Abstract
Human middle phalanges were tissue-engineered with midshaft scaffolds of poly(L-lactide-ɛ-caprolactone) [P(LA-CL)], hydroxyapatite-P(LA-CL), or β-tricalcium phosphate-P(LA-CL) and end plate scaffolds of bovine chondrocyte-seeded polyglycolic acid. Midshafts were either wrapped with bovine periosteum or left uncovered. Constructs implanted in nude mice for up to 20 weeks were examined for cartilage and bone development as well as gene expression and protein secretion, which are important in extracellular matrix (ECM) formation and mineralization. Harvested 10- and 20-week constructs without periosteum maintained end plate cartilage but no growth plate formation. They also consisted of chondrocytes secreting type II collagen and proteoglycan, and they were composed of midshaft regions devoid of bone. In all periosteum-wrapped constructs at like times, end plate scaffolds held chondrocytes elaborating type II collagen and proteoglycan and cartilage growth plates resembling normal tissue. Chondrocyte gene expression of type II collagen, aggrecan, and bone sialoprotein varied depending on midshaft composition, presence of periosteum, and length of implantation time. Periosteum produced additional cells, ECM, and mineral formation within the different midshaft scaffolds. Periosteum thus induces midshaft development and mediates chondrocyte gene expression and growth plate formation in cartilage regions of phalanges. This work is important for understanding developmental principles of tissue-engineered phalanges and by extension those of normal growth plate cartilage and bone.
Introduction
Since Langer and Vacanti proposed the concept in 1993,1 tissue engineering has made rapid progress toward regenerating mineralizing connective tissues such as cartilage and bone. Numerous basic science and clinical studies have been performed to apply tissue engineering in this context, including development of biodegradable polymers as scaffolds for cultured cells,2 modification of culture systems for enhancing cell numbers,3 stem cell isolation and proliferation,4,5 and cell growth factor release.6 Specifically regarding advancement of the difficult engineering of hard tissues possessing three-dimensional (3D) structure, Isogai et al.7 prepared a complex by combining calf chondrocytes, tendon cells, and periosteum with the biodegradable polymers poly(L-lactide) (PLLA) and polyglycolic acid (PGA). This complex was shaped into different 3D forms, and, following implantation under the dorsum of nude (athymic) mice, it induced bone and cartilage development in constructs modeling a distal phalanx, a middle phalanx, or a distal interphalangeal joint.7 In this experimental series, bone, cartilage, tendon, and a rudimentary joint were regenerated, and the 3D shape and anatomy of the normal human phalanx and finger joint were faithfully reproduced.7 The results broadened tissue engineering possibilities to formation of complicated constructs sutured from three different types of cell-scaffolds. Subsequent long-term experimentation of other human phalanx and joint models using the regeneration technique led to understanding the cellular origin, microstructure, gene expression, and composition of developed bone and cartilage, which maintained their initial bovine phenotype and constituted the regenerated phalanges.8–11
While these novel and interesting models of human phalanges and small joints are consistently reproducible and have been investigated by various means, they do not possess appreciable intrinsic mechanical strength and their processes of development are not completely understood. In this regard, the use of porous ceramics with superior biocompatibility has made recent progress. Biomaterials such as a bioactive ceramic (hydroxyapatite [HA]) and a bioabsorbable ceramic (β-tricalcium phosphate [β-TCP]) have been clinically applied as reconstruction materials for bone defects.12–14 HA [Ca10(PO4)6(OH)2] is minimally degraded in the body, and on implantation into a bone defect it induces formation of new bone by osteoblasts proliferating in contact with the HA implant surface.15 β-TCP [Ca3(PO4)2], a possible precursor of HA, has high osteoconductivity like HA but is relatively rapidly degraded and absorbed with time in the body.15 The osteoconductivity as well as higher mechanical strengths of these ceramics relative to organic polymers are important in their application in bone regeneration.12–15 However, neither ceramic is entirely suitable in this context because both are physically brittle or fragile, difficult to shape for complex 3D construct formation, and reported to yield limited bone mass formation in tissue engineering approaches.16,17
Poly(L-lactide-ɛ-caprolactone) [P(LA-CL)] is a biodegradable block copolymer of PLLA and poly-ɛ-caprolactone [PCL] that has been used for bone and cartilage induction in tissue engineering applications.18–21 P(LA-CL) has a relatively long degradation time of 4–6 months in vivo, causes limited inflammation, has superior tissue compatibility, and is soft and flexible.18,19 To enhance potential suitability for engineered bone and cartilage regeneration and to sustain mechanical strength and long-term maintenance of 3D construct shape, the HA and β-TCP ceramics described above were added as particulates to P(LA-CL) to create two new scaffold materials. These were compared in model human middle phalanx constructs whose midshafts were wrapped with bovine periosteum or left uncovered and then sutured at midshaft ends to small PGA sheets seeded with bovine chondrocytes. Following harvest from nude mice after up to 20 weeks of implantation, the constructs were assessed in terms of gene expression of selected markers; composition, structure, and extent of development related to the presence of periosteum; and scaffold nature. Observed differences indicated that periosteum generates midshaft cells, extracellular matrix (ECM), and mineral deposition and also appears unexpectedly to mediate growth plate formation in separate segments of middle phalanx models. The two ceramic-based copolymers also permit osteogenesis of constructs that compare favorably with those composed of P(LA-CL) alone, but have properties enhanced by ceramic inclusions. Distinct from previous studies, the present work, then, has examined potential interaction between periosteum, growth plate cartilage, and midshaft scaffold composition in the models, and it provides further insight into principles directing development of tissue-engineered phalanges and relating to those of normal bone and growth plate cartilage.
Materials and Methods
In the present study, the tissue engineering approach to human middle phalanx modeling was adapted from the original work of Isogai et al.,7 using bovine cells and tissues and biodegradable polymer scaffolds. In brief, specific cell types were seeded onto such scaffolds to form cell-scaffold constructs viable in vitro. Growth and development of constructs were continued by implanting them into athymic (nude) mice for periods exceeding a year. Mice were sacrificed at intervals, and retrieved constructs were investigated analytically.10,20–24 The various aspects of cell and copolymer preparation, construct formation and implantation, and analyses are described in separate sections below.
Isolation of tissues and cells
Bovine tissues (shoulder joints and radii) from calves less than 1 month old were obtained fresh on sacrifice (Mahan Packing Co., Warren, OH) and transported on ice to the laboratory for dissection. Under sterile conditions, periosteum was lifted with an elevator and strips (∼1–2 cm in length and 1 cm in width) were excised from radial diaphyses and placed in M199 culture medium (Mediatech, Inc., Herndon, VA) containing 10% fetal bovine serum (FBS; Hyclone/Thermo-Fisher Scientific, Pittsburgh, PA) and antibiotic/antimycotic (100 U/mL penicillin, 100 μg/mL streptomycin, and 0.25 μg/mL amphotericin B; Mediatech). Cartilage was dissected with a scalpel from calf shoulder joints, and, according to the method of Klagsbrun,25 small pieces (∼5 × 5 × 2 mm) were digested in 0.3% type II collagenase (Worthington Biochemical, Freehold, NJ) at 37°C for 14 hours. Processing the resulting cell suspension to obtain and count chondrocytes followed the detail of Kusuhara et al.22 Final chondrocyte concentrations were adjusted to 100 × 106 cells/mL.
Scaffold fabrication
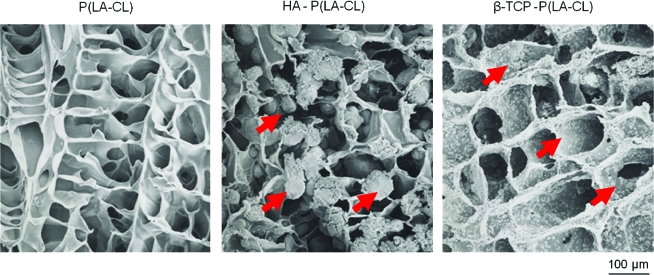
Biodegradable scaffolds used for chondrocyte seeding in this study consisted of PGA (Gunze, Ltd., Kyoto, Japan), having dimensions of ∼1 × 1 × 0.2 cm in length, width and thickness, respectively. Scaffolds for construct midshafts were initiated with PLLA (Gunze) and PCL (Gunze) mixtures (75:25) as described previously.22 P(LA-CL) copolymer was developed as before22 to form the final 3D biodegradable rectangular solid (∼2 × 0.7 × 0.5 cm in length, width and thickness, respectively) to approximate the dimensions of the midshaft of an adult human middle phalanx. Prepared P(LA-CL) scaffolds had a molecular weight of 367 kDa and a sponge-like internal appearance (Fig. 1). The pore size, porosity, and biodegradation rate of these scaffolds were designed to be 50–100 μm, 95%, and 4–6 months, respectively (Gunze). Two additional copolymers for construct midshaft use were developed (Gunze), in which P(LA-CL) was admixed (30:70 wt %) with HA particles 30 μm in diameter [HA-P(LA-CL)] or with β-TCP particles (30:70 wt %) having 2 μm diameter [β-TCP-P(LA-CL)].
FIG. 1.
Scanning electron photomicrographs of representative copolymer scaffolds, air dried and carbon coated for imaging. Poly(L-lactide-ɛ-caprolactone) [P(LA-CL)] (left panel; 75:25 ratio of PLLA:PCL) is composed of thin layers of material that form small fenestrations or pores ∼50–100 μm in diameter throughout the scaffold. Hydroxyapatite (HA) added to P(LA-CL) in a wt % ratio of 70:30 is incorporated into the scaffolds as small spherules of diameter ∼30 μm (arrows; middle panel). β-tricalcium phosphate (β-TCP) mixed with P(LA-CL) in a wt % ratio of 70:30 appears as fine particulates of diameter ∼3 μm that decorate the P(LA-CL) layers rather uniformly (arrows; right panel). Magnification = 230 ×. Color images available online at www.liebertonline.com/ten.
Scanning electron microscopy was used to investigate the fine structure of the three copolymers constituting scaffold midshafts. A JEOL model JSM-5800LV (JEOL, Inc., Tokyo, Japan) instrument was operated at 5–25 kV for this purpose. The different-sized HA and β-TCP particles were found to be incorporated into the structure of the P(LA-CL) copolymer (Fig. 1).
Tissue-engineered design, fabrication, and development of 3D model human middle phalanx constructs
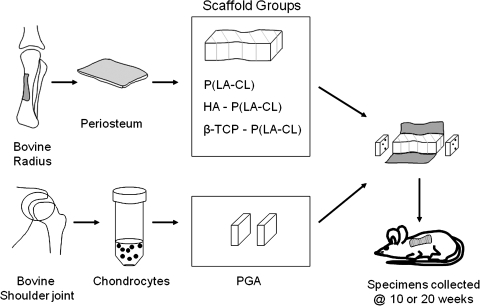
The form of tissue-engineered middle phalanx models consisted of three components, a single midshaft of P(LA-CL), HA-P(LA-CL), or β-TCP-P(LA-CL) sutured with absorbable thread (5-0 Vicryl®; Johnson & Johnson, Langhorne, PA) to single PGA sheets at each of its two ends.7 Prior to suturing the scaffold components together, a single strip of periosteum was wrapped around the entire circumference of an individual midshaft (composed of one of the three different copolymers above) and sutured with Vicryl across the line of tissue contact. To allow osteoblasts and undifferentiated cells constituting the cambium layer of periosteum to migrate into the scaffold, the cambium layer was placed in close contact with the copolymer surface. Each periosteum-wrapped midshaft scaffold was placed in M199 culture medium supplemented as detailed previously and transferred for 5 days to an incubator maintained at 37°C and 5% CO2. Several midshaft scaffolds of the various copolymers were not wrapped with periosteum and were incubated in identical fashion to those covered by the tissue.
While midshaft scaffolds just described were prepared, 100 μL aliquots of chondrocyte suspensions (100 ×106 cells/mL in Ham's F12 medium) were seeded onto individual PGA sheets. Chondrocyte-seeded scaffolds were then placed in 6-well plates and moved to an incubator (37°C, 5% CO2) for 4 hours to provide time for cell adhesion onto PGA surfaces. After this time, supplemented Ham's F12 was added to the wells and the cell-seeded scaffolds were further incubated under the same conditions for 5 days.
After 5 days of culture for periosteum-wrapped midshaft scaffolds, midshaft scaffolds without periosteum, and chondrocyte-seeded scaffolds, models of human middle phalanges were fabricated using a midshaft sutured with Vicryl at its two ends to a chondrocyte-seeded PGA sheet as described above. These various constructs were placed in M199 medium in an incubator (37°C, 5% CO2) for 24 hours. The constructs were then implanted into individual athymic (nude) mice. For this purpose, male, 4- to 6-week-old nude mice (Harlan Sprague Dawley, Indianapolis, IN) were maintained in compliance with the regulations of the Institutional Animal Care and Use Committee of the Northeastern Ohio Universities Colleges of Medicine and Pharmacy and under conditions earlier noted.22 The general experimental protocol including construct implantation is presented in Figure 2. Tissue-engineered models of the human middle phalanges were implanted subcutaneously into the dorsum of the animals following procedures previously reported.8,10,11,21,22
FIG. 2.
A schematic experimental design for tissue engineering model human middle phalanges. Small strips of periosteum, including its cambium layer of pluripotent cells, and cartilage were obtained from calf radii and shoulders, respectively. Intact periosteum was wrapped about each of the three different scaffold composites to fabricate construct midshafts, and chondrocytes isolated from cartilage were seeded onto polyglycolic acid (PGA) used as construct end plates. Some midshaft scaffolds were left uncovered. Middle phalanx models were implanted into nude mice for as long as 20 weeks.
Construct analyses
Mice were sacrificed, and engineered constructs were excised 10 (n = 6) or 20 (n = 6) weeks after implantation. Specimens were retrieved for experimental study by X-ray radiography, histology, and gene expression with particular attention to their development of periosteum and cartilage and the onset and progression of mineralization. On harvest from mice, constructs were bisected longitudinally with half being fixed in 10% neutral-buffered formalin for 24 hours for radiography and histology and half stored in RNAlater™ (Ambion, Inc., Austin, TX) for gene expression analysis. Data from the same and different samples were correlated. During the 24-hour fixation, specimens were imaged and photographed first by radiography and then returned to histological processing.
X-ray radiographs were acquired at 25 kV, 2.4 mA, and 0.2 sec exposure using an X-ray generator (Lorad MIIE; Mammoview, Bedford, MA), and areas of calcification in the specimens were recorded. For comparing retrieved implants under identical X-ray conditions, some fabricated constructs were examined without implantation. These control constructs consisted of PGA scaffolds sutured to midshaft scaffolds composed of each of the three different copolymers described previously.
After 24-hour fixation in formalin, specimens were dehydrated through graded ethanols, embedded in paraffin, and cut into serial sections 6 μm thick on a microtome.7 Sections were stained with toluidine blue to show construct general morphology, Safranin-O red to demonstrate proteoglycans secreted by chondrocytes, and Alizarin red (calcium) and von Kossa (phosphorus) solutions to reveal the presence or absence of areas of calcification.
The specimens placed in RNAlater were frozen at −20°C for subsequent RNA isolation and expression analysis of three genes mediating normal ECM formation and mineralization of vertebrate cartilage: type II collagen, aggrecan, and bone sialoprotein (BSP). The investigative method involved chondrocyte isolation from cartilage regions of constructs by laser capture microdissection (LCM)26 and analysis by quantitative reverse transcription-polymerase chain reaction (QRT-PCR). The approach has been described in detail24 and applied in other studies from this laboratory.22,24,27,28 In summary, construct halves stored in RNAlater were cryosectioned, fixed in ethanol, stained with eosin, and dehydrated. Clusters of ∼200 chondrocytes/section were captured (microdissected) from cartilage adjacent to periosteum-wrapped or uncovered midshaft scaffolds of middle phalanx models harvested after implantation for 10 or 20 weeks. Typically, 30–40 sections were prepared for LCM of the cartilage areas in the three different midshaft scaffold groups (n = 3 for each cell-scaffold group for either 10 or 20 weeks of implantation). The clusters of cells comprising chondrocyte-seeded cartilage portions of constructs were captured using a 30 μm diameter spot size with the PixCell II laser capture system24 (Arcturus Engineering, Mountain View, CA).
Cells captured on PixCell cap surfaces were lysed with guanidine thiocyanate buffer and RNA isolated as described earlier.24 All RNA samples were DNase-treated and reverse-transcribed.24 Bovine-specific primers were designed using Primer Express 1.0 software (Applied Biosystems, Foster City, CA), verified and published previously.22 Gene expression was analyzed with an ABI Prism 7700 Sequence Detector (Applied Biosystems) following the methodology for relative standard curves29 as previously detailed.22,24 Expression levels of type II collagen, aggrecan, and BSP were normalized to that of the housekeeping reference gene, GAPdH. The normalized amount of target gene expression was used to compare the relative amount of target in different samples.
Results
Scanning electron microscopy of scaffolds and macroscopic character and X-ray radiography of constructs
Scaffold microscopy
Ultrastructurally, midshaft scaffolds composed of P(LA-CL) demonstrated a porous, fenestrated appearance on scanning electron microscopy, and those with either HA or β-TCP ceramic added were found to incorporate the particles into the P(LA-CL) framework (Fig. 1). PGA scaffolds consisted of intermeshed, cylindrically shaped, narrow polymer threads having irregular spaces (∼50–100 μm in largest dimension) between them.7
Macroscopic characterization of constructs
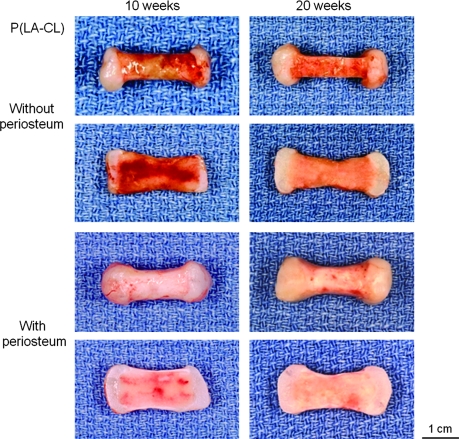
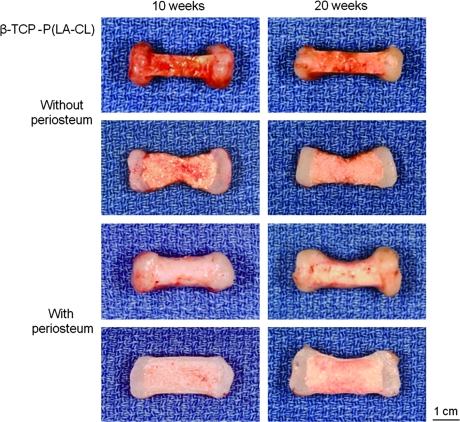
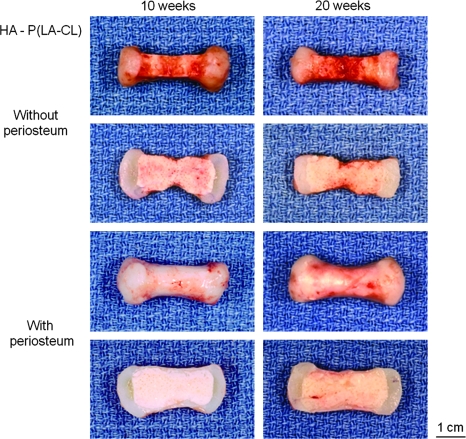
On retrieval after 10 and 20 weeks of implantation, all constructs retained the shape of the human middle phalanx initially designed by tissue engineering (Figs. 3–5, upper panels). Chondrocyte-seeded PGA sheets were white, lustrous, and flexible, resembling cartilage in vivo, and these cell-scaffolds integrated well with their various midshaft scaffold counterparts, whether wrapped with periosteum or uncovered. Chondrocyte-seeded scaffolds thickened with time of implantation. Midshafts varied in the degree of surface reddening resulting from the presence of vascularity contributed by the host mice. In particular, midshafts not covered by periosteum were relatively highly vascularized compared to those wrapped by the tissue, regardless of the copolymer used for fabrication (Figs. 3–5). Further, nonwrapped midshafts were thinner than the qualitatively firmer periosteum-wrapped midshafts, again without consideration of copolymer type (Figs. 3–5). On bisection revealing the internal appearance of specimens, vascularity persisted and was more evident within the midshafts of most nonwrapped constructs and especially those harvested after 10, rather than 20, weeks of implantation (Figs. 3–5).
FIG. 3.
A series of panels showing intact (rows 1 and 3) and bisected (rows 2 and 4) representative middle phalanx specimens harvested after 10 (left four panels) and 20 (right four panels) weeks of implantation in nude mice. Construct midshafts were composed of P(LA-CL) and were either uncovered (upper four panels) or wrapped with calf periosteum (lower four panels). With time of implantation, there were noticeable differences between constructs with and without periosteum in terms of midshaft vascularization and thickness. Magnification = 1.6×.
FIG. 5.
Panels of constructs as described in Figure 3 but with midshafts composed of β-TCP-P(LA-CL). Magnification = 1.6×.
FIG. 4.
Panels of constructs as described in Figure 3 but with midshafts composed of HA-P(LA-CL). Magnification = 1.6×.
Construct X-ray analysis
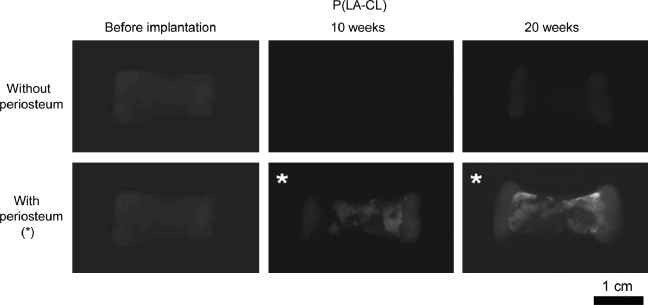
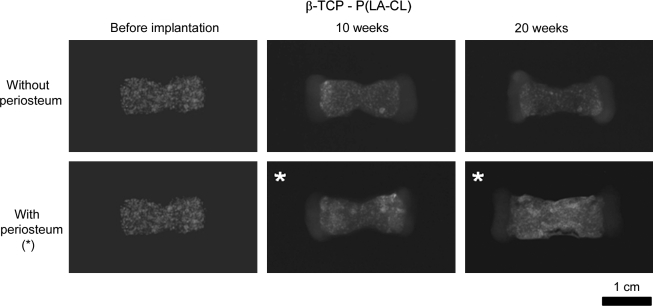
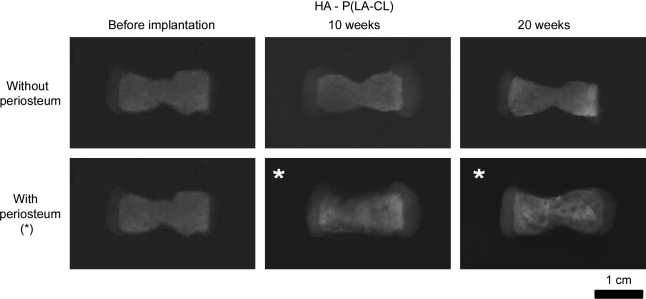
X-ray radiography showed clear images of constructs composed of PGA/P(LA-CL) copolymers and definitive opacity in both PGA/HA-P(LA-CL) and PGA/β-TCP-P(LA-CL) scaffolds as they were first fabricated without seeded chondrocytes or wrapped periosteum (Figs. 6–8). Each of these constructs with chondrocyte-seeded end plates and nonwrapped midshaft copolymers showed no change in radiographic character following implantation in nude mice for up to 20 weeks. On the other hand, constructs seeded with chondrocytes and having periosteum wrapped about their midshaft scaffolds showed higher opacity in midshaft regions over the same time course (Figs. 6–8).
FIG. 6.
X-ray radiographs showing representative middle phalanx constructs with PGA end plates and midshaft compositions of P(LA-CL), imaged before implantation and following 10- and 20-week harvests from nude mice. Constructs with no periosteum wrapped about midshafts failed to mineralize, while those with periosteum (*) developed increasing mineral opacity over midshaft regions with time of implantation. All images were normalized to the density of the unimplanted construct without periosteum (upper far left panel). Magnification = 1.6×.
FIG. 8.
Panels of constructs as presented in Figure 6 but with midshafts composed of β-TCP-P(LA-CL). Intrinsic mineral opacity remained unchanged with time in constructs without periosteum, while in constructs with periosteum-wrapped midshafts (*) opacity increased with implantation time compared to unimplanted specimens. Baseline opacity (far left panels) of these samples shows a punctuated or mottled mineral appearance on imaging compared to specimens with midshafts composed of HA-P(LA-CL) (Figure 7; far left panels). Images were normalized as described in Figure 6. Magnification = 1.6×.
FIG. 7.
Panels of constructs as detailed in Figure 6 but with midshafts composed of HA-P(LA-CL). On X-ray imaging, apatite mineral in nonwrapped constructs yielded intrinsic opacity effectively unchanging with time. Opacity progressively increased in midshafts covered with periosteum at 10- and 20-week harvests (*) compared to baseline opacity of unimplanted models. Images were normalized as described in Figure 6. Magnification = 1.6×.
Histology of constructs
General features
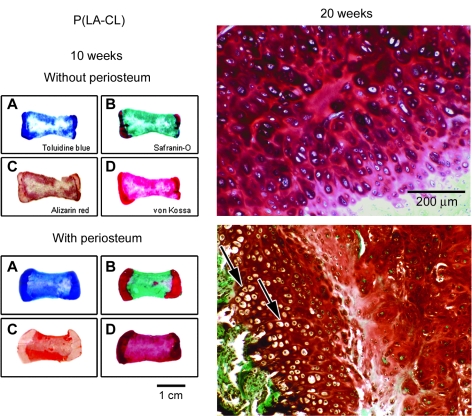
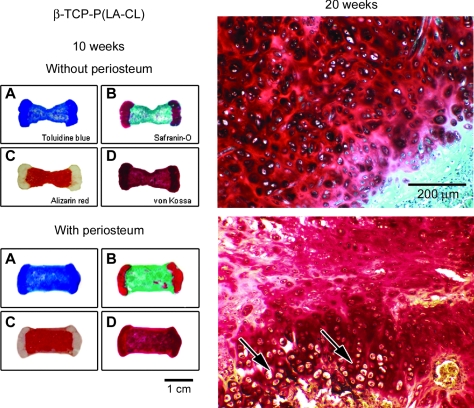
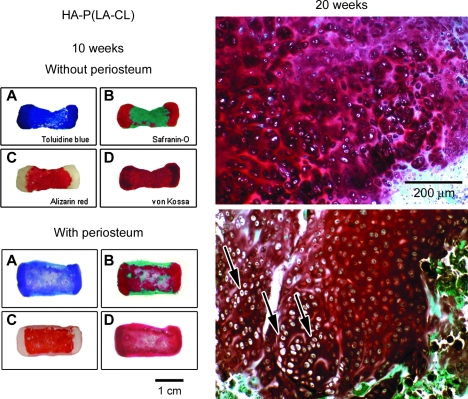
Figures 9–11 provide histological comparisons among constructs composed of P(LA-CL), HA-P(LA-CL), and β-TCP-P(LA-CL) at their midshafts, respectively. Images are representative of three constructs harvested from each midshaft group. The figures illustrate two sets of four panels of stained sections of a single fixed construct retrieved from nude mice after 10 weeks of implantation. All specimens were composed of two end plates of chondrocyte-seeded PGA sheets, but one specimen was wrapped completely about its midshaft scaffold with a strip of periosteum and the other was left uncovered at its midshaft. Sections were stained with toluidine blue, Safranin-O red, Alizarin red, and von Kossa solution as described above. Enlargements of the end plate cartilage of periosteum-wrapped and nonwrapped constructs are shown after these specimens were harvested at 20 weeks. Compared to the general histology of 10-week samples with periosteum, counterpart constructs obtained at 20 weeks were more mineralized in midshaft regions (see below and Fig. 12).
FIG. 9.
Panels showing histological staining of paraffin-embedded sections of constructs composed of midshafts of P(LA-CL). Constructs were prepared with or without periosteum and retrieved from nude mice after 10 (sets A–D of eight small images) or 20 (two enlargements on the right) weeks of implantation. Staining is as noted for specimens harvested at 10 weeks. Samples obtained at 20 weeks represent end plate cartilage of constructs stained with Safranin-O red. Histological stains distinguish between midshaft and end plate regions of each construct (panels A–D). Specimens implanted for 20 weeks with or without periosteum have viable chondrocytes in end plate cartilage as detected by Safranin-O staining (enlargements). Samples containing periosteal tissue (lower right panel) are composed of end plate chondrocytes of different phenotypes, arranged in columnar fashion (arrows) and organized into a rudimentary growth plate like that in vivo. Specimens without periosteum have no distinct chondrocyte organization in end plates (upper right panel). Magnification = 1× (left panels A–D) and 100× (right panels).
FIG. 11.
Panels of construct sections as in Figures 9 and 10 but having midshafts composed of β-TCP-P(LA-CL). After 20 weeks of implantation, chondrocytes in end plate cartilage of constructs with periosteum are phenotypically distinguishable and become progressively hypertrophic and organized in columns like a growth plate in vivo (arrows; lower right panel). Specimens without periosteum have little end plate resemblance to the structure of a normal growth plate (upper right panel). Magnification = 1× (left panels A–D) and 100× (right panels).
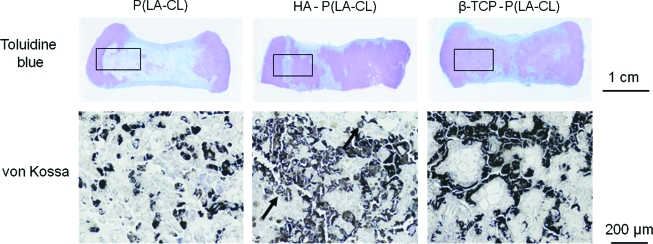
FIG. 12.
Sections of middle phalanx models stained with von Kossa solution to show phosphate as an indicator of mineral formation when correlated with Alizarin red staining for calcium. Constructs were implanted for 20 weeks and have midshafts of P(LA-CL), HA-P(LA-CL), or β-TCP-P(LA-CL). Patterns of phosphate are different for each type of midshaft, and enlargements of specific regions (boxed outlines) illustrate varying staining distributions (black deposits indicated by arrows, for example, in the lower middle panel). Magnification = 1.8× (upper panels) and 70 × (lower panels). Color images available online at www.liebertonline.com/ten.
Emphasis of differences between constructs with and without periosteum
For all 10-week constructs without periosteum (upper panels A–D, Figs. 9–11), toluidine blue clearly marks the end plate and midshaft regions and is of highest metachromatic intensity over end plates. Safranin-O red heavily stains all end plates with some occasional staining observed in midshaft areas of specimens composed of HA-P(LA-CL) (Fig. 10) and β-TCP-P(LA-CL) (Fig. 11). Alizarin red is scattered about construct midshafts of P(LA-CL) (Fig. 9), and it enhances constructs with midshafts of HA-P(LA-CL) (Fig. 10) and β-TCP-P(LA-CL) (Fig. 11). Staining with von Kossa solution is somewhat apparent in end plates of constructs having midshafts of P(LA-CL) (Fig. 9) but is more intense in end plate regions adjacent to midshafts with HA-P(LA-CL) (Fig. 10) and β-TCP-P(LA-CL) (Fig. 11). The latter two midshaft copolymers are also enhanced with von Kossa staining (Figs. 10 and 11) but not P(LA-CL) midshafts (Fig. 9). For all 20-week constructs without periosteum, enlargements of end plate sections treated with Safranin-O red show highly stained ECM secreted by chondrocytes in no apparent arrangement or organization (Figs. 9–11).
FIG. 10.
As described in Figure 9 but with histological stained sections of constructs composed of midshafts of HA-P(LA-CL). Enlargement of representative cartilage end plates from phalanx constructs whose midshafts are wrapped with periosteum and harvested after 20 weeks of implantation (lower right panel) shows chondrocytes of distinct phenotypes in columnar arrangement resembling normal growth plate tissue (arrows). Cartilage chondrocytes from constructs without periosteal wrapping are viable, as judged by Safranin-O staining, but they are not organized as in a true growth plate (upper right panel). Magnification = 1× (left panels A–D) and 100 × (right panels).
For all 10-week constructs wrapped with periosteum (lower panels A–D, Figs. 9–11), toluidine blue again discriminates between end plate and midshaft regions and is deepest in metachromasia over end plates. Safranin-O red stains all end plates most heavily and appears as well in midshafts of all samples, particularly within those composed of HA-P(LA-CL) (Fig. 10). Alizarin red is observed in all construct midshafts, enhancing especially those with midshafts of HA-P(LA-CL) (Fig. 10) and β-TCP-P(LA-CL) (Fig. 11). Staining with von Kossa solution is found in end plates and midshafts of all constructs, with relatively more intensity in the end plates of constructs having midshafts of P(LA-CL) (Fig. 9). For all 20-week constructs covered with periosteum, end plate sections treated with Safranin-O red show well stained ECM and chondrocytes residing in clear lacunae. The cells are phenotypically different, undergo progressive hypertrophy in deeper end plate regions, and are organized in somewhat extended parallel columns (Figs. 9–11).
Figure 12 presents toluidine blue- and von Kossa-stained sections from middle phalanx models implanted for 20 weeks and having periosteum-wrapped midshafts composed of P(LA-CL), HA-P(LA-CL), and β-TCP-P(LA-CL). The staining follows different distribution patterns in each midshaft with no apparent trabecular organization at this stage of bone development. The degree of von Kossa staining is greater at 20 weeks than at 10 weeks (Figs. 3–5) and corresponds with Alizarin red staining (data not shown) and X-ray radiography (Figs. 6–8), indicative of increasing mineralization with time of implantation.
Analyses of chondrocyte gene expression
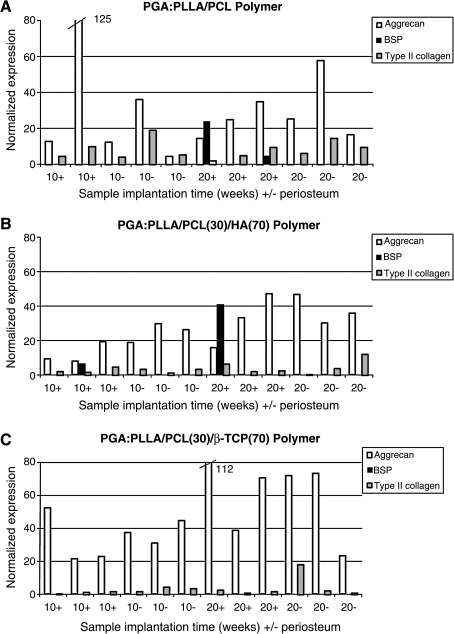
Figure 13 shows the results of gene expression analyses of cartilaginous end plates from constructs retrieved after implantation for 10 and 20 weeks and whose midshafts were composed of either P(LA-CL), HA-P(LA-CL), or β-TCP-P(LA-CL). Midshafts were either wrapped with periosteum or left uncovered. All end plates of the constructs were composed of PGA, onto which chondrocytes were seeded. In these investigations, LCM was used to isolate groups of ∼200 chondrocytes from frozen sections of deeper end plate regions of constructs (n = 3). QRT-PCR data indicate several aspects of expression of type II collagen, aggrecan, and BSP. A comparison of Figures 13A, B, and C demonstrates that normalized expression of each of these genes obtained from chondrocytes is different and varies according to midshaft composition of a particular construct, presence of periosteum, and length of construct implantation time. Aggrecan expression is most conspicuous and type II collagen expression is detectable to a lesser extent in all end plate cartilage regardless of midshaft composition of the constructs and presence of periosteum. BSP expression is found in end plates retrieved after 20 weeks of implantation in constructs whose midshafts are composed of P(LA-CL) and wrapped with periosteum and also in cartilage retrieved after both 10 and 20 weeks of implantation in constructs with midshaft composition of HA-P(LA-CL) and wrapped with periosteum. Regardless of midshaft composition, it is difficult to determine whether the presence or absence of periosteum in each construct results in significant differences in the expression of aggrecan obtained from end plate cartilage. The same can be said for expression of type II collagen in each construct end plate, but expression of BSP is detected only when periosteum is present. In general for all constructs, whether covered with periosteum or not, longer implantation times of 20 weeks compared to 10 weeks yield greater gene expression levels for aggrecan. BSP expression follows similarly with respect to constructs of HA-P(LA-CL). Time of implantation and presence of periosteum appear to have little effect on gene expression levels for type II collagen.
FIG. 13.
Gene expression profiles of type II collagen, aggrecan, and bone sialoprotein obtained from ∼6000–8000 chondrocytes constituting the cartilaginous end plates of individual tissue-engineered human middle phalanx models. Cells residing in deeper regions of end plates were isolated by laser capture microdissection from construct frozen sections. Chondrocytes in all models were initially seeded onto PGA scaffolds. Midshafts of the models were composed of (A) P(LA-CL), (B) HA-P(LA-CL), or (C) β-TCP-P(LA-CL). Admixtures of P(LA-CL) with HA or β-TCP both had the wt % ratio 30:70. Midshafts were either wrapped by periosteum (+) or left uncovered (−) and implanted for 10 or 20 weeks before retrieval from nude mice. Each bar represents a single middle phalanx construct, and expression levels determined by QRT-PCR analysis for the three genes of interest are normalized to GAPdH. Values for normalized expression beyond the plotted scale are given numerically adjacent to the truncated bar for two aggrecan measurements.
Discussion
Models of human distal and middle phalanges and a distal interphalangeal joint have been successfully tissue-engineered in this laboratory and reported in several studies.7–11,21 Such constructs recapitulate new bone and cartilage formation in vivo when periosteum is wrapped about the model midshafts and chondrocytes are seeded to scaffolds sutured to the midshaft end(s). The most recent of these investigations briefly examined possible interaction between P(LA-CL) midshafts wrapped with periosteum and adjacent cartilaginous end plates of middle phalanx specimens.21 As an extension of this work, two types of ceramic particles, HA or β-TCP, were added to the basic P(LA-CL) copolymer and the resulting HA-P(LA-CL) or β-TCP-P(LA-CL) scaffolds were compared to P(LA-CL) alone, either wrapped with bovine periosteum (typically 0.2–0.4 mm thick) or left completely uncovered. Both HA and β-TCP have been used as additives to biodegradable polymers,12,14,17,30–33 and osteoblasts appear to respond to the presence of HA by proliferating and forming bone on this scaffold because of its compositional similarities to bone tissue in vivo.34 In experiments with β-TCP in polymer scaffolds, osteoblasts adhered to the surfaces of this ceramic and secreted collagen deep into the pores of these construct supports.33 Thus, HA and β-TCP in earlier tissue engineering applications were associated with increased osteoconductive and osteogenic capacities.
The present experiments show that periosteum orchestrates events leading to mineralization of midshafts of the middle phalanx model, as has been documented,7,11 and that the HA and β-TCP scaffolds are each osteoconductive like P(LA-CL) itself. Thus, copolymers of HA or β-TCP admixed with P(LA-CL) can serve as scaffolds for phalanx constructs with chondrocyte-seeded PGA sutured to them. In addition, and unexpectedly, periosteum appears to affect profoundly development of adjacent chondrocyte-seeded PGA scaffolds in tissue-engineered middle phalanx constructs. Cells of these cartilage end plates synthesize and secrete type II collagen and proteoglycans as do cells of cartilage end plates in constructs without midshaft periosteum. In the presence of midshaft periosteal tissue, however, chondrocytes also become disposed and distinguishable phenotypically in the end plates. They become progressively hypertrophic in deeper regions of the cartilage, where they assemble and organize in slightly extended, approximately parallel, columnar arrangements. These biochemical and structural features closely resemble the characteristics and appearance of normal cartilaginous growth plates in vivo.
Regardless of midshaft copolymer composition, these cartilage end plate results can be reproduced as long as periosteum is included in phalanx constructs. However, midshaft composition, as well as the presence of periosteum, is important to end plate features since P(LA-CL), HA-P(LA-CL), and β-TCP-P(LA-CL) each evoke a different chondrocyte gene expression profile for type II collagen, aggrecan, and BSP, markers used for assessing cartilage matrix formation and subsequent mineralization as in growth plate cartilage in vivo.35,36
Both the presence of periosteum and midshaft scaffold composition suggest that cells of periosteum and cartilage in tissue-engineered middle phalanx models interact with each other in some as yet unknown manner. Putative interaction between periosteal cells and their supporting scaffold also mediates cartilage effects. Explanations for these results can only be conjectural at this point. Possibly a signaling regime exists, for example, between cells constituting the periosteum and those of the adjacent cartilage similar to that involving Indian hedgehog, parathyroid hormone-related peptide, and transforming growth factor-β within perichondrium and growth plate cartilage in vivo.35–38 In addition, periosteal cell receptors may be sensitive to the specific chemical nature and composition, texture, particle size, porosity, or some other factor(s) of the midshaft scaffolds. Transduction of these types of signals may also introduce changes in gene expression of the periosteal cells, leading ultimately to the reported changes in genotype and phenotype of end plate chondrocytes in the middle phalanx models.
Such cross-talk between the various cells of the constructs or the response(s) of periosteal cells to their underlying scaffold support may be fundamental in the development of their respective components. The engineered specimens may also serve as critical means for understanding events in vivo of both phalanx growth plate and midshaft formation. Studies in progress are examining, for example, possible effects of periosteum as well as midshaft scaffold composition on genes and proteins involved in signaling events between growth plate cartilage and perichondrium leading to growth plate regulation.35–43 Other molecular, biochemical, or structural aspects mediating growth and development of middle phalanx models are also being investigated using constructs whose midshafts are wrapped with periosteum over only half their length.21
Several specific results of the present work are worth considering. First, the use of two scaffolds each containing mineral and inducing additional mineral formation is important where tissue engineering may require physically substantive cell support greater than that afforded by the organic copolymer P(LA-CL) alone. HA or β-TCP admixed with P(LA-CL) may provide a stronger scaffold through mineral addition, which may support and sustain greater masses of cells or larger amounts of periosteum. Further, these initially mineralized scaffolds constituting phalanx model midshafts may be more advantageous in their biochemical nature compared to P(LA-CL) itself since HA or β-TCP addition to P(LA-CL) may enhance the development of pluripotent periosteal mesenchymal cells, osteoblasts, or chondrocytes to a greater degree than P(LA-CL) alone. Precise means underlying the osteoconductive and osteogenic capacity of HA-P(LA-CL) and β-TCP-P(LA-CL) remain to be explained.
von Kossa staining has been used in some instances as an indicator of mineral formation in periosteum-wrapped midshafts of the model phalanges fabricated with different scaffolds. The variability in observed mineral distribution may result because the periosteal sheets wrapping the midshaft scaffolds are not identical and the periosteum, as it elaborates new cells and ECMs, would be expected to be distinct in its response to the different scaffold compositions.
Regarding the staining character of midshafts devoid of periosteum, there is clear evidence for the presence of cells, ECM proteoglycans, and calcium and phosphate ions in this region of these phalanx models. These results may be a consequence of cells or cellular elements introduced into midshafts by vascularity of the nude mice harboring the implanted constructs and the possible migration to midshaft regions of some of the viable chondrocytes composing the cartilage end plates of the specimens. Such chondrocytes would presumably secrete proteoglycans, type II collagen, and other molecules in midshafts, which would lead to toluidine blue and Safranin-O red stained construct sections.
The basis is not known for the observed differences in chondrocyte gene expression of type II collagen, aggrecan, and BSP as the scaffold copolymer composition is changed beneath midshaft-wrapped constructs. As noted above, these results would suggest that periosteal cells are affected in specific ways by their underlying scaffold and these cells in turn communicate to chondrocytes of adjacent construct cartilage, or the various scaffold compositions of midshafts are sensed by chondrocytes directly. Detection of gene expression levels of type II collagen, aggrecan, and BSP indicates that chondrocytes initially seeded onto construct end plates of PGA remain viable after implantation for at least 20 weeks. Aggrecan gene expression levels are maintained most conspicuously compared to those of type II collagen and BSP, regardless of midshaft composition or presence of periosteum. In all constructs, aggrecan gene expression also increases noticeably by 20 compared to 10 weeks of implantation, again whether periosteum is present or not. Such increasing expression levels may be correlated with increasing cartilage end plate development in the various constructs and the appearance of Safranin-O staining of proteoglycan presented here. The presence of BSP gene expression in cartilage end plates of constructs composed of P(LA-CL) and HA-P(LA-CL) scaffolds wrapped with periosteum may be correlated with the putative developmental onset and progression of mineralization of these regions of the specimens. Such mineralization events have been observed in cartilage end plates of related constructs whose P(LA-GA) midshafts were wrapped with periosteum7 and that are well documented in growth plate cartilage in vivo.35,36 The reasons for the absence of BSP expression by chondrocytes in constructs having midshafts composed of β-TCP-P(LA-CL) are conjectural. BSP may simply not be expressed by chondrocytes in these specimens, or the gene may appear at implantation times longer than 20 weeks.
The putative interrelationships between midshaft scaffold composition, presence of periosteum, chondrocyte gene expression, secretion of ECM molecules, and cartilage growth plate formation in tissue-engineered human phalanx models are still being investigated. Results are potentially fundamental with regard to more complete understanding of the basic principles guiding the formation and development of phalanx constructs as well as bone and growth plate cartilage in vivo.
Acknowledgments
The authors are grateful for the assistance, care, and dedication regarding animal use and care practiced by Walter Horne, D.V.M., director, Comparative Medicine Unit, Northeastern Ohio Universities Colleges of Medicine and Pharmacy, and his staff members, Ms. Deborah Dutton, Ms. Cindy Fobes, Ms. Lora Nicholson, and Mr. John Ryznar. This work was supported by grant AR41452 from the National Institutes of Health, Washington, DC (to WJL), and a Hitech Research Center grant from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (to NI).
Disclosure Statement
No competing financial interests exist.
References
- 1.Langer R. Vacanti J.P. Tissue engineering. Science. 1993;260:920. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 2.Banu N. Banu Y. Sakai M. Mashino T. Tsuchiya T. Biodegradable polymers in chondrogenesis of human articular chondrocytes. J Artif Organs. 2005;8:184. doi: 10.1007/s10047-005-0302-3. [DOI] [PubMed] [Google Scholar]
- 3.Wendt D. Jakob M. Martin I. Bioreactor-based engineering of osteochondral grafts: from model systems to tissue manufacturing. J Biosci Bioeng. 2005;100:489. doi: 10.1263/jbb.100.489. [DOI] [PubMed] [Google Scholar]
- 4.Noel D. Djouad F. Jorgense C. Regenerative medicine through mesenchymal stem cells for bone and cartilage repair. Curr Opin Investig Drugs. 2002;3:1000. [PubMed] [Google Scholar]
- 5.Dragoo J.L. Samimi B. Zhu M. Hame S.L. Thomas B.J. Lieberman J.R. Hedrick M.H. Benhaim P. Tissue-engineered cartilage and bone using stem cells from human infrapatellar fat pads. J Bone Joint Surg Br. 2003;85:740. [PubMed] [Google Scholar]
- 6.Isogai N. Morotomi T. Hayakawa S. Munakata H. Tabata Y. Ikada Y. Kamiishi H. Combined chondrocyte-copolymer implantation with slow release of basic fibroblast growth factor for tissue engineering an auricular cartilage construct. J Biomed Mater Res A. 2005;74:408. doi: 10.1002/jbm.a.30343. [DOI] [PubMed] [Google Scholar]
- 7.Isogai N. Landis W.J. Kim T.H. Gerstenfeld L.C. Upton J. Vacanti J.P. Formation of phalanges and small joints by tissue engineering. J Bone Joint Surg Am. 1999;81A:306. doi: 10.2106/00004623-199903000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Isogai N. Landis W.J. Phalanges and small joints. In: Atala A., editor; Lanza R., editor. Methods of Tissue Engineering. San Diego, CA: Academic Press; 2001. pp. 1041–1047. [Google Scholar]
- 9.Chubinskaya S. Jacquet R. Isogai N. Asamura S. Landis W.J. Characterization of the cellular origin of a tissue-engineered human phalanx model by in situ hybridization. Tissue Eng. 2004;10:1204. doi: 10.1089/ten.2004.10.1204. [DOI] [PubMed] [Google Scholar]
- 10.Landis W.J. Jacquet R. Hillyer J. Zhang J. Siperko L. Chubinskaya S. Asamura S. Isogai N. The potential of tissue engineering in orthopedics. Orthop Clin North Am. 2005;36:97. doi: 10.1016/j.ocl.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Potter K. Sweet D.E. Anderson P. Davis G.R. Isogai N. Asamura S. Kusuhara H. Landis W.J. Non-destructive studies of tissue-engineered phalanges by magnetic resonance microscopy and X-ray microtomography. Bone. 2006;38:350. doi: 10.1016/j.bone.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 12.Yaszemski M.J. Payne R.G. Hayes W.C. Langer R. Mikos A.G. Evolution of bone transplantation: molecular, cellular and tissue strategies to engineer human bone. Biomaterials. 1996;17:175. doi: 10.1016/0142-9612(96)85762-0. [DOI] [PubMed] [Google Scholar]
- 13.Metsger D.S. Driskell T.D. Paulsrud J.R. Tricalcium phosphate ceramic—a resorbable bone implant: review and current status. J Am Dent Assoc. 1982;105:1035. doi: 10.14219/jada.archive.1982.0408. [DOI] [PubMed] [Google Scholar]
- 14.Hollinger J.O. Brekke J. Gruskin E. Lee D. Role of bone substitutes. Clin Orthop Relat Res. 1996;324:55. doi: 10.1097/00003086-199603000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Helm G.A. Graft substitutes for use in spinal fusions. Clin Neurosurg. 2004;52:142. [PubMed] [Google Scholar]
- 16.Hoogendoorn H.A. Renooij W. Akkermans L.M. Visser W. Wittebol P. Long-term study of large ceramic implants (porous hydroxyapatite) in dog femora. Clin Orthop Relat Res. 1984;187:281. [PubMed] [Google Scholar]
- 17.Kitsugi T. Yamamuro T. Nakamura T. Kotani S. Kokubo T. Takeuchi H. Four calcium phosphate ceramics as bone substitutes for non-weight-bearing. Biomaterials. 1993;14:216. doi: 10.1016/0142-9612(93)90026-x. [DOI] [PubMed] [Google Scholar]
- 18.Yamada K. Miyamoto S. Nagata I. Kikuchi H. Ikada Y. Iwata H. Yamamoto K. Development of a dural substitute from synthetic bioabsorbable polymers. J Neurosurg. 1997;86:1012. doi: 10.3171/jns.1997.86.6.1012. [DOI] [PubMed] [Google Scholar]
- 19.Yamada K. Miyamoto S. Takayama M. Nagata I. Hashimoto N. Ikada Y. Kikuchi H. Clinical application of a new bioabsorbable artificial dura mater. J Neurosurg. 2002;96:731. doi: 10.3171/jns.2002.96.4.0731. [DOI] [PubMed] [Google Scholar]
- 20.Isogai N. Kusuhara H. Ikada Y. Otani H. Jacquet R. Hillyer J. Lowder E. Landis W.J. Comparison of different chondrocytes for use in tissue engineering of cartilage model structures. Tissue Eng. 2006;12:691. doi: 10.1089/ten.2006.12.691. [DOI] [PubMed] [Google Scholar]
- 21.Landis W.J. Jacquet R. Lowder E. Enjo M. Wada Y. Isogai N. Tissue engineering models of human digits: effect of periosteum on growth plate cartilage development. Cells Tissues Organs. 2009;189:241. doi: 10.1159/000151432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kusuhara H. Isogai N. Enjo M. Otani H. Ikada Y. Jacquet R. Lowder E. Landis W.J. Tissue engineering a model for the human ear: assessment of size, shape, morphology, and gene expression following seeding of different chondrocytes. Wound Repair Regen. 2009;17:136. doi: 10.1111/j.1524-475X.2008.00451.x. [DOI] [PubMed] [Google Scholar]
- 23.Isogai N. Asamura S. Higashi T. Ikada Y. Morita S. Hillyer J. Jacquet R. Landis W.J. Tissue engineering of an auricular cartilage model utilizing cultured chondrocyte-poly(L-lactide-ɛ-caprolactone) scaffolds. Tissue Eng. 2004;10:673. doi: 10.1089/1076327041348527. [DOI] [PubMed] [Google Scholar]
- 24.Jacquet R. Hillyer J. Landis W.J. Analysis of connective tissues by laser capture microdissection and reverse transcriptase-polymerase chain reaction. Anal Biochem. 2005;337:22. doi: 10.1016/j.ab.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 25.Klagsbrun M. Large-scale preparation of chondrocytes. Methods Enzymol. 1979;58:560. doi: 10.1016/s0076-6879(79)58171-3. [DOI] [PubMed] [Google Scholar]
- 26.Emmert-Buck M.R. Bonner R.F. Smith P.D. Chauqui R.F. Zhuang Z. Goldstein S.R. Weiss R.A. Liotta L. Laser capture microdissection. Science. 1996;274:998. doi: 10.1126/science.274.5289.998. [DOI] [PubMed] [Google Scholar]
- 27.Scharschmidt T. Jacquet R. Laskovski J. Lowder E. Weiner S. Landis W.J. Analysis of human osteoarthritic connective tissue by laser capture microdissection and QRT-PCR. Connect Tissue Res. 2007;48:316. doi: 10.1080/03008200701692685. [DOI] [PubMed] [Google Scholar]
- 28.Scharschmidt T. Jacquet R. Weiner D. Lowder E. Schrickel T. Landis W.J. Gene expression in slipped capital femoral epiphysis. Evaluation with laser capture microdissection and quantitative reverse transcription-polymerase chain reaction. J Bone Joint Surg. 2009;91:366. doi: 10.2106/JBJS.G.00039. [DOI] [PubMed] [Google Scholar]
- 29.User Bulletin #2. Foster City, CA: Applied Biosystems; 1997. ABI PRISM 7700 Sequence Detection System. [Google Scholar]
- 30.Ozawa M. Experimental study on bone conductivity and absorbability of the pure β-TCP. J Jpn Soc Biomater. 1995;13:167. [Google Scholar]
- 31.Saito M. Shimizu H. Beppu M. Takagi M. The role of β-tricalcium phosphate in vascularized periosteum. J Orthop Sci. 2000;5:275. doi: 10.1007/s007760050163. [DOI] [PubMed] [Google Scholar]
- 32.Dong J. Uemura T. Shirasaki Y. Tateishi T. Promotion of bone formation using highly pure porous β-TCP combined with bone marrow-derived osteogenitor cells. Biomaterials. 2002;23:4493. doi: 10.1016/s0142-9612(02)00193-x. [DOI] [PubMed] [Google Scholar]
- 33.Chazono M. Tanaka T. Komaki H. Fujii K. Bone formation and bioresorption after implantation of injectable β-tricalcium phosphate granules–hyaluronate complex in rabbit bone defects. J Biomed Mater Res A. 2004;70:542. doi: 10.1002/jbm.a.30094. [DOI] [PubMed] [Google Scholar]
- 34.Glimcher M.J. Composition, structure and organization of bone and other mineralized tissues and the mechanism of calcification. In: Greep R.O., editor; Astwood E.B., editor. Handbook of Physiology. Section 7: Endocrinology. Baltimore, MD: American Physiological Society; 1976. pp. 25–116. [Google Scholar]
- 35.Ballock R.T. O'Keefe R.J. Physiology and pathophysiology of the growth plate. Birth Defects Res C. 2003;69:123. doi: 10.1002/bdrc.10014. [DOI] [PubMed] [Google Scholar]
- 36.Ballock R.T. O'Keefe R.J. The biology of the growth plate. J Bone Joint Surg. 2003;85:715. [PubMed] [Google Scholar]
- 37.Kronenberg H.M. Lee K. Lanske B. Segre G.V. Parathyroid hormone-related protein and Indian hedgehog control the pace of cartilage differentiation. J Endocrinol. 1997;154:S39. [PubMed] [Google Scholar]
- 38.Eames B.F. de la Fuente L. Helms J.A. Molecular ontogeny of the skeleton. Birth Defects Res C. 2003;69:93. doi: 10.1002/bdrc.10016. [DOI] [PubMed] [Google Scholar]
- 39.Maeda Y. Nakamura E. Nguyen M.T. Suva L.J. Swain F.L. Razzaque M.S. Mackem S. Lanske B. Indian hedgehog produced by postnatal chondrocytes is essential for maintaining a growth plate and trabecular bone. Proc Natl Acad Sci USA. 2007;104:6382. doi: 10.1073/pnas.0608449104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mak K.K. Kronenberg H.M. Chuang P.T. Mackem S. Yang Y. Indian hedgehog signals independently of PTHrP to promote chondrocyte hypertrophy. Development. 2008;135:1947. doi: 10.1242/dev.018044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dong Y.-F. Soung D.Y. Chang Y. Enomoto-Iwamoto M. Paris M. O'Keefe R.J. Schwarz E.M. Drissi H. Transforming growth factor-β and Wnt signals regulate chondrocyte differentiation through Twist1 in a stage-specific manner. Mol Endocrinol. 2007;21:2805. doi: 10.1210/me.2007-0199. [DOI] [PubMed] [Google Scholar]
- 42.Leucht P. Minear S. Ten Berge D. Nusse R. Helms J.A. Translating insights from development into regenerative medicine: the function of Wnts in bone biology. Semin Cell Dev Biol. 2008;19:434. doi: 10.1016/j.semcdb.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 43.Day T.F. Yang Y. Wnt and hedgehog signaling pathways in bone development. J Bone Joint Surg Am. 2008;90:19. doi: 10.2106/JBJS.G.01174. [DOI] [PubMed] [Google Scholar]