooth muscle cells from human and rabbit blood vessels. Exp Cell Res. 1998;241:309. doi: 10.1006/excr.1998.4035. [DOI] [PubMed] [Google Scholar]
- 33.Allen J. Khan S. Serrano M. Ameer G. Characterization of porcine circulating progenitor cells: toward a functional endothelium. Tissue Eng Part A. 2008;14:183. doi: 10.1089/ten.a.2007.0265. [DOI] [PubMed] [Google Scholar]
- 34.Griese D.P. Ehsan A. Melo L.G. Kong D. Zhang L. Mann M.J. Pratt R.E. Mulligan R.C. Dzau V.J. Isolation and transplantation of autologous circulating endothelial cells into denuded vessels and prosthetic grafts: implications for cell-based vascular therapy. Circulation. 2003;108:2710. doi: 10.1161/01.CIR.0000096490.16596.A6. [DOI] [PubMed] [Google Scholar]
- 35.Kaushal S. Amiel G.E. Guleserian K.J. Shapira O.M. Perry T. Sutherland F.W. Rabkin E. Moran A.M. Schoen F.J. Atala A. Soker S. Bischoff J. Mayer J.E. Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat Med. 2001;7:1035. doi: 10.1038/nm0901-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shirota T. He H. Yasui H. Matsuda T. Human endothelial progenitor cell-seeded hybrid graft: proliferative and antithrombogenic potentials in vitro and fabrication processing. Tissue Eng. 2003;9:127. doi: 10.1089/107632703762687609. [DOI] [PubMed] [Google Scholar]
- 37.Shirota T. Yasui H. Matsuda T. Intralumenal tissue-engineered therapeutic stent using endothelial progenitor cell-inoculated hybrid tissue and in vitro performance. Tissue Eng. 2003;9:473. doi: 10.1089/107632703322066651. [DOI] [PubMed] [Google Scholar]
- 38.Guven H. Shepherd R.M. Bach R.G. Capoccia B.J. Link D.C. The number of endothelial progenitor cell colonies in the blood is increased in patients with angiographically significant coronary artery disease. J Am Coll Cardiol. 2006;48:1579. doi: 10.1016/j.jacc.2006.04.101. [DOI] [PubMed] [Google Scholar]
- 39.Thill M. Strunnikova N.V. Berna M.J. Gordiyenko N. Schmid K. Cousins S.W. Thompson D.J.S. Csaky K.G. Late outgrowth endothelial progenitor cells in patients with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008;49:2696. doi: 10.1167/iovs.07-0955. [DOI] [PubMed] [Google Scholar]
- 40.Rosenman J.E. Kempczinski R.F. Pearce W.H. Silberstein E.B. Kinetics of endothelial cell seeding. J Vasc Surg. 1985;2:778. [PubMed] [Google Scholar]
- 41.Magometschnigg H. Kadletz M. Vodrazka M. Grabenwöger M. Moritz A. Grimm M. Böck P. Leukauf C. Trubel W. Wolner E. Changes following in vitro endothelial cell lining of ePTFE prostheses: late morphologic evaluation of six failed grafts. Eur J Vasc Surg. 1994;8:502. doi: 10.1016/s0950-821x(05)80972-7. [DOI] [PubMed] [Google Scholar]
- 42.Melero-Martin J.M. Khan Z.A. Picard A. Wu X. Paruchuri S. Bischoff J. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood. 2007;109:4761. doi: 10.1182/blood-2006-12-062471. [DOI] [PubMed] [Google Scholar]
- 43.Walter D.H. Rittig K. Bahlmann F.H. Kirchmair R. Silver M. Murayama T. Nishimura H. Losordo D.W. Asahara T. Isner J.M. Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation. 2002;105:3017. doi: 10.1161/01.cir.0000018166.84319.55. [DOI] [PubMed] [Google Scholar]
- 44.Brooks P.C. Clark R.A. Cheresh D.A. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264:569. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 45.Davids L. Dower T. Zilla P. The lack of healing in conventional vascular grafts. In: Zilla P, editor; Greisler H, editor. Tissue Engineering of Prosthetic Vascular Grafts. Austin, TX: RG Landes Company; 1999. pp. 3–44. [Google Scholar]
- 46.Ingram D.A. Krier T.R. Mead L,E. McGuire C. Prater D.N. Bhavsar J. Saadatzadeh M.R. Bijangi-Vishehsaraei K. Li F. Yoder M.C. Haneline L.S. Clonogenic endothelial progenitor cells are sensitive to oxidative stress. Stem Cells. 2007;25:297. doi: 10.1634/stemcells.2006-0340. [DOI] [PubMed] [Google Scholar]
- 47.Sieveking D.P. Buckle A. Celermajer D.S. Ng M.K. Strikingly different angiogenic properties of endothelial progenitor cell subpopulations: insights from a novel human angiogenesis assay. J Am Coll Cardiol. 2008;51:660. doi: 10.1016/j.jacc.2007.09.059. [DOI] [PubMed] [Google Scholar]
- 48.Naseem K.M. The role of nitric oxide in cardiovascular diseases. Mol Aspects Med. 2005;26:33. doi: 10.1016/j.mam.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 49.Dekker R.J. Boon R.A. Rondaij M.G. Kragt A. Volger O.L. Elderkamp Y.W. Meijers J.C.M. Voorberg J. Pannekoek H. Horrevoets A.J.G. KLF2 provokes a gene expression pattern that establishes functional quiescent differentiation of the endothelium. Blood. 2006;107:4354. doi: 10.1182/blood-2005-08-3465. [DOI] [PubMed] [Google Scholar]
- 50.Lin Z. Kumar A. SenBanerjee S. Staniszewski K. Parmar K. Vaughan D.E. Gimbrone M.A., Jr. Balasubramanian V. Garcia-Cardena G. Jain M.K. Kruppel-like factor 2 (KLF2) regulates endothelial thrombotic function. Circ Res 2005. 2005;96:e48. doi: 10.1161/01.RES.0000159707.05637.a1. [DOI] [PubMed] [Google Scholar]
- 51.Case J. Mead L.E. Bessler W.K. Prater D. White H.A. Saadatzadeh M.R. Bhavsar J.R. Yoder M.C. Haneline L.S. Ingram D.A. Human CD34+ AC133+ VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp Hematol. 2007;35:1109. doi: 10.1016/j.exphem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 52.Veleva A. Cooper S. Patterson C. Selection and initial characterization of novel peptide ligands that bind specifically to human blood outgrowth endothelial cells. Biotechnol Bioeng. 2007;98:306. doi: 10.1002/bit.21420. [DOI] [PubMed] [Google Scholar]
- 53.Massa M. Campanelli R. Bonetti E. Ferrario M. Marinoni B. Rosti V. Rapid and large increase of the frequency of circulating endothelial colony-forming cells (ECFCs) generating late outgrowth endothelial cells in patients with acute myocardial infarction. Exp Hematol. 2009;37:8. doi: 10.1016/j.exphem.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 54.Huang L. Hou D. Thompson M.A. Baysden S.E. Shelley W.C. Ingram D.A. March K.L. Yoder M.C. Acute myocardial infarction in swine rapidly and selectively releases highly proliferative endothelial colony forming cells (ECFCs) into circulation. Cell Transplant. 2007;16:887. doi: 10.3727/096368907783338181. [DOI] [PubMed] [Google Scholar]
- 55.Deschaseaux F. Selmani Z. Falcoz P-E. Mersin N. Meneveau N. Penfornis A. Kleinclauss C. Chocron S. Etievent J-P. Tiberghien P. Kantelip J-P. Davani S. Two types of circulating endothelial progenitor cells in patients receiving long term therapy by HMG-CoA reductase inhibitors. Eur J Pharmacol 2007. 2007;562:111. doi: 10.1016/j.ejphar.2007.01.045. [DOI] [PubMed] [Google Scholar]
- 56.Xing Z. Ryan M.A. Daria D. Nattamai K.J. Van Zant G. Wang L. Zheng Y. Geiger H. Increased hematopoietic stem cell mobilization in aged mice. Blood. 2006;108:2190. doi: 10.1182/blood-2005-12-010272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aranguren X.L. Verfaillie C.M. Luttun A. Emerging hurdles in stem cell therapy for peripheral vascular disease. J Mol Med. 2009;87:3. doi: 10.1007/s00109-008-0394-3. [DOI] [PubMed] [Google Scholar]
- 58.Reyes M. Dudek A. Jahagirdar B. Koodie L. Marker P.H. Verfaillie C.M. Origin of endothelial progenitors in human postnatal bone marrow. J Clin Invest. 2002;109:337. doi: 10.1172/JCI14327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ross J.J. Hong Z. Willenbring B. Zeng L. Isenberg B. Lee E.H. Reyes M. Keirstead S.A. Weir E.K. Tranquillo R.T. Verfaillie C.M. Cytokine-induced differentiation of multipotent adult progenitor cells into functional smooth muscle cells. J Clin Invest. 2006;116:3139. doi: 10.1172/JCI28184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Amatschek S. Kriehuber E. Bauer W. Reininger B. Meraner P. Wolpl A. Schweifer N. Haslinger C. Stingl G. Maurer D. Blood and lymphatic endothelial cell-specific differentiation programs are stringently controlled by the tissue environment. Blood. 2007;109:4777. doi: 10.1182/blood-2006-10-053280. [DOI] [PubMed] [Google Scholar]