Abstract
The use of pluripotent human embryonic stem (hES) cells for tissue engineering may provide advantages over traditional sources of progenitor cells because of their ability to give rise to multiple cell types and their unlimited expansion potential. We derived cell populations with properties of ectodermal and mesenchymal cells in two-dimensional culture and incorporated these divergent cell populations into three-dimensional (3D) epithelial tissues. When grown in specific media and substrate conditions, two-dimensional cultures were enriched in cells (EDK1) with mesenchymal morphology and surface markers. Cells with a distinct epithelial morphology (HDE1) that expressed cytokeratin 12 and β-catenin at cell junctions became the predominant cell type when EDK1 were grown on surfaces enriched in keratinocyte-derived extracellular matrix proteins. When these cells were incorporated into the stromal and epithelial tissue compartments of 3D tissues, they generated multilayer epithelia similar to those generated with foreskin-derived epithelium and fibroblasts. Three-dimensional tissues demonstrated stromal cells with morphologic features of mature fibroblasts, type IV collagen deposition in the basement membrane, and a stratified epithelium that expressed cytokeratin 12. By deriving two distinct cell lineages from a common hES cell source to fabricate complex tissues, it is possible to explore environmental cues that will direct hES-derived cells toward optimal tissue form and function.
Introduction
Ex vivo regeneration of tissues that mimic the form and function of their in vivo counterparts holds great promise for new therapeutic strategies to replace damaged or diseased tissues. Human embryonic stem (hES) cells can provide significant advantages over adult stem cells (ASCs) as an unlimited, pluripotent source of the multiple cell types needed to fabricate such complex tissues.1,2 In addition, the isolation and enrichment of progenitor cells for tissue engineering has been limited by the lack of well-defined phenotypic markers.3 Direct differentiation of hES cells now provides a novel source of pluripotent progenitor cells that may help overcome these obstacles in engineering a variety of tissues.
hES cells were initially isolated from the inner cell mass of preimplantation blastocysts generated by in vitro fertilization.4 It has been shown that these cells can subsequently undergo directed differentiation to specified lineage fates that will develop into a variety of cell types by altering culture substrate and growth conditions. A common method for such directed differentiation involves the use of embryoid bodies (EBs) that resemble the structure of embryos during their development. This has successfully generated multiple cell lineages, including mesenchymal stem cells (MSCs),5 yet these populations often need to be sorted to obtain homogeneous cultures. More recently, the use of a more uniform growth environment that includes substrates like Matrigel,6 gelatin,7 and feeder layers8 have resulted in the efficient, directed differentiation of hES cells to derive a number of mature cell types from all embryonic layers. Specifically, this approach has been used for the directed differentiation of hES cells toward ectodermal lineages including neural lineage derivation.9 In addition, recent studies have shown the derivation of definitive ectoderm, including keratinocytes10 and corneal epithelial cells.11 However, cells derived in this way have demonstrated limited expansion potential, highlighting the need to develop new differentiation techniques that more closely mimic the microenvironment found during normal development that enables derivation of more pure populations of proliferative progenitor cells. This will be essential to optimize the hES-derived cells for tissue engineering applications in the future.
hES cells are capable of organizing into multicellular structures during their in vitro differentiation within EBs while retaining their growth potential.12 Further, growth of hES cells in 3D scaffolds has resulted in the generation of organ-like structures that resembled their in vivo counterparts.13 The aggregate effect of appropriate microenvironmental factors, such as 3D scaffolds and growth factors, has been found to support lineage commitment and differentiation of hES cells.14,15 In transplantation studies, hES cells are capable of differentiating within scaffolds into a variety of cell types that demonstrate features of normal tissues, although they also formed teratomas that limit their use for transplantation.16 In this light, properly harnessing the potential of hES cells to develop into functional tissues in an engineered 3D tissue environment would provide an important means of generating complex tissues for transplantation, to study developmental biology, and to develop platforms for drug discovery and screening.3
hES cells provide an opportunity to derive both ectodermal and mesenchymal progenitor cells from a common pluripotent source and to combine these two cell types into distinct, yet interactive tissue compartments. However, current regenerative technologies have yet to show that multiple cell types, whose crosstalk is critical for normal tissue formation and organogenesis, can be derived from a single source of pluripotent cells. This approach has great potential for streamlining progenitor cell acquisition and tissue fabrication.
We have developed a strategy to derive multiple cell lineages needed for fabrication of stratified epithelial tissues from a common hES cell source (H9). Successful isolation of these hES-derived progenitor cells was accomplished by varying extracellular matrix (ECM) substrate and medium conditions that resulted in enrichment of divergent cell populations with morphologic features and protein expression characteristic of distinct ectodermal and mesenchymal cell types. hES-derived ectodermal cells expressed cytokeratins and β-catenin, while mesenchymal-like cells expressed surface markers characteristic of MSCs and mature fibroblasts. When these mesenchymal and ectodermal cells were incorporated into stromal and epithelial tissue compartments, respectively, and grown as 3D tissue constructs at an air–liquid interface, they generated stratified epithelia with features similar to tissues generated with foreskin-derived epithelium and fibroblasts. hES cell–derived tissues demonstrated deposition of the basement membrane (BM) component type IV collagen at the epithelial–stromal interface, and stromal cells showed features of mature fibroblasts. By deriving two cell lineages from the same hES cell source, it is now possible to further explore the environmental cues in 3D tissues that are needed to sustain the growth and maturation of mesenchymal and ectodermal lineages that will support tissue assembly and organization.
Materials and Methods
Cell culture
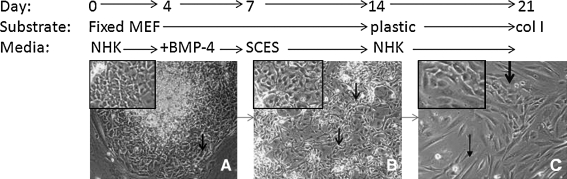
Conditions for directed differentiation of H9-hES cells are shown schematically in Figure 1. hES cell line H9 were maintained in culture as described,4 on irradiated feeder layers of mouse embryonic fibroblasts (MEFs). For directed differentiation, hES cells were first passaged at a ratio of 1:6 onto MEFs that were fixed in 4% formaldehyde and cultured in normal human keratinocyte (NHK) medium containing 3:1 Dulbecco's modified Eagle's medium (DMEM):F12 (Invitrogen, Carlsbad, CA), 5% FCII (Hyclone, Logan, UT), 0.18 mM adenine, 8 mM HEPES, 0.5 μg/mL hydrocortisone, 10−10 M cholera toxin, 10 ng/mL epidermal growth factor, and 5 μg/mL insulin (NHK medium). At day 4, 0.5 nM human bone morphogenetic protein (BMP)-4 (R&D Systems, Minneapolis, MN) was added for 3 days. At day 7, cells were passaged 1:3 onto a fresh fixed MEF feeder layer and cultured in 1:1 DMEM:F12 (Invitrogen), 5% FCII (Hyclone), and 1% non-essential amino acids (NEAA) (serum-containing ES medium) and grown for 7 days. At day 14, cells were passaged 1:3 onto plastic and grown in NHK medium. Cells were expanded on day 21 using type I collagen–coated plates (BD Biosciences, San Jose, CA). EDK1 cells were trypsinized and plated onto type I collagen–coated plates for 20 passages.
FIG. 1.
Stages of development of ES-derived progenitor cells (EDK1). H9 human embryonic stem cells were seeded onto fixed mouse embryonic fibroblasts and grown in the normal human keratinocyte (NHK) medium for 1 week while supplemented with 0.5 nM bone morphogenetic protein-4 from days 4 to 7. Appearance of cells on day 1 indicated morphologically distinct cells on the periphery of ES colonies (A, arrow). On day 12, cells had lost their tightly aggregated appearance in focal areas (B, arrows). After 2 weeks, cells passaged onto tissue culture plastic generated cell populations that showed features of two distinct morphologic cell types, epithelial-like cells (C, thick arrow and inset) and elongated spindle-shaped cells (C, thin arrow). On day 21 cells were further expanded on type I collagen–coated plates.
Real-time reverse transcriptase–polymerase chain reaction
RNA was extracted using the Qiagen RNeasy kit. The cDNA was transcribed with 1 μg RNA using the Quantiscript Reverse Transcriptase (Qiagen, Valencia, CA). For each real-time reverse transcriptase–polymerase chain reaction (RT-PCR), we used 20 ng of cDNA, 200 nM of each primer, and 2 × SYBRgreen (Applied Biosystems, Foster City, CA), and samples were run on the Bio-Rad iQ5 Real-Time PCR Detection System according to manufacturers' instructions. The relative level of gene expression was assessed using the 2−ΔΔCt method, and results are presented as an average of three experiments. The following oligonucleotide primer sequences were used: K18-F1, 5′ ccgtcttgctgctgatgact 3′; K18-R1, 5′ ggccttttacttcctcttcgt 3′; nestin-F1, 5′ tgaagggcaatcacaacagg; nestin-R1, 5′ tgaccccaacatgacctctg 3′; GAPDH-F1, 5′ tcgacagtcagccgcatcttcttt 3′; and GAPDH-R1, 5′ accaaatccgttgacctt 3′.
Three-dimensional tissue fabrication
Three-dimensional control tissues were grown using human keratinocytes (NHK) derived from foreskin that were first cultured in two-dimensional (2D), monolayer culture on irradiated 3T3 fibroblasts. Foreskin fibroblasts were grown in a medium containing DMEM and 10% fetal calf serum. Tissues were grown by adding foreskin fibroblasts or ES-derived EDK1 cells to neutralized type I collagen (Organogenesis, Canton, MA) to a final concentration of 2.5 × 104 cells/mL. Three milliliters of this mixture was added to each 35 mm well of a six-well plate (Organogenesis) and incubated for 7 days in the medium containing DMEM and 10% fetal calf serum, until the collagen matrix showed no further shrinkage. About 5 × 105 NHK or 1 × 106 EDK1 cells were seeded onto the collagen matrix, and tissues were then maintained submerged in the low-calcium epidermal growth medium for 2 days, submerged for 2 days in the normal-calcium medium, and raised to the air–liquid interface for an additional 8 days by feeding from below as previously described.17
Teratoma formation
To assess teratoma formation, hES or EDK1 cells were trypsinized, counted, and resuspended in 0.5 mL of phosphate-buffered saline. Four mice were injected with 3 × 106 of either cell type in the rear leg muscle of SCID-beige mice. Mice were sacrificed after 2 and 4 months, tissues were harvested, and tissue morphology was analyzed after hematoxylin and eosin staining. Two of four mice injected with H9 cells developed tumors, while none of the mice injected with EDK1 developed teratomas.
Immunocytochemistry and frozen section staining
EDK1 and HDE1 cells were grown to confluence on type I collagen–coated coverslips, fixed in 4% paraformaldehyde, and permeabilized using 0.1% triton X-100. Cells were stained using primary antibodies directed against cytokeratin 18 (Abcam, Cambridge, MA), vimentin (Abcam), cytokeratin 12 (SantaCruz, Santa Cruz, CA), p63 (Santa Cruz), and keratin 14 (K14) (Millipore, Billerica, MA) or β-catenin (Millipore) for 1 h followed by Alexa 488–conjugated goat anti-rabbit or Alexa 594–conjugated goat anti-mouse secondary antibodies (Invitrogen) for 1 h. For immunohistochemistry, tissues were frozen in O.C.T. compound (Sakura Finetek USA, Torrance, CA), and 8 μm sections were fixed in 4% paraformaldehyde and immunostained using the same protocol as above. Coverslips and tissues were counterstained with DAPI in Vectashield mounting medium (Vector, Burlingame, CA). Hematoxylin and eosin staining for morphologic analysis was performed on paraffin-embedded tissues that were sectioned at 8 μm thickness. Images were captured using a SPOT RT camera and a Nikon Eclipse 80i microscope.
Flow cytometry
EDK1 and HDE1 cells were harvested and divided into 11 FACS tubes (BD Biosciences) at 2 × 105 cells/tube and stained with PE-conjugated anti-CD73, -CD10, -CD13, -CD105, -CD106, -CD44, -CD34-, -CD45, -CD31, and -IgG1k (BD Biosciences, San Jose, CA). Cells were incubated for 40 min at 4°C in the dark and washed with 2% fetal bovine serum in phosphate-buffered saline solution. All data were acquired using a FACSCalibur (BD Biosciences) and analyzed using CellQuest (BD Biosciences) and Summit V4.3 software (Dako). Analysis was performed on 20,000 cells per sample, and positive expression was defined as the level of fluorescence greater than 99% of the corresponding isotype control IgG1k (BD Biosciences) or the corresponding unstained cell samples.
Results
hES cells undergo commitment to ectodermal lineage fate when grown in specific feeder layer and medium conditions
H9 hES cells were first induced to differentiate by growth in serum-containing keratinocyte medium (NHK) on MEFs that were formaldehyde fixed. Within 24 h, ES colonies had begun to undergo morphologic changes at their periphery, characterized by enlarged cells with a cobblestone appearance (Fig. 1A, arrows and inset). To block outgrowth of neural precursors, BMP-4 was added to the medium on days 4–7.18 At day 7, differentiating cells were split onto fixed MEFs, and the medium was changed to a serum-containing ES medium, in which cell populations that demonstrated focal areas of loosely aggregated, irregularly shaped cells expanded without evidence of further morphologic changes (Fig. 1B, arrows and inset). On day 14, cells were passaged to uncoated plastic plates and returned to NHK growth medium. During this time, colonies appeared that contained cells with an epithelial morphology (Fig. 1C, thick arrow and inset). These cells were further expanded by seeding them on plates coated with type I collagen. The resulting cultures, known as EDK1, had acquired either polygonal or elongated (Fig. 1C, thin arrow) morphology. EDK1 cells did not express Oct-4 or SSEA4 and were expanded for 20 passages without decreased growth or senescence. Two and 4 months after injection of either 3 × 106 hES or EDK1 cells into the rear leg muscle of SCID-beige mice, teratomas were only seen in mice injected with hES cells and not with EDK1.
ES-derived progenitor cells demonstrate markers of ectodermal phenotype
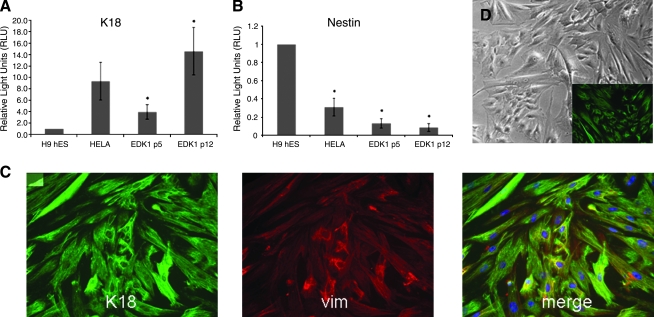
To characterize phenotypic properties of EDK1 cells, patterns of gene expression were analyzed by real-time RT-PCR after RNA extraction from cells at both early (p5) and later (p12) passages. Expression levels of the ectodermal marker cytokeratin 18 (K18) and nestin, a marker of neural progenitors, were analyzed by real-time RT-PCR. Expression of K18 was elevated in both early and late passage cells (Fig. 2A), indicating that cells were stable under culture conditions used for cell expansion. Nestin expression was significantly lower than that seen in hES cells (Fig. 2B), suggesting that cells had not differentiated into a neural lineage. These findings were confirmed by immunohistochemical staining that demonstrated coexpression of both K18 and vimentin in all EDK1 cells. These results indicated that the differentiation protocol used yielded cell populations that were highly enriched in keratin-expressing cells that demonstrated the morphologic and molecular characteristics of commitment toward an ectodermal lineage fate. These ectodermal features of EDK1 were similar to those previously described using a similar protocol.18 We analyzed the morphologic appearance and expression of K18 in EDK1 cells that were cultured for prolonged passages. Passage 19 EDK1 showed no change in morphology (Fig. 2D) and maintained expression of K18 (Fig. 2D, inset).
FIG. 2.
Expression of both K18 and vimentin suggests a primitive population of ectodermal cells. EDK1 cells were characterized by real-time reverse transcriptase–polymerase chain reaction (RT-PCR) at early passage (p5) and later passage (p12). Cells expressed cytokeratin 18 (A), a marker of early ectoderm, and expressed low levels of nestin (B), a marker of neuroectoderm. HeLa cell RNA was used as a positive control for K18 expression and also a negative control for nestin. Ct values were normalized to 18S rRNA levels, and significance was determined by one-tailed T-test. One hundred percent of EDK1 population stained positive for K18 (C, green) and vimentin (vim) (C, red) by immunofluorescence. Cell nuclei were stained with DAPI (C, blue). No significant change in morphology (D) or expression of K18 (D, inset) was seen after 19 passages in culture.
Further commitment to epithelial fate by culturing EDK1 on a substrate enriched in ECM proteins
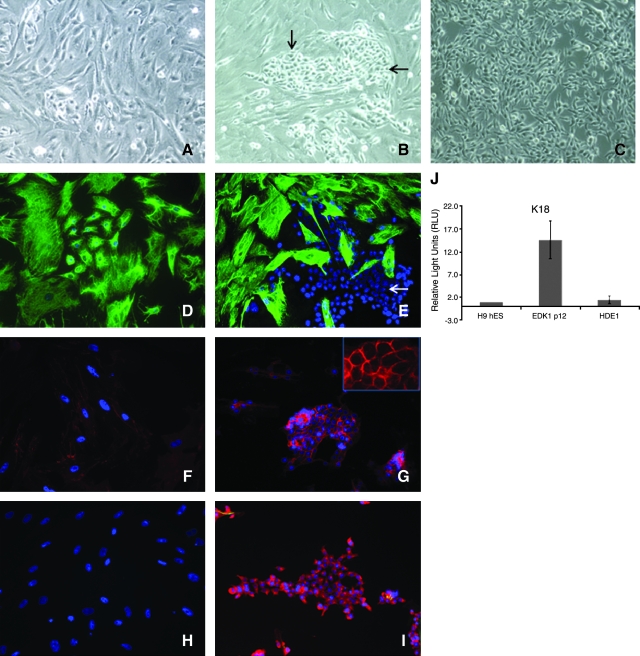
To establish the potential of EDK1 cells to undergo further commitment to specific cell fates, we next cultured these cells on plates coated with ECM proteins deposited by an epithelial cell line (HaCaT). We first prepared a surface enriched in ECM proteins by growing HaCaT cells to confluence on tissue culture plastic. Cells were then removed using ethylenediaminetetraacetic acid to create a surface highly enriched in laminin 5 (data not shown). When seeded on this surface for several passages, the morphology (Fig. 3A) and expression of cytokeratins (data not shown) did not change. However, when these cells were next seeded onto on a surface coated with type I collagen, a population of cells with an epithelial morphology emerged that grew as compact colonies (Fig. 3B, arrows) and became the predominant cell type in these cultures as they were expanded (HDE1) (Fig. 3C). HDE1 cells demonstrated loss of K18 expression (Fig. 3E, arrows), acquisition of K12 (Fig. 3I), and elevated β-catenin expression that localized to cell membranes (Fig. 3G). The downregulation of K18 expression in HDE1 cells was confirmed at the RNA level (Fig. 3J). We also performed immunocytochemical stains for markers of basal keratinocytes, cytokeratin 14 (K14), and p63, which are normally found in mature epithelial tissues. Expression of K14 and p63 were not detected in this cell population, suggesting that these ectodermal cells have not acquired the phenotype of basal keratinocytes. These results demonstrated that growth on ECM proteins could direct ES-derived cells to further differentiate to a cell lineage with characteristics of epithelial cells.
FIG. 3.
Emergence of compact, epithelial-like colonies when EDK1 cells were grown on extracellular matrix (ECM)–coated plates. EDK1 underwent further differentiation to morphologically distinct cell populations that expressed K12, but not K18, after sequential seeding on ECM-coated and type I collagen–coated plates. EDK1 cells were grown for several passages on HaCat-CM plates, without change in morphology (A). Growth on type I collagen resulted in emergence of fast-growing, epithelial-like cells that grew as compact colonies (B, arrows) that became the predominant cell type after passaging (C). Immunofluorescent characterization of emergent colonies showed K18 expressed homogeneously in EDK1 (D), which was absent in HDE1 (E, arrow). We observed a low-level, diffuse staining of β-catenin that was diffuse in EDK1 cells (F), while β-catenin expression was elevated in HDE1 (G), and was localized to cell membranes (inset). The downregulation of K18 expression in HDE1 cells was confirmed by RT-PCR analysis to determine at the RNA levels (J). Cytokeratin 12 was not expressed in EDK1 (H) but was highly expressed in HDE1 cells (I). All images were taken at 10 × magnification.
EDK1 cells are enriched in cells with surface markers characteristic of mesenchymal cells
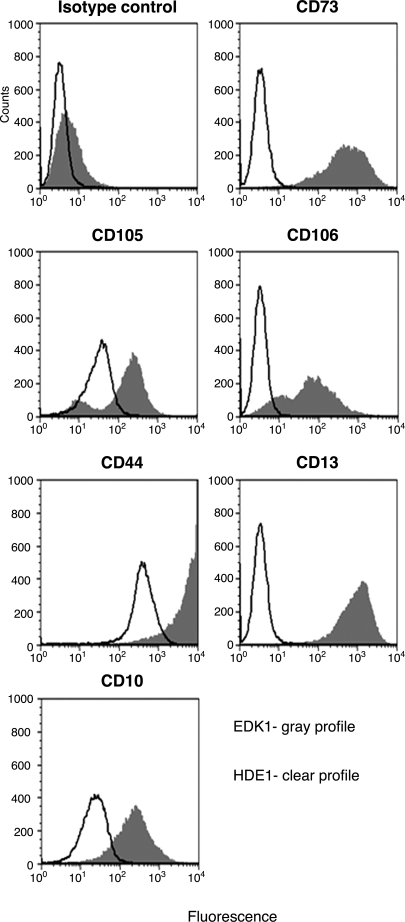
The presence of cell subpopulations with morphologic features of mesenchymal-like cells that were predominant in EDK1 cultures and less common in HDE1 cells suggested that EDK1 cells were enriched in cells with a mesenchymal phenotype. We therefore compared EDK1 to HDE1 cells by flow cytometry using a panel of nine monoclonal antibodies to determine their surface marker expression. Both cell types were analyzed by flow cytometry for surface markers known to identify human MSCs, including surface enzymes (CD73, CD10, and CD13), adhesion molecules (CD105 and CD106), and a receptor molecule (CD44). In addition, flow cytometry was also performed to rule out the presence of hematopoietic or endothelial cell types using markers that would identify these cells (CD34, CD45, and CD31).
Flow cytometric analysis of the labeled cells showed that large numbers (80–99.5%) of EDK1 cells strongly expressed the MSC-associated antigens CD73, CD105, CD106, CD44, CD10, and CD13, indicating their mesenchymal phenotype. HDE1 cells demonstrated a dramatic reduction of CD73-, CD106-, and CD10-positive cells and similar levels of expression for CD105 and CD44 (Table 1), although EDK1 and HDE1 featured notable differences with respect to fluorescence intensity of CD105 and CD44 markers (Fig. 4). Both cell types lacked expression of CD31, CD45, and CD34, suggesting the absence of cells of hematopoietic and endothelial lineages in either of these populations (Table 1). These findings suggest that EDK1 cells were enriched in populations that express markers of mesenchymal precursor cells. In contrast, HDE1 cells did not sustain this expression profile after their sequential passage on HaCaT cell–derived ECM and type I collagen surfaces. This indicated that HDE1 cells underwent a transition from cells with mesenchymal features toward cells displaying an ectodermal phenotype.
Table 1.
Surface Marker Expression in EDK1 and HDE1 Cell Lines by Flow Cytometric Analysis of 20,000 Cells
| Surface protein | Antigen | EDK1 | HDE1 |
|---|---|---|---|
| CD73 | SH3, ecto-5′-nucleotidase | 99.0 ± 0.2 | 4.6 ± 5.2 |
| CD105 | SH2, endoglin | 82.4 ± 1.7 | 88.2 ± 4.2 |
| CD106 | VCAM-1 | 80.2 ± 0.1 | 1.4 ± 1.7 |
| CD44 | Hyaluronate, HCAM | 99.0 ± 0.6 | 93.8 ± 3.1 |
| CD10 | CALLA | 99.5 ± 0.1 | 2.4 ± 0.7 |
| CD13 | Aminopeptidase N | 92.2 ± 9.3 | 85.8 ± 2.5 |
| CD45 | Leucocyte common antigen | 5.0 ± 1.9 | 4.4 ± 4.2 |
| CD34 | Hematopoietic marker | 1.5 ± 1.6 | 0.6 ± 0.4 |
| CD31 | PECAM | 4.8 ± 5.2 | 0.9 ± 1.1 |
Results are displayed as means of the surface marker expression in percent ± standard deviation (n = 2).
CALLA, common leucocyte lymphocytic leukemia antigen; PECAM, platelet endothelial cell adhesion molecule; HCAM, lymphocyte homing-associated cell adhesion molecule; VCAM, vascular cell adhesion molecule.
FIG. 4.
Comparative flow cytometric analysis of mesenchymal stem cell-associated antigens. Representative histograms show the fluorescence intensity of EDK1 cells (gray profiles) and HDE1 cells (clear profiles) stained with isotype control antibodies and antibodies against the surface proteins.
HDE1 cells form a 3D multilayer epithelium when grown at the air–liquid interface on collagen gels populated with EDK1 cells
In light of the emergence of stable ES-derived cell populations with features of either ectodermal or mesenchymal-like cells, we next determined if these distinct cell populations could be cocultured in 3D engineered tissue constructs to form complex tissues. EDK1 were embedded into collagen gels and allowed to contract the gel for 7 days. During this time, these cells acquired an elongated and stellate morphology (Fig. 5D, G, insets) that resembled that of foreskin fibroblasts grown under similar conditions with foreskin-derived keratinocytes (Fig. 5A, inset). EDK1 or HDE1 cells were then seeded onto the surface of collagen gels, and tissues were grown at an air–liquid interface for an additional 7 days to compare their capacity to form a multilayer epithelium (Fig. 5A).
FIG. 5.
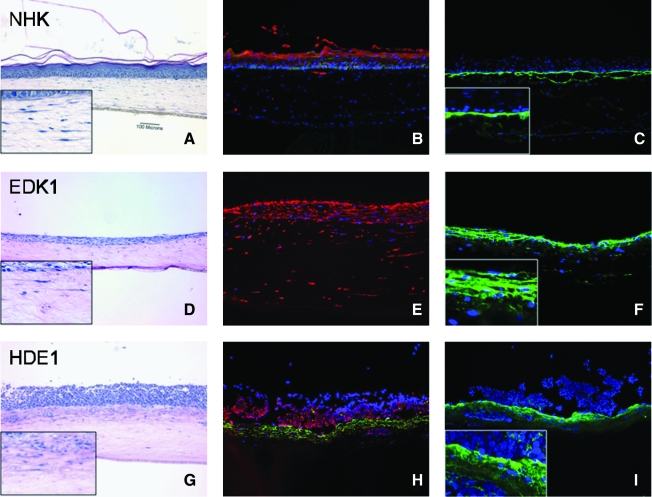
HDE1 form a well-stratified three-dimensional (3D) tissue when grown on collagen gels harboring mesenchymal-like EDK1 cells. Two cell types were combined to form 3D tissues by incorporation of one cell type into the stroma and the other cell type on the surface. This combination of cells included human foreskin fibroblasts (HFF) in the stroma and NHK on the surface (A), EDK1 in the stroma and EDK1 on the surface (D), and EDK1 in the stroma and HDE1 on the surface (G). Hematoxylin and eosin staining of tissues showed that HDE1 cells formed a well-stratified 3D tissue when grown in the presence of EDK1 cells in collagen gels. EDK1 cells also expressed K18 (E) and type IV collagen (F). HDE1 cells on the surface were positive for K12 (H, red), while EDK1 cells in the collagen gel maintained expression of K18 (H, green). Deposition of collagen type IV in HDE1 tissues (I, inset) at the dermal–epithelial junction was similar to that in keratinocyte cultures (C). Tissues were counterstained with DAPI to view nuclei (blue). All images are 10 × magnification.
EDK1 cells grown at an air–liquid interface showed only a minimal degree of stratification resulting in a surface layer that was only two to three cell layers thick (Fig. 5D). EDK1 cells expressed K18 and type IV collagen that was retained in the cell cytoplasm and not deposited in the BM zone (Fig. 5F, inset). In contrast, when HDE1 cells were grown on the surface of collagen gels in which EDK1 cells were present, tissues demonstrated a significantly thickened epithelium (Fig. 5G) that approximated the number of cell layers seen in tissues constructed with NHK (Fig. 5A). Immunofluorescent staining for localization of K18 (Fig. 5H, green) and K12 (Fig. 5H, red) showed that these proteins were localized in distinct compartments of the developing tissue. These multilayer tissues were well attached to the underlying collagen gel (Fig. 5G). This may be explained by the linear deposition of type IV collagen that was localized in the upper part of the collagen gel at the connective tissue–epithelial interface (Fig. 5I). This pattern of deposition was similar to that seen at this interface when control tissues harboring NHKs were grown on collagen gels containing foreskin fibroblasts (Fig. 5C).
Discussion
Stem cell fate is directed during early development by the integration of numerous signals that are mediated by paracrine-acting, growth-regulatory factors and structural proteins that provide dynamic crosstalk between the ectodermal, mesodermal, and endodermal tissues.19 A goal of research at the interface of tissue engineering and stem cell biology is to fabricate in vitro tissues that can simulate the complexity of these in vivo developmental cues, to better understand how intercellular interactions stimulate tissue assembly and organization to generate functional tissues. In our current study, we demonstrate the directed differentiation of H9 ES cells to derive cell populations with properties of ectodermal and mesenchymal cells that, for the first time, have been incorporated into in a 3D tissue environment to generate complex stratified epithelium.
Significant progress has been made in the commitment and differentiation of pluripotent hES cells toward a broad variety of cell types. Initial attempts to generate keratinocytes using EBs successfully generated keratinocytes expressing cytokeratins 5 and 14, but these cells had limited expansion potential.20,21 The efficiency of this technique was improved by bypassing EB formation and treating cells with BMP-4, which resulted in generation of K18- and K14-expressing cells with many characteristics of ectodermal cell lineages.18,22 While cells generated in this manner displayed markers of keratinocyte differentiation, the low expansion potential and colony-forming efficiencies limited their usefulness in tissue engineering applications, where high growth fraction is critical for tissue formation.17 In addition, while mesenchymal progenitors have been previously identified in skin-like tissues constructed from murine ES cells,23 it has not been shown that can give rise to cells with features of mature fibroblasts that can provide crosstalk needed to support the maturation of adjacent epithelial cells. We have demonstrated the generation of distinct mesenchymal- and ectodermal-like ES-derived cell populations that formed complex tissues comprised of both stromal and epithelial compartments.
To derive the cell populations used to fabricate tissues, we adapted previously described protocols for ectodermal differentiation18 using a fixed MEF feeder layer. K18 was used as a marker of ectodermal commitment, as it is the first cytoplasmic intermediate filament to be expressed in the developing embryo and in primitive ectoderm.24 A number of sequential substrate and medium conditions used in our experiments, such as serum-free medium, and adult fibroblast and NIH-3T3 fibroblasts, were able to enrich for K18-expressing cells. While most of these previous attempts generated cell populations that demonstrated only a brief period of K18 expression before growth arrest, we describe an approach (Fig. 1) that successfully generated ectodermal cells (EDK1). Our protocol for the directed differentiation of hES cells was designed to recapitulate the environment that hES cells are exposed to during early development. This was accomplished by growing cells on a feeder layer of fixed fibroblasts and subsequently on plate coated with type I collagen, the major collagenous component of stroma within developing tissues. The addition of BMP-4 during a critical time for cell commitment (days 4–7) has previously been shown to suppress neural outgrowth of differentiating hES (Gambaro).22 Supplements included in the medium such as insulin, epidermal growth factor, and cholera toxin have previously been shown to support growth of mature, epidermal keratinocytes in 2D culture. We demonstrate that these additional factors can also support the differentiation of cells toward ectodermal lineages. The resulting EDK1 population was highly proliferative and could be expanded for 18–20 passages without change in morphology. Despite this growth potential, EDK1 cells did not stratify within 3D tissues but rather generated a thin tissue, suggesting that they required additional developmental stimulus to generate a multilayer epithelium. It is possible that paracrine-acting factors or other ECM elements are critical cues needed to direct the fate of epithelial cells in complex tissues.
The specification of cell types giving rise to stratified epithelia is determined in the embryo by precise cues from the underlying mesenchyme that direct development of complex epithelial tissues.25–27 Therefore, an important goal of our study was to derive and incorporate cells with mesenchymal features into engineered 3D epithelial tissues in a way that would enable crosstalk between stromal and epithelial compartments needed for tissue development.28,29 Similarly, cells with mesenchymal features were also seen when mouse ES cells were used to generate a multilayer epithelium by growth on ECM proteins secreted by fibroblasts.23 Cells with a mesenchymal phenotype were the predominant cell population in EDK1 cells but significantly diminished in numbers when these cells were passaged onto an ECM-coated substrate. However, the ability of EDK1 to generate HDE1 populations was indeed maintained over passage in culture. While passage 18 EDK1 cells were used in our experiments to generate HDE1 cells, we also generated HDE1-like cells from EDK1 cells at passage 5. HDE1 cells generated a morphologically distinct subpopulation that lost MSC surface marker expression and acquired epithelial morphology, demonstrating the plasticity of these hES-derived cells and their dynamic response to variations in ECM substrate and growth conditions. This enrichment for ectodermal-like cells in the HDE1 population was further seen by the significantly greater tissue thickness when compared to EDK1 cells in 3D tissues. HDE1 cells also appeared to have acquired the capacity to deposit BM proteins at the stromal–epithelial interface in 3D tissues. While EDK1 cells expressed type IV collagen in the absence or presence of HDE1 cells, this protein was only localized to the BM interface when both cell types were present. HDE1 cells do not synthesize this BM protein although it is possible that other BM proteins may be synthesized by HDE1 that enable EDK1-derived type IV collagen to incorporate into an assembling BM. This raises the possibility that such localization of type IV collagen in 3D tissues is linked to the initiation of BM organization, as it is known that interactions between type IV collagen and other BM components at the stromal–epithelial interface provide an early scaffold for BM organization.30 Thus, future optimization of tissue morphogenesis from hES cell-derived precursors may require the development of normalized BM assembly.
We have adapted our approach for the fabrication of epithelial tissues using ASCs31 to develop tissues using the hES cells. The gold standard for generation of 3D epithelial tissues are human skin equivalents, that incorporate dermal fibroblasts and keratinocytes that contain ASCs derived from human skin. While hES cells can be reproducibly obtained from blastocysts, ASCs are considerably more difficult to isolate. This is because of the fact that ASCs do not have unique surface markers that allow their efficient isolation. As a result, ASCs are usually defined by functional assays such as adherence in 2D culture or colony-forming efficiency. In addition, the existence of heterogeneous and poorly defined subpopulations of ASCs contributes to the complexity of using these cells as growth conditions and may select for subpopulations of cells with specific growth characteristics.32 In this light, it is important to optimize and better define specific protocols for the isolation of progenitor cells from hES cells that will offer more reproducible stem cell sources for tissue fabrication.
The integrated events that occur during tissue morphogenesis need to be studied in biological systems in which a high degree of tissue complexity can be achieved. Biologically meaningful signaling pathways, including stem cell differentiation, function optimally when cells are spatially organized in 3D tissues rather than in rudimentary 2D culture systems.33 In this light, the application of 3D tissues will play a pioneering role in moving tissue engineering and discovery science into this paradigm that now provides a focus on translational and clinical relevance. The combinatorial model we present here, using both 2D and 3D techniques to first commit hES cells to multiple lineages and then to incorporate these cells into bioengineered 3D tissues, provides a novel paradigm to study ectodermal–mesodermal crosstalk during epithelial tissue fabrication. Further manipulation of the 3D tissue environment, mediated by epithelial–mesenchymal crosstalk and the presence of structured BM, will identify the microenvironmental cues that can normalize epithelial tissue outcomes in engineered tissues. Once we better understand the signals communicated by ES-derived progenitors in distinct tissue compartments, the application of newly discovered pluripotent stem cell sources such as induced pluripotent stem cells to generate patient-specific tissues may provide new tissue engineering approaches to fabricate complex tissues.
Acknowledgments
We thank Tessa DesRochers, Addy Alt-Holland, Shumin Dong and Christophe Egles for their contributions during the progress of this work and Judith Edwards for assistance in the preparation of the manuscript. We thank the laboratory of Larry Feig for HeLa cells. This work was supported by grant #R01-DE017413 to J.A.G. from the National Institute for Dental and Craniofacial Research (NIDCR).
Disclosure Statement
No competing financial interests exist.
References
- 1.Metallo C.M. Azarin S.M. Ji L. de Pablo J.J. Palecek S.P. Engineering tissue from human embryonic stem cells. J Cell Mol Med. 2008;12:709. doi: 10.1111/j.1582-4934.2008.00228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Godier A.F. Marolt D. Gerecht S. Tajnsek U. Martens T.P. Vunjak-Novakovic G. Engineered microenvironments for human stem cells. Birth Defects Res C Embryol Today. 2008;84:335. doi: 10.1002/bdrc.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riazi A.M. Kwon S.Y. Stanford W.L. Stem cell sources for regenerative medicine. Methods Mol Biol. 2009;482:55. doi: 10.1007/978-1-59745-060-7_5. [DOI] [PubMed] [Google Scholar]
- 4.Thomson J.A. Itskovitz-Eldor J. Shapiro S.S. Waknitz M.A. Swiergiel J.J. Marshall V.S. Jones J.M. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 5.Hwang N.S. Varghese S. Lee H.J. Zhang Z.J. Ye Z.H. Bae J. Cheng L.Z. Elisseeff J. In vivo commitment and functional tissue regeneration using human embryonic stem cell-derived mesenchymal cells. Proc Natl Acad Sci USA. 2008;105:20641. doi: 10.1073/pnas.0809680106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma W. Tavakoli T. Derby E. Serebryakova Y. Rao M.S. Mattson M.P. Cell-extracellular matrix interactions regulate neural differentiation of human embryonic stem cells. BMC Dev Biol. 2008;8:90. doi: 10.1186/1471-213X-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saeki K. Saeki K. Nakahara M. Matsuyama S. Nakamura N. Yogiashi Y. Yoneda A. Koyanagi M. Kondo Y. Yuo A. Feeder-free and efficient production of functional neutrophils from human embryonic stem cells. Stem Cells. 2009;27:59. doi: 10.1634/stemcells.2007-0980. [DOI] [PubMed] [Google Scholar]
- 8.Correia A.S. Anisimov S.V. Li J.Y. Brundin P. Growth factors and feeder cells promote differentiation of human embryonic stem cells into dopaminergic neurons: a novel role for fibroblast growth factor-20. Front Neurosci. 2008;2:26. doi: 10.3389/neuro.01.011.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie J. Willerth S.M. Li X. Macewan M.R. Rader A. Sakiyama-Elbert S.E. Xia Y. The differentiation of embryonic stem cells seeded on electrospun nanofibers into neural lineages. Biomaterials. 2009;30:354. doi: 10.1016/j.biomaterials.2008.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Metallo C.M. Ji L. de Pablo J.J. Palecek S.P. Retinoic acid and bone morphogenetic protein signaling synergize to efficiently direct epithelial differentiation of human embryonic stem cells. Stem Cells. 2007;26:372. doi: 10.1634/stemcells.2007-0501. [DOI] [PubMed] [Google Scholar]
- 11.Ahmad S. Stewart R. Yung S. Kolli S. Armstrong L. Stojkovic M. Figueiredo F. Lako M. Differentiation of human embryonic stem cells into corneal epithelial-like cells by in vitro replication of the corneal epithelial stem cell niche. Stem Cells. 2007;25:1145. doi: 10.1634/stemcells.2006-0516. [DOI] [PubMed] [Google Scholar]
- 12.Itskovitz-Eldor J. Schuldiner M. Karsenti D. Eden A. Yanuka O. Amit M. Soreq H. Benvenisty N. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol Med. 2000;6:88. [PMC free article] [PubMed] [Google Scholar]
- 13.Levenberg S. Huang N.F. Lavik E. Rogers A.B. Itskovitz-Eldor J. Langer R. Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. Proc Natl Acad Sci USA. 2003;100:12741. doi: 10.1073/pnas.1735463100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zoldan J. Levenberg S. Engineering three-dimensional tissue structures using stem cells. Methods Enzymol. 2006;420:381. doi: 10.1016/S0076-6879(06)20018-1. [DOI] [PubMed] [Google Scholar]
- 15.Inanc B. Elcin A.E. Elcin Y.M. Human embryonic stem cell differentiation on tissue engineering scaffolds: effects of ngf and retinoic acid induction. Tissue Eng Part A. 2008;6:955. doi: 10.1089/ten.tea.2007.0213. [DOI] [PubMed] [Google Scholar]
- 16.Lees J.G. Lim S.A. Croll T. Williams G. Lui S. Cooper-White J. McQuade L.R. Mathiyalagan B. Tuch B.E. Transplantation of 3D scaffolds seeded with human embryonic stem cells: biological features of surrogate tissue and teratoma-forming potential. Regen Med. 2007;2:289. doi: 10.2217/17460751.2.3.289. [DOI] [PubMed] [Google Scholar]
- 17.Margulis A. Zhang W. Garlick J.A. In vitro fabrication of engineered human skin. Methods Mol Biol. 2005;289:61. doi: 10.1385/1-59259-830-7:061. [DOI] [PubMed] [Google Scholar]
- 18.Aberdam E. Barak E. Rouleau M. De LaForest S. Berrih-Aknin S. Suter D.M. Krause K.H. Amit M. Itskovitz-Eldor J. Aberdam D. A pure population of ectodermal cells derived from human embryonic stem cells. Stem Cells. 2008;26:440. doi: 10.1634/stemcells.2007-0588. [DOI] [PubMed] [Google Scholar]
- 19.Murry C.E. Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Green H. Easley K. Iuchi S. Marker succession during the development of keratinocytes from cultured human embryonic stem cells. Proc Natl Acad Sci USA. 2003;100:15625. doi: 10.1073/pnas.0307226100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ji L. Allen-Hoffmann B.L. de Pablo J.J. Palecek S.P. Generation and differentiation of human embryonic stem cell-derived keratinocyte precursors. Tissue Eng. 2006;12:665. doi: 10.1089/ten.2006.12.665. [DOI] [PubMed] [Google Scholar]
- 22.Gambaro K. Aberdam E. Virolle T. Aberdam D. Rouleau M. BMP-4 induces a Smad-dependent apoptotic cell death of mouse embryonic stem cell-derived neural precursors. Cell Death Differ. 2006;13:1075. doi: 10.1038/sj.cdd.4401799. [DOI] [PubMed] [Google Scholar]
- 23.Coraux C. Hilmi C. Rouleau M. Spadafora A. Hinnrasky J. Ortonne J.P. Dani C. Aberdam D. Reconstituted skin from murine embryonic stem cells. Curr Biol. 2003;13:849. doi: 10.1016/s0960-9822(03)00296-3. [DOI] [PubMed] [Google Scholar]
- 24.Owens D.W. Lane E.B. The quest for the function of simple epithelial keratins. Bioessays. 2003;25:748. doi: 10.1002/bies.10316. [DOI] [PubMed] [Google Scholar]
- 25.Fuchs E. Scratching the surface of skin development. Nature. 2007;445:834. doi: 10.1038/nature05659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koster M.I. Roop D.R. Mechanisms regulating epithelial stratification. Annu Rev Cell Dev Biol. 2007;23:93. doi: 10.1146/annurev.cellbio.23.090506.123357. [DOI] [PubMed] [Google Scholar]
- 27.Hardin J. Armstrong N. Short-range cell-cell signals control ectodermal patterning in the oral region of the sea urchin embryo. Dev Biol. 1997;182:134. doi: 10.1006/dbio.1996.8436. [DOI] [PubMed] [Google Scholar]
- 28.Smola H. Thiekotter G. Fusenig N.E. Mutual induction of growth factor gene expression by epidermal-dermal cell interaction. J Cell Biol. 1993;122:417. doi: 10.1083/jcb.122.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szabowski A. Maas-Szabowski N. Andrecht S. Kolbus A. Schorpp-Kistner M. Fusenig N.E. Angel P. c-jun and JunB antagonistically control cytokine-regulated mesenchymal-epidermal interaction in skin. Cell. 2000;103:745. doi: 10.1016/s0092-8674(00)00178-1. [DOI] [PubMed] [Google Scholar]
- 30.Fleischmajer R. Utani A. Macdonald E.D. Perlish J.S. Pan T.C. Chu M.L. Nomizu M. Ninomiya Y. Yamada Y. Initiation of skin basement membrane formation at the epidermo-dermal interface involves assembly of laminins through binding to cell membrane receptors. J Cell Sci. 1998;111:1929. doi: 10.1242/jcs.111.14.1929. [DOI] [PubMed] [Google Scholar]
- 31.Andriani F. Margulis A. Lin N. Griffey S. Garlick J.A. Analysis of microenvironmental factors contributing to basement membrane assembly and normalized epidermal phenotype. J Investig Dermatol. 2003;120:923. doi: 10.1046/j.1523-1747.2003.12235.x. [DOI] [PubMed] [Google Scholar]
- 32.Meirelles L.D. Caplan A.I. Nardi N.B. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26:2287. doi: 10.1634/stemcells.2007-1122. [DOI] [PubMed] [Google Scholar]
- 33.Alcaraz J. Xu R. Mori H. Nelson C.M. Mroue R. Spencer V.A. Brownfield D. Radisky D.C. Bustamante C. Bissell M.J. Laminin and biomimetic extracellular elasticity enhance functional differentiation in mammary epithelia. EMBO J. 2008;27:2829. doi: 10.1038/emboj.2008.206. [DOI] [PMC free article] [PubMed] [Google Scholar]