Abstract
Endothelial progenitor cells isolated from umbilical cord blood (CB-EPCs) represent a promising source of endothelial cells for synthetic vascular grafts and tissue-engineered blood vessels since they are readily attainable, can be easily isolated, and possess a high proliferation potential. The objective of this study was to compare the functional behavior of late outgrowth CB-EPCs with human aortic endothelial cells (HAECs). CB-EPCs and HAECs were cultured on either smooth muscle cells in a coculture model of a tissue-engineered blood vessels or fibronectin adsorbed to Teflon-AF™–coated glass slides. Late outgrowth CB-EPCs expressed endothelial cell–specific markers and were negative for the monocytic marker CD14. CB-EPCs have higher proliferation rates than HAECs, but are slightly smaller in size. CB-EPCs remained adherent under supraphysiological shear stresses, oriented and elongated in the direction of flow, and expressed similar numbers of α5β1 and αvβ3 integrins and antithrombotic genes compared to HAECs. There were some differences in mRNA levels of E-selectin and vascular cell adhesion molecule 1 between CB-EPCs and HAECs; however, protein levels were similar on the two cell types, and CB-EPCs did not support adhesion of monocytes in the absence of tumor necrosis factor-α stimulation. Although CB-EPCs expressed significantly less endothelial nitric oxide synthase protein after exposure to flow than HAECs, nitric oxide levels induced by flow were not significantly different. These results suggest that late outgrowth CB-EPCs are functionally similar to HAECs under flow conditions and are a promising cell source for cardiovascular therapies.
Introduction
To increase options for arterial repair, researchers are developing small-diameter synthetic vascular grafts1 and tissue-engineered blood vessels (TEBVs).2 For vascular grafts and TEBVs to remain patent long-term, they must be lined with functional endothelial cells (ECs). ECs are normally antithrombotic and inhibit smooth muscle cell (SMC) growth, thereby preventing intimal hyperplasia.3–7 Autologous vascular–derived ECs would be ideal candidates for these applications; however, their use is not practical, and hence alternative cell sources are needed. Five criteria must first be satisfied to consider use of specific ECs or endothelial progenitor cells (EPCs) for vascular repair: (1) they must come from an easily obtainable and reliable source, (2) they must exhibit EC-specific markers without monocytic markers, (3) they must have strong adhesion to the underlying material, (4) they must exhibit antithrombotic and antiinflammatory properties characteristic of native ECs, and (5) they must inhibit neointimal hyperplasia and thrombosis.
Many EC sources have been investigated, including vascular-derived ECs and adipose-derived microvascular ECs; however, both these cell types are isolated using methods that require an invasive surgical procedure and/or extensive in vitro expansion.1,8 Thus, these cells can only be used in elective procedures and not for emergency vessel replacement. While adipose-derived ECs are more abundant than vessel wall cells, extensive isolation must be performed to remove contaminating cells that promote neointimal hyperplasia.9 Autologous blood-derived EPCs are an attractive cell source for cardiovascular therapies,10–12 but patients in need of replacement vessels are typically elderly and often suffer from vascular diseases, which leads to a reduced number of circulating EPCs capable of expansion and seeding onto grafts or TEBVs.2,13–18 In contrast, umbilical cord blood–derived endothelial progenitor cells (CB-EPCs) are easily obtained and have a high proliferative potential,19 enabling the cells to be cultured to sufficient numbers so that they can be available at the time of the procedure. CB-EPCs may be suitable for transplantation if the recipient can be matched to a donor's human leukocyte antigen as performed with current organ transplantations.20–23
The potential of EPCs isolated from peripheral and umbilical cord blood to become fully functional ECs depends upon the method of isolation.1,24–26 There are at least two distinct populations of EPCs isolated from peripheral and cord blood: early outgrowth colony-forming unit ECs (CFU-ECs) and late outgrowth endothelial colony forming cells (ECFCs). Most studies have focused on CFU-ECs, which are of a myeloid lineage and have a limited capacity to proliferate.27 In contrast, ECFCs do not have markers for monocytic cells or macrophages and exhibit more EC-specific markers than CFU-ECs.27 In addition, late outgrowth EPCs from umbilical cord blood are distinct from the EPCs isolated from peripheral blood (PB-EPCs); for example, CB-EPCs appear sooner after isolation, have a shorter doubling time, and have higher proliferation potential probably because they have an increased telomerase activity.19,26
Prior studies have examined the potential of CFU-ECs, derived from peripheral and umbilical cord blood, as well as ECFCs from peripheral blood for TEBVs8,28,29 or synthetic grafts.1,10,30 Initial results are very promising; however, little is known about the functional properties of ECFCs isolated from umbilical cord blood,27 especially after exposure to physiological flow conditions. To address this issue, we investigated whether late outgrowth CB-EPCs are functionally similar to native ECs. We compared adhesion, function, and responses to flow of late outgrowth CB-EPCs with human aortic endothelial cells (HAECs) on both fibronectin (FN)–coated Teflon-AF, to model synthetic vascular grafts,31 and SMCs, to mimic TEBVs. We used FN because of its role in promoting EC adhesion to materials. Functional properties examined include growth rates, spreading, strength of adhesion, integrin expression, integrins involved in initial adhesion, and flow-induced responses on gene, and protein levels were measured.
Materials and Methods
Cell culture
CB-EPCs were isolated as previously described by Ingram et al.19 Umbilical cord blood was obtained from the Carolina Cord Blood Bank at Duke University per protocols approved by the Duke University Institutional Review Board. Before receipt, all patient identifiers were removed. As determined by the Duke University Institutional Review Board, the use of these blood samples is exempt from human subjects' approval as defined by 45 CFR 46.102(f) and is not subject to the Privacy Rule (45 CFR 164.500[a]). After collection, blood was diluted 1:1 with Hanks balanced salt solution (HBSS; Gibco, Carlsbad, CA), placed onto Histopaque 1077 (Sigma), and centrifuged at 740 g for 30 min. Buffy coat mononuclear cells were collected and washed three times with a complete medium, which was composed of endothelial basal media-2 (Cambrex, Walkersville, MD), supplemented with endothelial growth media-2 SingleQuots (Cambrex), 10% fetal bovine serum (Gibco), and 1 × antibiotic–antimycotic solution (Gibco). Mononuclear cells were plated on collagen I (rat tail; Becton Dickinson, San Jose, CA) coated onto tissue culture plastic. The medium was exchanged 24 h after the initial plating to remove nonadherent cells and was exchanged everyday for the first week. Colonies of CB-EPCs appeared 7–10 days after the initial isolation. The cells grew to confluence and were serially passaged onto collagen I tissue–coated surfaces. CB-EPCs were used at passages 4–9 for all experiments, although the cells were still healthy and continued to proliferate at passage 10 and above.
HAECs (Cambrex) were maintained in the HAEC growth medium (endothelial basal media-2 supplemented with endothelial growth media-2 SingleQuots and 1 × antibiotic–antimycotic solution). HAECs were used at passage 7–10 for all experiments.
Human aortic SMCs (Cambrex) were expanded with the SMC growth medium containing smooth muscle basal medium (Cambrex) supplemented with smooth muscle growth media-2 SingleQuots (Cambrex) and 1 × antibiotic–antimycotic solution. The serum-free quiescent medium was composed of Dulbecco's modified Eagle's medium/F12 (Gibco) supplemented with 1 × insulin-transferrin-selenium (Gibco), and 1 × antibiotic–antimycotic solution. SMCs were used at passages 8–11 for all experiments.
All static and flow experiments were performed with the flow medium, which consisted of endothelial growth medium (phenol red free), 3.3% fetal bovine serum, 1 × insulin, transferrin and selenium, and 1 × antibiotic–antimycotic solution.
All cells were cultured in a tissue culture incubator with 95% air/5% CO2 at 37°C. Unless otherwise described, cells were detached from surfaces by incubating with 0.025% trypsin–ethylenediaminetetraacetic acid (Cambrex) for 5 min at 37°C and neutralized with trypsin neutralizing solution (Cambrex) at twice the volume of the trypsin.
Substrates for EC culture
All experiments with CB-EPCs or HAECs alone were performed on Teflon-A (DuPont, Wilmington, DE) films spun-cast onto standard glass microscope slides (ThermoScientific, Waltham, MA), which were cleaned by sonication with 2% PCC-54 solution (Pierce, Rockford, IL) in a 1:1 mixture of MeOH:HCl. After air-drying, Teflon-AF was spun-cast onto the slides.32 Teflon-AF–coated slides were incubated with 3.3 μg/mL human plasma FN (Sigma) in Dulbecco's phosphate buffered saline (DPBS; Gibco) for 1 h at 37°C.
Cocultures were prepared as previously described.10 Briefly, SMCs were plated at 80,000 cells/cm2, with SMC growth medium on polystyrene Slideflasks (Nunc, Rochester, NY) that were incubated with 3.3 μg/mL FN for 1 h. One day after seeding, the growth medium was switched to SMC quiescent medium. SMCs were maintained with quiescent medium for 2 days before the HAECs or CB-EPCs were seeded at a confluent density (125,000 HAECs/cm2 and 200,000 CB-EPCs/cm2) directly on top of the quiescent SMCs in HAEC growth medium. After 24 h, experiments were performed.
Cell staining
CB-EPCs and HAECs were stained with DiI-acetylated-LDL (Biomedical Technologies, Stoughton, MA) by adding the 5 μg/mL solution directly into cell culture medium and incubating for 4 h at 37°C. Cells were immunolabeled by fixing the cells in 3.7% paraformaldehyde for 10 min at 37°C, incubating with 0.2% Triton for 5 min at room temperature, blocking with 10% goat serum for 30 min at 37°C, and incubating with CD31 (1:100; Serotec, Kidlington, United Kingdom), von Willebrand Factor (vWF, 1:1000; Santa Cruz, Santa Cruz, CA), or VE-cadherin (6:200; Santa Cruz) for 1 h. After rinsing, cells were incubated with Alexa Fluor 488 (1:500; Invitrogen, Grand Island, NY) for 45 min at 37°C. Images were taken using an epi-fluorescence microscope (Ziess Axiovert S100, Carl Zeiss Inc., Thornwood, NY).
Cell spreading
To determine the projected cell area, both CB-EPCs and HAECs were stained with Cell Tracker Orange (2 μM, 15 min, 37°C; Invitrogen) and seeded onto either a confluent layer of quiescent SMCs or FN-coated Teflon-AF at a subconfluent density (approximately 10–20,000 cells/cm2). One hour after attachment, the medium was changed to the HAEC growth medium. The projected cell area was measured using ImageJ (version 1.36, National Institutes of Health). Approximately 75 cells were examined and averaged at each time point for each condition. Three independent experiments were performed for all conditions.
Strength of adhesion
Cell detachment as a function of shear stress was determined as previously described.32 Cell nuclei were labeled with Hoescht 33342 (1 μM, 5 min, 37°C; Invitrogen). Cells were then seeded onto quiescent SMCs in slideflasks or FN adsorbed to Teflon-AF–coated slides for 10 min at room temperature. Next, slides were gently rinsed three times with DPBS to remove nonspecifically adherent cells. After rinsing, slides were placed in a variable height flow chamber,33 and 5 images were taken at 5 different channel heights along the chamber for a total of 25 images per experiment. Steady laminar flow was applied for 2 min. The total elapsed time from initial cell attachment to the onset of flow was 20 min. The flow medium consisted of DPBS with varying amounts of dextran (2 × 106 kDa; Sigma) yielding fluid viscosities from 1 to 5 centipoise. The shear stress was computed by
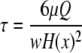 |
where μ is the fluid viscosity, w is the width of the flow channel, Q is the volumetric flow rate, and H(x) is the height of the flow chamber as a function of position along the flow chamber.33 Shear stresses typically ranged from 100 to 285 dyn/cm2. After flow exposure, images were captured at exactly the same positions along the slide as the preflow images, and the number of cells that remained adherent postflow was determined. Experiments were performed in triplicate.
Flow cytometry
The surface expression of α5β1 and αVβ3 integrins present on CB-EPCs and HAECs was determined by flow cytometry. Cells were trypsinized, incubated with 10 μg/mL (approximate value) mouse-anti-α5β1, 20 μg/mL mouse-anti-αVβ3 antibodies (Chemicon), 1 μg/mL CD31, 1 μg/mL VE-cadherein (Santa Cruz), or 2.5 μg/mL CD14 (Santa Cruz) for 1 h, rinsed and pelleted, incubated with Alexa fluor 488 goat-anti-mouse secondary antibody (1:500; Invitrogen), rinsed and pelleted, and fixed in 3.7% paraformaldehyde. For each antibody, preliminary experiments were performed to determine the antibody concentration necessary to saturate the integrin binding sites.
Fluorescence intensity per cell produced by the bound antibodies was measured using a FACSCalibur flow cytometer (Becton Dickinson). Typically, 10,000 CB-EPCs and HAECs were measured for fluorescent intensity per experiment. In addition, isotype controls (Mouse IgG; Caltag, Burlingame, CA) were performed for each sample condition, and the mean fluorescent intensity found for the isotype control was subtracted from the mean fluorescent intensity of the antibody-bound cells.
Blocking studies
CB-EPCs or HAECs were stained with Cell Tracker Orange, trypsinized, and resuspended in DPBS. Cells were then incubated with 10 μg/mL mouse-anti-α5β1, 20 μg/mL mouse-anti-αVβ3 antibodies, alone or in combination, or no antibodies (control) for 30 min at 37°C with gentle rotation. The cells were seeded onto either confluent layers of SMCs, or FN and allowed to adhere for 15 min. After incubation, nonadherent cells were rinsed off the surface and the cells that adhered were imaged and counted using ImageJ software. Experiments were performed in triplicate.
Long-term flow experiments
CB-EPCs or HAECs stained with Cell Tracker Orange were seeded at 100,000 cells/cm2 onto FN-coated Teflon-AF or SMCs as previously described (in Substrates for EC Culture section). After 24 h of adhesion, the slides were placed in a parallel plate flow chamber and connected to a circular flow loop as described previously.33 Cells were exposed to a shear stress of 15 dyn/cm2 for 24 or 48 h with the flow medium, which had a viscosity of 1.05 centipoise at 37°C. Controls consisted of cells under identical culture conditions, but not exposed to flow (static).
Five random images of the Cell Tracker Orange–positive cells were captured at each condition after the 48 h experiment. ImageJ software was used to trace the cells and measure the area (A), maximum chord length (L), and angle of cell orientation with flow (0° corresponds to the direction of flow). The roundness of the cells was calculated using:
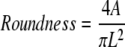 |
The roundness ranges from 1.0, for a circle, to 0, for a straight line; therefore, the closer to 0 the more elongated the cells. On average, 75 cells were examined for each condition and 4 independent experiments were completed. After imaging the cell cultures, the cells were detached and CB-EPCs/HAECs were separated from SMCs as previously described and used for quantitative real-time reverse transcriptase–polymerase chain reaction (RT-PCR).34
Quantitative real-time RT-PCR
RNA was isolated from the purified HAECs or CB-EPCs using an RNA isolation kit (High-Pure Total RNA Isolation Kit; Roche Applied Science, Indianapolis, IN), and real-time quantitative RT-PCR was performed as described previously.34 The 2−ΔΔCT method was used to determine the relative gene expression.35 The primers used, selected based on the gene sequence of interest, were
beta 2-microglobulin: 5′-GGC TAT CCA GCG TAC TCC AAA G-3′ and 5′-CAA CTT CAA TGT CGG ATG GAT G-3′;
endothelial nitric oxide synthase (eNOS): 5′-GTG ATG GCG AAG CGA GTG AAG-3′ and 5′CCG AGC CCG AAC ACA CAG AAC 3′;
cyclooxygenase-2 (COX2): 5′-TGA GCA TGT ACG GTT TGC TG-3′ and 5′-TGC TTG TCT GGA ACA ACT GC-3′;
endothelin (ET-1): 5′-TCC TCT GCT GGT TCC TGA CT-3′ and 5′-CAG AAA CTC CAC CCC TGT GT-3′;
tissue plasminogen activator (tPA): 5′-CAA GTG TCC TTC CCC TTT CC-3′ and 5′-GGG TTG TGG CAA CAG AAA GT-3′;
thrombomodulin: 5′-TAC GGG AGA CAA CAA CAC CA-3′ and 5′-AAG TGG AAC TCG CAG AGG AA-3′;
Kruppel-like factor-2 (KLF-2): 5′-GCA CGC ACA CAG GTC AGA AG-3′ and 5′-ACC AGT CAC AGT TTG GGA GGG-3′;
cyclin D: 5′-GAG GAA GAG GAG GAG GAG GA-3′ and 5′-GAG ATG GAA GGG GGA AAG AG-3′;
vascular cell adhesion molecule 1 (VCAM-1): 5′-GGG CTT TCC TGC TGC GAA-3′ and 5′-AAG AGG CTG TAG CTC CCC G-3′;
intracellular adhesion molecule 1 (ICAM-1): CAG TGA CCA TCT ACA GCT TTC CGG-3′ and 5′-GCT GCT ACC ACA GTG ATG ATG ACA-3′; and
E-selectin: 5′-GAT GTG GGC ATG TGG AAT GAT G-3′ and 5′-AGG TAC ACT GAA GGC TCT GG-3′.
Beta 2-microglobulin was the housekeeping gene used for the 2−ΔΔCT method. Melting curves were obtained after each reaction to verify that only the target cDNA was amplified.
Adhesion protein measurements and monocyte adhesion
CB-EPCs and HAECs were stained with Cell Tracker Green, seeded onto SMCs or Teflon-AF, and either exposed to long-term flow or cultured under static conditions as previously described. To examine the inflammatory response, tumor necrosis factor (TNF)-α (5 Units/mL; Sigma) was added for the last 4.5 h of the 24 h experiment. At the end of the experiment, CB-EPCs or HAECS were stained with mouse anti-human ICAM-1, VCAM-1, E-Selectin (BD Biosciences, Bedford, MA), or Mouse IgG (Caltag) at a 1:200 dilution and prepared for flow cytometry. Cell Tracker Green intensity was used to distinguish CB-EPCs and HAECs from SMCs, and the protein expression (as determined by PE intensity) was only measured for the Cell Tracker Green–positive cells. Monocyte adhesion experiments were performed at a shear stress of 1 dyn/cm2 as described by Wallace.36
Western blot of eNOS
Cells were removed from the FN-coated slides by scraping into ice-cold DPBS. Samples were centrifuged and resuspended into Cell Lytic M (Sigma), supplemented 1:10 with Protease Inhibitors Cocktail (Sigma). Samples were incubated on ice, vortexed periodically for 15 min, and centrifuged for 15 min to remove the cell debris. The supernatant was collected and stored at −20°C.
Protein concentrations were measured using the bicinchoninic acid protein assay (Thermo Scientific). Ten micrograms of protein lysate was added to a loading buffer (Laemelli buffer; Bio-Rad, Chicago, IL, and Cleland's Reagent; Calbiochem, San Diego, CA) in a 1:1 ratio and boiled for 5 min. The protein solution was loaded into 4–15% Tris-HCl Ready Made gel (Bio-Rad), and electrophoresis was performed at 150 V for approximately 40–45 min. A wet transfer (Tris-Glycine; Bio-Rad) was completed at 40 W, 500 mA, and 200 V for 2.5 h to transfer the protein from the gel to a polyvinylidene fluoride membrane (Bio-Rad). After blocking with 5% milk in Tris-buffered saline + 0.05% Tween (TBST) for 1 h at room temperature, the membrane was incubated with a rabbit anti-human eNOS antibody (1:200; Santa Cruz) in 1% milk in TBST overnight at 4°C, rinsed, incubated with a goat anti-rabbit secondary horseradish peroxidase (HRP) (1:5000; Santa Cruz) for 45 min at room temperature, rinsed, covered with enhanced chemiluminescence solution (ECL; Pierce) for 5 min, and then exposed to CL-Xposure Film (Pierce). The membrane was then stripped (Restore Plus Western Stripping Buffer; Pierce), blocked with 5% milk in TBST, and incubated with a mouse anti-human glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody (1:5000; Imgenex, San Diego, CA) and incubated overnight at 4°C. Membranes were then incubated with goat anti-mouse secondary HRP (1:5000; Santa Cruz) for 45 min at room temperature, rinsed, and exposed to detect bands as described above.
Bands were quantified using densitometry, eNOS molecular weight was about 135 kDa, and GAPDH molecular weight was about 37 kDa. The films were scanned into the computer, and ImageJ was used to quantify the band density. The eNOS values were normalized to the loading controls (GAPDH).
Measurement of nitric oxide production
After 24 h of flow, medium samples were frozen at −80°C and lyophilized (Appropriate Technical Resources, Laurel, MD) for 20–24 h. The lyophilized medium was resuspended into 1/5 of its original volume of assay buffer provided by the Nitric Oxide Quantification Kit (Active Motif, Carlsbad, CA), and the kit's protocol was followed to determine amount of nitrites and nitrates in the original medium samples.
Statistical analysis
Statview 5.0.1 was used to compare data to determine the statistical differences between cell sources and conditions used. Analysis of variance (ANOVA) and Tukey-Kramer post hoc analysis were conducted to determine significance for all experiments. All values are reported as means ± standard error of the means.
Results
CB-EPC culture and characterization
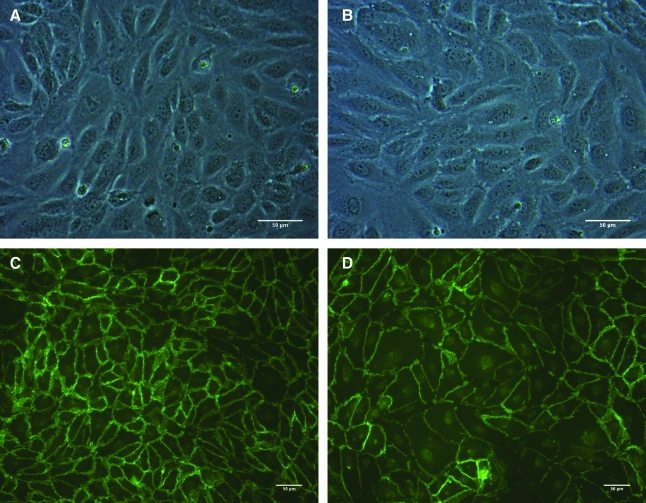
CB-EPCs appeared as early as 7 days after isolation and reached confluency and were passaged once before characterization. As viewed under phase contrast microscopy, CB-EPCs and HAECs (Fig. 1A, B) have a typical EC cobblestone morphology. Both cell types were positive for VE-cadherin and demonstrate cell-to-cell contact (Fig. 1C, D). The staining pattern for platelet endothelial cell adhesion molecule (PECAM) was similar to the pattern observed with VE-cadherin (images not shown). Both CB-EPCs and HAECs incorporated DiI-acetylated LDL and stained positively for CD31 and vWF. As demonstrated by flow cytometry, CB-EPCs had similar levels of CD31 and VE-cadherin surface expression as HAECs and did not express CD14, a monocyte/macrophage marker (not shown). Monocytes (U937, American Type Culture Collection (ATCC)) were used as a control for all experiments and did not express any EC markers, while positively expressing CD14.
FIG. 1.
Morphology of CB-EPCs compared to HAECs. (A) Phase contrast image of CB-EPCs seeded onto FN-coated Teflon-AF. (B) Phase contrast image of HAECs seeded onto FN-coated Teflon-AF. (C) CB-EPCs stained with VE-cadherin. (D) HAECs stained with VE-cadherin. CB-EPCs, umbilical cord blood–derived endothelial progenitor cells; HAEC, human aortic endothelial cells; FN, fibronectin.
The CB-EPCs had a doubling time of 1.25 ± 0.12 days, while HAECs had a doubling time of 2.32 ± 1.15 days (p < 0.015, n = 6).
Cell spreading
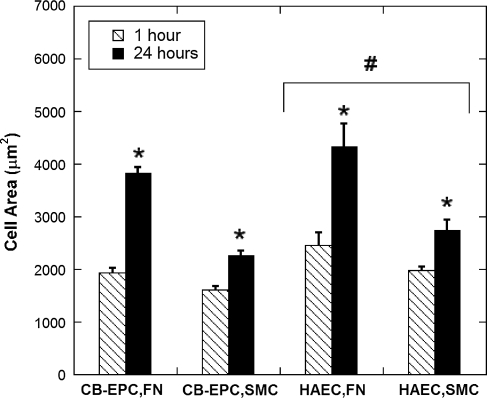
Both CB-EPCs and HAECs had similar projected areas, spread significantly more on FN-coated Teflon-AF compared to SMCs (p < 0.001), and were larger at 24 h compared to 1 h (p < 0.001) (Fig. 2, n = 3). After 1 h both CB-EPCs and HAECs were roughly 50% of their final size when seeded on FN-coated Teflon-AF and 70% of their final size when seeded on SMCs; this suggests that CB-EPCs spread at a similar rate compared to HAECs.
FIG. 2.
CB-EPC and HAEC spreading. Spreading of CB-EPCs and HAECs on either FN-coated Teflon-AF or SMCs at 1 and 24 h. Cell area is greater at 24 h (*p < 0.001) and on FN-coated Teflon-AF compared to SMCs (p < 0.01), and, overall, HAECs are significantly larger than CB-EPCs (#p < 0.05; n = 3, standard error based off of the n = 3). SMC, smooth muscle cell.
Strength of adhesion
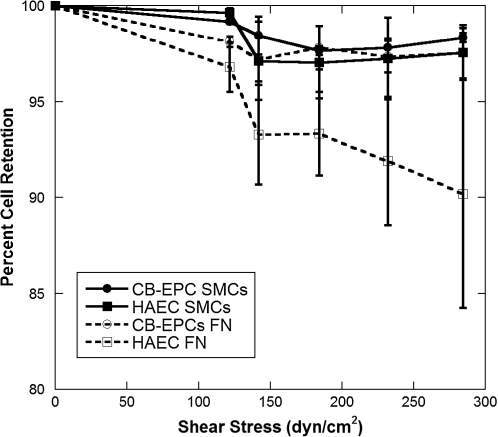
CB-EPCs and HAECs adhered firmly to both FN-coated Teflon-AF and SMCs (Fig. 3, n = 4) when exposed to high shear stresses. Shear stresses as high as 285 dyn/cm2, approximately 20 times greater than normal physiological shear stress, were applied after 20 min of cell attachment. At the highest shear stress, less than 5% of CB-EPCs on FN Teflon-AF or SMCs detached, while 10% of HAECs on FN-coated Teflon-AF detached. This high strength of adhesion has only been observed a few times for such short attachment times.32,37
FIG. 3.
CB-EPC and HAEC strength of adhesion. CB-EPCs and HAECs were adhered to either FN-coated Teflon-AF or SMCs for 20 min before exposure to 2 min of shear stress, and the percentage of cells that remained adherent was determined (n = 4).
Expression and blocking of α5β1 and αVβ3 integrins
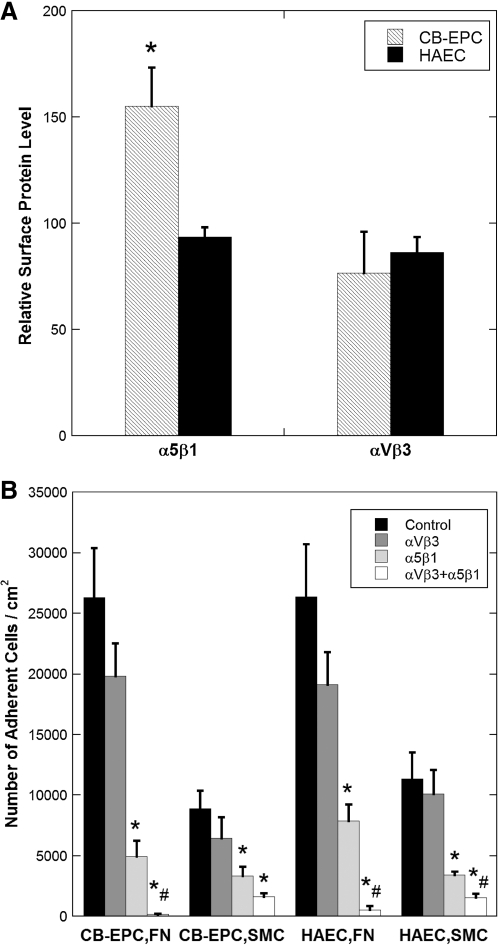
Both α5β1 and αVβ3 integrins affect EC adhesion to FN,32,34 and expression of these integrins may affect the adhesion strength. Both cell types have similar expression of αVβ3, but CB-EPCs express a significantly higher level of α5β1 (Fig. 4A, p < 0.05, n = 4). Antibodies to α5β1 alone significantly decreased adhesion to both FN and SMCs (p < 0.05, n = 4), antibodies to αVβ3 did not decrease adhesion, and the combination of the two antibodies significantly decreased adhesion (p < 0.01) to a greater extent than the decrease caused by α5β1 alone for all conditions except for CB-EPCs on SMCs (p < 0.05) (Fig. 4D).
FIG. 4.
Integrin expression and blocking by CB-EPCs and HAECs. (A) Relative amount of adhesion integrins present on CB-EPCs and HAECs determined by flow cytometry. CB-EPCs express a significantly higher level of α5β1 compared to HAECs (*p < 0.05; n = 4). (B) Number of adherent cells after blocking α5β1, αVβ3, or both α5β1 and αVβ3 integrins with blocking antibodies. α5β1 significantly decreased adhesion compared to the control (no blocking antibodies) (*p < 0.05), both α5β1 and αVβ3 significantly decreased adhesion compared to the control (*p < 0.01), and both α5β1 and αVβ3 significantly decreased adhesion compared to α5β1 (#p < 0.05).
CB-EPC alignment under flow
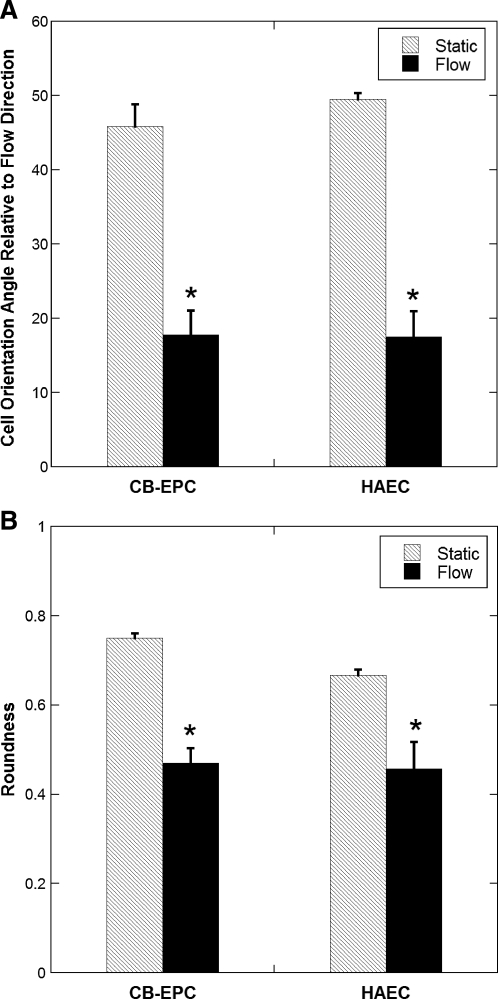
After exposure of CB-EPCs and HAECs seeded on FN-coated Teflon-AF to a shear stress of 15 dyn/cm2 for 48 h, both cell types oriented and elongated in the direction of flow compared to static conditions (Fig. 5, p < 0.001, n = 4). This behavior is characteristic of most native ECs exposed to steady, fully developed laminar flow. No significant differences were observed in the orientation or roundness between HAECs and CB-EPCs exposed to long-term flow.
FIG. 5.
CB-EPC and HAEC alignment under long-term flow. (A) Orientation and (B) elongation of CB-EPCs and HAECs on FN-coated Teflon-AF surfaces when exposed to 48 h of 15 dyn/cm2 shear stress and under static conditions (0° is perfectly aligned with the direction of flow, 0 is a perfect line, and 1 is a perfect circle), *p < 0.001 compared to static conditions (n = 4).
Effect of flow on antithrombogenic and prothrombogenic gene expression
To determine if CB-EPCs behaved similar to HAECs under static and flow conditions, we examined the expression of 10 genes involved in antithrombogenic and prothrombogenic EC function: eNOS, COX2, ET-1, tPA, thrombomodulin, KLF-2, cyclin D1, VCAM-1, ICAM-1, and E-selectin on FN-coated Teflon-AF or cocultured on SMCs (Table 1, n = 4). The differences found between the two cell types are underlined on the table, and the significance level is denoted with superscript “e”. On FN under static conditions, the only difference between the two cell types was a higher level of ET-1 mRNA on CB-EPCs. Flow caused a significant increase in eNOS, COX2, KLF-2, VCAM-1, and thrombomodulin for both cell types and ICAM-1 and E-selectin in CB-EPCs. After exposure to flow, only COX2 mRNA levels were significantly higher in CB-EPCs than HAECs. ET-1 mRNA levels were depressed slightly by flow, but the effect was not significant. Flow caused a suppression of cyclin D-1 on HAECs but not CB-EPCs. For cells grown on SMCs, the trends were generally similar to those observed on FN, although tPA and ET-1 levels were not affected by flow. The other major difference between cells grown on SMCs is the significant elevation of VCAM-1, ICAM-1, and E-selectin in HAECs exposed to flow.
Table 1.
Antithrombotic and Prothrombotic Gene Expression in Umbilical Cord Blood–Derived Endothelial Progenitor Cells and Human Aortic Endothelial Cells
|
Surface |
FN |
SMC |
||||||
|---|---|---|---|---|---|---|---|---|
|
Flow conditions |
Static |
Flow |
Static |
Flow |
||||
| Gene | CB-EPC | HAEC | CB-EPC | HAEC | CB-EPC | HAEC | CB-EPC | HAEC |
| eNOS | 1.0 ± 0.2 | 1.4 ± 0.4 | 6.0 ± 0.8a | 4.9 ± 1.1a | 1.5 ± 0.2 | 1.1 ± 0.2 | 5.9 ± 0.9a | 3.8 ± 0.6a |
| COX2 | 1.2 ± 0.2 | 0.9 ± 0.3 | 6.7 ± 0.9a,b,c | 2.2 ± 0.2 | 0.8 ± 0.2 | 0.5 ± 0.1 | 12.5 ± 0.7a,b | 8.9 ± 0.2a,c |
| KLF-2 | 1.5 ± 0.6 | 0.6 ± 0.3 | 9.1 ± 1.2a | 5.2 ± 1.8d | 1.9 ± 0.5e | 0.6 ± 0.3 | 7.3 ± 1.8a | 4.0 ± 0.4a |
| Thrombomodulin | 2 ± 0.1 | 2.1 ± 0.4 | 10.6 ± 1.9a | 10.7 ± 1.9d | 2.6 ± 0.4 | 5.8 ± 0.3 | 8.5 ± 1.6d | 10.6 ± 1.7d |
| tPA | 0.7 ± 0.1c | 0.5 ± 0.1c | 0.5 ± 0.1c | 1.4 ± 0.1c | 1.3 ± 0.04 | 1.1 ± 0.1 | 0.9 ± 0.08 | 0.9 ± 0.1 |
| ET-1 | 1.0 ± 0.1c,e | 0.4 ± 0.1 | 0.5 ± 0.1 | 0.3 ± 0.1 | 0.4 ± 0.04 | 0.3 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 |
| Cyclin D | 0.9 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.2 | 0.5 ± 0.1d | 0.7 ± 0.1 | 0.9 ± 0.04 | 0.7 ± 0.1 | 0.6 ± 0.04a |
| VCAM-1 | 0.4 ± 0.1 | 0.2 ± 0.03 | 2.0 ± 0.8 | 0.9 ± 0.2d | 0.2 ± 0.1 | 0.1 ± 0.02 | 1.1 ± 0.6 | 2.1 ± 0.5a |
| ICAM-1 | 0.5 ± 0.1 | 0.7 ± 0.1 | 4.8 ± 1.3a | 0.5 ± 0.1c | 0.2 ± 0.1 | 0.2 ± 0.1 | 4.3 ± 2.2a | 5.9 ± 0.9a |
| E-selectin | 0.9 ± 0.2 | 1.7 ± 1.1 | 5.2 ± 3.4 | 1.7 ± 0.8c | 0.5 ± 0.3 | 0.2 ± 0.1b | 12.2 ± 11 | 90.7 ± 9.2a |
Comparison of antithrombotic and prothrombotic genes in CB-EPCs and HAECs on either FN or SMC surfaces exposed to static or flow conditions. The underlined values represent differences in the two cell types, and the boldfaced values are the significant findings.
p < 0.01 and dp < 0.05, compared to equivalent static conditions.
p < 0.01 and ep < 0.05, compared to equivalent HAEC conditions.
p < 0.05, compared to equivalent SMC conditions.
CB-EPCs, umbilical cord blood–derived endothelial progenitor cells; HAEC, human aortic endothelial cells; FN, fibronectin; SMC, smooth muscle cell; eNOS, endothelial nitric oxide synthase; COX2, cyclooxygenase-2; ET-1, endothelin-1; tPA, tissue plasminogen activator; KLF-2, Kruppel-like factor-2; VCAM-1, vascular cell adhesion molecule 1; ICAM-1, intracellular adhesion molecule 1.
Leukocyte adhesion molecule protein levels and monocyte adhesion
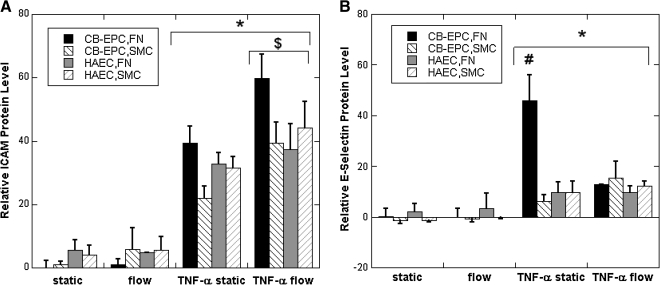
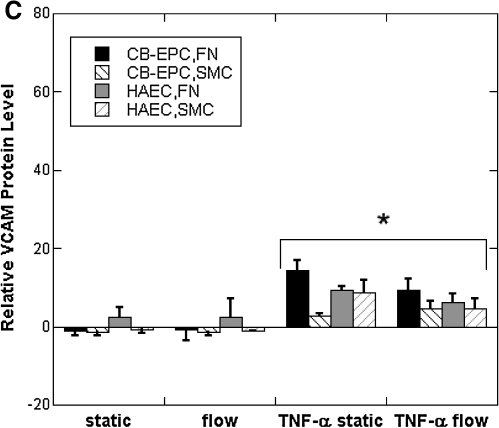
While mRNA levels of VCAM-1, ICAM-1, and E-selectin were elevated under flow for CB-EPCs on FN and both cell types on SMCs, the mRNA for these conditions was much less than we had previously observed after stimulation with 5 U/mL TNF-α.36 We did note that mRNA levels of VCAM-1 and E-selectin under static conditions were much lower than other genes, suggesting that these responses to flow may not lead to significant protein expression. To assess the functional implications of VCAM-1, ICAM-1, and E-selectin mRNA levels, we measured protein expression by flow cytometry for both CB-EPCs and HAECs seeded onto SMCs and/or FN-coated Teflon-AF that underwent static and flow conditions for 24 h. The expression of all three adhesion molecules was very low for all conditions and in fact could not be distinguished from nonspecific binding with mouse IgG for most cases (Fig. 6A–C). Monocyte adhesion experiments did not detect any adhesion at a shear stress of 1 dyn/cm2.
FIG. 6.
CB-EPC and HAEC expression of adhesion molecules as measured by flow cytometry after exposure to static and/or flow conditions on both FN-coated Teflon-AF or SMCs and either unstimulated or stimulated with TNF-α for 4.5 h. (A) Expression of intracellular adhesion molecule 1. (B) Expression of E-Selectin. (C) Expression of vascular cell adhesion molecule: *p < 0.01 compared to non-TNF-α stimulated conditions, #p < 0.01 compared to all other conditions, and $p < 0.01 compared to static conditions (n = 4). TNF, tumor necrosis factor.
To assess any differences between CB-EPCs and HAECs after exposure to an inflammatory stimulus, we exposed the cells to 5 U/mL TNF-α for the final 4.5 h of the 24 h experiment. The presence of TNF-α caused an overall increase in all three adhesion molecules (n = 4). An ANOVA tests revealed the following differences between the two cell types after exposure to TNF-α. E-selectin levels on CB-EPCs seeded on FN under static conditions were significantly elevated. The elevated level of E-selectin on CB-EPCs was diminished when the cells were exposed to flow conditions. The expression of VCAM-1 was significantly less for CB-EPCs cocultured with SMCs under static conditions compared to culture on FN. ICAM expression was increased for flow conditions versus static for both cell types on both surfaces. As expected, we did observe monocyte adhesion to CB-EPCs and HAECs treated with 5 U/mL TNF-α at a shear stress of 1 dyn/cm2 (data not shown).
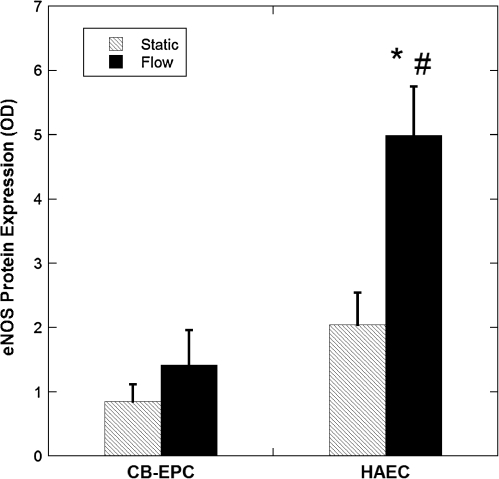
eNOS protein expression and NO production
Total eNOS protein expression was determined after 24 h of exposure to 15 dyn/cm2. Flow induced an overall increase in eNOS protein expression, but the increase was not statistically significant for CB-EPCs, while it was for HAECs (p < 0.05, n = 4) (Fig. 7). eNOS protein expression was found to be significantly higher in the HAECs compared to the CB-EPCs (p < 0.05). Possibly due to the trypsin levels used and the long procedure necessary to separate the ECs with SMCs, we were unsuccessful in measuring eNOS in coculture although we have measured cell surface proteins and other intracellular proteins in ECs in coculture.34
FIG. 7.
CB-EPC and HAEC eNOS protein expression. eNOS protein expression was measured from CB-EPCs and HAECs on FN-coated Teflon-AF either exposed to 15 dyn/cm2 for 24 h or under static conditions: *p < 0.05, compared to static conditions, and #p < 0.05, compared to CB-EPCs (n = 4). eNOS, endothelial nitric oxide synthase.
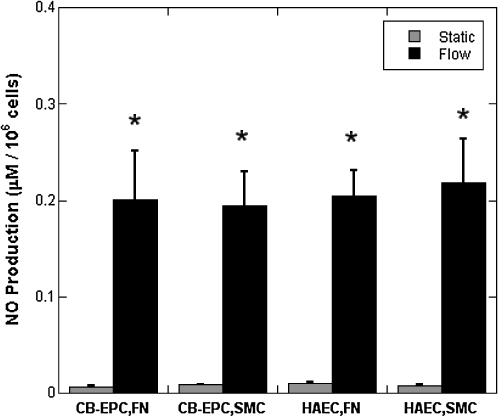
Thus, to assess the effect of the reduced eNOS levels in CB-EPCs and the effect of coculture, we measured NO after 24 h of exposure to 15 dyn/cm2. Flow induced a significant increase in NO production for both cell types on both FN-coated Teflon-AF and SMCs (Fig. 8, n = 4, p < 0.001). There were no significant differences in NO levels between cell types.
FIG. 8.
NO produced by CB-EPCs and HAECs. NO was measured, by total amount of nitrites and nitrates, in the supernatant of CB-EPCs and HAECs on FN-coated Teflon-AF or SMCs either exposed to 15 dyn/cm2 of shear stress for 24 h or under static conditions, *p < 0.001, compared to static conditions (n = 4).
Discussion
CB-EPCs are a promising cell source for many cardiovascular therapeutic applications; however, the extent to which they behave like vascular endothelium and respond to flow has yet to be investigated. We isolated late outgrowth CB-EPCs from umbilical cord blood and compared EC functional properties of CB-EPCs with adult HAECs under laminar flow conditions to determine their ability to meet the five criteria we set for use as replacement cells for synthetic vascular grafts and TEBVs.
Late outgrowth CB-EPCs were easily isolated and grew to large numbers with a doubling time approximately twice as fast as the doubling time of HAECs, satisfying the first criterion that the EC replacement comes from an easily obtainable and reliable source.
We showed that late outgrowth CB-EPCs resembled HAECs in morphology, expression of CD31, VE-cadherein, vWF, ability to bind and internalize DiI-acetylated-LDL, and absence of CD14. These results are consistent with the description of late outgrowth CB-EPCs by Yoder and colleagues, which they defined as ECFCs.27 CB-EPCs meet our second criterion for EC replacement cells in vascular therapies, which is that the cells display EC-specific markers and lack monocyte characteristics. This important criterion is based on the presence of two distinct EPCs, ECFCs and CFU-ECs, found within peripheral and umbilical cord blood.27 CFU-ECs have limited proliferation, express monocytic/macrophage markers, do not adopt a cobblestone morphology, differentiate into phagocytic macrophages, and display myeloid progenitor cell activity.27 ECFCs do not exhibit these monocytic properties, which are unfavorable for a vessel lining.
An ideal synthetic graft or TEBV needs a lining of ECs that can withstand physiological levels of shear stress to maintain a confluent endothelium. Through the use of a mild trypsinization techniques32 we found that CB-EPCs have similar adhesion strength compared to HAECs when seeded onto FN-coated Teflon-AF or SMCs (Fig. 3). In fact, both cell types remained adherent when exposed to shear stresses much higher than the physiological range and have some of the highest adhesion strength ever reported for such short attachment times.32 The ability of CB-EPCs to remain adherent when exposed to supraphysiological shear stresses after only 20 min of attachment suggests that they can be seeded onto synthetic grafts or TEBVs within a short time period before use. Such rapid and firm adhesion may permit rapid seeding and graft implantation, in which the stored CB-EPCs could be available and matched from donor to recipient and then seeded onto the graft during the surgical procedure.
Both cell types maintained confluent monolayers on SMCs or FN-coated Teflon-AF when exposed 15 dyn/cm2 for 24–48 h. CB-EPCs adapted to long-term flow conditions by elongating and aligning with the direction of flow similar to native ECs. PB-EPCs also exhibit this characteristic.1,38
EC adhesion to materials is mediated by the cell adhesion proteins bound to the surface of the material, such as FN or vitronectin.39,40 FN and vitronectin form bonds with the cell's surface integrins, specifically α5β1 and αVβ3, inducing firm adhesion at the cell–substrate interface. Cell adhesion strength is initially dependent on integrin– ligand interactions, which is later strengthened by focal contact formation.41
CB-EPCs and HAECs have a similar expression level of αVβ3; however, CB-EPCs have a significantly higher level of α5β1 expression compared to HAECs. Both CB-EPCs and HAECs had less adhesion to both substrates when α5β1 was blocked, and adhesion was further hindered when both α5β1 and αVβ3 were blocked; however, when only αVβ3 is blocked, the reduction in adhesion was not significant. These findings are similar to what we previously found with HAECs.34 Thus, α5β1 may play the dominant role in adhesion of both cell types, but αVβ3 integrins also contribute to adhesion. These results indicate that CB-EPCs satisfy our third criterion of strong adhesion necessary for successful grafts or TEBVs.
ECs lining synthetic grafts and TEBVs must be antithrombotic, which is our fourth criterion an EC source must meet to be used. ECs pro- and antithrombotic nature varies depending on the shear stress acting on the cells. The cells are more thrombotic at lower shear stresses and more antithrombotic at higher shear stress values. For instance, eNOS gene expression and NO production increase when shear stress increased.42 We compared the two cell types at only one laminar shear stress, 15 dyn/cm2.
The functional properties of ECs are required to maintain homeostasis in the vasculature; therefore, we examined a number of key antithrombotic and prothrombotic genes that are known to regulate vessel function. Three important molecules secreted by ECs that influence vascular hemodynamics are NO, prostacyclin, and ET-1.43 NO promotes vasodilation of the blood vessel and platelet disaggregation, while also inhibiting leukocyte adhesion and SMC migration and proliferation.5,43,44 NO is constitutively secreted by ECs primarily by eNOS,43 and its production is modulated by exogenous chemical and physical stimuli, such as shear stress.43,44 Prostacyclin, which is produced by COX2, can stimulate vasodilation and prevent platelet aggregation and deposition.5,43 In contrast, ET-1 is a very potent vasoconstrictor and promotes SMC proliferation.43 We found similar gene expression levels of eNOS, COX2, and ET-1 in both CB-EPCs and HAECs under static and long-term physiological flow conditions. The decrease in ET-1 expression for both cell types on FN is consistent with other reports for ECs seeded on FN-coated surfaces and exposed to laminar flow.45,46 The slight increase in ET-1 mRNA when CB-EPCs and HAECs were grown on SMCs and exposed to flow demonstrates the importance of the underlying substrate upon EC phenotype. We also determined that eNOS protein expression increased under flow conditions in HAECs and was significantly less abundant in CB-EPCs compared to HAECs, while other studies have not found detectable levels of eNOS in either CB-EPCs or PB-EPCS (CFU-ECs) through Western blotting.47 However, the eNOS protein expression does not directly correlate with NO production since the protein must be activated to produce NO.48 The amount of NO produced by CB-EPCs and HAECs increased significantly after exposure to fluid shear stress for both substrates, and there was no significant difference in the amount of NO produced by the two cell types. While cultured SMCs alone do produce NO in response to fluid shear stress by neuronal NOS,49 SMCs in coculture are not exposed to shear stress directly and the similarity in the levels of NO produced with and without SMC coculture suggests that SMC NO production was not significant.
Although tPA levels did increase with HAEC cultured on FN, which correlates with previous findings on synthetic grafts,50 the ANOVA was not significant. tPA mRNA levels in CB-EPCs did not increase with shear stress. However, when cultured on SMCs, both cell types had slightly higher levels of tPA mRNA and did not increase tPA mRNA after exposure to flow, further demonstrating the importance of the substrate.
CB-EPCs and HAECs expressed similar levels of the transcription factor KLF-2, which is exclusively expressed within the endothelium in the vasculature.51 KLF-2 is a key regulator of oxidative and antithrombotic responses to shear stress.51 KLF-2 is known to induce eNOS and thrombomodulin expression and activity, reduce ET-1 expression, inhibit the inflammatory cytokine-mediated induction of adhesion molecule expression, and inhibit leukocyte adhesion.34 CB-EPCs and HAECs have a significantly higher expression level of KLF-2 under flow.
To assess the effect of flow on the growth potential of the highly proliferative CB-EPCs, we measured gene expression of cyclin D1, which is involved with proliferation.52 However, we did not find any significant difference between cyclin D1 levels in CB-EPCs and HAECs, and there was a slight decrease in cyclin D1 when the cells were exposed to flow. The cells were seeded at confluent density to simulate in vivo conditions and hence would not be likely to proliferate extensively, which may be the reason we do not see a difference between the two cell types.
VCAM-1, ICAM-1, and E-selectin are major players in monocyte and leukocyte adhesion to the endothelium. E-selectin interacts with a ligand on the leukocyte surface, which captures the leukocyte and induces it to roll on the endothelium. Then, VCAM-1 and ICAM-1 bind to leukocyte integrins causing firm adhesions of the leukocyte to the endothelium surface.53 There were some notable differences in mRNA expression on FN and SMCs. VCAM-1 on CB-EPCs attached to FN increased under flow, and VCAM-1, ICAM-1, and E-selectin increased in HAECs exposed to flow. This increase in VCAM-1 and E-selectin mRNA after flow exposure differs from the results of Brooks et al.45 for HAEC grown on FN-coated plastic and exposed to laminar shear stress for 24 h. However, we did find the effect of flow on ICAM-1 mRNA for HAECs on FN to be similar to previously reported trends.45 The increased mRNA levels with flow for cells grown on FN are much less than the increase in VCAM-1, ICAM-1, and E-selectin mRNA in the presence of TNF-α, which increased VCAM-1 and E-selectin 44–112-fold at 5 U/mL.36 The increase in E-selectin level for HAECs grown on SMCs and exposed to flow is comparable to low levels of TNF-α stimulation, but the increase for CB-EPCs grown on SMCs is about seven times smaller. We found that the expression of all three of these proteins to be very low, with no significant difference between HAECs and CB-EPCs found (Fig. 6a–c). At 1 dyn/cm2 monocytes did not adhere to CB-EC or HAECs previously cultured under static or flow conditions. Therefore, CB-EPCs have a small amount of adhesion proteins on their surface and do not promote monocyte adhesion similar to HAECs.
Overall, we found few differences between the two cell types in protein levels of VCAM-1, ICAM-1, and E-selectin after stimulation with TNF-α. CB-EPCs on FN under static conditions and exposed to TNF-α did have a significantly higher amount of E-selectin compared to all other conditions; however, this protein expression was significantly down-regulated under flow conditions. The effect of flow on adhesion molecule protein levels after TNF-α stimulation was similar to that reported by Chiu et al.54 Overall, these results suggest that CB-EPCs are similar when compared to HAECs' protein expression of important monocyte adhesion molecules, and prevent monocyte adhesion in vitro.
In conclusion, these results suggest that CB-EPCs meet most of the criteria for a suitable cell source for lining synthetic vascular grafts and TEBVs to obtain a firmly adherent, confluent, and antithrombotic endothelium. CB-EPCs (1) are easily obtainable, (2) exhibit EC-specific markers and do not exhibit monocytic markers, (3) have a high strength of adhesion, and (4) possess a number of antithrombotic qualities when exposed to physiological laminar shear stress. Future studies will determine whether CB-EPCs prevent neointimal thickening and thrombosis in vivo. The effect of other flow conditions (e.g., low shear stresses and oscillatory shear stress) in combination with cytokine treatment needs to be assessed to determine how CB-EPCs behave under athero-prone conditions. However, the functional properties of CB-EPCs found in this study provide initial support for the use CB-EPCs as an EC source for replacement vessels that will remain patent under flow conditions.
Acknowledgments
The authors would like to thank Laura Mead from Indiana University School of Medicine for valuable advice in the isolation of cord blood–derived endothelial progenitor cells and Yihua Loo from Duke University for assistance with the lyophilization process. The work was supported by NIH Grants HL-44972 and HL-88825, an NIH Biotechnology Training Grant (T31GM8555) fellowship to M.A.B., and a graduate fellowship to C.S.W. (F31EB006298).
Disclosure Statement
No competing financial interests exist.
References
- 1.Shirota T. He H. Yasui H. Matsuda T. Human endothelial progenitor cell-seeded hybrid graft: proliferative and antithrombogenic potentials in vitro and fabrication processing. Tissue Eng. 2003;9:127. doi: 10.1089/107632703762687609. [DOI] [PubMed] [Google Scholar]
- 2.Gong Z. Niklason L.E. Blood vessels engineered from human cells. Trends Cardiovasc Med. 2006;16:153. doi: 10.1016/j.tcm.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Shirota T. Yasui H. Matsuda T. Intralumenal tissue-engineered therapeutic stent using endothelial progenitor cell-inoculated hybrid tissue and in vitro performance. Tissue Eng. 2003;9:473. doi: 10.1089/107632703322066651. [DOI] [PubMed] [Google Scholar]
- 4.Thompson M.M. Budd J.S. Eady S.L. Underwood M.J. James R.F. Bell P.R. The effect of transluminal endothelial seeding on myointimal hyperplasia following angioplasty. Eur J Vasc Surg. 1994;8:423. doi: 10.1016/s0950-821x(05)80961-2. [DOI] [PubMed] [Google Scholar]
- 5.Brown M. Wallace C.S. Truskey G.A. Vascular and capillary endothelium. In: Metin Akay., editor. Wiley Encyclopedia of Biomedical Engineering. John Wiley & Sons I; 2006. DOI: 10.1002/9780471740360.ebs0436. [Google Scholar]
- 6.Hutter R. Carrick F.E. Valdiviezo C. Wolinsky C. Rudge J.S. Wiegand S.J. Fuster V. Badimon J.J. Sauter B.V. Vascular endothelial growth factor regulates reendothelialization and neointima formation in a mouse model of arterial injury. Circulation. 2004;110:2430. doi: 10.1161/01.CIR.0000145120.37891.8A. [DOI] [PubMed] [Google Scholar]
- 7.Consigny P.M. Vitali N.J. Resistance of freshly adherent endothelial cells to detachment by shear stress is matrix and time dependent. J Vasc Interv Radiol. 1998;9:479. doi: 10.1016/s1051-0443(98)70303-3. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt D. Breymann C. Weber A. Guenter C.I. Neuenschwander S. Zund G. Turina M. Hoerstrup S.P. Umbilical cord blood derived endothelial progenitor cells for tissue engineering of vascular grafts. Ann Thorac Surg. 2004;78:2094. doi: 10.1016/j.athoracsur.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 9.Arts C.H. Blankensteijn J.D. Heijnen-Snyder G.J. Verhagen H.J. Hedeman Joosten P.P. Sixma J.J. Eikelboom B.C. de Groot P.G. Reduction of non-endothelial cell contamination of microvascular endothelial cell seeded grafts decreases thrombogenicity and intimal hyperplasia. Eur J Vasc Endovasc Surg. 2002;23:404. doi: 10.1053/ejvs.2002.1604. [DOI] [PubMed] [Google Scholar]
- 10.He H. Shirota T. Yasui H. Matsuda T. Canine endothelial progenitor cell-lined hybrid vascular graft with nonthrombogenic potential. J Thorac Cardiovasc Surg. 2003;126:455. doi: 10.1016/s0022-5223(02)73264-9. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs S. Hermanns M.I. Kirkpatrick C.J. Retention of a differentiated endothelial phenotype by outgrowth endothelial cells isolated from human peripheral blood and expanded in long-term cultures. Cell Tissue Res. 2006;326:79. doi: 10.1007/s00441-006-0222-4. [DOI] [PubMed] [Google Scholar]
- 12.Zammaretti P. Zisch A.H. Adult endothelial progenitor cells. Renewing vasculature. Int J Biochem Cell Biol. 2005;37:493. doi: 10.1016/j.biocel.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 13.Luttun A. Carmeliet G. Carmeliet P. Vascular progenitors: from biology to treatment. Trends Cardiovasc Med. 2002;12:88. doi: 10.1016/s1050-1738(01)00152-9. [DOI] [PubMed] [Google Scholar]
- 14.Dimmeler S. Zeiher A.M. Vascular repair by circulating endothelial progenitor cells: the missing link in atherosclerosis? J Mol Med. 2004;82:671. doi: 10.1007/s00109-004-0580-x. [DOI] [PubMed] [Google Scholar]
- 15.Wassmann S. Werner N. Czech T. Nickenig G. Improvement of endothelial function by systemic transfusion of vascular progenitor cells. Circ Res. 2006;99:e74. doi: 10.1161/01.RES.0000246095.90247.d4. [DOI] [PubMed] [Google Scholar]
- 16.Vemulapalli S. Metzler S.D. Akabani G. Petry N.A. Niehaus N.J. Liu X. Patil N.H. Greer K.L. Jaszczak R.J. Coleman R.E. Dong C. Goldschmidt-Clermont P.J. Chin B.B. Cell therapy in murine atherosclerosis: in vivo imaging with high-resolution helical SPECT. Radiology. 2007;242:198. doi: 10.1148/radiol.2421051461. [DOI] [PubMed] [Google Scholar]
- 17.Rauscher F.M. Goldschmidt-Clermont P.J. Davis B.H. Wang T. Gregg D. Ramaswami P. Pippen A.M. Annex B.H. Dong C. Taylor D.A. Aging, progenitor cell exhaustion, and atherosclerosis. Circulation. 2003;108:457. doi: 10.1161/01.CIR.0000082924.75945.48. [DOI] [PubMed] [Google Scholar]
- 18.Dzau V.J. Gnecchi M. Pachori A.S. Morello F. Melo L.G. Therapeutic potential of endothelial progenitor cells in cardiovascular diseases. Hypertension. 2005;46:7. doi: 10.1161/01.HYP.0000168923.92885.f7. [DOI] [PubMed] [Google Scholar]
- 19.Ingram D.A. Mead L.E. Tanaka H. Meade V. Fenoglio A. Mortell K. Pollok J. Ferkowicz M.J. Gilley D. Yoder M.C. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 20.Ghen M.J. Roshan R. Roshan R.O. Blyweiss D.J. Corso N. Khalili B. Zenga W.T. Potential clinical applications using stem cells derived from human umbilical cord blood. Reprod Biomed Online. 2006;13:562. doi: 10.1016/s1472-6483(10)60646-3. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L. Yang R. Han Z.C. Transplantation of umbilical cord blood-derived endothelial progenitor cells: a promising method of therapeutic revascularisation. Eur J Haematol. 2006;76:1. doi: 10.1111/j.1600-0609.2005.00579.x. [DOI] [PubMed] [Google Scholar]
- 22.Jaing T.H. Yang C.P. Hung I.J. Chen S.H. Sun C.F. Chow R. Transplantation of unrelated donor umbilical cord blood utilizing double-unit grafts for five teenagers with transfusion-dependent thalassemia. Bone Marrow Transplant. 2007;40:307. doi: 10.1038/sj.bmt.1705737. [DOI] [PubMed] [Google Scholar]
- 23.Suarez Y. Shepherd B.R. Rao D.A. Pober J.S. Alloimmunity to human endothelial cells derived from cord blood progenitors. J Immunol. 2007;179:7488. doi: 10.4049/jimmunol.179.11.7488. [DOI] [PubMed] [Google Scholar]
- 24.Yang Z. Tao J. Wang J.M. Tu C. Xu M.G. Wang Y. Pan S.R. Shear stress contributes to t-PA mRNA expression in human endothelial progenitor cells and nonthrombogenic potential of small diameter artificial vessels. Biochem Biophys Res Commun. 2006;342:577. doi: 10.1016/j.bbrc.2006.01.172. [DOI] [PubMed] [Google Scholar]
- 25.Ingram D.A. Krier T.R. Mead L.E. McGuire C. Prater D.N. Bhavsar J. Saadatzaden M.R. Bijangi-Vishehsaraei K. Yoder M.C. Haneline L.S. Clonogenic endothelial progenitor cells are sensitive to oxidative stress. Stem Cells. 2007;25:297. doi: 10.1634/stemcells.2006-0340. [DOI] [PubMed] [Google Scholar]
- 26.Ingram D.A. Caplice N.M. Yoder M.C. Unresolved questions, changing definitions, and novel paradigms for defining endothelial progenitor cells. Blood. 2005;106:1525. doi: 10.1182/blood-2005-04-1509. [DOI] [PubMed] [Google Scholar]
- 27.Yoder M.C. Mead L.E. Prater D. Krier T.R. Mroueh K.N. Li F. Krasich R. Temm C.J. Prchal J.T. Ingram D.A. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt D. Asmis L.M. Odermatt B. Kelm J. Breymann C. Gossi M. Genoni M. Zund G. Hoerstrup S.P. Engineered living blood vessels: functional endothelia generated from human umbilical cord-derived progenitors. Ann Thorac Surg. 2006;82:1465. doi: 10.1016/j.athoracsur.2006.05.066. discussion 71. [DOI] [PubMed] [Google Scholar]
- 29.Kaushal S. Amiel G.E. Guleserian K.J. Shapira O.M. Perry T. Sutherland F.W. Rabkin E. Moran A.M. Schoen F.J. Atala A. Soker S. Bischoff J. Mayer J.E., Jr. Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat Med. 2001;7:1035. doi: 10.1038/nm0901-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Griese D.P. Ehsan A. Melo L.G. Kong D. Zhang L. Mann M.J. Pratt R.E. Mulligan R.C. Dzau V.J. Isolation and transplantation of autologous circulating endothelial cells into denuded vessels and prosthetic grafts: implications for cell-based vascular therapy. Circulation. 2003;108:2710. doi: 10.1161/01.CIR.0000096490.16596.A6. [DOI] [PubMed] [Google Scholar]
- 31.Anamelechi C.C. Truskey G.A. Reichert W.M. Mylar and Teflon-AF as cell culture substrates for studying endothelial cell adhesion. Biomaterials. 2005;26:6887. doi: 10.1016/j.biomaterials.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 32.Brown M.A. Wallace C.S. Anamelechi C.C. Clermont E. Reichert W.M. Truskey G.A. The use of mild trypsinization conditions in the detachment of endothelial cells to promote subsequent endothelialization on synthetic surfaces. Biomaterials. 2007;28:3928. doi: 10.1016/j.biomaterials.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Truskey G.A. Proulx T.L. Relationship between 3T3 cell spreading and the strength of adhesion on glass and silane surfaces. Biomaterials. 1993;14:243. doi: 10.1016/0142-9612(93)90114-h. [DOI] [PubMed] [Google Scholar]
- 34.Wallace C.S. Strike S.A. Truskey G.A. Smooth muscle cell rigidity and extracellular matrix organization influence endothelial cell spreading and adhesion formation in coculture. Am J Physiol Heart Circ Physiol. 2007;293:H1978. doi: 10.1152/ajpheart.00618.2007. [DOI] [PubMed] [Google Scholar]
- 35.Livak K.J. Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 36.Wallace C.S. Department of Biomedical Engineering. Durham: Duke University; 2008. Endothelial Cell Adhesion and Function when Co-cultured with Smooth Muscle Cells. [Google Scholar]
- 37.Wallace C.S. Champion J.C. Truskey G.A. Adhesion and function of human endothelial cells co-cultured on smooth muscle cells. Ann Biomed Eng. 2007;35:375. doi: 10.1007/s10439-006-9230-5. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto K. Takahashi T. Asahara T. Ohura N. Sokabe T. Kamiya A. Ando J. Proliferation, differentiation, and tube formation by endothelial progenitor cells in response to shear stress. J Appl Physiol. 2003;95:2081. doi: 10.1152/japplphysiol.00232.2003. [DOI] [PubMed] [Google Scholar]
- 39.Ellis P.D. Metcalfe J.C. Hyvonen M. Kemp P.R. Adhesion of endothelial cells to NOV is mediated by the integrins alphavbeta3 and alpha5beta1. J Vasc Res. 2003;40:234. doi: 10.1159/000071887. [DOI] [PubMed] [Google Scholar]
- 40.Mathur A.B. Chan B.P. Truskey G.A. Reichert W.M. High-affinity augmentation of endothelial cell attachment: long-term effects on focal contact and actin filament formation. J Biomed Mater Res A. 2003;66:729. doi: 10.1002/jbm.a.10581. [DOI] [PubMed] [Google Scholar]
- 41.Gallant N.D. Michael K.E. Garcia A.J. Cell adhesion strengthening: contributions of adhesive area, integrin binding, and focal adhesion assembly. Mol Biol Cell. 2005;16:4329. doi: 10.1091/mbc.E05-02-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tao J. Yang Z. Wang J.M. Tu C. Pan S.R. Effects of fluid shear stress on eNOS mRNA expression and NO production in human endothelial progenitor cells. Cardiology. 2006;106:82. doi: 10.1159/000092636. [DOI] [PubMed] [Google Scholar]
- 43.Cines D.B. Pollak E.S. Buck C.A. Loscalzo J. Zimmerman G.A. McEver R.P. Pober J.S. Wick T.M. Konkle B.A. Schwartz B.S. Barnathan E.S. McCrae K.R. Hug B.A. Schmidt A.M. Stern D.M. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527. [PubMed] [Google Scholar]
- 44.Mattsson E.J. Kohler T.R. Vergel S.M. Clowes A.W. Increased blood flow induces regression of intimal hyperplasia. Arterioscler Thromb Vasc Biol. 1997;17:2245. doi: 10.1161/01.atv.17.10.2245. [DOI] [PubMed] [Google Scholar]
- 45.Brooks A.R. Lelkes P.I. Rubanyi G.M. Gene expression profiling of human aortic endothelial cells exposed to disturbed flow and steady laminar flow. Physiol Genomics. 2002;9:27. doi: 10.1152/physiolgenomics.00075.2001. [DOI] [PubMed] [Google Scholar]
- 46.McCormick S.M. Eskin S.G. McIntire L.V. Teng C.L. Lu C.M. Russell C.G. Chittur K.K. DNA microarray reveals changes in gene expression of shear stressed human umbilical vein endothelial cells. Proc Natl Acad Sci USA. 2001;98:8955. doi: 10.1073/pnas.171259298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muscari C. Gamberini C. Carboni M. Basile I. Farruggia G. Bonafe F. Giordano E. Caldarera C.M. Guarnieri C. Different expression of NOS isoforms in early endothelial progenitor cells derived from peripheral and cord blood. J Cell Biochem. 2007;102:992. doi: 10.1002/jcb.21338. [DOI] [PubMed] [Google Scholar]
- 48.Motley E.D. Eguchi K. Patterson M.M. Palmer P.D. Suzuki H. Eguchi S. Mechanism of endothelial nitric oxide synthase phosphorylation and activation by thrombin. Hypertension. 2007;49:577. doi: 10.1161/01.HYP.0000255954.80025.34. [DOI] [PubMed] [Google Scholar]
- 49.Papadaki M. Tilton R.G. Eskin S.G. McIntire L.V. Nitric oxide production by cultured human aortic smooth muscle cells: stimulation by fluid flow. Am J Physiol. 1998;274:H616. doi: 10.1152/ajpheart.1998.274.2.h616. [DOI] [PubMed] [Google Scholar]
- 50.Fernandez P. Bourget C. Bareille R. Daculsi R. Bordenave L. Gene response in endothelial cells cultured on engineered surfaces is regulated by shear stress. Tissue Eng. 2007;13:1607. doi: 10.1089/ten.2006.0399. [DOI] [PubMed] [Google Scholar]
- 51.Lin Z. Kumar A. SenBanerjee S. Staniszewski K. Parmar K. Vaughan D.E. Gimbrone M.A., Jr. Balasubramanian V. Garcia-Cardena G. Jain M.K. Kruppel-like factor 2 (KLF2) regulates endothelial thrombotic function. Circ Res. 2005;96:e48. doi: 10.1161/01.RES.0000159707.05637.a1. [DOI] [PubMed] [Google Scholar]
- 52.Yasui M. Yamamoto H. Ngan C.Y. Damdinsuren B. Sugita Y. Fukunaga H. Gu J. Maeda M. Takemasa I. Ikeda M. Fujio Y. Sekimoto M. Matsuura N. Weinstein I.B. Monden M. Antisense to cyclin D1 inhibits vascular endothelial growth factor-stimulated growth of vascular endothelial cells: implication of tumor vascularization. Clin Cancer Res. 2006;12:4720. doi: 10.1158/1078-0432.CCR-05-1213. [DOI] [PubMed] [Google Scholar]
- 53.Ley K. Laudanna C. Cybulsky M.I. Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 54.Chiu J.J. Lee P.L. Chen C.N. Lee C.I. Chang S.F. Chen L.J. Lien S.C. Ko Y.C. Usami S. Chien S. Shear stress increases ICAM-1 and decreases VCAM-1 and E-selectin expressions induced by tumor necrosis factor-[alpha] in endothelial cells. Arterioscler Thromb Vasc Biol. 2004;24:73. doi: 10.1161/01.ATV.0000106321.63667.24. [DOI] [PubMed] [Google Scholar]