Abstract
The objective of this study was to determine if three different biomimetic approaches could facilitate tissue regeneration and improve viscoelastic properties in the scarred vocal fold lamina propria extracellular matrix (ECM). Twenty rabbit vocal folds were biopsied bilaterally; 2 months postinjury rabbits were unilaterally treated with (i) autologous fibroblasts, (ii) a semisynthetic ECM (sECM), or (iii) autologous fibroblasts encapsulated in sECM. Saline was injected as a control into the contralateral fold. Animals were sacrificed 2 months after treatment. Outcomes measured were procollagen, collagen, and fibronectin levels in the lamina propria, and tissue viscosity and elasticity across three frequency decades. All treatment groups demonstrated accelerated proliferation of the ECM. Vocal fold lamina propria treated with autologous fibroblasts were found to have significantly improved viscosity (p = 0.0077) and elasticity (p = 0.0081) compared to saline. This treatment group had significantly elevated fibronectin levels. sECM and autologous fibroblasts/sECM groups had significantly elevated levels of procollagen, collagen, and fibronectin, indicating abundant matrix production as compared to saline with viscoelastic measures that did not differ statistically from controls. The use of autologous fibroblasts led to better restoration of the vocal fold lamina propria biomechanical properties. Optimization of cell–scaffold interactions and subsequent cell behavior is necessary for utilization of scaffold and scaffold–cell approaches.
Introduction
Biomimetic tissue engineering strategies focus on the function of cells and the role of biomaterials to actively stimulate tissue healing and regeneration. Cell-based and bioactive biomaterials and tissue engineering tools and methods create exciting new opportunities that may be useful in laryngology for treatment of vocal fold scarring and other existing extracellular matrix (ECM) defects of the lamina propria, exclusively for the superficial and middle layers. Another approach for engineering, the vocal fold lamina propria may be important such that tissue-specific scaffolds may provide a vehicle for delivery of cells into the graft site, facilitating their retention and distribution throughout the region where new tissue is desired.
For vocal fold lamina propria, two tissue engineering approaches have predominately been reported for potential treatment of vocal fold scar: injection of cells and injection of scaffolds. Cell approaches have included autologous and nonautologous mesenchymal stem cells,1–3 autologous fibroblasts,4 and human embryonic stem cells.5 Various engineered scaffolds tested include acellular xenogeneic scaffolds,6,7 collagen hydrogels,8 and hyaluronic acid (HA)–based hydrogels.9–12 A regenerative medicine approach, that is, cells injected concurrently with an in situ crosslinkable scaffolding, has not been reported to date as an approach for vocal fold regeneration.
Use of cell-seeded, resorbable synthetic scaffolds may serve as a tissue substitute for facilitation of tissue regeneration. Resorbable hydrogels that can be crosslinked in situ provide a vehicle for introducing and retaining cells into the defect site and for facilitating recruitment of new cells to the injured area. Tissue-specific scaffolds can be engineered to optimize the shear thinning and viscoelastic properties to best meet the vibrational frequencies encountered in phonation and to minimize degradation and resorption. As scaffolds degrade, they allow for transfer of mechanical stress to the newly formed tissue. Chemically modified HA hydrogels have been engineered to have a longer residence time in the tissues,13 and have been engineered specifically for translational use in three-dimensional cell and cell therapy.14 Further, HA hydrogels have been shown to have viscoelastic properties similar to those of human vocal fold mucosa,10–12,15 improve wound healing in a prophylactic rabbit wound healing model, and are biocompatible.16 Specific compositions of an HA-based semisynthetic mimic of the native ECM have been optimized for human fibroblasts.17 To further build on our previous research demonstrating the effectiveness of HA hydrogels in a prophylactic injury model of the vocal fold lamina propria, we developed a rabbit vocal fold scar model to assess three approaches for tissue regeneration. The purpose of this study was to determine if the biomechanical and biological properties of vocal fold lamina propria scar could be improved with injection of (i) autologous fibroblasts, (ii) a semisynthetic ECM (sECM), or (iii) autologous fibroblasts encapsulated into the sECM when compared to saline-treated controls.
Materials and Methods
Vocal fold biopsy
All animal procedures followed an approved protocol of Institutional Animal Care and Use Committee at The University of Utah. Twenty 4-month-old male New Zealand White breeder rabbits (Western Oregon Rabbit Company, Philomath, OR), each weighing 2.6–3.0 kg, were sedated intramuscularly with xylazine 5 mg/kg and ketamine 35 mg/kg. To create vocal fold scar, vocal fold biopsies were made as previously described.12,18 With the animal supine, microscopic direct laryngoscopy was completed using a Pillings pediatric endoscope (Pilling, Horsham, PN). Once visualized, a 2 mm forceps biopsy (Microfrance, Montreal, Canada) was removed from the midmembraneous point of the vocal fold bilaterally. Postoperatively, buprenorphine (0.05 mg/kg) subcutaneous was provided for analgesia.
Autologous fibroblast isolation and expansion
Vocal fold biopsies from 10 rabbits were used for fibroblast isolation and in vitro expansion as described previously.19 Careful notation and handling were required to ensure that cells from each animal were cultured and grown separately. Vocal fold biopsies were transported in Dulbecco's modified Eagle's medium/nutrient mixture F-12 (DMEM/F-12, 1 : 1 mixture; Gibco, Rockville, MD) supplemented with 10% fetal bovine serum (Gibco; growth medium used throughout the study). Vocal fold biopsies were rinsed once with 70% alcohol and twice with Dulbecco's phosphate-buffered saline (DPBS; Gibco), cut into small fragments under sterile conditions, and seeded on 60-mm cell culture dishes that were precoated with medium. Medium was added gently to the top of the tissue pieces. The medium was changed every 3 days for 14 days, after which fibroblasts had grown from the tissue. Tissues were removed, and cells were grown to 80% confluence and then passaged to T75 flasks (passage 1). Passage treatment involved 0.125% trypsin with division of the cells into eight equal subsets. Those cells were plated and grown to 100% confluence, at which time they were passaged once more (passage 2, 1:8). Every 7 days thereafter, 100% confluent cells were trypsinized and passed (1:8), with the medium changed every second day. Passage 4 cells were used for this study; fibroblast expansion took 70 days.
Treatment
Three months after biopsy, rabbits were anesthetized and visualized as above. Five rabbits were randomly assigned to each of the four treatment groups: (i) saline treated, (ii) synthetic ECM treated (sECM), (iii) autologous fibroblast treated, and (iv) autologous fibroblasts + sECM treated. Rabbits were bilaterally injected using a 26-gauge needle (Microfrance, Terrebonne, QC, Canada). Control rabbits were injected with 0.30 mL of sterile saline. For the second treatment group (sECM), 0.30 mL HA-DTPH (half 3′3′ dithiobis [propanoic hydrazide])/gelatin-DTPH/poly(ethylene glycol) dia-crylate (PEGDA) gel (HA-DTPH, 42% modification, i.e., 42 thiol groups per 100 disaccharide units; Mw 158 kDa, Mn 78 kDa, polydispersity index = 2.03) was synthesized and injected as described.20–22 Gelatin-DTPH was prepared as described for HA-DTPH. The HA-DTPH and gelatin-DTPH were dissolved in serum-free medium at a concentration of 1.25 and 3.0% (w/v), and the pH was adjusted to 7.4 by the addition of 1.0 N sodium hydroxide. A solution of 4% PEGDA (3400 kDa; Nektar Therapeutics, Huntsville, AL) was prepared by dissolving PEGDA in DPBS. All solutions were sterilized by filtration (pore size 0.45 μm). The third group was injected with autologous fibroblasts (0.30 mL) that were harvested and suspended in serum-free medium at 50 × 106 cells/mL. The last group was injected with autologous fibroblast–loaded HA-DTPH/gelatin-DTPH/PEGDA gel (0.30 mL). In vitro–expanded fibroblasts (50 × 106 cells/mL) were suspended in ungeled sECM solution. The HA hydrogels were prepared as above and were allowed to partially gel for 2 min at room temperature in a 26-gauge needle before injection. Gelling minimized any leakage of the hydrogel out of the puncture site at the time of injection. All animals were provided with Buprenex (0.05 mg/kg) postoperatively for pain management. All animals were sacrificed 2 months after unilateral intervention (5 months after initial injury) by an IV administration of Beuthanasia-D (0.05 mg/kg). The larynx and vocal folds were removed.
Histology
Right vocal folds from each animal were placed in 10% formaldehyde for a minimum of 24 h and a maximum of 48 h. Routine processing, including alcohol dehydration and paraffin embedding, was completed. Vocal folds were cut into 5-μm-thick coronal sections, dehydrated for 30 min in a 37°C oven, incubated in acetone for 10 min, and incubated in Morphosave (Ventana Medical Systems, Tucson, AZ) for 15 min and in 3% hydrogen peroxide (Dako, Carpinteria, CA) for 5 and 10 min in Sniper block (Biomedical Medical, Walnut Creek, CA). Hematoxylin and eosin stain was used for orientation and morphology. Trichrome stain was used to stain collagen. Immunohistochemical analyses were undertaken with mouse monoclonal antibodies directed against human fibronectin monoclonal antibodies, IST-3 (Sigma, St. Louis, MO) at a concentration of 1:20, and monoclonal antibodies (SP1.D8; Hybridoma Bank, Iowa City, IA) directed against procollagen 1 (concentration 1:100). Rabbit skin and mitral valve were used as controls to determine appropriate dilution and developing time for all antibodies. Primary antibodies were incubated for 60 min at room temperature, washed with phosphate-buffered saline (PBS), and further incubated for 60 min with mouse anti-human IgG antibodies conjugated with streptavidin (Dako) at room temperature. The streptavidin label was located with 3-amino-9-ethylcarbazole for 10 min before washing, counter staining with hematoxylin, and mounting. Primary fibromodulin antibodies were incubated for overnight at 4°C. Primary IgG were detected using goat anti-mouse secondaries conjugated to Envision+ (Dako), a dextran alkaline phosphatase backbone for 30 min. Colorimetric reagent was diaminobenzidine chromogen, and all sections were counterstained with hematoxylin. To perform negative controls, the specific first antibody was substituted with an IgG primary for all proteins measured. No positive reaction was measured.
Images were captured with the use of a Nikon Eclipse E600 microscope (Nikon, Melville, NY) and a Pixera color camera (Pixera, Los Gatos, CA). The intensity (amount of stain per unit area) of the ECM molecule of interest was measured and recorded by Metamorph Image Analysis Software (Universal Imaging, West Chester, PA). The lamina propria of each vocal fold was examined starting at the superficial layer and progressing to the border of the vocalis muscle. There was no overlap of frames. Each sample was normalized to the total area to allow comparison between the different samples, regardless of size of the lamina propria. For each vocal fold, a total of 10 sections were analyzed. Ten percent of the slides were remeasured for interrater reliability (p = 0.58, paired t-test).
Vocal fold tissue rheology
Viscoelastic properties of the left vocal fold lamina propria from each animal were determined using dynamic rheometry, including elastic shear moduli (G′) and viscous moduli (G″) as a function of oscillatory frequency (0.01–10 Hz), as described by Klemuk and Titze.23 All rheological testing was carried out using a controlled stress rheometer, Bohlin CVO120 (Malvern Instruments, Worcestershire, UK). Vocal folds were immediately flash frozen when removed from the larynx.24 All samples were stored at −80°C until testing, at which time each was thawed at room temperature and hydrated with PBS. All rheological measurements were taken without knowledge of treatment condition. Gaps were 0.1–0.6 mm. A parallel plate setup was used, with a stationary lower plate and a rotating upper plate (8 mm diameter). Wet–dry sandpaper was affixed to both the attachment and the base plate to avoid slippage between the sample and the rheometer surfaces. Temperature during testing was maintained at 37°C ± 0.5°C through the use of a water-jacketed base plate. Shear stress, shear strain, and strain rate associated with the oscillatory shear deformation were computed from the prescribed torque and the measured angular velocity by a computer, and viscoelastic data were obtained based on these functions.
Statistical analysis
Histological image analysis data were analyzed with analysis of variance to examine differences between the four groups. Fisher's protected least significant difference tests were used for pair-wise comparisons. Rheological data were analyzed utilizing a repeated measures analysis of covariance assuming a first-order autoregressive error structure within a subject using SAS PROC MIXED (SAS Institute, Cary, NC). p-Values less than 0.05 were considered statistically significant.
Results
Histology
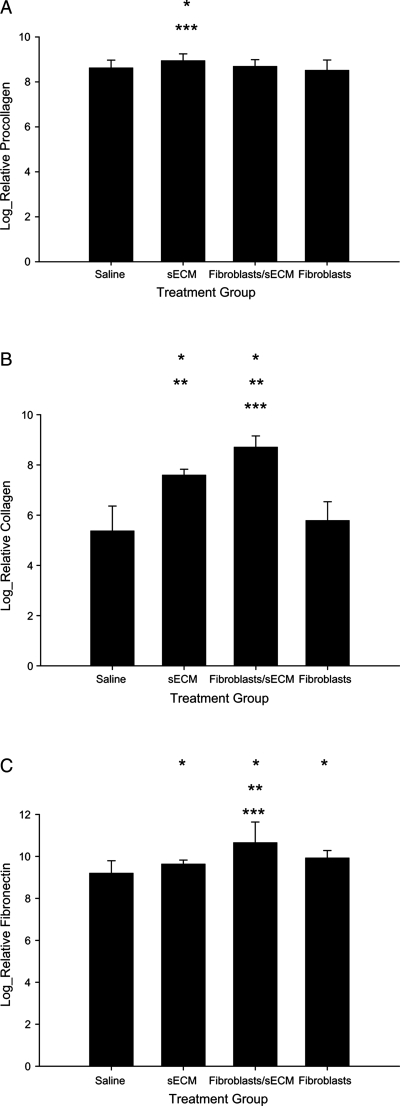
Collagen, procollagen, and fibronectin were observed in all vocal fold samples and in all layers of the lamina propria. Log-transformed means and standard deviations for each of the proteins are in Figure 1. Normalized procollagen intensity was significant between groups [F(3,71), p = 0.0224], with procollagen being higher in the sECM group as compared to saline-treated group (p = 0.015) and for the sECM group as compared to the autologous fibroblast–treated group (p = 0.0025). Significant differences for procollagen intensity were not found when comparing the autologous fibroblasts or the autologous fibroblast–treated/sECM-treated group to saline-treated group (p = 0.3141 and p = 0.6157, respectively), or to each other (p = 0.2171).
FIG. 1.
Histological levels for (A) procollagen, (B) collagen, and (C) fibronectin for four conditions—saline treated, autologous fibroblast treated, sECM treated, and sECM/autologous fibroblast treated. *p < 0.05 compared to saline treated; **p < 0.05 compared to sECM; ***p < 0.05 compared to autologous fibroblast treated.
For collagen (Fig. 1B), significant differences were measured between the treatment groups [F(3,57), p < 0.0001] with the autologous fibroblast/sECM group had significantly greater levels than saline-treated (p < 0.0001), sECM-treated (p = 0.02), and autologous fibroblast–treated (p < 0.0001) groups. Further significant differences were measured between autologous fibroblast and sECM (p < 0.0001), where collagen intensity for the autologous fibroblast condition was less than that of the sECM condition, and sECM collagen intensity was great than the saline treat group (p < 0.0001). Nonsignificant differences in density were measured between autologous fibroblast–treated and saline-treated (p = 0.0839) groups.
Significant differences were measured for fibronectin between treatment groups [F(3,54), p < 0.0001]. The highest density of fibronectin was measured in the autologous fibroblast–treated/sECM-treated group, and it was significantly higher than saline-treated (p ≤ 0.0001), sECM-treated (p = 0.0004), and autologous fibroblast–treated (p = 0.0034) groups (Fig. 1C). Autologous fibroblast–treated tissue had significantly greater fibronectin than saline-treated tissue (p < 0.0001), and the sECM-treated group has significantly greater fibronectin than saline-treated group (p = 0.0352).
Rheology
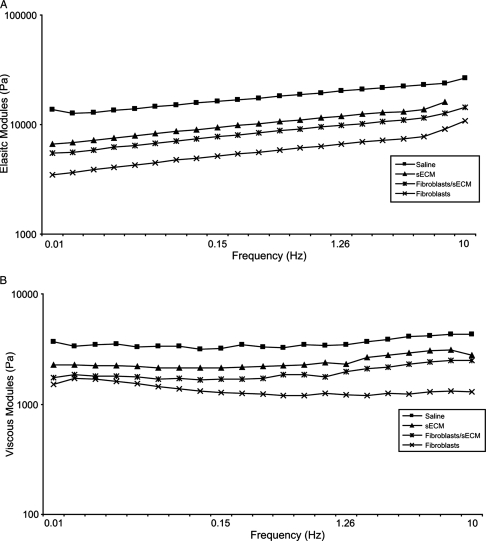
Elastic shear modulus (G′) and viscous modulus (G″) of treated vocal fold tissues were measured as a function of frequency using a stress-controlled rheometer. The results of the rheological analysis (G′ and G″) are shown in Figure 2. The mean magnitude of the elastic and viscous moduli across frequency decreased monotonically according to the following treatment conditions: saline, sECM, autologous fibroblasts/sECM, and autologous fibroblasts. All three treated conditions resulted in a decline in both elastic and viscous moduli and were more than one standard deviation away from mean values of untreated, scarred vocal folds. Logarithmic and power law fit for G′ and the dynamic viscosity η′, where η′ is equal to G″ divided by the angular frequency, are shown in Tables 1 and 2. R2-values were greater than 0.91 for all conditions. All tissue samples demonstrated monotonic decreases in dynamic viscosity as frequency increased, which is indicative of shear thinning. Slope and intercept of G′ fits, a and b, respectively, and intercept of η′ fits, a, decreased following the same treatment conditions as in Figure 2. The declination rate of η′ fits, b, however, was nearly the same for all treatment conditions. For G′, the saline-treated group was significantly stiffer than the autologous fibroblast–treated group (p = 0.0081). No significant differences were found between saline and the other two treatment groups—autologous fibroblasts/sECM (p = 0.0965) and sECM (p = 0.3560). For G″, the saline-treated group was significantly more viscous than the autologous fibroblast–treated group (p = 0.0077). No significant differences were found between saline and the other two treatment groups—autologous fibroblasts/sECM (p = 0.1319) and sECM (p = 0.3709).
FIG. 2.
(A) Elastic modulus (G′) and (B) viscous modulus (G″) for four conditions—saline treated, autologous fibroblast treated, sECM treated, and sECM/autologous fibroblast treated.
Table 1.
Coefficient a and Constant b and Corresponding Coefficient of Determination R2 for Curve Fitting for Elastic Moduli (G′ = a ln x + b)
| Treatment group | a | b | R2 |
|---|---|---|---|
| Saline | 1679 | 18594 | 0.94 |
| sECM | 1390 | 12216 | 0.92 |
| Autologous fibroblasts/sECM | 1114 | 10008 | 0.95 |
| Autologous fibroblasts | 827.9 | 6844 | 0.91 |
x = x intercept.
Table 2.
Coefficient a and Exponent b and Corresponding Coefficient of Determination R2 for Curve Fitting for Dynamic Moduli (η = axb)
| Treatment group | a | b | R2 |
|---|---|---|---|
| Saline | 543.6 | −1.021 | 0.98 |
| sECM | 401.3 | −0.9526 | 0.999 |
| Autologous fibroblasts/sECM | 322.8 | −0.949 | 0.998 |
| Autologous fibroblasts | 224.2 | −0.959 | 0.999 |
Discussion
Treatment of vocal fold scarring represents a clinical challenge distinct from injury prevention. Prophylaxis, which has been shown to enhance wound repair and minimize scar formation after injury, has already been identified and has been a focus of the vocal fold scarring literature.3,10–12,25 It would be expected that the two approaches for treating vocal fold scarring—early treatment versus late treatment–would have mechanistically different biological responses. The cellular response would be phenotypically different for tissue that has been recently injured versus a chronic injury. The motivation for the present study is to investigate three approaches of tissue restoration in tissue with established ECM injury.
The functional consequence of the three treatments was measured by rheology. There were statistically significant improvements in the viscoelastic measures for the autologous fibroblast–treated group when compared to saline-treated controls. The controls had significantly elevated rheological values for both dynamic modulus and elastic shear modulus, which may be attributed to their greater levels of fibrosis noted in vocal fold scar as previously published,11,12,18,26 but contradicts collagen levels of the present study. When comparing our results with the literature for chronic injury, there are similar fits for dynamic moduli for all treatment groups. When comparing to a prophylaxic study,12 where HA hydrogels were injected immediately after injury and animals were sacrificed 21–24 days later, curve fit coefficients of G′ and η′ of saline controls were quite similar to saline controls of the present study as were coefficients of our sECM and their hydrogel treatment. Viscoelastic measurements for saline controls in this study were also similar to untreated vocal fold scar.18
All groups demonstrated an increase in matrix accumulation. Histological levels of procollagen 1, collagen, and fibronectin were similar to or greater than saline control for all treatment groups. Autologous fibroblast–treated vocal folds have elevated fibronectin; autologous fibroblasts/sECM has elevated collagen and fibronectin; sECM has fibronectin, collagen, and procollagen at significantly higher levels. It is still unclear what role collagen content plays in the viscoelastic function of vocal fold tissues. There have been a number of research papers in laryngology that have measured collagen levels in a chronic model, with a functional outcome measure, in attempts to determine if collagen is responsible for changes in tissue viscoelasticity. Previous work by Chhetri et al.4 in a canine model, demonstrated increased collagen levels after autologous fibroblast injections in scarred vocal folds. Increased levels of collagen were related to improved mucosal wave as measured by stroboscopy. Hertegard and coworkers5 utilized human embryonic stem cells in a rabbit acute model and found improved biomechanical properties without a concomitant change in collagen levels. Compared to normal tissue,18,26 untreated vocal fold scar, with increased viscosity and decreased elasticity in a rabbit model, has been characterized by increased procollagen and fibronectin without changes in collagen levels. Using human cadaver tissue, Chan et al.27 did not find a relationship between tensile elastic modulus of the vocal fold cover or ligament and relative collagen density.
The best biomechanical outcomes—autologous fibroblast–treated group—were characterized with increased fibronectin and the lowest collagen staining of the three experimental conditions. Increased levels of fibronectin have been reported in untreated scar26 and have been related to increased viscosity in benign lesions. Guarnieri et al.28 reported that fibronectin organizes in aggregates, intersperses in collagen, and in thin fibrils, distributes along collagen fibers. High fibronectin concentrations affected collagen fiber assembly and structure leading to drastic effects on rheological properties. The improved rheological properties in this investigation could be related to varying orientations of the proteins, augmented by the upregulation of fibronectin. Our present histological image analysis is inadequate to study such relationships and orientation. Further studies are necessary for assessing the organization and relationship between the proteins to better understand the contribution of specific proteins to ECM rheological properties. To provide an understanding of the full mechanism, it would be beneficial to measure tissue changes earlier after treatment.
Using autologous fibroblasts has led to better tissue restoration in vocal folds1,2,4 and dermal skin.29,30 Autologous fibroblasts allow for permanent engraftment. Use of autologous fibroblasts encapsulated in a scaffold would seem ideal. In this study, even though not statistically significant, the autologous fibroblast–treated/sECM-treated vocal folds had a more favorable biomechanical outcome than sECM alone. Previous research demonstrated cytocompatibility of cells in sECM,31 and improved viscoelasticity and less fibrosis in an acute wound rabbit model for the HA hydrogels were utilized in this research.12 With the addition of autologous cells, viscoelasticity was further improved. Despite collagen levels being higher, the increase in fibronectin of the fibroblast/sECM condition over the sECM condition may have compensated. Fibroblasts may very well have altered gene and protein expression in the presence of the sECM. Further, in vitro studies are necessary to determine the effects of sECM on cell behavior. Moreover, vocal fold fibroblasts may not be an option for the clinical setting, given lack of availability; subsequently, further investigation utilizing more accessible fibroblasts, that is, dermis and/or gingival, is warranted.
In summary, findings of this study indicate that injections of autologous fibroblast into established vocal fold scar significantly improved tissue biomechanical properties closer to normal levels over the injection of autologous fibroblasts with sECM or sECM alone, as evaluated 2 months after injury. Our results also provide insight into the relative contribution of collagen, procollagen, and fibronectin to tissue biomechanics. Further investigation is necessary to understand the underlying biology of the chronic vocal fold scar, the relationship between scaffolds and cells in vitro and in vivo, and the relationship between the protein constituents of the vocal fold and rheological outcomes. Optimization of cell–scaffold interactions and subsequent cell behavior is necessary for utilization of scaffold and scaffold–cell approaches.
Acknowledgments
NIH Grant R01 DC4336 from the National Institute of Deafness and Other Communicative Disorders funded this study, with additional financial contributions from the Centers of Excellence Program of the State of Utah. We thank Glen Leverson, Ph.D., Department of Surgery, University of Wisconsin–Madison, for statistical assistance and Dr. Xiao-Zheng Shu, University of Utah, for providing materials.
Disclosure Statement
G.D. Prestwich holds equity in Carbylan Biosurgery and Glycosan Biosystems. The other writers have no competing financial interests.
References
- 1.Kanemaru S. Nakamura T. Omori K. Kojima H. Magrufov A. Hiratsuka Y. Hirano S. Ito J. Shimizu Y. Regeneration of the vocal fold using autologous mesenchymal stem cells. Ann Otol Rhinol Laryngol. 2003;112:915. doi: 10.1177/000348940311201101. [DOI] [PubMed] [Google Scholar]
- 2.Kanemaru S. Nakamura T. Yamashita M. Magrufov A. Kita T. Tamaki H. Tamura Y. Iguchi F. Kim T.S. Kishimoto M. Omori K. Ito J. Destiny of autologous bone marrow-derived stromal cells implanted in the vocal fold. Ann Otol Rhinol Laryngol. 2005;114:907. doi: 10.1177/000348940511401203. [DOI] [PubMed] [Google Scholar]
- 3.Hertegard S. Cedervall J. Svensson B. Forsberg K. Maurer F.H. Vidovska D. Olivius P. Ahrlund-Richter L. Le Blanc K. Viscoelastic and histologic properties in scarred rabbit vocal folds after mesenchymal stem cell injection. Laryngoscope. 2006;116:1248. doi: 10.1097/01.mlg.0000224548.68499.35. [DOI] [PubMed] [Google Scholar]
- 4.Chhetri D.K. Head C. Revazova E. Hart S. Bhuta S. Berke G.S. Lamina propria replacement therapy with cultured autologous fibroblasts for vocal fold scars. Otolaryngol Head Neck Surg. 2004;131:864. doi: 10.1016/j.otohns.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Cedervall J. Ahrlund-Richter L. Svensson B. Forsgren K. Maurer F.H. Vidovska D. Hertegard S. Injection of embryonic stem cells into scarred rabbit vocal folds enhances healing and improves viscoelasticity: short-term results. Laryngoscope. 2007;117:2075. doi: 10.1097/MLG.0b013e3181379c7c. [DOI] [PubMed] [Google Scholar]
- 6.Xu C.C. Chan R.W. Tirunagari N. A biodegradable, acellular xenogeneic scaffold for regeneration of the vocal fold lamina propria. Tissue Eng. 2007;13:551. doi: 10.1089/ten.2006.0169. [DOI] [PubMed] [Google Scholar]
- 7.Ringel R.L. Kahane J.C. Hillsamer P.J. Lee A.S. Badylak S.F. The application of tissue engineering procedures to repair the larynx. J Speech Lang Hear Res. 2006;49:194. doi: 10.1044/1092-4388(2006/016). [DOI] [PubMed] [Google Scholar]
- 8.Hahn M.S. Teply B.A. Stevens M.M. Zeitels S.M. Langer R. Collagen composite hydrogels for vocal fold lamina propria restoration. Biomaterials. 2006;27:1104. doi: 10.1016/j.biomaterials.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 9.Jia X. Yeo Y. Clifton R.J. Jiao T. Kohane D.S. Kobler J.B. Zeitels S.M. Langer R. Hyaluronic acid-based microgels and microgel networks for vocal fold regeneration. Biomacromolecules. 2006;7:3336. doi: 10.1021/bm0604956. [DOI] [PubMed] [Google Scholar]
- 10.Duflo S. Thibeault S.L. Li W. Shu X.Z. Prestwich G. Effect of a synthetic extracellular matrix on vocal fold lamina propria gene expression in early wound healing. Tissue Eng. 2006a;12:3201. doi: 10.1089/ten.2006.12.3201. [DOI] [PubMed] [Google Scholar]
- 11.Duflo S. Thibeault S.L. Li W. Shu X.Z. Prestwich G.D. Vocal fold tissue repair in vivo using a synthetic extracellular matrix. Tissue Eng. 2006b;12:2171. doi: 10.1089/ten.2006.12.2171. [DOI] [PubMed] [Google Scholar]
- 12.Hansen J.K. Thibeault S.L. Walsh J.F. Shu X.Z. Prestwich G.D. In vivo engineering of the vocal fold extracellular matrix with injectable hyaluronic acid hydrogels: early effects on tissue repair and biomechanics in a rabbit model. Ann Otol Rhinol Laryngol. 2005;114:662. doi: 10.1177/000348940511400902. [DOI] [PubMed] [Google Scholar]
- 13.Prestwich G.D. Shu X.Z. Liu Y. Cai S. Walsh J.F. Hughes C.W. Ahmad S. Kirker K.R. Yu B. Orlandi R.R. Park A.H. Thibeault S.L. Duflo S. Smith M.E. Injectable synthetic extracellular matrices for tissue engineering and repair. Adv Exp Med Biol. 2006;585:125. doi: 10.1007/978-0-387-34133-0_9. [DOI] [PubMed] [Google Scholar]
- 14.Prestwich G.D. Liu Y. Yu B. Shu X.Z. Scott A. 3-D culture in synthetic extracellular matrices: new tissue models for drug toxicology and cancer drug discovery. Adv Enzyme Regul. 2007;47:196. doi: 10.1016/j.advenzreg.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 15.Roychowdhury P. Klemuk S. Titze I. Kumar V. Effects of fabrication parameters on viscoelastic shear modulus of 2,3-dialdehydecellulose membranes-Potential scaffolds for vocal fold lamina propria tissue engineering. J Biomed Mater Res A. 2008. (in press). [DOI] [PubMed]
- 16.Thibeault S.L. Duflo S. Inflammatory cytokine responses to synthetic extracellular matrix injection to the vocal fold lamina propria. Ann Otol Rhinol Laryngol. 2008;117:221. doi: 10.1177/000348940811700310. [DOI] [PubMed] [Google Scholar]
- 17.Serban M.A. Liu Y. Prestwich G.D. Effects of extracellular matrix analogues on primary human fibroblast behavior. Acta Biomater. 2008;4:67. doi: 10.1016/j.actbio.2007.09.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thibeault S.L. Gray S.D. Bless D.M. Chan R.W. Ford C. Histologic and rheologic characterization of vocal fold scarring. J Voice. 2002a;16:96. doi: 10.1016/s0892-1997(02)00078-4. [DOI] [PubMed] [Google Scholar]
- 19.Thibeault S.L. Li W. Gray S.D. Chen Z. Instability of extracellular matrix gene expression in primary cell culture of fibroblasts from human vocal fold lamina propria and tracheal scar. Ann Otol Rhinol Laryngol. 2002b;111:8. doi: 10.1177/000348940211100102. [DOI] [PubMed] [Google Scholar]
- 20.Shu X.Z. Liu Y. Palumbo F. Prestwich G.D. Disulfide-crosslinked hyaluronan-gelatin hydrogel films: a covalent mimic of the extracellular matrix for in vitro cell growth. Biomaterials. 2003;24:3825. doi: 10.1016/s0142-9612(03)00267-9. [DOI] [PubMed] [Google Scholar]
- 21.Shu X.Z. Liu Y. Luo Y. Roberts M.C. Prestwich G.D. Disulfide cross-linked hyaluronan hydrogels. Biomacromolecules. 2002;3:1304. doi: 10.1021/bm025603c. [DOI] [PubMed] [Google Scholar]
- 22.Shu X.Z. Ahmad S. Liu Y. Prestwich G.D. Synthesis and evaluation of injectable, in situ crosslinkable synthetic extracellular matrices for tissue engineering. J Biomed Mater Res A. 2006;79:902. doi: 10.1002/jbm.a.30831. [DOI] [PubMed] [Google Scholar]
- 23.Klemuk S.A. Titze I.R. Viscoelastic properties of three biomaterials at low audio frequencies. Laryngoscope. 2004;114:1597. doi: 10.1097/00005537-200409000-00018. [DOI] [PubMed] [Google Scholar]
- 24.Chan R.W. Titze I.R. Effect of postmortem changes and freezing on the viscoelastic properties of vocal fold tissues. Ann Biomed Eng. 2003;31:482. doi: 10.1114/1.1561287. [DOI] [PubMed] [Google Scholar]
- 25.Hirano S. Bless D.M. Rousseau B. Welham N. Montequin D. Chan R.W. Ford C.N. Prevention of vocal fold scarring by topical injection of hepatocyte growth factor in a rabbit model. Laryngoscope. 2004;114:548. doi: 10.1097/00005537-200403000-00030. [DOI] [PubMed] [Google Scholar]
- 26.Thibeault S.L. Bless D.M. Gray S.D. Interstitial protein alterations in rabbit vocal fold with scar. J Voice. 2003;17:377. doi: 10.1067/s0892-1997(03)00064-x. [DOI] [PubMed] [Google Scholar]
- 27.Chan R.W. Fu M. Young L. Tirunagari N. Relative contributions of collagen and elastin to elasticity of the vocal fold under tension. Ann Biomed Eng. 2007;35:1471. doi: 10.1007/s10439-007-9314-x. [DOI] [PubMed] [Google Scholar]
- 28.Guarnieri D. Battista S. Borzacchiello A. Mayol L. de Rosa E. Keene D.R. Muscariello L. Barbarisi A. Netti P.A. Effects of fibronectin and laminin on structural, mechanical and transport properties of 3D collageneous network. J Mater Sci Mater Med. 2007;18:245. doi: 10.1007/s10856-006-0686-5. [DOI] [PubMed] [Google Scholar]
- 29.Weiss R.A. Weiss M.A. Beasley K.L. Munavalli G. Autologous cultured fibroblast injection for facial contour deformities: a prospective, placebo-controlled, Phase III clinical trial. Dermatol Surg. 2007;33:263. doi: 10.1111/j.1524-4725.2007.33060.x. [DOI] [PubMed] [Google Scholar]
- 30.Fujimori Y. Ueda K. Fumimoto H. Kubo K. Kuroyanagi Y. Skin regeneration for children with burn scar contracture using autologous cultured dermal substitutes and superthin auto-skin grafts: preliminary clinical study. Ann Plast Surg. 2006;57:408. doi: 10.1097/01.sap.0000237057.49772.4f. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y. Zheng Shu X. Prestwich G.D. Biocompatibility and stability of disulfide-crosslinked hyaluronan films. Biomaterials. 2005;26:4737. doi: 10.1016/j.biomaterials.2005.01.003. [DOI] [PubMed] [Google Scholar]