Abstract
Objective
Proinflammatory cytokines are known to provoke degradative signaling cascades that promote extracellular matrix disintegration in articular cartilage. Because integration of the repair tissue into the surrounding native cartilage to produce a mechanically stable interface has a profound impact on the viability and functionality of the restored joint surface, this study examined the effects of proinflammatory cytokines on the properties of tissue-engineered cartilage in the context of integration. Methods: Using an established in vitro cartilage defect model, we examined the integration of chondrocyte-laden agarose constructs into native articular cartilage and the biochemical and biomechanical alterations of these implants upon treatment with interleukin 1-β (IL1-β) and tumor necrosis factor-α (TNF-α). Additionally, we probed extracellular regulated kinase (ERK) signaling involvement in response to proinflammatory cytokines. Results: The time-dependent accumulation of extracellular matrix and concomitant increase in Young's modulus observed in the absence of cytokines was significantly decreased upon IL1-β and TNF-α treatment. Push-out test showed the highest interface strength in hybrid cultures maintained without cytokines, which was significantly lowered with IL1-β and TNF-α treatment. Histological characteristics of the interface region are consistent with the biochemical findings. Treatment with an inhibitor of ERK pathway antagonized the deleterious effects caused by both cytokines. Conclusion: This study is the first to show the functional catastrophic effects of IL1-β and TNF-α on the biochemical, structural, and integrative properties of tissue-engineered cartilage and their significant counteraction by the blockade of ERK signaling pathway. With the discovery of new potential chemical entities, ERK inhibitor may emerge as a new therapeutic approach for functional integration and mechanical integrity of an engineered cartilage to the host tissue and, therefore, enhance long-term viability and functionality of the restored joint surface.
Introduction
The common threat linking the two major arthritic diseases, rheumatoid arthritis (RA) and osteoarthritis (OA), is irreversible destruction of the cartilage in the affected joints. Cartilage tissue engineering has been widely proposed as a promising approach to repair injured or diseased cartilage. As articular surfaces are subject to constant mechanical forces, the strength of engineered implant and its stable anchorage to the injured site are essential for successful joint function. Currently, considerable efforts in this field are directed toward achieving long-term maintenance of regenerated hyaline cartilage tissue under conditions of normal joint loading. Moreover, without a functional integrated interface, the junction between native and engineered cartilage will exhibit limited mechanical stability; lack of integration constitutes the primary cause of long-term failure in cartilage repair.1 The functional integration of an engineered tissue to the host tissue is dependent on proper interaction of the tissues in terms of both cell activities and matrix distribution and assembly.
Interleukin 1-β (IL1-β) and tumor necrosis factor-α (TNF-α) play a pivotal role in arthritic diseases by inducing and sustaining cartilage damage by negatively influencing the balance between cartilage destruction and cartilage repair (for review, see Goldring and Berenbaum2 and Lianxu et al.3). IL1-β and TNF-α have been shown to affect chondrocyte phenotype4 and to enhance extracellular matrix (ECM) degradation through up-regulation of metalloproteinase (MMP) production.3,5 In arthritic joints, MMPs are predominantly responsible for connective tissue destruction. Two MMP families are the major mediators of cartilage destruction: a disintegrin and MMP with thrombospondin motifs (ADAMTSs) and the matrix MMPs.6 The production of MMPs is tightly regulated at the gene expression level. In normal connective tissues their expression is low, but increases considerably under the pathologic conditions of OA and RA.
The mitogen-activated protein kinases (MAPKs) and extracellular regulated kinases (ERKs) 1 and 2 are activated by a wide range of stimuli, including cytokines such as IL1-β and TNF-α.7 Further, ERK pathway is involved in IL1-β and TNF-α induction of MMP-1, MMP-13, and ADAMTS-4 expression in chondrocytes.8–10 In these studies, ERK1/2 pathway blockade prevents the production of proteinases induced by proinflammatory cytokine treatment.
The present study for the first time explored the impact of proinflammatory cytokines on the integration of tissue-engineered cartilage constructs into native articular cartilage, and analyzed the biochemical and biomechanical alterations of these implants in response to varying doses of IL1-β and TNF-α, using an established bovine in vitro cartilage defect model.11 Because proinflammatory cytokines are known to activate ERK signaling in chondrocytes, we also investigated the involvement of this pathway in the IL1-β– and TNF-α–induced altered equilibrium between the catabolic and anabolic activities of the chondrocytes in compromising functional integration between the chondrocyte-laden agarose constructs and native articular cartilage.
Materials and Methods
Cartilage construct assembly and culture
An established “construct in cartilage-ring model” was used as described previously.12 Circular native articular cartilage rings (Ø 8 × 2.25 mm thickness) with a centered 4-mm hole were prepared from knee cartilage of 6-month-old calves. Chondrocyte-seeded agarose hydrogels were fabricated as described previously.13–15 Chondrocytes were isolated from carpometacarpal joint cartilage of the forelimbs of young calves (6 months old). Cell suspensions were mixed in equal parts with 4% agarose (type VII; Sigma, St. Louis, MO) in phosphate-buffered saline to produce a final concentration of 20 × 106 chondrocytes/mL in 2% agarose. After 20 min gelation at room temperature, cylindrical samples were cored using a dermal punch. Cartilage rings and chondrocytes were harvested from three different animals, and were mixed randomly for each construct (n = 5). In one group, chondrocyte-laden agarose disks (Ø 4.1 × 2.25 mm thickness), freshly obtained, were press-fit implanted into the cartilage rings. These hybrid constructs were then cultured for 28 days in a serum-free medium, designated as CM−, containing Dulbecco's modified Eagle's medium, 50 μg/mL ascorbate, 0.1 μM dexamethasone, 40 μg/mL L-proline, 100 μg/mL sodium pyruvate, insulin-transferrin-selenium-plus, and antibiotics. In another group, samples of the above stated cell-laden disks were initially kept as free-swelling culture in CM− supplemented with 10 ng/mL TGF-β3 (R&D Systems, Minneapolis, MN), designated as CM+, for 21 days before implantation into the cartilage rings and subsequent culture for 28 days in CM−. Hybrid constructs from the two different groups were treated with or without varying doses of IL1-β or TNF-α (1 and 10 ng/mL) (R&D) for 28 days with medium change twice weekly. In some cases, articular cartilage rings were precultured for 10 days with IL1-β or TNF-α (1 and 10 ng/mL). The precultured cartilage rings were then washed three times in CM− before their centered perforation and the implantation of the fresh, chondrocyte-laden agarose disks. Hybrid constructs from this group were cultured for 28 days in CM−. In some experiments, the cultures were treated with the ERK inhibitor U0126 (Cat. No. 662005; Calbiochem, San Diego, CA), which was added to the culture medium at a final concentration of 25 μM 30 min before the addition of IL1-β or TNF-α, for 28 days with medium change twice weekly.
Biochemical composition
The biochemical composition of samples at various times was analyzed based on assays of sulfated glycosaminoglycan (sGAG), DNA, and bulk collagen ortho-hydroxyproline (OHP) content. After wet weight (ww) was measured, samples were homogenized in papain digest buffer (Sigma). Aliquots were analyzed for sGAG content using the 1,9-dimethylmethylene blue dye-binding assay,16 for DNA content using the PicoGreen dsDNA Quantification kit (Molecular Probes, Eugene, OR), and for OHP content (after acid hydrolysis) by reaction with chloramine T and dimethylaminobenzaldehyde.17 OHP was converted to collagen content using a 1:10 ratio of OHP:collagen.18 Each constituent (DNA, sGAG, and collagen) was normalized to the ww.
Mechanical testing
A custom-designed apparatus15,19 was used for mechanical testing. Chondrocyte-laden agarose disks were carefully removed from the cartilage rings, and their thickness and diameter measured with a digital micrometer. The disks were tested under unconfined compression between two smooth impermeable surfaces in phosphate-buffered saline at room temperature as previously described.20 Briefly, samples were first tested in creep under a tare load of 0.02 N applied until equilibrium was achieved (∼300 s). Subsequently, stress relaxation tests were carried out with a compressive deformation of 1 μm/s to 10%, after which samples were allowed to relax to equilibrium (1200 s). The equilibrium compressive Young's modulus (Eγ) was determined from the equilibrium force normalized to the original cross-sectional area divided by the equilibrium compressive strain.
Interface strength
The strength of the native/engineered tissue interface after 28 days was measured by push-out tests performed using the ELF3200 system (Enduratec, Minnetonka, MN). Failure stress (kPa) was computed from the maximum load that led to interface disruption and the surface area of integration.12,21,22 The core was pushed out with a plunger with a diameter (3.5 mm) slightly less than that of the core, while the annulus of native cartilage rested on a rigid ring with a center hole diameter (5.0 mm) slightly larger than that of the core. The maximum force achieved before separation of the materials was normalized to the lateral surface area of the core (height times circumference), and the resulting value was considered the failure stress.
Histology and immunohistochemistry
Samples for histology were fixed in 4% buffered paraformaldehyde, ethanol dehydrated, paraffin embedded (Tissue Prep; Fisher Scientific, Hampton, NH), and sectioned at 8 μm thickness. Sections were stained with Alcian blue (pH 1.0) to detect sulfated proteoglycans, and with Picrosirius red (0.1% w/v in saturated picric acid) to examine collagen deposition. For immunohistochemistry, sections were epitope-exposed by digestion with 1 mg/mL bovine testicular hyaluronidase (Cat. No. H 3506; Sigma) or 20 μg/mL proteinase K (Roche Diagnostics, Indianapolis, IN) in 10 mM Tris-HCl (pH 7.5) for 30 min at 37°C. The sections were then incubated overnight at room temperature with the following antibodies in Tris-buffered saline containing 0.1% bovine serum albumin: antibodies to aggrecan (AGN) and collagen type II (Col2) (Developmental Studies Hybridoma Bank, Iowa City, IA), and antibody to fibronectin (R&D). Immunostaining was detected histochemically using the streptavidin-peroxidase Histostain SP Kit for DAB (Zymed Laboratories, San Francisco, CA), and imaged with a color charge–coupled device camera and an inverted microscope.
MMP-2 and MMP-9 assays
Aliquots of 3-day culture supernatant of hybrid constructs were collected at the end of the experiment (day 28). Both total and active MMP-2 and MMP-9 activities were detected using the SensoLyte™ 490 MMP-2 or SensoLyte MMP-9 assay kits, respectively, according to the manufacturer's instructions (AnaSpec, San Jose, CA). Pro-MMP-2 and -9 were activated with 1 mM p-aminophenylmercuric acetate (APMA). MMP activity was expressed as a change in fluorescence intensity (relative fluorescence units, RFU; excitation at 355 nm and emission at 460 nm) measured using a fluorescence plate reader.
Western blot
First-passage monolayer carpometacarpal chondrocyte cultures were treated with cytokines (IL1-β or TNF-α), with or without addition of U0126 (25 μM) 30 min before cytokine treatment, and homogenized in RIPA lysis buffer (Sigma) supplemented with protease and phosphatase inhibitors. Extracted proteins were separated on 12% polyacrylamide gels and electroblotted. The blots were immunoprobed for total ERK1/2 and dual phosphorylated ERK1/2 (the respective polyclonal antibodies from Cell Signaling Technology, Danvers, MA). Immune detection was performed using horseradish peroxidase–conjugated secondary goat antibodies to rabbit IgG.
Gene expression analysis by real-time reverse transcription–polymerase chain reaction (RT-PCR)
Bovine chondrocytes were isolated from the carpometacarpal joints, seeded as monolayer cultures at a density of 15,000 cells/cm2, and used at passage 1. RNA was extracted from monolayer cultures of bovine chondrocytes using the RNeasy Kit (Qiagen, Valencia, CA). Total RNA (1 μg) was reverse transcribed using the Multiscribe reverse transcriptase (Applied Biosystems, Foster City, CA). Real-time PCR reactions were performed as previously described.23 Gene-specific primers for glyceraldehyde 3-phosphate dehydrogenase (GAPDH), TNF-α, IL1-β, MMP-1, MMP-3, MMP-13, ADAMTS-4, ADAMTS-5, AGN, and Col2 (forward and reverse) were designed based on GenBank cDNA sequences. Primer sequences are available on request. Quantitative analyses were performed using BioRad iCycler software, and standard curves were generated using 10-fold serial dilutions of cDNA with a correlation coefficient of ≥0.9 and a PCR efficiency of ≥80%.
Statistical analyses
Statistical significance was determined by Student's t-test with significance at p ≤ 0.05.
Results
Effects of proinflammatory cytokines on the biochemical properties of engineered cartilage
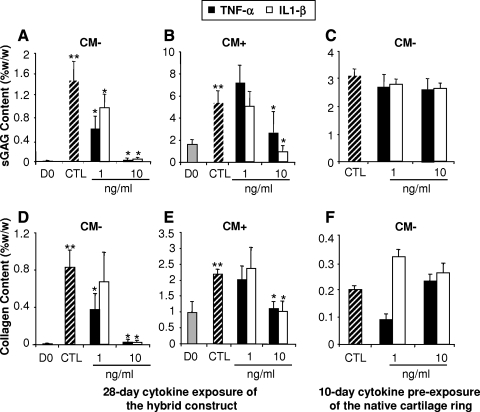
Engineered agarose disks fabricated by encapsulation of chondrocytes were press-fit implanted into native cartilage rings immediately or after 21 days of culture in CM+. The hybrid constructs were then cultured for 28 days in CM− containing varying concentrations of IL1-β or TNF-α (1 and 10 ng/mL) or without cytokines as control (CTL). The biochemical properties of the cell-laden agarose disks were determined. By day 28, sGAG and collagen contents (normalized to ww) significantly increased in chondrocyte-laden disks cultured without cytokine in CM− (Fig. 1A, D) or CM+ (Fig. 1B, E) groups, compared to the fresh disks at day 0 (p < 0.05). However, in CM− groups, treatment with IL1-β or TNF-α at both 1 and 10 ng/mL significantly diminished sGAG and collagen deposition (p < 0.05) (Fig. 1A, D). In contrast, implants precultured in CM+ medium for 21 days before implantation in cartilage rings were less sensitive to cytokine treatment, exhibiting significantly decreased sGAG and collagen contents only at the higher cytokine concentration of 10 ng/mL, with no effect observed at 1 ng/mL (Fig. 1B, E). In CM− groups, real-time PCR analysis showed more than a threefold decrease in AGN mRNA level in constructs cultured with 1 and 10 ng/mL of both cytokines, while Col2 mRNA expression was also suppressed except at 1 ng/mL of TNF-α (results not shown). When the cartilage rings were pretreated for 10 days with IL1-β or TNF-α before the implantation of the agarose disks, no significant additional decrease in sGAG and collagen deposition resulted in the cartilage construct upon further culture in CM− medium for 28 days (Fig. 1C, F). Importantly, under the conditions tested, IL1-β and TNF-α treatment did not alter the total DNA content of the chondrocyte-laden constructs, suggesting that the effects of IL1-β and TNF-α were unlikely to be related to changes in cell number (Fig. 5A).
FIG. 1.
(A–C) Sulfated glycosaminoglycan (sGAG) content (%ww) and (D–F) bulk collagen content (%ww) of chondrocyte-laden constructs in response to cytokine treatment (1 and 10 ng/mL). Control (CTL), no cytokine treatment. (A, D) After 28 days in CM− medium, sGAG and collagen contents of chondrocyte-laden constructs (CTL), removed from the cartilage rings, were significantly increased compared to day 0 (D0) freshly prepared constructs (p < 0.05). The 28-day interleukin 1-β (IL1-β) and tumor necrosis factor-α (TNF-α) treatments resulted in a dose-dependent decrease in sGAG and collagen contents compared to the CTL group. (B, E) Compared to the freshly prepared 21-day CM+ precultured chondrocyte-laden constructs (D0), the chondrocyte-laden constructs removed from the cartilage rings cultured for another 28 days in CM− (CTL) displayed a significant increase in sGAG and collagen contents. A 21-day CM+ preculture of chondrocyte-laden constructs blocked the decrease in sGAG and collagen contents induced by the 1 ng/mL IL1-β or TNF-α treatment. However, at the higher dose tested (10 ng/mL), sGAG and collagen contents were reduced to baseline (D0) levels. (C, F) No significant effect of a 10-day preexposure of the cartilage rings to IL1-β and TNF-α (1 and 10 ng/mL) on sGAG and collagen contents of chondrocyte-laden constructs before a 28-day culture period in CM− in the absence of cytokines. **p < 0.05 versus D0; *p < 0.05 versus CTL. Values are mean ± SD (n = 5).
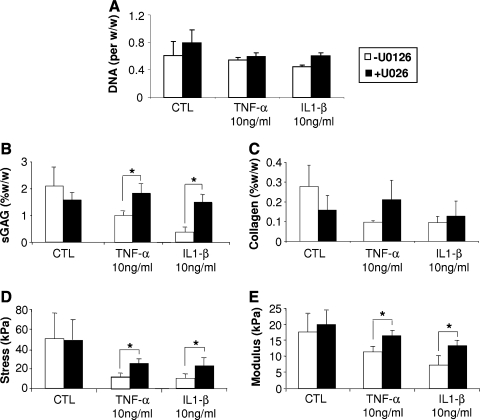
FIG. 5.
ERK pathway involvement in IL1-β– and TNF-α–induced deleterious effects on cartilage matrix production and integration of engineered cartilage. (A) DNA content (DNA per ww) in chondrocyte-laden agarose constructs. (B, C) After 28 days of culture in CM−, sGAG and collagen contents of chondrocyte-laden constructs removed from the cartilage rings were assessed. IL1-β and TNF-α treatment decreased sGAG and collagen contents compared to the CTL group, which was reversed by U0126. (D, E) U0126 prevented (D) the decrease in adhesive strength of the integration interface recorded after the 28-day treatment with IL1-β and TNF-α, as well as (E) the compromised Equilibrium Young's modulus resulting from cytokine treatment. Values are mean ± SD (n = 5). *p < 0.05.
Effects of proinflammatory cytokines on the biomechanical properties of engineered cartilage
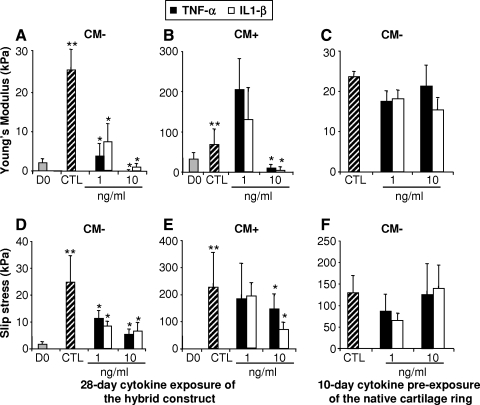
After 28 days in CM− culture medium, the Young's modulus of the constructs precultured in CM+ for 21 days before implantation into the native cartilage rings was significantly higher than that of the constructs freshly implanted into the native cartilage rings (68.89 ± 35.53 kPa and 25.80 ± 4.97 kPa, respectively) (p < 0.05) (Fig. 2A, B). Treatment with cytokines at 1 ng/mL showed no significant effect on the mechanical properties of the CM+ constructs, but 10 ng/mL of IL1-β and TNF-α drastically reduced the Young's modulus values for both CM− and CM+ constructs (Fig. 2A, B). Culturing with native cartilage rings pretreated with 1 and 10 ng/mL of both cytokines had no additional effect on the dynamic modulus of the chondrocyte-laden constructs maintained in CM− medium for 28 days (Fig. 2C).
FIG. 2.
(A–C) Equilibrium Young's modulus (Eγ) for chondrocyte-seeded agarose constructs, and (D–F) adhesive strength of the integration interface, as a function of interleukin 1-β (IL1-β) and TNF-α treatment. Experimental scheme was similar to that in Figure 1. Treatment with 10 ng/mL of IL1-β and TNF-α significantly reduced the mechanical properties of both CM− and CM+ constructs as well as their integration to the native cartilage. **p < 0.05 versus D0; *p < 0.05 versus CTL. Values are mean ± SD (n = 5).
Effects of proinflammatory cytokines on adhesive strength of the integration interface
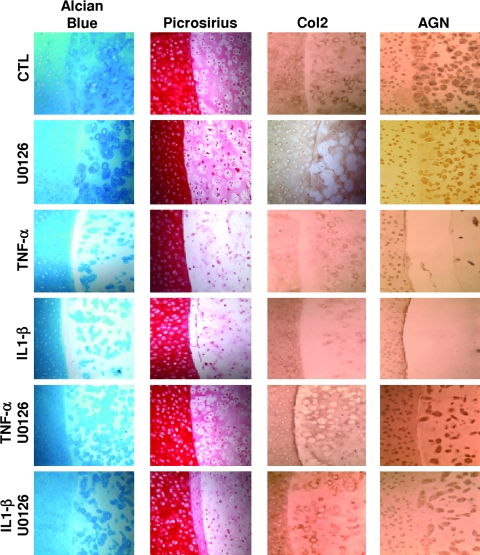
Hybrid constructs consisting of engineered cartilage disks that were precultured for 21 days in CM+ and then maintained in cytokine-free CM− medium for another 28 days postimplantation in the cartilage ring exhibited higher interface strength during push-out test (failure stress value of 226.7 ± 127.1 kPa vs. 24.8 ± 9.6 kPa for CM− group) (Fig. 2D, E). Cytokine treatment at all concentrations tested significantly lowered interface strength (Fig. 2D) for the CM− group, whereas 21-day preculture in CM+ overcame the effect of the cytokines at a concentration of 1 ng/mL (Fig. 2E). Histological staining confirmed the negative effect of the proinflammatory cytokines on matrix deposition. Indeed, compared to the CTL group, treatment of the hybrid constructs for 28 days with 10 ng/mL of IL1-β or TNF-α decreased both Picrosirius red and Alcian blue staining in the constructs (Fig. 6). Similarly, immunohistochemistry revealed that cytokine treatment resulted in marked decrease in Col2 and AGN protein levels (Fig. 6). In addition, there appeared to be a complete disappearance of the adhesive integrating interface (Fig. 6), paralleling the dramatic decrease in strength of the native/engineered interface (Fig. 2D).
FIG. 6.
Involvement of extracellular regulated kinase (ERK) signaling pathway in the deleterious effects on cartilage matrix deposition at the interface zone induced by IL1-β and TNF-α. Alcian blue and Picrosirius red staining as well as immunostaining for Col2 and AGN at the integration interface area revealed reduced cartilage matrix components upon exposure to the proinflammatory cytokines, which was blocked by co-treatment with U0126.
In constructs where the cartilage rings were precultured with IL1-β and TNF-α 10 days before implantation of the chondrocyte-laden constructs, continued treatment with cytokines did significantly further reduced the adhesive strength of the integration interface (Fig. 2F), as observed with mechanical properties (Fig. 2C).
Effects of proinflammatory cytokines on MMP-2 and MMP-9 secretion
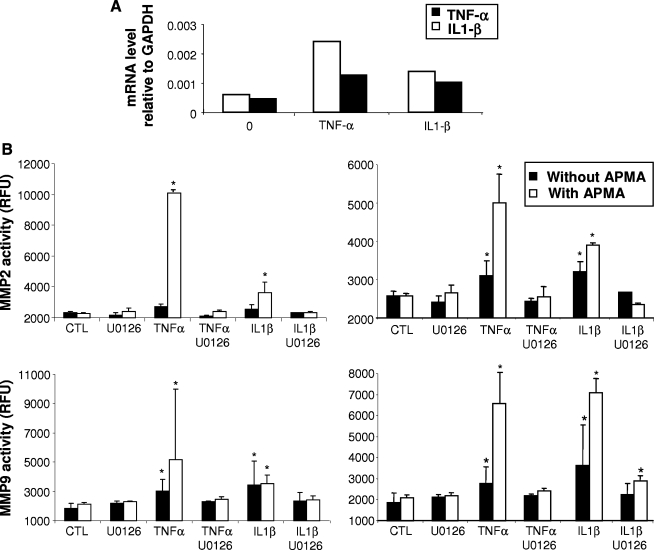
We next assessed the mechanism of action of IL1-β and TNF-α using monolayer cultures of bovine chondrocytes. A 48 h treatment with IL1-β or TNF-α resulted in increased mRNA expression levels of both cytokines, supporting the cross-interacting nature of these cytokines (Fig. 3A).
FIG. 3.
Effect of IL1-β and TNF-α (10 ng/mL) treatment on their self-expression and on metalloproteinase (MMP)-2 and MMP-9 activities in chondrocytes in monolayer cultured for 72 h and in chondrocyte-laden agarose constructs cultured for 28 days. (A) mRNA levels of IL1-β and TNF-α induced by 10 ng/mL of both cytokines in monolayer cultures of chondrocytes. (B) MMP-2 and MMP-9 activities measured in a 72 h culture supernatant of chondrocytes (on the left) and in a 72 h culture supernatant of hybrid constructs at day 28 (on the right). In both culture conditions, at 10 ng/mL, the cytokines induced an increase in MMP-2 and MMP-9 activities. IL1-β– and TNF-α–induced MMP-2 and MMP-9 activities were significantly reduced when cultures were co-treated with U0126. Values correspond to the mean ± SD of three independent experiments. *p < 0.05 versus CTL. APMA, p-aminophenylmercuric acetate.
As IL1-β and TNF-α are known to increase MMP production (reviewed in Ries and Petrides24), we analyzed their effects on MMP-2 and MMP-9 secretion both in chondrocytes in monolayer and in the hybrid cartilage constructs. Fresh chondrocyte-laden agarose disks were implanted in native cartilage rings and cultured for 28 days in CM−. Active and total (i.e., proplus active) activities of MMP-2 and MMP-9 were quantified in 72 h culture supernatants harvested in both chondrocytes monolayer cultures and at the end of the experiment involving hybrid cartilage constructs, that is, at day 28, and treated without or with APMA, respectively. IL1-β and TNF-α treatment resulted in a dose-responsive increase in MMP-2 and MMP-9 activities (Fig. 3B).
ERK pathway inhibition suppresses IL1-β– and TNF-α–induced MMP-2, MMP-9 activities
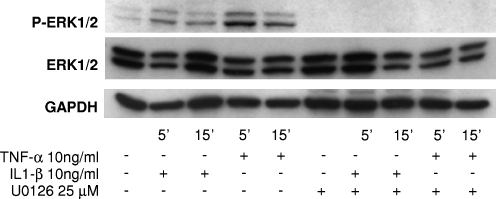
Chondrocyte monolayer cultures treated with the proinflammatory cytokines at 10 ng/mL showed ERK1/2 phosphorylation by 5 min in immunoblot analysis (Fig. 4). ERK phosphorylation was maximal by 5 min (Fig. 4) for both cytokines, and declined gradually over time to baseline level by 24 h after the stimulation (data not shown). By 15 min after IL1-β or TNF-α treatment, ERK phosphorylation decreased substantially (Fig. 4), with no appreciable changes in total ERK1 or ERK2 protein levels. Treatment with 25 μM U0126, a highly selective and potent inhibitor of MEK,25 the upstream activator of ERK1/2, added 30 min before IL1-β or TNF-α treatment, completely inhibited ERK1/2 phosphorylation without affecting total levels of ERK (Fig. 4).
FIG. 4.
Time course of extracellular regulated kinase (ERK)1/2 activation after IL1-β or TNF-α treatment. Representative Western blots obtained from cultures derived from six different bovine carpometacarpal joints are shown. Phosphorylated ERK1/2 was detected rapidly after treatment with IL1-β or TNF-α, and levels remained elevated for up to 15 min. Total levels of ERK protein were unchanged with cytokine treatment. At 25 μM, U0126 completely inhibited ERK1/2 phosphorylation.
The role of ERK1/2 activation on MMP-2 and MMP-9 activities in response to IL1-β or TNF-α was also assessed in chondrocytes in monolayer and in hybrid construct cultures. In both cases, co-treatment of the constructs with U0126 significantly decreased total MMP-2 and MMP-9 activities induced by both IL1-β and TNF-α (Fig. 3B). Importantly, under the conditions tested, U0126 treatment did not alter the DNA content (normalized to ww) of the chondrocyte-laden constructs, suggesting that treatment with the MEK inhibitor was unlikely to affect cell proliferation (Fig. 5A).
Effects of IL1-β and TNF-α on mRNA expression of MMPs and ADAMTSs: involvement of ERK1/2 pathway
In cultured bovine chondrocytes, increased IL1-β and TNF-α mRNA expression levels in response to IL1-β or TNF-α stimulation were partially or completely blocked in the presence of U0126 (Table 1).
Table 1.
Expression of Metalloproteinases (MMPs) and ADAMTSs in Monolayer Cultures of Primary Chondrocytes in Response to Interleukin 1-β (IL1-β) and Necrosis Factor-α (TNF-α), and Co-treatment with U0126
| MMP-1 | MMP-3 | MMP-13 | ADAMTS4 | ADAMTS5 | |
|---|---|---|---|---|---|
| CTL | 0 | 0.01 | 0 | 0 | 0.29 |
| IL1-β | 0.02 | 32.00 | 13.93 | 0.08 | 16.00 |
| TNF-α | 0.02 | 274.37 | 27.86 | 0.40 | 18.38 |
| IL1-β + U0126 | 0 | 19.70 | 2.30 | 0.04 | 6.96 |
| TNF-α + U0126 | 0 | 168.90 | 2.83 | 0.12 | 6.50 |
Chondrocytes were cultured for 48 h with or without 10 ng/mL IL1-β or TNF-α, and the effect of the ERK inhibitor U0126 was examined. Gene expression was analyzed by real-time RT-PCR using a mixture of articular chondrocytes harvested from six different carpometacarpal joints of the forelimbs of young calves. Values represent the relative levels of the respective transcripts normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA.
Real-time RT-PCR analysis of cultures treated with 10 ng/mL of IL1-β or TNF-α for 48 h revealed that both cytokines increased the mRNA expression levels of MMP-1, MMP-13, and MMP-3. ADAMTS-4 and ADAMTS-5 mRNA expression was potently induced by a 48 h treatment with IL1-β (∼38-fold and ∼56-fold, respectively) or TNF-α (∼203-fold and ∼64-fold, respectively) (Table 1).
Upon inhibition of the ERK pathway (25 μM U0126), real-time RT-PCR showed suppression of IL1-β- and TNF-α–stimulated up-regulation of MMP-3 by 38.5% and 38.5%, respectively. More importantly, ERK inhibition suppressed IL1-β and TNF-α stimulation of MMP-1 by 93.2% and 91.2%, respectively, and of MMP-13 by 83.6% and 89.9%, respectively.
Similarly, with U0126 pretreatment, the increase in ADAMTS-4 and ADAMTS-5 mRNA levels potently induced by IL1-β and TNF-α was reduced by 42% and 69% for ADAMTS-4, and by 56.5% and 64.7% for ADAMTS-5, respectively.
ERK pathway inhibition suppresses deleterious effects of IL1-β and TNF-α on integration
Hybrid constructs were cultured in CM− with or without IL1-β or TNF-α (10 ng/mL) and with or without U0126 (25 μM) for 28 days. On day 28, the sGAG content of the chondrocyte-seeded agarose constructs removed from the cartilage rings (2.1 ± 0.7%ww) was significantly affected by treatment with IL1-β (0.4 ± 0.1%ww) or TNF-α (1 ± 0.1%ww). U0126 treatment blocked the reduction of sGAG content induced by the proinflammatory cytokines (p < 0.05) (Fig. 5B). The collagen content of constructs (0.3 ± 0.1% w/w in CTL group) was also affected by TNF-α (0.1 ± 0.01% w/w) and IL1-β (0.1 ± 0.03% w/w) treatment, which was slightly but not significantly reversed by co-treatment with U0126 (0.2 ± 0.1% w/w and 0.13 ± 0.08% w/w, respectively) (Fig. 5C). Real-time RT-PCR showed a down-regulation of the mRNA levels of AGN and Col2 in the constructs cultured in the presence of IL1-β and TNF-α, which was partially inhibited upon inhibition of ERK activation (Table 2). By day 28, histological staining showed a clear decrease in sulfated proteoglycan content and in fibrous collagen matrix, as assessed by Alcian blue and Picrosirius staining, respectively, particularly in the interface zone for constructs cultured with proinflammatory cytokines (Fig. 6). In both cases, U0126 substantially suppressed the down-regulation of ECM production induced by both cytokines (Fig. 6).
Table 2.
Expression of Aggrecan (AGN) and Collagen Type II (Col2) by Chondrocyte-Laden Agarose Constructs
| AGN | Col2 | |
|---|---|---|
| CTL | 0.06 | 0.9 |
| IL1-β | 0.01 | 0.2 |
| TNF-α | 0.02 | 0.1 |
| IL1-β + U0126 | 0.06 | 0.5 |
| TNF-α + U0126 | 0.04 | 0.2 |
Constructs were cultured for 28 days in CM− with or without 10 ng/mL IL1-β or TNF-α, and the effect of the ERK inhibitor U0126 was analyzed. Real-time RT-PCR analysis of gene expression was performed using a mixture of constructs (n = 5). Values represent the relative levels of the respective transcripts (× 10) normalized to that of GAPDH mRNA.
Interestingly, ERK inhibition also significantly blocked the IL1-β– and TNF-α–induced compromised integration between the engineered and the native tissue in terms of interfacial mechanical properties: IL1-β (9.9 ± 3.9 kPa to 23.1 ± 6.8 kPa) and TNF-α (11.9 ± 3.1 kPa to 24.9 ± 3.9 kPa) (p < 0.05) (Fig. 5D). In parallel, ERK inhibition improved the mechanical properties of the constructs in terms of dynamic modulus. Specifically, on day 28, CTL constructs reached a Young's modulus of 17.7 ± 5.5 kPa, while IL1-β– and TNF-α–treated samples reached 7.30 ± 2.8 kPa and 11.5 ± 1.7 kPa, respectively; this cytokine-induced decrease in compressive stiffness was significantly reversed by treatment with U0126 (p < 0.05) (Fig. 5E).
Discussion
Proinflammatory cytokine treatment has profound impact on the integration/maturation of chondrocyte-laden hydrogels after implantation into native cartilage in vitro. Using an established bovine in vitro cartilage defect model, we showed that both IL1-β and TNF-α have a catastrophic effect on the biochemical and biomechanical properties of the engineered cartilage and on the development of a sufficient native cartilage–construct interface. Moreover, we demonstrated for the first time that the blockade of IL1-β and TNF-α signaling and their molecular targets using an inhibitor of ERK pathway is beneficial for restoring the biosynthetic activity of chondrocytes, for maintaining the composition and structural organization of the cartilaginous ECM, and for allowing integrative cartilage repair.
Preculturing constructs in TGF-β3–containing medium (CM+) before their implantation in native cartilage rings promotes ECM deposition and enhances the establishment of a functional biological interface between the engineered and the native tissue. TGF-β3 is likely to act via enhanced ECM synthesis by the chondrocytes to yield an appropriate spatial distribution of cells and an appropriate ECM arrangement within the agarose scaffold to approximate that of native cartilage, thereby strengthening the interface. This postulate would be consistent with previous studies showing that fusion between two pieces of cartilage occurred only when they were from the same developmental origin but not when the origins differed.26,27 Thus, the differences in integration observed between the nonprecultured and precultured constructs in CM+ may be attributed to a greater similarity in structure and composition of the latter to native cartilage.
ECM accumulation and subsequent increase in compressive Young's modulus of the chondrocyte-laden constructs, evident after 28 days of culture in both CM− and CM+ groups, were significantly decreased upon IL1-β and TNF-α treatment. Interestingly, while cultures treated at both low and high cytokine concentrations showed significantly lower interface strength, 21-day preculture in CM+ prevented the deleterious effect of the cytokines at a concentration of 1 ng/mL. Thus, enhancement of cartilaginous ECM production, mechanical properties, and failure stress values by preculturing with TGF-β3 should provide protection from cytokine exposure. Moreover, our study underscores the effect of the 28-day preculture in CM− on matrix accumulation at a similar level as 21-day preculture constructs in CM+. This observation raises the possible importance of two variables, preculture time and TGF-β3, in providing the constructs protection from the deleterious effects of the cytokines; the nature of this protection will be investigated in the future. However, at a higher concentration of 10 ng/mL, both cytokines significantly affect ECM content and distribution as well as the equilibrium Young's modulus and strength of the integration. The antagonistic activities between TGF-β3, which enhances ECM deposition by inducing collagen gene expression,28 and the proinflammatory cytokines have previously been proposed.29 Our results are also supported in light of the traditional view that TGF-β plays a protective role in matrix metabolism.30,31 Moreover, TGF-β has been described to inhibit IL1-β–induced chondrocyte protease activity and cartilage proteoglycan degradation, and promote proteoglycan synthesis in cartilage.32 However, our data point out that the balance between the catabolic versus anabolic stimuli provided by proinflammatory cytokines and TGF-β3, observed in the presence of 1 ng/mL of both cytokines, is unstable. Thus, at a higher cytokine concentration, the loss of chondrocytic phenotype and the associated compromised structure and composition of the cartilage ECM resulted in an inferior construct with poor integration efficiency.
Overall, the histological integration patterns correlated with the measured adhesive strength of the tissue interface, which was markedly lowered by exposure to IL1-β and TNF-α. Functional integration is thus dependent on both deposition of ECM as well as active tissue remodeling by proliferative cells.
To mimic the pathological, cytokine-rich environmental characteristic of OA or RA, we preexposed the cartilage rings to various concentrations of IL1-β and TNF-α for 10 days before insertional implantation of the chondrocyte-laden disks, and the composites were then cultured in CM− for 28 days in the absence of cytokines. In this setup, the transient exposure of the cartilage rings to cytokines was insufficient to stimulate catabolic mediator secretion by chondrocytes resident inside the host native cartilage at a level that might affect the biochemical and biomechanical properties of the constructs or their capacity to integrate with the native cartilage. This preexposure to cytokines also failed to disturb the balance between catabolism and anabolism or to induce a sustained activation of signals to result in deleterious effects, such as observed for continuous treatment, and also did not affect the host cartilage tissue receptivity to integration in term of matrix metabolism and content. Thus, repeated exposures are necessary to maintain active IL1-β and TNF-α pathways.
IL1-β and TNF-α activate MMP gene expression in chondrocytes through signal transduction pathways, such as MAPKs.6 We have targeted here ERK because ERK signaling pathway was shown to play a major role in the antianabolic and catabolic effects induced by proinflammatory cytokines on articular chondrocytes, specifically on Col2 repression and MMP-1 and MMP-13 up-regulation.10 We observed increased ERK1/2 phosphorylation 5 min after the stimulation of cultured chondrocytes with IL1-β and TNF-α. In parallel, we showed that after 48 h treatment, mRNA expression levels of MMP-1, MMP-3, and MMP-13 as well as ADAMTS-4 and ADAMTS-5, known to be involved in cartilage destruction, were also increased in response to both cytokines. U0126, a selective inhibitor of ERK signaling, effectively blocked cytokine induction of these MMPs (Table 1). Moreover, analysis of hybrid culture supernatant showed that MMP-2 and MMP-9 activities induced by the long-term chronic treatment with IL1-β and TNF-α were significantly decreased by U0126 co-treatment (Fig. 3B). Taken together, these data strongly suggest that ERK1/2 activation in response to IL1-β and TNF-α is needed for the expression of these major cartilage-damaging proteases.
In conclusion, our findings strongly suggest that the ERK pathway is critical in transducing the IL1-β and TNF-α signal to induce expression of MMPs responsible for cartilage destruction. In parallel, we have demonstrated for the first time that the dramatic deleterious effects caused by IL1-β and TNF-α on the integration/maturation of chondrocyte-laden hydrogels after implantation into native cartilage in vitro are reversed by the blockade of ERK signaling pathway. As inflammatory cytokines are involved in the pathogenesis of OA and RA, our results raise the importance of adjuvant antiinflammatory interventions in the framework of tissue engineering–based cartilage repair strategies, as well as the development of inhibitor(s) of downstream target genes and pathway-specific therapeutic agents to ensure long-term viability and functionality of the restored joint surface.33,34
Acknowledgment
Supported by NIAMS Intramural Research Program (NIH ZO1 AR 41131).
Authors' Contributions
F.D. performed experimental work, and analyzed and prepared the data and manuscript. L.R. participated in the experimental work and the analysis of the data. Y.S. performed the histology and the immunohistochemistry. S.J. helped in the analysis of the data. R.S.T. participated in experimental design and data analysis, prepared the manuscript, and supervised the project. All authors read and approved the final manuscript.
Disclosure Statement
The authors declare that they have no competing interests.
References
- 1.Spalazzi J.P. Doty S.B. Moffat K.L. Levine W.N. Lu H.H. Development of controlled matrix heterogeneity on a triphasic scaffold for orthopedic interface tissue engineering. Tissue Eng. 2006;12:3497. doi: 10.1089/ten.2006.12.3497. [DOI] [PubMed] [Google Scholar]
- 2.Goldring M.B. Berenbaum F. The regulation of chondrocyte function by proinflammatory mediators: prostaglandins and nitric oxide. Clin Orthop Relat Res. 2004;(467 Suppl):S37. doi: 10.1097/01.blo.0000144484.69656.e4. [DOI] [PubMed] [Google Scholar]
- 3.Lianxu C. Hongti J. Changlong Y. NF-kappaBp65-specific siRNA inhibits expression of genes of COX-2, NOS-2 and MMP-9 in rat IL-1beta-induced and TNF-alpha-induced chondrocytes. Osteoarthritis Cartilage. 2006;14:367. doi: 10.1016/j.joca.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Horiguchi M. Akiyama H. Ito H. Shigeno C. Nakamura T. Tumour necrosis factor-alpha up-regulates the expression of BMP-4 mRNA but inhibits chondrogenesis in mouse clonal chondrogenic EC cells, ATDC5. Cytokine. 2000;12:526. doi: 10.1006/cyto.1999.0577. [DOI] [PubMed] [Google Scholar]
- 5.van de Loo F.A. Joosten L.A. van Lent P.L. Arntz O.J. van den Berg W.B. Role of interleukin-1, tumor necrosis factor alpha, and interleukin-6 in cartilage proteoglycan metabolism and destruction. Effect of in situ blocking in murine antigen- and zymosan-induced arthritis. Arthritis Rheum. 1995;38:164. doi: 10.1002/art.1780380204. [DOI] [PubMed] [Google Scholar]
- 6.Burrage P.S. Mix K.S. Brinckerhoff C.E. Matrix metalloproteinases: role in arthritis. Front Biosci. 2006;11:529. doi: 10.2741/1817. [DOI] [PubMed] [Google Scholar]
- 7.Saklatvala J. Inflammatory signaling in cartilage: MAPK and NF-kappaB pathways in chondrocytes and the use of inhibitors for research into pathogenesis and therapy of osteoarthritis. Curr Drug Targets. 2007;8:305. doi: 10.2174/138945007779940115. [DOI] [PubMed] [Google Scholar]
- 8.Liacini A. Sylvester J. Li W.Q. Huang W. Dehnade F. Ahmad M. Zafarullah M. Induction of matrix metalloproteinase-13 gene expression by TNF-alpha is mediated by MAP kinases, AP-1, and NF-kappaB transcription factors in articular chondrocytes. Exp Cell Res. 2003;288:208. doi: 10.1016/s0014-4827(03)00180-0. [DOI] [PubMed] [Google Scholar]
- 9.El Mabrouk M. Sylvester J. Zafarullah M. Signaling pathways implicated in oncostatin M-induced aggrecanase-1 and matrix metalloproteinase-13 expression in human articular chondrocytes. Biochim Biophys Acta. 2007;1773:309. doi: 10.1016/j.bbamcr.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 10.Fan Z. Yang H. Bau B. Soder S. Aigner T. Role of mitogen-activated protein kinases and NFkappaB on IL-1beta-induced effects on collagen type II, MMP-1 and 13 mRNA expression in normal articular human chondrocytes. Rheumatol Int. 2006;26:900. doi: 10.1007/s00296-006-0114-7. [DOI] [PubMed] [Google Scholar]
- 11.Obradovic B. Martin I. Padera R.F. Treppo S. Freed L.E. Vunjak-Novakovic G. Integration of engineered cartilage. J Orthop Res. 2001;19:1089. doi: 10.1016/S0736-0266(01)00030-4. [DOI] [PubMed] [Google Scholar]
- 12.Hunter C.J. Levenston M.E. Maturation and integration of tissue-engineered cartilages within an in vitro defect repair model. Tissue Eng. 2004;10:736. doi: 10.1089/1076327041348310. [DOI] [PubMed] [Google Scholar]
- 13.Mauck R.L. Nicoll S.B. Seyhan S.L. Ateshian G.A. Hung C.T. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng. 2003;9:597. doi: 10.1089/107632703768247304. [DOI] [PubMed] [Google Scholar]
- 14.Mauck R.L. Seyhan S.L. Ateshian G.A. Hung C.T. Influence of seeding density and dynamic deformational loading on the developing structure/function relationships of chondrocyte-seeded agarose hydrogels. Ann Biomed Eng. 2002;30:1046. doi: 10.1114/1.1512676. [DOI] [PubMed] [Google Scholar]
- 15.Mauck R.L. Soltz M.A. Wang C.C. Wong D.D. Chao P.H. Valhmu W.B. Hung C.T. Ateshian G.A. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122:252. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 16.Farndale R.W. Buttle D.J. Barrett A.J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 17.Stegemann H. Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18:267. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- 18.Vunjak-Novakovic G. Martin I. Obradovic B. Treppo S. Grodzinsky A.J. Langer R. Freed L.E. Bioreactor cultivation conditions modulate the composition and mechanical properties of tissue-engineered cartilage. J Orthop Res. 1999;17:130. doi: 10.1002/jor.1100170119. [DOI] [PubMed] [Google Scholar]
- 19.Soltz M.A. Ateshian G.A. Experimental verification and theoretical prediction of cartilage interstitial fluid pressurization at an impermeable contact interface in confined compression. J Biomech. 1998;31:927. doi: 10.1016/s0021-9290(98)00105-5. [DOI] [PubMed] [Google Scholar]
- 20.Mauck R.L. Yuan X. Tuan R.S. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthritis Cartilage. 2006;14:179. doi: 10.1016/j.joca.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 21.An Y.H. Friedman R.J. Jiang M. LaBreck J.C. Draughn R.A. Butehorn H.F., 3rd Bauer T.W. Bone ingrowth to implant surfaces in an inflammatory arthritis model. J Orthop Res. 1998;16:576. doi: 10.1002/jor.1100160509. [DOI] [PubMed] [Google Scholar]
- 22.Dhert W.J. Verheyen C.C. Braak L.H. de Wijn J.R. Klein C.P. de Groot K. Rozing P.M. A finite element analysis of the push-out test: influence of test conditions. J Biomed Mater Res. 1992;26:119. doi: 10.1002/jbm.820260111. [DOI] [PubMed] [Google Scholar]
- 23.Derfoul A. Miyoshi A.D. Freeman D.E. Tuan R.S. Glucosamine promotes chondrogenic phenotype in both chondrocytes and mesenchymal stem cells and inhibits MMP-13 expression and matrix degradation. Osteoarthritis Cartilage. 2007;15:646. doi: 10.1016/j.joca.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 24.Ries C. Petrides P.E. Cytokine regulation of matrix metalloproteinase activity and its regulatory dysfunction in disease. Biol Chem Hoppe Seyler. 1995;376:345. [PubMed] [Google Scholar]
- 25.Duncia J.V. Santella J.B., 3rd Higley C.A. Pitts W.J. Wityak J. Frietze W.E. Rankin F.W. Sun J.H. Earl R.A. Tabaka A.C. Teleha C.A. Blom K.F. Favata M.F. Manos E.J. Daulerio A.J. Stradley D.A. Horiuchi K. Copeland R.A. Scherle P.A. Trzaskos J.M. Magolda R.L. Trainor G.L. Wexler R.R. Hobbs F.W. Olson R.E. MEK inhibitors: the chemistry and biological activity of U0126, its analogs, and cyclization products. Bioorg Med Chem Lett. 1998;8:2839. doi: 10.1016/s0960-894x(98)00522-8. [DOI] [PubMed] [Google Scholar]
- 26.Fyfe D.M. Hall B.K. Lack of association between avian cartilages of different embryological origins when maintained in vitro. Am J Anat. 1979;154:485. doi: 10.1002/aja.1001540404. [DOI] [PubMed] [Google Scholar]
- 27.Archer C.W. Redman S. Khan I. Bishop J. Richardson K. Enhancing tissue integration in cartilage repair procedures. J Anat. 2006;209:481. doi: 10.1111/j.1469-7580.2006.00624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ignotz R.A. Massague J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986;261:4337. [PubMed] [Google Scholar]
- 29.Verrecchia F. Mauviel A. TGF-beta and TNF-alpha: antagonistic cytokines controlling type I collagen gene expression. Cell Signal. 2004;16:873. doi: 10.1016/j.cellsig.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 30.Kuruvilla A.P. Shah R. Hochwald G.M. Liggitt H.D. Palladino M.A. Thorbecke G.J. Protective effect of transforming growth factor beta 1 on experimental autoimmune diseases in mice. Proc Natl Acad Sci USA. 1991;88:2918. doi: 10.1073/pnas.88.7.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brandes M.E. Allen J.B. Ogawa Y. Wahl S.M. Transforming growth factor beta 1 suppresses acute and chronic arthritis in experimental animals. J Clin Investig. 1991;87:1108. doi: 10.1172/JCI115073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Beuningen H.M. van der Kraan P.M. Arntz O.J. van den Berg W.B. Protection from interleukin 1 induced destruction of articular cartilage by transforming growth factor beta: studies in anatomically intact cartilage in vitro and in vivo. Ann Rheum Dis. 1993;52:185. doi: 10.1136/ard.52.3.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen F.H. Tuan R.S. Mesenchymal stem cells in arthritic diseases. Arth Res Ther. 2008;10:223. doi: 10.1186/ar2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steinert A.F. Noth U. Tuan R.S. Concepts in gene therapy for cartilage repair. Injury. 2008;39(S1):S97. doi: 10.1016/j.injury.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]