Abstract
Contrast-enhanced ultrasound (CEUS) has unique advantages over contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) in the characterization of hepatic tumors. These include the capability of real-time dynamic imaging depicting the enhancement pattern of tumors regardless of its rapidity, purely intravascular properties of the microbubble contrast agents more consistently demonstrating washout of malignancy, and capability of repetitive observation of tumor vascularity with multiple injections of microbubbles with an excellent safety profile and no nephrotoxicity. For an indeterminate mass detected on an ultrasound scan, an immediate benign diagnosis reduces the necessity of costly further imaging as well as patients’ anxiety and an immediate malignant diagnosis prompts the proper work-up and management. CEUS is often served as a problem-solving tool for indeterminate lesions on prior CT or MRI scans, obviating further invasive steps. CEUS offers excellent visualization of peripheral nodular enhancement in even flash-filling or very slow-filling hemangiomas. Careful observation of early arterial filling pattern is helpful in the differentiation of focal nodular hyperplasia versus adenoma. Hepatocellular carcinoma is typically characterized by arterial hypervascularity and often late, partial washout. Metastasis shows brief arterial hypervascularity and complete rapid washout, which can improve its detection during a portal phase survey. The washout phenomenon of malignant tumors in general is useful to differentiate them from benign lesions.
Keywords: Ultrasound, liver tumors, liver neoplasms, contrast-enhanced ultrasound, microbubbles
Introduction
non-invasive characterization of hepatic tumors is largely based on their enhancement patterns on contrast-enhanced imaging. There has been increasing consensus that microbubble contrast-enhanced ultrasound (CEUS) with low-mechanical index (MI) imaging techniques is an excellent tool for assessment of the vascular characteristics of focal hepatic lesions and current results indicate great potential in the detection and characterization of hepatic tumors.
CEUS has several advantages over contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) in the evaluation of hepatic tumors[1]. First, CEUS provides real-time dynamic imaging, useful to visualize a very early or late enhancement pattern of tumors that may not occur at the predetermined timing of CT or MRI scans. Second, unique intravascular properties of the microbubbles are often beneficial for CEUS to characterize malignant tumors with increased vascular permeability and a large interstitial space; CEUS demonstrates the washout phenomenon clearly and consistently, whereas CT or MRI may show prolonged enhancement due to contrast leakage into the tumor interstitium. Third, multiple injections of microbubbles are allowed and repetitive observation of tumor enhancement pattern is possible in a single CEUS examination. Fourth, in addition to the excellent safety profile with a low rate of adverse reactions, microbubble contrast agents can be used in patients with decreased renal function who are not suitable for contrast-enhanced CT or MRI[2].
This article illustrates the findings on CEUS of commonly encountered benign and malignant hepatic tumors with an emphasis on unique capability and appropriate indications of CEUS in the detection and characterization of hepatic tumors.
Hemangioma
The enhancement pattern of hemangioma on CEUS is similar to that seen on CT or MRI[3–5]. In the arterial phase, hemangiomas usually demonstrate strong, nodular enhancement at the periphery of the lesion (Fig. 1). The pools of enhancement expand in a centripetal pattern during the portal venous phase and beyond, progressing to complete fill-in of the lesion except areas of thrombosis or fibrosis. Sustained enhancement through the portal venous phase and beyond is a prerequisite for confident diagnosis[6]; however, hemangiomas infrequently show an echogenicity slightly lower than that of liver (washout) in the late phase. The diagnostic accuracy of hemangioma on CEUS is comparable with that of MRI[7–9] including those of very small size, removing the necessity for further imaging for confirmation of diagnosis when CEUS shows characteristic findings.
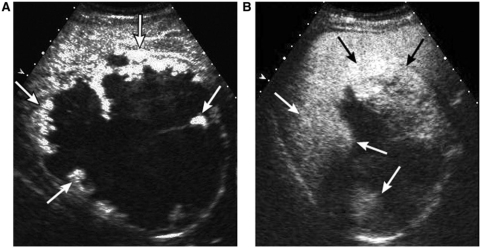
Figure 1.
A 37-year-old female with a giant hemangioma. A contrast-enhanced sonogram shows a mass with peripheral nodular enhancement (arrows) on the arterial phase (A) and gradual centripetal filling-in of enhancement (arrows) on the portal venous phase (B).
The rapidity of enhancement of hemangioma varies greatly. A rapid-filling hemangioma is frequently seen as a complete enhancing nodule without the appearance of a peripheral nodular enhancement in the arterial phase of the CT scan. In these cases, it may be difficult to make a specific diagnosis of hemangioma because a hepatocellular carcinoma (HCC) or hypervascular metastasis may show similar findings. On the other hand, slow-filling hemangiomas may be seen as indeterminate hypoattenuating lesions in the arterial and portal venous phases of the CT scan. High temporal resolution and the real-time nature of CEUS often enable depiction of very early or very late nodular enhancement initiating from the periphery regardless of its rapidity, providing a confident diagnosis (Fig. 2)[4].
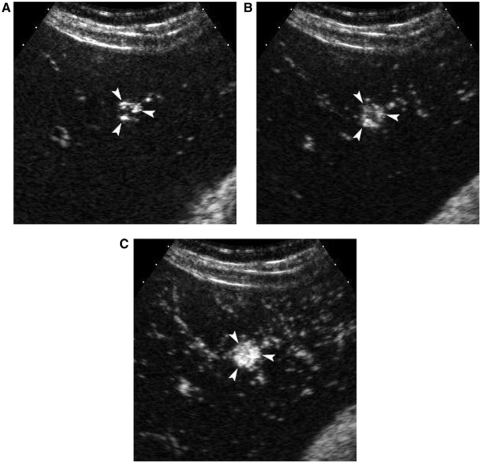
Figure 2.
A 37-year-old female with a small fast-filling hemangioma. Serial contrast-enhanced sonograms at (A) 6 s, (B) 7 s and (C) 8 s show peripheral nodular enhancement with rapid filling-in of the nodule (arrowheads).
Focal nodular hyperplasia
Focal nodular hyperplasia (FNH) is proliferation of non-neoplastic hepatocytes with an abnormal arrangement and is frequently associated with a central fibrous scar and anomalous arteries[10]. FNH most commonly occurs in young asymptomatic females. Differentiation from hepatic adenoma and hypervascular malignancies such as HCC is important. In contrast to other significant masses, FNH is managed conservatively irrespective of its size because it is not at risk for malignant transformation or bleeding[11].
On CEUS, FNH typically shows strong, brisk, homogeneous enhancement in the arterial phase. CEUS frequently demonstrates central stellate arteries in the early arterial phase[12,13], and the direction of intratumoral enhancement is typically centrifugal, from the center to the periphery over time. Maximum intensity processing techniques are particularly useful to visualize central stellate arteries[14]. FNH mostly shows sustained enhancement in the portal phase; however, mild negative enhancement of the lesion compared with the liver in the portal venous phase is sometimes seen. The central scar is frequently seen as a hypoechoic area within the lesion, more conspicuously in the portal venous phase than in the arterial phase[12]. The central scar may be disproportionately large in a small FNH and may mimic a central washout pattern of malignancy. In these cases, recognition of initial arterial vascular morphology and sustained enhancement of periphery is important for the correct diagnosis of FNH.
Adenoma
Hepatic adenoma is a true neoplasm caused by proliferative stimulation of hepatocytes. Its association with the use of oral contraceptives and glycogen storage disease is well known. Surgical resection or local ablation therapy is advocated in the case of a large adenoma to reduce the risk of rupture and malignant degeneration[11].
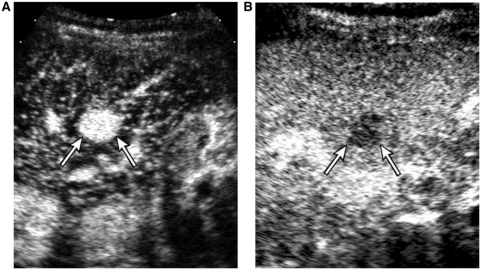
Adenomas have clinical and imaging similarities to FNH; mostly found in women of child-bearing age and hypervascular in the arterial phase. On CEUS, the majorities of adenomas, which are detected incidentally, show homogeneous arterial enhancement. A large lesion can show heterogeneous enhancement. Intratumoral non-enhancing areas which may represent necrosis or hemorrhage are occasionally seen and may be mistaken as a central scar of FNH if they are central in location. Adenomas usually show sustained enhancement in the portal venous phase, but occasionally show mild washout more commonly than FNH. Careful observation of the early arterial phase filling pattern is important to characterize these masses; adenoma typically shows peritumoral arteries with centripetal or diffuse filling of intratumoral enhancement (Fig. 3), in contrast to a central stellate artery with centrifugal filling of FNH (Fig. 4)[12]. Real-time evaluation with high temporal resolution of CEUS allows visualization of the arterial phase filling pattern, which occurs within a few seconds.
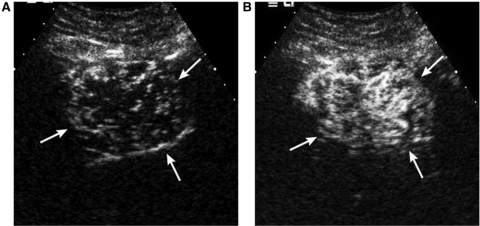
Figure 3.
A 45-year-old female with hepatic adenoma. Contrast-enhanced sonograms. The mass shows tumor vessels initiating from the periphery (arrows in (A)) at 6 s (A) with centripetal filling of intratumoral enhancement at 7 s delay (B).
Figure 4.
A 37-year-old female with FNH. (A) Contrast-enhanced sonogram at 8 s delay shows a mass (arrows) with central stellate arteries (arrowhead). (B) At 11 s delay, note the centrifugal progression of enhancement of the mass (arrows).
Complex cyst
CEUS is useful to characterize indeterminate complex cysts. Neoplastic cysts such as cystic metastasis or biliary cystic neoplasm can be clearly characterized on CEUS by sensitive real-time demonstration of vascular flow within the septa or solid component. Non-neoplastic complex cysts such as hemorrhagic cysts or hydatid cysts show the absence of intralesional enhancement on CEUS, confirming their non-neoplastic nature[4].
Hepatocellular carcinoma
CEUS has recently been established as a useful imaging technique in the characterization of HCCs and their differentiation from various nodules related to cirrhosis[9,15,16]. Evaluation of the blood supply in a hepatocellular nodule is extremely important for characterization of the lesion since there are sequential changes in the supplying vessels and hemodynamic state during hepatocarcinogenesis. The real-time capability of CEUS along with high contrast resolution makes it feasible to focus on a small indeterminate nodule from wash-in to washout of contrast and also provide better understanding of complex hemodynamic changes of a nodule. The contribution of CEUS to the imaging of livers at risks for HCC is most significant in the following areas: immediate confirmation of hemangioma detected on screening sonography reduces further costly investigation as well as patient anxiety; exclusion of infiltrative HCC or multifocal HCCs when baseline sonography shows extremely coarse liver or indeterminate portal venous thrombosis; and further characterization of indeterminate small nodules seen on the prior CT or MR scan[17].
The enhancement patterns of HCC are linked to the degree of histologic differentiation. The majority of moderately differentiated HCCs show classic enhancement features of arterial phase hypervascularity and later washout; well-differentiated and poorly differentiated HCC account for the majority of iso- or hypovascular variations of enhancement. Extended observation over 3 min is important to characterize HCC by demonstrating eventual washout, as more than half of the washout occurs after a 90-s delay (Fig. 5). Absence of portal venous washout is rare in advanced HCC. However, lack of washout should not be considered diagnostic of a benign lesion in a cirrhotic liver since approximately half of well-differentiated tumors fail to show washout[15].
Figure 5.
A 74-year-old female with HCC. (A) Contrast-enhanced sonogram at 24 s delay shows hypervascularity of the nodule (arrows). (B) Late washout of the mass typical of HCC is seen at 169 s delay (arrows).
Non-specific small arterial enhancing foci with no washout are frequently seen on CT and MRI in liver cirrhosis and studies have shown that the majority of them are not HCC[18,19]. On the other hand, on CEUS, without features of hemangioma, arterial hypervascularity alone raises a high suspicion of HCC in the setting of cirrhosis. Hypervascular pseudolesions are not seen on baseline scan and therefore do not cause pseudolesions on CEUS, as CEUS is generally performed for a targeted lesion identified on a baseline scan[9].
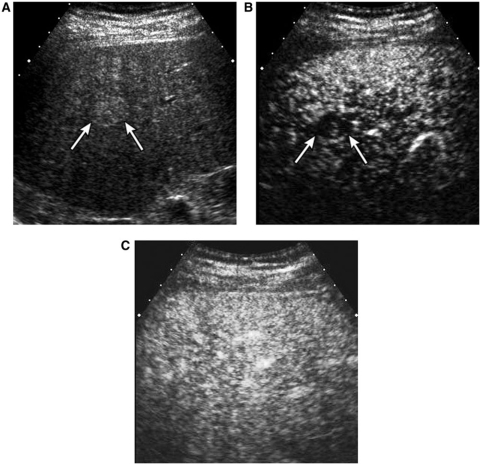
Differential diagnosis between HCC and benign cirrhosis-related nodules is very important clinically. There are significant overlaps of vascular supply between dysplastic nodules and well-differentiated HCCs. CEUS, CT, and MRI all suffer from similar problems in the imaging of these nodules. Regenerative and dysplastic nodules generally show no hypervascularity in the arterial phase. They are usually isoechoic to the parenchyma during all phases on CEUS with or without transient arterial hypovascularity (Fig. 6). They may show mild negative enhancement during the portal venous phase. However, increasing hypoechogenicity, or progressive washout, in the portal venous phase even in the absence of prior arterial hypervascularity is a highly suspicious finding for HCC[15].
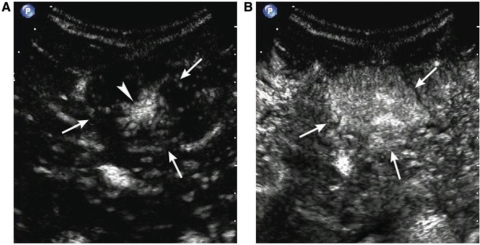
Figure 6.
A 39-year-old male with a regenerative nodule. (A) Oblique sonogram shows a well-defined hyperechoic nodule (arrows). (B) Contrast-enhanced sonogram at 13 s delay shows transient arterial hypovascularity of the nodule (arrows). (C) The nodule is isoechoic to surrounding liver parenchyma in the portal phase.
Peripheral cholangiocarcinoma (CCA)
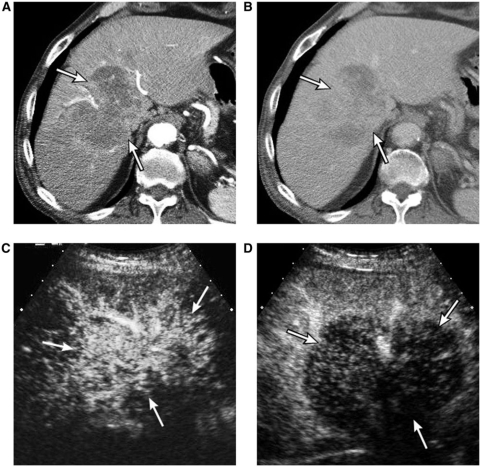
The enhancement pattern on imaging is similar to metastatic adenocarcinomas as is the microscopic morphology. On dynamic CT or MRI scan, marked hypoattenuation or hypointensity with thin, incomplete peripheral enhancement in the arterial phase and peripheral washout (areas of viable cancer cells) with centripetal progression of enhancement over time (fibrous component) are typical. On CEUS, on the other hand, CCA shows rapid and complete washout within 60 s regardless of arterial phase vascularity[17]. CCA is one of the lesions that can produce the most striking discordance in enhancement patterns with CT or MRI, probably attributable to its exuberant fibrous component (Fig. 7)[1].
Figure 7.
An 82-year-old male with cholangiocarcinoma. (A) Arterial phase CT scan shows a large mass (arrows) with irregular peripheral enhancement. (B) Three-minute delay phase CT scan shows progression of enhancement within the mass (arrows). (C) Contrast-enhanced sonogram at 19 s delay shows hypervascularity of the mass (arrows). (D) Contrast-enhanced sonogram at 34 s delay shows early complete washout of enhancement of the mass (arrows), discordant with the CT scan (B).
Metastasis
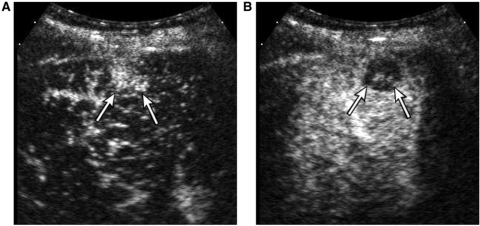
Hepatic metastases in general are considered hypovascular on dynamic CT and MRI. On CEUS, however, metastases typically show very brief arterial enhancement, peripheral or diffuse, followed by rapid washout in the early portal phase (Fig. 8). The mean peak enhancement of metastasis on CEUS is reported at 15 s delay and washout commences earlier than 25 s delay after the bolus injection[20]. The capability of CEUS to depict such a rapid dynamic change is largely attributed to the real-time scanning capability and high temporal resolution; arterial phase images obtained at a single time point on CT and MRI often miss the early enhancement[1]. The sensitive detection of arterial vascularity in metastases suggests the potential usefulness of CEUS in the therapeutic assessment of metastasis after local ablation, which is often difficult on dynamic CT or MRI due to the hypoattenuating or hypointense appearance of recurrent tumor similar to that of an ablated zone.
Figure 8.
A 57-year-old male with metastasis from colon cancer. Contrast-enhanced sonogram at (A) 17 s and (B) 36 s delay demonstrates clear arterial enhancement of a nodule (arrows in (A)) and rapid washout (arrows in (B)), characteristic of metastasis.
In contrast to the tendency of late and variable washout of HCC, rapid and complete washout is an invariable characteristic of metastasis on CEUS. This is in contrast to CT and MRI in which the intravenous contrast leaks out into the interstitium of some fibrotic tumors leading to positive enhancement in the venous or delayed phases. Those consistent enhancement features of metastases are also helpful in distinguishing metastasis from benign lesions especially in the characterization of small indeterminate lesion on CT or MRI in the oncologic patient. When a nodule shows sustained enhancement on CEUS, metastasis is virtually excluded.
Detection of malignant tumors
Many studies have demonstrated that the use of microbubble contrast substantially improves the detection of metastases compared with unenhanced ultrasound[21–23]. As virtually all metastases show early complete washout and are seen as punched out defects contrasted with the well-perfused background liver on CEUS, liver survey during portal venous phase is very effective for the detection of additional small metastases which may not seen on unenhanced ultrasound or prior imaging. There may be a role for CEUS in the surveillance of hepatic metastasis. However, it is not certain at this point if CEUS could serve as a standard imaging technique for the staging or follow-up for metastases, because of the relatively limited evaluation of extrahepatic findings and the relative difficulty of one-on-one comparison on follow-up scans.
In contrast with metastasis, it is difficult to confirm or exclude an additional HCC during a portal venous phase liver survey in a cirrhotic liver. A combination of a tendency for late washout of HCC and the relatively poor portal venous enhancement of a background cirrhotic liver, makes the washout of HCC less conspicuous than that of metastasis[15].
Conclusion
With several unique capabilities, CEUS has become an important non-invasive tool in characterizing both benign and malignant hepatic tumors, when found as an indeterminate lesion either on baseline sonography or on previous CT or MRI. Liver survey in the portal venous phase of CEUS is effective in the additional detection of hepatic metastasis, although it appears to be still challenging for HCC.
This paper is available online at http://www.cancerimaging.org. In the event of a change in the URL address, please use the DOI provided to locate the paper.
References
- 1.Wilson SR, Kim TK, Jang HJ, Burns PN. Enhancement patterns of focal liver masses: discordance between contrast-enhanced sonography and contrast-enhanced CT and MRI. AJR Am J Roentgenol. 2007;189:W7–12. doi: 10.2214/AJR.06.1060. doi:10.2214/AJR.06.1060. PMid:17579140. [DOI] [PubMed] [Google Scholar]
- 2.Piscaglia F, Bolondi L. The safety of Sonovue in abdominal applications: retrospective analysis of 23188 investigations. Ultrasound Med Biol. 2006;32:1369–75. doi: 10.1016/j.ultrasmedbio.2006.05.031. doi:10.1016/j.ultrasmedbio.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 3.Brannigan M, Burns PN, Wilson SR. Blood flow patterns in focal liver lesions at microbubble-enhanced US. Radiographics. 2004;24:921–35. doi: 10.1148/rg.244035158. doi:10.1148/rg.244035158. PMid:15256618. [DOI] [PubMed] [Google Scholar]
- 4.Kim TK, Jang HJ, Wilson SR. Benign liver masses: imaging with microbubble contrast agents. Ultrasound Q. 2006;22:31–9. [PubMed] [Google Scholar]
- 5.Quaia E, Calliada F, Bertolotto M, et al. Characterization of focal liver lesions with contrast-specific US modes and a sulfur hexafluoride-filled microbubble contrast agent: diagnostic performance and confidence. Radiology. 2004;232:420–30. doi: 10.1148/radiol.2322031401. doi:10.1148/radiol.2322031401. PMid:15286314. [DOI] [PubMed] [Google Scholar]
- 6.Wilson SR, Burns PN. An algorithm for the diagnosis of focal liver masses using microbubble contrast-enhanced pulse-inversion sonography. AJR Am J Roentgenol. 2006;186:1401–12. doi: 10.2214/AJR.04.1920. doi:10.2214/AJR.04.1920. PMid:16632737. [DOI] [PubMed] [Google Scholar]
- 7.Lee JY, Choi BI, Han JK, Kim AY, Shin SH, Moon SG. Improved sonographic imaging of hepatic hemangioma with contrast-enhanced coded harmonic angiography: comparison with MR imaging. Ultrasound Med Biol. 2002;28:287–95. doi: 10.1016/s0301-5629(01)00511-7. doi:10.1016/S0301-5629(01)00511-7. [DOI] [PubMed] [Google Scholar]
- 8.Dietrich CF, Mertens JC, Braden B, Schuessler G, Ott M, Ignee A. Contrast-enhanced ultrasound of histologically proven liver hemangiomas. Hepatology. 2007;45:1139–45. doi: 10.1002/hep.21615. doi:10.1002/hep.21615. PMid:17464990. [DOI] [PubMed] [Google Scholar]
- 9.Jang HJ, Kim TK, Wilson SR. Small nodules (1–2 cm) in liver cirrhosis: characterization with contrast-enhanced ultrasound. Eur J Radiol. 2008 doi: 10.1016/j.ejrad.2008.08.011. ; doi:10.1016/j.ejrad.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Wanless IR, Mawdsley C, Adams R. On the pathogenesis of focal nodular hyperplasia of the liver. Hepatology. 1985;5:1194–200. doi: 10.1002/hep.1840050622. doi:10.1002/hep.1840050622. PMid:4065824. [DOI] [PubMed] [Google Scholar]
- 11.Cobey FC, Salem RR. A review of liver masses in pregnancy and a proposed algorithm for their diagnosis and management. Am J Surg. 2004;187:181–91. doi: 10.1016/j.amjsurg.2003.11.016. doi:10.1016/j.amjsurg.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 12.Kim TK, Jang HJ, Burns PN, Murphy-Lavallee J, Wilson SR. Focal nodular hyperplasia and hepatic adenoma: differentiation with low-mechanical-index contrast-enhanced sonography. AJR Am J Roentgenol. 2008;190:58–66. doi: 10.2214/AJR.07.2493. doi:10.2214/AJR.07.2493. PMid:18094294. [DOI] [PubMed] [Google Scholar]
- 13.Dietrich CF, Schuessler G, Trojan J, Fellbaum C, Ignee A. Differentiation of focal nodular hyperplasia and hepatocellular adenoma by contrast-enhanced ultrasound. Br J Radiol. 2005;78:704–7. doi: 10.1259/bjr/88181612. doi:10.1259/bjr/88181612. PMid:16046421. [DOI] [PubMed] [Google Scholar]
- 14.Wilson SR, Jang HJ, Kim TK, Iijima H, Kamiyama N, Burns PN. Real-time temporal maximum-intensity-projection imaging of hepatic lesions with contrast-enhanced sonography. AJR Am J Roentgenol. 2008;190:691–5. doi: 10.2214/AJR.07.3116. doi:10.2214/AJR.07.3116. PMid:18287440. [DOI] [PubMed] [Google Scholar]
- 15.Jang HJ, Kim TK, Burns PN, Wilson SR. Enhancement patterns of hepatocellular carcinoma at contrast-enhanced US: comparison with histologic differentiation. Radiology. 2007;244:898–906. doi: 10.1148/radiol.2443061520. doi:10.1148/radiol.2443061520. PMid:17709836. [DOI] [PubMed] [Google Scholar]
- 16.Nicolau C, Catala V, Vilana R, et al. Evaluation of hepatocellular carcinoma using SonoVue, a second generation ultrasound contrast agent: correlation with cellular differentiation. Eur Radiol. 2004;14:1092–9. doi: 10.1007/s00330-004-2298-0. doi:10.1007/s00330-004-2298-0. PMid:15007620. [DOI] [PubMed] [Google Scholar]
- 17.Jang HJ, Kim TK, Wilson SR. Imaging of malignant liver masses: characterization and detection. Ultrasound Q. 2006;22:19–29. [PubMed] [Google Scholar]
- 18.Kim TK, Choi BI, Han JK, Chung JW, Park JH, Han MC. Nontumorous arterioportal shunt mimicking hypervascular tumor in cirrhotic liver: two-phase spiral CT findings. Radiology. 1998;208:597–603. doi: 10.1148/radiology.208.3.9722834. [DOI] [PubMed] [Google Scholar]
- 19.O'Malley ME, Takayama Y, Sherman M. Outcome of small (10–20 mm) arterial phase-enhancing nodules seen on triphasic liver CT in patients with cirrhosis or chronic liver disease. Am J Gastroenterol. 2005;100:1523–8. doi: 10.1111/j.1572-0241.2005.41814.x. doi:10.1111/j.1572-0241.2005.41814.x. PMid:15984975. [DOI] [PubMed] [Google Scholar]
- 20.Murphy-Lavallee J, Jang HJ, Kim TK, Burns PN, Wilson SR. Are metastases really hypovascular in the arterial phase? The perspective based on contrast-enhanced ultrasonography. J Ultrasound Med. 2007;26:1545–56. doi: 10.7863/jum.2007.26.11.1545. [DOI] [PubMed] [Google Scholar]
- 21.Kim TK, Choi BI, Hong HS, Choi BY, Han JK. Improved imaging of hepatic metastases with delayed pulse inversion harmonic imaging using a contrast agent SH U 508A: preliminary study. Ultrasound Med Biol. 2000;26:1439–44. doi: 10.1016/s0301-5629(00)00268-4. doi:10.1016/S0301-5629(00)00268-4. [DOI] [PubMed] [Google Scholar]
- 22.Oldenburg A, Hohmann J, Foert E, et al. Detection of hepatic metastases with low MI real time contrast enhanced sonography and SonoVue. Ultraschall Med. 2005;26:277–84. doi: 10.1055/s-2005-858526. doi:10.1055/s-2005-858526. PMid:16123921. [DOI] [PubMed] [Google Scholar]
- 23.Hohmann J, Albrecht T, Hoffmann CW, Wolf KJ. Ultrasonographic detection of focal liver lesions: increased sensitivity and specificity with microbubble contrast agents. Eur J Radiol. 2003;46:147–59. doi: 10.1016/s0720-048x(02)00053-0. doi:10.1016/S0720-048X(02)00053-0. PMid:12714231. [DOI] [PubMed] [Google Scholar]