Abstract
Our group has previously reported that in vitro mechanical stimulation of tissue-engineered tendon constructs significantly increases both construct stiffness and the biomechanical properties of the repair tissue after surgery. When optimized using response surface methodology, our results indicate that a mechanical stimulus with three components (2.4% strain, 3000 cycles/day, and one cycle repetition) produced the highest in vitro linear stiffness. Such positive correlations between construct and repair stiffness after surgery suggest that enhancing structural stiffness before surgery could not only accelerate repair stiffness but also prevent premature failures in culture due to poor mechanical integrity. In this study, we examined the combined effects of scaffold crosslinking and subsequent mechanical stimulation on construct mechanics and biology. Autologous tissue-engineered constructs were created by seeding mesenchymal stem cells (MSCs) from 15 New Zealand white rabbits on type I collagen sponges that had undergone additional dehydrothermal crosslinking (termed ADHT in this manuscript). Both constructs from each rabbit were mechanically stimulated for 8 h/day for 12 consecutive days with half receiving 100 cycles/day and the other half receiving 3000 cycles/day. These paired MSC–collagen autologous constructs were then implanted in bilateral full-thickness, full-length defects in the central third of rabbit patellar tendons. Increasing the number of in vitro cycles/day delivered to the ADHT constructs in culture produced no differences in stiffness or gene expression and no changes in biomechanical properties or histology 12 weeks after surgery. Compared to MSC-based repairs from a previous study that received no additional treatment in culture, ADHT crosslinking of the scaffolds actually lowered the 12-week repair stiffness. Thus, while ADHT crosslinking may initially stiffen a construct in culture, this specific treatment also appears to mask any benefits of stimulation among repairs postsurgery. Our findings emphasize the importance of properly preconditioning a scaffold to better control/modulate MSC differentiation in vitro and to further enhance repair outcome in vivo.
Introduction
In vitro mechanical stimulation of adult stem cells has been shown to direct differentiation and promote extracellular matrix (ECM) development.1,2 Altman et al. demonstrated that in vitro mechanical stimulation of mesenchymal stem cells (MSCs) embedded in collagen gel upregulates ligament fibroblast markers, including expression of collagen I and III genes as well as tenascin-C.1 In another study,2 mechanically stimulating bioartificial tendons composed of avian flexor tendon cells seeded in type I collagen gels (1 h/day to 1% elongation at 1 Hz for 8 days) resulted in phenotypic expression profiles for the predominant collagens found in tendon. Other investigators have also studied the effect of mechanical stimulation on human tendon fibroblasts.3,4 One group found that applying cyclic biaxial mechanical strain to human patellar tendon fibroblasts cultured on silicone dishes altered cellular proliferation depending on the duration of the mechanical stimulation.4 This stimulation pattern increased secretion of growth factors (transforming growth factor-β, platelet-derived growth factor, and basic fibroblast growth factor).3 Mechanical stimulation has also been shown to induce cell alignment1,5 and increase collagen production.1
However, translating these in vitro stimulation benefits into improved repair outcome has required new strategies like in vitro–to–in vivo correlation. To date, stimulation has often meant imposing a single mechanical treatment pattern to the construct with a fixed set of mechanical signal components (peak strain, frequency, duration, cycle number per day, cycle repetition, and rise and fall times). Our group has extended this strategy by developing in vitro correlates of in vivo outcome.6,7 We first harvested bone marrow–derived MSCs from adult female New Zealand white (NZW) rabbits and then created four cell–collagen sponge constructs per animal. Two constructs were mechanically stimulated (2.4% peak strain, 1 Hz, 100 cycles for 8 h/day for 14 days), while the other two served as nonstimulated controls. One treated and one control construct were mechanically failed in tension, while the other pair were reimplanted as autologous constructs in contralateral central patellar tendon defects in the rabbits. Not only did mechanical stimulation significantly increase construct stiffness, but also this increase was positively correlated with stimulation-induced increases in patellar tendon repair stiffness 12 weeks after implantation.6,7
Such in vitro predictors of repair outcome are exciting and can speed development time, but they do not necessarily optimize the in vitro or in vivo outcome. To assess the relative importance of the individual components of the mechanical signal, our group has been using response surface methodology8 to optimize construct stiffness by varying levels of the input peak strain, cycle number per day, and cycle repetition. These studies have revealed that constructs subjected to a single cycle repetition up to 2.4% peak strain (levels already used in our laboratory6,7) as well as a higher cycle number per day (3000 vs. 100 cycles/day) produced the highest tensile stiffness in culture.9
Despite the improved biomechanical response, the construct is still quite weak and compliant before surgery. Current constructs possess a stiffness that is three orders of magnitude lower than the repair stiffness 12 weeks after surgery.6,7 This biological augmentation strategy is satisfactory in the central patellar tendon wound site, a load-protected environment where even small improvements in construct stiffness can enhance repair stiffness after surgery. However, stiffer tissue-engineered constructs will soon be needed to function in more challenging loading environments.
Another way to improve construct and hopefully repair stiffness might be to stiffen the scaffold by physical or chemical crosslinking.10 Under appropriate conditions, dehydrothermal (DHT) or ultraviolet light physical crosslinking can significantly improve scaffold strength without introducing cytotoxic chemicals as occurs with glutaraldehyde chemical crosslinking.11,12 For example, Wang et al. reported that exposing reconstituted collagen fibers to 5 days of DHT crosslinking at elevated temperatures increased tensile strength and modulus values.13 Initial improvements in the structural and material properties of collagen fibers (e.g., by DHT crosslinking) will be critical to developing biodegradable materials that promote healing of orthopedic soft tissues.13 Applied to tendon tissue engineering, one might then question what added benefits might accrue to repair stiffness by additional DHT (i.e., ADHT) crosslinking the collagen scaffold before mechanically stimulating the entire MSC–scaffold construct in culture.
Therefore, the objectives of this study were to (1) determine how increasing the number of cycles delivered per day to an MSC-ADHT–crosslinked scaffold construct in culture affects repair outcome after surgical implantation into a rabbit central patellar tendon defect site and (2) discover how ADHT crosslinking of a mechanically stimulated construct (2.4% peak strain for 100 cycles/day in culture) influences repair biomechanics in the same rabbit tendon defect model. We first hypothesized that increasing cycle number from 100 to 3000 cycles would (a) significantly improve repair biomechanics and histological appearance, (b) better match tangent stiffness compared to values found from a previous study,6 and (c) upregulate collagen I and III gene expression. We also hypothesized that ADHT crosslinking would increase in vitro stiffness and improve in vivo repair biomechanics.
Experimental Design
Fifteen skeletally mature (5.7 ± 0.2 kg, mean ± SEM) female NZW rabbits (Myrtles Rabbitry, Thompson Station, TN) were used in this study. MSCs, isolated from bone marrow and expanded using previously published methods,14 were chosen based on their ability to rapidly expand and our prior success with them for tendon repair.6,7 Six cell–scaffold constructs from each cell line (i.e., cells from the same animal) were created by seeding the MSCs at one density (0.14 × 106 cells/construct) onto the surface of type I collagen sponge that was previously subjected to additional DHT (ADHT) crosslinking (see details below). All constructs were mechanically stimulated in culture (1-s pulse duration to 2.4% peak strain for 8 h/day for 14 days). Group 1 constructs (n = 3 per rabbit) received only 100 cycles over the 8-h period (with a 287-s rest period between pulses), while group 2 constructs (n = 3 per rabbit) received 3000 cycles over the same period (with an 8.6-s rest period between pulses). The first two constructs from each treatment group were assigned for in vitro biomechanical evaluation and gene expression analysis. Using a random, left-versus-right assignment strategy, the third constructs from the 100 and 3000 cycles/day groups were surgically reimplanted into the same rabbit in contralateral, full-length, central patellar tendon defects. Twelve of these animals were assigned for biomechanical evaluation of the repairs at 12 weeks postsurgery (see details below and Refs.15,16), while the remaining three were assigned for histological appearance (organization, integration, and inflammation) and relative amounts of ECM components (collagen types I, III, and V, fibronectin, and decorin). Within- and between-subject comparisons were made of the effects of the mechanical stimulus (cycle number per day) on in vitro construct biomechanical properties, gene expression, and the biomechanical properties of repair tissue. Repair biomechanical data from the current in vivo study were also compared to (a) data from a previous study6 in which non-ADHT–crosslinked constructs were exposed to 100 cycles/day of stimulation before surgery and (b) values for normal patellar tendon (PT) from another study.16
Materials and Methods
Mesenchymal stem cells
MSCs were isolated from the iliac crest and processed.16,17 Briefly, each aspirate was centrifuged at 2000 rpm for 6 min after mixing with 25 mL of MSC growth medium, advanced Dulbecco's modified Eagle's media (ADV-DMEM; Gibco BRL/Life Technologies, Gaithersburg, MD), supplemented with 1% antibiotic/antimycotic (Gibco BRL/Life Technologies), and 5% fetal bovine serum of selected lots (Hyclone Laboratories, Logan, UT).18 After aspirating the supernatant, the precipitating cell pellet was suspended in 10 mL of MSC growth media. Cells were counted, plated at 22 × 106 cells/100 mm dish, placed in an incubator, and fed for 12–14 days. MSCs proliferated to form colonies between 6 and 8 days in primary culture. After cells reached confluency, they were retrieved, counted, and subcultured again to passage 2.
Scaffold preparation
Type I collagen sponges (P1076; Kensey Nash, Exton, PA; 94% pore volume; 62 μm mean pore diameter) were additionally dehydrothermally crosslinked (ADHT crosslinked) for 16 h at 135°C. Sponges were then cut to fit in the base of each of four wells in a silicone dish containing two restraining posts protruding from the base.15 Two 4-mm-diameter holes were created, permitting the sponge to be positioned over the posts.15 Before making the constructs, sponges were disinfected with 70% ethanol (Ethanol 95%; Cabisco Chemicals, Burlington, NC) for 24 h, rinsed three times with phosphate-buffered saline (Gibco BRL/Life Technologies), and placed in the wells of the silicone dish.
Construct preparation
Constructs were created by seeding a cell suspension aliquot (0.4 mL) containing 0.14 × 106 MSCs on top of the type I collagen sponge that was subjected to ADHT crosslinking. All constructs were placed in an incubator (37°C, 5% CO2, 95% RH) for 2 weeks and fed three times weekly with ADV-DMEM supplemented with 5% ascorbic acid, 1% antibiotic/antimycotic, and 5% fetal bovine serum. The final dimensions (length, width, and thickness) of the constructs are listed in Table 1.
Table 1.
Increasing the Duty Cycle from 100 to 3000 Cycles/Day Did Not Alter the Dimensions or the Load-Related Biomechanical Properties of the ADHT-Crosslinked Constructs in Culture (Mean ± SEM)
| 100 Cycles/day ADHT crosslinked | 3000 Cycles/day ADHT crosslinked | 100 Cycles/day non-ADHT crosslinked6 | |
|---|---|---|---|
| Dimensions | |||
| Length (mm) | 22.1 ± 0.2 | 22.2 ± 0.3 | 21.2 ± 0.7 |
| Width (mm) | 8.5 ± 0.2 | 8.3 ± 0.2 | 5.0 ± 0.5a |
| Thickness (mm) | 2.6 ± 0.1 | 2.4 ± 0.1 | 2.1 ± 0.1 |
| Structural and mechanical properties | |||
| Linear stiffness (N/mm) | 0.068 ± 0.007 | 0.074 ± 0.01 | 0.05 ± 0.007a |
| Maximum force (N) | 0.34 ± 0.009 | 0.35 ± 0.051 | 0.11 ± 0.011a |
| Linear modulus (MPa) | 0.038 ± 0.004 | 0.046 ± 0.01 | 0.02 ± 0.004a |
| Maximum stress (MPa) | 0.015 ± 0.001 | 0.017 ± 0.003 | 0.005 ± 0.001a |
However, the load-related biomechanical properties of the ADHT-crosslinked repairs cycled 100 times/day were significantly greater than corresponding properties of non-ADHT–crosslinked constructs cycled 100 times/day.6
Significantly different from 100 cycles/day, ADHT-crosslinked group (p < 0.05).
Mechanical stimulation
After allowing the MSC–collagen sponge constructs to equilibrate in the incubator for 2 days between the nonmoving posts, the silicone dishes containing the constructs were transferred to a custom five-station pneumatic mechanical stimulation system housed in an incubator.6,7,15 Constructs assigned for group 1 (100 cycles/day) and group 2 (3000 cycles/day) were stretched after 2 days of equilibration as described above using previously published methods.6,7,15 After 12 days of stimulation, the dishes were removed from the stimulator, the constructs assigned for surgery were implanted in vivo, and the constructs assigned for in vitro biomechanical evaluation were placed in cryovials and stored in a −80°C freezer until mechanical testing. The constructs assigned for gene expression analysis were treated with RNAlater (Qiagen, Valencia, CA) for 6 h, snap-frozen in liquid nitrogen, and placed in a −80°C freezer to prevent RNA degradation.19
Biomechanical evaluation of the constructs
On the day of testing, constructs were slowly thawed to room temperature over an hour and failed in tension using a custom materials testing system (100R6; TestResources, Shakopee, MN) by following previously published procedures.6,20,21 Briefly, specimens were secured in grips covered with fine grit sandpaper and then failed in tension at a rate of 10%/s. Linear stiffness and linear modulus were calculated from the linear region of the force–elongation and stress–strain curves generated by the testing system.20,21
Real-time quantitative RT-PCR
Using previously published methods, RNA from each construct was extracted using an RNeasy mini kit (Qiagen).19 First-strand complementary deoxyribonucleic acid (cDNA) was generated using a reverse transcriptase (RT) reaction. Rabbit-specific primers22,23 were used for collagen type I, collagen type III, decorin, fibronectin, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Conventional PCR was performed according to the following protocol: denaturation at 94°C for 20 s; annealing at 60°C for 30 s; and extension at 72°C for 30 s, 35 cycles. Amplification products were then verified using electrophoresis with Tris-Acetate-EDTA 2% agarose gel and SYBR safe DNA gel stain (Invitrogen–Molecular probes, Eugene, OR).
To quantify mRNA levels of the genes, real-time quantitative RT-PCR was performed with a continuous fluorescence detector (DNA Engine Opticon 2 System; MJ Research, Waltham, MA) by monitoring SYBR Green fluorescent dye (SYBR Green PCR master mix; Applied Biosystems, Foster City, CA) incorporated in double-strand DNA. The 50 μL reaction included 25 μL 2× SYBR Green PCR master mix, 6 μL of primer mix, 14 μL RNAase-free water, and 5 μL cDNA template. Real-time PCR was run according to the following protocol: 50°C for 2 min, 95°C for 10 min, 40 cycles of 95°C for 15 s, and 60°C for 1 min. All samples were run in duplicates. Standard curves were created for each target gene following procedures described previously.19 Gene expression was normalized by calculating the ratio between each target gene and GAPDH for each sample.
Surgical implantation
Following a protocol approved by the Institutional Animal Care and Use Committee at the University of Cincinnati, each rabbit was anesthetized, and the knees were aseptically prepped by using previously published methods.6,15 Full-length and full-thickness defects measuring approximately 3 mm in width were created in the central third of each patellar tendon. Defects were extended into each bone (5 mm long, 1 mm deep, and 2 mm wide) using a pneumatic sagittal saw blade (MicroAire Surgical Instruments, Charlottesville, VA). Constructs exposed to 100 and 3000 cycles/day were placed into contralateral defects and anchored to the remaining medial and lateral struts of the native PT using polypropylene sutures (Prolene 4-0; Ethicon, Somerville, NJ). The skin was closed with a simple interrupted suture pattern. After recovery, animals were fed ad libitum and allowed unrestricted cage activity in individual 5-foot square cages. Twelve weeks postsurgery, animals were sacrificed and tendon–bone units were harvested for histological and biomechanical evaluations.
Histological evaluation
Tissues were processed as previously described.6,15 Briefly, tissues were fixed in 10% neutral-buffered formalin. The bone ends (patella and tibia) were separated from the soft connective tissue and decalcified for 12–48 h using Calrite decalcification solution. All tissues were then processed through a gradient of alcohols and embedded in paraffin blocks. Ten (10) serial longitudinal sections (each 5 μm thick) were cut in the coronal plane at 400-μm intervals to a total depth of 2 mm from the anterior surface. Each section was stained with hematoxylin and eosin.6 Selected serial sections were subjected to immunohistochemical staining for collagen types I, III, and V (Accurate Chemical, Westbury, NY), fibronectin, and decorin (Oncogene Research Products–Calbiochem, San Diego, CA). Repair organization, integration, foreign body (sponge) reaction, metaplasia, and inflammation were subjectively evaluated.
Biomechanical evaluation of the repair
Patella-patellar tendon–tibia specimens were dissected, and dimensions (length and width) of the whole tendon were measured using vernier calipers accurate to 0.1 mm. Normal tissue on either side of the repair region was removed using the sutures at the edge of the defect as guides. The respective length, thickness, and width of the repair region were then remeasured using vernier calipers and a light force (<0.15 N) digital indicator accurate to 0.01 mm (IDC-type Mitutoyo Digimatic Indicator; MTI, Aurora, IL). Cross-sectional area was computed by multiplying tissue width by tissue thickness at proximal, middle, and distal locations and averaging these three local areas.6,16,24 The patellar and tibial bone blocks at each end of the repair were fixed into special grips using polymethylmethacrylate cement. Each specimen was preconditioned in tension at a strain rate of 3%/s for 50 cycles and then failed in tension at a constant strain rate of 20%/s in a chamber of phosphate-buffered saline (pH 7.4) mounted on the testing system (100R6; Testresources). A recommended higher strain rate of 20%/s (compared to 10%/s for the constructs) has been used in this testing to avoid the failure through bone.25,26 The force–displacement and stress–strain curves were plotted to determine structural and material properties, including stiffness, maximum force, modulus, and maximum stress as previously described.6,15,16
Statistical analysis
In vitro constructs
A paired Student's t-test was performed to determine if increasing the duty cycle from 100 to 3000 cycles/day affected biomechanical properties and the expressions of collagen type I, collagen type III, decorin, and fibronectin genes. Treating animals as a random effect, biomechanical properties of the 100 cycle/day constructs were also compared with those of 100 cycle/day, non-ADHT crosslinked scaffold constructs from a previous study.6
In vivo repairs
Using a Student's t-test,8 biomechanical properties of 100 and 3000 cycles/day groups were directly contrasted for specific structural biomechanical properties (stiffness, maximum force, maximum elongation, and strain energy) and material properties (modulus, maximum stress, maximum strain, and strain energy density).14,16 Using one-way analysis of variance, biomechanical data from 100 and 3000 cycles/day repairs were also compared to the biomechanical data of 100 cycles/day, non-ADHT–crosslinked repair tissues from a previous study,6 and those of normal patellar tendon from another study16 that used NZW rabbits of equivalent age and gender. Post hoc, least square means comparisons were performed to test for significance, and Bonferroni adjustments were made to account for multiple comparisons among the contrasts.8 The residuals were tested for normality and homoscedasticity and found to satisfy these criteria at a 5% level of significance. All conclusions regarding the biomechanical significance of increasing the duty cycle from 100 to 3000 cycles/day as well as imposing additional DHT crosslinking were made at the α = 0.05 experiment-wise level.
Results
In vitro
Increasing the cycle number from 100 to 3000 cycles/day did not alter the dimensions of the ADHT-crosslinked constructs (Table 1). No difference was found for in vitro biomechanical properties between constructs exposed to 100 versus 3000 cycles/day (Table 1). Similarly, no differences were detected in collagen type I, collagen type III, decorin, and fibronectin gene expression relative to GAPDH between the two treatment groups (Table 2). The 100 cycle/day, ADHT-crosslinked constructs exhibited significantly higher in vitro structural and material properties than 100 cycle/day, non-ADHT–crosslinked constructs from a previous study6 (Table 1) (p < 0.05).
Table 2.
Increasing Cycle Number Produced No Significant Differences in Gene Expression (Mean ± SEM) for Collagen I and III, Decorin, or Fibronectin Relative to GAPDH (i.e., the Ratio Between Each Target Gene and GAPDH for Each Sample) of ADHT Constructs
| 100 Cycles/day, ADHT crosslinked | 3000 Cycles/day, ADHT crosslinked | |
|---|---|---|
| Collagen type I | 0.136 ± 0.069 | 0.054 ± 0.012 |
| Collagen type III | 0.029 ± 0.019 | 0.024 ± 0.016 |
| Decorin | 0.012 ± 0.005 | 0.010 ± 0.003 |
| Fibronectin | 0.056 ± 0.038 | 0.093 ± 0.054 |
In vivo
Only 10 of the 15 rabbits assigned to the study provided tissues for histological (n = 2 rabbit) and biomechanical evaluation (n = 8). Two other rabbits died during the 12-week healing period after surgery. In the remaining three rabbits, one or both of the tendons were observed during dissection to have ruptured or luxated in a medial–lateral direction to spread the repair. These tendons were not included in the analysis.
Increasing cycle number from 100 to 3000 cycles/day did not alter the length, width, or thickness of either the engineered construct (Table 1) or the corresponding repair (Table 3). However, the length, width, and thickness of repair tissue containing the 100 and 3000 cycle/day, ADHT-crosslinked constructs were significantly greater than corresponding dimensions for the normal tendons from a previous study16 (p < 0.05).
Table 3.
Increasing Cycle Number Had No Effect on the Dimensions (Mean ± SEM) of ADHT-Crosslinked Repairs at 12 Weeks Postsurgery
| 100 Cycles/day, ADHT crosslinked | 3000 Cycles/day, ADHT crosslinked | Normal PT16 | |
|---|---|---|---|
| Entire tissue | |||
| Length (mm) | 29.1 ± 1.6 | 28.7 ± 0.8 | 24.6 ± 0.5a |
| Width (mm) | 17.4 ± 0.8 | 17.0 ± 0.9 | 8.5 ± 0.9a |
| Thickness (mm) | 3.6 ± 0.3 | 3.4 ± 0.3 | 1.7 ± 0.2a |
| Central third region | |||
| Length (mm) | 28.5 ± 1.1 | 27.8 ± 0.9 | 24.1 ± 0.8a |
| Width (mm) | 6.3 ± 0.5 | 6.4 ± 0.5 | 3.1 ± 0.3a |
| Thickness (mm) | 3.0 ± 0.3 | 2.8 ± 0.3 | 1.5 ± 0.3a |
However, all three dimensions (length, width, and thickness) for both repairs were significantly greater than those for normal patellar tendon.16
Statistically different than values for 100 and 3000 cycles/day, ADHT-crosslinked repairs (p < 0.05).
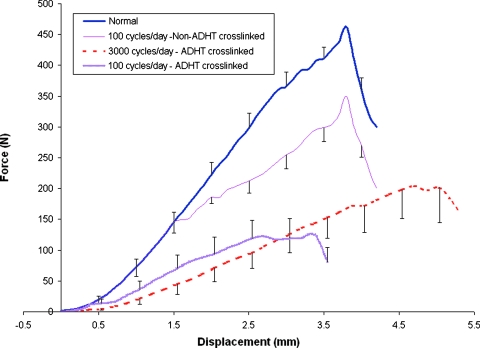
Increasing the duty cycle of mechanical stimulation applied to ADHT constructs produced different effects on repair biomechanics than did the application of ADHT crosslinking. Increasing the cycle number from 100 to 3000 cycles/day did not significantly affect any of the repair biomechanical response measures (Table 4 and Fig. 1; p > 0.05). However, ADHT crosslinking of the 100 cycle/day–stimulated constructs actually reduced both the structural biomechanical and material properties of the 12-week repairs. Whereas noncrosslinked scaffold repairs matched normal PT force–displacement response up to 32% of failure force,6,16 ADHT crosslinking significantly reduced the load-related parameters extracted from the force–elongation curve to failure (Fig. 1). Specifically, ADHT crosslinking reduced repair linear stiffness and maximum force as well as repair linear modulus, maximum stress, strain energy density, and maximum strain (Table 4; p < 0.05).
Table 4.
Increasing Cycle Number Produced No Significant Differences in Any Biomechanical Property (Mean ± SEM) for ADHT-Crosslinked Repair Tissues at 12 Weeks Postsurgery
| 100 Cycles/day, ADHT crosslinked | 3000 Cycles/day, ADHT crosslinked | Normal PT16 | 100 Cycles/day, non-ADHT crosslinked6 | |
|---|---|---|---|---|
| Structural properties | ||||
| Linear stiffness (N/mm) | 58.5 ± 11.3 | 59.8 ± 10.3 | 159.8 ± 10.5a | 141.6 ± 11.3a |
| Maximum force (N) | 166.4 ± 23.2 | 217.9 ± 35.4 | 470.7 ± 67.2a | 339.3 ± 11.4a |
| Strain energy (N-mm) | 360.1 ± 65.3 | 579.3 ± 112.5a | 875.7 ± 104.1a | 561.1 ± 22.2 |
| Maximum displacement (mm) | 4.7 ± 0.9 | 5.5 ± 0.9 | 4.0 ± 0.3a | 5.4 ± 1.2 |
| Material properties | ||||
| Linear modulus (MPa) | 47.0 ± 7.6 | 51.4 ± 12.1 | 861.4 ± 105.9a | 441.2 ± 26.3a |
| Maximum stress (MPa) | 9.7 ± 1.9 | 13.8 ± 3.8 | 100.7 ± 16.0a | 72.1 ± 11.1a |
| SED (N-mm/mm3) | 1.6 ± 0.5 | 2.6 ± 0.9 | 8.2 ± 1.7a | 6.9 ± 1.3a |
| Maximum strain (%) | 30.2 ± 5.0 | 36.5 ± 4.0 | 16.0 ± 1.5a | 19.3 ± 1.2a |
FIG. 1.
The force–displacement curves for repairs containing constructs that were ADHT crosslinked and cycled 100 versus 3000 times/day are significantly inferior to both repairs that were non-ADHT crosslinked and cycled 100 times/day6 as well as normal tendon16 (p < 0.05). Note that the ADHT-crosslinked repair tissues were tested at room temperature, and the remainder was tested at 37°C. Color images available online at www.liebertonline.com/ten.
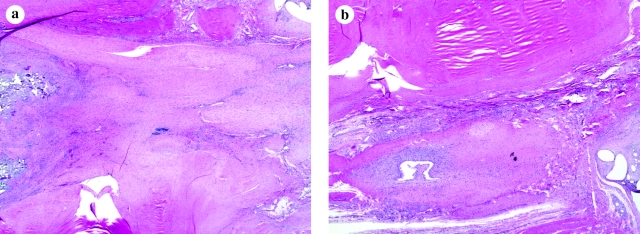
Repair histology was unaffected by increasing the cycle number of stimulation delivered to the constructs in culture. Increasing cycle number from 100 to 3000 cycles/day produced no subjective differences in immunohistochemical staining, tenocyte density, or cellular alignment (Fig. 2). Although tenocytes showed a preferred axis, large areas of the repairs showed tenocytes not oriented parallel to the long axis of force transmission. Repair sites also showed areas of severe mixed neutrophil and lymphocyte infiltration.
FIG. 2.
No differences in immunohistochemical staining, tenocyte density, or cellular alignment were detected between (a) 100 cycles/day and (b) 3000 cycles/day ADHT-crosslinked repair tissues (original magnification, × 2). Color images available online at www.liebertonline.com/ten.
Discussion
This study examined the individual and combined effects of daily cycles of mechanical stimulation and scaffold pretreatment with ADHT crosslinking to MSC–collagen sponge constructs in culture. We sought to judge the effects of both treatments by monitoring changes in construct stiffness in vitro and in biomechanical properties of the repairs 12 weeks after surgery. Our results showed no improvement in either in vitro or in vivo response measures after mechanically stimulating the MSC-ADHT–crosslinked scaffold constructs. Even though ADHT crosslinking of the scaffolds did improve in vitro biomechanical properties compared to no crosslinking, no corresponding in vivo benefits were observed. In fact, we observed an adverse effect of this crosslinking treatment postsurgery.
Findings from this study are inconsistent with those from previous studies that have used collagen sponges without ADHT crosslinking. We previously reported that the in vitro stiffness of non-ADHT–crosslinked constructs could be improved up to 50% by increasing the duty cycle from 100 to 3000 cycles/day (from 0.045 ± 0.007 to 0.068 ± 0.009 N/mm).9 Although ADHT crosslinking did increase average in vitro stiffness compared to constructs receiving no additional crosslinking,6,9 the corresponding repairs were not significantly enhanced. In fact, the biomechanical and material properties of the repairs containing the mechanically stimulated and ADHT-crosslinked scaffold constructs were inferior to corresponding 12-week repairs that received stimulation but no additional crosslinking.6 While such crosslinking may stiffen the construct so as to avoid premature failure before surgery, we conclude that it also produces the observed adverse effect on repair biomechanical properties because it was the only factor changed in this part of the study to avoid premature failure of constructs in culture before delivering mechanical stimulation.
Our additional crosslinking treatment may have contributed not only to the lack of effect of stimulation on in vitro or in vivo biomechanical properties, but also to a lack of effect on gene expression (collagen type I, collagen type III, decorin, and fibronectin). ADHT crosslinking could have stiffened the collagen sponge to such an extent that cells did not sense the imposed mechanical stimuli and synthesize new ECM to enhance stiffness. These cells may have become stress shielded from the mechanical signals, adversely affecting both gene and protein expression in culture and possibly even changing cell phenotype during repair. For example, we previously found that when MSCs were suspended in collagen gel, contracted around stiff sutures, and then placed in rabbit PT wound sites, approximately 28% of the 12-week repairs exhibited ectopic bone formation.16 Alkaline phosphatase, an early bone marker, was upregulated in the 3D cell–gel–suture constructs in culture but not when the cells were expanded in monolayer.27 Ectopic bone formation was eliminated when we reduced cell density and eliminated the suture. Reducing scaffold stiffness by controlling the degree of ADHT crosslinking might induce similar benefits (although the actual change in scaffold stiffness and cellular activity produced by ADHT crosslinking must still be quantified). Alternatively, ADHT crosslinking may have altered the scaffold pore size28,29 or cell attachment efficiency. Any reductions in pore size could have reduced the number of cells able to migrate into the scaffold of the implant used for surgery. Any surface changes induced by crosslinking may have also limited initial cellular attachment or decreased cell viability due to inadequate nutrient supply and waste removal. The lack of cellular infiltration may have thus produced a more natural host-induced rather than cell-assisted repair, resulting in a more inferior repair outcome. Studies to examine these possibilities are in process in our laboratory.
Although DHT crosslinking has been reported to improve scaffold strength more than other crosslinking techniques, the chemical changes produced by DHT may also adversely affect the cells embedded in the scaffold.10–13,30–32 If not applied appropriately, DHT crosslinking is well known to change the chemical composition of collagen-based membranes.10 DHT crosslinking–induced changes in chemical composition could alter the cytocompatibility of the scaffold to accept MSCs and thus compromise the cellular attachment.12,13,30 Any cytotoxicity of strongly crosslinked, collagen-based materials could modify cell shape and significantly reduce cell growth.31 DHT crosslinking has also been postulated to significantly decrease the rate of cell migration by masking the integrin binding sites that promote cellular attachment.11,32 One or more of these crosslinking-induced phenomena could have adversely affected repair tissue, while at the same time improved the construct's in vitro properties compared to non-ADHT–crosslinked constructs.
The results from this study both contradict and support important findings previously reported by our group and others. (1) Recently, we showed that the in vitro stiffness of MSC–collagen sponge constructs without additional crosslinking correlated with repair stiffness 12 weeks after surgery.6,7 We were surprised to find in the current study, however, that despite improvements in in vitro stiffness due to ADHT crosslinking, no such improvements occurred in repair stiffness 12 weeks after surgery. This lack of in vitro–to–in vivo correlation might be explained by the mechanisms of DHT crosslinking. DHT crosslinking causes dehydration of the fibers and in turn draws the collagen molecules closer together. While this action increases temperature stability, the act of crosslinking remains a delicate balance between removing intrafibrillar water content and maintaining thermal stability of collagen fibers, which can be exceeded at 54°C. Therefore, thermal damage (denaturation) to collagen fiber subunits could have occurred with our ADHT technique, thereby altering the potential of collagen to effectively bear load in this repair application. Future studies will need to be conducted to study not only the ability of in vitro measures to predict in vivo repair outcome, but also the role that crosslinking and other factors might have on such predictions. (2) Now that the cellular response to mechanical stimulation has been shown to be scaffold dependent, investigators will likely need to optimize these mechanical stimulation effects every time a new scaffold is identified.21 While optimizing the mechanical stimulus can significantly alter the stiffness of constructs containing collagen sponges with no additional crosslinking,9 the same mechanical stimulus produced no such effect in the presence of ADHT crosslinking. These findings emphasize the importance of identifying appropriate modification techniques so as to engineer the scaffold with sufficient mechanical integrity, and biologic activity to better modulate MSC differentiation in vitro before attempting to optimize constructs to create functionally efficacious tendon repairs. (3) Our in vivo results would also support recommendations by Weadock et al. that DHT treatment be avoided in load-bearing, collagen-based implant applications so as to minimize the denaturation effect of the treatment.29 These consistencies and inconsistencies between our results and those from other studies suggest that the specific ADHT crosslinking (16 h at 135°C) that we used in this study be avoided in future optimization studies or at least that the scaffold be minimally altered by the technique/process that we used. It is possible that the duration and the temperature we chose for ADHT crosslinking in this study were severe enough to adversely affect the collagen sponges. Therefore, future studies should identify the optimum ADHT crosslink conditions.
Our study is not without limitations. (1) We observed a relatively high incidence of tendon ruptures at the wound site during the healing period after surgery. Considering the load-protected nature of the central tendon wound site, these ruptures suggest insufficient construct stiffness at the time of implantation to withstand the forces acting at the repair site. We are currently examining stiffer scaffold materials to improve in vitro construct stiffness without stress shielding the cells and compromising the potential benefits of the mechanical stimulus. (2) We did not monitor cell viability in the constructs before surgery because insufficient cells were available at passage 2 to make additional constructs. In future studies, we plan to either try and increase seeding density at early passages or move beyond passage 2 for additional cells that would permit testing for both cell viability and crosslinking-related effects on cellular activity. (3) We did not determine what fraction of cells was participating in the repair after surgery. In the past, our group has added a DiI label (CM-DiI, CellTracker™; Molecular Probes, Eugene, OR) to the MSCs when creating the constructs, permitting us to sort host from introduced cells up to 12 weeks after surgery.15,16 Our future studies will again utilize this technique to better understand the effects of crosslinking on cell migration and binding. (4) Animal-to-animal variability as well as inter-study comparisons of treatment effect could have influenced our conclusions. Therefore, future studies will separately examine intra-animal effects of crosslinking-induced scaffold stiffening and increasing cycle number using well-controlled experimental designs. (5) The testing conditions that we used were also not identical in these two studies. Repair tissues from the current study were tested at room temperature, whereas the repair tissues from the previous studies were tested in 37°C.6,16 Tendons are known to exhibit lower stiffness at body temperature than at room temperature.33 The detrimental effects we observed at room temperature when testing both 100 and 3000 cycle/day, ADHT-crosslinked repairs might have been even more profound had we tested these repair tissues at 37°C. Thus, additional DHT crosslinking may even more adversely affect repair stiffness than what is reported in the current study. (6) We did not fully examine the causes of the histologic changes that occurred in the repair sites due to the treatment. The presence of mixed neutrophils and lymphocyte infiltration suggest a foreign body response to the sponge. This response would have greatly inhibited any tendon remodeling that would occur. Future studies will more closely examine the underlying causes of these histologic changes.
This study was designed to examine whether optimizing the mechanical stimulus in culture to improve in vitro biomechanical properties would improve repair biomechanical properties. Our results indicate that the level of ADHT crosslinking of the scaffold that we used in this study actually reduced the biomechanical properties of the repair tissue. Our results also demonstrate that altering cycling conditions with ADHT crosslinking had no significant impact on in vivo repairs. Results from this study and the previous study6 collectively suggest that only the improvements in the in vitro stiffness achieved through cellular activities (for example mechanical stimulation) would improve the repair outcome. However, this needs to be verified in future studies. These findings emphasize the importance of screening and appropriately altering scaffold mechanics so as to facilitate cell migration, proliferation, and differentiation in culture before performing more costly and time-consuming in vivo repair studies.
Acknowledgments
This research was supported by a grant from the National Institutes of Health (AR 46574) given to the University of Cincinnati and by a Merit Review grant from the Veteran's Administration to Gregory Boivin. The authors would also like to thank Kensey Nash for providing the collagen sponges.
Disclosure Statement
No competing financial interests exist.
References
- 1.Altman G.H. Horan R.L. Martin I. Farhadi J. Stark P.R. Volloch V. Richmond J.C. Vunjak-Novakovic G. Kaplan D.L. Cell differentiation by mechanical stress. FASEB J. 2002;16:270. doi: 10.1096/fj.01-0656fje. [DOI] [PubMed] [Google Scholar]
- 2.Garvin J. Qi J. Maloney M. Banes A.J. Novel system for engineering bioartificial tendons and application of mechanical load. Tissue Eng. 2003;9:967. doi: 10.1089/107632703322495619. [DOI] [PubMed] [Google Scholar]
- 3.Skutek M. van Griensven M. Zeichen J. Brauer N. Bosch U. Cyclic mechanical stretching modulates secretion pattern of growth factors in human tendon fibroblasts. Eur J Appl Physiol. 2001;86:48. doi: 10.1007/s004210100502. [DOI] [PubMed] [Google Scholar]
- 4.Zeichen J. van Griensven M. Bosch U. The proliferative response of isolated human tendon fibroblasts to cyclic biaxial mechanical strain. Am J Sports Med. 2000;28:888. doi: 10.1177/03635465000280061901. [DOI] [PubMed] [Google Scholar]
- 5.Wang H. Ip W. Boissy R. Grood E.S. Cell orientation response to cyclically deformed substrates: experimental validation of a cell model. J Biomech. 1995;28:1543. doi: 10.1016/0021-9290(95)00101-8. [DOI] [PubMed] [Google Scholar]
- 6.Juncosa-Melvin N. Shearn J.T. Boivin G.P. Gooch C. Galloway M.T. West J.R. Nirmalanandhan V.S. Bradica G. Butler D.L. Effects of mechanical stimulation on the biomechanics and histology of stem cell-collagen sponge constructs for rabbit patellar tendon repair. Tissue Eng. 2006;12:2291. doi: 10.1089/ten.2006.12.2291. [DOI] [PubMed] [Google Scholar]
- 7.Shearn J.T. Juncosa-Melvin N. Boivin G.P. Galloway M.T. Goodwin W. Gooch C. Dunn M.G. Butler D.L. Mechanical stimulation of tendon tissue engineered constructs: effects on construct stiffness, repair biomechanics and their correlation. J Biomech Eng. 2007;129:848. doi: 10.1115/1.2800769. [DOI] [PubMed] [Google Scholar]
- 8.Montgomery D. fifth edition. New York, NY: John Wiley and Sons; 2001. Design and Analysis of Experiments. [Google Scholar]
- 9.Nirmalanandhan V. Shearn J. Juncosa-Melvin N. Rao M. Gooch C. Bradica G. Butler D. Improving linear stiffness of the cell-seeded collagen sponge constructs by varying the components of the mechanical stimulus. Tissue Eng Part A. 2008;14:1883. doi: 10.1089/ten.tea.2007.0125. [DOI] [PubMed] [Google Scholar]
- 10.Yannas I.V. Burke J.F. Gordon P.L. Huang C. Rubenstein R.H. Design of an artificial skin. II. Control of chemical composition. J Biomed Mater Res. 1980;14:107. doi: 10.1002/jbm.820140203. [DOI] [PubMed] [Google Scholar]
- 11.Cornwell K.G. Lei P. Andreadis S.T. Pins G.D. Crosslinking of discrete self-assembled collagen threads: effects on mechanical strength and cell-matrix interactions. J Biomed Mater Res A. 2007;80:362. doi: 10.1002/jbm.a.30893. [DOI] [PubMed] [Google Scholar]
- 12.Weadock K. Olson R.M. Silver F.H. Evaluation of collagen crosslinking techniques. Biomater Med Devices Artif Organs. 1983;11:293. doi: 10.3109/10731198309118815. [DOI] [PubMed] [Google Scholar]
- 13.Wang M.C. Pins G.D. Silver F.H. Collagen fibres with improved strength for the repair of soft tissue injuries. Biomaterials. 1994;15:507. doi: 10.1016/0142-9612(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 14.Young R.G. Butler D.L. Weber W. Caplan A.I. Gordon S.L. Fink D.J. Use of mesenchymal stem cells in a collagen matrix for Achilles tendon repair. J Orthop Res. 1998;16:406. doi: 10.1002/jor.1100160403. [DOI] [PubMed] [Google Scholar]
- 15.Juncosa-Melvin N. Boivin G.P. Galloway M.T. Gooch C. West J.R. Sklenka A.M. Butler D.L. Effects of cell-to-collagen ratio in mesenchymal stem cell-seeded implants on tendon repair biomechanics and histology. Tissue Eng. 2005;11:448. doi: 10.1089/ten.2005.11.448. [DOI] [PubMed] [Google Scholar]
- 16.Awad H.A. Boivin G.P. Dressler M.R. Smith F.N. Young R.G. Butler D.L. Repair of patellar tendon injuries using a cell-collagen composite. J Orthop Res. 2003;21:420. doi: 10.1016/S0736-0266(02)00163-8. [DOI] [PubMed] [Google Scholar]
- 17.Awad H.A. Butler D.L. Boivin G.P. Smith F.N. Malaviya P. Huibregtse B. Caplan A.I. Autologous mesenchymal stem cell-mediated repair of tendon. Tissue Eng. 1999;5:267. doi: 10.1089/ten.1999.5.267. [DOI] [PubMed] [Google Scholar]
- 18.Lennon D.P. Haynesworth S.E. Young R.G. Dennis J.E. Caplan A.I. A chemically defined medium supports in vitro proliferation and maintains the osteochondral potential of rat marrow-derived mesenchymal stem cells. Exp Cell Res. 1995;219:211. doi: 10.1006/excr.1995.1221. [DOI] [PubMed] [Google Scholar]
- 19.Juncosa-Melvin N. Matlin K.S. Holdcraft R.W. Nirmalanandhan V.S. Butler D.L. Mechanical stimulation increases collagen type I and collagen type III gene expression of stem cell-collagen sponge constructs for patellar tendon repair. Tissue Eng. 2007;13:1219. doi: 10.1089/ten.2006.0339. [DOI] [PubMed] [Google Scholar]
- 20.Nirmalanandhan V.S. Rao M. Sacks M.S. Haridas B. Butler D.L. Effect of length of the engineered tendon construct on its structure-function relationships in culture. J Biomech. 2007;40:2523. doi: 10.1016/j.jbiomech.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 21.Nirmalanandhan V.S. Dressler M.R. Shearn J.T. Juncosa-Melvin N. Rao M. Gooch C. Bradica G. Butler D.L. Mechanical stimulation of tissue engineered tendon constructs; effect of scaffold materials. J Biomech Eng. 2007;129:919. doi: 10.1115/1.2800828. [DOI] [PubMed] [Google Scholar]
- 22.Boykiw R. Sciore P. Reno C. Marchuk L. Frank C.B. Hart D.A. Altered levels of extracellular matrix molecule mRNA in healing rabbit ligaments. Matrix Biol. 1998;17:371. doi: 10.1016/s0945-053x(98)90089-0. [DOI] [PubMed] [Google Scholar]
- 23.Guehring T. Omlor G.W. Lorenz H. Bertram H. Steck E. Richter W. Carstens C. Kroeber M. Stimulation of gene expression and loss of anular architecture caused by experimental disc degeneration—an in vivo animal study. Spine. 2005;30:2510. doi: 10.1097/01.brs.0000186591.17114.e9. [DOI] [PubMed] [Google Scholar]
- 24.Dressler M.R. Butler D.L. Boivin G.P. Effects of age on the repair ability of mesenchymal stem cells in rabbit tendon. J Orthop Res. 2005;23:287. doi: 10.1016/j.orthres.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 25.Noyes F.R. DeLucas J.L. Torvik P.J. Biomechanics of anterior cruciate ligament failure: an analysis of strain-rate sensitivity and mechanisms of failure in primates. J Bone Joint Surg Am. 1974;56:236. [PubMed] [Google Scholar]
- 26.Butler D.L. Grood E.S. Noyes F.R. Zernicke R.F. Brackett K. Effects of structure and strain measurement technique on the material properties of young human tendons and fascia. J Biomech. 1984;17:579. doi: 10.1016/0021-9290(84)90090-3. [DOI] [PubMed] [Google Scholar]
- 27.Harris M.T. Butler D.L. Boivin G.P. Florer J.B. Schantz E.J. Wenstrup R.J. Mesenchymal stem cells used for rabbit tendon repair can differentiate into osteoblasts and form ectopic bone. J Orthop Res. 2004;22:998. doi: 10.1016/j.orthres.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 28.Wahl D.A. Sachlos E. Liu C. Czernuszka J.T. Controlling the processing of collagen-hydroxyapatite scaffolds for bone tissue engineering. J Mater Sci Mater Med. 2007;18:201. doi: 10.1007/s10856-006-0682-9. [DOI] [PubMed] [Google Scholar]
- 29.Weadock K.S. Miller E.J. Keuffel E.L. Dunn M.G. Effect of physical crosslinking methods on collagen-fiber durability in proteolytic solutions. J Biomed Mater Res. 1996;32:221. doi: 10.1002/(SICI)1097-4636(199610)32:2<221::AID-JBM11>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 30.Bush K.A. Downing B.R. Walsh S.E. Pins G.D. Conjugation of extracellular matrix proteins to basal lamina analogs enhances keratinocyte attachment. J Biomed Mater Res A. 2007;80:444. doi: 10.1002/jbm.a.30933. [DOI] [PubMed] [Google Scholar]
- 31.Rousseau C.F. Gagnieu C.H. In vitro cytocompatibility of porcine type I atelocollagen crosslinked by oxidized glycogen. Biomaterials. 2002;23:1503. doi: 10.1016/s0142-9612(01)00276-9. [DOI] [PubMed] [Google Scholar]
- 32.Cornwell K.G. Downing B.R. Pins G.D. Characterizing fibroblast migration on discrete collagen threads for applications in tissue regeneration. J Biomed Mater Res A. 2004;71:55. doi: 10.1002/jbm.a.30132. [DOI] [PubMed] [Google Scholar]
- 33.Woo S.L. Lee T.Q. Gomez M.A. Sato S. Field F.P. Temperature dependent behavior of the canine medial collateral ligament. J Biomech Eng. 1987;109:68. doi: 10.1115/1.3138645. [DOI] [PubMed] [Google Scholar]