Abstract
The aim of this study was to determine the effects of bone morphogenetic protein-2 (BMP-2) on articular chondrocyte tissues grown as monolayers in vitro for up to 8 weeks. Articular chondrocytes were isolated from New Zealand White rabbits and plated in monolayer cultures. The cultures were supplemented with 100 ng/mL of BMP-2 for up to 8 weeks and the extracellular matrix (ECM) composition, material properties, and messenger RNA (mRNA) expression were analyzed. mRNA expression of cartilage-specific genes, type II collagen, and aggrecan showed that BMP-2 enhanced chondrocyte stability for up to 3 weeks. After 3 weeks in culture, there was substantially more type I collagen expression and more osteopontin and runt-related transcription factor 2 expression in 5- and 8-week cultures treated with BMP-2 than in controls. Additionally, matrix metalloproteinase-13 and ADAMTS-5 (A disintegrin-like and metalloproteinase with thrombospondin 5) were upregulated in 5- and 8-week cultures treated with BMP-2, coinciding with a loss of ECM density, collagen, and proteoglycan. Eight-week tissue stimulated with BMP-2 was more fragile and tore more easily when removed from the culture dish as compared to controls, suggesting temporal limitations to the effectiveness of BMP-2 in monolayer systems and perhaps other models to enhance the generation of a cartilage-like tissue for tissue engineering purposes.
Introduction
Articular cartilage is a specialized tissue found at the ends of bones in articulating joints. It is critical for resisting compression and reducing friction during joint movement. Articular cartilage functions in part due to cartilage specific components such as type II collagen and aggrecan located in the dense extracellular matrix (ECM).1,2 Differentiated mesenchymal cells called chondrocytes maintain the ECM composition and organization of articular cartilage. These cells make up less than 5% of the tissue volume in adult animals3 but are responsible for organization of the cartilage through synthesis and degradation of matrix components in response to molecular, electrical, and mechanical stimuli.4–6 Unfortunately, chondrocytes have a limited ability to repair cartilage defects, which are manifested in diseases such as osteoarthritis, in which cracks and fibrillations in the surface of the tissue can progress to full-thickness cartilage defects, resulting in severe joint pain.7
Cartilage tissue engineering is a promising technology for repairing or regenerating articular cartilage in the body. These techniques often involve growth of cells or tissue in vitro that are then implanted to initiate repair.8 Articular chondrocytes grown in monolayer cultures seldom result in a satisfactory cartilage-like tissue and tend to “dedifferentiate,” as indicated by a loss of type II collagen and aggrecan expression and an increase in type I collagen expression.9 Thus, various cellular stimuli such as growth factors and mechanical forces have been employed to regulate cellular phenotype and increase mechanical quality.10
BMP-2 is a growth factor that is currently being studied for its musculoskeletal applications. BMP-2 is a member of the transforming growth factor-beta (TGF-β) superfamily. Binding of BMP ligands to their membrane receptors induces phosphorylation of the intracellular signaling mediators Smads, which regulate BMP-responsive genes in concert with other transcription factors.11 BMP-2 is widely associated with osteogenic promotion in in vitro differentiation studies and in clinical applications;12,13 however, the use of BMP-2 for chondrocyte differentiation is also under investigation.14
BMP-2 promotes chondrogenesis of embryonic stem cells,15 mesenchymal stem cells,16 and synovium-derived progenitor cells17 in vitro, suggesting a beneficial aspect of using this growth factor for cartilage tissue engineering purposes. Additionally, there is evidence that BMP-2 maintains or enhances the differentiated primary chondrocyte phenotype in in vitro monolayer cultures. Sailor et al. showed that 100 ng/mL of BMP-2 was sufficient to maintain articular chondrocyte expression of type II collagen and aggrecan and suppress the expression of type I collagen in cultured monolayers for up to 4 weeks.18 Stewart et al. had similar results using 100 ng/mL of BMP-2 in their model and concluded that BMP-2 was a potent stimulator of chondrocyte differentiation in monolayer cultures for up to 7 days.19
Recently, it was found that chondrocytes grown in monolayer cultures lose endogenous BMP-2 expression in as little as 7 days, coinciding with a loss of chondrocyte-specific gene expression.20 These new findings add credibility to the idea of adding BMP-2 to monolayer cultures to enhance the chondrocyte-differentiated phenotype, yet the extent of the effectiveness of BMP-2 supplements in long-term primary chondrocyte cultures remains poorly understood.
In vivo and in vitro studies suggest that BMP-2 can mediate chondrogenesis, stabilize chondrocyte differentiation, and enhance cartilage matrix synthesis. However, there is little evidence available to show that chronic exposure of this growth factor can induce long-term phenotypic stability in primary chondrocyte cultures or how this growth factor affects the matrix composition and mechanical properties of the engineered tissue. The goal of this research was therefore to determine the effects of BMP-2 on long-term (up to 8 weeks) primary chondrocyte cultures as assessed by biochemical analysis of matrix composition, to determine gene expression according to quantitative reverse transcriptase polymerase chain reaction (RT-PCR), and to measure the mechanical properties of the tissues formed.
Methods
Cell culture
All tissue culture reagents were from Invitrogen (Carlsbad, CA) unless otherwise noted. Articular chondrocytes were isolated from the shoulders and knees of 2- to 3-pound New Zealand White rabbits as previously described.21 Briefly, articular cartilage was removed from rabbit joints, pooled, and minced with a scalpel blade into approximately 1-mm pieces. Chondrocytes were released from the minced articular cartilage after sequential digestions with 0.25% trypsin-ethylenediaminetetraacetic acid (EDTA) and collagenase type II (Sigma-Aldrich, St. Louis, MO). Released chondrocytes were pelleted using centrifugation at 855 relative centrifugal force for 5 min. The pellet was washed twice with serum-free Ham's F-12 medium to remove endogenous ECM and then cells counted using a hemacytometer. Cell viability was determined using trypan blue exclusion. After isolation of the chondrocytes, cells were plated on Vitrogen-coated (COHESION, Palo Alto, CA) 6-well Falcon plates at 2 × 105 cells/cm2 (except cultures analyzed for gene expression on weeks 1–4, which were grown on Vitrogen-coated 12-well Falcon plates at 2 × 105 cells/cm2). Cells were grown in Ham's F-12 medium, 1:100 penicillin:streptomycin, 1:100 Fungizone (Invitrogen), and 50 μg/mL of gentamicin supplemented with 15% fetal bovine serum. After 2 days in culture, penicillin:streptomycin and Fungizone were omitted from the cell culture medium. After 2 weeks, the medium was supplemented with 30 μg/mL of ascorbate (Sigma). The medium was exchanged every 2 to 3 days from the start of culture until the experiment terminated. One hundred ng/mL of rhBMP-2 (gift from John Stark, Department of Orthopaedic Surgery, University of Minnesota) was added immediately after plating cells and included in every medium exchange in BMP-2–treated cultures.
Biochemical analysis of 5- and 8-week monolayer tissues
The tissue generated in monolayer cultures at weeks 5 and 8 was removed from the culture wells (n = 3 per condition), washed two times with 1× Dulbecco's phosphate buffered saline (Invitrogen), and the thickness determined as described below. Tissue volume (mL = mL wet weight tissue) was determined by multiplyng the thickness of the tissue by the area. The tissue from each condition was pooled for biochemical analyses. Pooled samples were digested with 1 mg/mL of protease K (Pierce Chemical, Thermo Scientific, Rockford, IL) in 100 mM sodium phosphate, 50 mM EDTA, pH = 7.4, for 18 h at 56°C and analyzed for proteoglycan, DNA, and hydroxyproline content. Proteoglycan content was measured according to the binding of dimethylmethylene blue, as previously described,22 with purified proteoglycan monomer as the standard. DNA quantitation was performed using the Hoescht dye method, with calf thymus DNA as the standard.23 Hydroxyproline (OHPro), used as a measure of total collagen content, was determined following the chloramine T method as described after hydrolysis in 6 N hydrochloric acid (HCl) for 24 h at 120°C.24
Quantitative real-time PCR
Total RNA was isolated from chondrocyte cultures using Trizol reagent (Invitrogen) following the manufacturer's instructions. Five- and 8-week tissues were pooled as in the biochemistry experiments. RNA from cultures on weeks 1 to 4 was analyzed from individual wells with an n = 3 per condition. Complementary DNA (cDNA) was amplified using the Qiagen (Valencia, CA) Quantitect One-Step SYBR Green RT-PCR kit using gene-specific primers for type I collagen (5′-GATGCGTTCCAGTTCGAGTA-3′ and 5′-GGTCTTCCGGTGGTCTTGTA-3′), type II collagen (5′-GCACCCATGGACATTGGAGGG-3′ and 5′-GACACGGAGTAGCACCATCG-3′), type X collagen (5′-TGGCACAGAAATGCCAGCTG-3′ and 5′-CCAGTGAGAGGAGAACAAGG-3′), aggrecan (5′-GAG GAGATGGAGGGTGAGGTCTTT-3′ and 5′-CTTCGCCTGTGTAGCAGATG-3′), matrix metalloproteinase (MMP)-13 (5′-TTCGGCTTAGAGGTGACAGG-3′ and 5′-ACTCTTGCCGGTGTAGGTGT-3′), runt-related transcription factor 2 (Runx2) (5′-ACCATGGTGGAGATCATCGC-3′ and 5′-CATCAAGCTTCTGTCTGTGC-3′), 3-phosphate dehydrogenase (GAPDH) (5′-TCACCATCTTCCAGGAGCGA-3′ and 5′-CACAATGCCGAAGTGGTCGT),25 A disintegrin-like and metalloproteinase with thrombospondin 5 (ADAMTS5) (5′-ATGACCATGAGGAGCACTACGA-3′ and 5′-GGAGAACATATGGTCCCAACGA-3′),26 and osteopontin (5′-CCGATGACTCTCACCACTCC-3′ and 5′-CCTCTTCACTCTTCGGCTCG-3′)27 in a MyIQ Single Color Real-Time PCR Detection System (BioRad, Hercules, CA). Quantification and normalization to GAPDH amplicons were performed as previously described.28 All amplicons for each experiment were run on a 1% agarose gel and stained with ethidium bromide to verify the correct amplification product.
Western blot for type I and II collagen in 5- and 8-week monolayer tissues
Tissues from 5- and 8-week cultures were pooled, respectively, and the collagens extracted and resolved on a 7.5% sodium dodecyl sulfate Tris-HCl polyacrylamide gel, as previously described, with minor revision.29 Briefly, tissue was removed from the culture wells and washed two times with Dulbecco's phosphate buffered saline (PBS). Proteoglycans were removed using moderate agitation in 10 volumes of 4 M guanidine chloride, 250 mM sodium acetate, 10 mM EDTA, 5 mM tryptamine-HCl, 2.5 mM phenanthrolene, 10 mM N-dthylmaleimide, and 0.5 mM phenylmethylsulphonyl fluoride, pH 5.8, at 4°C for 24 h. After proteoglycan extraction, the tissue was removed and washed twice in ice-cold distilled water and twice in 0.5 M acetic acid and digested for 24 h in 10 volumes of 1 mg/mL pepsin in 0.5 M acetic acid at 4°C with mild agitation. The supernatant-containing collagen was dialyzed three times against 0.5 M acetic acid at 4°C and lyophilized to dryness. The lyophilized powder was worked up into 0.5 M acetic acid and the protein content determined using the BioRad protein assay. Two μg of protein was resolved on a polyacrylamide gel under non-reducing conditions. The resolved proteins were transferred to a nitrocellulose membrane (Millipore, Billerica, MA) and immunoblotted for type I collagen (Sigma, C2456) and type II collagen (Calbiochem, CP18L, Gibbstown, NJ) in 3% bovine serum albumin in PBS following the manufacturer's instructions. The immunoblots were washed extensively in PBS and blotted 1:5000 with anti-mouse horseradish peroxidase (Sigma) followed by signal development using Amersham's electrochemiluminescence detection kit following the manufacturer's instructions. Densitometry was used to determine the relative amounts of specific collagens in the tissue.
Mechanical tests
Tissue was tested in tension at 5 and 8 weeks. Tissue was removed from the culture flask, cut into 20-mm × 5-mm strips, and bathed in PBS at room temperature during all mechanical testing. Thickness was measured using a custom-made device equipped with a low-force load cell and a displacement transducer. A probe attached to the load cell was brought down onto the tissue sitting on a glass slide and the displacement measured; then the tissue was removed, and the probe was brought down onto the glass slide substrate and the displacement measured again. Tissue was then placed in sandpaper grips on a MicroBionix (MTS, Inc., Minneapolis, MN) test instrument equipped with a 5 N load cell. Nominal gage length was 11 mm. Displacement was adjusted so that there was approximately 0.1 mN on the sample and then 0.15 mm displacement applied at 9 mm/s as a preload and held for 180 s. The load was then ramped down to 0 N and held for 10 s and then a displacement of 1.1 mm applied at 8 mm/s and held for 300 s. Data were reduced to rapid modulus, defined as the equivalent elastic modulus during the ramp displacement of 1.1 mm, and normalized relaxation function R(t), defined as the load as a function of time divided by the maximum load for the 300 s after the 1.1-mm displacement application.
Immunohistochemistry
Immunohistochemical staining was performed on formalin-fixed, paraffin-embedded sections cut 4 μm thick. Sections were deparaffinized and rehydrated, followed by antigen retrieval with pronase 1 mg/mL (Calbiochem) for 15 min at room temperature. The sections were blocked using a universal Protein Block (Dako, Carpinteria, CA) for 10 min at room temperature. Mouse anti-collagen type I (Sigma) antibody was diluted at 1:350 and mouse anti-collagen type II (Hybridoma Bank, University of Iowa, Iowa City, IA) antibody was diluted at 1:10 and incubated at 4°C overnight. Biotinylated anti-mouse secondary (Biogenex, San Ramon, CA) was used at a dilution of 1:20 for 30 min at 30°C. A Streptavidin Alkaline Phosphatase (Biogenex) conjugated tertiary was applied for 30 min at 30°C at a dilution of 1:20. Specific labeling was developed using enzyme substrate (Vector Red Substrate Kit, Invitrogen) for 5 min at room temperature. Negative control included substituting the primary antibody with SuperSensitive mouse negative control serum (Biogenex).
Statistical Analysis
All statistical analyses were performed using an unpaired Student t-test assuming equal variances. Statistical significance was considered at p < 0.05.
Results
BMP-2 alters matrix deposition and cell density in 5- and 8-week chondrocyte cultures
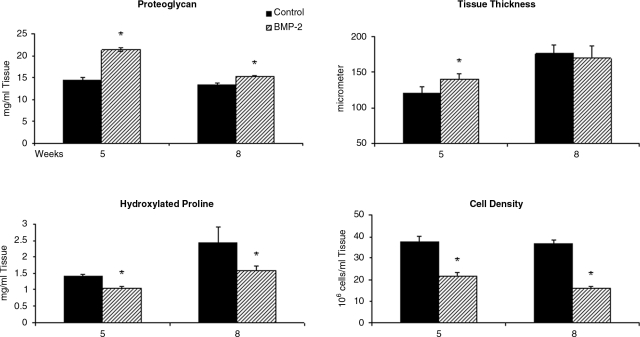
Primary articular chondrocytes were isolated from rabbit joints and grown in monolayer culture with medium containing 15% fetal bovine serum (FBS) in the presence or absence of 100 ng/mL of BMP-2 for 5 and 8 weeks. Biochemical analyses were used to measure the proteoglycan accumulation, OHPro (total collagen) content, and the cell density of the matrix formed. At 5 weeks in culture, the BMP-2–treated tissue was more proteoglycan rich, containing nearly 50% more proteoglycan than the untreated controls (mg proteoglycan/mL tissue: control = 14.3 ± 0.7; BMP-2 = 21.3 ± 0.5). However, the OHPro content and cell density were 74% (mg OHPro/mL tissue: control = 1.4 ± 0.1; BMP-2 = 1.0 ± 0.1) and 58% (106 cells/mL tissue: control = 37.3 ± 2.8; BMP-2 = 21.9 ± 1.4) of those of controls, respectively, in the BMP-2–treated tissue (Fig. 1). By 8 weeks in culture, the BMP-2–treated tissue had less additional proteoglycan accumulated in the matrix than controls (control = 13.3 ± 0.1; BMP-2 = 15.3 ± 0.2). The OHPro content and the cell density were an additional 67% (control =2.4 ± 0.5; BMP-2 = 1.6 ± 0.1) and 43% (control = 36.5 ± 1.9; BMP-2 = 16.3 ± 0.9) less, respectively, in BMP-2–treated cultures (Fig. 1).
FIG. 1.
Articular chondrocytes were plated in monolayer cultures and grown for 5 and 8 weeks in medium without bone morphogenetic protein (BMP)-2 (control: solid) or in medium containing 100 ng/mL BMP-2 (BMP-2: shaded). The resulting tissue was analyzed for proteoglycan and hydroxylated proline content, tissue thickness, and cell density. *Significant difference from controls with p < 0.05.
BMP-2 increases type II collagen relative to type I collagen deposition in the ECM in 5- but not 8-week cultures
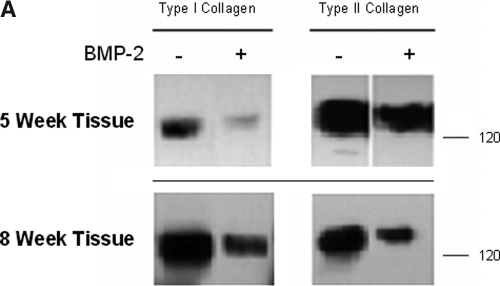
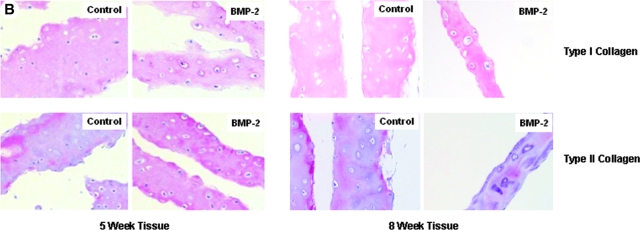
The predominance of type II collagen in the ECM of in vitro chondrocyte cultures is indicative of a stable chondrocyte phenotype and articular cartilage–like tissue. Types I and II collagen were in the matrix of controls and cultures treated with BMP-2; however, the amount of type I collagen per μg of extracted collagens in the ECM of BMP-2–treated samples was less than controls at 5 weeks (Fig. 2A). Moreover, densitometry measurements show that type II collagen was more abundant than type I collagen in BMP-2-treated tissues than in controls at 5 weeks in culture ((BMP-2: type I collagen/type II collagen)/(control: type I collagen/type II collagen) = 1.41). By 8 weeks, there was the same ratio of type I to type II collagen in the BMP-2 tissue as in controls ((BMP-2: type I collagen/type II collagen)/(control: type I collagen/type II collagen) = 1.01). Immunohistochemical analysis of these tissues is consistent with these data (Fig. 2B). There was intense vector red staining of type II collagen in 5-week tissue treated with BMP-2, but there was substantial loss of type II collagen staining in BMP-2–treated tissues at 8 weeks. Overall, these results suggest that BMP-2 may have enhanced the chondrogenic phenotype early in our model but, by 8 weeks in culture, had lost much of its effectiveness.
FIG. 2.
(A) Collagens were extracted from 5- and 8-week monolayer cultures grown in medium with or without 100 ng/mL of bone morphogenetic protein (BMP)-2. Two μg of total protein extracted was resolved on a 7.5% Tris-hydrochloric acid gel and western blotted for type I and type II collagen. (B) Immunohistochemical analysis for types I and II collagen in 5- and 8-week culture tissue from control and BMP-2–stimulated monolayer cultures. Specific labeling was developed using enzyme substrate vector red substrate kit. Images were taken at 10× magnification.
BMP-2 affects the mechanical properties of 8-week chondrocyte tissue
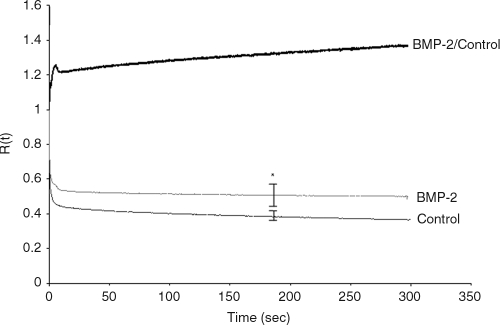
After 5 and 8 weeks in culture, control and BMP-2–treated tissue was removed from the tissue culture well and 5-mm × 20-mm tissue strips prepared. Thickness was measured, and tensile rapid modulus and relaxation function were determined. At 5 weeks, the BMP-2 tissue was slightly thicker than the untreated controls (BMP-2 = 141 ± 7 μm, Control =121 ± 8 μm); however, by 8 weeks in culture, there was no significant difference between the thickness of the BMP-2–treated and that of the control tissue (Fig. 1). There was no difference between BMP-2–treated tissues and controls in the rapid modulus (2.74 ± 0.29 MPa and 2.96 ± 0.17 MPa, respectively) or relaxation function after 5 weeks in culture, but mechanical analyses at 8 weeks showed less relaxation in the BMP-2–treated tissue than in controls (Fig. 3). This indicates that equilibrium stiffness was greater for the BMP-2–treated tissue and that there was less energy dissipation. These data suggest the BMP-2–treated tissue is less shock absorbing than the control tissue. In addition, the 8-week BMP-2–treated tissues were more fragile and tore more easily when removed from the tissue culture well (data not shown). By contrast, the control tissue could be easily removed from the flask without tearing.
FIG. 3.
The relaxation function of control and bone morphogenetic protein (BMP)-2 treated 8-week chondrocyte tissue. The upper curve is the R(t) for the BMP-2 tissue divided by the R(t) of the controls at each time point. *Significant difference from controls at p < 0.05.
BMP-2 decreases type II collagen message levels and induces proteolytic enzyme expression in 5- and 8-week tissues
The expression of articular chondrocyte–specific markers (type II collagen and aggrecan), typical chondrocyte dedifferentiation markers (type I collagen), chondrocyte hypertrophic and chondrocyte inflammation, and bone-like phenotypic markers (type X collagen and Runx230 or osteopontin31) were measured using quantitative RT-PCR (qRT-PCR) in 5- and 8-week chondrocyte cultures. Type II collagen expression was decreased to 85% and 35% in BMP-2 cultures at 5 and 8 weeks in culture, respectively, as compared to the controls. At 5 weeks, aggrecan expression in BMP-2 cultures was 1.25 times that in controls but was decreased to 71% by 8 weeks. Type I collagen in BMP-2–stimulated tissue was more than 7 times that of controls at 5 weeks and even more dramatically by 8 weeks, at which point BMP-2 treated tissue had 32 times higher expression of type I collagen than controls. Although there was no indication of type X collagen expression in the control or BMP-2–treated cultures (data not shown), osteopontin was approximately 24 and 7 times greater in 5- and 8-week BMP-2 cultures, respectively. Runx2 expression was also higher in BMP-2–treated cultures at 5 and 8 weeks than in controls (3.1 and 5.6 times, respectively (Fig. 4).
FIG. 4.
Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) analysis of type I and II collagen, aggrecan, runt-related transcription factor 2 (RunX2), osteopontins, ADAMTS-5, and matrix metalloproteinase (MMP)-13 in 5-week (solid) and 8-week (shaded) chondrocyte cultures grown in medium containing 100 ng/mL of bone morphogenetic protein (BMP)-2. Specific gene expression was normalized to internal 3-phosphate dehydrogenase expression. Values represent the fold change compared with controls (no BMP-2 added to medium) at the respective time point (solid line at 1). NA, not available.
To address the lower density of collagen (mg OHPro/mL of tissue) in cultures treated with BMP-2 than in controls and similarly in the lower proteoglycan content (mg proteoglycan/mL tissue) in 5- and 8-week cultures stimulated with BMP-2 (Fig. 1), MMP-13 and ADAMTS5 expression was analyzed. MMP-13, a protease with high enzymatic activity in response to type I and type II collagen,32 was more than 2 and 7 times as great in 5- and 8-week BMP-2–treated cultures, respectively, as in controls. Likewise, ADAMTS5, an enzyme that degrades aggrecan,33 was 21 times as great as that of controls by 8 weeks in culture (Fig. 4).
Chondrogenic promotion by BMP-2 in primary monolayer chondrocyte cultures is limiting
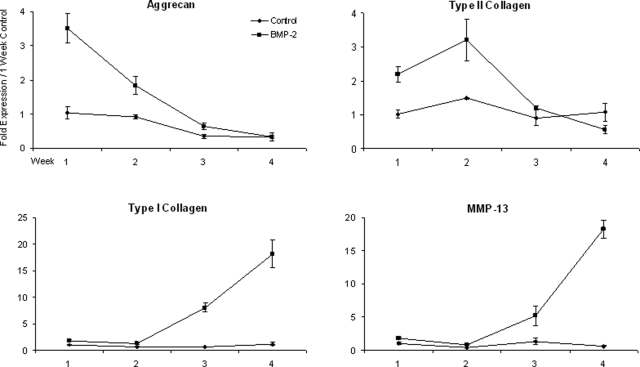
Biochemical, immunohistochemical, and western blot analyses of the 5-week chondrocyte tissues suggests that BMP-2 enhances, at least in part, the chondrocyte phenotype in primary chondrocyte monolayer cultures (i.e., greater amount of proteoglycan and type II collagen deposited in the matrix of BMP-2–stimulated cultures). To determine when this enhancement becomes limiting, primary articular chondrocytes were cultured in medium containing 15% FBS with and without BMP-2 for up to 4 weeks, and message levels for aggrecan, type I and II collagen, and MMP-13 were measured weekly. Type II collagen expression was greater in BMP-2–treated cultures for the first 3 weeks than in controls; however, by 4 weeks, the effectiveness of BMP-2 in promoting type II collagen expression had been lost (Fig. 5). Aggrecan expression levels were also greater in BMP-2–treated cultures during the first 3 weeks, but this enhancement was lost by the 4-week time point. Type I collagen levels were similar to those in controls for the first 2 weeks but were dramatically greater (nearly 20 times those of controls by 4 weeks). Additionally, MMP-13 was upregulated by BMP-2 by 3 weeks in culture, again nearly 20 times greater than in controls by the 4-week time point (Fig. 5).
FIG. 5.
Type I and II collagen, aggrecan, and matrix metalloproteinase (MMP)-13 messenger RNA in control (♦) and bone morphogenetic protein (BMP)-2–treated (▪)chondrocyte cultures at weeks 1 to 4. Gene expression was normalized to internal 3-phosphate dehydrogenase values. Fold expression was based on control values at the 1-week time point.
BMP-2 promotes moderate long-term chondrocyte stability in 5% serum but increases type I collagen expression
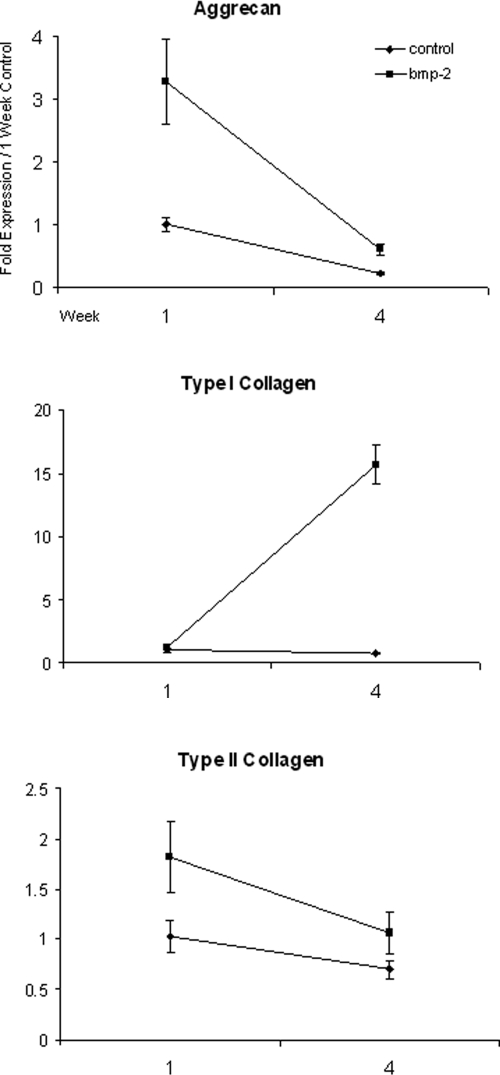
The effectiveness of BMP-2 in enhancing chondrocyte-specific gene expression has been shown to be dependent on the addition of serum to the medium.34 In an effort to determine whether the response of chondrocytes to BMP-2 in our model was dependent on the amount of serum in the medium, chondrocytes were cultured for 1 or 4 weeks in 5% FBS-supplemented media with or without 100 ng/mL of BMP-2. Type I and type II collagen and aggrecan mRNA levels were measured using qRT-PCR. After 1 week in culture, BMP-2 upregulated type II collagen and aggrecan mRNA to levels similar to cultures grown in the presence of 15% serum. However, in contrast to 15% serum-supplemented cultures, BMP-2 in the presence of 5% serum maintained moderately more type II collagen and aggrecan expression than controls at 4 weeks. Type I collagen mRNA levels in cultures with 5% serum plus BMP-2 at 1 week were similar to untreated controls, but by 4 weeks, type I collagen message levels had increased dramatically to 21 times those of the controls, consistent with the BMP-2–dependent upregulation of type I collagen seen in cultures supplemented with 15% FBS (Fig. 6).
FIG. 6.
The effects of serum on bone morphogenetic protein (BMP)-2 stimulation in primary chondrocyte cultures. Type I and II collagen and aggrecan expression in control (♦) and BMP-2-stimulated (▪) cultures in the presence of 5% betal bovine serum at weeks 1 and 4. Expression was normalized to internal 3-phosphate dehydrogenase values. Fold expression was based on control values at the 1-week time point.
Discussion
The role of BMP-2 in skelatogenesis is not completely understood. Historically, BMPs were found to be potent osteoinductive agents and were originally described as proteins capable of inducing ectopic endochondral bone formation in subcutaneous implants.35,36 More recently, BMPs were shown to play critical roles in a variety of different cell types and tissues, including neural and epidermal induction,37 differentiation of chondrocytes in the growth plate,38 and enhancing maturation in growth plate chondrocytes cultured in vitro.39 Current evidence suggests that BMP-2 can play pivotal roles in chondrogenesis40 and promote retention18,19 or reversion41 of chondrocyte differentiation in vitro. The goal of this study was therefore to examine the ability of BMP-2 to promote chondrocyte differentiation in long-term monolayer cultures up to 8 weeks.
Previous studies suggest a beneficial effect of adding BMP-2 to articular chondrocytes in in vitro monolayer cultures, enhancing production of matrix molecules indicative of articular cartilage and reducing production of molecules indicative of the differentiated state, such as type I collagen. In this report, initial enhancement of proteoglycan and type II collagen expression in BMP-2 treated cultures and their accumulation in the ECM of BMP-2–stimulated 5-week monolayer cultures is consistent with these reports. However, mRNA analyses suggest that the beneficial effect of adding BMP-2 to chondrocyte cultures is limiting for up to approximately 3 weeks after the initial plating, after which the protective effect of BMP-2 on chondrocyte stability in monolayer cultures begins to decrease, with a loss of chondrocyte-specific genes, type II collagen, and aggrecan and an increase in expression of type I collagen. Additionally, in our model, sustained BMP-2 stimulation resulted in an increase in osteopontin and Runx2 message levels, markers typically associated with bone and pre-hypertrophic chondrocytes,42 respectively. These deviations from a stable chondrocyte phenotype are evident in the poor mechanical quality of our 8-week BMP-2–treated tissue, contrasting the assumed purpose of using the engineered tissue for cartilage repair.
These results are not unique to our model system. BMP-2 stimulation has been shown to have desirable and undesirable effects in other cartilage tissue-engineering models. BMP-2 was shown to promote the expression of chondrocyte-specific genes by adipose-derived stem cells, but there was not only an upregulation of type II collagen expression and chondroitin-4-sulfate (a measure of proteoglycan synthesis), but also an induction of alkaline phosphatase expression and activity and Runx-2 expression.43 Synovial explants treated with BMP-2 expressed the cartilage-specific markers type II collagen and proteoglycan, but by 6 weeks in culture, the authors noted the grossly hypertrophic state of the differentiated synovial cells.44 In a more recent experiment, ATDC5 cells, a chondrogenic cell line from the AT805 mouse teratocarcinoma, were shown to have enhanced proteoglycan synthesis up to 4 weeks in cultures in which BMP-2 was stably over-expressed, but the same study found that alkaline phosphatase expression and activity also increased in response to BMP-2 in as little as 9 days.45 Similarly, BMP-2 promoted hypertrophy of primary chondrocytes grown for 4 weeks on substrates that support three-dimensional cell morphology.46 Collectively, these data indicate that BMP-2 is a potent growth factor that can influence a multiplex of genes formally associated with chondrogenesis15-17 and chondrocyte differentiation or are involved in osteogenesis.42,47
Recent evidence proposes that BMP-2 is a mediator of articular cartilage repair and remodeling in vivo. Davidson et al. showed that BMP-2 stimulation increased matrix synthesis and catabolism in articular cartilage.48 Our data, notably the initial increased deposition of cartilage-specific molecules and then loss of ECM components coinciding with an upregulation of collagen and proteoglycan proteases, are consistent with a bimodal or biphasic BMP-2 metabolic model.
The lower cell density of our BMP-2–treated 5- and 8-week cultures than in controls may also indicate an undesirable aspect of using BMP-2 for cartilage tissue engineering purposes. Although articular cartilage is a relatively acellular tissue, a presumed purpose for placing primary cells in monolayer cultures for tissue engineering is to increase cell density. Some have found BMP-2 to be associated with an increase and decrease in cell proliferation,49,50 whereas others found no significant difference in cell proliferation or cell death with concentrations of BMP-2 similar to those used in our model.18 It may be of interest to further investigate cell proliferation or apoptosis at earlier time points than in this study.
Our results do not exclude the possibility that BMP-2 may play an important role in cartilage tissue engineering. Our data are consistent with other studies that BMP-2 helps retain the chondrocyte phenotype with enhancement of type II collagen and proteoglycan expression in monolayers for the first few weeks in culture, after which the cells may be readily used for cartilage repair or for further exploitation in other model systems to generate tissue suitable for repair of cartilage defects.
Addition of BMP-2 to cell cultures has shown some promise in maintaining the chondrocyte phenotype in vitro; however, the present study implies limitations to these beneficial effects on chondrocyte monolayer cultures, resulting in a loss of chondrocyte stability. It is likely that these results are dependent on the specifics of the culture conditions and model systems, but they suggest that long-term exposure to BMP-2 does not enhance the quality of tissue-engineered cartilage and consequently may have detrimental effects.
Acknowledgments
The authors would like to thank Anne Undersander and Josh Parker in the Comparative Pathology Shared Resource center at the University of Minnesota for their histochemical expertise.
Disclosure Statement
No competing financial interests exist.
References
- 1.Silver F. Bradica G. Tria A. Relationship among biomechanical, biochemical, and cellular changes associated with osteoarthritis. Crit Rev Biomed Eng. 2001;29:373. doi: 10.1615/critrevbiomedeng.v29.i4.10. [DOI] [PubMed] [Google Scholar]
- 2.Watanabe H. Yamada Y. Kimata K. Roles of aggrecan, a large chondroitin sulfate proteoglycan, in cartilage structure and function. J Biochem. 1998;124:687. doi: 10.1093/oxfordjournals.jbchem.a022166. [DOI] [PubMed] [Google Scholar]
- 3.Stockwell R. The cell density of human articular and costal cartilage. J Anat. 1967;101:753. [PMC free article] [PubMed] [Google Scholar]
- 4.Chandrasekhar S. Harvey A. Differential regulation of metalloprotease steady state mRNA levels by IL-1 and FGF in rabbit articular chondrocytes. FEBS Lett. 1992;296:195. doi: 10.1016/0014-5793(92)80378-t. [DOI] [PubMed] [Google Scholar]
- 5.Massari L. Benazzo F. De Mattei M. Setti S. Fini M CRES Study Group. Effects of electrical physical stimuli on articular cartilage. J Bone Joint Surg Am. 2007;89 Suppl 3:152. doi: 10.2106/JBJS.G.00581. [DOI] [PubMed] [Google Scholar]
- 6.Huang J. Ballou L. Hasty K. Cyclic equibiaxial tensile strain induces both anabolic and catabolic responses in articular chondrocytes. Gene. 2007;404:101. doi: 10.1016/j.gene.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Lebaron R. Athanasiou K. Ex vivo synthesis of articular cartilage. Biomaterials. 2000;21:2575. doi: 10.1016/s0142-9612(00)00125-3. [DOI] [PubMed] [Google Scholar]
- 8.Peterson L. Minas T. Brittberg M. Nilsson Sjögren-Jansson E. Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;374:212. doi: 10.1097/00003086-200005000-00020. [DOI] [PubMed] [Google Scholar]
- 9.Schnabel M. Marlovits S. Eckhoff G. Fichtel I. Gotzen L. Vecsei V. Schlegel J. Dedifferentiation-associated changes in morphology and gene expression in primary human articular chondrocytes in cell culture. Osteoarthritis Cartilage. 2002;10:62. doi: 10.1053/joca.2001.0482. [DOI] [PubMed] [Google Scholar]
- 10.Chung C. Burdick J. Engineering cartilage tissue. Adv Drug Deliv Rev. 2008;60:243. doi: 10.1016/j.addr.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ebara S. Nakayama K. Mechanisms for the action of bone morphogenetic proteins and regulation of their activity. Spine. 2002;27:S10. doi: 10.1097/00007632-200208151-00004. [DOI] [PubMed] [Google Scholar]
- 12.Katagiri T. Yamaguchi A. Komaki M. Abe E. Takahashi N. Ikeda T. Rosen V. Wozney J. Fujisawa-Sehara A. Suda T. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol. 1994;127:1755. doi: 10.1083/jcb.127.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlisle E. Fischgrund J. Bone morphogenetic proteins for spinal fusion. Spine. 2005;5:S240. doi: 10.1016/j.spinee.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Oshin A. Stewart M. The role of bone morphogenetic proteins in articular cartilage development, homeostasis and repair. Vet Comp Orthop Traumatol. 2007;20:151. doi: 10.1160/vcot-07-02-0018. [DOI] [PubMed] [Google Scholar]
- 15.Zur Nieden N. Kempka G. Rancourt D. Ahr HJ. Induction of chondro-, osteo- and adipogenesis in embryonic stem cells by bone morphogenetic protein-2: effect of cofactors on differentiating lineages. BMC Dev Biol. 2005;5:1. doi: 10.1186/1471-213X-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nöth U. Rackwitz L. Heymer A. Weber M. Baumann B. Steinert A. Schutze N. Jakob F. Eulert J. Chondrogenic differentiation of human mesenchymal stem cells in collagen type I hydrogels. J Biomed Mater Res A. 2007;83:626. doi: 10.1002/jbm.a.31254. [DOI] [PubMed] [Google Scholar]
- 17.Park Y. Sugimoto M. Watrin A. Chiquet M. Hunziker E. BMP-2 induces the expression of chondrocyte-specific genes in bovine synovium-derived progenitor cells cultured in three-dimensional alginate hydrogel. Osteoarthritis Cartilage. 2005;13:527. doi: 10.1016/j.joca.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Sailor L. Hewick R. Morris E. Recombinant human bone morphogenetic protein-2 maintains the articular chondrocyte phenotype in long-term culture. J Orthop Res. 1996;14:937. doi: 10.1002/jor.1100140614. [DOI] [PubMed] [Google Scholar]
- 19.Stewart M. Saunders K. Burton-Wurster N. MacLeod J. Phenotypic stability of articular chondrocytes in vitro: The effects of culture models, bone morphogenetic protein 2, and serum supplementation. J Bone Miner Res. 2000;15:166. doi: 10.1359/jbmr.2000.15.1.166. [DOI] [PubMed] [Google Scholar]
- 20.Oshin A. Caporali E. Byron C. Stewart A. Stewart M. Phenotypic maintenance of articular chondrocytes in vitro requires BMP activity. Vet Comp Orthop Traumatol. 2007;20:185. doi: 10.1160/vcot-06-07-0061. [DOI] [PubMed] [Google Scholar]
- 21.Fedewa M. Oegema T. Schwartz M. MacLeod A. Lewis J. Chondrocytes in culture produce a mechanically functional tissue. J Orthop Res. 1998;16:227. doi: 10.1002/jor.1100160210. [DOI] [PubMed] [Google Scholar]
- 22.Chandrasekhar S. Esterman M. Hoffman H. Microdetermination of proteoglycans and glycosaminoglycans in the presence of guanidine hydrochloride. Anal Biochem. 1987;161:103. doi: 10.1016/0003-2697(87)90658-0. [DOI] [PubMed] [Google Scholar]
- 23.Labarca C. Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980;102:344. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- 24.Keane M. Belperio J. Arenberg D. Burdick M. Xu Z. Xue Y. Strieter R. IFN-gamma-inducible protein-10 attenuates bleomycin-induced pulmonary fibrosis via inhibition of angiogenesis. J Immunol. 1999;163:5686. [PubMed] [Google Scholar]
- 25.Reno C. Marchuk L. Sciore P. Frank C. Hart D. Rapid isolation of total RNA from small samples of hypocellular, dense connective tissues. Biotechniques. 1997;22:1082. doi: 10.2144/97226bm16. [DOI] [PubMed] [Google Scholar]
- 26.Tsuji T. Chiba K. Imabayashi H. Fujita Y. Hosogane N. Okada Y. Toyama Y. Age-related changes in expression of tissue inhibitor of metalloproteinase-2 associated with transition from the notochordal nuclease pulposus to the fibrocartilaginous nucleus pulposus in rabbit intervetebral disc. Spine. 2007;32:849. doi: 10.1097/01.brs.0000259804.39881.62. [DOI] [PubMed] [Google Scholar]
- 27.Achari Y. Reno C. Tsao H. Morck D. Hart D. Influence of timing (pre-puberty or skeletal maturity) on ovariohysterectomy on mRNA levels in corneal tissues of rabbits. Mol Vis. 2008;14:443. [PMC free article] [PubMed] [Google Scholar]
- 28.Schroeder T. Kahler R. Li X. Westendorf J. Histone deacetylase 3 interacts with runx2 to repress the osteocalcin promoter and regulate osteoblast differentiation. J Biol Chem. 2004;279:41998. doi: 10.1074/jbc.M403702200. [DOI] [PubMed] [Google Scholar]
- 29.Wardale R. Duance V. Quantification and immunolocalisation of porcine articular and growth plate cartilage collagens. J Cell Sci. 1993;105:975. doi: 10.1242/jcs.105.4.975. [DOI] [PubMed] [Google Scholar]
- 30.Schroeder T. Jensen E. Westendorf J. Runx2: a master organizer of gene transcription in developing and maturing osteoblasts. Birth Defects Res C Embryo Today. 2005;75:213. doi: 10.1002/bdrc.20043. [DOI] [PubMed] [Google Scholar]
- 31.Attur M. Dave M. Stuchin S. Kowalski A. Steiner G. Abramson S. Denhardt D. Amin A. Osteopontin. Arthritis Rheum. 2001;44:578. doi: 10.1002/1529-0131(200103)44:3<578::AID-ANR106>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell P. Magna H. Reeves L. Lopresti-Morrow L. Yocum S. Rosner P. Geoghegan K. Hambor J. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest. 1996;97:761. doi: 10.1172/JCI118475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stanton H. Rogerson F. East C. Golub S. Lawlor K. Meeker C. Little C. Last K. Farmer P. Campbell I. Fourie A. Fosang A. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005;434:648. doi: 10.1038/nature03417. [DOI] [PubMed] [Google Scholar]
- 34.Frenkel S. Saadeh P. Mehrara B. Chin G. Steinbrech D. Brent B. Gittes G. Longaker M. Transforming growth factor beta superfamily members: role in cartilage remodeling. Plast Reconstr Surg. 2000;105:980. doi: 10.1097/00006534-200003000-00022. [DOI] [PubMed] [Google Scholar]
- 35.Urist M. Mikulski A. Lietze A. Solubilized and insolubilized bone morphogenetic protein. Proc Natl Acad Sci U S A. 1979;76:1828. doi: 10.1073/pnas.76.4.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sampath T. Reddi A. Dissociative extraction and reconstitution of extracellular matrix components involved in local bone differentiation. Proc Natl Acad Sci U S A. 1981;78:7599. doi: 10.1073/pnas.78.12.7599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Christiansen J. Coles E. Wilkinson D. Molecular control of neural crest formation, migration and differentiation. Curr Opin Cell Biol. 2000;12:719. doi: 10.1016/s0955-0674(00)00158-7. [DOI] [PubMed] [Google Scholar]
- 38.Nilsson O. Parker E. Hegde A. Chau M. Barnes K. Baron J. Gradients in bone morphogenetic protein-related gene expression across the growth plate. J Endocrinol. 2007;193:75. doi: 10.1677/joe.1.07099. [DOI] [PubMed] [Google Scholar]
- 39.Hiraki Y. Inoue H. Shigeno C. Sanma Y. Bentz H. Rosen D. Asada A. Suzuki F. Bone morphogenetic proteins (BMP-2 and BMP-3) promote growth and expression of the differentiated phenotype of rabbit chondrocytes and osteoblastic MC3T3-E1 cells in vitro. J Bone Miner Res. 1991;6:1376. doi: 10.1002/jbmr.5650061215. [DOI] [PubMed] [Google Scholar]
- 40.Pufe T. Petersen W. Fändrich F. Varoga D. Wruck C. Mentlein R. Helfenstein A. Hoseas D. Dressel S. Tillmann B. Ruhnke M. Programmable cells of monocytic origin (PCMO): A source of peripheral blood stem cells that generate collagen type II-producing chondrocytes. J Orthop Res. 2008;26:304. doi: 10.1002/jor.20516. [DOI] [PubMed] [Google Scholar]
- 41.Gründer T. Gaissmaier C. Fritz J. Stoop R. Hortschansky P. Mollenhauer J. Aicher W. Bone morphogenetic protein (BMP)-2 enhances the expression of type II collagen and aggrecan in chondrocytes embedded in alginate beads. Osteoarthritis Cartilage. 2004;12:559. doi: 10.1016/j.joca.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 42.Dong Y. Soung do Y. Schwarz EM. O'Keef R. Drissi H. Wnt induction of chondrocyte hypertrophy through the Runx2 transcription factor. J Cell Physiol. 2006;208:77. doi: 10.1002/jcp.20656. [DOI] [PubMed] [Google Scholar]
- 43.Mehlhorn A. Niemeyer P. Kaschte K. Muller L. Finkenzeller G. Hart D. Sudkamp N. Schmal H. Differential effects of BMP-2 and TGF-β1 on chondrogenic differentiation of adipose derived stem cells. Cell Prolif. 2007;40:809. doi: 10.1111/j.1365-2184.2007.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shintani N. Hunziker E. Chondrogenic differentiation of bovine synovium. Bone morphogenetic proteins 2 and 7 and transforming growth factor β1 induce the formation of different types of cartilaginous tissue. Arthritis Rheum. 2007;56:1869. doi: 10.1002/art.22701. [DOI] [PubMed] [Google Scholar]
- 45.Vogt S. Ueblacker P. Geis C. Wagner B. Wexel G. Tischer T. Kruger A. Plank C. Anton M. Martinek V. Imhoff A. Gansbacher B. Efficient and stable gene transfer of growth factors into chondrogenic cells and primary articular chondrocytes using VSV.G pseudotyped retroviral vector. Biomaterials. 2008;29:1242. doi: 10.1016/j.biomaterials.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 46.Gooch K. Blunk T. Courter D. Sieminski A. Vunjak-Novakovic G. Freed L. Bone morphogenetic proteins-2, -12, and -13 modulate in vitro development of engineered cartilage. Tissue Eng. 2002;8:591. doi: 10.1089/107632702760240517. [DOI] [PubMed] [Google Scholar]
- 47.Kishimoto K. Kitazawa R. Kurosaka M. Maeda S. Kitazawa S. Expression profile of genes related to osteoclastogenesis in mouse growth plate and articular cartilage. Histochem Cell Biol. 2006;125:593. doi: 10.1007/s00418-005-0103-z. [DOI] [PubMed] [Google Scholar]
- 48.Blaney Davidson EN. Vitters E. van Lent P. van de Loo F. van den Berg F. van der Kraan P. Elevated extracellular matrix production and degradation upon BMP-2 stimulation point towards a role for BMP-2 in cartilage repair and remodeling. Arthritis Res Ther. 2007;9:R102. doi: 10.1186/ar2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Luca F. Barnes K. Uyeda J. De-levi S. Abad V. Palese T. Mericq V. Baron J. Regulation of growth plate chondrogenesis by bone morphogenetic protein-2. Endocrinology. 2001;142:430. doi: 10.1210/endo.142.1.7901. [DOI] [PubMed] [Google Scholar]
- 50.Wong G. Tang V. El-sabeawy F. Weiss R. BMP-2 inhibits proliferation of human aortic smooth muscle cells via p21Cip1/Waf1. Am J Physiol Endocrinol Metab. 2003;284:E972. doi: 10.1152/ajpendo.00385.2002. [DOI] [PubMed] [Google Scholar]