Abstract
The ability to encapsulate cells over a range of cell densities is important toward mimicking cell densities of native tissues and rationally designing strategies where cell source and/or cell numbers are clinically limited. Our preliminary findings demonstrate that survival of freshly isolated adult bovine chondrocytes dramatically decreases when photoencapsulated in poly(ethylene glycol) hydrogels at low densities (4 million cells/mL). During enzymatic digestion of cartilage, chondrocytes undergo a harsh change in their microenvironment. We hypothesize that the absence of exogenous antioxidants, the hyposmotic environment, and the loss of a protective pericellular matrix (PCM) increase chondrocytes' susceptibility to free radical damage during photoencapsulation. Incorporation of antioxidants and serum into the encapsulation medium improved cell survival twofold compared to phosphate-buffered saline. Increasing medium osmolarity from 330 to 400 mOsm (physiological) improved cell survival by 40% and resulted in ∼2-fold increase in adenosine triphosphate (ATP) production 24 h postencapsulation. However, cell survival was only temporary. Allowing cells to reproduce some PCM before photoencapsulation in 400 mOsm medium resulted in superior cell survival during and postencapsulation for up to 15 days. In summary, the combination of antioxidants, physiological osmolarity, and the development of some PCM result in an improved robustness against free radical damage during photoencapsulation.
Introduction
Tissue engineering holds great promise for replacing damaged and/or diseased tissues with regenerated healthy living tissues.1 One attractive approach to engineering living tissues involves the encapsulation of cells in 3D hydrogels.2 Hydrogels are characterized by their high water contents and tissue-like elastic properties making them ideal environments for cell and tissue growth. In addition, the gelation process is often mild permitting in vivo delivery of cells. Hydrogels formed via photopolymerization are particularly attractive because the process occurs on clinically relevant time scales, allows for spatial and temporal control over the polymerization reaction, and can be tuned to obtain a range of macroscopic properties and degradation profiles.3 Furthermore, synthetic and natural polymers have been modified with polymerizable functionalities [e.g., (meth)acrylate] to create 3D environments suited for a range of cell encapsulation and tissue engineering applications, including encapsulation of osteoblasts,4 islets of Langerhans,5 chondrocytes,6,7 and mesenchymal stem cells.8
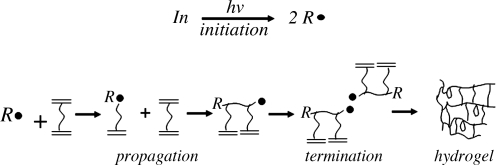
Photopolymerization of hydrogels occurs through a photoinitiated free radical chain polymerization involving initiation, propagation, and termination. The process is described by Figure 1. For cell encapsulation strategies, the precursors include multifunctional macromolecular monomers (i.e., macromers), photoinitiator molecules, and light. Upon exposure to light, photoinitiator molecules absorb photons of light energy and dissociate into radicals that initiate the polymerization reaction to form growing kinetic chains. During propagation, the rate of polymerization increases dramatically with conversion as a result of diffusion-controlled termination kinetics leading to autoacceleration.9 During autoacceleration, there is a large increase in the concentration of propagating chains, that is, macroradicals. Termination occurs through bimolecular termination or chain transfer between two propagating chains. Chain transfer may also occur with other solutes or molecules present in the polymerization medium, including proteins and/or molecules associated with cells.
FIG. 1.
A schematic of the process for fabricating hydrogels by photopolymerization, which are used in cell encapsulation strategies. The photopolymerization process occurs via a photoinitiated free radical chain polymerization involving initiation, propagation, and termination. Photoinitiator molecules (In) absorb photons of light energy (hv) and dissociate into radicals (R•) (initiation). The initiator radicals react with unreacted double bonds (C = C) on macromolecular monomers [e.g., poly(ethylene glycol) dimethacrylate] to form growing kinetic chains (propagation). Termination occurs through either bimolecular termination or chain transfer between two propagating chains.
For cell encapsulation strategies, the photopolymerization conditions must be carefully chosen to minimize cellular damage from free radicals associated with the initiating radicals and propagating macroradicals. It is well known that free radicals can damage cell membranes, nucleic acids, and proteins that can ultimately lead to cell death.10–12 Several studies have examined the cytocompatibility of different photoinitiators and their resulting radicals from exposure to light.13–16 Earlier work by Bryant et al.15 demonstrated that initiator chemistry, initiator concentration, and their resulting radicals dramatically affected cell viability of NIH/3T3 fibroblasts. The initiating system, 2-hydroxy-1-[4-(hydroxyethoxy) phenyl]-2-methyl-1-propanone) or Irgacure 2959® under low-intensity UV light (365 nm), was determined to be cytocompatible. This initiating system has since been used to encapsulate a number of different cell types in photopolymerized hydrogels without adversely affecting cell viability or cellular functions.8,15,16
To develop a clinically relevant tissue engineering strategy for cartilage regeneration, cell source and availability are important considerations. In addition, one of the limitations involving the use of primary chondrocytes is the fact that chondrocytes are known to de-differentiate rapidly when expanded in 2D cultures.17–19 Therefore, developing successful strategies that employ low cell densities are attractive from a clinical perspective.
Our lab focuses on encapsulating chondrocytes in photopolymerized poly(ethylene glycol) (PEG) hydrogels fabricated from PEG dimethacrylate (PEGDM) macromers and the Irgacure 2959 initiating system toward engineering functional cartilage tissues. PEG hydrogels provide a 3D environment that maintains the chondrocyte phenotype, promotes cartilage-specific matrix synthesis, and when designed to biodegrade leads to macroscopic tissue development.6,20–22 Traditionally, to enhance matrix deposition, we and others have employed high cell concentrations (∼50 ×106 cells/mL) when encapsulating primary chondrocytes in hydrogels for cartilage regeneration.6,7,20–22 Toward developing strategies that employ low cell densities, unpublished observations from our lab have found that when freshly isolated bovine chondrocytes are photoencapsulated in PEG hydrogels at cell densities similar to adult cartilage (i.e., ∼4 × 106 cells/mL), very few cells survived the encapsulation process. This finding was particularly surprising because other cell types have been successfully encapsulated in photopolymerized PEG hydrogels at low cell densities, including bone marrow stromal cells,23 endothelial cells,24 and osteoblasts.4
Primary chondrocytes are obtained from enzymatic digestion of cartilage tissue explants. The collagenase digestion process dissolves the extracellular matrix (ECM) and strips the cells of their pericellular matrix (PCM) resulting in a suspension of isolated single cells.25–27 In addition, the isolation process for chondrocytes is typically performed in standard culture medium with osmolarities that are lower than native cartilage resulting in a hyposmotic environment.28 We hypothesize that this harsh change in the microenvironment surrounding the chondrocyte enhances their susceptibility to free radical damage associated with radicals of the polymerization. These negative effects may be more pronounced when low cell densities are employed due to the higher concentrations of free radicals per cell.
Because free radicals are normally present in cells and tissues, cells inherently have protective capabilities against free radical damage. For example, the unsaturated bonds in lipids that are present on the cell membrane are key targets for free radical reactions and oxidative damage, which can lead to adverse cellular functions and even cell death.10,29 To prevent oxidative damage to the cell membrane, antioxidants released by the cell and/or present exogenously serve to quench and scavenge free radicals.29 The ECM may also have potential to act as radical scavengers where previous studies have reported the ability of collagen type I to scavenge radicals in vitro.30 Furthermore, the change in the osmotic environment during the isolation can result in rapid cell swelling that can deplete the cell of important osmolytes, such as potassium, which has been shown to inhibit free radical formation in other cell types.31 Therefore, the loss of PCM, the hyposmotic environment, and the absence of exogenous antioxidants may act to increase the chondrocytes susceptibility to free radical damage.
Therefore, this study explores the role of the encapsulation medium and the importance of a PCM on cell survival and function during photoencapsulation of primary bovine chondrocytes in PEG hydrogels. Specifically, we examine several different media including (i) a basic phosphate-buffered saline (PBS) solution, which does not provide any protective components against radical damage, (ii) PBS supplemented with medium nutrients, which include exogenous antioxidants, such as ascorbic acid and serum, which has antioxidant capabilities,32 (iii) standard culture medium, which contains nutrients and serum, and (iv) media with varying osmolarities. To assess the importance of a PCM, isolated chondrocytes were allowed to reform some of the PCM before encapsulation. To assess cell survival, we assessed cell viability semiquantitatively with fluorescence microscopy and metabolic activity quantitatively by ATP production. Chondrocyte function was assessed through proteoglycan production. Our findings indicate that a combination of physiological osmolarity and restoration of some of the PCM is necessary to maintain chondrocyte survival during photoencapsulation when low cell densities are employed, and this environment promotes cartilage-specific matrix deposition in long-term culture.
Materials and Methods
Synthesis of PEGDM
PEGDM was synthesized by reacting linear PEG (3000MW; Fluka, Milwaukee, WI) with methacrylic anhydride (Sigma, St. Louis, MO) at a molar ratio of 1:10 using microwave irradiation.33 The final product was purified by dissolution in methylene chloride followed by precipitation in cold ethyl ether. The degree of methacrylate substitution for PEGDM was 90% as determined through 1H NMR (Varian VYR-500 MHz) by comparing the area under the integral for the vinyl resonances (δ = 5.7 ppm and δ = 6.1 ppm) to that of the methylene protons (δ = 4.3 ppm) in the PEG backbone.
Chondrocyte isolation
Bovine articular cartilage (1–2 years; Arapahoe Foods, Lafayette, CO/Sigma, St. Louis, MO) was removed within several hours of slaughter under sterile conditions from metacarpalphalangeal joints of eight steers in two separate isolations. The cartilage was washed in PBS supplemented with 1% penicillin streptomycin (PBS-P/S; Invitrogen), diced finely, and digested in 0.2% collagenase type II (Worthington Biochemical, Lakewood, NJ) in Dulbecco's modified Eagle's medium (DMEM; Invitrogen) and 10% fetal bovine serum (FBS; Invitrogen) for 16 h at 37°C. Isolated chondrocytes were washed in PBS/PS + 0.02% ethylenediaminetetraacetic acid (EDTA) to deactivate the collagenase followed by centrifugation at 1200 rpm for 10 min. Cell viability was determined by the trypan blue exclusion to be ∼97%.
Chondrocyte encapsulation
Immediately after isolation, chondrocytes were maintained in PBS-P/S for ∼30 min at 37°C. After which time, chondrocytes were recovered by centrifugation and gently mixed with macromer solution at concentrations of 4, 10, or 50 million cells/mL. The macromer solution consisted of 10% w/v PEGDM, 0.05% w/w photoinitiator (1-[4-(2-hydroxyethoxy)-phenyl]-2-hydroxy-2-methyl-1-propane-1-one, Irgacure 2959; Ciba Specialty Chemical, Basel, Switzerland) in an encapsulation medium. Encapsulation media included (Table 2) (i) PBS, (ii) PBS + medium nutrients (10 mM HEPES, 0.1 M nonessential amino acids, 0.4 mM L-proline, 50 mg/L L-ascorbic acid, 1% P/S, and 0.5 μg/mL of amphoterecin B (all from Invitrogen), (iii) basal medium (high-glucose DMEM + medium nutrients) supplemented with varying concentrations of FBS (0–5%), (iv) standard chondrocyte medium (basal medium + 10% FBS), or (v) standard chondrocyte medium supplemented with salts to adjust the medium osmolarity. Based on the assumption that standard chondrocyte medium has an osmolarity of 330 mOsm (per manufacturer), the addition of salts (potassium chloride [2.6, 2.9, 3.3 mg/mL] and sodium chloride [5.2, 5.9, 6.6 mg/mL]) will increase the medium osmolarity to 400, 450, or 500 mOsm, respectively.28 The isolated cells were mixed with PBS-P/S for 30 min at 37°C before combining with their respective macromer solution. The cell-macromer solution was exposed to 365 nm light (∼4 mW/cm2) for 10 min to form cylindrical hydrogel constructs (5 mm in diameter and height). Postencapsulation, the cell–laden constructs were cultured in standard chondrocyte medium with the appropriate osmolarity.
Table 2.
Recipes for Encapsulation and Culture Media
| Encapsulation medium | Medium recipea |
|---|---|
| PBS | PBS, pH 7.4, 330 mOsm |
| PBS + medium nutrients | PBS supplemented with HEPES, nonessential amino acids, l-proline, L-ascorbic acid, penicillin, streptomycin, and amphoterecin B |
| Basal medium | DMEM + medium nutrients |
| Basal medium + FBS | DMEM + medium nutrients + 0–5% FBS |
| Standard chondrocyte medium (330 mOsm) | Basal medium supplemented with 10% FBS |
| Chondrocyte medium (400 mOsm) | Standard chondrocyte medium supplemented with potassium chloride (2.6 mg/mL) and sodium chloride (5.2 mg/mL) |
| Chondrocyte medium (450 mOsm) | Standard chondrocyte medium supplemented with potassium chloride (2.9 mg/mL) and sodium chloride (5.9 mg/mL) |
| Chondrocyte medium (500 mOsm) | Standard chondrocyte medium supplemented with potassium chloride (3.3 mg/mL) and sodium chloride (6.6 mg/mL) |
See Chondrocyte encapsulation section for concentrations for the different medium components.
PBS, phosphate-buffered saline; FBS, fetal bovine serum; DMEM, Dulbecco's modified Eagle's medium.
Cell viability
At specified time points, PEG constructs (n = 3) were sliced in half lengthwise, and chondrocyte viability assessed using a LIVE/DEAD® membrane integrity assay (Invitrogen, Carlsbad, CA), in which live cells fluoresce green and dead cells fluoresce red. Three regions were randomly selected from the cut side of the construct representing the edge and interior regions of the gel. Images were acquired using an inverted confocal laser scanning microscope (CLSM; Zeiss LSM 510, Thornwood, NY) equipped with a 10× water immersion objective. Percent cell viability was semiquantified by counting manually live and dead cells in each image.
Metabolic activity assay based on ATP production
At specified time points, PEG constructs (n = 5–8) were removed and immediately frozen in liquid nitrogen. PEG constructs were crushed using a tissue homogenizer in 200 μL of lysis buffer (20 mM Tris, 2 mM EDTA, 150 mM NaCl, and 0.5% Triton-X-100 in DI water). The solution was transferred into individual wells of a 96-well plate, combined with an equal volume of the CytoTox-Glo™ cytotoxicity substrate solution (Promega, Madison, WI/Chemicon, Billerica, MA), and incubated at 37°C for 10 min. ATP production was measured following the manufacturer's protocol by luminescence (Fluostar Optima, BMG Labtech, Durham, North Carolina) and normalized to gel wet weight.
Caspase-3/7 apoptosis assay
PEG constructs photopolymerized in PBS (n = 3) were frozen in liquid nitrogen immediately postencapsulation and crushed using a tissue homogenizer in 200 μL of lysis buffer described above. Apoptosis was detected by measuring for caspase-3/7 activity using the Apo-ONE® (Promega) assay following manufacturer's protocol. Cell pellets (n = 3) comprised of the same number of cells as in the PEG constructs (320,000 cells) were used as the control. The cell pellets were formed by centrifugation and cultured for 24 h.
PCM development and observation
Isolated chondrocytes (10 million/dish) were placed in 100 mm tissue culture dishes supplemented with 10 mL of standard chondrocyte medium (330 or 400 mOsm) for 24 h to allow the cells to deposit some of their own PCM before encapsulation. Chondrocytes that did not attach to the surface of the tissue culture dish were recovered, and viability was assessed by the trypan blue exclusion test. The cells were then encapsulated at a concentration of 4 × 106 cells/mL as described above in standard chondrocyte medium (330 or 400 mOsm). The hydrogel constructs were cultured in their respective culture media. The PCM was assessed immediately postencapsulation by immunohistochemistry. The PCM was also assessed immediately after encapsulation for freshly isolated chondrocytes, that is, which were not preplated, to assess whether there was any PCM present postisolation. Hydrogel constructs were cut in half and placed in PBS supplemented with 0.5 units/mL Chondroitinase ABC (Sigma) and 1% bovine serum albumin solution for 30 min followed by treatment with mouse anti-chondroitin-6-sulfate (clone MK302; Chemicon, Billerica, MA) (1:50) in DMEM + 20% FBS for 1 h. Each construct was rinsed with Earle's balanced salt solution without phenol red (Invitrogen) and placed in DMEM + 20% FBS supplemented with goat anti-mouse Alexa Fluor 546 (1:20) (Invitrogen) for 1 h. The cytoplasm of live cells was counterstained using 2 μL of calcein AM (Invitrogen) for 30 min. Constructs were imaged by CLSM with a 40× oil immersion objective. The cytoplasm of live cells stains green and chondroitin-6-sulfate stains red providing an indication of PCM development.
Matrix synthesis and deposition
To assess matrix synthesis, hydrogel constructs were cultured in medium supplemented with 10 μCi/mL 35SO42− (Perkin Elmer, Shelton, CT) for 24 h. The constructs were removed, crushed using a tissue homogenizer, and digested by papain (125 mg/mL papain [Worthington Biochemical], 10 mM of L-cysteine-HCl [Sigma], 100 mM of phosphate [Sigma], and 10 mM EDTA [Biorad, Hercules, CA] at a pH of 6.3) for 16–17 h at 60°C. Incorporation of [35SO42−] into newly synthesized proteoglycans (cpm/g gel wet weight) was determined using alcian blue precipitation.34 A sample size of 3 was used. To assess long-term matrix deposition, constructs cultured for 1 day, 1 week, or 2 weeks were digested in papain. Sulfated glycosaminoglycan (GAG) content was assayed by dimethylmethylene blue dye35 in the papain digests. Total DNA content was determined by Hoeschst 33258 (Polysciences, Warrington, PA) in the digest.36 GAG production was normalized to its corresponding DNA content at each time point. A sample size of 6 was used.
Statistical analysis
Data are reported as mean ± standard deviation. Single-factor analysis of variance (ANOVA) was used, and a confidence level of 0.95 was considered significant.
Results
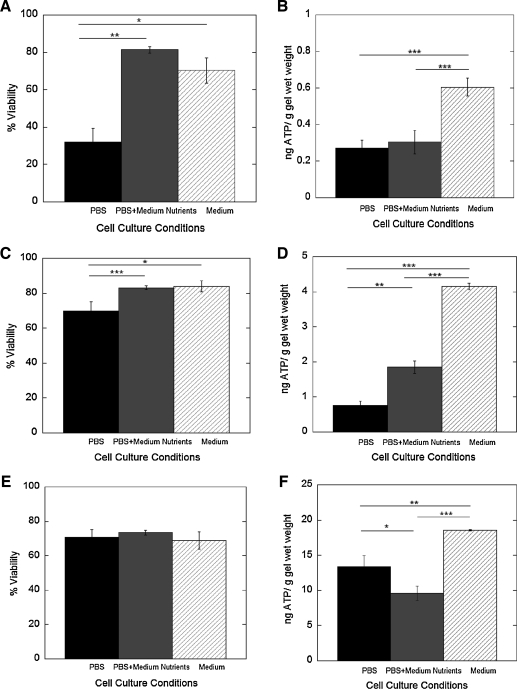
Bovine articular chondrocytes were seeded at low, intermediate, and high cell encapsulation densities (Table 1) in PEG hydrogels in one of three encapsulation media: (i) PBS, (ii) PBS + medium nutrients, and (iii) standard chondrocyte medium containing 10% FBS (Table 2). Cellular viability was assessed by a membrane integrity assay, while metabolic activity was assessed by ATP production. Before encapsulation, cell viability was ∼97%. Upon encapsulation in photopolymerized hydrogels with PBS, cell viability was dramatically reduced to 37% with low cell encapsulation densities (Fig. 2A). However, incorporation of medium nutrients to the PBS or employing standard chondrocyte medium enhanced cell survival resulting in cell viabilities that were twofold higher compared to encapsulation in PBS. ATP production was similar for PBS and PBS supplemented with media nutrients in PBS, but was twofold higher in standard chondrocyte medium (Fig. 2B).
Table 1.
Cell Encapsulation Densities for Chondrocytes Photoencapsulated in Poly(Ethylene Glycol) Hydrogels
| Description | Cell encapsulation density (cells/mL macromer solution) |
|---|---|
| Low | 4 × 106 |
| Intermediate | 10 × 106 |
| High | 50 × 106 |
FIG. 2.
The effects of cell density and encapsulation medium on cell viability (A, C, E) and adenosine triphosphate (ATP) production (B, D, F) immediately after encapsulation in photopolymerized poly(ethylene glycol) (PEG) hydrogels. Three cell encapsulation densities were studied: 4 × 106 cells/mL (low; A, B), 10 × 106 cells/mL (intermediate; C, D), and 50 × 106 cells/mL (high; E, F). Three different encapsulation media were studied: phosphate-buffered saline (PBS, pH 7.4), PBS + medium nutrients, and standard culture medium. ATP production (ng) for each gel was normalized to its respective gel wet weight (g). Percent cell viability (n = 3) and ATP production (n = 5–8) are given by mean ± standard deviation; *p < 0.05, **p < 0.01, and ***p < 0.001.
For the intermediate cell encapsulation densities, cell viability was markedly improved in PBS compared to lower cell encapsulation densities. Cell viability (Fig. 2C) was significantly higher when medium nutrients were incorporated into the PBS and in standard chondrocyte medium. ATP production (Fig. 2D) was twofold higher in the PBS + medium nutrients threefold higher in standard chondrocyte media.
In the high cell encapsulation density, cell viability was not affected by the encapsulation medium (Fig. 2E). However, ATP production (Fig. 2F) was ∼30% lower in the PBS + medium nutrients compared to PBS, while encapsulation in standard chondrocyte media resulted in the highest ATP production.
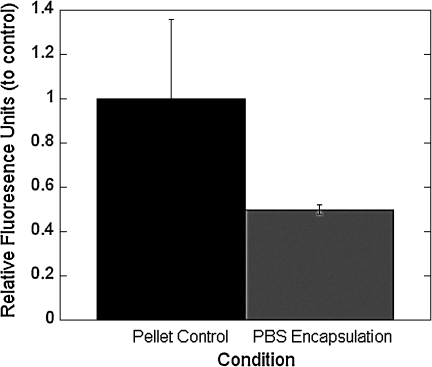
To assess the mechanism of cell death for chondrocytes photoencapsulated at low densities and in PBS, caspase-3/7 activity was assessed (Fig. 3). As a control, chondrocytes were cultured in a cell pellet, which served as a 3D control to compare the 3D culture environment within PEG hydrogels. The mean relative fluorescence decreased by ∼50% for chondrocytes encapsulated within PEG hydrogels when compared to the cell pellet control, but was not statistically significant.
FIG. 3.
Caspase-3/7 activity for chondrocytes photoencapsulated in PEG hydrogels using PBS as the encapsulation medium and for the low cell encapsulation density. The relative fluorescence is directly associated with caspase-3/7 activity where activity in chondrocytes encapsulated in PEG hydrogel constructs was normalized to the activity of chondrocytes cultured in a three-dimensional (3D) cell pellet (control). The total number of cells was the same for both the control and the samples. Data are given by mean ± standard deviation (n = 3).
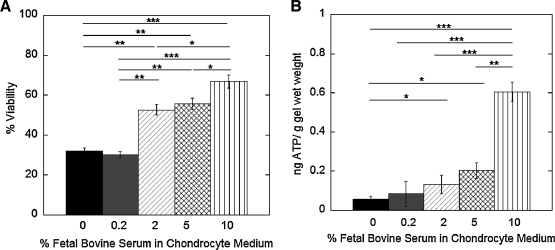
The effect of serum concentration on chondrocyte survival was assessed under low cell encapsulation densities. Chondrocytes were photopolymerized in basal medium supplemented with 0%, 0.2%, 2%, 5%, or 10% FBS (Fig. 4). An increase in FBS concentration resulted in higher cell viabilities immediately postencapsulation (p < 0.001). Similarly, an increase in serum concentration led to higher ATP levels (p < 0.001, Fig. 4B).
FIG. 4.
The effect of serum concentration on cell viability (A) and ATP production (B) immediately after photoencapsulation in basal medium containing 0%, 0.2%, 2%, 5%, or 10% fetal bovine serum for the low cell encapsulation density. ATP production (ng) for each gel was normalized to its respective gel wet weight (g). Percent cell viability (n = 3) and ATP production (n = 5–7) are given by mean ± standard deviation; *p < 0.05, **p < 0.01, and ***p < 0.001.
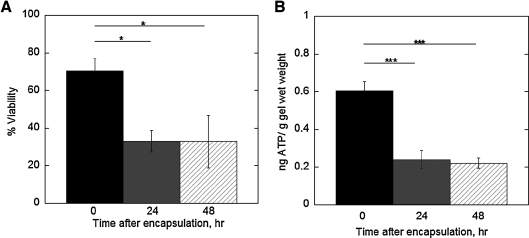
To assess cell survival post cell encapsulation, chondrocytes encapsulated at low densities in standard chondrocyte medium (i.e., containing 10% serum) were cultured for 0, 24, or 48 h (Fig. 5). After 24 h of culture, cell viability decreased by 30% and remained low after 48 h (Fig. 5A). Similarly, ATP production was highest immediately after encapsulation, but decreased threefold 24 h postencapsulation and remained low after 48 h (Fig. 5B).
FIG. 5.
The effect of culture time on cell viability (A) and ATP production (B) for chondrocytes photoencapsulated in PEG hydrogels at the low cell encapsulation density using standard chondrocyte medium (330 mOsm). The constructs were cultured for 0, 24, or 48 h in similar medium. ATP production (ng) for each gel was normalized to its respective gel wet weight (g). Percent cell viability (n = 3–5) and ATP production (n = 5–8) are given by mean ± standard deviation; *p < 0.05, ***p < 0.001.
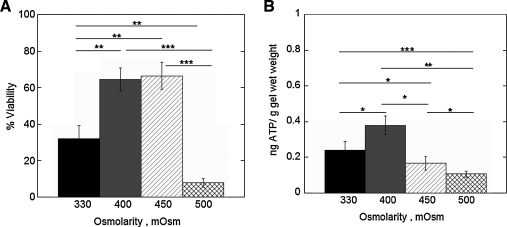
The effect of medium osmolarity on cell viability (Fig. 6A) and ATP production (Fig. 6B) was assessed for chondrocytes encapsulated in low densities and in standard chondrocyte medium with osmolarities ranging from 330 to 500 mOsm. After 24 h of culture postencapsulation, an increase in medium osmolarity from 330 to 400 or 450 mOsm resulted in 40% higher cell viabilities with only ∼8% of chondrocytes surviving under 500 mOsm medium. The 400 mOsm medium resulted in the highest ATP levels, while 500 mOsm chondrocyte medium resulted in the lowest ATP levels.
FIG. 6.
The effect of medium osmolarity on cell viability (A) and ATP production (B) for chondrocytes isolated, encapsulated at the low cell encapsulation density, and cultured for 24 h in chondrocyte medium at 330 (i.e., standard chondrocyte medium), 400, 450, and 500 mOsm. Medium osmolarity was adjusted by the addition of potassium chloride and sodium chloride. ATP production (ng) for each gel was normalized to its respective gel wet weight (g). Percent cell viability (n = 3) and ATP production (n = 5–8) are given by mean ± standard deviation; *p < 0.05, **p < 0.01, and ***p < 0.001.
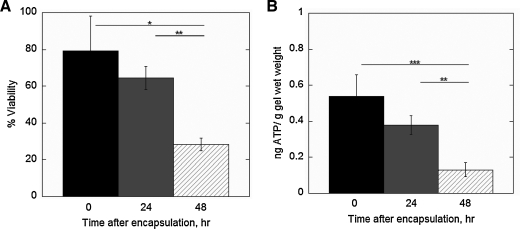
For the 400 mOsm chondrocyte medium, cell viability and ATP production were assessed as a function of culture time (Fig. 7A and B, respectively). Immediately after encapsulation, cell viability was ∼80% and did not show a significant change in viability after a 24-h culture period. After 48 h of culture, cell viability decreased by ∼63%. Similarly, ATP production was highest immediately postencapsulation and did not significantly change after 24 h of culture. However, after 48 h of culture ATP production decreased by 90%.
FIG. 7.
The effect of culture time on cell viability (A) and ATP production (B) for chondrocytes photoencapsulated in PEG hydrogels at the low cell encapsulation density using chondrocyte medium at 400 mOsm, representative of the physiological osmolarity of native cartilage. The constructs were cultured for 0, 24, or 48 h in similar culture medium. ATP production (ng) for each gel was normalized to its respective gel wet weight (g). Percent cell viability (n = 3) and ATP production (n = 5–10) are given by mean ± standard deviation; *p < 0.05, **p < 0.01, and ***p < 0.001.
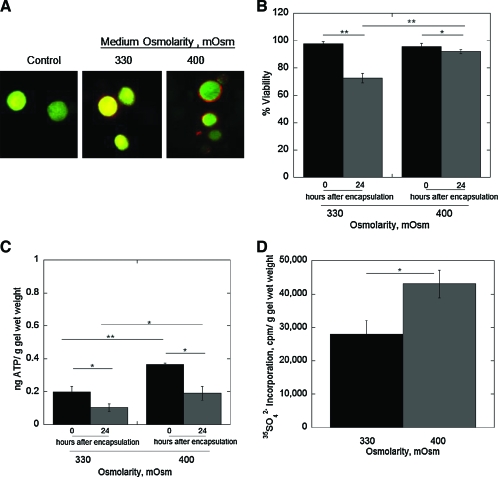
To assess the role of the PCM in mediating the negative effects due to the photoencapsulation process, freshly isolated chondrocytes, which were devoid of any PCM, were allowed to reform some of their own PCM before encapsulation under 330 or 400 mOsm medium. Immediately postencapsulation, the presence of a PCM was confirmed by positive staining for chondroitin sulfate surrounding the chondrocytes (Fig. 8A). Cell viability was high at ∼98% for both culture media immediately postencapsulation. After 24 h of culture, cell viability declined by ∼25% and ∼3% in the 330 and 400 mOsm medium, respectively. At 0 and 24 h postencapsulation, ATP production was higher in the 400 mOsm medium compared to the 300 mOsm. However, for both culture media, ATP production decreased significantly with culture time. We additionally assessed the chondrocytes' ability to function and produce cartilage-specific matrix, specifically through proteoglycan synthesis, when encapsulated with a PCM. Proteoglycan synthesis was evident under both culture media with the 400 mOsm medium resulting in 35% more matrix deposition (Fig. 8D).
FIG. 8.
(A) Representative confocal microscopy images of chondrocytes and their surrounding pericellular matrix immediately after encapsulation. The left image is a representative image of freshly isolated chondrocytes immediately after encapsulation indicating the lack of pericellular matrix, The two images on the right are of chondrocytes that were cultured in monolayer for 24 hours in either 330 or 400 mOsm culture medium to allow them to re-form some of their own pericellular matrix prior to photoencapsulation at the low cell encapsulation density. The far right image (400mOsm) shows cells with positive staining for chondroitin sulfate, a major glycosaminoglycan found in aggrecan. Original magnification is 40 × oil. For chondrocytes, which were pre-plated prior to encapsulation, their cell viability (B) and ATP production (C) were assessed immediately after encapsulation (0 hr) and 24 hours post-encapsulation for the 330 or 400 mOsm medium. ATP production (ng) for each gel was normalized to its respective gel wet weight (g). Proteoglycan synthesis (D) was assessed by 35SO42− incorporation normalized to total DNA content during the first 24 hour of culture post-encapsulation in the 330 or 400 mOsm medium. Percent cell viability (n = 3), ATP production (n = 5–8), and proteoglycan synthesis (n = 3) are given by mean ± standard deviation; *p < 0.05, **p < 0.01. Color images available online at www.liebertonline.com/ten.
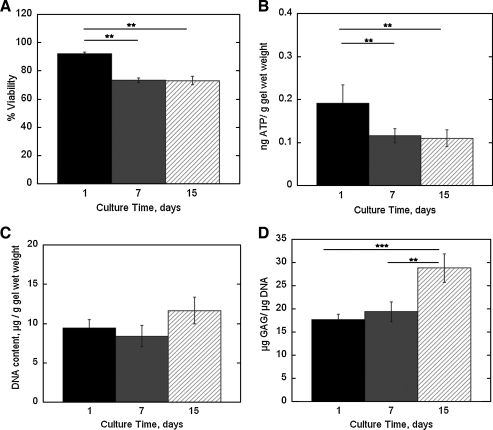
Long-term chondrocyte survival and function were assessed under the best encapsulation condition where chondrocytes were preplated for 24 h and encapsulated in 400mOsm chondrocyte medium (Fig. 9). The cell–laden constructs were cultured in the 400 mOsm medium for 1, 7, or 15 days. Cell viability (Fig. 9A) decreased by 21% after 7 days of culture from day 1, but was not affected by longer culture times of 15 days. Similarly, ATP production (Fig. 9B) decreased by 26% after 7 days of culture but no further changes were found after 15 days. Total DNA content did not change as a function of culture time (Fig. 9C). Chondrocyte function was assessed by matrix deposition for sulfated GAG (Fig. 9D). GAG content increased by ∼35% after 15 days of culture.
FIG. 9.
The effects of long-term culture on chondrocyte cell viability (A), ATP production (B), total DNA content (C), and glycosaminoglycan (GAG) production (D) for chondrocytes that were preplated for 24 h then photoencapsulated and cultured in 400 mOsm medium for 1, 7, and 15 days. ATP production (ng) for each gel was normalized to its respective gel wet weight. GAG production was normalized to total DNA content. Percent cell viability (n = 3), ATP production (n = 3), and GAG production (n = 5–6) are given by mean ± standard deviation **p < 0.01, and ***p < 0.001.
Discussion
In this study, we have shown that when freshly isolated bovine chondrocytes are photoencapsulated in PEG hydrogels via free radical photoinitiated polymerization, their viability and metabolic activity are dramatically influenced by the cell density employed during encapsulation, encapsulation medium, and presence of a PCM.
When high cell encapsulation densities (10–50 millions cells/mL) were employed, the percentage of cells that remained viable immediately after encapsulation was greater than 70% in the different encapsulation mediums. High cell encapsulation densities of 50 million cells/mL have been used successfully to grow cartilaginous tissue within similar photopolymerized PEG and biodegradable PEG hydrogels.6,7,37 However, only ∼35% of chondrocytes survived when a low cell encapsulation density and PBS were used. For cell encapsulation strategies involving photopolymerization, the polymerization must occur in the presence of oxygen to maintain cell survival. However, oxygen is a well-known inhibitor of free radical polymerizations where radicals react with molecular oxygen to produce a less reactive peroxy radical.10,29 Oxygen radicals, however, can be highly toxic to cells most notably through damage to cellular membranes by lipid peroxidation, which can lead to numerous adverse effects, including altered membrane permeability and, if severe, cell death via necrosis. The rapid cell death that occurred during the 10 min encapsulation process, as measured by a membrane integrity assay, indicates that cell death is likely through necrosis. The 50% mean decrease observed in caspase-3/7 activity also suggests necrosis.
For each encapsulation condition, the concentrations of photoinitiator and polymerizable double bonds were similar, suggesting that the number of initiator radicals and macroradicals generated during the polymerization process was similar. Based on the percentage of viable cells and the cell density at encapsulation, the number of cells that did not survive the encapsulation process in PBS was similar for the 4 and 10 million cells/mL conditions (∼220,000 cells/gel). Interestingly, the total number of cells that did not survive the encapsulation process for the 50 million cells/mL density was approximately five times higher. During necrosis, dying cells release a plethora of enzymes, which can trigger a chain reaction of cell death. It is possible that this phenomenon may have occurred due to the high cell concentration and proximity of neighboring cells to a dying cell. Alternatively, the higher cell concentration may increase the viscosity of the solution enhancing the autoacceleration effect and leading to an overall higher concentration of macroradicals during polymerization.38 Taken together, our findings indicate that high cell encapsulation densities lead to significantly more cell death when assessing total numbers of dead cells, suggesting that either high cell density and/or alterations in the polymerization reaction enhance cellular death via free radical damage. Nonetheless, the number of cells that remain viable under the high cell encapsulation densities is sufficient to promote macroscopic tissue development.39
At the low cell concentration, the presence of medium nutrients, which include ascorbic acid and HEPES, and serum in a concentration-dependent manner, significantly improved cell viability immediately postencapsulation. All of these components have known antioxidant capabilities. For example, ascorbic acid reacts directly with superoxides, radicals, and singlet oxygen.29 The presence of HEPES was found to reduce DNA damage during photoencapsulation of plasmid DNA due to its radical scavenging abilities.40 Serum has been shown to protect cells against damage due to oxidative stress.41 In general, metabolic activity as measured by ATP production mirrored cell viability with the exception of when chondrocytes were encapsulated in the presence of glucose and serum, precursors of cellular respiration, and ATP production. As expected, ATP production was significantly higher in chondrocytes encapsulated in standard culture medium compared to PBS supplemented with medium nutrients even though cell viability was similar (i.e., Fig. 2). Nonetheless, cell viability and metabolic activity were not maintained with culture time resulting in a 50% and 60% decline in cell viability and ATP production, respectively, after 1 day of culture. This finding suggests that the presence of antioxidants in medium nutrients and serum minimize acute cellular damage during encapsulation, but are insufficient at preventing radical damage and cell death.
As noted earlier, the typical isolation process for chondrocyte occurs in a hyposmotic environment and leaves the chondrocyte deprived of any protective PCM. The hyposmotic environment initially causes cell swelling, but through regulatory volume decrease mechanisms, chondrocytes are able to respond quickly by counteracting cell swelling through the removal of intracellular osmolytes, typically potassium chloride and organic solutes.28 Prolonged exposure to nonphysiological osmotic environments and long-term loss of osmolytes, however, can be detrimental to cells.28 This phenomenon is often characteristic of many pathological conditions, including osteoarthritis.42 We hypothesized that this decrease in osmolytes associated with the hyposmotic environment may increase the cells' susceptibility to oxidative degradation. After 24 h postencapsulation, cell viability and metabolic activity was highest under physiological medium osmolarity of 400 mOsm, while chondrocyte survival was significantly compromised under hyposmotic and hyperosmotic media. However, by 48 h viability and ATP production decreased markedly, suggesting that free radical damage during encapsulation still occurred.
In native cartilage, chondrocytes interact directly with their pericellular microenvironment to receive biomechanical and biochemical signals.43 Freshly isolated chondrocytes, although deprived of their PCM, are known to begin reforming a PCM within 24 h after their isolation.44 Here, we demonstrate that after 24 h in monolayer culture, freshly isolated chondrocytes have deposited pericellular chondroitin sulfate, one of the major GAGs in aggrecan. Previous studies have reported collagen type VI and keratan sulfate deposition in the pericellular regions of chondrocytes 24 h postisolation.44 The presence of some of the PCM components improved cell viability when encapsulated and cultured in a hyposmotic environment, which is likely due to the antioxidant capabilities of many ECM molecules.31 The combination of PCM and a physiological osmotic environment, however, resulted in superior cell survival after 24 h postencapsulation. Chondrocyte function was also enhanced by the isotonic culture environment as evident by increased proteoglycan synthesis during the first 24 h of encapsulation compared to the hyposmotic environment. Previous studies have reported that a physiological osmotic environment for chondrocytes results in the highest GAG production.45 Over long-term cell cultures (of 15 days), cell viability, metabolic activity, and matrix deposition were maintained under these encapsulation and culture conditions.
In summary, for chondrocytes that were photoencapsulated in low cell encapsulation densities in PEG hydrogels, the combination of antioxidants, physiological osmolarity, and the development of some PCM resulted in an improved robustness against free radical damage during photoencapsulation. Our findings indicate that primary isolated cells, particularly chondrocytes, are more susceptible to free radical damage. These findings may be important in other tissue engineering applications where freshly isolated cells are employed with photoencapsulation strategies at low cell densities. Nonetheless, we report suitable encapsulation conditions that maintain chondrocyte survival and function at least for several weeks postencapsulation towards developing strategies that employ low encapsulation densities and photopolymerization. Furthermore, the encapsulation conditions identified here can readily be translated to in vivo applications where cells can be delivered in a milieu of hydrogel precursors and antioxidants and photopolymerized in situ. Once the cells have survived the photoencapsulation process, the in vivo environment or in vitro culture conditions mimicking the in vivo environment with physiological osmolarities will promote long-term cell survival and tissue production.
Acknowledgments
This work was supported by a research grant from the NIH (K22 DE016608), NIH Pharmaceutical Biotechnology Training Fellowship to N.L.B., and a NASA Harriett Jenkins Predoctoral Fellowship and a Department of Education's Graduate Assistantship in Areas of National Need Fellowship to I.V. Confocal microscopy was performed at the Nanomaterials Characterization Facility at the University of Colorado. A very special thanks to Sara K. Gladem for her technical assistance in this study.
Disclosure Statement
No competing financial interests exist.
References
- 1.Mikos A.G. Herring S.W. Ochareon P. Elisseeff J. Lu H.H. Kandel R. Schoen F.J. Toner M. Mooney D. Atala A. van Dyke M.E. Kaplan D. Vunjak-Novakovic G. Engineering complex tissues. Tissue Eng. 2006;12:3307. doi: 10.1089/ten.2006.12.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicodemus G.D. Bryant S.J. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng. 2008;14:149. doi: 10.1089/ten.teb.2007.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ifcovits J.L. Burdick J.A. Review: photopolymerizable and degradable biomaterials for tissue engineering applications. Tissue Eng. 2007;13:2369. doi: 10.1089/ten.2007.0093. [DOI] [PubMed] [Google Scholar]
- 4.Burdick J.A. Anseth K.S. Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials. 2002;23:4315. doi: 10.1016/s0142-9612(02)00176-x. [DOI] [PubMed] [Google Scholar]
- 5.Sawhney A.S. Pathak C.P. Hubbell J.A. Modification of islet of Langerhans surfaces with immunoprotective poly(ethylene glycol) coatings via interfacial photopolymerization. Biotechnol Bioeng. 1994;44:383. doi: 10.1002/bit.260440317. [DOI] [PubMed] [Google Scholar]
- 6.Elisseeff J. McIntosh W. Anseth K. Riley S. Ragan P. Langer R. Photoencapsulation of chondrocytes in poly(ethylene oxide)-based semi-interpenetrating networks. J Biomed Mater Res. 2000;51:164. doi: 10.1002/(sici)1097-4636(200008)51:2<164::aid-jbm4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 7.Bryant S.J. Anseth K.S. The effects of scaffold thickness on tissue engineered cartilage in photocrosslinked poly(ethylene oxide) hydrogels. Biomaterials. 2001;22:619. doi: 10.1016/s0142-9612(00)00225-8. [DOI] [PubMed] [Google Scholar]
- 8.Nuttelman C.R. Tripodi M.C. Anseth K.S. In vitro osteogenic differentiation of human mesenchymal stem cells photoencapsulated in PEG hydrogels. J Biomed Mater Res A. 2004;68A:773. doi: 10.1002/jbm.a.20112. [DOI] [PubMed] [Google Scholar]
- 9.Goodner M.D. Lee H.R. Bowman C.N. Method for determining the kinetic parameters in diffusion-controlled free-radical homopolymerizations. Ind Eng Chem Res. 1997;36:1247. [Google Scholar]
- 10.Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 11.Halliwell B. Chirico S. Lipid peroxidation: its mechanism, measurement and significance. Am J Clin Nutr. 1993;57:715S. doi: 10.1093/ajcn/57.5.715S. [DOI] [PubMed] [Google Scholar]
- 12.Terakado M. Yamazaki M. Tsujimoto Y. Kawashima T. Nagashima K. Ogawa J. Fujita Y. Sugiya H. Sakai T. Furuyama S. Lipid peroxidation as a possible cause of benzoyl peroxide toxicity in rabbit dental pulp—a microsomal lipid peroxidation in vitro. J Dent Res. 1984;63:901. doi: 10.1177/00220345840630061801. [DOI] [PubMed] [Google Scholar]
- 13.Hanks C.T. Strawn S.E. Wataha J.C. Craig R.G. Cytotoxic effects of resin components on cultured mammalian fibroblasts. J Dent Res. 1991;70:1450. doi: 10.1177/00220345910700111201. [DOI] [PubMed] [Google Scholar]
- 14.Atsumi T. Murata J. Kamiyanagi I. Fujisawa S. Ueha T. Cytotoxicity of photosensitizers camphorquinone and 9-fluorenone with visible light irradiation on a human submandibular-duct cell line in vitro. Arch Oral Biol. 1998;43:73. doi: 10.1016/s0003-9969(97)00073-3. [DOI] [PubMed] [Google Scholar]
- 15.Bryant S.J. Nuttelman C.R. Anseth K.S. Cytocompatibility of UV and visible light photoinitiating systems on cultured NIH/3T3 fibroblasts in vitro. J Biomater Sci Polymer Ed. 2000;11:439. doi: 10.1163/156856200743805. [DOI] [PubMed] [Google Scholar]
- 16.Fedorovich N.E. Oudshoorn M.H. van Geemen D. Hennink W.E. Alblas J. Dhert W.J. The effect of photopolymerization on stem cells embedded in hydrogels. Biomaterials. 2009;30:344. doi: 10.1016/j.biomaterials.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 17.Mayne R. Vail M.S. Mayne P.M. Miller E.J. Changes in type of collagen synthesized as clones of chick chondrocytes grow and eventually lose division capacity. Proc Natl Acad Sci USA. 1976;73:1674. doi: 10.1073/pnas.73.5.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aulthouse A.L. Beck M. Griffey E. Sanford J. Arden K. Machado M.A. Horton W.A. Expression of the human chondrocyte phenotype in vitro. In Vitro Cell Dev Biol. 1989;25:659. doi: 10.1007/BF02623638. [DOI] [PubMed] [Google Scholar]
- 19.de Haart M. Marijnissen W.J. van Osch G.J. Verhaar J.A. Optimisation of chondrocyte expansion in culture. Effect of TGFβ2, bFGF and L-ascorbic acid on bovine articular chondrocytes. Acta Orthop Scand. 1999;70:55. doi: 10.3109/17453679909000959. [DOI] [PubMed] [Google Scholar]
- 20.Bryant S.J. Anseth K.S. Controlling the spatial distribution of ECM components in degradable PEG hydrogels for tissue engineering cartilage. J Biomed Mater Res. 2003;64:70. doi: 10.1002/jbm.a.10319. [DOI] [PubMed] [Google Scholar]
- 21.Nicodemus G.D. Villanueva I. Bryant S.J. Mechanical stimulation of TMJ condylar chondrocytes encapsulated in PEG hydrogels. J Biomed Mater Res A. 2007;83A:323. doi: 10.1002/jbm.a.31251. [DOI] [PubMed] [Google Scholar]
- 22.Villanueva I. Hauschulz D.S. Mejic D. Bryant S.J. Static and dynamic compressive strains influence nitric oxide production and chondrocyte bioactivity when encapsulated in PEG hydrogels of different crosslinking densities. Osteoarthritis Cartilage. 2008;16:909. doi: 10.1016/j.joca.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Temenoff J.S. Park H. Jabbari E. Sheffield T.L. LeBaron R.G. Ambrose C.G. Mikos A.G. In vitro osteogenic differentiation of marrow stromal cells encapsulated in biodegradable hydrogels. J Biomed Mater Res A. 2004;70A:235. doi: 10.1002/jbm.a.30064. [DOI] [PubMed] [Google Scholar]
- 24.Suggs L.J. Mikos A.G. Development of poly(propylene fumarate-co-ethylene glycol) as an injectable carrier for endothelial cells. Cell Transplant. 1999;8:345. doi: 10.1177/096368979900800402. [DOI] [PubMed] [Google Scholar]
- 25.Knight M.M. Lee D.A. Bader D.L. Distribution of chondrocyte deformation in compressed agarose gel using confocal microscopy. J Cell Eng. 1996;1:97. [Google Scholar]
- 26.Lee D.A. Bader D.L. The development and characterisation of an in vitro system to study strain induced cell deformation in isolated chondrocytes. In Vitro Cell Dev Biol. 1995;31:828. doi: 10.1007/BF02634565. [DOI] [PubMed] [Google Scholar]
- 27.Knight M.M. Ghori S.A. Lee D.A. Bader D.L. Measurement of the deformation of isolated chondrocytes in agarose subjected to cyclic compression. Med Eng Phys. 1998;20:684. doi: 10.1016/s1350-4533(98)00080-0. [DOI] [PubMed] [Google Scholar]
- 28.Urban J.P.G. Hall A.C. Gehl K.A. Regulation of matrix synthesis rates by the ionic and osmotic environment of articular chondrocytes. J Cell Physiol. 1993;154:262. doi: 10.1002/jcp.1041540208. [DOI] [PubMed] [Google Scholar]
- 29.Machlin L.J. Bendich A. Free radical tissue damage: protective role of antioxidant nutrients. FASEB J. 1987;1:441. [PubMed] [Google Scholar]
- 30.Xiao H.L. Cai G.P. Liu M.Y. Hydroxyl radical induced structural changes of collagen. Spectrosc Int J. 2007;21:91. [Google Scholar]
- 31.McCabe R.D. Bakarich M.A. Srivastava K. Young D.B. Potassium inhibits free radical formation. Hypertension. 1994;24:77. doi: 10.1161/01.hyp.24.1.77. [DOI] [PubMed] [Google Scholar]
- 32.Burton G.W. Ingold K.V. Beta carotene: an unusual type of lipid antioxidant. Science. 1984;224:569. doi: 10.1126/science.6710156. [DOI] [PubMed] [Google Scholar]
- 33.Lin-Gibson S. Bencherif S. Cooper J.A. Wetzel S.J. Antonucci J.M. Vogel B.M. Horkay F. Washburn N.R. Synthesis and characterization of PEG dimethacrylates and their hydrogels. Biomacromolecules. 2004;5:1280. doi: 10.1021/bm0498777. [DOI] [PubMed] [Google Scholar]
- 34.Lee D.A. Bader D.L. Compressive strains at physiological frequencies influence the metabolism of chondrocytes seeded in agarose. J Orthop Res. 1997;15:181. doi: 10.1002/jor.1100150205. [DOI] [PubMed] [Google Scholar]
- 35.Farndale R.W. Buttle D.J. Barrett A.J. Improved quantitation and discrimination of sulfated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 36.Kim Y.J. Sah R.L.Y. Doong J.Y.H. Grodzinsky A.J. Fluorometric assay of DNA in cartilage explants using Hoechst-33258. Anal Biochem. 1988;174:168. doi: 10.1016/0003-2697(88)90532-5. [DOI] [PubMed] [Google Scholar]
- 37.Bryant S.J. Anseth K.S. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels. J Biomed Mater Res. 2001;59:63. doi: 10.1002/jbm.1217. [DOI] [PubMed] [Google Scholar]
- 38.Lovell L.G. Newman S.M. Bowman C.N. The effects of light intensity, temperature, and comonomer composition on the polymerization behavior of dimethacrylate dental resins. J Dent Res. 1999;78:1469. doi: 10.1177/00220345990780081301. [DOI] [PubMed] [Google Scholar]
- 39.Bryant S.J. Anseth K.S. Hydrogel properties influence ECM production by chondrocyte photoencapsulated in poly(ethylene glycol) hydrogels. J Biomed Mater Res. 2002;59:63. doi: 10.1002/jbm.1217. [DOI] [PubMed] [Google Scholar]
- 40.Quick D.J. Anseth K.S. DNA delivery from photocrosslinked PEG hydrogels: encapsulation efficiency, release profiles, and DNA quality. J Control Release. 2004;96:341. doi: 10.1016/j.jconrel.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 41.Odland L. Wallin S. Walum E. Lipid peroxidation and activities of aminotransferase and glutamine synthetase in hepatoma and glioma cells grown in bovine colostrum-supplemented medium. In Vitro. 1986;22:259. doi: 10.1007/BF02621228. [DOI] [PubMed] [Google Scholar]
- 42.Okada Y. Ion channels and transporters involved in cell volume regulation and sensor mechanisms. Cell Biochem Biophys. 2004;41:233. doi: 10.1385/CBB:41:2:233. [DOI] [PubMed] [Google Scholar]
- 43.Guilak F. Alexopoulos L.G. Upton M.L. Youn I. Choi J.B. Cao L. Setton L.A. Haider M.A. The pericellular matrix as a transducer of biomechanical and biochemical signals in articular cartilage. Ann NY Acad Sci. 2006;1068:498. doi: 10.1196/annals.1346.011. [DOI] [PubMed] [Google Scholar]
- 44.Hing W.A. Sherwin A.F. Ross J.M. Poole C.A. The influence of the pericellular microenvironment on the chondrocyte response to osmotic challenge. Osteoarthritis Cartilage. 2002;10:297. doi: 10.1053/joca.2002.0517. [DOI] [PubMed] [Google Scholar]
- 45.Negoro K. Kobayashi S. Takeno K. Uchida K. Baba H. Effect of osmolarity on glycosaminoglycan production and cell metabolism of articular chondrocyte under three-dimensional culture system. Clin Exp Rheumatol. 2008;26:534. [PubMed] [Google Scholar]