Abstract
The effective delivery of bioactive molecules to wound sites hasten repair. Cellular therapies provide a means for the targeted delivery of a complex, multiple arrays of bioactive factors to wound sites. Thus, the identification of ideal therapeutic populations is an essential aspect of this approach. In vitro assays can provide an important first step toward this goal by selecting populations that are likely suitable for more expensive and time-consuming in vivo assays. In this study, bone marrow–derived mesenchymal stem cells (BM-MSCs) were integrated into a three-dimensional coculture system that supports the development and stabilization of vascular tube-like structures. The presence of a limited number of BM-MSCs resulted in their coalignment with vascular structures, and it further resulted in increased tubule numbers and complexity. Thus, these studies suggest that BM-MSCs functionally interacted with and were attracted to in vitro formed vascular structures. Further, these cells also provided sufficient bioactive factors and matrix molecules to support the formation of tubular arrays and the stabilization of these arrays. This in vitro system provides a means for assessing the function of BM-MSCs in aspects of the angiogenic component of wound repair.
Introduction
Angiogenesis at wound sites provides a patent vascular supply that can deliver diverse cellular populations and bioactive factors that initiate and support tissue restoration.1,2 Our expanded understanding of the events that occur during inflammation and repair has lead to the concept that wound repair can be hastened and improved through the ectopic delivery of specific growth factors such as fibroblast growth factors (FGFs) and platelet-derived growth factors (PDGFs).3–6 However, superior results have been obtained through the application of multiple factors rather than the introduction of a single factor.7 In this regard, cellular therapy has been proposed as an effective means for the delivery of multiple factors.8–12 The introduction of selected cellular populations into wound sites would enable these cells to interact in a paracrine manner with other cellular residents. The consequent release of bioactive factors should hasten vascular and tissue repair processes.8 The issue then becomes, first, the identification of potential therapeutic cellular populations, second, the application of a suitable experimental model to assess cellular effectiveness, and, third, to develop efficient delivery methods.
In recent years, a number of studies have identified two unique subpopulations of bone marrow–derived cells that home to wound sites. The bone marrow–derived mesenchymal stem cell (BM-MSC) and the fibrocyte travel to wound sites via the circulatory system, and both sets of these cells have been proposed to influence repair processes.2,13–15 Both populations of cells express fibroblast-like characteristics in that they produce type I collagen and presumably other extracellular matrix molecules. However, they differ in that fibrocytes also express hematopoietic cell markers and also express major histocompatibility markers that make them effective antigen-presenting cells.14 Both of these immigrant populations appear to influence repair events.16,17 The delivery of MSCs to wound sites has been shown to be therapeutic for repair events in experimental animal models.9–12,18,19 Thus, this population of cells has been chosen for this study. However, the mechanisms by which MSCs exert their influence on repair are not yet fully understood.
In vitro systems cannot fully replicate the repertoire of cellular and molecular events that constitute in vivo wound repair. However, it is possible to devise approaches that mimic essential elements of these repair processes.2,20,21 In this study, an in vitro assay is introduced to assess cellular interactions between MSCs and vascular elements to determine whether these cells might play an effective role in angiogenesis at wound sites. This coculture system consists of a thin, self-assembled, three-dimensional supportive lawn of fibroblasts onto which human umbilical vein endothelial cells (HUVECs) are seeded.22,23 HUVECs attach to the lawn matrix and rapidly begin to migrate, align end-to-end, and subsequently form stable tube-like structures. These events occur in a culture medium that contains low serum levels and which is not supplemented with the major angiogenic factors vascular endothelial growth factor-A (VEGF-A) and FGF-2.22 The second in vitro system was set up by seeding human dermal microvascular endothelial cells (HDMVECs) either alone or in combination with MSCs from bone marrow or adipose tissue onto growth factor–depleted Matrigel.24,25 This model made it possible to assess direct interactions between MSCs and vascular endothelial cells without the additional influences provided by dermal fibroblasts.
The BM-MSCs coaligned with tube-like structures in both in vitro systems in a pericyte-like manner,26 suggesting that these cells may have been attracted to the vascular structures. In addition, some populations of BM-MSCs enhanced the complexity and extent of tubule formation. This observation suggested that these BM-MSCs released angiogenic factors or otherwise communicated in a paracrine manner with vascular structures to regulate vessel formation. Thus, these in vitro platforms provide a means to experimentally assess critical interactions between BM-MSCs and other cellular populations that are present at sites of wound repair in vivo. These results also raise the possibility that at least one of the functions of BM-MSCs is to home to wound sites and function as pericytes.
Materials and Methods
Reagents
Anti-human monoclonal antibody to platelet and endothelial cell adhesion molecule-1 (PECAM-1) was purchased from Chemicon–Millipore (Temecula, CA). Anti-mouse antibody conjugated with fluorescein isothiocyanate (FITC) was purchased from Southern Biotechnology (Birmingham, AL). The vital dye CM-DiI and 4′,6-diamidino-2-phenylindole (DAPI) were purchased from Molecular Probes–Invitrogen (Carlsbad, CA). Growth factor–depleted Matrigel was purchased from BD Biosciences (San Jose, CA). Murine anti-human type IV collagen and laminin were purchased from Sigma Chemical (St. Louis, MO). Anti-human FGF-2 was purchased from R&D Systems (Minneapolis, MN). The CD44 antibody used in this study recognizes all splice-variants and was a gift from Dr. David Naor, Hebrew University Jerusalem. The 6B6 decorin antibody produced for Seikagaku America was purchased from Cape Cod Associates (Falmouth, MA).
Cells
Fibroblasts
The fibroblasts, at fifth to ninth passages, used for this study were established as explant cultures obtained from the papillary dermis of normal adult human skin as previously described.27 All dermal fibroblasts and BM-MSCs were cultured in the same medium, Dulbecco's modified Eagle's medium with low glucose (DMEM-lg) containing 10% of a selected lot of fetal bovine serum (FBS) purchased from Gibco–Invitrogen (Carlsbad, CA).28 This medium also contained 1% of stock antibiotic–antimycotic (catalog #15240; Gibco–Invitrogen). All fibroblasts used in these studies were obtained from discarded surgical tissue.
BM-MSCs
The MSCs used for this study were obtained from healthy adult human bone marrow donors as previously described by Lennon et al.28 Briefly, bone marrow aspirates obtained from the iliac crests were diluted with DMEM-lg containing 10% of a selected lot28 of FBS. After centrifugation at 500 g for 5 min, the pelleted cells were fractionated by centrifugation on a 63% solution of Percoll in Tyrode's salt solution. The top 25% of the centrifuge contents was harvested and mixed with DMEM-lg and 10% FBS. The cells were pelleted by centrifugation, resuspended in medium, and plated onto plastic culture dishes. Cells at the first through fourth passages were used for this study. Although the specific MSCs used in this assay were not assayed for multilineage potential, this procedure has previously been used to obtain MSCs that differentiated into osteogenic and chondrogenic lineages.28 A list of MSCs obtained from consenting donors and used in this study can be found in Table 1.
Table 1.
BM-MSC Populations Used for the Study
| Donor ID number | Donor age in years | Donor gender |
|---|---|---|
| 1282 | 27 | Male |
| 1284 | 26 | Male |
| 1286 | 23 | Female |
| 1291 | 41 | Male |
| 1364 | 26 | Female |
| 1376 | 56 | Female |
| 1411 | 44 | Male |
| 1435 | 29 | Male |
| 1446 | 33 | Male |
| 1456 | 45 | Female |
| 1458 | 19 | Male |
| 1490 | 26 | Female |
| 1493 | 51 | Male |
| 1494 | 51 | Male |
| 1495 | 27 | Female |
| 1496 | 43 | Male |
| 1533 | 42 | Male |
| 1535 | 47 | Male |
Adipose tissue–derived MSCs
The 2008A and 2008B cell lines used in these studies were obtained from Farshid Guilak's Laboratory, Duke University, Durham, NC.29 These cells were culture expanded in a medium consisting of DMEM-lg and F-12 Nutrient broth (1:1 mixture), 5 ng/mL epidermal growth factor, 1 ng/mL FGF-2, 0.25 ng/mL transforming growth factor (TGF)-β1, antibiotic–antimycotic, and 10% growth selected FBS that was provided by the Guilak Laboratory.29 After detachment from the culture plate using trypsin, the cells were mixed with HDMVECs and seeded onto Matrigel as described below.
Human endothelial cells
HUVECs were purchased as frozen pooled cell populations from Lonza Biologicals (Walkersville, MD). Frozen cells were cultured in endothelial cell basal medium (EBM)-2 medium supplemented with angiogenic and other factors provided by Lonza Biologicals (hydrocortisone, FGF-2/heparin, VEGF-A, insulin-like growth factor, ascorbate, epithelial growth factor, and gentamicin) and with 5% endothelial cell growth-tested FBS that was provided with the EBM-2 medium. HUVECs were employed at the first to fourth passages for these studies. Adult HDMVECs were also purchased from Lonza Biologicals and cultured in the same medium used for HUVECs. The use of human tissue and cells as described above was approved by the Institutional Review Board of the University Hospitals of Cleveland and Case Western Reserve University.
Labeling MSCs with CM-DiI
The CM-DiI was dissolved in dimethyl sulfoxide to give a stock of 1 mg/mL. The stock was aliquoted and frozen. This stock was thawed and diluted immediately before use 1/1000 in Tyrode's solution. The MSCs and, where relevant, vascular endothelial cells were labeled while they were attached to culture dishes. The attached cells were washed twice with Tyrode's solution, and the diluted dye was added to the culture dish for 20–30 min at 37°C. The cells were rinsed three times with the Tyrode's solution, detached from the culture with trypsin, and counted. Fibroblasts and MSCs require a slightly longer labeling time than do endothelial cells (30 min compared to 20 min for endothelial cells).
Coculture conditions
The coculture conditions have been previously reported.23 Briefly, the first step was the preparation of confluent multilayer human papillary dermal fibroblast lawns either on 24-well culture plates or on 35-mm culture dishes (BD Falcon–BD Biosciences, San Jose, CA). The fibroblasts were seeded at a density of 37,500 cells/cm2 and were cultured in DMEM-lg containing 10% FBS (Gibco–Invitrogen) for 4–5 days. At this point, the culture medium was removed, and the cell layer was washed with Tyrode's solution. HUVECs were seeded onto fibroblast lawns at a density of 10,000 cells/cm2. In studies where HUVECs and MSCs were mixed, HUVECs were seeded at the same density as above; however, MSCs were seeded at a density of 2500 cells/cm2. This coculture system was incubated at 37°C with 5% CO2 in a medium that consisted of the EBM-2 base medium supplemented with 2% FBS (Lonza Biologicals, supplied with the base medium as a kit). Epidermal growth factor, ascorbate, and gentamicin were also added to the medium at levels recommended by the manufacturer (Lonza Biologicals). The angiogenic factors VEGF-A, FGF-2, and insulin-like growth factor-1 were not included in the coculture medium. Human BM-MSCs were treated in the same manner as described above for dermal fibroblasts to create high-density three-dimensional lawns onto which HUVECs were seeded. Either human BM-MSCs at a density of (2500 cells/cm2) were mixed with HUVECs (at a ratio of 1 BM-MSC to 4 HUVECs), or, in some studies, the BM-MSCs were seeded 5 days after the seeding of the HUVECs. The same EBM-2 culture medium as described above was used in cocultures containing BM-MSCs.
Observation of cultures
Live cocultures were observed daily and photographed using an Olympus IX71 inverted tissue culture microscope equipped with phase contrast and fluorescent optics (Olympus America, Melville, NY). Tube-like structures formed by the HUVECs could be visualized by phase contrast optics at about day 7 after the seeding of the HUVECs. The CM-DiI–labeled BM-MSCs could be visualized using fluorescent optics. It was possible to simultaneously visualize both the tube-like structures and MSCs by reducing the direct illumination to low levels and using UV illumination. At the termination of the cultures, typically at days 10–15 after the seeding of HUVECs, the cells were fixed at room temperature for 15 min using 60% acetone in water, a procedure that does not affect tissue culture plastic. The acetone was removed, and the cultures were washed twice with Tyrode's solution. The cultures were then immunostained in situ for 1 h at room temperature using a murine monoclonal antibody for human PECAM-1 (1/200 dilution of stock in 0.01 M phosphate-buffered saline [PBS] containing 0.05% bovine serum albumin [BSA]) and a second anti-mouse antibody conjugated with FITC (1/500 dilution of stock in 0.01 M PBS containing 0.05% BSA) for 1 h at room temperature. Nonfixed intact cultures were immunostained for CD44 (undiluted hybridoma medium) and decorin (1/200 dilution of stock in 0.01 M PBS containing 0.05% BSA) as described above. Before taking photographs, a fluorescence antifade solution30 was diluted 1:1 with Tyrode's solution and added to the wells. Photographs were taken using an Olympus DP Controller image capture system and saved as RGB color mode TIFF format images. Conversion to grayscale images and image exposure adjustments were made using Adobe Photoshop CS3. Image labeling was performed using Adobe Illustrator CS3, and the finished product was saved in TIFF format. Specificity for PECAM immunostaining was tested using nonrelevant murine antibodies.
For some studies, cultures were gently detached from the plastic dishes and snap frozen in O.C.T. compound (Sakura Finetek USA, Torrance, CA) and sectioned at a thickness of 8 μm for immunohistochemical analyses. Negative controls were incubated with nonimmune serum and FITC-conjugated second antibody for the same time intervals indicated above. Primary antibodies were diluted 1/100 and the second antibody was diluted 1/500 in the buffer indicated above. DAPI staining was performed on acetone-fixed sections. A coverglass was mounted on both whole-mount cultures and sections using a mounting medium that suppresses fading of fluorochromes.30 Digital photographs were taken on a Leica DML Microscope (Wetzlar, Germany) using SPOT Image Capture System (Diagnostic Instruments, Sterling Heights, MI) and saved in RBG color mode TIFF format. Color images were converted to grayscale images, and exposure was enhanced using Adobe Photoshop. Labeled composite images were created using Adobe Illustrator.
Matrigel assays
HDMVECs were seeded onto growth factor–depleted Matrigel either alone or mixed with DiI-labeled MSCs at a density of 10,000 cells/cm2 for the endothelial cells and 2500 cells/cm2 for MSCs. The HDMVECs seeded alone where cultured in EBM-2 base medium supplemented with 2% FBS, the recommended complement of proangiogenic factors provided by the manufacturer (Lonza Biologicals). Cultures that contained mixtures of MSCs and endothelial cells were cultured in EBM-2 base medium without proangiogenic factors.22,23 The cultures were monitored daily using an inverted tissue culture microscope with phase contrast optics (HDMVECs alone) or with combined phase contrast and fluorescence optics (mixed cell populations). Mixed cultures were assayed for MSC alignment with tubules and stability of these tubules.
Computer-assisted morphometric analyses
For analytical morphometric studies, cocultures were established in wells of 35-mm culture plates. Endothelial cells and tubular structures were visualized by fixing and immunostaining with antibody against PECAM-1. Digital images in the TIFF format were taken on a Leica microscope equipped with fluorescent optics using a 5 × objective lens. Eight photographs were taken in different regions of the culture dish. Quantitative measurements were restricted to tube-like structures; undifferentiated endothelial cells that were not part of tube-like structures were eliminated from the analyses. The extent to which tube-like structures formed was expressed in the terms of percent area occupied by these structures that form within the extracellular matrix elaborated by papillary dermal fibroblasts. These measurements were determined using Image Pro Plus 5.0 software in each of the eight fields. The percent areas occupied in the eight fields were then averaged and presented along with the standard deviation of the mean. The paired t-test was used to determine statistical significance in comparing specific parameters. p-Values of 0.05 or less were considered significant.
Results
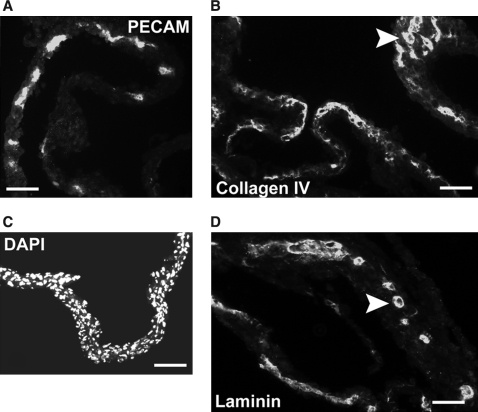
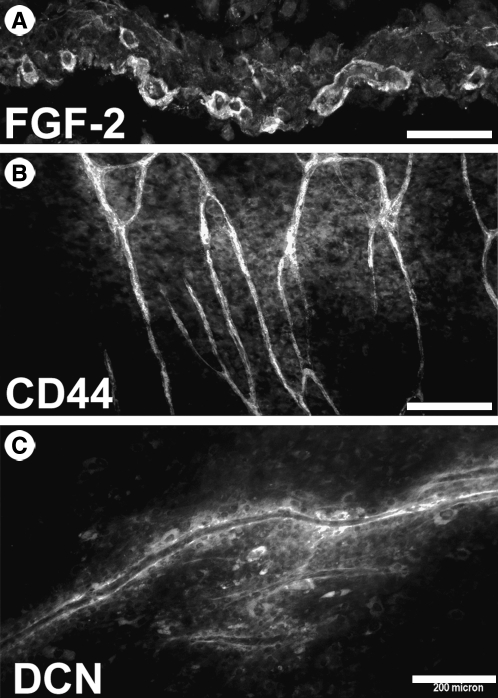
The studies reported here employed a coculture system in which adult human papillary dermal fibroblasts were seeded at high density to generate a multilayered, self-assembled lawn. HUVECs seeded onto this lawn differentiated and organized into tube-like vascular structures that matured and persisted for up to 2 weeks.22,23 The three-dimensional nature of these cocultures is evident from sections of these cultures that were lifted from the culture dish (Fig. 1). Vascular tube-like structures (Fig. 1A) were embedded in the fibroblast lawn (Fig. 1B). Bishop et al.22 have previously demonstrated by transmission electron microscopy the presence of lumens in these tubules. These structures were also surrounded by basement membrane proteins type IV collagen (Fig. 1C) and laminin (Fig. 1D). The extracellular matrix in the immediate vicinity of the tubules differed substantially from the rest of the culture matrix. FGF-2 was concentrated around tubules (Fig. 2A), presumably being bound to elements of the basement membrane. In whole-mount samples, the tubules, but not undifferentiated vascular endothelial cells, expressed CD44 (Fig. 2B) and were surrounded by a matrix enriched in the small dermatan sulfate proteoglycan decorin (Fig. 2C).
FIG. 1.
Fibroblast–HUVEC coculture sections. Frozen sections were cut from cocultures lifted intact from culture dishes. (A) PECAM-1 immunostaining shows tube-like structures embedded in fibroblast lawns. (B) DAPI staining of nuclei emphasizes the three-dimensional nature of the fibroblast lawns. (C) Type IV collagen immunostaining demonstrates the formation of basement membrane around tube-like structures (arrowhead). (D) Laminin immunostaining demonstrates the formation of basement membrane around tube-like structures (arrowhead). Scale bars: (A, C, D) 200 μm and (B) 500 μm.
FIG. 2.
Microenvironment of tube-like structures. (A) Frozen sections of cocultures were immunostained using an antibody against FGF-2. (B) Intact cultures were immunostained with an antibody that recognizes all splice-variants of CD44. (C) Intact cocultures were immunostained with an antibody that recognizes the small proteoglycan decorin (DCN). Scale bars: (A) 40 μm, (B) 300 μm, and (C) 200 μm.
Culture-expanded MSCs were labeled with CM-DiI before their seeding onto fibroblast lawns at a density of 5000 cells/well of a 24-well plate (2500 cells/cm2). The number of these cells was kept low to minimize random associations between these cells and tube-like structures. Although cell counts were not performed, there was no indication of a significant increase in the number of MSCs during their residence in cocultures. HUVECs were seeded onto fibroblast lawns at a standard density of 10,000 cells/cm2.
In the first set of studies, MSCs were mixed with HUVECs at a ratio of 1:4 and seeded randomly into wells that contained fibroblast lawns. The distribution of MSCs was followed in live cultures at daily intervals. However, it was not until approximately day 7 that tube-like structures were sufficiently large to be easily identified by phase contrast microscopy. These cultures were followed until day 10. At this point, one set of photographs was taken of live cultures using combined phase contrast optics and fluorescent optics. Labeled MSCs (Fig. 3A) were observed aligned with tube-like structures. Such cells were always elongated in the axis of the tubule. Nonaligned cells tended to be rounded. Because the CM-DiI label was restricted to the perinuclear region, it was not possible to appreciate the full extent of cellular interactions. At approximately 2 weeks, the cultures were terminated and immunostained for PECAM-1 to visualize the tube-like structures. Photographs were taken of CM-DiI–labeled cells and PECAM-labeled cells, and these images were merged to demonstrate cellular interactions (Fig. 3B).
FIG. 3.
BM-MSCs and HUVECs seeded together at day 0. (A) This photograph was taken using combined phase contrast and fluorescent optics on day 11. Arrows indicate CM-DiI–labeled MSCs aligned with tube-like structures. The circled cell is an example of a nonaligned BM-MSC. (B) This is a merged image taken at day 14, which shows CM-DiI (red)–labeled MSCs (arrows) aligned with PECAM-1 immunostained tube-like structures (green). Scale bars: (A) 500 μm and (B) 200 μm.
Live cultures observed daily gave no indication of physical interactions between coseeded HUVECs and BM-MSCs during the first 5–7 days of culture, because the marrow cells appeared to be distributed in a random manner during this period. However, the possibility of interactions could not be totally excluded because the unlabeled HUVECs were difficult to identify at these early time points. Therefore, a new set of studies was set up to determine whether BM-MSCs responded to organized vascular tubules. This was accomplished by seeding 5000 labeled BM-MSCs per well of 24-well plates on day 5, which is the time that previous studies have shown that tubules have begun to form.23 As shown in Figure 4A, coalignment occurred, but only after a delay of approximately 4 days. These cultures were terminated at day 13, and merged images were prepared to demonstrate the association between BM-MSCs and tube-like structures (Fig. 4B). Thus, similar results were obtained for both early and later seeding of BM-MSCs. This suggests that the MSCs may be interacting with tubules rather than individual endothelial cells.
FIG. 4.
BM-MSCs seeded on day 5. (A) Combined phase and fluorescent optics show aligned MSCs on day 9 (arrows). (B) The merged image for CM-DiI (red) BM-MSCs and PECAM-1 (green) immunostained tube-like structures shows alignment of BM-MSCs (arrows). Scale bars: (A) 500 μm and (B) 200 μm.
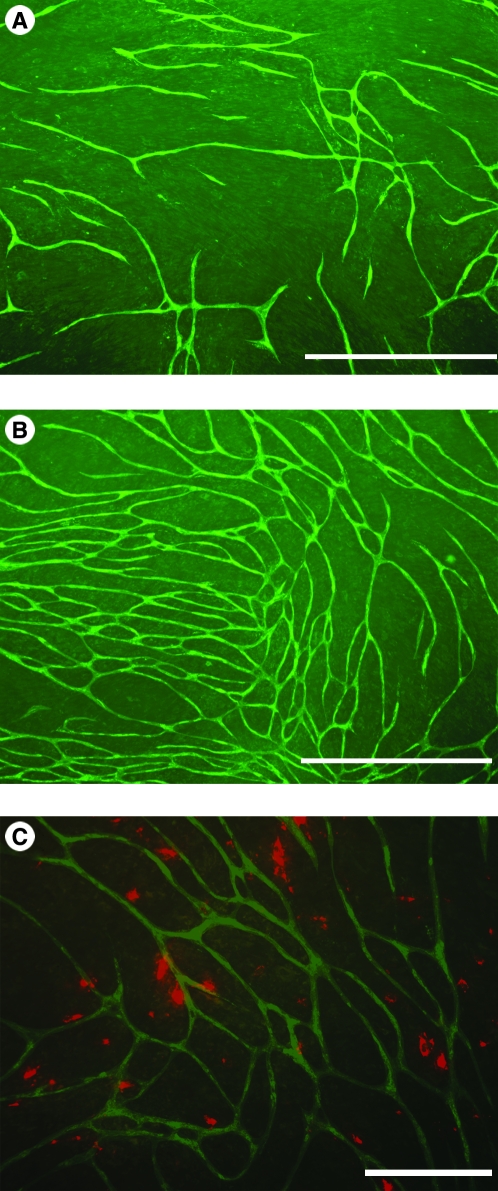
Previous studies have shown that the number and complexity of tube-like structures can be modulated by supplementing the culture medium with angiogenic factors.23,31 Thus, if BM-MSCs are producing significant amounts of angiogenic factors, they may also influence tubule formation. This was tested by seeding BM-MSCs from three different donors at a density of 5000 cells/well in 24-well plates (as described above) and quantifying the percent area occupied by tubules in a photographic field on day 13. As shown in Table 2, the presence of BM-MSCs from three different donors significantly increased the percent areas occupied by tubules. As shown for donor 1291, fewer tubules formed (Fig. 5A) in the absence of MSCs than formed when these cells were present (Fig. 5B). This effect on tubule formation was mediated by a relatively small number of cells (Fig. 5C).
Table 2.
MSC Effect on the Formation of Tube-Like Structures
| Control | Donor 1282a | Donor 1286a | Donor 1291a | |
|---|---|---|---|---|
| Percent areab | 3.97 | 6.98 | 8.60 | 10.81 |
| SD | 1.02 | 0.78 | 2.37 | 1.41 |
Indicates that the value is statistically different (p < 0.05 by the paired t-test) from the control value.
The mean value for the percent area occupied by tube-like structures.
SD, the standard deviation of the mean values.
FIG. 5.
Effect of BM-MSCs on the formation of vascular tube-like structures. (A) Tube-like structures formed in the absence of BM-MSCs (day 13). (B) Increased numbers of tube-like structure formed when BM-MSCs were coplated with HUVECs. There is also increased branching of tube-like structures. (C) The merged image shows the presence of CM-DiI–labeled MSCs in the culture photographed in (B). Scale bars: (A, B) 500 μm and (C) 200 μm.
The results presented above suggest that BM-MSCs communicated with HUVECs. The nature of this communication was assessed by creating dense BM-MSC lawns and seeding these lawns with HUVECs in the same manner as described above for dermal fibroblasts. Figure 6A–C demonstrates that tube-like structures formed on BM-MSC lawns established from three separate donors. All BM-MSC lawns supported tubule formation; however, there was substantial variability for the different lawns. BM-MSCs for the same three donors were also seeded onto papillary fibroblast lawns simultaneously with HUVECs (Fig. 6A’–C’). The presence of a limited number of BM-MSCs increased tubule formation on these lawns compared with HUVECs seeded alone onto the same lawn (Fig. 6D).
FIG. 6.

BM-MSC lawns support the formation and stability of tube-like structures. (A–C) Tube-like structures at day 13 on BM-MSC lawns immunostained with PECAM-1. (A) Donor 1490; (B) donor 1494; (C) donor 1496. (A′–C′) Tube-like structures formed at day 13 on papillary dermal fibroblast lawns seeded with mixtures of HUVECs and the same BM-MSC populations used for (A–C). Panel (D) shows tube-like structures formed at day 13 when HUVECs were seeded alone onto papillary dermal fibroblast lawns. Scale bars: 2 mm.
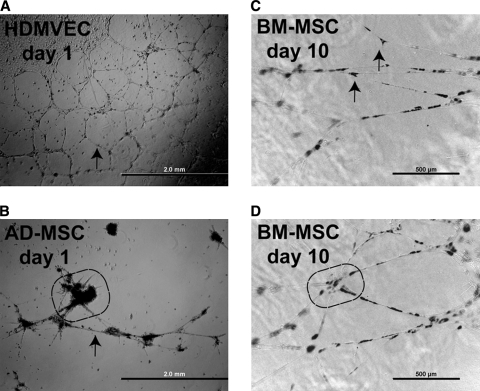
These results suggest that the MSCs provided factors that stabilized the tubules. However, it is clear that the dermal fibroblasts also provided similar factors. Therefore, the ability of MSCs to stabilize vascular structures was assessed on a Matrigel model where dermal fibroblasts were not present. This model has also been used by others to demonstrate stabilization effects by putative pericyte populations.25,32 Vascular endothelial cells such as HDMVECs (Fig. 7A) spontaneously organize into tubular structures when cultured in the presence of potent proangiogenic factors such as VEGF-A and FGF-2.24,25,32 These structures typically survive 2–3 days before disintegrating. However, the incorporation of pericytes has been shown to prolong stability for 7 days or longer.25 In this study, DiI-labeled MSCs that were derived either from adult human adipose tissue or from bone marrow from different donors were mixed with HDMVECs at a ratio of one MSC to four endothelial cells to assess the coalignment of these cells with tubular structures and to assess the effects of these cells on tubular stability. These mixed populations of cells were cultured in a medium that lacked VEGF-A and FGF-2.22,23 When HDMVECs were mixed with adipose tissue–derived MSCs (AD-MSCs), only sparse, rudimentary tubule formation occurred (Fig. 7B). The AD-MSCs formed large clusters, but otherwise displayed poor coalignment characteristics. The few vascular tubules that formed on day 1 disintegrated by day 2. Thus, these AD-MSCs failed to stabilize tubules. In contrast, BM-MSCs coaligned with tubules by day 1. Further, these tubules remained stable for 10 days in culture in the absence of VEGF-A or FGF-2 in the culture medium (Fig. 7C, D). The BM-MSCs frequently appeared at sites of tubule bifurcations (Fig. 7C). In addition, larger clusters of these BM-MSCs appeared at the hub of multiple branches (Fig. 7D). Thus, the BM-MSCs stabilize tubules in a manner like that of pericyte-like cells.25
FIG. 7.
BM-MSCs stabilize tubules formed on Matrigel. (A) Adult HDMVECs spontaneously form tubules on Matrigel when cultured in the presence of medium that contains proangiogenic factors, phase contrast optics, day 2 postseeding. (B) AD-MSCs coseeded with HDMVECs failed to stabilize tubules on Matrigel in the absence of exogenous proangiogenic factors, day 2 postseeding. The MSCs formed dense clusters on Matrigel (circled region) and failed to align. Combined phase contrast and fluorescence optics. (C) BM-MSCs coseeded with HDMVECs aligned with tubules and stabilized these structures when cultured in medium that lacked proangiogenic factors, day 10 postseeding. Arrows indicate BM-MSCs at sites of tubule branching. Combined phase contrast and fluorescence optics. (D) Multiple branches occurred at sites of higher concentrations of BM-MSCs, day 10 postseeding (circled region). Combined phase contrast and fluorescence optics. Scale bars: (A, B) 2 mm and (C, D) 500 μm.
Discussion
Cellular therapy models have been proposed as a means for efficiently delivering a complex repertoire of bioactive molecules to wound sites to hasten tissue restoration.8 This raises the issue of identifying putative therapeutic cellular populations and testing their effectiveness. To date, in vivo models have been primarily used for such identification purposes.9,11,12,33–35 Such models, while necessary, are expensive and lengthy endeavors. Thus, it would be helpful to develop in vitro models that would provide a preliminary screen for cellular populations that are likely to exert an in vivo effect.
At the minimum, a suitable in vitro model should provide extracellular matrix molecules and angiogenic factors, both of which are necessary for in vitro angiogenesis.24,36 Essential extracellular matrix formats include type I collagen gels, fibrin gels, or Matrigel.24,36 However, matrix molecules alone do not induce the differentiation events exhibited by vascular endothelial cells. Potent proangiogenic factors, such as VEGFs and FGFs, are equally essential.36 Thus, a suitable in vitro model should provide both essential matrix molecules and proangiogenic factors.
The Matrigel model has been used extensively for in vitro angiogenic assays because it provides essential extracellular matrix molecules and required growth factors can be added to the culture medium.24 The system can be further modified through the inclusion of mesenchymal cells in lieu of growth factors. Mesenchymal cells such as pericytes, MSCs, or fibroblasts coalign in this model.25 However, the critical issue is whether these cells provide sufficient quantities and varieties of growth factors to sustain vascular cords beyond 2–3 days. In this study, BM-MSCs, but not AD-MSCs, provided this additional structural stability. The inability of AD-MSCs to establish a perivascular phenotype is contrary to the observations of Zannetino et al.32 Clonal analyses of AD-MSCs have shown that multiple phenotypes can be derived from this population.29 Thus, the population used for this study may represent one that is incapable of interacting with vascular endothelial cells. In this study, BM-MSCs, but not AD-MSCs, provided a stabilizing influence that lasted for up to 10 days in culture in a culture medium that lacked VEGF-A and FGF-2. These results suggest that the BM-MSCs released both angiogenic and stabilization factors.
Due to limitations imposed by the Matrigel assay such as vascular instability and absence of tube-like structures,24 a second in vitro model was employed. In this system, both extracellular matrix molecules and required growth factors were produced by adult human dermal fibroblasts,23 making this system more akin to the in vivo situation. This three-dimensional platform allowed individual HUVECs to assemble into tube-like structures that have been reported to contain lumens22 in a period of 5–7 days.
Interactions between fibroblasts and vascular endothelial cells resulted in the formation of a basement membrane around tube-like structures,37 which again reprised a situation that occurs in vivo. The apparent association of FGF-2 with this basement membrane suggested the presence of heparan sulfate proteoglycans because this factor is known to bind heparan sulfate in tissues.38 In addition, FGF-2 also binds to dermatan sulfate chains such as those attached to the proteoglycan decorin39 that has been found in the perivascular regions of tube-like structures. The concentration of heparin-binding growth factors such as FGFs and PDGFs near these structures could affect not only angiogenic events but also interactions between vascular structures and mesenchymal cells.40 Decorin, which does not interact with hyaluronan, has previously been reported at sites of angiogenesis during inflammation.41 Decorin interacts with two proangiogenic growth factors, FGF-2 (Fig. 2A) and TGF-β1.42 Decorin binds and concentrates TGF-β1 in an inactive form; however, once released, this factor becomes functional. This factor has been proposed to play a role in the induction of the pericyte phenotype.43 Thus, the peritubular domains established in these cocultures create a microenvironment that differs from the surrounding matrix.
Hyaluronan, which has been proposed to engender a stem cell niche for both hematopoietic stem cells and MSCs, was not assayed in this study. Others have shown that MSCs that express CD44 migrate into regions with a high hyaluronan concentration.44,45 However, tube-like structures expressed the CD44 receptor that raises the possibility that hyaluronan may be localized in this region.
For this study, a third population of cells was introduced into the fibroblast–vascular endothelial cell coculture system. The BM-MSCs were either mixed with HUVECs for seeding on day 0, or they were seeded alone on day 5 after seeding HUVECs. The two time points were chosen to assess possible interactions between BM-MSCs and HUVECs that might occur during the early migration and alignment events that are typically completed by day 5. Other studies have shown that pericytes respond to newly formed vascular tubules rather than interact with individual vascular endothelial cells.46 Thus, coalignment of BM-MSCs would be expected to occur when these cells were added at both time points, and the results confirmed this expectation.
The BM-MSCs used in these studies were isolated from adult human bone marrow aspirates and cultured in exactly the same manner as were cells previously shown to differentiate along osteogenic, chondrogenic, and adipose lineages.13,28 This population of cells was chosen for this study due to recent reports in which similar cells have been shown to play a role in wound repair in various organs.9,11,12,16,33–35,47,48 The mechanisms by which MSCs accelerate wound repair are currently under investigation. They may involve complex paracrine interactions between the MSCs and various other cellular populations at wound sites.8 The potential angiogenic association of these cells may be related to their origin in the bone marrow where Bianco and others49–54 have proposed that these cells might arise from perivascular stromal cells. Consequently, these cells might be expected to interact with vascular structures elsewhere in the body. Additional evidence indicates that BM-MSCs can circulate and home to wound sites where they may assume a perivascular orientation.51 In this study, a subset of BM-MSCs elongated and coaligned in close proximity with vascular tube-like structures. Other studies in this laboratory (data not shown) have also indicated that these cells express α-smooth muscle actin, which is one of the markers to define pericytes.55,56 Au et al.54 have demonstrated that BM-MSCs produce mRNAs for α-smooth muscle actin, desmin, calponin, smoothelin, and the NG2 protein, all known pericyte markers. Neither Ziegelhoeffer et al.57 nor Au et al.54 found evidence that MSCs formed vascular endothelial cells. Likewise, no such evidence was found in this study.
The bone marrow contains a second population of fibroblast-like cells that have been shown to circulate and home to wound sites. Fibrocytes differ from MSCs in that they express hematopoietic markers such as CD34 and major histocompatibility markers that make these cells resemble antigen-presenting cells.2,14 Pericytes are mesenchymal cells that reside along the abluminal surfaces of the microvasculature where they form N-cadherin junctional complexes with vascular endothelial cells.15,26,55 The origin of pericytes is still obscure. This population may arise from multiple cellular populations that include other pericytes, myofibroblasts, fibrocytes, and MSCs.15,49–54 In vivo studies indicate that pericytes migrate to neovasculature subsequent to its formation, and they prevent the regression of those vessels that they contact.46 In this study, BM-MSCs appeared to be attracted to vascular tube-like structures and a subset coaligns with vascular structures. Au et al.54 recently found that BM-MSC migration toward vascular cells was dependent upon PDGF-BB signaling, a mechanism that has also been proposed to direct pericyte migration. In vivo data suggest that pericytes are attracted to newly formed vessels.46 This evidence also indicates that migration occurred only after tubule formation. In this study, coalignment occurred only after tubule formation, suggesting that it was intact tubules that attracted these cells.
The papillary dermal fibroblasts in this coculture system did not coalign with vascular structures. Nevertheless, these dermal fibroblasts provided essential growth factors and matrix molecules necessary for tubule formation and stabilization. The observation of interactions between BM-MSCs and vascular endothelial cells in both in vitro assays implies a level of communication between these two cellular populations. The nature of these communications remains to be determined. The release of soluble factors by MSCs has been demonstrated in studies by Gruber et al.,58 who demonstrated that gelatin sponges soaked in MSC-conditioned medium enhanced vascular development using a chick chorioallantoic membrane assay. Conditioned medium from BM-MSCs has been shown to induce the vascular-like organization of endothelial cells on Matrigel or collagen substrates.59,60 Tille and Pepper59 also found that murine MSCs produced factors other than VEGF-A or FGF-2 that were essential for angiogenesis. These studies indicate that BM-MSCs fulfill at least one aspect of therapeutically delivered cells in that they release multiple factors that may exert influences over angiogenic events. It is not clear in the current study whether BM-MSCs merely augment factors already released by dermal fibroblasts or whether they release unique factors.
This study provides evidence that adult human BM-MSCs act as facilitator cells with respect to the angiogenic process. This raises the issue whether these cells might be employed in a tissue-engineered setting to promote and stabilize in vitro produced vasculature. Au et al.54 have recently published evidence supporting this notion. This raises the possibility that a variety of multicellular strategies might be developed to help to promote a more rapid and effective vascularization of grafted implants.
Acknowledgments
We gratefully acknowledge Dr. Donald Lennon for providing the adult human BM-MSCs used for these studies, and we would also like to thank Dr. Farshid Guilak for the AD-MSCs used in these studies. These studies were supported, in part, by the L. David and Virginia Baldwin Foundation and by NIH Grant AG021542.
Disclosure Statement
None of the authors have a financial conflict of interest associated with the contents of this manuscript.
References
- 1.Tonnessen M.G. Feng X. Clark R.A.F. Angiogenesis in wound healing. J Investig Dermatol Symp Proc. 2000;5:34. doi: 10.1046/j.1087-0024.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- 2.Metcalfe A.D. Ferguson M.W.J. Bioengineering skin using mechanisms of regeneration and repair. Biomaterials. 2007;28:100. doi: 10.1016/j.biomaterials.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 3.Pierce G.F. Tarpley J.E. Tseng J. Bready J. Chang D. Kenney W.C. Rudolph R. Robson M.C. Vande Berge J. Reid P. Kaufman S. Farrell C. Detection of platelet-derived growth factor (PDGF)-AA in actively healing human wounds treated with recombinant PDGF-BB and absence of PDGF in chronic nonhealing wounds. J Clin Invest. 1995;96:1336. doi: 10.1172/JCI118169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Obara K. Ishihara M. Fujita M. Kanatani Y. Hattori H. Matsui T. Takas B. Ozeki Y. Nakamura S. Ishizuka T. Tominaga S. Hiroi S. Kawai T. Maehara T. Acceleration of wound healing in healing-impaired db/db mice with a photocrosslinkable chitosan hydrogel containing fibroblast growth factor-2. Wound Repair Regen. 2005;13:390. doi: 10.1111/j.1067-1927.2005.130406.x. [DOI] [PubMed] [Google Scholar]
- 5.Zymek P. Bujak M. Chatila K. Cieslak A. Thakker G. Entman M.L. Frangogiannis N.G. The role of platelet-derived growth factor signaling in healing myocardial infarcts. J Am Coll Cardiol. 2006;48:2315. doi: 10.1016/j.jacc.2006.07.060. [DOI] [PubMed] [Google Scholar]
- 6.Li J.L. Chen J. Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol. 2007;25:9. doi: 10.1016/j.clindermatol.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Cao R. Brakenhielm E. Pawliuk R. Wariaro D. Post M.J. Wahlberg E. Leboulch P. Cao Y. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat Med. 2003;9:604. doi: 10.1038/nm848. [DOI] [PubMed] [Google Scholar]
- 8.Caplan A.I. Dennis J.E. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 9.Badiavas E.V. Abedi M. Butmarc J. Falanga V. Quesenberry P. Participation of bone marrow derived cells in cutaneous wound healing. J Cell Physiol. 2003;196:245. doi: 10.1002/jcp.10260. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y. Dulchavsky D.S. Gao X. Kwon D. Chopp M. Dulchavsky S. Gautam S.C. Wound repair by bone marrow stromal cells through growth factor production. J Surg Res. 2006;136:336. doi: 10.1016/j.jss.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 11.Falanga V. Iwamoto S. Charter M. Yufit T. Butmarc J. Kouttab N. Shrayer D. Carson P. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007;13:1299. doi: 10.1089/ten.2006.0278. [DOI] [PubMed] [Google Scholar]
- 12.Tögel F. Weiss K. Yang Y. Hu Z. Zhang P. Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol. 2007;292:F1626. doi: 10.1152/ajprenal.00339.2006. [DOI] [PubMed] [Google Scholar]
- 13.Caplan A.I. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 14.Quan T.E. Cowper S. Wu S.-P. Bockenstedt L.K. Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol. 2004;36:598. doi: 10.1016/j.biocel.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Xueyong L. Shaozong C. Wangzhou L. Yuejun L. Xiaoxing L. Jing L. Jinqing L. Differentiation of the pericyte in wound healing: the precursor, the process, and the role of the vascular endothelial cell. Wound Repair Regen. 2008;16:346. doi: 10.1111/j.1524-475X.2008.00374.x. [DOI] [PubMed] [Google Scholar]
- 16.Wu Y. Chen L. Scott P.G. Tredget E.E. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 17.Wang J.F. Jiao H. Stewart T.L. Shankowsky H.A. Scott P.G. Tredget E.E. Fibrocytes from burn patients regulate the activities of fibroblasts. Wound Repair Regen. 2006;15:113. doi: 10.1111/j.1524-475X.2006.00192.x. [DOI] [PubMed] [Google Scholar]
- 18.Opalenik S.R. Davidson J.M. Fibroblast differentiation of bone marrow-derived cells during wound repair. FASEB J. 2005;19:1561. doi: 10.1096/fj.04-2978fje. [DOI] [PubMed] [Google Scholar]
- 19.Chamberlain G. Fox J. Ashton B. Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 20.MacNeil S. Progress and opportunities for tissue-engineered skin. Nature. 2007;445:874. doi: 10.1038/nature05664. [DOI] [PubMed] [Google Scholar]
- 21.Clark R.A.F. Ghosh K. Tonnesen M.G. Tissue engineering for cutaneous wounds. J Invest Dermatol. 2007;127:1018. doi: 10.1038/sj.jid.5700715. [DOI] [PubMed] [Google Scholar]
- 22.Bishop E.T. Bell G.T. Bloor S. Broom I.J. Hendry N.F.K. Wheatley D.N. An in vitro model of angiogenesis: basic features. Angiogenesis. 1999;3:335. doi: 10.1023/a:1026546219962. [DOI] [PubMed] [Google Scholar]
- 23.Sorrell J.M. Baber M.A. Caplan A.I. Human dermal fibroblast subpopulations; differential interactions with vascular endothelial cells in co-culture. Non-soluble factors in the extracellular matrix influence interactions. Wound Repair Regen. 2008;16:300. doi: 10.1111/j.1524-475X.2008.00369.x. [DOI] [PubMed] [Google Scholar]
- 24.Staton C.A. Stribbling S.M. Tazzyman S. Hughes R. Brown N.J. Lewis C.E. Current methods for assaying angiogenesis in vitro and in vivo. Int J Expt Pathol. 2004;85:233. doi: 10.1111/j.0959-9673.2004.00396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song S. Ewald A.J. Stallcup W. Werb Z. Bergers G. PDGFRβ+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat Cell Biol. 2005;7:870. doi: 10.1038/ncb1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armulik A. Abramsson A. Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 27.Sorrell J.M. Baber M.A. Caplan A.I. Site-matched papillary and reticular human dermal fibroblasts differ in their release of specific growth factors/cytokines and in their interaction with keratinocytes. J Cell Physiol. 2004;200:134. doi: 10.1002/jcp.10474. [DOI] [PubMed] [Google Scholar]
- 28.Lennon D.P. Haynesworth S.E. Arm D.M. Baber M.A. Caplan A.I. Dilution of human mesenchymal stem cells with dermal fibroblasts and the effects on in vitro and in vivo osteochondrogenesis. Dev Dyn. 2000;219:50. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1037>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 29.Guilak F. Lott K.E. Awad H.A. Cao Q. Hicok K.C. Fermor B. Gimble J.M. Clonal analysis of the differentiation potential of human adipose-derived adult stem cells. J Cell Physiol. 2006;206:229. doi: 10.1002/jcp.20463. [DOI] [PubMed] [Google Scholar]
- 30.Johnson G.D. Davidson R.S. McNamee K.C. Russell G. Goodwin D. Holborow E.J. Fading of immunofluorescence during microscopy: a study of the phenomenon and its remedy. J Immunol Methods. 1982;55:231. doi: 10.1016/0022-1759(82)90035-7. [DOI] [PubMed] [Google Scholar]
- 31.Beilmann M. Birk G. Lenter M.C. Human primary co-culture angiogenesis assay reveals additive stimulation and different angiogenic properties of VEGF and HGF. Cytokine. 2004;26:178. doi: 10.1016/j.cyto.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 32.Zannetino A.C.W. Paton S.A.A. Khor F. Itescu S. Gimble J.M. Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J Cell Physiol. 2008;214:413. doi: 10.1002/jcp.21210. [DOI] [PubMed] [Google Scholar]
- 33.Kinnaird T. Stabile E. Burnett M.S. Shou M. Lee C.W. Fuchs S. Epstein S.E. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 34.Nagaya N. Fujii T. Iwase T. Ohgushi H. Itoh T. Uematsu M. Yamagishi M. Mori H. Kangawa K. Kitamura S. Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocardial infarction through angiogenesis and myogenesis. Am J Physiol. 2004;287:H2670. doi: 10.1152/ajpheart.01071.2003. [DOI] [PubMed] [Google Scholar]
- 35.Tang Y. Zhao Q. Qin X. Shen L. Cheng L. Ge J. Phillips M.I. Paracrine action enhances the effects of autologous mesenchymal stem cell transplantation on vascular regeneration in rat model of myocardial infarction. Ann Thorac Surg. 2005;80:229. doi: 10.1016/j.athoracsur.2005.02.072. [DOI] [PubMed] [Google Scholar]
- 36.Jain R.K. Booth M.F. What brings pericytes to tumor vessels? J Clin Invest. 2003;112:1134. doi: 10.1172/JCI20087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sorrell J.M. Baber M.A. Caplan A.I. A self-assembled fibroblast-endothelial cell co-culture system that supports in vitro vasculogenesis by both human umbilical vein endothelial cells and human dermal microvascular endothelial cells. Cells Tissues Organs. 2007;186:157. doi: 10.1159/000106670. [DOI] [PubMed] [Google Scholar]
- 38.Klagsbrun M. The affinity of fibroblast growth factors (FGFs) for heparin: FGF-heparan sulfate interactions in cells and extracellular matrix. Curr Opin Cell Biol. 1990;2:857. doi: 10.1016/0955-0674(90)90084-r. [DOI] [PubMed] [Google Scholar]
- 39.Taylor K.R. Gallo R.L. Glycosaminoglycans and their proteoglycans: host-associated molecular patterns for initiation and modulation of inflammation. FASEB J. 2006;20:9. doi: 10.1096/fj.05-4682rev. [DOI] [PubMed] [Google Scholar]
- 40.Kano M.R. Morishita Y. Iwata C. Iwasaka S. Watabe T. Ouchi Y. Miyazono K. Miyazawa K. VEGF-A and FGF-2 synergistically promote neoangiogenesis through enhancement of endogenous PDGF-B-PDGFR beta signaling. J Cell Sci. 2005;118:3759. doi: 10.1242/jcs.02483. [DOI] [PubMed] [Google Scholar]
- 41.Nelimarkka L. Salminen H. Kuopio T. Nikkari S. Ekfors T. Laine J. Peliniemi L. Jarvelainen H. Decorin is produced by capillary endothelial cells in inflammation-associated angiogenesis. Am J Pathol. 2001;158:345. doi: 10.1016/S0002-9440(10)63975-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iozzo R.V. The biology of the small leucine-rich proteoglycans. J Biol Chem. 1999;274:18843. doi: 10.1074/jbc.274.27.18843. [DOI] [PubMed] [Google Scholar]
- 43.Hirschi K.K. Rohovsky S.A. D'Amore P.A. PDGF, TGF-β, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol. 1998;141:805. doi: 10.1083/jcb.141.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu H. Mitsuhashi N. Klein A. Barsky L.W. Weinberg K. Barr M.L. Demetriou A. Wu G.D. The role of the hyaluronan receptor CD44 in mesenchymal stem cell migration in the extracellular matrix. Stem Cells. 2006;24:928. doi: 10.1634/stemcells.2005-0186. [DOI] [PubMed] [Google Scholar]
- 45.Herrera M.B. Bussolati B. Bruno S. Morando L. MaurielloRomanazzi G. Sanavio F. Stamenkovic I. Biancone L. Camussi G. Exogenous mesenchymal stem cells localize to the kidney by means of CD44 following acute tubular injury. Kidney Int. 2007;72:430. doi: 10.1038/sj.ki.5002334. [DOI] [PubMed] [Google Scholar]
- 46.Benjamin L.E. Hemo I. Keshet E. A plasticity window for blood vessel remodeling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development. 1998;125:1591. doi: 10.1242/dev.125.9.1591. [DOI] [PubMed] [Google Scholar]
- 47.Cui X. Chen J. Zacharek A. Li Y. Roberts C. Kapke A. Savert-Bansale S. Chopp M. Nitric oxide donor upregulation of stromal cell-derived factor-1/chemokine (CXC motif ) receptor 4 enhances bone marrow stromal cell migration into ischemic brain after stroke. Stem Cells. 2007;11:2777. doi: 10.1634/stemcells.2007-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parr A.M. Tator C.H. Keating A. Bone marrow-derived mesenchymal stromal cells for the repair of central nervous system injury. Bone Marrow Transplant. 2007;40:609. doi: 10.1038/sj.bmt.1705757. [DOI] [PubMed] [Google Scholar]
- 49.Bianco P. Riminucci M. Gronthos S. Robey P.G. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 50.Rajantie I. Ilmonen M. Alminaite A. Ozerdem U. Alitalo K. Salven P. Adult bone marrow-derived cells recruited during angiogenesis comprise precursors for periendothelial vascular mural cells. Blood. 2004;104:2084. doi: 10.1182/blood-2004-01-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ozerdem U. Alitalo K. Salven P. Li A. Contribution of bone marrow-derived pericyte precursor cells to corneal vasculogenesis. Invest Ophthalmol Vis Sci. 2005;46:3502. doi: 10.1167/iovs.05-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kokovay E. Li L. Cummingham L.A. Angiogenic recruitment of pericytes from bone marrow after stroke. J Cereb Blood Flow Metab. 2006;26:545. doi: 10.1038/sj.jcbfm.9600214. [DOI] [PubMed] [Google Scholar]
- 53.Lamagna C. Bergers G. The bone marrow constitutes a reservoir of pericyte progenitors. J Leukoc Biol. 2006;80:677. doi: 10.1189/jlb.0506309. [DOI] [PubMed] [Google Scholar]
- 54.Au P. Tam J. Fukumura D. Jain R.K. Bone marrow-derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood. 2008;111:4551. doi: 10.1182/blood-2007-10-118273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gerhardt H. Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003;314:15. doi: 10.1007/s00441-003-0745-x. [DOI] [PubMed] [Google Scholar]
- 56.Sundberg C. Kowanetz M. Brown L.F. Detmar M. Dvorak H.F. Stable expression of angiopoietin-1 and other markers by cultured pericytes: phenotypic similarities to a subpopulation of cells in maturing vessels during later stages of angiogenesis in vivo. Lab Invest. 2002;82:387. doi: 10.1038/labinvest.3780433. [DOI] [PubMed] [Google Scholar]
- 57.Ziegelhoeffer T. Fernandez B. Kostin S. Heil M. Voswinckel R. Helisch A. Schaper W. Bone-marrow derived cells do not incorporate into the adult growing vasculature. Circ Res. 2004;94:230. doi: 10.1161/01.RES.0000110419.50982.1C. [DOI] [PubMed] [Google Scholar]
- 58.Gruber R. Kandler B. Holzmann P. Vögele-Kadleetz M. Losert U. Fisher M.B. Watzek G. Bone marrow stromal cells can provide a local environment that favors migration and formation of tubular structures of endothelial cells. Tissue Eng. 2005;11:896. doi: 10.1089/ten.2005.11.896. [DOI] [PubMed] [Google Scholar]
- 59.Tille J.-C. Pepper M.S. Mesenchymal cells potentiate vascular endothelial growth factor-induced angiogenesis in vitro. Exp Cell Res. 2002;280:179. doi: 10.1006/excr.2002.5635. [DOI] [PubMed] [Google Scholar]
- 60.Hung S.-C. Pochampally R.A. Chen S.-C. Hsu S.-C. Prockop D.J. Angiogenic effects of human multipotent stromal cell conditioned medium activate the PI3K-Akt pathway in hypoxic endothelial cells to inhibit apoptosis, increase survival, and stimulate angiogenesis. Stem Cells. 2007;25:2364. doi: 10.1634/stemcells.2006-0686. [DOI] [PubMed] [Google Scholar]