Abstract
The in vitro production of highly organized collagen fibrils by corneal keratocytes in a three-dimensional scaffold-free culture system presents a unique opportunity for the direct observation of organized matrix formation. The objective of this investigation was to develop such a culture system in a glass substrate (for optical accessibility) and to directly examine the effect of reducing serum and/or increasing insulin on the stratification and secretion of aligned matrix by fourth- to fifth-passage bovine corneal stromal keratocytes. Medium concentrations of 0%, 1%, or 10% fetal bovine serum and 0% or 1% insulin–transferrin–selenium were investigated. High-resolution differential interference contrast microscopy, quick-freeze/deep-etch, and conventional transmission electron microscopy were used to monitor the evolution, morphology, and ultrastructure of the cell–matrix constructs. In a medium containing 1% each of serum and insulin–transferrin–selenium, stromal cells stratified and secreted abundant and locally aligned matrix, generating the thickest cell–matrix constructs (allowing handling with forceps). The results of this study have the potential to significantly advance the field of developmental functional engineering of load-bearing tissues by (i) elucidating cues that modulate in vitro cell secretion of organized matrix and (ii) establishing an optically accessible cell culture system for investigating the mechanism of cell secretion of aligned collagen fibrils.
Introduction
Keratocytes are the principal cellular component of the adult corneal stroma. They express aldehyde dehydrogenase and keratocan,1,2 exhibit a dendritic/stellate morphology, interconnect using multiple long cellular processes,2,3 and help maintain the extracellular matrix (ECM) by synthesizing its components.4–8 After injury to the corneal stroma, keratocytes transform via a wound healing response into the more active phenotype of fibroblasts,9 and from there sometimes into myofibroblasts.10,11 These repair cells migrate to the wound area and secrete a repair ECM (collagen and proteoglycans)2 that is often fibrotic, fails to restore normal function, and may cause scarring and contraction of the cornea.12 Repair fibrotic tissue contains collagen types I and III and fibronectin,13,14 and altered proportions of proteoglycans.15,16 Stromal fibroblasts are spindle shaped, exhibit long cellular processes,17 actively secrete matrix metalloproteinases (MMPs), fibronectin, and α5 integrin,18–20 and are associated with corneal ulceration,21 low contractility, and lack of α-smooth muscle actin (α-SMA) expression or organized focal adhesions (reviewed in Refs.14,22). On the other hand, myofibroblasts actively express α-SMA and α5β1 integrin, exhibit pronounced stress fibers and focal adhesions,11,17,18,22 synthesize collagen types I and III and fibronectin,23,24 exhibit strong biomechanical activity,22 and when compared with fibroblasts show reduced expression of MMPs25,26 and are larger during in vitro culture.27
Precisely how the exquisite two-dimensional plus organization of the corneal stroma arises during development is not well understood.28 Two competing theories address the deposition of organized collagen. The first suggests that collagen is directly placed (via fibripositors) by the invading neural crest–derived mesenchymal cells,29 while the second suggests that collagen fibrils condense from concentrated solutions of collagen monomer.30 Determining the method by which fibroblastic cells produce organized collagen arrays is not only an important basic science question, but can also inform efforts to engineer load-bearing tissue in general.28 In a recent investigation by our laboratory, dedifferentiated human stromal keratocytes stratify and secrete a corneal-like organization of type I/V collagen lamellae in vitro in a scaffold-free tissue engineering system.31 To our knowledge, this is one of the first culture system capable of reproducing alternating arrays of collagen with organization similar to the corneal stroma. The principal purpose of this investigation is to robustly transfer the scaffold-free system onto a glass surface that will allow multiple differential interference contrast (DIC) investigations of the same culture at a high-temporal resolution and over long periods. We also are interested in reducing the dependence of culture on serum, which represents an uncontrolled variable. Due to the quiescent nature of stromal keratocytes, transformation into a more active phenotype (such as fibroblast or myofibroblast) must occur for sufficient ECM secretion. One way to guide in vitro stromal keratocyte transformation toward cell stratification and secretion of organized collagen is through the formulation of the culture medium.10,22,27,32,33 For example, keratocytes transform into myofibroblasts in vitro in the presence of transforming growth factor β (TGF-β). However, myofibroblasts produced via TGF-β–induced transformation of corneal stromal keratocytes exhibit contractile activity in vitro, which occasionally leads to a pulling23,34 of the construct into highly cellular clumps. This pulling is detrimental to our ultimate goal of generating organized arrays of collagen similar to those in the corneal stroma, and therefore we avoid the addition of exogenous TGF-β to the culture medium in the present study.
Stromal keratocytes undergo fibroblastic transformation in vitro in the presence of serum,23,35 while myofibroblastic transformation occurs in the presence of TGF-β1, regardless of serum concentration.23,35 Myofibroblastic transformation can be suppressed by the addition of basic fibroblast growth factor (bFGF)23 or entirely prevented by cultivation of primary keratocytes in the serum-free medium.23,36 However, for corneal stromal keratocytes, serum stimulates proliferation by 5- to 10-fold.23 It is important to minimize the concentration of serum because the exact composition of serum is both undefined and variable and because low-serum and serum-free culture media reduce the risk of contamination by pathogenic agents such as viruses and mycoplasma. Insulin, transferrin, and selenium have been shown to be critical components of serum-free media.37,38 Insulin promotes the uptake of glucose and amino acids and the synthesis of proteins and nucleic acids39; transferrin acts as an iron carrier (reviewed in Ref.40), and selenium is a powerful anti-oxidant (reviewed in Ref.41). Insulin–transferrin–selenium (ITS) is a precisely defined medium supplement that enhances the proliferation and differentiation of several cell types,42–48 increases the contraction of collagen gels by rat cardiac fibroblasts,49 and the synthesis of collagen by avian tendon cells.44 However, elimination of serum by replacement with insulin or ITS is often detrimental to cell survival, differentiation, and/or proliferation.42,50–52 Although the mechanisms of ITS action on in vitro cell proliferation and ECM synthesis are not entirely understood, its main component, insulin, increases type I collagen synthesis,53,54 and maintains keratocyte proliferation and morphology in the serum-free medium.32 It has been postulated that ascorbic acid, a cofactor in the production of collagen (for review, see Refs.55–56), and glucose share structural similarities that cause their metabolisms to interact during membrane transport and cellular processes. Thus, high glucose concentrations may inhibit the stimulatory action of ascorbic acid in the secretion of collagen, an effect that can be abolished by insulin.57
In this study, the effect of culture medium component modulation (serum 1–10%; ITS 0–1%) on cell stratification and secretion of aligned matrix by bovine corneal stromal fibroblasts cultured on glass was examined. It was hypothesized that the low-serum medium supplemented with ITS/bFGF could maintain organized collagen synthesis while converting the cells to a nonfibrotic phenotype.
Materials and Methods
Materials
Unless otherwise indicated, all materials and reagents were from Fisher Bioreagents (Fair Lawn, NJ). Sigma Aldrich (St. Louis, MO) was the source of antibiotic–antimycotic, bovine serum albumin (BSA), goat serum, and trypsin–ethylenediaminetetraacetic acid (EDTA). L-Ascorbic acid phosphate (a stable form of vitamin C) was from Wako Pure Chemical Industries (Osaka, Japan), bovine brain-derived bFGF and anti-human α-SMA phycoerythrin monoclonal antibody were from R&D Systems (Minneapolis, MN), ITS-A and rhodamine phalloidin were supplied by Invitrogen (Carlsbad, CA), and rabbit anti-bovine type I collagen polyclonal antibody and fluorescein isothiocyanate–conjugated sheep anti-rabbit IgG were from Chemicon International (Temecula, CA).
Cell culture
Bovine eyes were delivered within 8 h of slaughter from a local abattoir. The epithelium was scraped, and a square incision was made to remove the cornea. The endothelium and remaining limbus were then gently scraped. Corneas were rinsed several times with phosphate-buffered saline (PBS) with 1% antibiotic/antimycotic and minced into approximately 10 equally sized pieces. Excess liquid was removed, and corneal segments were allowed to attach to the bottom of six-well plastic culture dishes for 10 min. A small volume (0.04 mL/cm2 surface area) of the culture medium (Dulbecco's modified Eagle's medium with 1% antibiotic/antimycotic and 10% fetal bovine serum [FBS]) was added to the wells, and the corneas were cultivated overnight at standard incubator conditions (37°C, 5% CO2). Two milliliters of the culture medium was added to each well on the next day, and the corneas were cultivated in this manner for approximately 2 weeks, exchanging the medium every 5 days. After this period of time, the corneas were discarded and the wells were rinsed with PBS and treated with trypsin-EDTA (2.5 g trypsin/L and 2 g EDTA/L) for 5 min to release the cells that had migrated out of the corneas onto the tissue culture plastic. Cells were centrifuged, resuspended in the culture medium, and cultivated in standard tissue culture flasks. Fourth or fifth passage bovine corneal fibroblasts thus obtained were seeded on 1-cm-diameter glass-bottom cell culture dishes (MatTek, Ashland, MA) at 3 × 105 cells/cm2. Cultures were fed with 2 mL of basal culture medium (Dulbecco's modified Eagle's medium, 50 mg/L vitamin C, 10 ng/mL bFGF, and 1% antibiotic/antimycotic) supplemented with one of five different formulations described in Table 1. Cells were thus cultivated for 3 weeks with 100% medium exchanges every 3–4 days (i.e., twice a week).
Table 1.
Formulations of Culture Media
| Group | % FBS in medium | % ITS in medium |
|---|---|---|
| A | 10 | 0 |
| B | 1 | 0 |
| C | 0 | 0 |
| D | 1 | 1 |
| E | 0 | 1 |
ITS, insulin–transferrin–selenium; FBS, fetal bovine serum.
DIC microscopy
Cells were monitored using DIC microscopy every 3–4 days. DIC microscopy has excellent out-of-plane noise rejection (clearly resolved focal depths of ∼200 nm) and is capable of detecting organized fibrillar structures with x–y resolution on the order of 1/10th the wavelength of light. DIC allows for a clear observation of the cell morphology and reveals the organization of the aligned fibrils surrounding each individual cell.58 The procedure has been described elsewhere.58–60 Axial scans and snapshots were acquired using a Nikon TE2000U inverted microscope fitted with a 60 × 1.45 NA oil-immersion objective (Nikon, Melville, NY), a Coolsnap EZ camera (Photometrics, Tucson, AZ), and a Dell desktop computer. The thickness of the cell layer was determined from the axial scans and the microscope calibration. The number of cell layers was quantified by manual examination of at least six axial scans per time point and medium formulation.
Transmission electron microscopy
Samples for transmission electron microscopy (TEM) were prepared in two different ways: traditional preparation and quick-freeze/deep-etch (QFDE). Each of these types of sample preparation allowed for distinctive qualities of detail preservation and observation. In the traditional sample preparation, samples were immersed in ½ strength Karnovsky's fixative (2% paraformaldehyde, 2.5% glutaraldehyde, and 0.1 M cacodylate buffer, pH 7.4) for 1 h. Postfixation in 2% osmium tetroxide and serial dehydration in graded ethanol followed. After dehydration, constructs were embedded in Epon-Araldite and thin-sectioned en face or in cross section. After staining with uranyl acetate and lead citrate in methanol, construct sections were viewed and photographed on a JEOL JEM-1000 TEM (JEOL, Tokyo, Japan).
For QFDE sample preparation, after 3 weeks of cultivation the glass bottom of culture dishes was carefully removed from its plastic frame, and the cell sheets peeled off and cut into small pieces that were then preserved by slam freezing onto a copper block cooled to −194°C using a portable cryogun (Delaware Diamond Knives, Dover, DE). Frozen cell sheets were transferred under liquid nitrogen into a modified CFE-40 Freeze fracture/freeze etch apparatus (Cressington Scientific, Watford, United Kingdom). The cell sheets were superficially fractured at −150°C and etched for 15 min at −95°C. They were then rotary-shadowed at a 20° angle with Pt/C followed by a carbon coating applied at a 90° angle. After removal from the CFE-40, the sheets were digested in household bleach for 24 h, picked up on 600-mesh grids, and viewed at an acceleration of 70 kV on a JEOL JEM-1000 TEM.
In situ immunofluorescence
Cell constructs were stained in situ to investigate actin filament organization, detect α-SMA, and verify the secretion and large-scale organization of type I collagen. Compared to fibroblasts, myofibroblasts exhibit stronger stain for organized α-SMA and f-actin filaments. Cells for immunofluorescence were rinsed in PBS, fixed in 3% formalin for 10 min, and rinsed three times in PBS. For intracellular staining, fixed cells were permeabilized with 0.5% Triton X-100 and thoroughly rinsed with PBS. Cells were then treated according to one of the following protocols: (i) incubation with 1:20 phycoerythrin-conjugated antibody for α-SMA (1 h, room temperature), (ii) incubation with 1:40 rabbit anti-bovine collagen type I polyclonal antibody (1 h room temperature) followed by blocking with 1% goat serum (30 min, room temperature), wash and exposure to 33 μg/mL fluorescein isothiocyanate–conjugated sheep anti-rabbit IgG (1 h, room temperature), or (iii) blocking with 1% BSA (10 min, room temperature) followed by wash and incubation with 100 μL of rhodamine phalloidin in BSA (1:40) (20 min, room temperature). After three 5-min washes in PBS, all stained cells were observed on a Nikon TE2000E inverted microscope fitted with an epifluorescence attachment.
Statistics
An independent experiment was defined as the use of cells from one bovine specimen to seed a set of culture dishes at a minimum of one dish per culture medium formulation. The results are presented as average ± standard error. Student's t-test assuming equal variances was used to determine significant differences across groups. Significance was defined as p < 0.05.
Results
Cell proliferation and survival
In all cultures where serum was added to the medium (groups A, B, and D in Table 1), cells reached confluence within 1 week of cultivation. In contrast, cells cultured in the serum-free medium (groups C and E in Table 1) did not show appreciable proliferation, and failed to survive after approximately 1.5 weeks.
Culture microscopic morphology (DIC imaging)
Stratification and thickness
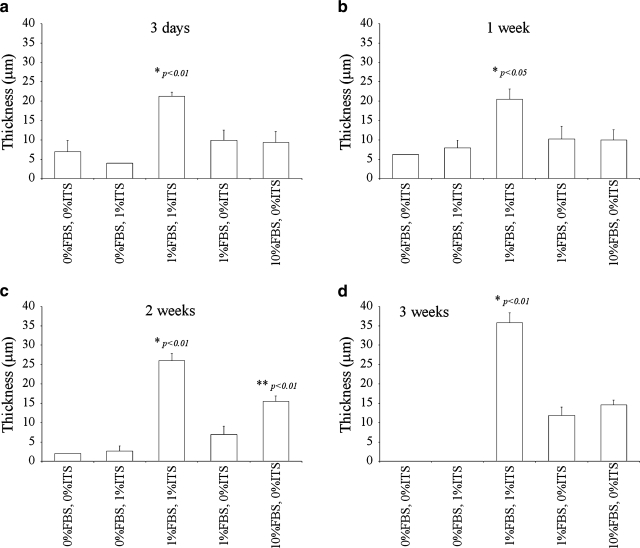
Multiple layers of cells were observed as early as the third day in cultures supplemented with 1% FBS + 1% ITS (group D in Table 1) and in cultures supplemented with either 1% or 10% FBS but no ITS (groups A and B in Table 1) (results not shown). The thickness of the cell sheets was significantly higher in cultures supplemented with 1% FBS + 1% ITS than in all other cultures throughout the culture period (Fig. 1). Cell sheets cultivated with 1% FBS + 1% ITS were 21.3 ± 1.0 μm thick on day 3 (Fig. 1a), and continued to thicken with time, reaching 35.8 ± 0.5 μm thickness at the end of the third week (Fig. 1d). This corresponds to a 1.7-fold increase in cell sheet thickness within an 18-day period. In comparison, cells cultivated with 10% FBS (group A in Table 1) formed sheets that were 9.3 ± 2.8 μm thick on day 3 (Fig. 1a) and had grown to 14.6 ± 1.2 μm at 3 weeks (Fig. 1d), corresponding to a 1.6-fold increase in thickness. Finally, 1% FBS cultures (group B in Table 1) exhibited less stratification, with an increase in thickness from 9.9 ± 2.7 μm to 11.9 ± 2.1 μm between the 3rd day (Fig. 1a) and 21st day (Fig. 1d) of cultivation (1.2-fold increase in thickness within the time frame). Three-week ITS-free cultures supplemented with 1% FBS or 10% FBS (groups A and B in Table 1) were only 33.1 ± 8.3 or 40.6 ± 6.2% (respectively) as thick as cultures supplemented with 1% FBS + 1% ITS at the same time point (Fig. 1d). The same comparison (thickness with respect to 1% FBS + 1% ITS culture) at 2 weeks yielded 26.7 ± 10.2% and 59.4 ± 9.6% for groups A and B in Table 1, respectively (Fig. 1c), and at 1 week it was 50.0 ± 22.5% and 48.8 ± 19.2% (Fig. 1b).
FIG. 1.
Thickness of constructs for the culture medium formulations described in Table 1, measured through differential interference contrast (DIC) microscopy. Results are average ± standard error of a minimum of four and a maximum of six independent experiments. *Significantly different to all other groups and **significantly different to serum-free groups. (a) 3 days, (b) 1 week, (c) 2 weeks, and (d) 3 weeks.
Cultures supplemented with 10% FBS were also significantly thicker than serum-free ones (groups C and E in Table 1) and thicker than those supplemented with 1% FBS but no ITS (group B in Table 1) on the second week of culture (Fig. 1c). At that particular time point, serum-free, ITS-free cultures (group C in Table 1) were only 12.9 ± 1.2% as thick as 10% FBS cultures, whereas for serum-free, 1% ITS cultures (group E in Table 1) and ITS-free, 1% FBS cultures (group B in Table 1) the same comparison yielded 17.2 ± 10.2% and 44.9 ± 18.0%. Interestingly, past the second week of cultivation, the thickness of cell sheets cultivated with 1% or 10% FBS but no ITS (groups A and B in Table 1) was statistically similar.
Cell morphology
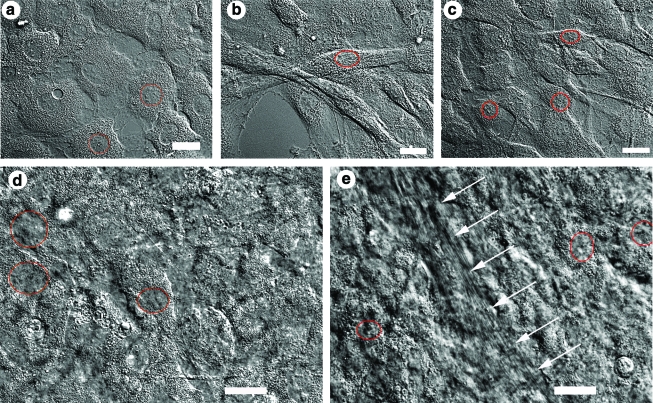
Cells cultivated without FBS and ITS appeared round with round nuclei during the first week of cultivation (Fig. 2a). On the second week of cultivation, these cells became more dendritic and in some cases assumed a “T” shape. An appreciable decrease in cell number was observed, such that by the third week of cultivation there were virtually no cells left on the culture surface (not shown). Cells cultivated with 1% ITS and no FBS (group E in Table 1) were confluent during the first week of cultivation (Fig. 2b). They were elongated with elliptical nuclei. During the second week of cultivation, the cell number started to decrease, such that there were virtually no cells left in the culture area on the third week of cultivation (not shown).
FIG. 2.
DIC microscopy images illustrating the differences in the morphology of bovine corneal fibroblasts cultivated for 1 week with the basic culture medium supplemented with (a) 0% fetal bovine serum (FBS) and 0% insulin–transferrin–selenium (ITS), (b) 0% FBS and 1% ITS, (c) 1% FBS and 0% ITS, (d) 10% FBS and 0% ITS, and (e) 1% FBS and 1% ITS; white arrows indicate streak of aligned matrix (most likely deposited collagen). Scale bars are 20 μm. Drawn circles or ellipses indicate the contour of some cell nuclei and are used to illustrate shape and size. Color images available online at www.liebertonline.com/ten.
In contrast, cells cultivated with 1% FBS and 0% ITS (group B in Table 1) proliferated actively, and the cell sheet was confluent by the end of the first week. Initially, these cells exhibited round nuclei and elongated cell bodies with long processes (Fig. 2c). A few ellipsoid cells were also observed (not shown). By the second week of cultivation, about one half of the cells were round, while the other half were ellipsoidal.
Cells cultivated in the presence of 10% FBS and 0% ITS (group A in Table 1) were confluent throughout the cultivation period (Fig. 2d). Due to a dense cell and ECM content, cellular features are not easily appreciable on the DIC micrographs in these cultures (Fig. 2d). However, it was observed that the nuclei of these cells were round, with cell bodies that were elongated with numerous processes during the first week of cultivation and that became more spread out by the end of the second week of cultivation (not shown).
Finally, cells cultivated simultaneously with 1% each of FBS and ITS (group D in Table 1) were confluent throughout the cultivation period, and similarly to the case of group A, dense cellular and ECM content obscured their cellular features in DIC images (Fig. 2e). Nonetheless, cells in the 1% FBS + 1% ITS group appeared less spread out than in all other groups and displayed round or elliptical nuclei. Cultures appeared to comprise more cell layers (i.e., increased cell stratification) than those in the other experimental groups, as assessed by qualitative examination of the library of DIC z-scans such as those in Supplemental Movies 1 and 2 (available online at www.liebertonline.com/ten). Although the previous statement does not convey statistical significance, it is supported by the observation of significantly increased construct thickness (Fig. 1). Cells in the 1% FBS + 1% ITS group did not exhibit numerous processes as did those cultivated in 10% FBS, nor did they possess a dendritic appearance upon visual inspection.
Extracellular fibrillar structures
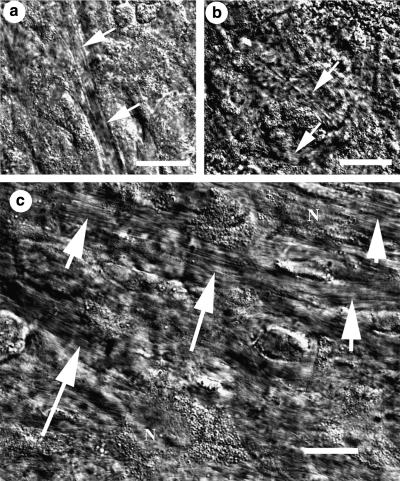
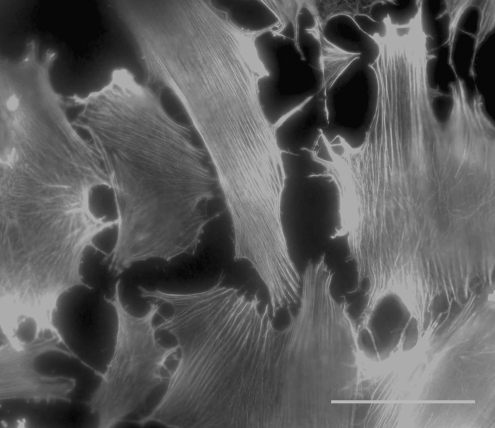
Aligned ECM (presumably bundles of collagen fibrils) was detected by DIC microscopy, which has very high spatial resolution in the z-plane, as early as the third day in cultivation for cultures supplemented with 1% FBS + 1% ITS (arrows in Fig. 3a). Matrix fibrils were detected after 7–10 days in those supplemented with either 1 or 10% FBS (arrows in Fig. 3b–10% FBS shown). Of these early fibrillar structures, only those in 1% FBS + 1% ITS cultures were visibly aligned (Fig. 3a). After 3 weeks, fibrillar ECM in 1% FBS + 1% ITS cultures was densely packed and demonstrated uniaxial alignment over reasonably long lateral length scales (∼100 μm; Fig. 3c), whereas in 10% FBS cultures the synthesized ECM remained sparse and disorganized (not shown).
FIG. 3.
DIC microscopy image of (a) 3-day cultures in 1% FBS + 1% ITS, (b) 10-day cultures in 10% FBS, and (c) 3-week cultures in 1% FBS + 1% ITS. Arrows point to what we believe are aligned collagen fibrillar structures in the extracellular matrix. N, cell nucleus. Scale bars are 20 μm.
Spatial organization of constructs
Representative z-scan DIC microscopy of culture systems that demonstrated appreciable stratification is provided in two Supplemental Movies (available online at www.liebertonline.com/ten). The z-scans begin at the glass substrate surface and move toward the culture medium–air interface. For the 10% FBS cultures, z-scans reveal a mostly cellular stratified construct interspersed with a loose fibrillar matrix. The 1% ITS + 1% FBS culture system typically comprised a dense matrix–cellular construct in which highly aligned fibrillar matrix occupied the center part of the constructs. Matrix fibrils were observed to align typically only in one direction throughout the axial scans of these cultures, suggesting that alternating orthogonal arrays of collagen similar to those found in the cornea were either not present or not discernible under DIC microscopy.
Cell contractility and pulling-off of the cell layer into highly cellular clumps was observed in n = 2 cultures that used 1% FBS + 1% ITS supplementation, in n = 5 cultures supplemented with 1% FBS but no ITS, and in n = 1 cultures supplemented with 10% FBS (not shown).
Biochemistry/immunostaining
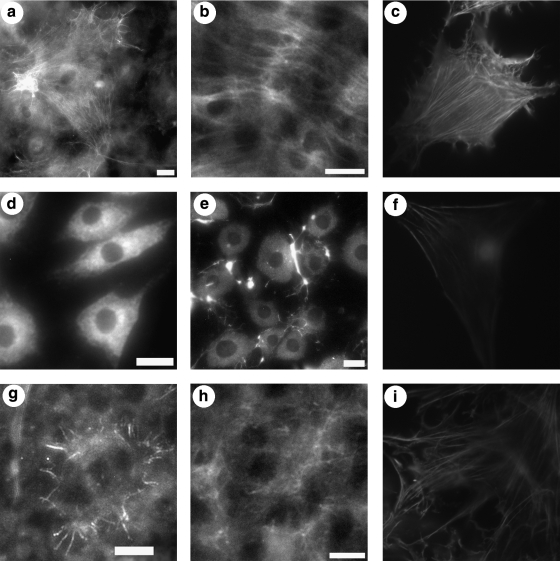
To detect differences in the density and/or organization of type I collagen and to investigate myofibroblastic transformation, 4-week cultures of dedifferentiated bovine corneal stromal keratocytes were stained for α-SMA, f-actin, and type I collagen. Cells in 1% FBS + 1% ITS showed prominent organization of actin filaments and intracellular α-SMA, suggestive of stress-fiber formation and myofibroblastic transformation. These cells also showed organized extracellular type I collagen (Figs. 4a–c and 5). 1% FBS + 0% ITS cultures did not stain robustly for f-actin, α-SMA, or type I collagen (Fig. 4d–f).Cells cultivated with 10% FBS + 0% ITS stained only peripherally for f-actin or α-SMA, and showed a diffuse type I collagen network (Fig. 4g–i).
FIG. 4.
Fluorescent images of 4-week cell sheets cultivated in 1% FBS + 1% ITS (a–c), 1% FBS (d–f), or 10% FBS (g–i) and stained with a phycoerythrin-conjugated antibody for α-smooth muscle actin (a, d, g), a collagen type I polyclonal antibody and a fluorescein isothiocyanate–conjugated secondary antibody (b, e, h), or phalloidin-rhodamine (c, f, i). All images were acquired using a 20 × objective; however, some images were cropped to remove artifacts. Scale bars are 20 μm. Asterisks in bindicate cell processes or filipodia.
FIG. 5.
Rhodamine phalloidin staining of 4-week cell sheet cultivated in 1% FBS + 1% ITS. Scale bar is 100 μm.
Cell and ECM ultrastructure
While DIC microscopy was used to observe large scale cell–matrix interaction and organization, standard preparation TEM (sTEM) micrographs provided a more detailed local account of the cell–matrix interaction, matrix organization, and fibrillar structure (e.g., collagen banding and periodicity). In addition to sTEM, we also used QFDE imaging to examine the fibroblasts and their synthesized ECM with higher fidelity and with fewer fixation and processing artifacts (although QFDE is not artifact free).
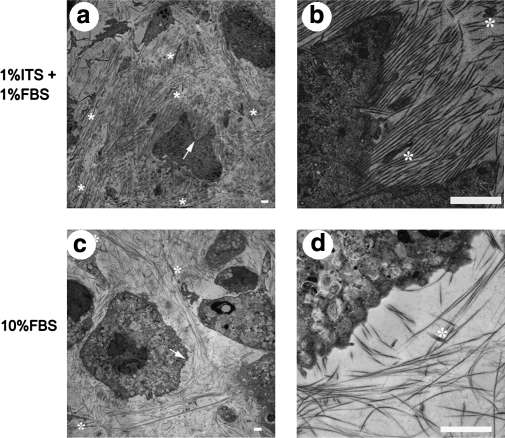
sTEM images from sections obtained at a distance of approximately 10 μm from the glass culture substrate corroborated the observations made using DIC microscopy. Measurements of n = 40 individual fibrils pooled from >10 TEM images served to positively identify ECM fibrils as collagenous due to their size and to the presence of characteristic banding (see Fig. 6 for representative high magnification TEM of cell-derived collagen). Though it is known that sTEM affects fibril diameter due to the dehydration processing, we felt that it was acceptable to determine fibril diameters directly from micrographs for direct comparison. Measurements of the smallest dimension of collagen fibrillar cross sections from sTEM images revealed a fibril diameter of 61.2 ± 0.9 nm for 1% FBS + 1% ITS cultures and 32.2 ± 0.7 nm for 10% FBS cultures, and this difference was significant (p < 1 × 10−10). Banding periodicity was 68.7 ± 2.2 nm and 60.3 ± 0.7 nm for 1% FBS + 1% ITS and 10% FBS cultures, respectively, and this difference was significant as well (p < 0.05). In 1% FBS + 1% ITS cultures, a higher density of collagen fibrils was observed, and these fibrils were generally oriented unidirectionally over length scales of 10–50 μm (Figs. 7a, b and 8a, b). In contrast, collagen fibrils were less dense in 10% FBS cultures, and did not show unidirectional alignment over the same length scale (Figs. 7c, d and 8c, d). However, occasional orthogonality in the direction of collagen layers separated by one cell layer was observed in these cultures (Fig. 8d).
FIG. 6.
Transmission electron microscopy (TEM) of bovine corneal stroma fibroblast cultures showing extracellular matrix fibrils with diameter (∼60 nm) and banding (arrowheads) characteristic of collagen. Scale bar is 500 nm.
FIG. 7.
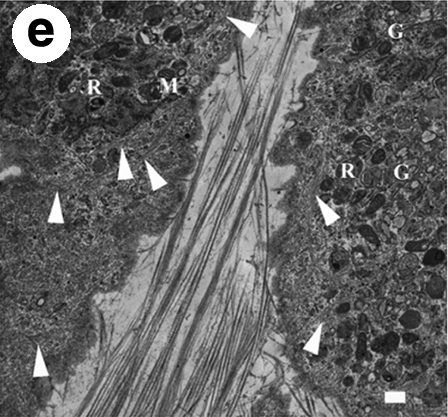
TEM images of en face sections from 3-week bovine corneal stroma fibroblasts cultivated in the culture medium supplemented with 1% FBS + 1% ITS (a, b, e) and 10% FBS (c, d). Panels (b) and (d) were taken at higher magnification at the same spot indicated by arrows in (a) and (c), respectively. Asterisks are used to indicate the presence of filopodia aligned in the same direction as surrounding collagen fibrils. Mitochondria (M), Golgi apparatus (G), residual bodies (R), and intracellular fibril bundles that may be actin or procollagen (arrowheads) are indicated in (e). Scale bars are 2 μm in (a–d) and 500 nm in (e).
FIG. 8.
TEM images of cross sections from 3-week bovine corneal stroma fibroblasts cultivated in the culture medium supplemented with 1% FBS + 1% ITS (a, b) and 10% FBS (c, d). Scale bars are 500 nm.
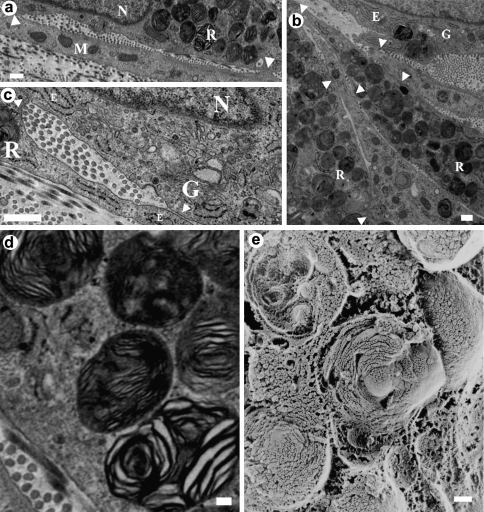
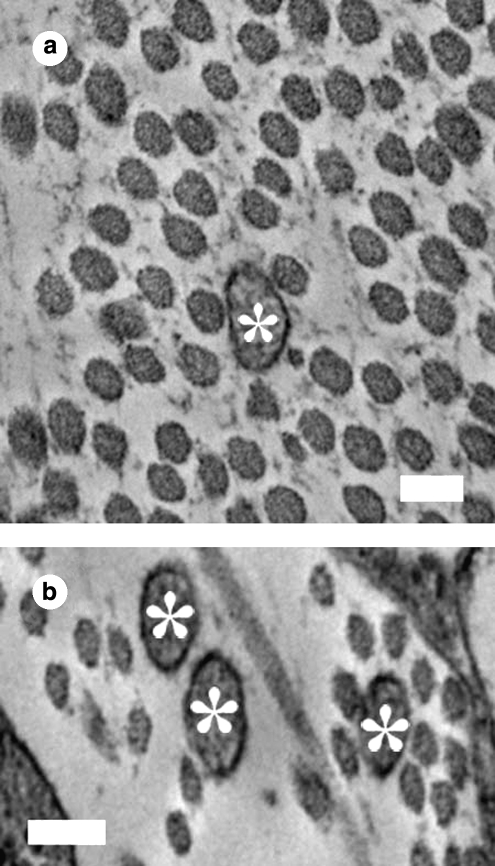
Interesting cellular features observed via traditional TEM included collagen fibrils appearing to emerge through the cell's membrane (Fig. 7b). In addition, extensive mitochondria, lysosomes, and residual bodies and what is believed to be either intracellular actin or collagen fibril bundles (Fig. 7e) were observed. Numerous cell–cell interactions and cell junctions between lamellar layers were discerned, in some cases the junctions produced a confined space between two cells where aligned collagen fibrils were present (Fig. 9a, c). In the intracellular space, there could be observed an abundance of residual bodies (Fig. 9b, d, and e). Further, abundant filopodia were observed within the extracellular space, and they appeared to orient in the same direction as surrounding collagen fibrils (Fig. 10).
FIG. 9.
Images from 3-week bovine corneal stroma fibroblasts cultivated in the culture medium supplemented with 1% FBS + 1% ITS: (a–d) cross-sectional traditional TEM and (e) quick-freeze/deep-etch (QFDE)/TEM. Arrowheads point to cell–cell junctions. Note the presence of matrix fibrils in between contacting cells. M, mitochondria; G, Golgi apparatus; E, rough endoplasmic reticulum; R, residual bodies; N, cell nucleus. (d, e) Close-up details of intracellular residual bodies with a concentric-ring shape observed in a traditional cross section. Scale bars are 500 nm in (a–c) and 100 nm in (d, e).
FIG. 10.
High-magnification TEM images of cross sections from 3-week bovine corneal stroma fibroblasts cultivated in the culture medium supplemented with 1% FBS + 1% ITS (a) or 10% FBS (b). Asterisks indicate filopodia in alignment with surrounding collagen fibrils. Scale bars are 100 nm.
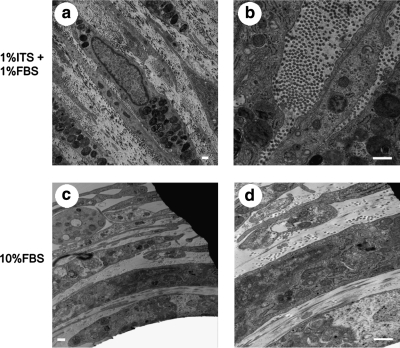
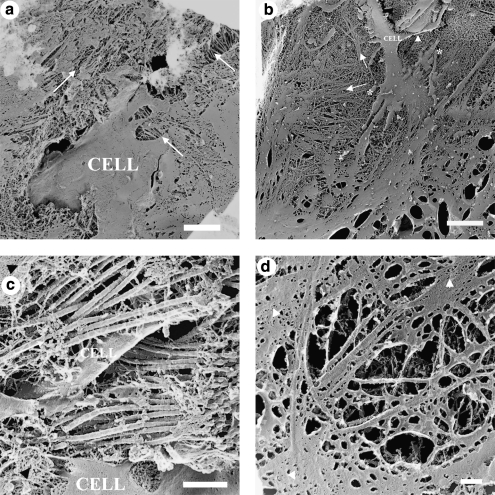
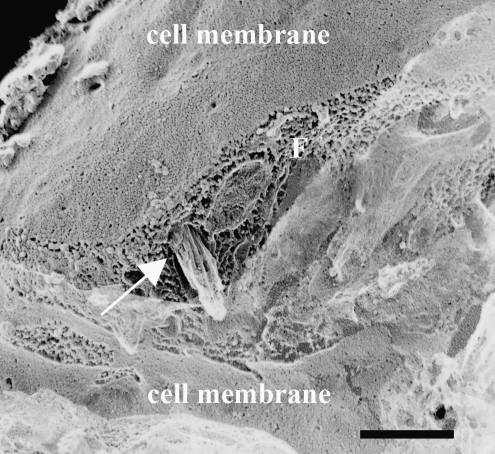
The TEM images obtained from samples prepared via QFDE (Figs. 11 and 12) corroborated the observations made on traditionally embedded TEM samples (Figs. 6–10) and DIC microscopy (Figs. 2 and 3; Supplemental Movies 1 and 2, available online at www.liebertonline.com/ten), as well as provided further insight into microstructural differences in the matrix secreted by cells cultivated with 10% FBS-supplemented or 1% FBS + 1% ITS-supplemented medium. Increased density of collagen fibrils was observed in 1% FBS + 1% ITS cultures (Fig. 11a vs. b, c vs. d). An increased number of cell processes (Fig. 11b) were observed in 10% FBS cultures. In both culture systems, collagen fibrils appeared in close relationship or attached to a secondary matrix (Fig. 11c, d), which may be material left behind after cell fracture by the knife during QFDE sample processing. In addition, a fibripositor-like structure was observed in a cell membrane. What appears to be a bundle of collagen fibrils is observed exiting the fibripositor-like structure (Fig. 12). In addition, the differences in collagen fibril diameter reported for traditionally prepared TEM images (Figs. 6–10) persisted in the QFDE specimens (Figs. 11 and 12), with 61.3 ± 1.1 nm and 42.1 ± 7.7 nm for 1% FBS + 1% ITS and 10% FBS cultures, respectively (p < 1 × 10−10). Banding of collagen fibrils in the QFDE specimens (such as those in Figs. 11 and 12) had a periodicity of 66.4 ± 1.8 nm and 63.6 ± 1.6 nm for 1% FBS + 1% ITS and 10% FBS cultures, respectively (p > 0.05). Fibril diameters measured using traditionally prepared versus QFDE-prepared TEM samples were statistically similar for 1% FBS + 1% ITS cultures (p = 0.09), but significantly different for 10% FBS cultures (p = 6 × 10−7), with fibrils in traditionally prepared TEM samples showing approximately 77% of the diameter of those in QFDE-prepared TEM samples.
FIG. 11.
TEM images showing fine cellular and matrix structures in culture samples that were prepared via QFDE. Panels (a) and (c) correspond to 1% FBS + 1% ITS cultures, (b) and (d) correspond to 10% FBS cultures. Remaining portions of the cell membrane in the fractured replicas are indicated by the word “CELL”; collagen fibrils are indicated by long arrows drawn parallel to the apparent direction of fibril orientation, and arrowheads point to what appears to be secondary matrix associated with the collagen fibrils. Scale bars are 2 μm in (a, b), and 500 nm in (c, d). Asterisks in b indicate cell processes or filipodia.
FIG. 12.
TEM image showing a fibripositor-like structure in a 1% FBS + 1% ITS culture sample that was prepared via QFDE. Remaining portions of the cell membrane in the fractured replica are indicated; “F” indicates the plane of fracture across the cell membrane. A fibripositor-like pore of approximately 530-nm-diameter is indicated by the arrow, with a tight bundle of fibrils extruding through the pore. Each fibril is approximately 30–40 nm in diameter. The bundle appears to be covered in a ground substance. Scale bar is 500 nm.
Discussion
During the in vivo development of the mammalian corneal stroma, cell stratification and organization occur before organized ECM production. Similarly, using an in vitro developmental corneal tissue engineering model, we recently reported that the stratification and organization of human corneal fibroblastic cells was an important factor preceding the production of organized collagenous lamellae.31 In the current investigation, both cell stratification and synthesis of aligned bundles of collagen by dedifferentiated bovine corneal stromal keratocytes cultivated on glass substrates were appreciably enhanced by using the culture medium supplemented with 1% each serum and ITS. However, this medium formulation induced unexpected phenotypic changes consistent with myofibroblastic transformation, and the resulting cell–matrix constructs were less mimetic to the ultrastructure of the cornea with respect to those in our previous study31 because they were not as thick and did not contain prolific alternating arrays of organized collagen lamella. The morphology of the collagen fibrils and the myofibroblastic transformation of the cells suggested that matrix production was more similar to scar formation than to developmental histogenesis. Cultures supplemented with 10% FBS only (no ITS) stratified and produced fine collagen that had individual fibril morphology similar to that found in the adult cornea. However, this collagen was not as prolific or locally aligned as that found in the combined serum/ITS cultures. The relative success in producing stratified cultures containing collagenous matrix on glass substrates in a scaffold-free system provides a unique opportunity to further observe matrix synthesis in vitro, even though it remains unclear how the observed myofibroblastic transformation in cell phenotype relates to what takes place during wound healing or developmental histogenesis in vivo.
Serum-free versus serum-supplemented medium
The inherent variability of serum composition and its potential for pathogenic agent contamination make its elimination from the culture medium highly desirable. A beneficial effect of serum is that it mediates the transformation of corneal stromal keratocytes into repair fibroblasts.23 However, the TGF-β found in serum61 has the potential to facilitate their further, undesirable transformation into myofibroblasts.23,35,62 The presence of bFGF in media can suppress fibroblast to myofibroblast transition.23 In an attempt to preclude the formation of scar-like tissue containing significant numbers of contractile myofibroblasts, a basal medium containing bFGF and no TGF-β was employed. A 10-fold reduction in the serum concentration was achieved (compared to our previous model31) while maintaining cell stratification and substantial aligned collagen secretion (if the medium contained 1% ITS). Interestingly, the use of 10% FBS culture medium (which mimicked that used in our scaffold-free corneal developmental model31) resulted in less fibrotic cultures (small, cornea-like collagen fibrils and dendritic cells, weak f-actin, and α-SMA staining) than the reduced serum culture system with ITS, which exhibited scar-like behavior (large fibrils and strong phalloidin and α-SMA staining consistent with the formation of stress fibers and myofibroblastic transformation). This result was unexpected and counterintuitive, given what is known about the role of TGF-β in mediating myofibroblastic transformation both in vivo and in vitro. Although no exogenous TGF-β was purposely added to any of the cultures, serum contains unspecified amounts of this growth factor, and thus higher serum concentrations should have resulted in increased myofibroblastic transformation.
Complete deletion of serum from our medium was detrimental to cell viability in the longer-term cultures (1.5–3 weeks). Others have maintained viability (and consistently reported quiescence) of bovine corneal stromal cells in serum-free cultivation for up to 11 days;1,36,63–65 however, they have used mostly plastic culture substrates (in some cases treated to enhance cell attachment), they did not stimulate the culture with ascorbic acid and they used primary cells, compared with our uncoated glass substrate and fourth- to fifth-passage cells.
ITS stimulated organized collagenous matrix secretion and organization
ITS in the reduced-serum culture medium significantly improved cell stratification and organized ECM secretion relative to the other medium formulations. This is consistent with reports of in vitro cell stratification in the insulin-supplemented culture medium.66–68 ECM fibrils were collagenous showing typical crossbanding in TEM images. ITS in low-serum cultures induced increased staining of organized actin filament bundles and α-SMA as well as organized extracellular type I collagen (Fig. 4), suggesting that these cells underwent myofibroblastic transformation.11,17,18 Although double labeling was not carried out in the present study, we expect that actin filaments and α-SMA would colocalize as they did in a study that reported myofibroblastic transformation in cultures of human corneal stromal keratocytes in the serum-free medium containing TGF-β.69 Compared with fibroblasts, myofibroblasts are more active in the production of α-SMA, collagen, and fibronectin, and in the formation of organized intracellular actin filaments and focal adhesions,11,17,18,23 and less active in the secretion of matrix-digesting MMPs.25,26 It is thus possible that cells in 1% FBS + 1% ITS medium retained more ECM than did their 10% FBS or 1% FBS counterparts, although MMP expression was not quantified.
Most available literature reports stimulation of collagen synthesis in vitro by ITS's main component, insulin,32,53,70,71 with few exceptions.57 It is possible that cellular uptake of ascorbic acid (∼0.07–1 mM) may be inhibited by glucose (∼1–25 mM), preventing its stimulatory effect on collagen synthesis; this inhibition may be reduced or abolished by insulin (∼10−9 to 10−6 mM).57,72,73 Ascorbic acid concentration accumulates in the intracellular space to a level 100-fold that in the medium, and this uptake directly correlates to intracellular hydroxyproline synthesis (reviewed in Ref.74). During wound healing, ascorbic acid autooxidates into dehydroascorbate,75,76 which is cytotoxic73 but can be reduced to ascorbate inside the cell. Insulin stimulates the uptake of dehydroascorbic acid by the cells under high glucose conditions.73 Thus, it is likely that in the present study (i) ascorbic acid stimulated collagen synthesis, (ii) glucose may have partially inhibited ascorbic acid cellular uptake, and (iii) insulin (1% ITS) abolished this effect.
Intracellular structures
Intracellular 8–12-nm-diameter fibrils (Fig. 7e) were similar to procollagen filaments or thin collagen fibrils reported elsewhere.77,78 Intracellular collagen fibrillogenesis can occur in activated fibroblasts when collagen production exceeds the cell's excretion capabilities.77,78 Thus, Figure 7e may suggest overstimulation of collagen synthesis in 1% FBS + 1% ITS cultures. Some of the smaller-diameter (∼7 nm) intracellular fibrils may correspond to actin microfilaments consistent with myofibroblastic transformation. Concentric-ring residual bodies (Fig. 9) may correspond to packed lipid membranes of incompletely digested aged organelles,79 and their abundance also suggests elevated synthetic activity.
ECM structures
Our observations agree with recent literature,80 where stem cells from human corneal stroma cultured in the serum-free medium secreted fibrillar collagen only if cultured in three-dimensional pellets (i.e., forced stratification). Collagen fibrils produced in the stratified 1% ITS + 1% FBS culture appear denser and more aligned than those of Du et al.'s pellet cultures.80 This difference may be due to our culture substratum (glass instead of pellet), medium formulation (we employed more glucose and ascorbic acid than Du et al., plus insulin and serum), or cell type.
The diameter of the collagen synthesized by the 1% FBS + 1% ITS system was larger than that produced in the normal bovine cornea, while the collagen produced by the 10% FBS system had nearly the correct diameter. Corneal fibroblasts and myofibroblasts express type III and type V collagen in vitro,2,81,82 both of which influence fibril diameter. Collagen types I and III and types I and V form heterotypic fibrils, with an inverse correlation between the concentrations of types III/I and V/I and the fibril diameter.83–91 Although the diameter of type I/V collagen fibrils in the bovine corneal stroma in vivo is 38.2 nm,92 in vitro cell-secreted collagen fibrils may be larger and/or more broadly distributed.93 Cells and fibrils appeared embedded in a matrix that we tentatively categorized as fibronectin-like (Fig. 11), based on its similarity to QFDE images of Zea-Aragon et al.,94 who identified similar structures via immunogold. Qualitatively, our QFDE image library showed that more of this fibronectin-like matrix was present in the 1% ITS + 1% FBS culture (not shown).
Figures 7b and 12 are consistent with the fibripositor mechanism of aligned collagen secretion.95,96 Fibripositors are invaginations in the plasma membrane that align parallel to the axis of tendons and vectorially discharge collagen fibrils into the ECM. To our knowledge, Figure 12 constitutes the first three-dimensional observation of a cell-membrane fibripositor extruding a bundle of collagen fibrils (although the fibrils in this structure were not positively identified). We hold the opinion that fibripositors are more likely utilized by cells in wound healing mode because they permit tight control of the fibril forming environment. We found much more evidence of fibripositors in the 1% ITS + 1% FBS system that we did in the 10% FBS system. The observation of filopodia in close relation to and aligned in the same direction as collagen fibrils in Figures 7 and 10 is similar to that made in developing chick corneas.97
Concluding remarks
Few corneal tissue engineering studies have focused on discerning the details of organized matrix production by fibroblasts. Because matrix production is a temporally varying event, it is critical to develop a highly controlled system that produces organized collagen while permitting maximum microscopic accessibility. In this study, bovine corneal fibroblasts were grown and stimulated to produce collagen in various culture medium formulations on glass substrates. The most successful of these (1% FBS + 1% ITS) produced highly aligned collagen but also promoted a myofibroblastic-like transformation in the cells. The other successful culture medium (10% FBS) retained a nonfibrotic cell phenotype but produced fewer, less regular (but more cornea-like) collagen fibrils. Although the 1% ITS + 1% FBS culture system did not result in the in vitro utter replication the ultrastructure of the cornea, it constituted an important advance toward the developing of highly controlled cell culture systems that permit the direct observation of the production of organized collagen secretion with high-spatial and temporal resolution.
Supplementary Material
Footnotes
All the work in this study was carried out at the Department of Mechanical and Industrial Engineering, Northeastern University, Boston, Massachusetts.
Acknowledgments
The authors wish to thank William Fowle, M.S., for assistance with preparation of TEM samples. This work was funded by NIH/NEI R01-EY015500-01A1, NIH/NEI R01-EY015500-01A1S, and NIH/NIBIB R21-EB007317-01.
Disclosure Statement
No competing financial interests exist.
References
- 1.Berryhill B.L. Kader R. Kane B. Birk David E. Feng J. Hassell John R. Partial restoration of the keratocyte phenotype to bovine keratocytes made fibroblastic by serum. Invest Ophthalmol Vis Sci. 2002;43:3416. [PubMed] [Google Scholar]
- 2.Funderburgh J.L. Mann M.M. Funderburgh M.L. Keratocyte phenotype mediates proteoglycan structure: a role for fibroblasts in corneal fibrosis. J Biol Chem. 2003;278:45629. doi: 10.1074/jbc.M303292200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watsky M.A. Keratocyte gap junctional communication in normal and wounded rabbit corneas and human corneas. Invest Ophthalmol Vis Sci. 1995;36:2568. [PubMed] [Google Scholar]
- 4.Brown D. Chwa M. Escobar M. Kenney M.C. Characterization of the major matrix degrading metalloproteinase of human corneal stroma. Evidence for an enzyme/inhibitor complex. Exp Eye Res. 1991;52:5. doi: 10.1016/0014-4835(91)90123-v. [DOI] [PubMed] [Google Scholar]
- 5.Funderburgh M.L. Du Y. Mann M.M. SundarRaj N. Funderburgh J.L. PAX6 expression identifies progenitor cells for corneal keratocytes. FASEB J. 2005;19:1371. doi: 10.1096/fj.04-2770fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snyder M.C. Bergmanson J.P. Doughty M.J. Keratocytes: no more the quiet cells. J Am Optom Assoc. 1998;69:180. [PubMed] [Google Scholar]
- 7.West-Mays J.A. Dwivedi D.J. The keratocyte: corneal stromal cell with variable repair phenotypes. Int J Biochem Cell B. 2006;38:1625. doi: 10.1016/j.biocel.2006.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imanishi J. Kamiyama K. Iguchi I. Kita M. Sotozono C. Kinoshita S. Growth factors: importance in wound healing and maintenance of transparency of the cornea. Prog Retin Eye Res. 1999;19:113. doi: 10.1016/s1350-9462(99)00007-5. [DOI] [PubMed] [Google Scholar]
- 9.Matsuda H. Smelser G.K. Electron microscopy of corneal wound healing. Exp Eye Res. 1973;16:427. doi: 10.1016/0014-4835(73)90100-0. [DOI] [PubMed] [Google Scholar]
- 10.Jester J.V. Huang J. Barry-Lane P.A. Kao W.W. Petroll W.M. Cavanagh H.D. Transforming growth factor(beta)-mediated corneal myofibroblast differentiation requires actin and fibronectin assembly. Invest Ophthalmol Vis Sci. 1999;40:1959. [PubMed] [Google Scholar]
- 11.Jester J.V. Rodrigues M.M. Herman I.M. Characterization of avascular corneal wound healing fibroblasts. New insights into the myofibroblast. Am J Pathol. 1987;127:140. [PMC free article] [PubMed] [Google Scholar]
- 12.Fini M.E. Stramer Brian M. How the cornea heals: cornea-specific repair mechanisms affecting surgical outcomes. Cornea. 2005;24:S2. doi: 10.1097/01.ico.0000178743.06340.2c. [DOI] [PubMed] [Google Scholar]
- 13.Friedman S.L. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med. 1993;328:1828. doi: 10.1056/NEJM199306243282508. [DOI] [PubMed] [Google Scholar]
- 14.Tomasek J.J. Gabbiani G. Hinz B. Chaponnier C. Brown R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 15.Funderburgh J.L. Hevelone N.D. Roth M.R. Funderburgh M.L. Rodrigues M.R. Nirankari V.S. Conrad G.W. Decorin and biglycan of normal and pathologic human corneas. Invest Ophthalmol Vis Sci. 1998;39:1957. [PubMed] [Google Scholar]
- 16.Hassell J.R. Cintron C. Kublin C. Newsome D.A. Proteoglycan changes during restoration of transparency in corneal scars. Arch Biochem Biophys. 1983;222:362. doi: 10.1016/0003-9861(83)90532-5. [DOI] [PubMed] [Google Scholar]
- 17.Jester J.V. Petroll W.M. Barry P.A. Cavanagh H.D. Expression of alpha-smooth muscle (alpha-SM) actin during corneal stromal wound healing. Invest Ophthalmol Vis Sci. 1995;36:809. [PubMed] [Google Scholar]
- 18.Garana R.M. Petroll W.M. Chen W.T. Herman I.M. Barry P. Andrews P. Cavanagh H.D. Jester J.V. Radial keratotomy. II. Role of the myofibroblast in corneal wound contraction. Invest Ophthalmol Vis Sci. 1992;33:3271. [PubMed] [Google Scholar]
- 19.Girard M.T. Matsubara M. Kublin C. Tessier M.J. Cintron C. Fini M.E. Stromal fibroblasts synthesize collagenase and stromelysin during long-term tissue remodeling. J Cell Sci. 1993;104:1001. doi: 10.1242/jcs.104.4.1001. [DOI] [PubMed] [Google Scholar]
- 20.Matsubara M. Girard M.T. Kublin C.L. Cintron C. Fini M.E. Differential roles for two gelatinolytic enzymes of the matrix metalloproteinase family in the remodeling cornea. Dev Biol. 1991;147:425. doi: 10.1016/0012-1606(91)90300-r. [DOI] [PubMed] [Google Scholar]
- 21.Matsubara M. Zieske J.D. Fini M.E. Mechanism of basement membrane dissolution preceding corneal ulceration. Invest Ophthalmol Vis Sci. 1991;32:3221. [PubMed] [Google Scholar]
- 22.Jester J.V. Ho-Chang J. Modulation of cultured corneal keratocyte phenotype by growth factors/cytokines control in vitro contractility and extracellular matrix contraction. Exp Eye Res. 2003;77:581. doi: 10.1016/s0014-4835(03)00188-x. [DOI] [PubMed] [Google Scholar]
- 23.Jester J.V. Barry-Lane P.A. Cavanagh H.D. Petroll W.M. Induction of alpha-smooth muscle actin expression and myofibroblast transformation in cultured corneal keratocytes. Cornea. 1996;15:505. [PubMed] [Google Scholar]
- 24.Yoshikawa H. Fukuda T. Kikuchi M. Oyamada T. Yoshikawa T. The relevance of myoid cells and myofibroblasts in testicular fibrosis in two horses. J Equine Sci. 2001;12:9. [Google Scholar]
- 25.Girard M.T. Matsubara M. Fini M.E. Transforming growth factor-beta and interleukin-1 modulate metalloproteinase expression by corneal stromal cells. Invest Ophthalmol Vis Sci. 1991;32:2441. [PubMed] [Google Scholar]
- 26.Fini M.E. Girard M.T. Matsubara M. Bartlett J.D. Unique regulation of the matrix metalloproteinase, gelatinase B. Invest Ophthalmol Vis Sci. 1995;36:622. [PubMed] [Google Scholar]
- 27.Masur S.K. Dewal H.S. Dinh T.T. Erenburg I. Petridou S. Myofibroblasts differentiate from fibroblasts when plated at low density. Proc Natl Acad Sci USA. 1996;93:4219. doi: 10.1073/pnas.93.9.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruberti J.W. Zieske J. Prelude to corneal tissue engineering—gaining control of collagen organization. Prog Retin Eye Res. 2008;27:549. doi: 10.1016/j.preteyeres.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Birk D.E. Trelstad R.L. Extracellular compartments in matrix morphogenesis: collagen fibril, bundle, and lamellar formation by corneal fibroblasts. J Cell Biol. 1984;99:2024. doi: 10.1083/jcb.99.6.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giraud-Guille M.M. Besseau L. Martin R. Liquid crystalline assemblies of collagen in bone and in vitro systems. J Biomech. 2003;36:1571. doi: 10.1016/s0021-9290(03)00134-9. [DOI] [PubMed] [Google Scholar]
- 31.Guo X. Hutcheon Audrey E.K. Melotti Suzanna A. Zieske James D. Trinkaus-Randall V. Ruberti Jeffrey W. Morphologic characterization of organized extracellular matrix deposition by ascorbic acid stimulated human corneal fibroblasts. Invest Ophthalmol Vis Sci. 2007;48:4050. doi: 10.1167/iovs.06-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Musselmann K. Alexandrou B. Kane B. Hassell J.R. Maintenance of the keratocyte phenotype during cell proliferation stimulated by insulin. J Biol Chem. 2005;280:32634. doi: 10.1074/jbc.M504724200. [DOI] [PubMed] [Google Scholar]
- 33.Maltseva O. Folger P. Zekaria D. Petridou S. Masur S.K. Fibroblast growth factor reversal of the corneal myofibroblast phenotype. Invest Ophthalmol Vis Sci. 2001;42:2490. [PubMed] [Google Scholar]
- 34.Fukamizu H. Grinnell F. Spatial organization of extracellular matrix and fibroblast activity: effects of serum, transforming growth factor b, and fibronectin. Exp Cell Res. 1990;190:276. doi: 10.1016/0014-4827(90)90197-i. [DOI] [PubMed] [Google Scholar]
- 35.Anderson S. DiCesare L. Tan I. Leung T. SundarRaj N. Rho-mediated assembly of stress fibers is differentially regulated in corneal fibroblasts and myofibroblasts. Exp Cell Res. 2004;298:574. doi: 10.1016/j.yexcr.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 36.Beales M.P. Funderburgh J.L. Jester J.V. Hassell J.R. Proteoglycan synthesis by bovine keratocytes and corneal fibroblasts: maintenance of the keratocyte phenotype in culture. Invest Ophthalmol Vis Sci. 1999;40:1658. [PubMed] [Google Scholar]
- 37.Bottenstein J. Hayashi I. Hutchings S. Masui H. Mather J. McClure D.B. Ohasa S. Rizzino A. Sato G. Serrero G. Wolfe R. Wu R. Methods in Enzymology. New York: Academic Press; 1979. [DOI] [PubMed] [Google Scholar]
- 38.Guilbert L.J. Iscove N.N. Partial replacement of serum by selenite, transferrin, albumin and lecithin in haemopoietic cell cultures. Nature. 1976;263:594. doi: 10.1038/263594a0. [DOI] [PubMed] [Google Scholar]
- 39.White A. Handler P. Smith E.L. Principles of Biochemistry. New York: McGraw Hill; 1973. [Google Scholar]
- 40.Testa U. Pelosi E. Peschle C. The transferrin receptor. Crit Rev Oncol. 1993;4:241. [PubMed] [Google Scholar]
- 41.Stadtman T.C. Selenium biochemistry. Annu Rev Biochem. 1990;59:111. doi: 10.1146/annurev.bi.59.070190.000551. [DOI] [PubMed] [Google Scholar]
- 42.Planz B. Kirley S.D. Wang Q. Tabatabaei S. Aretz H.T. McDougal W.S. Characterization of a stromal cell model of the human benign and malignant prostate from explant culture. J Urol. 1999;161:1329. [PubMed] [Google Scholar]
- 43.Lee J.-J. Kwon J.-H. Park Y.K. Kwon O. Yoon T.-W. The effects of various hormones and growth factors on the growth of human insulin-producing cell line in serum-free medium. Exp Mol Med. 1997;29:209. [Google Scholar]
- 44.Gauger A. Robertson C. Greenlee T.K., Jr. Riederer-Henderson M.A. A low-serum medium for tendon cells: effects of growth factors on tendon cell growth and collagen production. In Vitro. 1985;21:291. doi: 10.1007/BF02620945. [DOI] [PubMed] [Google Scholar]
- 45.Ostlund R.E., Jr. Yang J.W. Effect of cholesterol and growth factors on the proliferation of cultured human skin fibroblasts. Exp Cell Res. 1985;161:509. doi: 10.1016/0014-4827(85)90105-3. [DOI] [PubMed] [Google Scholar]
- 46.Chua K.H. Aminuddin B.S. Fuzina N.H. Ruszymah B.H.I. Insulin-transferrin-selenium prevent human chondrocyte differentiation and promote the formation of high quality tissue engineered human hyaline cartilage. Eur Cells Mater. 2005;9:58. doi: 10.22203/ecm.v009a08. [DOI] [PubMed] [Google Scholar]
- 47.Kisiday J.D. Kurz B. DiMicco M.A. Grodzinsky A.J. Evaluation of medium supplemented with insulin-transferrin-selenium for culture of primary bovine calf chondrocytes in three-dimensional hydrogel scaffolds. Tissue Eng. 2005;11:141. doi: 10.1089/ten.2005.11.141. [DOI] [PubMed] [Google Scholar]
- 48.Woost P.G. Jumblatt M.M. Eiferman R.A. Schultz G.S. Growth factors and corneal endothelial cells: I. Stimulation of bovine corneal endothelial cell DNA synthesis by defined growth factors. Cornea. 1992;11:1. doi: 10.1097/00003226-199201000-00001. [DOI] [PubMed] [Google Scholar]
- 49.Lijnen P. Petrov V. Fagard R. In vitro assay of collagen gel contraction by cardiac fibroblasts in serum-free conditions. Methods Find Exp Clin. 2001;23:377. doi: 10.1358/mf.2001.23.7.662122. [DOI] [PubMed] [Google Scholar]
- 50.Yates K.E. Allemann F. Glowacki J. Phenotypic analysis of bovine chondrocytes cultured in 3D collagen sponges: effect of serum substitutes. Cell Tissue Bank. 2005;6:45. doi: 10.1007/s10561-005-5810-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quarto R. Campanile G. Cancedda R. Dozin B. Thyroid hormone, insulin, and glucocorticoids are sufficient to support chondrocyte differentiation to hypertrophy: a serum-free analysis. J Cell Biol. 1992;119:989. doi: 10.1083/jcb.119.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Engelmann K. Friedl P. Growth of human corneal endothelial cells in a serum-reduced medium. Cornea. 1995;14:62. [PubMed] [Google Scholar]
- 53.Canalis E. Effect of hormones and growth factors on alkaline phosphatase activity and collagen synthesis in cultured rat calvariae. Metabolism. 1983;32:14. doi: 10.1016/0026-0495(83)90149-x. [DOI] [PubMed] [Google Scholar]
- 54.Abrahamsson S.O. Matrix metabolism and healing in the flexor tendon. Experimental studies on rabbit tendon. Scand J Plast Recons. 1991;S23:1. [PubMed] [Google Scholar]
- 55.Alcain F.J. Buron M.I. Ascorbate on cell growth and differentiation. J Bioenerg Biomembr. 1994;26:393. doi: 10.1007/BF00762780. [DOI] [PubMed] [Google Scholar]
- 56.Barnes M.J. Kodicek E. Biological hydroxylations and ascorbic acid with special regard to collagen metabolism. Vitam Horm. 1972;30:1. doi: 10.1016/s0083-6729(08)60793-1. [DOI] [PubMed] [Google Scholar]
- 57.Fisher E. McLennan S.V. Tada H. Heffernan S. Yue D.K. Turtle J.R. Interaction of ascorbic acid and glucose on production of collagen and proteoglycan by fibroblasts. Diabetes. 1991;40:371. doi: 10.2337/diab.40.3.371. [DOI] [PubMed] [Google Scholar]
- 58.Petroll W.M. Ma L. Direct, dynamic assessment of cell-matrix interactions inside fibrillar collagen lattices. Cell Motil Cytoskelet. 2003;55:254. doi: 10.1002/cm.10126. [DOI] [PubMed] [Google Scholar]
- 59.Petroll W.M. Ma L. Jester J.V. Direct correlation of collagen matrix deformation with focal adhesion dynamics in living corneal fibroblasts. J Cell Sci. 2003;116:1481. doi: 10.1242/jcs.00357. [DOI] [PubMed] [Google Scholar]
- 60.Vishwanath M. Ma L. Otey Carol A. Jester James V. Petroll W.M. Modulation of corneal fibroblast contractility within fibrillar collagen matrices. Invest Ophthalmol Vis Sci. 2003;44:4724. doi: 10.1167/iovs.03-0513. [DOI] [PubMed] [Google Scholar]
- 61.Childs C.B. Proper J.A. Tucker R.F. Moses H.L. Serum contains a platelet-derived transforming growth factor. Proc Natl Acad Sci USA. 1982;79:5312. doi: 10.1073/pnas.79.17.5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jester J.V. Huang J. Petroll W.M. Cavanagh H.D. TGFbeta induced myofibroblast differentiation of rabbit keratocytes requires synergistic TGFbeta, PDGF and integrin signaling. Exp Eye Res. 2002;75:645. doi: 10.1006/exer.2002.2066. [DOI] [PubMed] [Google Scholar]
- 63.Musselman K. Kane B.P. Hassell J.R. Isolation of a putative keratocyte activating factor from the corneal stroma. Exp Eye Res. 2003;77:273. doi: 10.1016/s0014-4835(03)00160-x. [DOI] [PubMed] [Google Scholar]
- 64.Guerriero E. Chen J. Sado Y. Mohan R.R. Wilson S.E. Funderburgh J.L. Sundarraj N. Loss of alpha3(IV) collagen expression associated with corneal keratocyte activation. Invest Ophthalmol Vis Sci. 2007;48:627. doi: 10.1167/iovs.06-0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Denk P.O. Wunderlich K. Knorr M. Serum-free cultivation of bovine stromal fibroblasts. Ophthalmologe. 1998;95:148. doi: 10.1007/s003470050253. [DOI] [PubMed] [Google Scholar]
- 66.Galfi P. Gabel G. Martens H. Influences of extracellular matrix components on the growth and differentiation of ruminal epithelial cells in primary culture. Res Vet Sci. 1993;54:102. doi: 10.1016/0034-5288(93)90018-b. [DOI] [PubMed] [Google Scholar]
- 67.Boisseau A.M. Donatien P. Surleve-Bazeille J.E. Amedee J. Harmand M.F. Bezian J.H. Maleville J. Taieb A. Production of epidermal sheets in a serum free culture system: a further appraisal of the role of extracellular calcium. J Dermatol Sci. 1992;3:111. doi: 10.1016/0923-1811(92)90044-c. [DOI] [PubMed] [Google Scholar]
- 68.Chlapowski F.J. Long term growth and maintenance of stratified rat urothelium in vitro. Cell Tissue Kinet. 1989;22:245. doi: 10.1111/j.1365-2184.1989.tb00210.x. [DOI] [PubMed] [Google Scholar]
- 69.Jester J.V. Huang J. Fisher S. Spiekerman J. Chang J.H. Wright W.E. Shay J.W. Myofibroblast differentiation of normal human keratocytes and hTERT, extended-life human corneal fibroblasts. Invest Ophthalmol Vis Sci. 2003;44:1850. doi: 10.1167/iovs.02-0973. [DOI] [PubMed] [Google Scholar]
- 70.Tokudome T. Horio T. Yoshihara F. Suga S.-i. Kawano Y. Kohno M. Kangawa K. Direct effects of high glucose and insulin on protein synthesis in cultured cardiac myocytes and DNA and collagen synthesis in cardiac fibroblasts. Metabolism. 2004;53:710. doi: 10.1016/j.metabol.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 71.Musselmann K. Kane B. Alexandrou B. Hassell John R. Stimulation of collagen synthesis by insulin and proteoglycan accumulation by ascorbate in bovine keratocytes in vitro. Invest Ophthalmol Vis Sci. 2006;47:5260. doi: 10.1167/iovs.06-0612. [DOI] [PubMed] [Google Scholar]
- 72.Kapeghian J.C. Verlangieri A.J. The effects of glucose on ascorbic acid uptake in heart endothelial cells: possible pathogenesis of diabetic angiopathies. Life Sci. 1984;34:577. doi: 10.1016/0024-3205(84)90491-0. [DOI] [PubMed] [Google Scholar]
- 73.Qutob S. Dixon S.J. Wilson J.X. Insulin stimulates vitamin C recycling and ascorbate accumulation in osteoblastic cells. Endocrinology. 1998;139:51. doi: 10.1210/endo.139.1.5659. [DOI] [PubMed] [Google Scholar]
- 74.Wolf G. The mechanism of uptake of ascorbic acid into osteoblasts and leukocytes. Nutr Rev. 1996;54:150. doi: 10.1111/j.1753-4887.1996.tb03918.x. [DOI] [PubMed] [Google Scholar]
- 75.Winkler B.S. In vitro oxidation of ascorbic acid and its prevention by GSH. Biochim Biophys Acta. 1987;925:258. doi: 10.1016/0304-4165(87)90190-5. [DOI] [PubMed] [Google Scholar]
- 76.Kim M. Otsuka M. Yu R. Kurata T. Arakawa N. The distribution of ascorbic acid and dehydroascorbic acid during tissue regeneration in wounded dorsal skin of guinea pigs. Int J Vitam Nutr Res. 1994;64:56. [PubMed] [Google Scholar]
- 77.Gonzalez Santander R. Plasencia Arriba M.A. Martinez Cuadrado G. Lopez Alonso A. Gonzalez-Santander Martinez M. Martinez Alonso F.J. Monteagudo M. Toledo Lobo M.V. Intracellular biogenesis of collagen fibrils in “activated fibroblasts” of tendo Achillis. An ultrastructural study in the New Zealand rabbit. J Bone Joint Surg Br. 1999;81:522. doi: 10.1302/0301-620x.81b3.9159. [DOI] [PubMed] [Google Scholar]
- 78.Michna H. Intracellular collagen fibrils: evidence of an intracellular source from experiments with tendon fibroblasts and fibroblastic tumour cells. J Anat. 1988;158:1. [PMC free article] [PubMed] [Google Scholar]
- 79.Cross P.C. Mercer K.L. A Functional Perspective. New York: W.H. Freeman and Company; 1993. Cell and Tissue Ultrastructure. [Google Scholar]
- 80.Du Y. Sundarraj N. Funderburgh Martha L. Harvey Stephen A. Birk David E. Funderburgh James L. Secretion and organization of a cornea-like tissue in vitro by stem cells from human corneal stroma. Invest Ophthalmol Vis Sci. 2007;48:5038. doi: 10.1167/iovs.07-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Evans R.A. Tian Y.C. Steadman R. Phillips A.O. TGF-b1-mediated fibroblast-myofibroblast terminal differentiation-the role of Smad proteins. Exp Cell Res. 2003;282:90. doi: 10.1016/s0014-4827(02)00015-0. [DOI] [PubMed] [Google Scholar]
- 82.Doane K.J. Babiarz J.P. Fitch J.M. Linsenmayer T.F. Birk D.E. Collagen fibril assembly by corneal fibroblasts in three-dimensional collagen gel cultures: small-diameter heterotypic fibrils are deposited in the absence of keratan sulfate proteoglycan. Exp Cell Res. 1992;202:113. doi: 10.1016/0014-4827(92)90410-a. [DOI] [PubMed] [Google Scholar]
- 83.Wenstrup R.J. Florer J.B. Brunskill E.W. Bell S.M. Chervoneva I. Birk D.E. Type V collagen controls the initiation of collagen fibril assembly. J Biol Chem. 2004;279:53331. doi: 10.1074/jbc.M409622200. [DOI] [PubMed] [Google Scholar]
- 84.Lapiere C.M. Nusgens B. Pierard G.E. Interaction between collagen type I and type III in conditioning bundles organization. Connect Tissue Res. 1977;5:21. doi: 10.3109/03008207709152608. [DOI] [PubMed] [Google Scholar]
- 85.Birk D.E. Fitch J.M. Babiarz J.P. Doane K.J. Linsenmayer T.F. Collagen fibrillogenesis in vitro: interaction of types I and V collagen regulates fibril diameter. J Cell Sci. 1990;95:649. doi: 10.1242/jcs.95.4.649. [DOI] [PubMed] [Google Scholar]
- 86.Whitby D.J. Ferguson M.W.J. Immunohistochemical localization of growth factors in fetal wound healing. Dev Biol. 1991;147:207. doi: 10.1016/s0012-1606(05)80018-1. [DOI] [PubMed] [Google Scholar]
- 87.Birk D.E. Mayne R. Localization of collagen types I, III and V during tendon development. Changes in collagen types I and III are correlated with changes in fibril diameter. Eur J Cell Biol. 1997;72:352. [PubMed] [Google Scholar]
- 88.Marchant J.K. Hahn R.A. Linesenmayer T.F. Birk D.E. Reduction of type V collagen using a dominant-negative strategy alters the regulation of fibrillogenesis and results in the loss of corneal-specific fibril morphology. J Cell Biol. 1996;135:1415. doi: 10.1083/jcb.135.5.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wenstrup R.J. Florer J.B. Cole W.G. Willing M.C. Birk D.E. Reduced type I collagen utilization: a pathogenic mechanism in COL5A1 haplo-insufficient Ehlers-Danlos syndrome. J Cell Biochem. 2004;92:113. doi: 10.1002/jcb.20024. [DOI] [PubMed] [Google Scholar]
- 90.Kypreos K.E. Birk D. Trinkaus-Randall V. Hartmann D.J. Sonenshein G.E. Type V collagen regulates the assembly of collagen fibrils in cultures of bovine vascular smooth muscle cells. J Cell Biochem. 2000;80:146. doi: 10.1002/1097-4644(20010101)80:1<146::aid-jcb140>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 91.Fichard A. Kleman J.-P. Ruggiero F. Another look at collagen V and XI molecules. Matrix Biol. 1995;14:515. doi: 10.1016/s0945-053x(05)80001-0. [DOI] [PubMed] [Google Scholar]
- 92.Meek K.M. Leonard D.W. Ultrastructure of the corneal stroma: a comparative study. Biophys J. 1993;64:273. doi: 10.1016/S0006-3495(93)81364-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Crabb Rachael A.B. Chau Eric P. Decoteau Danya M. Hubel A. Microstructural characteristics of extracellular matrix produced by stromal fibroblasts. Ann Biomed Eng. 2006;34:1615. doi: 10.1007/s10439-006-9181-x. [DOI] [PubMed] [Google Scholar]
- 94.Zea-Aragon Z. Terada N. Ohno N. Fujii Y. Baba T. Yoshida M. Ohtsuki K. Ohnishi M. Ohno S. Replica immunoelectron microscopic study of the upper surface layer in rat mandibular condylar cartilage by a quick-freezing method. Histochem Cell Biol. 2004;121:255. doi: 10.1007/s00418-004-0634-8. [DOI] [PubMed] [Google Scholar]
- 95.Canty E.G. Lu Y. Meadows R.S. Shaw M.K. Holmes D.F. Kadler K.E. Coalignment of plasma membrane channels and protrusions (fibripositors) specifies the parallelism of tendon. J Cell Biol. 2004;165:553. doi: 10.1083/jcb.200312071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Birk D.E. Trelstad R.L. Fibroblasts create compartments in the extracellular space where collagen polymerizes into fibrils and fibrils associate into bundles. Ann NY Acad Sci. 1985;460:258. doi: 10.1111/j.1749-6632.1985.tb51173.x. [DOI] [PubMed] [Google Scholar]
- 97.Hasty D.L. Hay E.D. Freeze-fracture studies of the developing cell surface. I. The plasmalemma of the corneal fibroblast. J Cell Biol. 1977;72:667. doi: 10.1083/jcb.72.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.