Abstract
Biologic scaffolds composed of extracellular matrix (ECM) are widely used to facilitate remodeling and reconstruction of a variety of tissues in both preclinical animal studies and human clinical applications. The mechanisms by which such scaffolds influence the host tissue response are only partially understood, but it is logical that the mononuclear macrophage cell population plays a central role. The present study evaluated the role of macrophages that derive from peripheral blood in the degradation of ECM scaffolds. An established rat body wall reconstruction model was used to evaluate the degradation of carbodiimide (CDI)–crosslinked scaffolds composed of porcine small intestinal submucosa (SIS), noncrosslinked SIS, and autologous body wall. To assess the role of circulating macrophages in the degradation process, the degradation of each scaffold was assessed with and without macrophage depletion caused by administration of clodronate-containing liposomes. Results showed that peripheral blood monocytes are required for the early and rapid degradation of both SIS scaffolds and autologous body wall, and that CDI crosslinked SIS is resistant to macrophage-mediated degradation.
Introduction
Biologic scaffolds composed of extracellular matrix (ECM) can promote the constructive remodeling of injured tissues through mechanisms that include angiogenesis,1–5 the recruitment of multipotential progenitor cells to the site of tissue reconstruction,6–9 the release of antimicrobial peptides,10–15 and activation of the alternative pathway of immunity.16–20 There is convincing evidence that host-mediated degradation of ECM scaffolds is typically completed within 8–12 weeks and is necessary to realize the full beneficial effects of ECM-mediated tissue remodeling,21–23 but the mechanisms by which such scaffold degradation occurs have been largely ignored.
It is intuitive and logical that phagocytic cells such as macrophages would play an important role in ECM scaffold degradation, but definitive studies to address the issue have not been reported. The concept of ECM scaffold degradation as a critical determinant of constructive tissue remodeling becomes even more clinically relevant when considering the widespread use of commercially available ECM materials that have been subjected to chemical and physical methods designed specifically to prevent scaffold degradation and increase the mechanical strength of the materials.24 A recent study that compares several commercially available ECM scaffold materials has shown that the methods used to process these scaffolds, especially chemical methods to create molecular crosslinks, have marked effects upon host macrophage phenotype surrounding the ECM scaffold and downstream remodeling events.18
The objective of the present study was to determine the contribution of mononuclear macrophages to the degradation and early remodeling events that follow the implantation of ECM scaffolds for the purpose of tissue reconstruction. This objective was accomplished by evaluating the degradation and remodeling response of native and chemically crosslinked ECM in a rat model of abdominal wall repair with and without macrophage depletion. Both the scaffolds tested in the study mimic products that have been used clinically for augmentation of the rotator cuff.25,26 Macrophage depletion was accomplished by administration of clodronate-containing liposomes that are subjected to phagocytosis by circulating macrophages. Apoptosis of macrophages occurs when intracellular clodronate reaches a threshold concentration.27–29 Liposomes do not cross the vascular–endothelial barrier, so only circulating macrophages are affected. Stated differently, resident tissue macrophages remain unaffected by the clodronate treatment.
Materials and Methods
Experimental design
Forty-eight adult rats were randomly divided into three equal groups (n = 16 for each group). A 1.5 cm × 1.5 cm partial thickness defect was created in the ventro-lateral abdominal wall of each animal. The defect was replaced with one of three different materials: a 10 layer configuration of an ECM biologic scaffold composed of porcine-derived small intestinal submucosa radiolabeled with 14C (14C-SIS); a 10-layer configuration of the same form of porcine-derived 14C-SIS test article except for the use of carbodiimide (CDI) as a crosslinking agent (14C-X-SIS); or autologous body wall tissue. Each group was divided into three subgroups. Subgroup no. 1 was administered clodronate-containing liposomes twice weekly (n = 8). Subgroup no. 2 was administered phosphate-buffered saline (PBS)–containing liposomes twice weekly (n = 4). Subgroup no. 3 was administered twice-weekly treatments with saline vehicle (n = 4). One half of the animals in each subgroup were sacrificed 1 week after surgery. The remaining animals were sacrificed 2 weeks after surgery. The implanted scaffold materials plus surrounding host native tissue were explanted and examined by (1) histologic and immunohistochemical methods to determine the participation of blood-derived macrophages in the host response and to characterize the scaffold remodeling response, and (2) by liquid scintillation counting (LSC) for 14C content to quantify the extent of SIS scaffold degradation. All procedures were performed in accordance with the National Institutes of Health (NIH) guidelines for care and use of laboratory animals, and with approval of the Institutional Animal Care and Use Committee (IACUC) and Radiation Safety Office (RSO) at the University of Pittsburgh.
Test article preparation
Preparation of 14C-SIS
SIS was manufactured from the small intestine of 26-week-old pigs that received weekly injections of 10 μCi of 14C-labeled proline from 3 to 26 weeks of age.22,30 The injected proline (or as modified in vivo to form hydroxyproline) is integrally incorporated into collagen. Proline and hydroxyproline account for approximately 10% of the amino acids in collagen, and collagen accounts for 90% of the dry weight of SIS. This protocol of 14C-labeled proline administration results in a two-log to four-log increase in 14C content within porcine ECM and thus serves as a tool for quantification of labeled ECM at the site of interest.22,30
The isolation of SIS from the small intestine has been previously described,31 but is summarized briefly herein. Immediately after euthanasia, the small intestine was harvested and the mesenteric tissues were removed. After rinsing, the tunica serosa, tunica muscularis externa, and the luminal portion of the tunica mucosa including most of the lamina propria were mechanically removed. The remaining layers represented 14C-SIS, and consisted of the tunica submucosa and the basilar portion of the tunica mucosa including the muscularis mucosa and the stratum compactum of the lamina propria. The 14C-SIS material was disinfected and decellularized with 0.1% peracetic acid and rinsed in hypotonic saline. The amount of 14C incorporated in SIS was 3500 counts per minute (CPM) per gram, compared to a background level of 25 CPM.30
The 14C-SIS scaffolds were constructed by stacking 10 layers of the ECM and subjecting them to a vacuum of 710–740 mmHg for 10–12 h to remove the water and form a tightly coupled multilaminate device. Ethylene oxide was used for terminal sterilization (500 mg/L/h for 16 h).
Preparation of CDI crosslinked 14C-SIS
The SIS scaffolds designated as 14C-X-SIS were prepared by immersing the multilaminate 14C-SIS scaffolds in a solution of 10 mM CDI (Sigma, St. Louis, MO) in 90% acetone/10% deionized water (v/v) for 24 h. The chemical crosslinking agent used was CDI, which has been used widely used to crosslink commercially available ECM scaffolds.32–34 To determine crosslinking efficacy, eight samples each of 14C-SIS and 14C-X-SIS were digested in triplicate in 27.3 U/mL bacterial collagenase type I (Sigma) for 72 h, and the amino acids released in the supernatant were quantified by the ninhydrin assay according to the manufacturer's protocol. The 14C-X-SIS scaffolds were terminally sterilized by exposure to ethylene oxide at a dose of 500 mg/L/h for a 16 h cycle.
Experimental animals and husbandry
Forty-eight adult male Sprague-Dawley rats weighing approximately 300 g were purchased from Charles River Laboratory (Wilmington, MA). Each animal was housed individually in sterile microisolator cages. The rats were fed a diet of γ-irradiated Purina rodent chow (LabDiet ProLab Isopro RMH 3000; PMI Nutrition International, St. Louis, MO) and sterile autoclaved water, and were housed individually in an environment maintained at 68–76°C for 24 h a day and with a light:dark cycle of 12:12 h.
Surgical procedure
The abdominal wall defect model has been previously described.23 Surgical plane anesthesia in each animal was induced and maintained with 2% isoflurane in oxygen. The ventral abdomen was prepared for sterile surgery in sterile fashion. A midline incision was made, and the underlying muscle tissue lateral to the linea alba was separated from the skin and subcutaneous tissues. A 1.5 × 1.5 cm partial thickness segment consisting of the oblique layers of the abdominal wall was excised and replaced with a size-matched graft of the 14C-SIS device, the 14C-X-SIS device, or the autologous abdominal wall tissue. The underlying transversalis fascia and peritoneum were left intact; therefore, the abdominal cavity was not penetrated. The grafts were sutured in place at each corner with 4–0 Prolene to secure and demarcate the boundaries of the device. The skin incision was then closed with a subcuticular stitch using 4–0 Vicryl. The animals were recovered from anesthesia on a heating pad and returned to the housing unit.
Ketoprofen (5 mg/kg of body weight) was administered by subcutaneous injection the day of surgery and for two additional days. Baytril (20 mg) was given orally the day of surgery and for two additional days. The dietary habits, general health status, and the surgical site were monitored daily. Any animal showing signs of infection or uncontrollable pain was immediately euthanized and removed from the study.
In vivo macrophage depletion
Clodronate (dichloromethylene bisphosphate, Cl2MBP; Sigma–Aldrich, St. Louis, MO) was encapsulated in multilamellar liposomes, as described.27,35 Clodronate was prepared at a concentration of 2.5 g per 10 mL of PBS solution.35 In the present study, clodronate liposomes were injected IV in the lateral tail vein to deplete macrophages from the peripheral blood. PBS containing liposomes labeled with the fluorochrome DiI36 (Molecular Probes, Eugene, OR) or saline served as the control injections. The volume of each injection was 0.1 mL liposome solution or saline solution per 10 g of body weight. Injections were given 2 days and 2 h before surgery. Subsequent injections were then given twice weekly until the predetermined time of sacrifice.
Euthanasia and specimen harvest
At the predetermined time of sacrifice, the animals were anesthetized with 5% isoflurane and euthanized by an intracardiac bolus injection of potassium chloride to induce cardiac arrest. The surgical site was explanted with an equal amount of adjacent native abdominal wall tissue. The specimens were either fixed in 10% neutral buffered formalin for histologic and immunohistochemical analysis or frozen for both immunofluorescent imaging studies (i.e., animals treated with DiI-labeled PBS liposomes) and 14C quantification (i.e., animals that received the 14C-SIS and 14C-X-SIS scaffolds). Liver and spleen specimens were also harvested and prepared for histology and immunoanalysis to monitor the efficacy of the macrophage depletion protocol.
Quantification of degradation
The 14C radioactivity of the specimens from animals that received the 14C-SIS and 14C-X-SIS scaffolds was determined by LSC using an LSI1800 B-counter (Beckman Coulter, Somerset, NJ). Approximately 80 mg of each specimen was incubated in Solvable (PerkinElmer, Waltham, MA) for 2–4 h at 50–60°C until solubilized. Ten milliliters of scintillation fluid (Ultima Gold; PerkinElmer) was added to each sample, and quantification of 14C was recorded as CPM. Background 14C activity was subtracted from the recorded activity, and the CPM was determined for each scaffold.
Histology and immunohistochemistry
The specimens that were fixed in neutral buffered formalin were embedded in paraffin, and 6 μm sections were cut and mounted for hematoxylin and eosin (H&E) staining and immunohistochemical analysis. The primary antibody used for immunohistochemical staining was mouse anti-rat CD68 (Clone ED1; Serotec, Raleigh, NC) at 1:50 dilution in PBS. The CD68 surface marker is an indicator of a macrophage phenotype. The secondary antibody used was biotinylated anti-mouse IgG (H+L) (Vector, Burlingham, CA) at 1:50 dilution. Formalin-fixed rat spleen from animals not treated with any test solutions was used as the positive control tissue.
Unstained sections were deparaffinized with xylene and rehydrated through a graded ethanol series. Heat-mediated antigen retrieval was performed with 0.01 M citrate buffer (Spectrum, New Brunswick, NJ), pH 6.0, at 95–100°C for 20 min. After cooling, two separate 5-min washes in Tris-buffered saline (TBS)/Tween20, pH 7.4, followed by three washes in PBS were performed. The slides were incubated for 30 min with 2% normal horse serum (Vector) at room temperature to prevent nonspecific antibody binding. To inhibit endogenous peroxidase activity, the slides were incubated with 3% hydrogen peroxide (Spectrum) in methanol for 30 min at room temperature. The slides were incubated with the secondary antibody for 30 min at room temperature, followed by horseradish peroxidase solution (Vector) for 30 min at 37°C. Diaminobenzidine (Vector) was applied to detect positive staining cells, and the slides were counterstained with hematoxylin.
The frozen tissue sections from animals that received DiI-labeled PBS liposomes were analyzed by fluorescent microscopy to determine the colocalization of DiI-labeled cells with CD68-labeled cells, presumably macrophages that had phagocytosed liposomes. The primary antibody was mouse anti-rat CD68 (Clone ED1; Serotec) at 1:100 dilution in PBS. The secondary antibody used was Alexafluor 488-labeled goat anti-mouse IgG (H+L) (Invitrogen, Carlsbad, CA) at 1:250 dilution. Frozen sections were fixed in ice-cold acetone for 5 min. After air-drying the tissue sections, two separate 5-min washes in PBS, followed by three washes in PBS-0.1% Tween 20 (Sigma), were conducted. The slides were incubated for 30 min with 2% normal goat serum (Vector), and incubated with the primary antibody for 60 min at room temperature. The slides were incubated with the secondary antibody for 30 min at room temperature, and dehydrated through a graded series of ethanol. The slides were cover-slipped with an aqueous medium containing DAPI (Vector).
Quantitative analysis of macrophages
Quantitative analysis of CD68+ cells was conducted independently by two investigators who were blinded to the identity of the tissue specimen. Cells immunopositive for CD68 were counted for each tissue specimen in 10 microscope fields at 400 × magnification at the host–implant interface.
Statistical analysis
The mean values for CD68+ cells for each group and subgroup were determined, and the group values compared using Student's t-test and one-way analysis of variance. Data were reported as mean ± standard deviation. Results were considered significant for p < 0.05.
Results
Crosslinking efficacy
The ninhydrin assay showed a greater concentration of amino acids in the supernatants from 14C-SIS compared to 14C-X-SIS (523.5 ± 40.0 vs. 263.3 ± 74.9 μM/mg, p < 0.01), confirming that the 14C-X-SIS scaffolds were more resistant to collagenase degradation than the 14C-SIS scaffolds.
Surgical and postoperative outcomes
No complications occurred during the surgical procedures for any animal in this study. All animals assigned to the 1-week time point survived without complication. Five out of the 24 animals with a survival time point of 2 weeks died before the scheduled date of sacrifice. All the animals that died prematurely had been administered clodronate liposomes. None of the animals that were administered the PBS liposomes or saline vector died prematurely. This outcome was not specific to any particular scaffold group. Histopathologic analysis of lung, spleen, and liver tissue specimens from the animals that died prematurely showed widely disseminated foci of parenchymal necrosis associated with colonies of bacteria and an absence of inflammatory cells. These findings were consistent with overwhelming sepsis secondary to clodronate-induced phagocyte depletion. The animals that died prematurely were replaced in the study, and these replacement animals survived until the study endpoint.
Quantification of degradation
At 1 and 2 weeks, LSC CPM of the 14C-SIS graft sites from clodronate-treated animals showed higher levels of 14C when compared to graft sites from PBS liposome or saline-treated animals (p < 0.05). At 2 weeks, CPM of PBS liposome–treated animals were less than the CPM from saline-treated animals (p < 0.05). CPM of 14C-X-SIS graft sites from clodronate liposome, PBS liposome, and saline-treated animals were all similar at 1 and 2 weeks, and these 14C levels were also similar to the nonimplanted scaffold (Table 1).
Table 1.
Quantification of 14C-SIS and 14C-X-SIS Scaffold Degradation, as Measured by LSC
| Clodronate liposomes | PBS liposomes | Saline | ||
|---|---|---|---|---|
| 14C-SIS | 1 week | 1273.0 ± 63.3a–c | 683.8 ± 134.9c | 468.0 ± 227.6c |
| 2 weeks | 1214.2 ± 407.3a,b | 41.5 ± 47.3b–d | 786.0 ± 215.78a,c | |
| 14C-X-SIS | 1 week | 1115.1 ± 453.5 | 1281.0 ± 204.6 | 1026.6 ± 536.3 |
| 2 weeks | 1115.8 ± 355.8 | 1130.4 ± 156.7 | 1349.0 ± 204.6 | |
| Nonimplanted 14C-SIS | 1081.5 ± 112.8 (n = 7) |
The CPM were measured for each scaffold. Values reported as mean ± standard deviation.
Significant compared with PBS liposome control (p < 0.05).
Significant compared with saline control (p < 0.05).
Significant compared with nonimplanted 14C-SIS scaffold (p < 0.05).
Significant compared with 1-week data from the same treatment group (p < 0.05).
Histology and immunohistochemistry
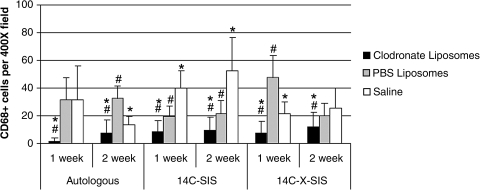
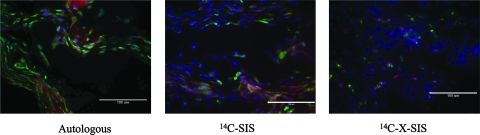
Macrophage depletion was noted in the spleen and liver of all animals treated with clodronate liposomes at 1 and 2 weeks postimplantation, confirming the effectiveness of the depletion procedure. For each graft type at 1 and 2 weeks, the surgical sites from the clodronate liposome–treated animals showed fewer macrophages (CD68+ cells) than graft sites from PBS liposome and saline-treated animals at both time points for all graft materials (p < 0.001) (Fig. 1). Animals treated with the DiI-labeled PBS liposomes showed DiI-labeled cells that colocalized with CD68+ cells at the site of tissue remodeling, verifying that liposomes were engulfed by circulating mononuclear cells and that these circulating cells subsequently populated the remodeling site in animals not treated with clodronate (Fig. 2).
FIG. 1.
CD68+ cells per 400 × field, 10 fields per graft site, examined at the host–implant interface. Data represent mean ± SD. *Significant compared with PBS liposome control (p < 0.001); #significant compared with saline control (p < 0.001).
FIG. 2.
Immunofluorescent images of scaffold sites 2 weeks postimplantation from animals that received IV injections of DiI-labeled PBS liposomes. The distribution of DiI-labeled cells (red), CD68+ macrophages (Alexafluor 488, green), and cell nuclei (DAPI, blue) are shown in merged images. Scale bar = 100 μm.
Autologous tissue graft
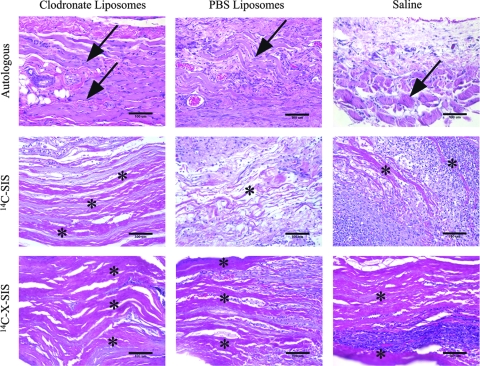
The autologous tissue graft from animals treated with clodronate-containing liposomes showed mild coagulative necrosis of the muscle fibers that were part of the autologous graft tissue, and these fibers were surrounded by partially organized collagenous tissue. Widely scattered mononuclear cells and neutrophils were noted at both the 1- and 2-week time points. The animals that received the saline or PBS liposomes treatment showed that the autologous graft tissue was infiltrated with a dense population of mononuclear cells and a smaller number of neutrophils at 1 and 2 weeks postimplantation. The muscle fiber bundles of the autologous graft tissue showed degeneration and necrosis at 1 week postsurgery. By 2 weeks, the tissue specimens of saline-treated animals showed that the tissue graft was almost totally replaced with collagenous tissue with remnants of degenerative or necrotic muscle remaining. The tissues from PBS liposome–treated animals showed remnants of skeletal muscle fibers dispersed within new host ECM and scattered accumulations of adipose tissue (Fig. 3).
FIG. 3.
Representative images of autologous, 14C-SIS, and 14C-X-SIS scaffold implantation sites 2 weeks postimplantation. Graft sites from animals treated with clodronate liposomes showed almost no scaffold degradation. Graft sites from animals treated with PBS liposomes or saline show dense accumulations of mononuclear cells for all graft types and scaffold degradation for the autologous and 14C-SIS scaffolds. Arrows point to muscle cells that remained in the autologous tissue graft site. For the 14C-SIS and 14C-X-SIS scaffolds, asterisks (*) indicate the layers of the original multilaminate scaffolds that remain in the implantation site. Sections were stained with H&E. Scale bar = 100 μm.
14C-SIS scaffold
The animals treated with the clodronate liposomes showed a markedly reduced mononuclear cell response for both time points. At 1 week, the scaffold was not adherent to the surrounding host tissue at the time of sacrifice, and at 2 weeks the scaffold was characterized histologically by mononuclear cells in a loosely organized matrix. Neutrophils were present adjacent to the scaffold surface and were occasionally present between the individual layers of the delaminating scaffold.
The surgical sites in which the 14C-SIS device was placed in rats that received either PBS liposomes or saline showed an abundant mononuclear cell response at 1 and 2 weeks. Animals treated with PBS liposomes showed that most of the 14C-SIS scaffold was degraded by 2 weeks, a finding that correlated with the 14C levels found at the graft site. The scaffold was replaced with new host ECM and was densely populated by mononuclear cells and blood vessels.
For the saline-treated animals, by 2 weeks the scaffold was delaminated and the individual layers at the edges of the scaffold were surrounded by new host ECM. Mononuclear cells were abundant, and these cells surrounded and infiltrated the entire scaffold implantation area (Fig. 3).
14C-X-SIS scaffold
Specimens from animals treated with the clodronate liposomes showed almost complete absence of mononuclear cells and a dense population of neutrophils at the implantation site. The scaffold showed no evidence of delamination and appeared to be completely intact. At 1 and 2 weeks the scaffold was loosely attached to the surrounding host tissue.
The animals that received the saline or PBS liposomes showed a dense population of mononuclear cells and neutrophils around the 14C-X-SIS material at 1 and 2 weeks. These cells were present at the periphery of the scaffold material with little to no infiltration of the cells into the scaffold itself. The layers of the scaffold remained essentially intact, and showed no morphologic evidence of degradation, a finding that was consistent with the LSC data (Fig. 3).
Discussion
The results of the present study confirm the intuitive concept that blood-derived mononuclear macrophages play a necessary and important role in the early in vivo degradation process of ECM scaffolds and autologous tissue grafts, and that chemical crosslinking of the ECM by compounds such as CDI effectively inhibits the degradation process. These findings are important in the context of regenerative medicine and biologic scaffolds because the proposed mechanisms of the constructive remodeling response typically require scaffold degradation to realize the optimal beneficial effects. Because the presence of mononuclear macrophages is usually associated with inflammatory processes and the expected downstream consequences of tissue necrosis and scar tissue formation, the findings of the present study emphasize the wisdom of evaluating the role of macrophages with an open mind at least with regard to ECM scaffold remodeling.
ECM scaffolds such as SIS-ECM and urinary bladder matrix (UBM-ECM) have been shown to degrade rapidly in vivo, typically within 8–10 weeks after implantation,21,22 and yet have been associated with long-term functional tissue remodeling in many anatomic locations.21,37,38 Degradation-dependent events such as the release of constituent growth factors, the production and release of matricryptic peptides, and the recruitment of host progenitor cells and stem cells contribute to the well-documented dynamic histomorphologic changes that characterize the host response to such scaffold materials during this early postoperative period.3,4,6,9,12,39–43 It is logical to speculate, based upon the results of the present study, that blood-derived mononuclear macrophages are at least important, if not essential, mediators of these degradation-dependent events. It is therefore reasonable to conclude that the degradation of an ECM scaffold is beneficial and necessary to tissue remodeling efforts. Such an interpretation is in stark contrast to the conclusion that a macrophage-rich tissue response after ECM scaffold implantation necessarily represents an adverse host–patient reaction.44
The clinical utility of ECM scaffolds depends upon the ability of the scaffold material to provide adequate mechanical support to the injured or missing tissue being repaired. The degradation rate of nonchemically crosslinked ECM appears to be compatible with the rate of new host tissue deposition, such that the scaffold retains sufficient strength to serve as a functional replacement until the host can deposit new host tissue.45–48 However, there is a measurable loss of strength that occurs before transfer of mechanical function to the new host tissue. A common approach for increasing the strength of biologic scaffolds composed of ECM or components of ECM (e.g., purified type I collagen) has been the use of chemical crosslinking agents such as CDI,24,32–34 isocyanate,49 or glutaraldehyde.50–52 While chemical crosslinking does maintain the mechanical strength of the scaffold, it also necessarily alters the kinetics of degradation and has a deleterious effect on the biologic activity of the degradation products. The present study showed that CDI effectively inhibited ECM scaffold degradation regardless of the presence of macrophages. This finding was not unexpected and was consistent with recent studies that show a markedly altered, proinflammatory host tissue response to ECM scaffolds that have been chemically crosslinked.23 In clinical applications, noncrosslinked ECM scaffolds are the only grafts that are indicated for contaminated wounds because of the antibacterial effects associated with their degradation.53 Taken together, the beneficial biologic effects of ECM scaffold degradation and the loss of scaffold mechanical strength associated with scaffold degradation would suggest that a balance of these seemingly mutually exclusive events is necessary to realize functional tissue reconstruction.
It has recently been shown that macrophage phenotype is an important determinant of tissue remodeling in the context of regenerative medicine.18 The M2 macrophage phenotype is considered to be an immunomodulatory and tissue remodeling phenotype. In contrast, proinflammatory macrophages, designated as an M1 phenotype, are associated with chronic inflammation and a foreign body reaction.54 Although not evaluated in the present study, the macrophage phenotype profile was characterized in a study that used the same animal model and showed the predominant macrophage phenotype to ECM scaffolds that are not chemically crosslinked to be M2 from 1 week postimplantation to at least 4 months postimplantation.18 In contrast, the predominant macrophage phenotype to CDI crosslinked ECM was M1 during the same time period. Therefore, it is important to consider not only the simple presence of macrophages at the site of scaffold remodeling but also the phenotype of this important cell population when attempting to predict downstream remodeling outcomes.
Although not directly investigated in this study, the depletion of macrophages would logically alter the temporal and spatial response of other cell types toward an implanted scaffold material, including ECM scaffolds. The acute host tissue response to an implanted foreign material, as all of these materials represent, is typically characterized by the presence of neutrophils. After 3–5 days, the neutrophil population decreases and the mononuclear macrophage population becomes the predominant cell response. This balance of neutrophil departure and mononuclear macrophage arrival was disrupted by the depletion of macrophages in the present study. Neutrophils were still the prominent feature of the host cell response 2 weeks after implantation in the animals that were implanted with the 14C-SIS scaffold and that received clodronate-containing liposomes. The present results show that neutrophils do indeed participate in ECM scaffold remodeling, but do not appear to be major determinants of early scaffold degradation based upon the quantitative 14C measurements. It is logical that at least a certain degree of neutrophil-mediated scaffold degradation should occur, but the ability of neutrophils alone to provide for all of the biologic effects that are associated with ECM scaffold degradation remains to be investigated.
The present study is limited by the fact that the results were confined to the early stages of scaffold remodeling, that is, 2 weeks. The marked depletion of mononuclear macrophages for longer periods of time places the host at great risk for sepsis and makes such studies difficult. Further, repeated administration of clodronate was performed to ensure that the results were not confounded by newly formed circulating macrophages later in the study period. It should also be noted that the present study is limited to a single type of biologic scaffold material, specifically the SIS-ECM, but the learned principles may be applicable to all biologic scaffold materials.
In summary, macrophages play an important and central role in biologic scaffold degradation and remodeling. Their presence within and surrounding scaffold materials after in vivo implantation should not only be expected but also perhaps be recognized as a favorable course of events and may predict a constructive remodeling outcome.
Conclusions
Macrophages that populated the 14C-SIS scaffold after in vivo placement were at least partially responsible for scaffold degradation. The CDI crosslinking agent used to manufacture 14C-X-SIS inhibited macrophage-mediated scaffold degradation. The depletion of macrophages at the sites of 14C-SIS, 14C-X-SIS, and autologous graft implantation resulted in an attenuation of the host inflammatory response and slowed the rate of scaffold degradation. These findings show that macrophages are important contributors to biologic scaffold degradation and early remodeling events.
Acknowledgments
The authors would like to thank Dr. Nico van Rooijen for his technical advice in the use of clodronate. Funding for this study was provided through a grant from the National Institutes of Health (NIH EB007678-01).
Disclosure Statement
The authors' have no professional or financial affiliations that would have biased this manuscript. No competing financial interests exist.
References
- 1.Chen F. Yoo J.J. Atala A. Acellular collagen matrix as a possible “off the shelf ” biomaterial for urethral repair. Urology. 1999;54:407. doi: 10.1016/s0090-4295(99)00179-x. [DOI] [PubMed] [Google Scholar]
- 2.Hodde J. Naturally occurring scaffolds for soft tissue repair and regeneration. Tissue Eng. 2002;8:295. doi: 10.1089/107632702753725058. [DOI] [PubMed] [Google Scholar]
- 3.Hodde J.P. Record R.D. Liang H.A. Badylak S.F. Vascular endothelial growth factor in porcine-derived extracellular matrix. Endothelium. 2001;8:11. doi: 10.3109/10623320109063154. [DOI] [PubMed] [Google Scholar]
- 4.Li F. Li W. Johnson S. Ingram D. Yoder M. Badylak S.F. Low-molecular-weight peptides derived from extracellular matrix as chemoattractants for primary endothelial cells. Endothelium. 2004;11:199. doi: 10.1080/10623320490512390. [DOI] [PubMed] [Google Scholar]
- 5.Nieponice A. Gilbert T.W. Badylak S.F. Reinforcement of esophageal anastomoses with an extracellular matrix scaffold in a canine model. Ann Thorac Surg. 2006;82:2050. doi: 10.1016/j.athoracsur.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 6.Badylak S.F. Park K. Peppas N. McCabe G. Yoder M. Marrow-derived cells populate scaffolds composed of xenogeneic extracellular matrix. Exp Hematol. 2001;29:1310. doi: 10.1016/s0301-472x(01)00729-9. [DOI] [PubMed] [Google Scholar]
- 7.Beattie A.J. Gilbert T.W. Guyot J.P. Yates A.J. Badylak S.F. Chemoattraction of progenitor cells by remodeling extracellular matrix scaffolds. Tissue Eng doi: 10.1089/ten.tea.2008.0162. 2008 doi: 10.1089/ten.tea.2008.0162. No additional information is available. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reing J.E. Zhang L. Myers-Irvin J. Cordero K.E. Freytes D.O. Heber-Katz E. Bedelbaeva K. McIntosh D. Dewilde A. Braunhut S.J. Badylak S.F. Degradation products of extracellular matrix affect cell migration and proliferation. Tissue Eng Part A doi: 10.1089/ten.tea.2007.0425. 2008 doi: 10.1089/ten.tea.2007.0425. No additional information is available. [DOI] [PubMed] [Google Scholar]
- 9.Zantop T. Gilbert T.W. Yoder M.C. Badylak S.F. Extracellular matrix scaffolds are repopulated by bone marrow-derived cells in a mouse model of Achilles tendon reconstruction. J Orthop Res. 2006;24:1299. doi: 10.1002/jor.20071. [DOI] [PubMed] [Google Scholar]
- 10.Badylak S.F. Coffey A.C. Lantz G.C. Tacker W.A. Geddes L.A. Comparison of the resistance to infection of intestinal submucosa arterial autografts versus polytetrafluoroethylene arterial prostheses in a dog model. J Vasc Surg. 1994;19:465. doi: 10.1016/s0741-5214(94)70073-7. [DOI] [PubMed] [Google Scholar]
- 11.Badylak S.F. Wu C.C. Bible M. McPherson E. Host protection against deliberate bacterial contamination of an extracellular matrix bioscaffold versus Dacron mesh in a dog model of orthopedic soft tissue repair. J Biomed Mater Res B Appl Biomater. 2003;67:648. doi: 10.1002/jbm.b.10062. [DOI] [PubMed] [Google Scholar]
- 12.Brennan E.P. Reing J. Chew D. Myers-Irvin J.M. Young E.J. Badylak S.F. Antibacterial activity within degradation products of biological scaffolds composed of extracellular matrix. Tissue Eng. 2006;12:2949. doi: 10.1089/ten.2006.12.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jernigan T.W. Croce M.A. Cagiannos C. Shell D.H. Handorf C.R. Fabian T.C. Small intestinal submucosa for vascular reconstruction in the presence of gastrointestinal contamination. Ann Surg. 2004;239:733. doi: 10.1097/01.sla.0000124447.30808.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarikaya A. Record R. Wu C.C. Tullius B. Badylak S.F. Ladisch M. Antimicrobial activity associated with extracellular matrices. Tissue Eng. 2002;8:63. doi: 10.1089/107632702753503063. [DOI] [PubMed] [Google Scholar]
- 15.Shell D.H. 4th, Croce M.A.Cagiannos C.Jernigan T.W.Edwards N.Fabian T.C.Comparison of small-intestinal submucosa and expanded polytetrafluoroethylene as a vascular conduit in the presence of gram-positive contamination Ann Surg 241995.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allman A.J. McPherson T.B. Badylak S.F. Merrill L.C. Kallakury B. Sheehan C. Raeder R.H. Metzger D.W. Xenogeneic extracellular matrix grafts elicit a TH2-restricted immune response. Transplantation. 2001;71:1631. doi: 10.1097/00007890-200106150-00024. [DOI] [PubMed] [Google Scholar]
- 17.Badylak S.F. Gilbert T.W. Immune response to biologic scaffold materials. Semin Immunol. 2008;20:109. doi: 10.1016/j.smim.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Badylak S.F. Valentin J.E. Ravindra A.K. McCabe G.P. Stewart-Akers A.M. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng Part A. 2008;14:1835. doi: 10.1089/ten.tea.2007.0264. [DOI] [PubMed] [Google Scholar]
- 19.Hodde J. Janis A. Hiles M. Effects of sterilization on an extracellular matrix scaffold: part II. Bioactivity and matrix interaction. J Mater Sci Mater Med. 2007;18:545. doi: 10.1007/s10856-007-2301-9. [DOI] [PubMed] [Google Scholar]
- 20.Whitlock P.W. Smith T.L. Poehling G.G. Shilt J.S. van Dyke M. A naturally derived, cytocompatible, and architecturally optimized scaffold for tendon and ligament regeneration. Biomaterials. 2007;28:4321. doi: 10.1016/j.biomaterials.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 21.Gilbert T.W. Stewart-Akers A.M. Simmons-Byrd A. Badylak S.F. Degradation and remodeling of small intestinal submucosa in canine Achilles tendon repair. J Bone Joint Surg Am. 2007;89:621. doi: 10.2106/JBJS.E.00742. [DOI] [PubMed] [Google Scholar]
- 22.Record R.D. Hillegonds D. Simmons C. Tullius R. Rickey F.A. Elmore D. Badylak S.F. In vivo degradation of 14C-labeled small intestinal submucosa (SIS) when used for urinary bladder repair. Biomaterials. 2001;22:2653. doi: 10.1016/s0142-9612(01)00007-2. [DOI] [PubMed] [Google Scholar]
- 23.Valentin J.E. Badylak J.S. McCabe G.P. Badylak S.F. Extracellular matrix bioscaffolds for orthopaedic applications. A comparative histologic study. J Bone Joint Surg Am. 2006;88:2673. doi: 10.2106/JBJS.E.01008. [DOI] [PubMed] [Google Scholar]
- 24.Billiar K. Murray J. Laude D. Abraham G. Bachrach N. Effects of carbodiimide crosslinking conditions on the physical properties of laminated intestinal submucosa. J Biomed Mater Res. 2001;56:101. doi: 10.1002/1097-4636(200107)56:1<101::aid-jbm1074>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 25.Coons D.A. Alan Barber F. Tendon graft substitutes-rotator cuff patches. Sports Med Arthrosc. 2006;14:185. doi: 10.1097/00132585-200609000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Metcalf M.H. Savoie F.H. Kellum B. Surgical technique for xenograft (SIS) augmentation of rotator-cuff repairs. Oper Tech Orthop. 2002;12:204. [Google Scholar]
- 27.van Rooijen N. Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 28.van Rooijen N. Sanders A. Elimination, blocking, and activation of macrophages: three of a kind? J Leukoc Biol. 1997;62:702. doi: 10.1002/jlb.62.6.702. [DOI] [PubMed] [Google Scholar]
- 29.van Rooijen N. Sanders A. van den Berg T.K. Apoptosis of macrophages induced by liposome-mediated intracellular delivery of clodronate and propamidine. J Immunol Methods. 1996;193:93. doi: 10.1016/0022-1759(96)00056-7. [DOI] [PubMed] [Google Scholar]
- 30.Gilbert T.W. Stewart-Akers A.M. Badylak S.F. A quantitative method for evaluating the degradation of biologic scaffold materials. Biomaterials. 2007;28:147. doi: 10.1016/j.biomaterials.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 31.Freytes D.O. Badylak S.F. Webster T.J. Geddes L.A. Rundell A.E. Biaxial strength of multilaminated extracellular matrix scaffolds. Biomaterials. 2004;25:2353. doi: 10.1016/j.biomaterials.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 32.Hafemann B. Ghofrani K. Gattner H.G. Stieve H. Pallua N. Cross-linking by 1-ethyl-3- (3-dimethylaminopropyl)-carbodiimide (EDC) of a collagen/elastin membrane meant to be used as a dermal substitute: effects on physical, biochemical and biological features in vitro. J Mater Sci Mater Med. 2001;12:437. doi: 10.1023/a:1011205221972. [DOI] [PubMed] [Google Scholar]
- 33.Kim M.S. Hong K.D. Shin H.W. Kim S.H. Lee M.S. Jang W.Y. Khang G. Lee H.B. Preparation of porcine small intestinal submucosa sponge and their application as a wound dressing in full-thickness skin defect of rat. Int J Biol Macromol. 2005;36:54. doi: 10.1016/j.ijbiomac.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 34.Markowicz M.P. Steffens G.C. Fuchs P.C. Pallua N. Enhanced dermal regeneration using modified collagen scaffolds: experimental porcine study. Int J Artif Organs. 2006;29:1167. doi: 10.1177/039139880602901210. [DOI] [PubMed] [Google Scholar]
- 35.van Rooijen N. van Kesteren-Hendrikx E. “In vivo” depletion of macrophages by liposome-mediated “suicide”. Methods Enzymol. 2003;373:3. doi: 10.1016/s0076-6879(03)73001-8. [DOI] [PubMed] [Google Scholar]
- 36.Frid M.G. Brunetti J.A. Burke D.L. Carpenter T.C. Davie N.J. Reeves J.T. Roedersheimer M.T. van Rooijen N. Stenmark K.R. Hypoxia-induced pulmonary vascular remodeling requires recruitment of circulating mesenchymal precursors of a monocyte/macrophage lineage. Am J Pathol. 2006;168:659. doi: 10.2353/ajpath.2006.050599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Badylak S.F. Vorp D.A. Spievack A.R. Simmons-Byrd A. Hanke J. Freytes D.O. Thapa A. Gilbert T.W. Nieponice A. Esophageal reconstruction with ECM and muscle tissue in a dog model. J Surg Res. 2005;128:87. doi: 10.1016/j.jss.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Kropp B.P. Sawyer B.D. Shannon H.E. Rippy M.K. Badylak S.F. Adams M.C. Keating M.A. Rink R.C. Thor K.B. Characterization of small intestinal submucosa regenerated canine detrusor: assessment of reinnervation, in vitro compliance and contractility. J Urol. 1996;156:599. doi: 10.1097/00005392-199608001-00008. [DOI] [PubMed] [Google Scholar]
- 39.Hodde J.P. Ernst D.M. Hiles M.C. An investigation of the long-term bioactivity of endogenous growth factor in OASIS wound matrix. J Wound Care. 2005;14:23. doi: 10.12968/jowc.2005.14.1.26721. [DOI] [PubMed] [Google Scholar]
- 40.McDevitt C.A. Wildey G.M. Cutrone R.M. Transforming growth factor-beta1 in a sterilized tissue derived from the pig small intestine submucosa. J Biomed Mater Res A. 2003;67:637. doi: 10.1002/jbm.a.10144. [DOI] [PubMed] [Google Scholar]
- 41.Voytik-Harbin S.L. Brightman A.O. Kraine M.R. Waisner B. Badylak S.F. Identification of extractable growth factors from small intestinal submucosa. J Cell Biochem. 1997;67:478. [PubMed] [Google Scholar]
- 42.Reing J.E. Zhang L. Myers-Irvin J. Cordero K.E. Freytes D.O. Heber-Katz E. Bedelbaeva K. McIntosh D. Dewilde A. Braunhut S.J. Badylak S.F. Degradation products of extracellular matrix affect cell migration and proliferation. Tissue Eng. 2008 doi: 10.1089/ten.tea.2007.0425. (In press). [DOI] [PubMed] [Google Scholar]
- 43.Badylak S.F. Kokini K. Tullius B. Simmons-Byrd A. Morff R. Morphologic study of small intestinal submucosa as a body wall repair device. J Surg Res. 2002;103:190. doi: 10.1006/jsre.2001.6349. [DOI] [PubMed] [Google Scholar]
- 44.Zheng M.H. Chen J. Kirilak Y. Willers C. Xu J. Wood D. Porcine small intestine submucosa (SIS) is not an acellular collagenous matrix and contains porcine DNA: possible implications in human implantation. J Biomed Mater Res B Appl Biomater. 2005;73:61. doi: 10.1002/jbm.b.30170. [DOI] [PubMed] [Google Scholar]
- 45.Badylak S.F. Arnoczky S. Plouhar P. Haut R. Mendenhall V. Clarke R. Horvath C. Naturally occurring extracellular matrix as a scaffold for musculoskeletal repair. Clin Orthop. 1999;367 Suppl:S333. doi: 10.1097/00003086-199910001-00032. [DOI] [PubMed] [Google Scholar]
- 46.Badylak S.F. Kochupura P.V. Cohen I.S. Doronin S.V. Saltman A.E. Gilbert T.W. Kelly D.J. Ignotz R.A. Gaudette G.R. The use of extracellular matrix as an inductive scaffold for the partial replacement of functional myocardium. Cell Transplant. 2006;15 Suppl 1:S29. doi: 10.3727/000000006783982368. [DOI] [PubMed] [Google Scholar]
- 47.Badylak S.F. Kokini K. Tullius B. Whitson B. Strength over time of a resorbable bioscaffold for body wall repair in a dog model. J Surg Res. 2001;99:282. doi: 10.1006/jsre.2001.6176. [DOI] [PubMed] [Google Scholar]
- 48.Dejardin L.M. Arnoczky S.P. Ewers B.J. Haut R.C. Clarke R.B. Tissue-engineered rotator cuff tendon using porcine small intestine submucosa. Histologic and mechanical evaluation in dogs. Am J Sports Med. 2001;29:175. doi: 10.1177/03635465010290021001. [DOI] [PubMed] [Google Scholar]
- 49.Harper C. Permacol: clinical experience with a new biomaterial. Hosp Med. 2001;62:90. doi: 10.12968/hosp.2001.62.2.2379. [DOI] [PubMed] [Google Scholar]
- 50.Kim K.M. Cells, rather than extracellular matrix, nucleate apatite in glutaraldehyde-treated vascular tissue. J Biomed Mater Res. 2002;59:639. doi: 10.1002/jbm.10038. [DOI] [PubMed] [Google Scholar]
- 51.Valente M. Laborde F. Thiene G. Milano A. Talenti E. Gallix P. Glutaraldehyde-fixed bovine iliac veins used as bioprosthetic conduits: an experimental animal study. J Card Surg. 1992;7:156. doi: 10.1111/j.1540-8191.1992.tb00791.x. [DOI] [PubMed] [Google Scholar]
- 52.Hsu S.Y. Cheng J.C. Chong Y.W. Leung P.C. Glutaraldehyde-treated bioprosthetic substitute for rabbit Achilles tendon. Biomaterials. 1989;10:258. doi: 10.1016/0142-9612(89)90102-6. [DOI] [PubMed] [Google Scholar]
- 53.Ueno T. Pickett L.C. de la Fuente S.G. Lawson D.C. Pappas T.N. Clinical application of porcine small intestinal submucosa in the management of infected or potentially contaminated abdominal defects. J Gastrointest Surg. 2004;8:109. doi: 10.1016/j.gassur.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 54.Mantovani A. Sica A. Sozzani S. Allavena P. Vecchi A. Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]