Abstract
Development of an alternative source of functional, transplantable β-cells to replace or supplement cadaveric tissue is critical to the future success of islet cell transplantation therapy. Embryonic pancreatic precursor cells are desirable as a renewable source of β-cells as they are both proliferative and inherently capable of pancreatic cell differentiation. We have previously shown that precursor cells undergo selective β-cell differentiation when dissociated and photoencapsulated in a polyethylene glycol (PEG) hydrogel network; however, these cells remained immature and were not glucose responsive. Collagen type 1 supports mature cell viability and function in many cell types and we hypothesized that incorporating it within our gels may support differentiating β-cells and facilitate β-cell maturation. For these studies, collagen-1 was entrapped with dissociated pancreatic precursor cells in a PEG hydrogel matrix (PEGCol) with the following key findings: (1) mature, glucose-responsive, islet-like structures differentiated from spontaneously forming precursor cell clusters in PEGCol, but not unmodified PEG, hydrogels; (2) a balance existed between providing sufficient collagen-1 signaling to support precursor cell development and providing an overabundance of adhesive sites allowing contaminating mesenchymal cells to thrive’ and (3) mechanical stability provided by the PEG hydrogel platform is important for successful precursor cell culture, as PEGCol hydrogels encourage glucose responsiveness and high-insulin gene expression, while pure collagen gel cultures, with the same collagen concentration, have negligible insulin gene expression. These results indicate that PEGCol hydrogels are a useful culture platform to promote differentiation of a glucose-responsive β-cell population from dissociated precursor cells.
Introduction
Islet cell transplantation has emerged as a potentially curative treatment for type 1 diabetes, a disease afflicting an estimated 500,000–1 million people in the United States; however, cadaveric tissue shortage remains a significant limitation for this therapy motivating the search for a renewable cell source.1–5 Embryonic pancreatic precursor cells are a potentially unlimited source of transplantable β-cells as they have been directed down the early stages of pancreatic development but remain proliferative and have the innate ability to differentiate into any of the cells found in the mature endocrine or exocrine pancreas. We have previously shown that dissociated precursor cells encapsulated in polyethylene glycol (PEG) hydrogels selectively differentiate into pancreatic β-cells, indicating that β-cell differentiation may be the default pathway in pancreatic development.6 One limitation of this system is that the differentiated cells in unmodified polyethylene glycol (PEG) hydrogels gels after 7 days of culture were immature and unable to release insulin in response to changes in media glucose concentrations. To build upon this encouraging foundation, we aim to move beyond using PEG hydrogels as a passive, protective three-dimensional culture platform by functionalizing the hydrogel with specific signaling molecules incorporated to selectively encourage desirable cell behavior, specifically the differentiation and maintenance of mature, glucose-responsive β-cells.
Collagen type 1 has been shown to support the survival and enhance differentiated function of many mature cell types, including smooth muscle cells, glomerular epithelial cells, and hepatocytes.7–10 When entrapped in PEG hydrogels along with neural precursor cells, collagen type 1 enhanced both cell viability and mature neuronal-specific differentiation.11 Further, collagen type 1 has proven beneficial for in vitro culture of pancreatic endocrine cells. Intact islets cultured in the presence of collagen-1 demonstrate enhanced survival and function, while blocking islet–matrix contacts with a disrupting β1 integrin antibody leads to a decrease in insulin gene expression and islet-cell apoptosis.12,13 Additionally, the presence of fibrillar collagen encouraged dedifferentiated pancreatic endocrine cells grown in monolayers to reestablish functional islet-like clusters.14,15
In this study, we explore the effect of entrapping collagen type 1 along with encapsulated embryonic pancreatic precursor cells in PEG hydrogels on precursor cell differentiation and maturation. The differentiation of entrapped cells was evaluated using gene expression analysis and immunohistochemistry, while functional maturity was determined by measuring insulin release in response to low- and high-glucose media. Hydrogels comprised of varying collagen concentrations were then tested to determine if an optimal collagen concentration could be identified to support β-cell differentiation, function, and maintenance. The results for PEG gels with entrapped collagen were then compared to both unmodified PEG hydrogels, providing structural integrity without the signaling molecule, and pure collagen gels, providing the signaling molecule without structural integrity, to determine if both structural integrity and collagen type 1 signaling capabilities would prove beneficial in a culture platform designed for the differentiation of embryonic pancreatic precursor cells into mature, glucose-responsive β-cells.
Materials and Methods
Pancreatic precursor cell isolation, encapsulation, and culture
PEG macromers were synthesized as previously described.6,16 Dorsal pancreatic buds were dissected from day 15 rat embryos, dissociated into single cells, and photoencapsulated into 7.5 wt% PEG hydrogels.6 Embryonic day 15 was selected because it is at the beginning of the secondary transition in rat pancreas development, where abundant multipotent precursor cells are present, but very little endocrine or exocrine differentiation has occurred.17 Final encapsulated cell concentration was 3 × 106 cells/mL. When collagen was entrapped in PEG hydrogel cultures, collagen type 1 was added to the precursor cell/PEG macromer solution to obtain final concentrations ranging from 0.25 to 1 mg/mL. PEGCol will be used to describe hydrogels with a collagen concentration of 0.5 mg/mL. For pure collagen gel cultures, cells were mixed with collagen (1 mg/mL final collagen concentration), and the collagen–cell solution was added to a 96-well conical bottom tissue culture plate, and gelation was allowed to proceed for 90 min before addition of sterile culture medium. Dissolution of degradable PEG and PEGCol hydrogels used in this study occurred in 12–14 days, and cell-loaded hydrogels were cultured for up to 10 days. Day 10 hydrogel mesh size for all PEG-based hydrogels used in this study (∼100Å), calculated using the volumetric swelling ratio as previously described,18 remained substantially smaller than collagen fibril diameter (30–70 nm),19 indicating stable collagen incorporation within PEGCol hydrogels over the entire culture period. This observation was corroborated via staining of day 10 hydrogels with an antibody directed against collagen.
Cells were maintained in RPMI-1640 culture medium (exchanged every 2–3 days) supplemented with 10% fetal bovine serum, 1% glutamate–penicillin–streptomycin, 0.5% fungizone (Sigma, St. Louis, MO), and 0.1% N2 for up to 10 days at 37°C. All medium components were purchased from Invitrogen (Carlsbad, CA) unless otherwise noted.
Cell differentiation
Gene expression by quantitative real-time polymerase chain reaction
Hydrogels were placed in TriReagent (Sigma) immediately following polymerization and on days 3, 7, and 10 of culture and stored at −20°C. RNA isolation, cDNA synthesis, polymerase chain reaction (PCR) amplification, and gene expression calculations were carried out as previously described.6 Total RNA was extracted from the hydrogel cultures via gel homogenization followed by standard phenol:chloroform extraction. DNase I treatment (Ambion, Austin, TX) was used to remove contaminating DNA, and the purified RNA was quantified using a RiboGreen assay (Invitrogen), after which all samples were diluted to 10 ng/μL. cDNA was generated from 100 ng purified RNA through reverse transcription (Reverse Transcription Kit; ABI, Foster City, CA) and stored at −20°C. Specific oligonucleotide sequences used for gene amplification have been published previously.6
Immunohistochemistry
Hydrogel cultures were fixed in 4% paraformaldehyde for 3–4 h followed by dehydration in a 30% sucrose solution. 40 μm gel sections were cut with a cryostat and processed with antibodies directed against insulin (β-cells; Sigma), glucagon (α-cells; Sigma), PDX-1 (undifferentiated precursor cells and differentiated β-cells; Abcam, Cambridge, MA), and vimentin (mesenchymal cells; Sigma) using standard immunohistochemical techniques. For PDX-1 antibody staining, slides with hydrogel sections were boiled in Retreivagen A solution (BD Biosciences, San Jose, CA) for 30 min and cooled to room temperature before staining. After primary antibody application, sections were rinsed and incubated with fluorophore-conjugated secondary antibodies (Molecular Probes, Carlsbad, CA). All images shown are projections of a series of 10–15 optical sections spaced 2 μm apart.
Cells staining positively for insulin, glucagon, PDX-1, or vimentin from both PEG and PEGCol day 0 sections were counted. This value was then compared to the total number of cells in the same section to determine the composition of the initial encapsulated cell population (≥20 sections from three or more hydrogels).
In addition to quantifying cell types in the initial cell population, tightly associated cell clusters greater than 20 μm in longest diameter (with nuclear staining alone) were also counted and their longest diameter was measured in sections from both PEG and PEGCol hydrogel conditions on days 0 and 7 (≥20 sections from three or more hydrogels for each condition). The number of clusters per hydrogel was estimated by using the conservative approximation that all clusters would appear in at most two consecutive 40 μm hydrogel sections. The number of sections/gel was calculated by dividing either the day 0 or 7 gel height by 40 μm. To determine the final cluster/gel estimate, the number of clusters/section was multiplied by the number of sections/gel and divided by 2 so that clusters appearing in consecutive sections would not be counted twice. Additionally, in sections stained with an antibody directed against insulin, it was noted whether or not the majority of cells (>50%) within a cluster stained positively for insulin. The preponderance of cells in a mature islet are insulin + β-cells, and therefore we used this criterion as an indication of islet-like differentiation. To better show collagen fiber distribution and continuity, intact hydrogels, as opposed to thin sections, were incubated with a monoclonal antibody directed against collagen type 1 (Sigma), followed by incubation with a fluorophore-conjugated secondary antibody. Images shown are a series of 21–25 optical sections spaced 15 μm apart.
Glucose challenge test
To determine if encapsulated precursor cells cultured in PEG, PEGCol, or collagen gels for 7 and 10 days were capable of glucose-stimulated insulin release (GSIR), hydrogels were incubated in either a low-glucose (1.1 mM) or high-glucose (16.7 mM) media. On days 7 and 10, hydrogel and collagen cultures were rinsed extensively then incubated for 2 h with either low- or high-glucose media, as previously described.6 Supernatant media insulin and C-peptide concentrations were measured using Rat Insulin and Rat C-peptide Elisa kits (Mercodia, Winston Salem, NC) according to the manufacturer's instructions.
Statistical analysis
Statistical significance was determined using two-tailed, unpaired Student's t-test with p < 0.05 significance. All data are presented as mean ± standard error of mean.
Results
Initial cell composition
PEG and PEGCol hydrogel discs fixed immediately following encapsulation of dissociated precursor cells (day 0) were sectioned and stained with antibodies directed against PDX-1, vimentin, insulin, and glucagon. As expected, cell populations in both the PEG and PEGCol conditions at this time were identical, and therefore images were used interchangeably for day 0 immunocytochemical analysis.
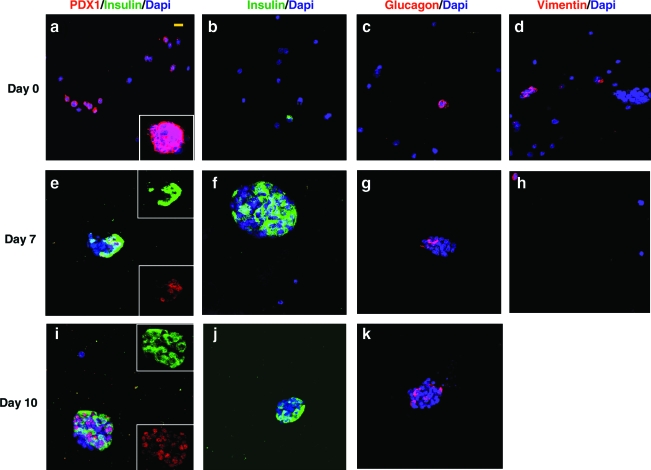
The majority of cells on day 0 stained positively for PDX-1 (65 ± 6%), a transcription factor present in undifferentiated pancreatic precursor cells that becomes restricted to maturing β-cells as precursor cell differentiation progresses.20,21 Another fraction of cells stained positively for vimentin (25 ± 3%), indicating the presence of mesenchymal cells (Table 1 and Fig. 1a, d). Only a few scattered insulin and glucagon cells were found (Fig. 1b, c), with each making up <1% of the initial encapsulated cell population.
Table 1.
Encapsulated Cell Composition at Day 0
| Antibody | Cell type | % positive staining |
|---|---|---|
| Anti-PDX-1 | Pancreatic precursor | 65 ± 6 |
| Anti-vimentin | Mesenchymal | 25 ± 3 |
| Anti-insulin | β-cell | <1 |
| Anti-glucagon | α-cell | <1 |
FIG. 1.
Confocal microscopy images of cells encapsulated in PEG hydrogels with entrapped collagen (PEGCol) on culture days 0 (a–d), 7 (e–h), and 10 (i–k). Images are of hydrogel sections incubated with antibodies directed against insulin and PDX-1 costained (a, e, i), insulin alone (b, f, j), glucagon (c, g, k), and vimentin (d, h). On day 0, 65 ± 5% of encapsulated cells stained positive for PDX-1 (a), including cell clusters (inset); however, no PDX-1+ cells were found to costain with insulin at this time. Insulin (b) and glucagon (c) staining was seen in only scattered single cells on day 0 and was not detected in cell clusters. On day 0, 25 ± 3% of the encapsulated cells were vimentin+ (d). Strong insulin and PDX-1 costaining was seen on both days 7 and 10 (e, i), where upper and lower insets show separated insulin and PDX-1 staining, respectively. Insulin staining was seen throughout the islet-like clusters present in PEGCol hydrogels at days 7 and 10 (f, j), while glucagon+ cells are predominantly found on the periphery of the clusters (g, k). By day 7 only a few scattered vimentin+ cells were present in PEGCol hydrogels (h) and none was found on day 10 (not shown). A 4′,6-diamidino-2-phenylindole (DAPI) nuclear counterstain was used in all images. Scale bar, 10 μm for all images. Color images available online at www.liebertonline.com/ten.
Differentiation of encapsulated pancreatic precursor cells in hydrogel cultures
PEG and PEGCol hydrogel cultures were followed for 10 days using a combination of immunohistochemistry and quantitative real-time PCR to determine the differentiation of encapsulated pancreatic precursor cells.
Immunohistochemistry
PEG and PEGCol hydrogels were sectioned and stained with antibodies directed against PDX-1, insulin, glucagon, and vimentin. Figure 1 shows representative images for PEGCol gels on days 0 (a–d), 7 (e–h), and 10 (i–k). Images from PEG hydrogels were in agreement with our previously published work and were not included in this manuscript.6 Upon initial encapsulation of the embryonic precursor cell population, PDX-1 + cells did not costain with insulin (Fig. 1a), indicating that these cells were undifferentiated pancreatic precursors. Only a few scattered insulin and glucagon + cells were seen on day 0 (Fig. 1b, c), while a larger fraction were vimentin + mesenchymal cells (Fig. 1d). In addition to single cells, spontaneously formed clusters of cells were also seen on day 0 and were comprised predominantly of PDX-1 + cells (Fig. 1a, inset). There were no insulin or glucagon + cells observed within these clusters on day 0. By days 7 and 10, however, the strongest insulin staining was observed within the cell clusters, while relatively weak insulin staining was observed in insulin + single cells in PEG and PEGCol cultures (not shown). No cell clusters were found remaining in unmodified PEG hydrogels. Insulin + cells within the clusters were also PDX-1 + (Fig. 1e, i), an expected result for differentiated β-cells. Dual staining was not observed in PEG hydrogels or single cells within PEGCol gels (not shown). Additionally, in PEGCol gels, glucagon + cells were found predominantly on the periphery of the clusters (Fig. 1g, k), similar to the pattern found in mature rodent islets22; however, glucagon staining was undetectable in PEG hydrogel cultures by day 7, consistent with previously published results.6 Although mesenchymal cells were a significant fraction of the initial cell population, only a few scattered cells remained vimentin + in PEGCol gels by day 7, and no vimentin + cells could be found on day 10 (not shown).
Gene expression analysis
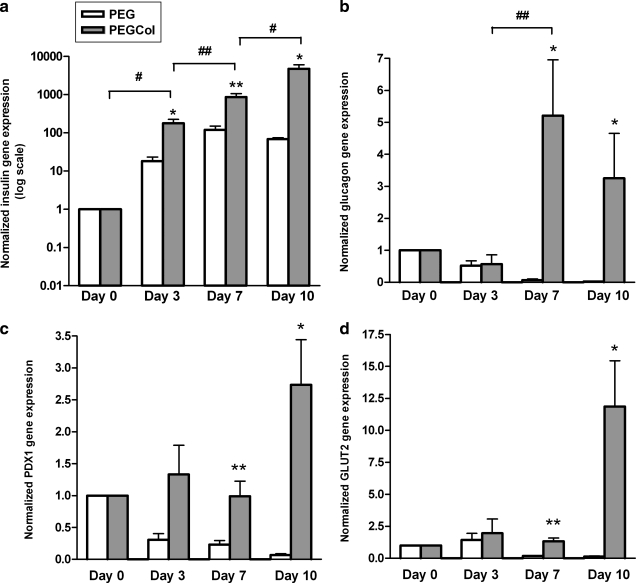
Quantitative real-time PCR was run on cell-loaded PEG and PEGCol hydrogels on culture days 0, 3, 7, and 10. Figure 2 shows relative fold-difference in insulin, glucagon, PDX-1, and Glut2 gene expression from day 0. Insulin gene expression is significantly greater in PEGCol hydrogel cultures compared to unmodified PEG cultures at all time points tested (Fig. 2a). Additionally, while relative insulin gene expression decreased in PEG hydrogels from days 7 to 10 (135- to 70-fold), insulin gene expression increased in PEGCol hydrogel cultures (800- to 4800-fold). Relative gene expression values for glucagon and PDX-1 were also significantly higher in PEGCol hydrogels when compared to time-matched PEG hydrogels on days 7 and 10. Insulin, glucagon, and PDX-1 gene expression results (Fig. 2) verified immunohistochemical images in Figure 1 and previously published work.6 Additionally, Glut2 gene expression levels were enhanced in PEGCol hydrogel cultures when compared to day 0 or time-matched PEG values. Glut2 is a glucose transporter found in the cell membrane of mature β-cells and is not only an important indicator of β-cell maturity but also is required for glucose-mediated insulin release. There was no evidence of exocrine differentiation in cell-loaded PEG or PEGCol hydrogels, as amylase gene expression was undetectable on culture days 7 and 10 (data not shown).
FIG. 2.
Real-time polymerase chain reaction obtained gene expression results for insulin (a), glucagon (b), PDX-1 (c), and GLUT2 (d) in both polyethylene glycol (PEG) hydrogels (white bars) and PEG with entrapped collagen (PEGCol) hydrogels (gray bars) over 10 days of culture. Insulin gene expression is shown on a log axis. All values are normalized to day 0. *p < 0.05 and **p < 0.01 compared to time-matched PEG hydrogel values. #p < 0.05 and ##p < 0.01 between indicated PEGCol time points.
Varying collagen composition in gel cultures
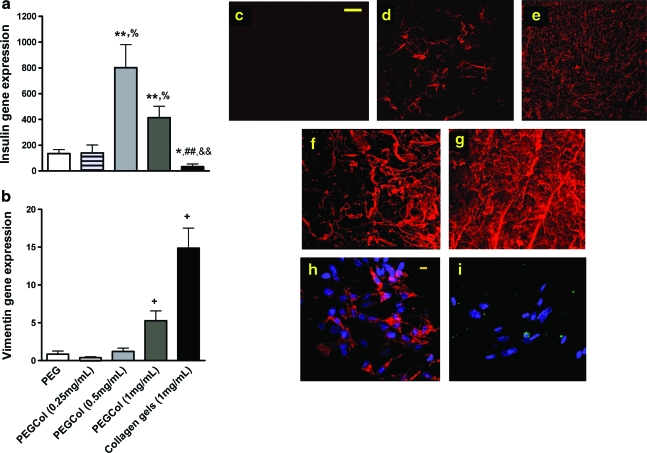
To determine if changes in collagen content in cell-loaded hydrogel cultures would alter insulin gene expression and β-cell differentiation, we encapsulated precursor cells in hydrogels with 0.25, 0.5, or 1 mg/mL collagen or in pure 1 mg/mL collagen gels. Interestingly, there was greater insulin gene expression in PEGCol (0.5 mg/mL) cultures when compared to all other conditions (Fig. 3a). Especially striking was the significant drop in insulin gene expression when changing culture platforms from the PEGCol (1 mg/mL) hydrogel to the pure 1 mg/mL collagen gel. Vimentin gene expression, however, was significantly higher in both pure collagen and PEGCol (1 mg/mL) cultures compared to time-matched PEG and PEGCol hydrogels (Fig. 3b), signifying an enhanced mesenchymal cell presence when collagen content was increased. To verify gene expression data, pure collagen gels at culture day 7 were sectioned and stained with antibodies directed against vimentin and insulin (Fig. 3h, i). The vast majority of cells cultured for 1 week in pure collagen gels were vimentin +mesenchymal cells, and only scattered single insulin + cells were detected.
FIG. 3.
Real-time polymerase chain reaction determined day 7 insulin (a) and vimentin (b) gene expression for PEG (white bars), PEGCol (0.25 mg/mL) (striped bars), PEGCol (0.5 mg/mL) (light gray bars), PEGCol (1 mg/mL) (dark gray bars), and 1 mg/mL collagen gels (black bars). All values are normalized to day 0. *p < 0.05 and **p < 0.01 compared to PEG hydrogel values. %p < 0.05 compared to PEGCol (0.25 mg/mL) hydrogel values. ##p < 0.01 compared to PEGCol (0.5 mg/mL). &&p < 0.01 compared to PEGCol (1 mg/mL) hydrogel values. +p < 0.05 compared to all other conditions. (c–g) Confocal microscopy images of PEG, (c) PEGCol (0.25 mg/mL), (d) PEGCol (0.5 mg/mL), (e) PEGCol (1 mg/mL), (f) and collagen (g) gels stained with an antibody directed against collagen type 1 (red). Scale bar, 100 μm. (h, i) Confocal microscopy image of cells in a 1 mg/mL collagen gel after 7 days of culture stained with antibodies directed against vimentin (h) and insulin (i). A 4′,6-diamidino-2-phenylindole (DAPI) nuclear counterstain was used. Scale bar, 10 μm. Color images available online at www.liebertonline.com/ten.
All gel conditions were also stained with an antibody directed against collagen type 1 for visual verification of collagen concentration and distribution (Fig. 3c–f).
No differences in measured volumetric swelling, mesh size, or compressive modulus were found between PEG and PEGCol gel conditions, indicating gene expression differences were due to collagen content and not changes in mechanical properties (data not shown). This result is not surprising since even at 1 mg/mL, collagen makes up only 0.1% of the total gel weight. The compressive modulus for a 1 mg/mL collagen gel was too low to be measured, demonstrating the lack of mechanical support provided to the pancreatic precursor cells entrapped within our pure collagen gels.
Functional β-cell differentiation in hydrogel and collagen gel cultures
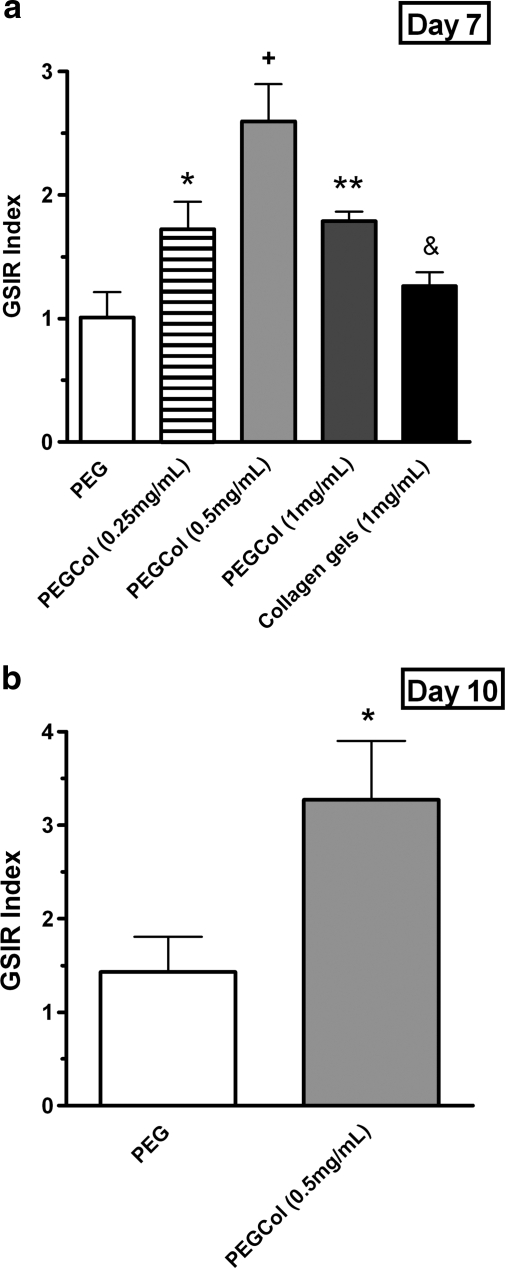
Functional differentiation of cells encapsulated in all culture platforms was tested on day 7 by incubating gels in low- and high-glucose media and measuring supernatant insulin content. High-glucose media insulin concentrations were normalized to low-glucose media insulin concentrations, resulting in GSIR values (Fig. 4a). Results in Figure 4a indicate a higher level of glucose responsiveness from the cell population in PEGCol (0.5 mg/mL) hydrogels (GSIR index of 2.6 ± 0.3) compared to all other culture platforms. Cells in PEGCol (0.25 and 1 mg/mL) gels did respond to high-glucose concentrations (GSIR index of 1.7 ± 0.2 and 1.8 ± 0.1, respectively), although to a lesser extent than PEGCol (0.5 mg/mL) cultures; however, cell populations cultured in unmodified PEG hydrogels and pure collagen gels were not glucose responsive. These results indicate that the decrease in insulin gene expression in PEGCol (1 mg/mL) hydrogels and pure collagen gels (Fig. 3a) was not simply a dilution effect due to the success of the mesenchymal cells and was instead an indication of decreased β-cell number or maturity compared to PEGCol (0.5 mg/mL) hydrogels.
FIG. 4.
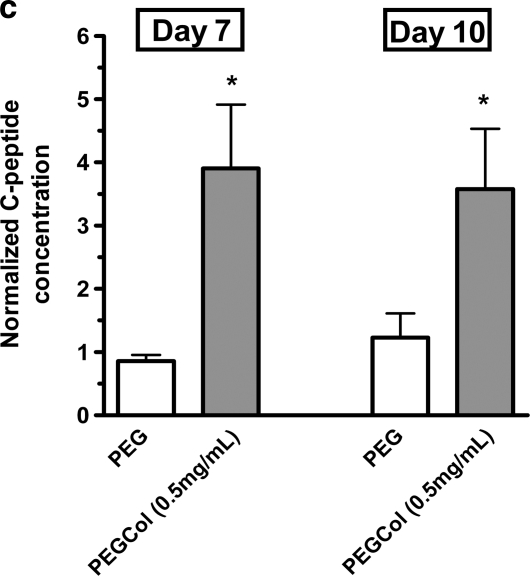
Insulin release from PEG, PEGCol (0.25 mg/mL), PEGCol (0.5 mg/mL), PEGCol (1 mg/mL), and 1 mg/mL collagen gels at 7 days of culture (a), PEG and PEGCol (0.5 mg/mL) at 10 days of culture (b), and C-peptide release for PEG and PEGCol (0.5 mg/mL) on days 7 and 10 of culture (c). Glucose-stimulated insulin release (GSIR) was calculated by normalizing the amount of insulin released in 16.7 mM glucose media to the amount of insulin released in 1.1 mM glucose media during a 2-h incubation. A GSIR value of 1 would indicate a culture that does not differentially release insulin in response to a higher glucose concentration. + designates statistical significance from all other conditions with p < 0.05 compared to PEGCol (0.25 and 1 mg/mL) gels and p < 0.005 compared to PEG and collagen gels. *p < 0.05 and **p < 0.01 compared to PEG hydrogel values. &p < 0.05 compared to PEGCol (1 mg/mL) hydrogels. (c) C-peptide release was also measured in low-glucose (1.1 mM) and high-glucose (16.7 mM) media and is presented as C-peptide concentration in high-glucose media normalized to the C-peptide concentration in low-glucose media. *p < 0.05 between low- and high-glucose concentrations.
To verify maintenance of a glucose-responsive cell population in PEGCol (0.5 mg/mL) gels, a glucose challenge test was performed on cell populations in both PEG and PEGCol hydrogels at 10 days of culture (Fig. 4b). PEGCol hydrogel cultures remain glucose responsive through day 10 (GSIR index of 3.3 ± 0.3), while PEG cultures were still unresponsive.
To ensure that released insulin was endogenously produced by the differentiating β-cells and not due to insulin uptake from media supplements, C-peptide concentrations in low- and high-glucose media were also measured on day 7 and 10 for PEG and PEGCol (0.5 mg/mL) cultures (Fig. 4c). Before secretory vesicle exocytosis, proinsulin is cleaved into insulin and C-peptide. C-peptide is therefore released in a 1:1 ratio with insulin and can be used to verify insulin synthesis within β-cells. Results in Figure 4c are shown as C-peptide concentrations measured in high-glucose media normalized to C-peptide concentrations measured in low-glucose media. These results corroborate insulin concentration measurements (Fig. 4a, b), with significantly more C-peptide released in high-glucose media when compared to low-glucose media for PEGCol (0.5 mg/mL) cultures on both days 7 and 10, and no differences between low-and high-glucose media concentrations for unmodified PEG hydrogels on either culture day were tested. Measurable C-peptide release that parallels the observed insulin release suggests that differentiating β-cells are releasing endogenously produced insulin.
Counts, sizes, and compositions of spontaneously formed clusters
The presence of islet-like clusters in PEGCol hydrogels on culture day 7, along with the conspicuous absence of similar clusters in unmodified PEG hydrogels, motivated an exploration of cell cluster number, size, and cell composition (Table 2). On day 0 there were no differences in cluster size or number between PEG and PEGCol hydrogels. The number of clusters remained constant in PEGCol hydrogels from days 0 to 7; however, in PEG hydrogels, there were no detectable clusters remaining after 1 week (Table 2). The average cluster size in PEGCol hydrogels increased from 36 to 51 μm from days 0 to 7 in PEGCol hydrogel cultures, potentially indicating a low level of proliferation within the clusters. However, as cells in clusters make up only a small fraction of the initial population (∼5%; estimated using single cell volume, cluster volumes, and encapsulated cell concentration), we were unable to detect any increases in DNA concentration to further verify proliferation (data not shown). Applying the same logic, it is not surprising that we were also unable to detect statistical differences when measuring DNA content in PEG and PEGCol hydrogel cultures even though clusters were lost by day 7 in unmodified PEG hydrogels, presumably due to apoptosis. As insulin + cells make up the majority of mature islets, we counted clusters in which greater than 50% of the cells were insulin + as an indication that these clusters were becoming islet-like. No cell clusters met this criterion in either PEG or PEGCol hydrogels on day 0 (Table 2). In fact, no cells within the spontaneously forming clusters were found to be insulin + at this initial time point and instead the vast majority of cells within these clusters stained positively for PDX-1 (Fig. 1a, inset), indicating that the clusters were comprised of undifferentiated, pancreatic precursor cells. By day 7, however, the majority of cells stained positive for insulin in every cell cluster in PEGCol hydrogels, indicating 100% differentiation of these spontaneously forming clusters into islet-like clusters (Fig. 1e, f).
Table 2.
Counts, Sizes, and Composition of Spontaneously Formed Clusters in PEG and PEGCol Hydrogels at Days 0 and 7
| Characteristic | Day | PEG | 0.5 mg/mL PEGCol |
|---|---|---|---|
| Cluster count (per hydrogel) | 0 | 39 ± 7 | 43 ± 5 |
| 7 | 0 | 38 ± 7 | |
| Average cluster size (high, low) (μm, largest diameter) | 0 | 41 (91.5, 20.6) | 36 (95.8, 20.2) |
| 7 | not applicable | 51a (100.6, 21.5) | |
| % clusters with >50% insulin + cells | 0 | 0 | 0 |
| 7 | not applicable | 100 |
p < 0.05 compared to day 0.
PEG, polyethylene glycol; PEGCol, PEG hydrogels with entrapped collagen type 1.
Discussion
Here we utilize biologically inert PEG hydrogels as mechanically supportive three-dimensional culture platforms to study the effects of collagen type 1 on encapsulated embryonic pancreatic precursor cells, a feat that would be impossible in Matrigel or collagen culture systems typically used to study the developing pancreas. We explored a variety of PEGCol hydrogel compositions, as well as pure PEG and pure collagen gels, to determine the hydrogel that would best support pancreatic precursor cell differentiation and maturation into glucose-responsive β-cells. Our results indicate a critical balance between providing sufficient numbers of collagen fibrils for adequate signaling to differentiating precursor cells and providing an overabundance of adhesive sites allowing for mesenchymal cell growth (Fig. 3). Increasing collagen content from pure PEG to PEGCol (0.5 mg/mL) enhances all measures of β-cell maturity (Figs. 2a, c, d and 3a) and function (Fig. 4a) without significantly enhancing mesenchymal cell growth (Fig. 3b); however, additional collagen (PEGCol 1 mg/mL) facilitates a significant increase in vimentin gene expression without a benefit to insulin gene expression (Fig. 3a, b) or glucose responsiveness (Fig. 4a). Providing mechanical stability with the PEG hydrogel platform appears to be important in maintaining a successful precursor cell population, as PEGCol (1 mg/mL) hydrogel cultures remain glucose responsive with a high level of insulin gene expression, while pure 1 mg/mL collagen gel cultures show negligible insulin gene expression (Figs. 3a and 4a). A higher concentration of collagen could be used to enhance mechanical stability in collagen gels; however, the overabundance of adhesive sites would still favor growth of the mesenchymal cells present in the initial cell population.
An interesting and important difference between PEG and PEGCol hydrogels was the survival and differentiation of spontaneously forming cell clusters found in both PEG and PEGCol gels immediately following polymerization. One possible explanation for spontaneous cluster formation is the presence of E-cadherin cell adhesion proteins on the surface of all epithelial cells of the developing and adult pancreas, including PDX-1 + embryonic pancreatic precursor cells.23 E-cadherin is known to mediate cell–cell interactions required for β-cell aggregation and islet formation in the developing pancreas,24 making it a likely facilitator of precursor cell clustering. On day 0 cluster number and size were the same in both gel conditions (Table 2), indicating that clusters formed in the medium before gelation via a collagen-independent mechanism. The same number of clusters remained in PEGCol gels on day 7 compared to day 0, indicating 100% cluster survival; however, no clusters survived in unmodified PEG hydrogels (Table 2). Cells within the clusters in PEGCol hydrogels were found to uniformly differentiate from groups of PDX-1 + /insulin− pancreatic precursor cells on day 0 (Table 1 and Fig. 1a, inset), to islet-like clusters on days 7 (Fig. 1e–g) and 10 (Fig. 1i–k), comprised mainly of cells costaining with insulin and PDX-1 and with glucagon + cells located primarily on the periphery. The majority of remaining single cells in both PEG and PEGCol hydrogels stained weakly for insulin on day 7 as previously described.6 As the presence or absence of clusters appeared to be the defining difference between the PEG and PEGCol conditions, enhanced insulin, glucagon, PDX-1, and Glut2 gene expression (Fig. 2a–d), as well as glucose responsiveness (Fig. 4), were all attributed to the presence of islet-like clusters in PEGCol hydrogels.
Collagen type 1 enhances mature islet viability and function,12,15,25 while blocking β1 integrin interactions between mature islets, and collagen type 1 decreases insulin gene expression and increases β-cell apoptosis.13 It appears as if the collagen in PEGCol hydrogels is providing the same necessary support to the differentiated islet-like clusters as it does to mature islets. Encapsulated cells in unmodified PEG hydrogels differentiate into insulin-expressing β-cells,6 but we believe maturing islet-like clusters are not provided the necessary signaling to maintain viability in the synthetic scaffold. This hypothesis is consistent with the fact that cell populations are weakly glucose responsive in PEG hydrogels on day 3, but lose this ability by day 7, indicating loss or dedifferentiation of maturing β-cells.6 When collagen is entrapped into the PEG matrix, the differentiating islet-like clusters maintain viability (Table 2), continue their maturation (Figs. 1 and 2), and become stably glucose responsive through 10 days of culture (Fig. 4). Our results indicate that immature, single cells may be more resilient to absent collagen–cell interactions as weakly staining single cells are present in both PEG6 and PEGCol cultures through day 7 (data not shown). These immature, PDX-1–negative β-cells are credited for the increase in insulin gene expression in PEG hydrogels through day 7 (Fig. 2a).
Although we have shown that the islet-like clusters have the characteristics of adult rodent islets, the GSIR index for day 10 PEGCol cultures (3.3 ± 0.3, Fig. 4b) is approximately 23% lower than reported values for mature rat islets (4.26 ± 0.34).26 One possible explanation is that the remaining single cells are contributing to basal insulin expression, but are not increasing their insulin release in response to high-glucose media conditions, a finding consistent with other published studies.27
In vivo, the development of mature islets begins as differentiated insulin + cells aggregate to form insulin cell clusters, while separately formed clusters of glucagon cells, originating from multihormonal cells,28 elongate and surround the insulin cell core.28,29 Because very little cell migration is expected to occur in hydrogels utilized in this study, in vitro development of undifferentiated PDX-1 + precursor cell clusters into islet-like structures within PEGCol hydrogels must proceed through a mechanism different from in vivo development. Previous studies demonstrated that induced clustering of adult pancreatic ductal epithelial cells enhanced β-cell differentiation and islet formation.30 Additionally, layering collagen or Matrigel on top of dissociated, dedifferentiated islet cells facilitates reaggregation of such cells into islet-like clusters,14,15,31 and islets which dedifferentiated while embedded within a collagen matrix have been made to reform islet-like clusters under specific culture conditions.32 Further, clusters of human fetal pancreatic cells differentiate in vivo into endocrine-rich islet-like clusters.33–35 Taken together, our work and previous findings demonstrate that clusters of un- or dedifferentiated cells have the inherent capability to dependably differentiate and organize into islet-like structures, with appropriate locations and numbers of α- and β-cells. One study hypothesized that cell–matrix contacts may direct non-β-cells toward the periphery of the reforming clusters; however, this theory was not explored further.15 Recapitulation of islet structures in our in vitro cultures is an important finding, as islet cell organization is crucial for islet function.36
In summary, results from this study highlight the permissive effect of entrapped collagen type 1 on both the differentiation of pancreatic precursor cell clusters into glucose-responsive, islet-like structures and the maintenance of differentiated clusters over several days in PEG hydrogel culture. Understanding how to generate a mature, glucose-responsive cell population from PDX-1 + precursor cells is an important step toward creating a cell source that can replace or augment cadaveric islet donors. Researchers working with embryonic stem cells are making advances toward the generation of a PDX-1 + precursor cell population, with the consensus that properly functioning islets must be generated through a cell type indistinguishable from pancreatic precursors found in the developing pancreatic bud.37–39 Recently, significant progress has been made toward the meaningful differentiation of human embryonic stem cells into functioning islets; however, the final stages of differentiation from a heterogeneous cell population highly enriched in PDX1 + pancreatic precursor-like cells to functioning islets have so far progressed in vivo.40 Transplanting a heterogeneous population of cells that are not fully differentiated poses potential risks to patients, as isolated pockets of unwanted ectoderm, mesoderm, and nonpancreatic endoderm have been found in vivo and contaminating undifferentiated, proliferative cells put the patient at risk for tumor formation.40,41 The research presented here focuses on the differentiation of PDX-1 + precursor cells into mature, functional islet-like clusters in vitro, which would alleviate much of the risk associated with the clinical use of embryonic tissue. Our study suggests a potential culture platform to facilitate the final differentiation step from clusters of undifferentiated PDX-1 + pancreatic precursor cells into mature, glucose-responsive, and transplantable islets. Further studies must be conducted to optimize the number of clusters formed and to test the capability of differentiated islet-like clusters to alleviate diabetes in animal models.
Acknowledgments
The project described was supported by Award Number F30DK081278 from the National Institute of Diabetes and Digestive and Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
Disclosure Statement
No competing financial interests exist.
References
- 1.Shapiro A.M. Lakey J.R. Ryan E.A. Korbutt G.S. Toth E. Warnock G.L. Kneteman N.M. Rajotte R.V. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 2.Ryan E.A. Lakey J.R. Paty B.W. Imes S. Korbutt G.S. Kneteman N.M., et al. Successful islet transplantation: continued insulin reserve provides long-term glycemic control. Diabetes. 2002;51:2148. doi: 10.2337/diabetes.51.7.2148. [DOI] [PubMed] [Google Scholar]
- 3.Lechner A. Habener J.F. Stem/progenitor cells derived from adult tissues: potential for the treatment of diabetes mellitus. Am J Physiol Endocrinol Metab. 2003;284:E259. doi: 10.1152/ajpendo.00393.2002. [DOI] [PubMed] [Google Scholar]
- 4.Serup P. Madsen O.D. Mandrup-Poulsen T. Islet and stem cell transplantation for treating diabetes. BMJ. 2001;322:29. doi: 10.1136/bmj.322.7277.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liao Y.H. Verchere C.B. Warnock G.L. Adult stem or progenitor cells in treatment for type 1 diabetes: current progress. Can J Surg. 2007;50:137. [PMC free article] [PubMed] [Google Scholar]
- 6.Mason M.N. Mahoney M.J. Selective beta-cell differentiation of dissociated embryonic pancreatic precursor cells cultured in synthetic PEG hydrogels. Tissue Eng Part A. 2008;15:1343. doi: 10.1089/ten.tea.2008.0290. [DOI] [PubMed] [Google Scholar]
- 7.Fassett J. Tobolt D. Hansen L. Type I collagen structure regulates cell morphology and EGF signaling in primary rat hepatocytes through cAMP-dependent protein kinase A. Mol Biol Cell. 2006;17:345. doi: 10.1091/mbc.E05-09-0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomez-Lechon M.J. Jover R. Donato T. Ponsoda X. Rodriguez C. Stenzel K.G., et al. Long-term expression of differentiated functions in hepatocytes cultured in three-dimensional collagen matrix. J Cell Physiol. 1998;177:553. doi: 10.1002/(SICI)1097-4652(199812)177:4<553::AID-JCP6>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 9.Koyama H. Raines E.W. Bornfeldt K.E. Roberts J.M. Ross R. Fibrillar collagen inhibits arterial smooth muscle proliferation through regulation of Cdk2 inhibitors. Cell. 1996;87:1069. doi: 10.1016/s0092-8674(00)81801-2. [DOI] [PubMed] [Google Scholar]
- 10.Schocklmann H.O. Lang S. Kralewski M. Hartner A. Ludke A. Sterzel R.B. Distinct structural forms of type I collagen modulate cell cycle regulatory proteins in mesangial cells. Kidney Int. 2000;58:1108. doi: 10.1046/j.1523-1755.2000.00268.x. [DOI] [PubMed] [Google Scholar]
- 11.Mahoney M.J. Anseth K.S. Contrasting effects of collagen and bFGF-2 on neural cell function in degradable synthetic PEG hydrogels. J Biomed Mater Res A. 2007;81:269. doi: 10.1002/jbm.a.30970. [DOI] [PubMed] [Google Scholar]
- 12.Wang R.N. Rosenberg L. Maintenance of beta-cell function and survival following islet isolation requires re-establishment of the islet-matrix relationship. J Endocrinol. 1999;163:181. doi: 10.1677/joe.0.1630181. [DOI] [PubMed] [Google Scholar]
- 13.Yashpal N.K. Li J. Wheeler M.B. Wang R. Expression of {beta}1 integrin receptors during rat pancreas development—sites and dynamics. Endocrinology. 2005;146:1798. doi: 10.1210/en.2004-1292. [DOI] [PubMed] [Google Scholar]
- 14.Lucas-Clerc C. Massart C. Campion J.P. Launois B. Nicol M. Long-term culture of human pancreatic islets in an extracellular matrix: morphological and metabolic effects. Mol Cell Endocrinol. 1993;94:9. doi: 10.1016/0303-7207(93)90046-m. [DOI] [PubMed] [Google Scholar]
- 15.Montesano R. Mouron P. Amherdt M. Orci L. Collagen matrix promotes reorganization of pancreatic endocrine cell monolayers into islet-like organoids. J Cell Biol. 1983;97:935. doi: 10.1083/jcb.97.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sawhney A. Pathak C. Hubbell J. Bioerodible hydrogels based on photopolymerized poly(ethylene glycol)-co-poly(alpha-hydroxy acid) diacrylate macromers. Macromolecules. 1993;26:581. [Google Scholar]
- 17.Rutter W.J. Pictet R.L. Harding J.D. Chirgwin J.M. MacDonald R.J. Przybyla A.E. An analysis of pancreatic development: role of mesenchymal factor and other extracellular factors. Symp Soc Dev Biol. 1978;35:205. doi: 10.1016/b978-0-12-612981-6.50019-3. [DOI] [PubMed] [Google Scholar]
- 18.Canal T. Peppas N.A. Correlation between mesh size and equilibrium degree of swelling of polymeric networks. J Biomed Mater Res. 1989;23:1183. doi: 10.1002/jbm.820231007. [DOI] [PubMed] [Google Scholar]
- 19.Cheema U. Chuo C. Sarathchandra P. Nazhat S. Brown R. Engineering functional collagen scaffolds: cyclical loading increases material strength and fibril aggregation. Adv Funct Mater. 2007;17:2426. [Google Scholar]
- 20.Le Lay J. Stein R. Involvement of PDX-1 in activation of human insulin gene transcription. J Endocrinol. 2006;188:287. doi: 10.1677/joe.1.06510. [DOI] [PubMed] [Google Scholar]
- 21.Edlund H. Developmental biology of the pancreas. Diabetes. 2001;50(Suppl 1):S5. doi: 10.2337/diabetes.50.2007.s5. [DOI] [PubMed] [Google Scholar]
- 22.Ge S. Crooks G.M. McNamara G. Wang X. Fluorescent immunohistochemistry and in situ hybridization analysis of mouse pancreas using low-power antigen-retrieval technique. J Histochem Cytochem. 2006;54:843. doi: 10.1369/jhc.5B6902.2006. [DOI] [PubMed] [Google Scholar]
- 23.Esni F. Stoffers D.A. Takeuchi T. Leach S.D. Origin of exocrine pancreatic cells from nestin-positive precursors in developing mouse pancreas. Mech Dev. 2004;121:15. doi: 10.1016/j.mod.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Dahl U. Sjodin A. Semb H. Cadherins regulate aggregation of pancreatic beta-cells in vivo. Development. 1996;122:2895. doi: 10.1242/dev.122.9.2895. [DOI] [PubMed] [Google Scholar]
- 25.Nagata N. Iwanaga A. Inoue K. Tabata Y. Co-culture of extracellular matrix suppresses the cell death of rat pancreatic islets. J Biomater Sci Polym Ed. 2002;13:579. doi: 10.1163/15685620260178418. [DOI] [PubMed] [Google Scholar]
- 26.Okamoto Y. Ishida H. Taminato T. Tsuji K. Kurose T. Tsuura Y., et al. Role of cytosolic Ca2 + in impaired sensitivity to glucose of rat pancreatic islets exposed to high glucose in vitro. Diabetes. 1992;41:1555. doi: 10.2337/diab.41.12.1555. [DOI] [PubMed] [Google Scholar]
- 27.Jaques F. Jousset H. Tomas A. Prost A.L. Wollheim C.B. Irminger J.C., et al. Dual effect of cell-cell contact disruption on cytosolic calcium and insulin secretion. Endocrinology. 2008;49:2494. doi: 10.1210/en.2007-0974. [DOI] [PubMed] [Google Scholar]
- 28.Larsson L.I. On the development of the islets of Langerhans. Microsc Res Tech. 1998;43:284. doi: 10.1002/(SICI)1097-0029(19981115)43:4<284::AID-JEMT2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 29.Oster A. Jensen J. Serup P. Galante P. Madsen O.D. Larsson L.I. Rat endocrine pancreatic development in relation to two homeobox gene products (Pdx-1 and Nkx 6.1) J Histochem Cytochem. 1998;6:707. doi: 10.1177/002215549804600602. [DOI] [PubMed] [Google Scholar]
- 30.Boretti M.I. Gooch K.J. Induced cell clustering enhances islet beta cell formation from human cultures enriched for pancreatic ductal epithelial cells. Tissue Eng. 2006;12:939. doi: 10.1089/ten.2006.12.939. [DOI] [PubMed] [Google Scholar]
- 31.Gao R. Ustinov J. Korsgren O. Otonkoski T. In vitro neogenesis of human islets reflects the plasticity of differentiated human pancreatic cells. Diabetologia. 2005;48:2296. doi: 10.1007/s00125-005-1935-8. [DOI] [PubMed] [Google Scholar]
- 32.Jamal A. Lipsett M. Sladek R. Laganiere S. Hanley S. Rosenberg L. Morphogenetic plasticity of adult human pancreatic islets of Langerhans. Cell Death Differ. 2005;12:702. doi: 10.1038/sj.cdd.4401617. [DOI] [PubMed] [Google Scholar]
- 33.Beattie G.M. Levine F. Mally M.I. Otonkoski T. O'Brien J.S. Salomon D.R., et al. Acid beta-galactosidase: a developmentally regulated marker of endocrine cell precursors in the human fetal pancreas. J Clin Endocrinol Metab. 1994;78:1232. doi: 10.1210/jcem.78.5.8175983. [DOI] [PubMed] [Google Scholar]
- 34.Otonkoski T. Beattie G.M. Mally M.I. Ricordi C. Hayek A. Nicotinamide is a potent inducer of endocrine differentiation in cultured human fetal pancreatic cells. J Clin Invest. 1993;92:1459. doi: 10.1172/JCI116723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beattie G.M. Rubin J.S. Mally M.I. Otonkoski T. Hayek A. Regulation of proliferation and differentiation of human fetal pancreatic islet cells by extracellular matrix, hepatocyte growth factor, and cell-cell contact. Diabetes. 1996;45:1223. doi: 10.2337/diab.45.9.1223. [DOI] [PubMed] [Google Scholar]
- 36.Unger R.H. Orci L. Glucagon and the A cell: physiology and pathophysiology (first two parts) N Engl J Med. 1981;304:1518. doi: 10.1056/NEJM198106183042504. [DOI] [PubMed] [Google Scholar]
- 37.D'Amour K.A. Bang A.G. Eliazer S. Kelly O.G. Agulnick A.D. Smart N.G., et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 38.Kahan B.W. Jacobson L.M. Hullett D.A. Ochoada J.M. Oberley T.D. Lang K.M., et al. Pancreatic precursors and differentiated islet cell types from murine embryonic stem cells: an in vitro model to study islet differentiation. Diabetes. 2003;52:2016. doi: 10.2337/diabetes.52.8.2016. [DOI] [PubMed] [Google Scholar]
- 39.Shiraki N. Yoshida T. Araki K. Umezawa A. Higuchi Y. Goto H., et al. Guided differentiation of embryonic stem cells into Pdx1-expressing regional-specific definitive endoderm. Stem Cells. 2008;26:874. doi: 10.1634/stemcells.2007-0608. [DOI] [PubMed] [Google Scholar]
- 40.Kroon E. Martinson L.A. Kadoya K. Bang A.G. Kelly O.G. Eliazer S., et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;6:443. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 41.Shi Y. Hou L. Tang F. Jiang W. Wang P. Ding M., et al. Inducing embryonic stem cells to differentiate into pancreatic beta cells by a novel three-step approach with activin A and all-trans retinoic acid. Stem Cells. 2005;23:656. doi: 10.1634/stemcells.2004-0241. [DOI] [PubMed] [Google Scholar]