Abstract
Successful approaches to tissue engineering smooth muscle tissues utilize biodegradable scaffolds seeded with autologous cells. One common problem in using biological scaffolds specifically is the difficulty of inducing cellular penetration and controlling de novo extracellular matrix deposition/remodeling in vitro. Our hypothesis was that small intestinal submucosa (SIS) exposed to specific mechanical stimulation regimes would modulate the synthesis of de novo collagen and elastin by bladder smooth muscle cells (BSMC) within the SIS matrix. We further hypothesized that the cytokines vascular endothelial growth factor (VEGF) and fibroblast growth factor-2 (FGF-2), two key growth factors involved in epithelial mesenchymal signaling, will promote the cellular penetration into SIS necessary for mechanical stimulation. BSMC were seeded at 0.5 × 106 cells/cm2 onto the luminal side of SIS specimens. VEGF (10 ng/mL) and FGF-2 (5 ng/mL) were added to each insert in the media every other day for up to 7 days in static culture. Following static culture, specimens were stretched strip-biaxially under 15% peak strain at either 0.5 or 0.1 Hz for an additional 7 days. Following the culture period, specimens were assayed histologically and biochemically for cellular penetration, proliferation, elastin, collagen, and protease activity. Histological analyses demonstrated that in standard culture media, BSMC remained on the surface of the SIS while both FGF-2 and VEGF profoundly promoted ingrowth of the BSMC into the SIS. The penetration of the cells in response to these cytokines was confirmed using a Transwell assay. Following cellular penetration, BSMC produced significant amounts of elastic fibers under cyclic mechanical stretching at 0.1 Hz under 15% stretch, as evidenced by colorimetric assay and histology using a Verhoeff-Van Gieson stain. Protease activity was assessed in the media and found to be statistically increased in static culture following FGF-2 treatment. These findings demonstrate, for the first time, the capability of BSMC to produce histologically apparent elastin fibers in vitro. Moreover, our results suggest that a strategy involving growth factors and controlled mechanical stimulation may be used to engineer functional, elastin-rich tissue replacements using decellularized biologically derived scaffolds.
Introduction
Current techniques of tissue engineering the urinary bladder wall using biodegradable scaffolds seeded with autologous cells have proven successful in increasing bladder capacity and decreasing the pressure build up that ultimately leads to kidney damage; however, problems remain with the lack of proper tissue organization after implantation, impairing the bladder's ability to maintain its full function.1 Small intestinal submucosa (SIS) has been utilized previously to engineer the urinary bladder wall with and without cell seeding. Previous studies have shown that to maintain graft size, cell seeding of the SIS prior to implantation is necessary.2 To engineer a functional tissue replacement for the bladder wall with controlled extracellular matrix (ECM) production and proper bladder smooth muscle cells' (BSMC) alignment for contraction, mechanical stimulation may be necessary. However, mechanical stimulation of cell-seeded SIS is difficult due to the long periods of time it takes for BSMC to penetrate the SIS so that it may be stretched.
Other studies utilizing BSMC seeded on an ECM scaffold (SIS or bladder acellular matrix) proved that cellular penetration was difficult to achieve in vitro without the use of coculture with urothelium.3,4 Gabouev et al.5 have also shown that cell penetration into SIS takes on the order of weeks. To obtain a construct that may be mechanically stimulated to promote ECM remodeling, cell penetration is necessary. Although the exact signaling mechanisms between the urothelium and BSMC in culture are unclear, it has been noted previously that soluble growth factors are likely involved.6,7 Burgu et al. demonstrated the importance of vascular endothelial growth factor (VEGF) in the development of murine embryonic bladders in culture.7 Further, Master et al.6 highlighted the importance of epithelial mesenchymal signaling in the ingrowth of fibroblasts into bladder acellular matrix. Therefore to increase cellular penetration, growth factors that are released in culture by the urothelium may be utilized.
SIS itself contains a number of growth factors and cytokines. Among the most abundant are basic fibroblast growth factor (bFGF or FGF-2) and transforming growth factor-beta (TGF-β).8 SIS also contains other factors such as VEGF, but VEGF is known to degrade in the processing of the matrix.9 These growth factors and cytokines likely aid in the remodeling response that occurs following implantation of SIS; however, in vitro, the inherent growth factors in the SIS may not be adequate to promote penetration of cell types other than fibroblasts.
FGF-2 is expressed in cell types from the mesoderm and neuroectoderm10 and has been shown to play a role in angiogenesis, proliferation, and differentiation in nearly every organ system.10 FGF-2 has been identified to play a crucial role for stimulating skeletal muscle regeneration.11 It has also been demonstrated that FGF-2 retains its bioactivity in SIS following processing.9 The growth factors FGF-2 and VEGF simulate urothelial cell presence,12 have been shown to increase proliferation in BSMC derived from neurogenic bladders,13 and have an antiapoptotic effect in culture of human BSMC.14 Additionally, VEGF plays a role in bladder development.7
During development, the urinary bladder undergoes repeated mechanical deformation that is believed to aid in the formation of the structural ECM components of the bladder wall.15 The arrangement of these structural components, mainly the ECM proteins' collagen types I and III and elastin, allows for the bladder to stretch to more than 15 times its original volume while filling and return to its original shape following voiding.16 These structural components need to be recapitulated in a tissue-engineered construct to replace portions of the bladder wall. Utilizing collagen-rich biological scaffolds such as SIS provides the structural support needed; however, the scaffolds itself does not inherently have elastin to provide the needed mechanical compliance and recoil for repeated filling and voiding. For these aforementioned reasons, we hypothesized that the growth factors VEGF and FGF-2 may be utilized to increase cellular penetration into the SIS. Further, we hypothesized that specific mechanical stimulation regimes would modulate the synthesis of de novo collagen and elastin by BSMC within the SIS matrix.
Materials and Methods
Cell culture
BSMC were isolated from female Sprague Dawley rat bladders as described previously17 and expanded in culture in Roswell Park Memorial Institute (RPMI) 1640 media with 10% fetal bovine serum (FBS) and 1% Pen/Strep (PS). All cells were used between passages 6 and 9 and seeded at 0.5 × 106 cells/cm2 onto the luminal side of SIS inserts (Cook Biotech, West Lafayette, IN). This seeding density was chosen based on the study by Gilbert et al. where fibroblasts were seeded on SIS and mechanically stimulated.18 Three separate lots of SIS were utilized for cell migration experiments and one lot of SIS was used for samples undergoing mechanical stimulation. A pilot study was performed wherein two concentrations of VEGF (low 10 ng/mL and high 20 ng/mL; Sigma-Aldrich, St. Louis, MO) and two concentrations of FGF-2 (low 5 ng/mL and high 10 ng/mL; Sigma-Aldrich, St. Louis, MO) were utilized based on the concentrations reported previously.19 A DNA quantification assay was performed at 7 days in culture, and no significant differences in cellular proliferation were observed between the low and high concentrations. Therefore, either VEGF (10 ng/mL) or FGF-2 (5 ng/mL) were added to each insert in the media every other day for up to 7 days in culture. Following culture in growth factor–treated media, samples were switched to regular culture media (RPMI 1640 supplemented with 10% FBS and 1% PS) and then either grown in static culture or dynamic culture for an additional 7 days.
Mechanical stimulation
Following 7 days in static culture with the exogenous growth factors, BSMC-seeded SIS was affixed with tissue grip springs to a tension bioreactor as described previously20 and stretched at 15%, 0.1 Hz or 15%, 0.5 Hz under strip biaxial stretch with the primary direction of stretch in the longitudinal direction, for an additional 7 days. These stretch conditions were within a range found to promote mRNA expression of various ECM genes in BSMC.21
The peak strain chosen in this study was based on a previous study by Adam et al.21 The study by Adam et al. utilized human cells as opposed to the rat bladder cells used in the present study. The human and rat bladder cells would potentially be different in physiology and genetic makeup; however, the approximation of 20% is thought to activate the contractile machinery of the smooth muscle cell.22 The level in the present study was limited to 15% stretch due to the stiffness of the SIS matrix.
DNA quantification
Following static and dynamic culture, samples were snap-frozen and stored at −80°C for biochemical assays. DNA quantification was performed as described previously.23 Each sample was cut into fourths and weighed prior to extraction. Samples were placed in a microcentrifuge tube and extracted in 1 mL of 0.125 mg/mL papain solution for 10 h in a 60°C water bath. Digested samples were analyzed with a PicoGreen dsDNA quantitation kit (Molecular Probes, Eugene, OR) as per the manufacturer's instructions and using a TBS-380 Mini-Fluorometer (Turner Biosystems, Sunnyvale, CA) at Ex 460 nm, Em 575 nm. There was a small amount of DNA found in the unseeded SIS scaffold of 8.02 ± 2.38 μg DNA/g wet weight, equivalent to that found in our previous study23; this amount was subtracted from the DNA found in each sample. The total DNA quantitation was done in triplicate with n = 3 per group.
Collagen and elastin assessment
Collagen and elastin concentrations were determined based on the techniques adapted from Brown et al.,24 which have previously been used to quantify ECM synthesis of ovine vascular smooth muscle cells under cyclic mechanical flexure.25 Soluble collagen was extracted from tissue samples using a solution of 0.5 M acetic acid (Sigma) and 1 mg/mL Pepsin A (Sigma). Each sample was placed in a microcentrifuge tube and incubated in 1 mL of extraction solution overnight (∼16 h) on a rocker table operating inside a refrigerator at 2–8°C. Elastin was extracted using a hot oxalic acid treatment at 95°C for 180 min (60 min × 3). The supernates from the oxalic acid treatments were loaded onto Centricon RC/YM-3 centrifugal filter units (Millipore, Bedford, MA) and centrifuged at 3000 g for an additional hour. The concentrate was then resuspended in cold (<5°C) Elastin Precipitating Reagent (UK Biocolor, Biocolor Ltd., County Antrim, United Kingdom). Soluble collagen from the collected media samples at days 2, 4, 6, 8, 10, 12, and 14 was precipitated with 4 M NaCl. Fresh medium was used as the control. Following the extraction steps, the collagen and elastin extracts were assayed according to the guidelines provided with the Sircol™ and Fastin™ assay kits, respectively (UK Biocolor).
Protease activity
The culture media were assayed for both collagen and bulk matrix metalloproteinase (MMP) activity. MMP activity was assayed from the conditioned media at days 2, 4, 6, 9, 11, and 13 utilizing a similar method to Aitken et al.26 Net activity was assayed using the EnzCheck collagenase/gelatinase assay kit (Invitrogen, Carlsbad, CA). DQ™-gelatin fluorescein conjugate (0.1 mg/mL) was incubated in Tris buffer (50 mM/L) with conditioned media for 2 h. The MMPs then released the quenched activity of the fluorescein isothiocyanate (FITC) from the FITC–gelatin. The released FITC was measured on a fluorescent microplate reader at 495 nm absorption and 525 nm excitation. Collagenase produced in Clostridium histolyticum provided in the kit was used as a positive control. Negative controls were performed with 20 μM/L of 1,10-phenanthroline to inhibit the MMP activity. Background from SIS incubated in media was subtracted from all samples at corresponding time points. Data are reported as a summed total of activity from each day media were changed (2, 4, 6, 9, 11, and 13).
Cell migration assays
Migration of the BSMC was assessed in two ways. First, BSMC were seeded at 0.5 × 106 cells/cm2 on SIS. Three samples at 2, 4, and 6 days following culture were fixed in 10% neutral buffered formalin for sectioning and nuclei staining to visualize nuclei distribution in the SIS. Additionally to quantify the effects of VEGF or FGF-2 on cellular migration without confounding effects of the inherent growth factors in SIS, cells were seeded on Costar Transwell Inserts (6.5 mm, 8 μm pore size; Fisher Scientific, Pittsburgh, PA) coated overnight at 4°C with type I collagen (PureCol, Sigma-Aldrich, St. Louis, MO). Following coating, the collagen was aspirated and inserts were dried under a laminar flow hood for 4 h. Cells were seeded at 4 × 104 cells/mL in either 10 ng/mL VEGF media or 5 ng/mL FGF-2 in RPMI-1640 media supplemented with 10% FBS and 1% PS (Invitrogen). Culture medium without growth factors was used as a negative control. Following 24 h, cells were scraped off the surface of the membrane, and fluorescent images were taken across the bottom of the membrane. Image analysis was performed with Sigmascan 4 to quantify the live cells that had migrated to the bottom of the membrane.
Histological staining
Three samples from each experimental group were fixed in 10% neutral buffered formalin, coated in 4% agar, paraffin embedded, and sectioned. Sections were stained with 4′,6-diamidino-2-phenylindole (DAPI) to visualize cell nuclei or stained with Masson's trichrome or Verhoeff-Van Gieson staining to visualize ECM components. Sections were imaged with light microscopy and captured with a digital camera (Nikon, Melville, NY).
Statistical analysis
All data are presented as mean ± standard error of mean. Analysis of data was performed using SigmaStat 3.0. One-way analysis of variance was performed followed by Tukey tests for pairwise comparisons. Data were considered statistically significantly different if p < 0.05.
Results
VEGF and FGF-2 promote mitogenesis
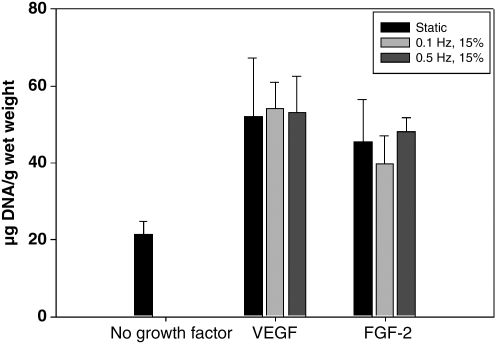
In static culture, VEGF and FGF-2 promote significantly higher (p < 0.05) BSMC proliferation than standard media alone (Fig. 1). Under dynamic culture, there were no statistical differences among the stretch or static cycles in the FGF-2– or VEGF-treated groups (Fig. 1). The regular media-treated group did not retain any attached cells when stretched during a preliminary experiment and therefore was not cycled as a control for the VEGF- or FGF-2–treated groups. This result was likely due to the cells on the surface of the SIS detaching with the application of mechanical stretch. Therefore, the DNA quantification of the dynamic cultures was that of the cells that had penetrated the SIS.
FIG. 1.
DNA quantification following 14 days culture with 7 days static growth factor treatment and 7 days no treatment static or stretched. Data are presented as mean ± SEM, n = 6 per group. All VEGF and FGF-2 groups are statistically significantly greater than the no growth factor–treated group, p < 0.01. SEM, standard error of mean; VEGF, vascular endothelial growth factor; FGF-2, fibroblast growth factor-2.
VEGF and FGF-2 promote cellular migration
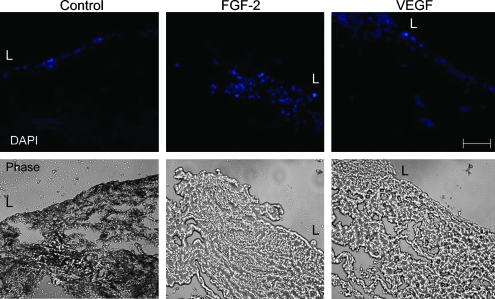
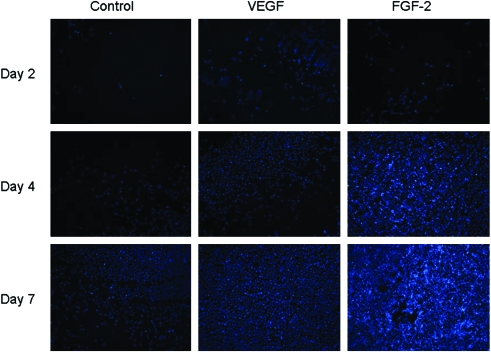
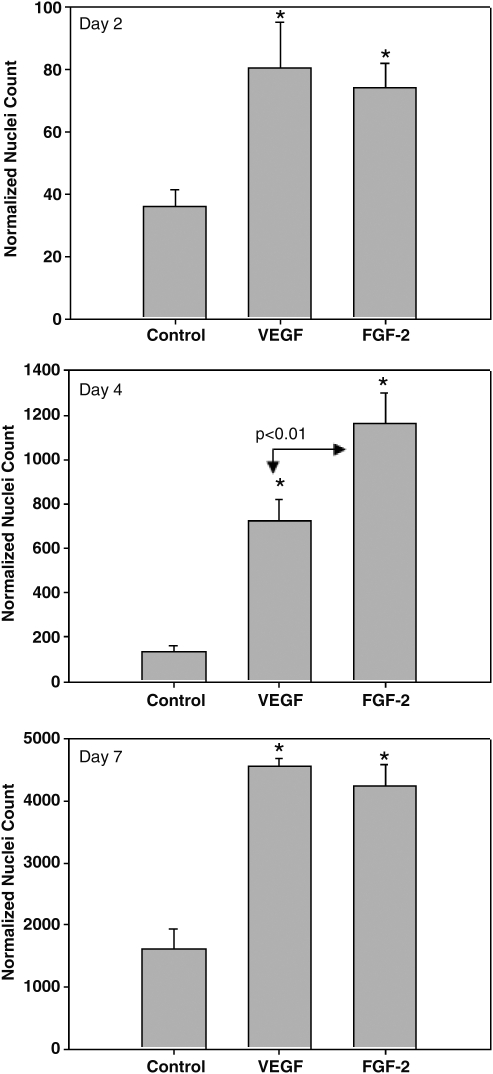
Histological analysis showed that in standard culture media, BSMC remained on the surface of the SIS while both FGF-2 and VEGF profoundly promoted ingrowth of the BSMC into the SIS (Fig. 2). In the FGF-2–treated group, the BSMC appeared to grow into the SIS in a cluster, whereas in the VEGF-treated group the cells were more spread throughout the tissue nonuniformly with small clusters seen more toward the surface (Fig. 2, upper left corner of VEGF DAPI image). In the transwell migration experiments, BSMC migrated more rapidly from one side of the culture insert to the other with the addition of the exogenous growth factors (Fig. 3). Further, the exogenous growth factors enhanced cellular migration into unoccupied space in the culture well with a significantly greater number of cells migrated in VEGF and FGF-2 than in standard media alone (Figs. 3 and 4).
FIG. 2.
Top panel, cross-section of DAPI-stained nuclei (blue) following 7 days with growth factor treatment on SIS. L indicates the luminal surface of the SIS where cells were seeded. Bottom panel, light microscopy image of SIS cross-section. Images are reduced from 400 ×. Scale bar represents 50 μm. DAPI, 4′,6-diamidino-2-phenylindole; SIS, small intestinal submucosa. Color images available online at www.liebertonline.com/ten.
FIG. 3.
DAPI-stained cell nuclei at days 2, 4, and 7. Images are reduced from 100 × . Color images available online at www.liebertonline.com/ten.
FIG. 4.
Normalized cell nuclei counts on the unseeded side of transwell inserts at 2, 4, and 7 days. n = 3 transwells per group with five images from each transwell analyzed. *p < 0.01 compared to regular media controls.
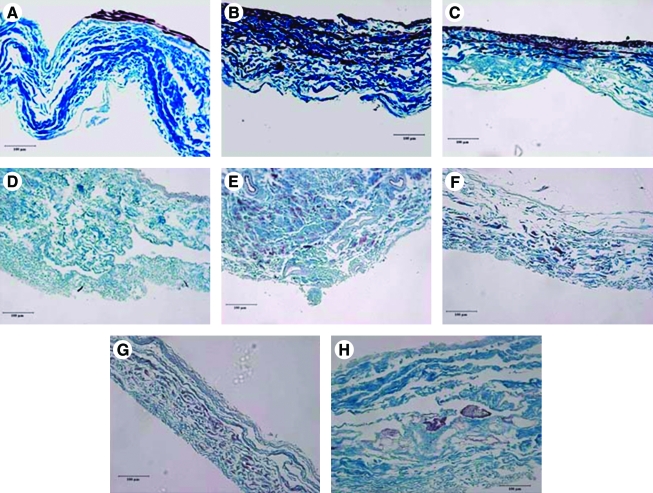
At 14 days total culture time, there still appeared to be more cellular penetration of the BSMC into the SIS in the VEGF- and FGF-2–treated groups compared to the regular media group in static culture (Fig. 5A–C). When constructs were cyclically stretched, there appeared to be fewer cells within the construct. However, upon further histological examination the cells were spread throughout the construct in the stretched groups (Fig. 5E–H) as opposed to the static groups where the cells remained in the central portion of the SIS where seeding occurred (Fig. 5B, C). Further, DNA quantification revealed that an equivalent number of cells were present in the constructs following stretch as in the statically cultured constructs (Fig. 1). In the FGF-2–treated group under 0.5 Hz stretch, groupings of BSMC were seen to congregate in clusters that resembled rudimentary smooth muscle fascicles (Fig. 5H). This observation has been shown previously with the treatment of TGF-β1 in bladders of rabbits27 as well as in our laboratory's previous work with the addition of TGF-β1 to BSMC seeded on collagen gels.28 Although there appear to be differences in the thickness of the samples in the histology, there were no measured differences in thickness of any of the samples with an average thickness of 120 μm. What appears to be thickness variability in the histological sectioning was due to artifact of the SIS shearing when sectioned.
FIG. 5.
Elastic trichrome staining of (A). No growth factor (NG) 14 day static (B). VEGF 7 day NG 7 day static (C). FGF-2 7 day NG 7 day static (D). Unseeded SIS (E). VEGF 7 day Stretch 7 day 0.1 Hz (F). FGF-2 7 day Stretch 7 day 0.1 Hz (G). VEGF 7 day 0.5 Hz 7 day (H). FGF-2 7 day 0.5 Hz 7 day. Images are reduced from 200 ×. Scale bar represents 100 μm. Color images available online at www.liebertonline.com/ten.
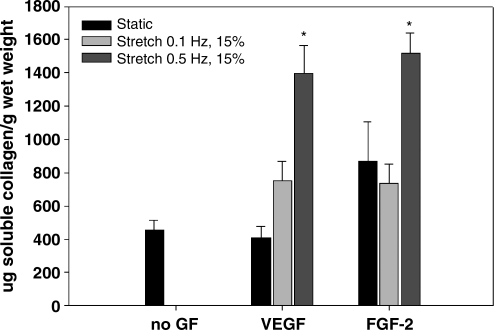
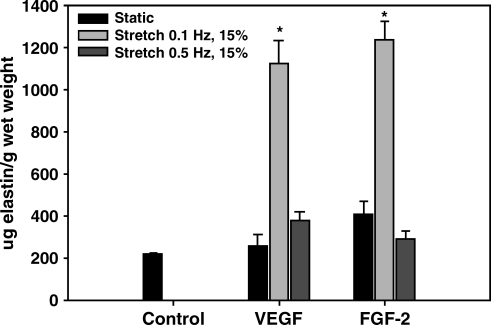
Dynamic culture at 0.1 Hz promotes elastogenesis
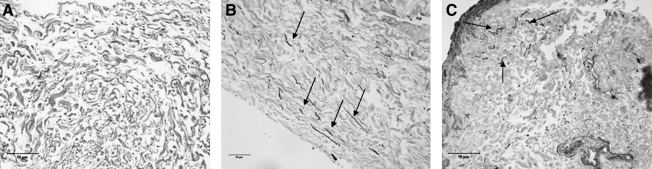
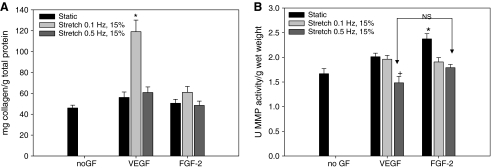
In static culture, there was no significant change in collagen content between treatment groups. Under dynamic culture, collagen content in the tissue was significantly higher in the 0.5-Hz stretch group in both the VEGF- and the FGF-2–treated groups compared to all other groups (Fig. 6). In contrast, the elastin content of the tissue was significantly higher in the 0.1-Hz stretch group in both the VEGF- and the FGF-2–treated groups compared to all other groups (Fig. 7). This elastin production was further confirmed with Verhoeff-Van Gieson staining (Fig. 8) where black staining of elastin was apparent throughout the VEGF- and FGF-2–treated tissues that were cyclically stretched at 0.1 Hz. Very small amounts of elastin were visible in the unseeded SIS (Fig. 8). Further, with elastic trichrome staining elastin fibrils were only visible in the 0.1-Hz stretch groups.
FIG. 6.
Elastin protein concentration per gram wet weight of BSMC-seeded SIS. Data are presented as mean ± SEM with n = 6 per group. * indicates statistical significance with p < 0.01 compared to all other groups. BSMC, bladder smooth muscle cell.
FIG. 7.
Soluble collagen concentration per gram wet weight of BSMC-seeded SIS. Data are presented as mean ± SEM with n = 6 per group. * indicates statistical significance with p < 0.05 compared to all other groups.
FIG. 8.
Verhoeff-Van Gieson staining of elastin fibrils (black) within: (A) SIS, (B) seeded SIS treated with VEGF for 7 days stretched at 0.1 Hz for 7 days, and (C) seeded SIS treated with FGF-2 for 7 days and then stretched at 0.1 Hz for 7 days. Black arrows indicate presence of elastin. Images are representative from n = 4 per group and are reduced from 400 ×. Scale bar represents 50 μm.
MMP activity and collagen in the media
Summed totals of soluble collagen within the media showed that there was a significantly higher amount of collagen in the media in the 0.1-Hz group that was treated with VEGF for the first 7 days of culture (Fig. 9). Summed totals of MMP activity at 14 days showed that static culture with the addition of FGF-2 for 7 days followed by regular culture media for 7 days had significantly higher amounts of active MMPs released into the media than all other groups (Fig. 9). Additionally, MMP activity was significantly lower at 14 days summed in the VEGF + 0.5 Hz group than all other groups except for the FGF-2 + 0.5 Hz stretch group (Fig. 9).
FIG. 9.
Summed totals of soluble collagen found in media (A) and bulk MMP activity found in media (B) of BSMC-seeded SIS. Data are presented as mean ± SEM with n = 6 per group. * indicates p < 0.05 compared to all other groups. + indicates p < 0.05 compared to all groups except FGF-2 at 0.5 Hz. MMP, matrix metalloproteinase.
Discussion
Exogenous growth factors VEGF and FGF-2
In the present study, the central hypothesis was that BSMC-seeded SIS exposed to mechanical stimulation will produce modulation of ECM components collagen and elastin, dependent on the frequency of stretch. To examine this hypothesis, it was necessary to utilize exogenous growth factors, VEGF and FGF-2, to promote cellular penetration into the SIS prior to mechanical simulation. Adding the exogenous growth factors VEGF and FGF-2 to culture improved migration of BSMC into SIS constructs. The migratory effect of the growth factors on the BSMC was confirmed using a transwell chamber assay. The relative quantities of VEGF and FGF-2 added to the media were chosen based on the previous results in the literature wherein VEGF and FGF-2 were added to culture vascular smooth muscle cells to evoke a response.29 These concentrations were also used in the ratio that they are released from the urothelium.12 The response of the BSMC to the growth factor groups is similar to that found previously in coculture of bladder urothelium with BSMC on SIS.3 This finding further confirms a report that states that VEGF and FGF-2 are two key growth factors released by the urothelium.12 Further, VEGF is a known promoter of mitogenesis and has been shown to increase proliferation in many cell types previously, whereas FGF-2 has been shown to up-regulate collagen type III production in BSMC.30 FGF-2 has previously been shown to decrease elastin mRNA expression in aortic smooth muscle cells.31 No differences were seen in the present study between groups treated with FGF-2 or VEGF in terms of elastogenesis.
Mechanical stimulation and ECM remodeling
The most interesting finding stemming from the central hypothesis of this study was that the capability of the BSMC to produce elastin fibers was captured with cyclic mechanical stretching once the BSMC were integrated into the SIS constructs. Interestingly, large amounts of elastin were produced under cyclic at 0.1 Hz with 15% stretch and not under 0.5 Hz 15% stretch as seen in the intact bladder strips in our previous study.32 These large levels of what appears to be fibrous elastin, produced by BSMC, have not previously been shown in tissue-engineered constructs in vitro.
Collagen remodeling in the constructs was dependent on the mechanical stretch frequency and the growth factors used to promote cell penetration. In static culture with no growth factors, increases in MMP-2 and -9 were not observed. This finding is in contrast to the increased MMP-1 activity found when adult muscle-derived stem cells are seeded on SIS.23 This finding gives some insight into the kinetics of the MMPs acting on SIS. It is known that MMP-1 is a main collagenase that breaks down collagen type I fibrils. It is possible that MMP-1 in the present study was increased early on in culture, whereas MMP-2 and -9 (the gelatinases) had impact on breaking down the collagen further. The addition of FGF-2 to the statically cultured constructs promoted increased bulk MMP activity, which may point to a release of MMPs as the mechanism used by the BSMC to grow into the tissue. Additionally collagen measured in the media during both the first 7 days and the last 7 days in culture varied dependent on what growth factors were used. Constructs treated with VEGF followed by mechanical stimulation at 0.1 Hz had significantly higher amounts of collagen within the media, yet there were no differences in the FGF-2–treated group at the same frequency. The lower MMP activity in the summed total of 14 days culture in the VEGF + 0.5 Hz stretch group corresponds to the VEGF + 0.5 Hz stretch group having a significantly greater amount of soluble collagen within the tissue. These differences demonstrate that the growth factors FGF-2 and VEGF impacted the cells during the first 7 days in culture and had persisting effects on the cells once the growth factors were removed from the system.
The stretch frequencies of the SIS were chosen to be nonphysiologic and to be within a range found to promote mRNA expression of various ECM genes.21 Additionally, the 0.5-Hz frequency has been shown by our laboratory to produce significant quantities of elastin in ex vivo organ culture.32 In the present study, the elastin production was found to be frequency dependent, only being produced in the 0.1-Hz condition. This difference is likely due to the environment of the cells either being in an intact tissue or an actively remodeling piece of ECM scaffold. SIS has been shown to remodel in a site-specific manner when used in vivo.33 Previous studies have speculated that mechanical stimulation of SIS may contribute to this site-specific tissue remodeling.18 Further, it is possible that frequency and stretch ratios both play a role in the BSMC production of elastin and collagen.
A previous study wherein BSMC were seeded on collagen and laminin-coated Flexcell™ (Flexcell Int., Hillsborough, NC) plates examined varying stretch frequencies.21 The study by Adam et al. showed that expression of collagen types I and III is dependent on the frequency of stretch.21 However, in this study the authors only examined one stretch level of 20% in the maximal direction. Isenberg and Tranquillo showed that vascular smooth muscle cells produce small quantities of elastin when cycled at 0.5 Hz at 5% stretch within collagen gels.34 Pulsitile flow in combination with endothelial cell seeding has also been found to promote elastin gene expression and positive staining in aortic smooth muscle cells.35 A study by Pattison et al. showed that BSMC are capable of producing elastin measured by the Fastin elastin assay when seeded on nanostructured poly(ether urethane) however, elastin fibers have not been produced in vitro until the present study. It is clear from these previous studies and the study presented here that the ECM production of the smooth muscle cell may depend on several factors of mechanical stimulation including the time of stretch, stretch rate, and extent of stretch.
Study limitations
It is common in utilizing exogenous growth factors to perform experiments in serum-free or low-serum media to clarify the biological impact of growth factors themselves. However, in the present study, the growth factors were utilized as an aid to promote enhanced penetration of the BSMC. Proliferation and enhanced cell survival were warranted to engineer a cellular construct for future use in bladder wall repair. Therefore, the addition of serum during growth was necessary to maintain high cell numbers in the construct, especially during the cyclic mechanical strain. The specific mechanisms by which the BSMC are coaxed to penetrate will be the focus of future studies. While it would be scientifically interesting to combine growth factors and mechanical stimulation, the amount of growth factor necessary to add to the bioreactors would significantly increase the cost of the experiment. In the present study, the primary use for the growth factors was to promote ingrowth of the BSMC into the SIS. Growth factors were not added to the media during the last 7 days of culture so that the effects of mechanical stimulation alone could be examined. An additional limitation to this study as well as many similar studies utilizing an ECM scaffold in vitro was the difficulty in histological sectioning. Reasonable images were obtained by coating the fixed SIS strips in a solution of 4% agar prior to paraffin embedding and sectioning. This coating aided in the sectioning of the SIS with limited shredding of the thin collagenous material; however, some shredding was noted in areas of cellular penetration.
Summary
Cultured BSMC integrated within SIS ECM matrices, when subjected to cyclic mechanical stretch, produced profound changes in collagen and elastin deposition, which was also dependent on the frequency of stretch. Moreover, it was demonstrated for the first time that BSMC are capable of producing elastin fibers in vitro. The BSMC within SIS matrix produced elastin fibers at 0.1 Hz cyclic stretch following a week of static culture with exogenous growth factors VEGF or FGF-2 to enhance cellular ingrowth. Collagen remodeling of the ECM constructs was dependent on both growth factor pretreatment and stretch history. These findings may lead to more functional tissue-engineered bladder wall replacements through de novo elastin deposition and collagen remodeling controlled by modulating in vitro culture conditions prior to implantation.
Acknowledgments
The authors kindly acknowledge Cook Biotech for providing the SIS and Silvia Wognum for her input. This work was supported by NIH R01-AR049398 01 and the W.K. Whiteford Professorship.
Disclosure Statement
No competing financial interests exist.
References
- 1.Brown A.L. Farhat W. Merguerian P.A. Wilson G.J. Khoury A.E. Woodhouse K.A. 22 week assessment of bladder acellular matrix as a bladder augmentation material in a porcine model. Biomaterials. 2002;23:2179. doi: 10.1016/s0142-9612(01)00350-7. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y. Frimberger D. Cheng E.Y. Lin H.K. Kropp B.P. Challenges in a larger bladder replacement with cell-seeded and unseeded small intestinal submucosa grafts in a subtotal cystectomy model. BJU Int. 2006;98:1100. doi: 10.1111/j.1464-410X.2006.06447.x. [DOI] [PubMed] [Google Scholar]
- 3.Brown A.L. Brook-Allred T.T. Waddell J.E. White J. Werkmeister J.A. Ramshaw J.A. Bagli D.J. Woodhouse K.A. Bladder acellular matrix as a substrate for studying in vitro bladder smooth muscle-urothelial cell interactions. Biomaterials. 2005;26:529. doi: 10.1016/j.biomaterials.2004.02.055. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y. Lin H.K. Frimberger D. Epstein R.B. Kropp B.P. Growth of bone marrow stromal cells on small intestinal submucosa: an alternative cell source for tissue engineered bladder. BJU Int. 2005;96:1120. doi: 10.1111/j.1464-410X.2005.05741.x. [DOI] [PubMed] [Google Scholar]
- 5.Gabouev AI. Schultheiss D. Mertsching H. Koppe M. Schlote N. Wefer J. Jonas U. Stief C.G. In vitro construction of urinary bladder wall using porcine primary cells reseeded on acellularized bladder matrix and small intestinal submucosa. Int J Artif Organs. 2003;26:935. doi: 10.1177/039139880302601011. [DOI] [PubMed] [Google Scholar]
- 6.Master V.A. Wei G. Liu W. Baskin L.S. Urothlelium facilitates the recruitment and trans-differentiation of fibroblasts into smooth muscle in acellular matrix. J Urol. 2003;170:1628. doi: 10.1097/01.ju.0000084407.24615.f8. [DOI] [PubMed] [Google Scholar]
- 7.Burgu B. McCarthy L.S. Shah V. Long D.A. Wilcox D.T. Woolf A.S. Vascular endothelial growth factor stimulates embryonic urinary bladder development in organ culture. BJU Int. 2006;98:217. doi: 10.1111/j.1464-410X.2006.06215.x. [DOI] [PubMed] [Google Scholar]
- 8.Voytik-Harbin S. Brightman A.O. Waisner B.Z. Robinson J.P. Lamar C.H. Small intestinal submucosa: a tissuederived extracellularmatrix that promotes tissue-specific growth and differentiation of cell in-vitro. Tissue Eng. 1998;4:157. [Google Scholar]
- 9.Hodde J. Janis A. Hiles M. Effects of sterilization on an extracellular matrix scaffold: part II. Bioactivity and matrix interaction. J Mater Sci Mater Med. 2007;18:545. doi: 10.1007/s10856-007-2301-9. [DOI] [PubMed] [Google Scholar]
- 10.Chen C.H. Poucher S.M. Lu J. Henry PD. Fibroblast growth factor 2: from laboratory evidence to clinical application. Curr Vasc Pharmacol. 2004;2:33. doi: 10.2174/1570161043476500. [DOI] [PubMed] [Google Scholar]
- 11.Liu H.Z. Li Q. Yang X.Y. Liu L. An X.R. Chen Y.F. Expression of basic fibroblast growth factor results in the decrease of myostatin mRNA in murine C2C12 myoblasts. Acta Biochim Biophys Sin. 2006;38:697. doi: 10.1111/j.1745-7270.2006.00215.x. [DOI] [PubMed] [Google Scholar]
- 12.Kanematsu A. Yamamoto S. Iwai-Kanai E. Kanatani I. Imamura M. Adam R.M. Tabata Y. Ogawa O. Induction of smooth muscle cell-like phenotype in marrow-derived cells among regenerating urinary bladder smooth muscle cells. Am J Pathol. 2005;166:565. doi: 10.1016/S0002-9440(10)62278-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beqaj S.H. Donovan J.L. Liu D.B. Harrington D.A. Alpert S.A. Cheng E.Y. Role of basic fibroblast growth factor in the neuropathic bladder phenotype. J Urol. 2005;174:1699. doi: 10.1097/01.ju.0000176633.92150.4e. [DOI] [PubMed] [Google Scholar]
- 14.Galvin D.J. Watson R.W. Gillespie J.I. Brady H. Fitzpatrick J.M. Mechanical stretch regulates cell survival in human bladder smooth muscle cells in vitro. Am J Physiol Renal Physiol. 2002;283:F1192. doi: 10.1152/ajprenal.00168.2002. [DOI] [PubMed] [Google Scholar]
- 15.Farhat W.A. Yeger H. Does mechanical stimulation have any role in urinary bladder tissue engineering? World J Urol. 2008;26:301. doi: 10.1007/s00345-008-0318-4. [DOI] [PubMed] [Google Scholar]
- 16.Korossis S. Bolland F. Ingham E. Fisher J. Kearney J. Southgate J. Review: tissue engineering of the urinary bladder: considering structure-function relationships and the role of mechanotransduction. Tissue Eng. 2006;12:635. doi: 10.1089/ten.2006.12.635. [DOI] [PubMed] [Google Scholar]
- 17.Kropp B.P. Zhang Y. Tomasek J.J. Cowan R. Furness P.D., 3rd. Vaughan M.B. Parizi M. Cheng E.Y. Characterization of cultured bladder smooth muscle cells: assessment of in vitro contractility. J Urol. 1999;162:1779. [PubMed] [Google Scholar]
- 18.Gilbert T.W. Stewart-Akers A.M. Sydeski J. Nguyen T.D. Badylak S.F. Woo S.L. Gene expression by fibroblasts seeded on small intestinal submucosa and subjected to cyclic stretching. Tissue Eng. 2007;13:1313. doi: 10.1089/ten.2006.0318. [DOI] [PubMed] [Google Scholar]
- 19.Cucina A. Borrelli V. Randone B. Coluccia P. Sapienza P. Cavallaro A. Vascular endothelial growth factor increases the migration and proliferation of smooth muscle cells through the mediation of growth factors released by endothelial cells. J Surg Res. 2003;109:16. doi: 10.1016/s0022-4804(02)00042-2. [DOI] [PubMed] [Google Scholar]
- 20.Merryman W.D. Lukoff H.D. Long R.A. Engelmayr G.C., Jr. Hopkins R.A. Sacks M.S. Synergistic effects of cyclic tension and transforming growth factor-beta1 on the aortic valve myofibroblast. Cardiovasc Pathol. 2007;16:268. doi: 10.1016/j.carpath.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adam R.M. Eaton S.H. Estrada C. Nimgaonkar A. Shih S.C. Smith L.E. Kohane I.S. Bägli D. Freeman M.R. Mechanical stretch is a highly selective regulator of gene expression in human bladder smooth muscle cells. Physiol Genomics. 2004;20:36. doi: 10.1152/physiolgenomics.00181.2004. [DOI] [PubMed] [Google Scholar]
- 22.Andersson K.E. Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev. 2004;84:935. doi: 10.1152/physrev.00038.2003. [DOI] [PubMed] [Google Scholar]
- 23.Long R.A. Nagatomi J. Chancellor M.B. Sacks M.S. The role of MMP-I up-regulation in the increased compliance in musclederived stem cell-seeded small intestinal submucosa. Biomaterials. 2006;27:2398. doi: 10.1016/j.biomaterials.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 24.Brown A.N. Kim B.S. Alsberg E. Mooney D.J. Combining chondrocytes and smooth muscle cells to engineer hybrid soft tissue constructs. Tissue Eng. 2000;6:297. doi: 10.1089/107632700418029. [DOI] [PubMed] [Google Scholar]
- 25.Engelmayr G.C., Jr. Rabkin E. Sutherland F.W. Schoen F.J. Mayer J.E., Jr. Sacks M.S. The independent role of cyclic flexure in the early in vitro development of an engineered heart valve tissue. Biomaterials. 2005;26:175. doi: 10.1016/j.biomaterials.2004.02.035. [DOI] [PubMed] [Google Scholar]
- 26.Aitken K.J. Block G. Lorenzo A. Herz D. Sabha N. Dessouki O. Fung F. Szybowska M. Craig L. Bägli D.J. Mechanotransduction of extracellular signal-regulated kinases 1 and 2 mitogen-activated protein kinase activity in smooth muscle is dependent on the extracellular matrix and regulated by matrix metalloproteinases. Am J Pathol. 2006;169:459. doi: 10.2353/ajpath.2006.050969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roelofs M. Faggian L. Pampinella F. Paulon T. Franch R. Chiavegato A. Sartore S. Transforming growth factor beta1 involvement in the conversion of fibroblasts to smooth muscle cells in the rabbit bladder serosa. Histochem J. 1998;30:393. doi: 10.1023/a:1003216124761. [DOI] [PubMed] [Google Scholar]
- 28.Parekh A.L. Long R.A. Chancellor M.B. Sacks M.S. Assessing the effects of TGF-b1 on bladder smooth muscle cell phenotype. II. Modulation of collagen organization. J Urol. 2009;182:1216. doi: 10.1016/j.juro.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Sung H.J. Johnson C.E. Lessner S.M. Magid R. Drury D.N. Galis Z.S. Matrix metalloproteinase 9 facilitates collagen remodeling and angiogenesis for vascular constructs. Tissue Eng. 2005;11:267. doi: 10.1089/ten.2005.11.267. [DOI] [PubMed] [Google Scholar]
- 30.Imamura M. Kanematsu A. Yamamoto S. Kimura Y. Kanatani I. Ito N. Tabata Y. Ogawa O. Basic fibroblast growth factor modulates proliferation and collagen expression in urinary bladder smooth muscle cells. Am J Physiol Renal Physiol. 2007;293:1007. doi: 10.1152/ajprenal.00107.2007. [DOI] [PubMed] [Google Scholar]
- 31.Carreras I. Rich C.B. Panchenko M.P. Foster J.A. Basic fibroblast growth factor decreases elastin gene transcription in aortic smooth muscle cells. J Cell Biochem. 2002;85:592. doi: 10.1002/jcb.10163. [DOI] [PubMed] [Google Scholar]
- 32.Long R.A. Parekh A. Sacks M.S. The effects of in-vitro mechanical stretch on urinary bladder wall extracellular matrix remodelling. Biomech Model Mechanobiol. [in revision]. [Google Scholar]
- 33.Badylak S.F. The extracellular matrix as a biologic scaffold material. Biomaterials. 2007;28:3587. doi: 10.1016/j.biomaterials.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 34.Isenberg B.C. Tranquillo R.T. Long-term cyclic distention enhances the mechanical properties of collagenbased media-equivalents. Ann Biomed Eng. 2003;31:937. doi: 10.1114/1.1590662. [DOI] [PubMed] [Google Scholar]
- 35.Bulick A.S. Muñoz-Pinto D.J. Qu X. Mani M. Cristancho D. Urban M. Hahn M.S. Impact of endothelial cells and mechanical conditioning on smooth muscle cell extracellular matrix production and differentiation. Tissue Eng Part A. 2008;15:815. doi: 10.1089/ten.tea.2008.0179. [DOI] [PubMed] [Google Scholar]
- 36.Pattison M.A. Webster T.J. Haberstroh K.M. Select bladder smooth muscle cell functions were enhanced on three-dimensional, nano-structured poly(ether urethane) scaffolds. J Biomater Sci Polym Ed. 2006;17:1307. doi: 10.1163/156856206778667460. [DOI] [PubMed] [Google Scholar]