Abstract
In this study, the bioactive effects of poly(ethylene glycol) (PEG) sebacic acid diacrylate (PEGSDA) hydrogels with or without RGD peptide modification on osteogenic differentiation and mineralization of marrow stromal cells (MSCs) were examined. In a separate experiment, the ability of PEGSDA hydrogel to serve as a delivery vehicle for bone morphogenetic protein 2 (BMP-2) was also investigated. As a scaffold, the attachment and proliferation of MSCs on PEGSDA hydrogel scaffolds with and without RGD peptide modification was similar to the control, tissue culture polystyrene. In contrast, cells were barely seen on unmodified PEG diacrylate (PEGDA) hydrogel throughout the culture period for up to 21 days. Osteogenic phenotypic expression such as alkaline phosphatase (ALP) of MSCs as well as mineralized calcium content were significantly higher on PEGSDA-based hydrogels than those on the control or PEGDA hydrogels. Potential use of PEGSDA scaffold as a delivery vehicle of osteogenic molecules such as BMP-2 was also evaluated. Initial burst release of BMP-2 from PEGSDA hydrogel scaffold (14.7%) was significantly reduced compared to PEGDA hydrogel scaffold (84.2%) during the first 3 days of a 21-day release period. ALP activity of an osteoblast was significantly higher in the presence of BMP-2 released from PEGSDA hydrogel scaffolds compared to that in the presence of BMP-2 released from PEGDA scaffolds, especially after 6 days of release. Overall, PEGSDA hydrogel scaffolds without further modification may be useful as orthopedic tissue engineering scaffolds as well as local drug carriers for prolonged sustained release of osteoinductive molecules.
Introduction
Although autograft, allograft, and bone graft substitutes such as metals or bone cement have been widely used for repairing critical-sized bone defects, other alternatives using tissue engineering strategies have been developed to overcome certain limitations associated with current therapies, such as donor-site morbidity, potential disease transmission, fatigue failure of the implant, among others.1,2 A number of biomaterials have been developed as bone tissue engineering scaffolds3–5 or delivery carriers of therapeutic agents such as osteoprogenitor cells6,7 or growth factors.8,9
Poly(ethylene glycol) (PEG)–based hydrogels have been developed for tissue engineering applications due to their excellent biocompatibility inherited from their bioinert nature.10,11 However, their lack of biomolecular recognition by cells prevents them from direct interaction with the cells, thus requiring further functionalization by biomimetic molecules to stimulate the interaction. Numerous technologies to render PEG-based hydrogels bioactive have been explored to make them more attractive candidates for tissue engineering applications.12,13 One of the most widely used strategies is to incorporate cell adhesion ligands such as Arg-Gly-Asp (RGD) peptide sequences derived from cell adhesion proteins such as fibronectin.14 For example, PEG-based hydrogels were successfully functionalized with N-hudroxysuccinimidyl–activated esters coupled with PEG spacer arm to enhance fibroblast cell attachment and spreading.15 Other approaches have been introduced to promote bioactivity of hydrogels such as incorporating positive charge,16 heparin,17 and enzymatic recognition sites to enhance specific types of enzyme activities such as matrix metalloproteinases.2,18 However, such approaches often involve complex and/or expensive procedures, which may not be favorable for large-scale applications.
Another strategy to make PEG-based hydrogels bioactive consists of incorporating biomolecules such as growth factors into the carriers.13 PEG-based hydrogels have been utilized as a delivery carrier of therapeutic biomolecules, including bone morphogenetic proteins (BMPs).8,9,19 However, traditional synthetic hydrogel scaffolds such as PEG diacrylate (PEGDA) often swell excessively, thus generating large pores to uptake considerable amount of water, leading to rapid initial burst release of incorporated bioactive molecules.20 Rapid initial burst release of such molecules is not desirable for continuous tissue ingrowth and regeneration during wound healing process over a reasonable time scale.21 For example, bone healing time varies from days to weeks to months, depending on the nature of bone defects.22,23 Extensive efforts have been focused on reducing the initial burst release of drug carriers by controlling formulation parameters of the carriers such as molecular weight, hydrophilicity, as well as drug loading amount.21
Recently, our laboratory has developed a biodegradable hydrogel of PEG sebacic acid diacrylate (PEGSDA), based on PEG and sebacic acid macromer terminated with acrylate groups.24 PEGSDA hydrogel has lower swelling ratio and is mechanically stronger than the conventional PEG-based hydrogel PEGDA, which could be beneficial to maintain structural integrity during tissue regeneration process. Previously, it was shown that PEGSDA hydrogel alone without any further modification can induce cell attachment as well as proliferation on the hydrogels using rat marrow stromal cells (MSCs).24 The primary goal of this study is to evaluate the potential of PEG-based hydrogels as scaffolds for orthopedic tissue engineering. We examined the effects of bioactive PEG-based hydrogels with or without covalently functionalized RGD peptide on osteogenic differentiation as well as mineralization of MSCs. Previous studies have shown that PEG-based hydrogels, notably PEG di(metha)acrylate or PEGD(M)A modified with biomimetic moieties such as RGD peptide, promote MSC attachment as well as osteogenic differentiation of MSCs.25,26 Interestingly, our recent study revealed that the PEG-based hydrogels incorporating sebacic acid (PECSDA) induced specific cell adhesion (e.g., MSCs) and proliferation up to 7 days regardless of RGD peptide modification in the polymer network. In the current study, we further examined if PEGSDA hydrogel itself can induce osteogenic differentiation as well as mineralization of MSCs.24
We also evaluated the PEGSDA hydrogel scaffolds as a delivery carrier of osteoinductive molecules such as BMP-2. The proof of concept is that this hydrogel is much less swellable, thus creating smaller mesh size that could prevent rapid initial burst release of encapsulated drugs, than traditional PEG-based hydrogels (e.g., PEGDA). In this study, without any further modification of the hydrogels, we directly compared the release profile of BMP-2 from the PEGSDA hydrogel with that of traditional PEG-based hydrogel (PEGDA) that has been extensively investigated as a carrier of such molecules.1,8
Materials and Methods
All chemicals were purchased from Sigma–Aldrich (St. Louis, MO) unless otherwise noted.
Preparation of PEGDA, PEGSDA, and PEG-RGD
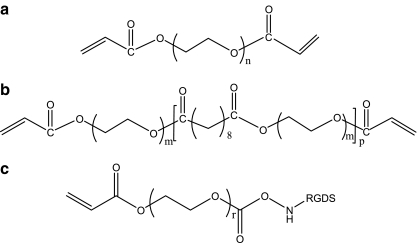
PEGDA and PEGSDA macromer were synthesized as described previously,24 and their chemical structures are shown in Figure 1. Acrylated peptide was synthesized by following previously established methods.14,27 Briefly, 1.0 mg/mL of RGDS peptide (American Peptide, Sunnyvale, CA) was dissolved in 50 mM of sodium bicarbonate buffer, pH 8.2. The acryloyl-PEG-N-hydroxysuccinimide ester (MW of PEG: 3400; Nektar Therapeutics, Huntsville, AZ) was separately dissolved in 50 mM of sodium bicarbonate buffer. The final molar ratio of acryloyl-PEG-N-hydroxysuccinimide to the peptide was two to minimize the production of acryloyl-PEG-OH.14 The PEG solution (200 μL) was added dropwise into the peptide solution (1 mg/mL) and reacted in the dark at room temperature for 2 h. The reaction mixture was dialyzed in distilled deionized water (DDW) for 2 days using a dialysis membrane (MWCO: 1000; Geno Technologies, St. Louis, MO) to remove any unreacted residues. The dialyzed polymer solution was lyophilized for at least 24 h and stored at −20°C until use.
FIG. 1.
Chemical structures of (a) poly(ethylene glycol) (PEG) diacrylate (PEGDA); (b) PEG sebacic acid diacrylate (PEGSDA), where molecular weights of PEG is 8000 and 1000 Da, respectively; and (c) acrylated PEG-RGDS.
Preparation and characterization of functionalized PEG-based hydrogels
To functionalize PEGSDA hydrogel, the acrylated peptide with MW 3400 spacer arm monomer was copolymerized with above-mentioned PEG-based macromers whose structures are shown in Figure 1. Briefly, 250 mg of PEGDA or PEGSDA prepolymer was dissolved at 25% (w/v) in DDW containing 0.05% of photoinitiator (Igracure D-2959; Ciba Geigy, Tarrytown, NY). One micromolar of acrylated peptide was added to the polymer solution. The peptide concentration was chosen based on previous studies that showed successful cell adhesion for different hydrogels.14,16,26 The solution was poured onto a glass plate and crosslinked under the UV light (λ = 310–400 nm; Blak-Ray®, Upland, CA) for 10 min. The hydrogel disc was then lifted from the plate and immersed in DDW overnight to remove any remaining unreacted oligomers. After equilibration, the fully swollen hydrogel films were cut using a cork borer (diameter 1.5 cm) to fit into the wells of tissue culture plates.
To characterize the surface chemistry of crosslinked functionalized PEGDA and PEGSDA hydrogels, Fourier transform infrared (FTIR) spectra were obtained using a Nicolet 8700 spectrophotometer (Nicolet, Madison, WI). Crosslinked PEGSDA hydrogel film was soaked in methylene chloride for 24 h at room temperature to completely remove all unreacted monomers, dried overnight under vacuum, and placed on a glass slide. FTIR spectra were obtained under a microscope by contacting the film onto zinc selenide attenuated total reflection (ATR) crystal.
Cell culture and attachment
MSCs were isolated from femurs of 5-month-old male Sprague–Dawley rats (Harlan, Indianapolis, IN) as described previously.24 The hydrogel discs were sterilized by soaking in 70% ethanol for 1 day and subsequently incubated in sterile phosphate buffered saline (PBS) solution for 2 days to remove residual ethanol. The sterilized PEGSDA hydrogels were then placed in a 24-well plate. MSCs were harvested, and the cells (passage 3) were seeded on each hydrogel at a density of 20,000 cells/cm2 and cultured in the osteogenic medium for up to 3 weeks at 37°C. The osteogenic medium contained Dulbecco's modified Eagle's medium (DMEM; Gibco, Grand Island, NY) supplemented with 10 nM dexmethasone, 50 μg/mL ascorbic acid 2-phosphate, 2 mM β-glycerophosphate, 10% fetal bovine serum, and 1% penicillin–streptomycin (Gibco). After 1, 2, and 3 weeks, the cells cultured on the hydrogels were rinsed twice with PBS; then, DNAase-free deionized water (Mediatech, Manassas, VA) was added, and the cells were stored at −20°C until use.
The cells were cultured in DMEM supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin. The morphology of adherent cells was evaluated after 24 h of incubation by phase contrast microscopy (Axiovert, Thornwood, NY) equipped with a CCD camera. For the analysis of cell density for longer time periods, DNA content, which is directly correlated to the cell number, was measured. The DNA content of each sample was measured using a fluorometric Picogreen® DNA quantification kit (Molecular Probes, Eugene, OR) by following the manufacturer's instruction. The fluorescent absorbance of the sample was measured by a fluorescent microplate reader (Molecular Devices, Sunnyvale, CA) equipped with a 480/525 nm (excitation/emission) filter set.
ALP activity and total protein
ALP activity was determined using a previously established protocol in our laboratory.28 Briefly, 20 μL of alkaline working buffer solution, 80 μL of the lysate solution, and 100 μL of substrate solution (5 mM p-nitrophenyl phosphate) were added to a 96-well plate. The plate was incubated at 37°C for 1 h, and the reaction was stopped by adding 100 μL of 0.3 N NaOH solution to each well. The absorbance of each well was measured at 405 nm and the value of ALP activity was normalized to the amount of total protein in the sample. Total protein in the cell lysate solution was quantified by the Bradford protein assay. Briefly, 200 μL of the assay reagent was added to 10 μL of the sample, and the absorbance of a differential color change was measured at 595 nm.
Mineralization assay
The calcium content of each cell layer was determined by following previously published method29 with a calcium assay kit (Bioassay, Hayward, CA). Each scaffold with or without cells was incubated for 30 min in 5% trichloroacetic acid (TCA), the extracts were collected, and UV absorbance of the extract was measured at 612 nm by microplate reader (Molecular Devices). Actual calcium content secreted by cells was then calculated by subtracting calcium content of each scaffold without cells from total calcium content from the cell layer on each scaffold.
BMP-2 radiolabeling and incorporation into the hydrogels
Human recombinant BMP-2 was radioiodinated according to the protocol provided by Radiation Safety at Mayo Clinic. Briefly, 1.0 mCi of Na125I was added to 50 μL of BMP-2 solution in the BMP-2 buffer (5 mM glutamic acid, 2.5 wt% glycine, 0.5 wt% sucrose, and 0.01 wt% Tween 80, pH 6.5). After gentle agitation at room temperature for 15 min, the reaction mixture was transferred to the dialysis tube and dialyzed for 24 h to remove the uncoupled, free 125I molecules from the radioiodinated BMP-2. The labeling efficiency was determined by TCA precipitation method and showed that more than 97% of BMP-2 was radioiodinated. BMP-2 solution containing radiolabeled BMP-2 (10 wt% of total BMP-2) was incorporated into the polymer solution, and the solution was photopolymerized in the presence of 0.05% initiator (Igracure D-2959) under the UV light for 10 min. To determine BMP-2 incorporation efficiency into the final scaffolds, the total incorporated radioactivity was compared to the initial activity of 125I-labeled BMP-2 before incorporation into the scaffolds by measuring the radioactivity on a gamma counter (PerkinElmer, Waltham, MA).
BMP-2 release profiles and cell culture in the presence of released BMP-2
To determine the bioactivity of BMP-2 released from PEG-based hydrogels, mouse bone MSC line W-20 clone 17 cells (W-20-17; Wyeth, Madison, NJ) were used. These cells are known to respond well to BMP-2 by sensitive changes in alkaline phosphatase production,28,30 one of the early bone markers, whereas MSCs in the presence of osteogenic molecules may not produce significant amount of ALP when compared with the control (tissue culture plates).8 The cells were plated at a density of 20,000 cells/cm2 on 24-well tissue culture plates (Corning, Cambridge, MA). After 24 h of incubation at 37°C, the media in each well were replaced with fresh media. Transwells (6.5 mm diameter, 3.0 μm pore size; Corning) containing the scaffolds incorporating BMP-2 (loaded at 20 μg/g scaffold) were transferred into the top of each tissue culture well, which had cells seeded on the bottom, while the scaffolds without BMP-2 were placed in the wells as controls. At day 1, the media were collected and radioactivity measured on a gamma counter to determine the BMP-2 release kinetics. At day 3, the media were collected again, the radioactivity was measured, all of the cells were rinsed with PBS, DNA-free deionized water was added, and the cells were frozen at −80°C until assaying. The transwells containing scaffolds were immediately transferred to new tissue culture wells that had been plated with the cells for 24 h and incubated for another 3 days. Above procedures were repeated up to 21 days. To determine the dose response to BMP-2, the cells were exposed to bolus of known concentrations of BMP-2 for 3 days and then assayed.
Statistical analyses
All data are represented as the mean and standard deviation. Statistical analysis was performed using either a Student's t-test for a direct comparison between a group and a control or single factor analysis of variance (ANOVA) with Tukey–Kramer method for a multiple comparison at a significance level of 0.05.
Results and Discussion
Characterization of the hydrogels
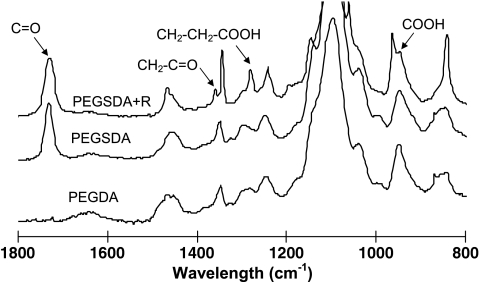
PEGDA and PEGSDA macromers were successfully synthesized, and the molecular weights of resulting PEGDA and PEGSDA polymers were similar (∼11,000 Da), which allows direct comparison of their properties.24 To analyze functional groups of the macromers, FTIR spectroscopy equipped with a zinc selenide ATR crystal was used. There was no other macroscopic difference in the FTIR spectra of PEGDA and PEGSDA hydrogels except strong characteristic band at ∼1730 cm−1 in the spectrum of PEGSDA due to the formation of ester bonds (Fig. 2). However, when additional functionality such as an RGD containing peptide was incorporated in the hydrogel network, the characteristic bands corresponding to the specific functional groups were observed in the FTIR spectra (Fig. 2). The absorbance peak at ∼1340 cm−1 was attributed to glycine (CH2C = O) and the peaks at ∼1280 and ∼960 cm−1 to aspartic acid (CH2CH2COOH and COOH) of RGDS peptide covalently bonded to the PEGSDA hydrogel surface,31,32 indicating that RGDS peptide was successfully incorporated into PEGSDA hydrogel.
FIG. 2.
Attenuated total reflection–Fourier transform infrared (ATR–FTIR) spectra of crosslinked PEGSDA hydrogel. Absorbance peaks at 1343, 1279, and 964 cm−1 were clearly seen on PEGSDA hydrogel incorporating RGDS peptide (PEGSDA+R) due to the presence of glycine and aspartic acid of RGDS peptide.
Attachment of mesenchymal stem cells (MSCs)
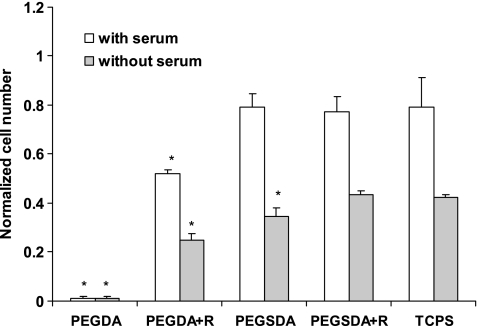
MSC attachment on each scaffold after 24 h of culture, in the presence or absence of fetal bovine serum (FBS), was measured and normalized to an initial cell seeding density (Fig. 3). In general, the cell attachment on each scaffold in the presence of FBS was nearly twofold greater than that in the absence of FBS. Cell densities after 24 h of culture in the presence of FBS on PEGSDA and RGDS peptide modified PEGSDA hydrogels were similar to the control tissue culture polystyrene (TCPS), whereas those on PEGDA hydrogel were significantly lower (p < 0.05). Thus, unlike PEGDA hydrogel, hydrophobic segment of PEGSDA hydrogel (sebacic acid) could attract cell adhesion proteins from the serum, such as fibronectin or vitronectin, likely due to the hydrophobic interaction between the substrate and the proteins.33 In a serum-free condition, similar significant differences were observed except for PEGSDA hydrogel without RGD modification, on which cell density was significantly lower than the control. Therefore, these results demonstrated that RGD peptide itself mediates cell adhesion without serum proteins resulting in similar cell attachment to the control (TCPS) and also indicate that PEGSDA hydrogel alone may need cell adhesion proteins to improve cell attachment in the absence of the serum. However, that requirement may not be necessary in the body where serum proteins are generally available, especially in blood-contacting conditions. The cell density on PEGDA hydrogel was negligible compared to all other substrates as previously shown by others.7
FIG. 3.
Initial mesenchymal stem cell (MSC) attachment on different PEG-based hydrogels and TCPS after 24 h of culture. Cell number at each time point was normalized to the number of initially seeded cells. Asterisk (*) represents significant difference (p < 0.05) compared to the control (TCPS). Error bars represent means ± standard deviation for n = 3.
MSC proliferation and differentiation
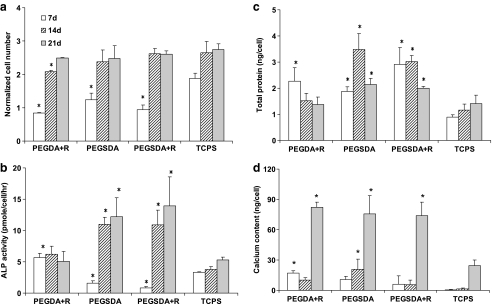
Cell densities on different hydrogel scaffolds were also measured to evaluate cell proliferations over longer period of time up to 3 weeks of culture in the presence of FBS at 37°C (Fig. 4a). In general, cell densities reached a plateau after 2 weeks and remained at similar level during later time periods. After 1 week of culture, cell densities on all tested hydrogels were significantly lower than the control. However, after 2 and 3 weeks of culture, cell densities on all substrates regardless of RGDS peptide modification were similar to the control and reached maximum, which was two to three times higher than initial cell seeding density. Unlike PEGDA hydrogel, there was no additional effect of the incorporation of RGD peptide into the PEGSDA hydrogel on the cell proliferation of MSCs throughout the tested time course. Overall, these results confirmed that PEGSDA hydrogel without any further modification could induce cell attachment as well as proliferation for longer period time in the presence of serum without substantial cell detachment.24
FIG. 4.
(a) Cell proliferation of MSCs cultured on PEG-based hydrogels after 7, 14, and 21 days of culture. Cell number at each time point was normalized to the number of initially seeded cells. (b) Alkaline phosphatase (ALP) activity, (c) total protein production, and (d) mineralized calcium deposition of MSCs cultured on the hydrogels. Asterisk (*) represents significant difference (p < 0.05) compared to the control (TCPS) at the corresponding time point. Error bars represent means ± standard deviation for n = 4.
ALP production in MSCs, which is known as an early marker of osteoblast phenotype,34 was measured for cells grown on each scaffold type and normalized to the cell number (Fig. 4b). It monotonically increased up to 2 weeks of cultivation and remained similar level thereafter. At week 1, ALP activities in MSCs grown on all hydrogels were significantly lower (p < 0.05) than the control (TCPS). However, after 2 and 3 weeks of culture, MSCs grown on PEGSDA hydrogels with or without RGD modification exhibited significantly higher (p < 0.05) ALP activity than the control, whereas the cells grown on PEGDA hydrogels produced minimal amount of ALP due to very limited cell adhesion on the scaffold as shown in Figure 3, which is consistent with the results previously reported by others.7 The cells grown on RGD peptide–modified PEGDA hydrogel did produce significantly greater amount of ALP than on unmodified PEGDA hydrogel, indicating that RGD peptide modification on the PEGDA hydrogel induced cell attachment and proliferation and subsequently initiated the osteogenic differentiation of MSCs on the hydrogel. However, there was no additional effect of RGD peptide incorporation into the PEGSDA hydrogel on ALP activity, which is consistent with the cell proliferation data (Fig. 4a).
Total protein level produced by the cells cultured for 3 weeks on the various substrates was measured to evaluate total protein production, including extracellular matrix (ECM) proteins by the MSCs (Fig. 4c). Throughout the culture period, total protein productions on all tested hydrogels were significantly higher (p < 0.05) than that on the control surface after 2 and 3 weeks of culture, except for the PEGDA+R hydrogel. For PEGSDA-based hydrogel with or without peptide modification, the significant increase of total protein production by MSCs indicates that the hydrophobic segment of PEGSDA backbone may initiate some protein production such as fibronectin, which could signal subsequent mineralization of osteoblast, likely due to strong hydrophobic interaction with osteoblasts. This trend was similarly found in osteogenic phenotypic expressions, especially ALP activity as shown in Figure 4b, indicating that ECM protein production may explain why the cells well attach, proliferate, and differentiate on PEGSDA hydrogel despite the absence of RGD peptide incorporation. Because ECM proteins, especially fibronectin and vitronectin, were well known to mediate osteoblastic–ECM interactions with biomaterials via integrin receptors such as α5β1 or αvβ3,35,36 it is possible to assume that the hydrophobic segment of PEGSDA hydrogel may initially attract cell adhesion proteins such as fibronectin from the surrounding (e.g., serum) and those proteins attach to the surface and extensively induce the cell adhesion and proliferation of MSCs. Therefore, the cells on PEGSDA hydrogel subsequently secreted significantly higher amounts of total proteins, including osteogenic proteins, which initiate differentiation of MSCs into osteoblasts, than the cells grown on TCPS.
Mineralization
To evaluate mineralization on PEG-based hydrogels, mineralized calcium content deposited on the hydrogels was determined (Fig. 4d). Because β-glycerophosphate (β-GP) is known to induce the mineralization of the ECM,37,38 minimum amount of β-GP (2 mM) was used to maximize the effect of PEG-based hydrogels on the mineraliztion.29 Up to 2 weeks of culture, mineralized calcium depositions on all PEG-based hydrogels were similar to the control. However, after 3 weeks of culture, the calcium depositions on all PEG-based hydrogels, except for PEGDA on which calcium depositions were not detectable throughout the culture periods, were significantly higher (p < 0.05) than the control. These results were also well correlated with the results for total protein production, except for the case of PEGDA+R hydrogel after 3 weeks of culture, further indicating that PEGSDA hydrogel itself can stimulate the cells to produce significant amount of cell adhesion proteins that modulate the differentiation as well as mineralization of MSCs. Although the reason is unknown, the calcium deposition on PEGGA+R hydrogel was significantly higher than the control, especially after 3 weeks of culture, in contrast to the phenotypic expression results, which were shown to be similar to the control.
PEG-based hydrogel has been widely regarded as a cell-repellent material, mainly due to its notable hydrophilicity. However, a previous study from the present team demonstrated that it can be bioactive to specific cell types if its backbone is incorporated with certain types of hydrophobic segment that render it bioresponsive to cell adhesion proteins and cell attachment.24 The current study further demonstrated that PEG-based hydrogels with sebacic acid incorporation can significantly induce specific differentiation of MSCs without any further modification. There was no additional effect of RGD peptide incorporation into the PEGSDA hydrogel on osteogenic differentiation of MSCs, although the concentration and orientation of cell adhesion peptides incorporated in the hydrogel may result in a different outcome of osteoblastic activities on the hydrogel as suggested elsewhere with other PEG-based hydrogels.25 Overall, it became evident that the PEGSDA hydrogel itself without functionalization can induce specific cell adhesion through the hydrophobic segment resulting in proliferation, differentiation, and mineralization as much as the PEGDA hydrogel functionalized with RGDS peptide or performed at least similarly to the control (TCPS). Further, this PEGSDA hydrogel has been proven to be biodegradable, which is an additional important advantage compared with traditional PEG-based hydrogels. These unique features of the PEGSDA hydrogel could be beneficial when used as orthopedic tissue engineering scaffold, especially in non-load-bearing applications, in which the need for osteoconductive materials has been tremendous to treat a variety of orthopedic defects.3
BMP-2 loading efficiency and release profiles
To examine the feasibility of PEGSDA hydrogel scaffold to also serve as a delivery vehicle for osteogenic molecules, the hydrogels were loaded with BMP-2. BMP-2 loading efficiency was measured by comparing the difference between the actual amounts of entrapped protein to its initial amount. BMP-2 was successfully encapsulated in the scaffolds with high efficiencies (85.6 ± 8.2% and 81.2 ± 9.6% for PEGSDA and PEGDA scaffolds, respectively; n = 4), and there was no significant difference in loading efficiency between the scaffolds.
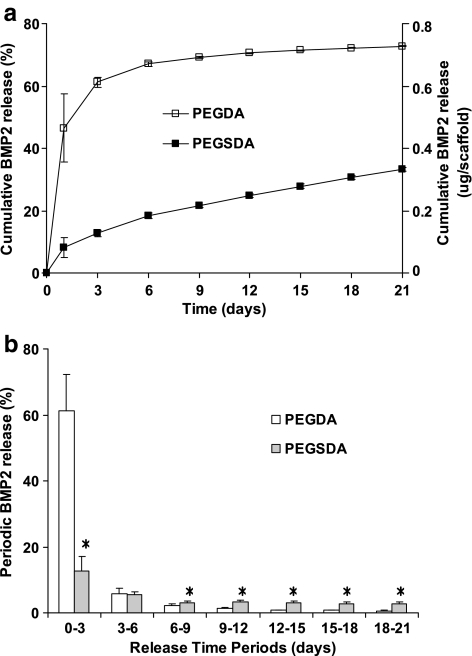
BMP-2 release profiles from PEG-based hydrogel scaffolds were evaluated by immersing the scaffolds containing the biomolecule in PBS at 37°C and measuring BMP-2 concentration over time (Fig. 5a). The results showed that the BMP-2 release profile from PEGDA scaffold had significantly greater initial burst release followed by limited further release of the protein over the rest of the time periods, whereas the profile from PEGSDA scaffold showed little initial burst release followed by continuous sustained release of BMP-2. For comparison, the percent released amounts of BMP-2 from PEGDA and PEGSDA hydrogel scaffolds for the first 3 days were 84.2% and 14.7% of total released amount, respectively (Fig. 5b). In the current study, the controlled release of BMP-2 was observed up to 21 days, indicating that the drug release is mainly dependent on the diffusion from the crosslinked network instead of the degradation of the polymer, especially for PEGDA hydrogel because it is considered as nonbiodegradable. However, for PEGSDA hydrogel, BMP-2 release depends not only on the diffusion, but also partially on the degradation, especially at later time periods (PEGSDA hydrogel degrade about 20% after 3 weeks at 37°C24). The reduction of initial burst of the drug from PEGSDA hydrogel scaffold was likely due to the decrease of mesh size of the polymer network resulting from lower swelling ratio of this hydrogel compared to PEGDA hydrogel. Since the PEGSDA hydrogel scaffold contains a hydrophobic segment (sebacic acid), it may also affect the release profile of BMP-2 from the scaffold possibly due to the hydrophobic interaction between the incorporated molecule and the scaffold, leading to molecule aggregation, thus decreasing the released amount of the molecule.21
FIG. 5.
(a) Percent and actual amount of cumulative release profiles of BMP-2 from PEG-based hydrogel scaffolds. (b) Periodic release of incorporated BMP-2 from different scaffolds. Asterisk (*) represents significant difference (p < 0.05) compared to PEGDA hydrogel. Error bars represent means ± standard deviation for n = 4.
Bioactivity of released BMP-2
The purpose of the BMP-2 release studies was to assess whether the released BMP-2 from different carriers at 3-day intervals for up to 21 days could induce osteogenic differentiation. For this purpose, we used W-20 cells due to its sensitive bio-response to BMP-2. Primary mesenchymal cells, including human MSCs, on the other hand, do not quickly produce significant amount of osteogenic proteins such as ALP in vitro.30
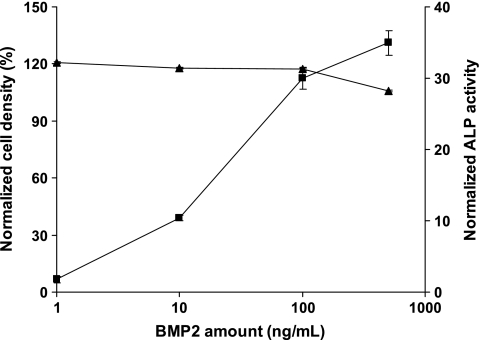
The dose responses for the cellular densities and ALP activities in the control (TCPS) were first evaluated after 3 days of cell culture (Fig. 6). For cellular density, there was no significant difference between the samples treated with different BMP-2 concentrations. However, ALP activity was increased with increasing BMP-2 concentration, which is consistent with the previous study.30
FIG. 6.
Cell density (▲) and ALP activity (■) of W-20-17 cells grown on TCPS for 3 days in response to different doses of BMP-2. Cell density on TCPS in the presence of BMP-2 was quantified by total DNA analysis and normalized to that on TCPS in the absence of BMP-2. ALP activity was also normalized in the same way. Error bars represent means ±standard deviation for n = 4.
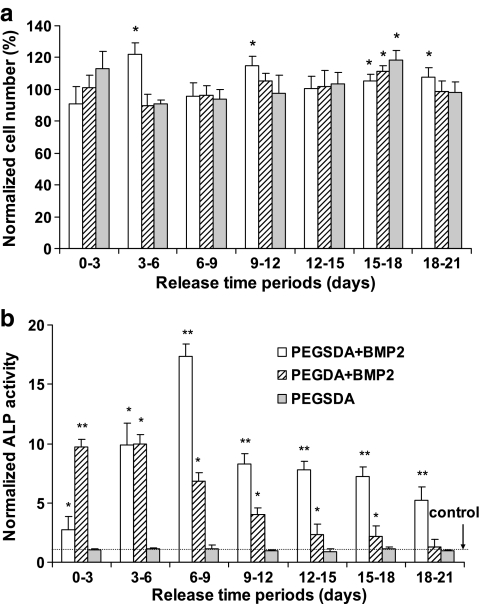
To compare the effect of released BMP-2 from different hydrogel scaffolds on the proliferation of W-20-17 cells, cell densities in different scaffolds were evaluated throughout 21 days and normalized to that on the control TCPS (Fig. 7a). The percent cell densities from tested scaffolds were between 90% and 120% of the control TCPS. The cells grown on TCPS in the presence of released BMP-2 from each scaffold after 3 days of culture appeared to be fully confluent throughout all time periods. There were no consistent trends or differences between the samples throughout that period. These results indicate that cell proliferation was not significantly affected by controlled release of BMP-2 over the time period mainly because BMP-2 does not induce the proliferation of W-20-17 cells significantly compared to the control (TCPS), confirming the previous findings.39 In addition, we also compared the cellular density from the PEGSDA scaffold in the absence of BMP-2, with those on TCPS to evaluate if there are any detrimental effects on the bioactivity of the cells in the presence of the PEGSDA hydrogel. As expected, there was no significant difference between the sample and the control, confirming that tested hydrogel was not cytotoxic and had no adverse effect on the proliferation of the cells in the presence of the scaffold.
FIG. 7.
(a) Periodic cell proliferation of W-20-17 cells on TCPS in the presence or absence of released BMP-2 from PEG SDA and PEGDA hydrogel scaffolds and (b) periodic ALP activity of W-20-17 cells in the presence of released BMP-2 from PEG-based hydrogel scaffolds during each time intervals. ALP activity from each treatment was normalized to the activity from the control (TCPS without BMP-2). Asterisk (*) and Double asterisks (**) represent significant difference (p < 0.05) compared to TCPS and all other scaffolds, respectively. Error bars represent means ± standard deviation for n = 4.
ALP activities of the cells treated with released BMP-2 from PEG-based hydrogel scaffolds were also determined over different time intervals (Fig. 7b). Peak ALP activities were observed at early time points for both scaffolds likely due to early burst release of BMP-2 from the scaffolds as seen in the release profiles (Fig. 5). ALP activities of the cells grown in the presence of released BMP-2 from PEGSDA hydrogel scaffold were significantly higher than from PEGDA scaffold, especially at later time intervals (after 6 days of release), and this trend was well correspondent to the BMP-2 release profiles after 6 days (Fig. 5b). Additionally, in the final 3 days BMP-2 release (the period from 18 to 21 days) from PEGDA was not significantly different from the control (TCPS), indicating that small amount of released BMP-2 (<0.6% out of total encapsulated BMP-2) from PEGDA hydrogel did not induce significant ALP activity, compared to the control. The PEGSDA hydrogel was expected to degrade more quickly after 21 days, thus continuing to release BMP-2 over a longer time period. In the absence of released BMP-2 from PEGSDA scaffold, ALP activities of the cells were similar to the control, indicating that PEGSDA scaffold itself did not compromise ALP activity during the tested period as seen in the result of cell proliferation.
Finally, one of the major concerns for use of synthetic polymers as tissue engineering scaffolds is biocompatibility.40 Ideally, synthetic polymeric delivery systems should not be carcinogenic or immunogenic due to cytotoxicity or unexpected inflammatory reaction when implanted into the host.41 Because PEGSDA polymer appeared to be biocompatible and its degradation products such as PEG, sebacic acid, and oligo(acrylic acid) have proven to be safe in other drug delivery systems,24,42 these PEG-based hydrogel may be used as tissue engineering scaffolds and/or delivery carriers of therapeutic molecules without any incorporation of bioactive moieties that are expensive and often involve complex process steps.
Conclusions
The potential of a biodegradable PEG-based hydrogel (PEGSDA) for use as substrate and delivery vehicles for orthopedic tissue engineering was evaluated. Osteogenic bioactivities of MSCs such as proliferation, ALP activity, and mineralized calcium content on PEGSDA-based hydrogel scaffolds were examined and compared to conventional hydrogel (PEGDA). Cellular densities, ALP activity on PEGSDA with or without RGD modification, and RGD modified PEGDA were similar or significantly higher than the control TCPS, whereas those on unmodified PEGDA hydrogel were minimal. Similar results were observed for mineralized calcium content on those hydrogels. There was no significant additional effect of RGD modification on osteogenic differentiation to PEGSDA hydrogel. BMP-2 release profiles and bioactivities of BMP-2 released from PEGSDA hydrogels were also examined. The BMP-2 release profile from PEGSDA scaffolds showed little burst release followed by sustained release over 21 days, whereas that from the control PEGDA hydrogel showed rapid initial burst release followed by little further release. ALP activity of osteoblast cell line cells from 6 to 21 days in the presence of released BMP-2 from PEGSDA scaffold was significantly greater than that from PEGDA scaffold. Overall, PEGSDA hydrogel alone can induce cell proliferation as well as osteogenic differentiation without modification. PEGSDA hydrogel scaffold may also serve as a delivery vehicle for BMP-2.
Acknowledgments
This study was funded by the Mayo Foundation and the National Institutes of Health (R01AR45871 and R01EB003060).
Disclosure Statement
No competing financial interests exist.
References
- 1.Winn S.R. Uludag H. Hollinger J.O. Sustained release emphasizing recombinant human bone morphogenetic protein-2. Adv Drug Deliver Rev. 1998;31:303. doi: 10.1016/s0169-409x(97)00126-9. [DOI] [PubMed] [Google Scholar]
- 2.Lutolf M.R. Weber F.E. Schmoekel H.G. Schense J.C. Kohler T. Muller R. Hubbell J.A. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat Biotechnol. 2003;21:513. doi: 10.1038/nbt818. [DOI] [PubMed] [Google Scholar]
- 3.Temenoff J.S. Mikos A.G. Injectable biodegradable materials for orthopedic tissue engineering. Biomaterials. 2000;21:2405. doi: 10.1016/s0142-9612(00)00108-3. [DOI] [PubMed] [Google Scholar]
- 4.Jabbari E. Wang S.F. Lu L.C. Gruetzmacher J.A. Ameenuddin S. Hefferan T.E. Currier B.L. Windebank A.J. Yaszemski M.J. Synthesis, material properties, and biocompatibility of a novel self-cross-linkable poly(caprolactone fumarate) as an injectable tissue engineering scaffold. Biomacromolecules. 2005;6:2503. doi: 10.1021/bm050206y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang S.F. Lu L.C. Gruetzmacher J.A. Currier B.L. Yaszemski M.J. A biodegradable and cross-linkable multiblock copolymer consisting of poly(propylene fumarate) and poly(epsilon-caprolactone): synthesis, characterization, and physical properties. Macromolecules. 2005;38:7358. [Google Scholar]
- 6.Yang F. Williams C.G. Wang D.A. Lee H. Manson P.N. Elisseeff J. The effect of incorporating RGD adhesive peptide in polyethylene glycol diacrylate hydrogel on osteogenesis of bone marrow stromal cells. Biomaterials. 2005;26:5991. doi: 10.1016/j.biomaterials.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 7.Burdick J.A. Anseth K.S. Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials. 2002;23:4315. doi: 10.1016/s0142-9612(02)00176-x. [DOI] [PubMed] [Google Scholar]
- 8.Burdick J.A. Mason M.N. Hinman A.D. Thorne K. Anseth K.S. Delivery of osteoinductive growth factors from degradable PEG hydrogels influences osteoblast differentiation and mineralization. J Control Release. 2002;83:53. doi: 10.1016/s0168-3659(02)00181-5. [DOI] [PubMed] [Google Scholar]
- 9.Saito N. Okada T. Horiuchi H. Murakami N. Takahashi J. Nawata M. Ota H. Nozaki K. Takaoka K. A biodegradable polymer as a cytokine delivery system for inducing bone formation. Nat Biotechnol. 2001;19:332. doi: 10.1038/86715. [DOI] [PubMed] [Google Scholar]
- 10.Jeon S.I. Andrade J.D. DeGennes P. Protein surface interactions in the presence of polyethylene oxide. 1. Simplified theory. J Colloid Interface Sci. 1991;142:149. [Google Scholar]
- 11.Harris J.M. New York: Plenum Press; 1992. Poly(ethylene glycol) Chemistry: Biotechnical and Biomedical Applications. [Google Scholar]
- 12.Hubbell J.A. Bioactive biomaterials. Curr Opin Biotechnol. 1999;10:123. doi: 10.1016/s0958-1669(99)80021-4. [DOI] [PubMed] [Google Scholar]
- 13.Shin H. Jo S. Mikos A.G. Biomimetic materials for tissue engineering. Biomaterials. 2003;24:4353. doi: 10.1016/s0142-9612(03)00339-9. [DOI] [PubMed] [Google Scholar]
- 14.Hern D.L. Hubbell J.A. Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue resurfacing. J Biomed Mater Res. 1998;39:266. doi: 10.1002/(sici)1097-4636(199802)39:2<266::aid-jbm14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 15.Massia S.P. Hubbell J.A. An RGD spacing of 440 nm is sufficient for integrin alpha V beta 3-mediated fibroblast spreading and 140 nm for focal contact and stress fiber formation. J Cell Biol. 1991;114:1089. doi: 10.1083/jcb.114.5.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneider G.B. English A. Abraham M. Zaharias R. Stanford C. Keller J. The effect of hydrogel charge density on cell attachment. Biomaterials. 2004;25:3023. doi: 10.1016/j.biomaterials.2003.09.084. [DOI] [PubMed] [Google Scholar]
- 17.Benoit D.S.W. Durney A.R. Anseth K.S. The effect of heparin-functionalized PEG hydrogels on three-dimensional human mesenchymal stem cell osteogenic differentiation. Biomaterials. 2007;28:66. doi: 10.1016/j.biomaterials.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 18.Lutolf M.P. Hubbell J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 19.Holland T.A. Tabata Y. Mikos A.G. In vitro release of transforming growth factor-beta 1 from gelatin microparticles encapsulated in biodegradable, injectable oligo(poly(ethylene glycol) fumarate) hydrogels. J Control Release. 2003;91:299. doi: 10.1016/s0168-3659(03)00258-x. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman A.S. Hydrogels for biomedical applications. Adv Drug Deliv Rev. 2002;54:3. doi: 10.1016/s0169-409x(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 21.Yeo Y. Park K. Control of encapsulation efficiency and initial burst in polymeric microparticle systems. Arch Pharm Res. 2004;27:1. doi: 10.1007/BF02980037. [DOI] [PubMed] [Google Scholar]
- 22.Jeon O. Kang S.W. Lim H.W. Chung J.H. Kim B.S. Long-term and zero-order release of basic fibroblast growth factor from heparin-conjugated poly(l-lactide-co-glycolide) nanospheres and fibrin gel. Biomaterials. 2006;27:1598. doi: 10.1016/j.biomaterials.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 23.Wuisman P.I.J.M. Smit T.H. Bioresorbable polymers: heading for a new generation of spinal cages. Eur Spine J. 2006;15:133. doi: 10.1007/s00586-005-1003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim J. Lee K.W. Hefferan T.E. Currier B.L. Yaszemski M.J. Lu L. Synthesis and evaluation of novel biodegradable hydrogels based on poly(ethylene glycol) and sebacic acid as tissue engineering scaffolds. Biomacromolecules. 2008;9:149. doi: 10.1021/bm700924n. [DOI] [PubMed] [Google Scholar]
- 25.Benoit D.S.W. Anseth K.S. The effect on osteoblast function of colocalized RGD and PHSRN epitopes on PEG surfaces. Biomaterials. 2005;26:5209. doi: 10.1016/j.biomaterials.2005.01.045. [DOI] [PubMed] [Google Scholar]
- 26.Shin H. Temenoff J.S. Bowden G.C. Zygourakis K. Farach-Carson M.C. Yaszemski M.J. Mikos A.G. Osteogenic differentiation of rat bone marrow stromal cells cultured on Arg-Gly-Asp modified hydrogels without dexamethasone and beta-glycerol phosphate. Biomaterials. 2005;26:3645. doi: 10.1016/j.biomaterials.2004.09.050. [DOI] [PubMed] [Google Scholar]
- 27.Shin H. Jo S. Mikos A.G. Modulation of marrow stromal osteoblast adhesion on biomimetic oligo[poly(ethylene glycol) fumarate] hydrogels modified with Arg-Gly-Asp peptides and a poly(ethylene glycol) spacer. J Biomed Mater Res. 2002;61:169. doi: 10.1002/jbm.10193. [DOI] [PubMed] [Google Scholar]
- 28.Kempen D.H.R. Lu L. Hefferan T.E. Creemers L.B. Maran A. Classic K.L. Dhert W.J.A. Retention of in vitro and in vivo BMP-2 bioactivities in sustained delivery vehicles for bone tissue engineering. Biomaterials. 2008;29:3245. doi: 10.1016/j.biomaterials.2008.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmad M. McCarthy M. Gronowicz G. An in vitro model for mineralization of human osteoblast-like cells on implant materials. Biomaterials. 1999;20:211. doi: 10.1016/s0142-9612(98)00152-5. [DOI] [PubMed] [Google Scholar]
- 30.Thies R.S. Bauduy M. Ashton B.A. Kurtzberg L. Wozney J.M. Rosen V. Recombinant human bone morphogenetic protein-2 induces osteoblastic differentiation in W-20-17 stromal cells. Endocrinology. 1992;130:1318. doi: 10.1210/endo.130.3.1311236. [DOI] [PubMed] [Google Scholar]
- 31.Lin Y.S. Wang S.S. Chung T.W. Wang Y.H. Chiou S.H. Hsu J.J. Chou N.K. Hsieh T.H. Chu S.H. Growth of endothelial cells on different concentrations of Gly-Arg-Gly-Asp photochemically grafted in polyethylene glycol modified polyurethane. Artif Organs. 2001;25:617. doi: 10.1046/j.1525-1594.2001.025008617.x. [DOI] [PubMed] [Google Scholar]
- 32.Cavalcanti-Adam E.A. Shapiro I.M. Composto R.J. Macarak E.J. Adams C.S. RGD peptides immobilized on a mechanically deformable surface promote osteoblast differentiation. J Bone Miner Res. 2002;17:2130. doi: 10.1359/jbmr.2002.17.12.2130. [DOI] [PubMed] [Google Scholar]
- 33.Horbett T.A. Proteins at interfaces—an overview. ACS Symp Ser. 1995;602:1. [Google Scholar]
- 34.Stein G.S. Lian J.B. Owen T.A. Relationship of cell growth to the regulation of tissue-specific gene expression during osteoblast differentiation. FASEB J. 1990;4:3111. doi: 10.1096/fasebj.4.13.2210157. [DOI] [PubMed] [Google Scholar]
- 35.Wilson C.J. Clegg R.E. Leavesley D.I. Pearcy M.J. Mediation of biomaterial–cell interactions by adsorbed proteins: a review. Tissue Eng. 2005;11:1. doi: 10.1089/ten.2005.11.1. [DOI] [PubMed] [Google Scholar]
- 36.Garcia A.J. Vega M.D. Boettiger D. Modulation of cell proliferation and differentiation through substrate-dependent changes in fibronectin conformation. Mol Biol Cell. 1999;10:785. doi: 10.1091/mbc.10.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bellows C.G. Aubin J.E. Heersche J.N. Antosz M.E. Mineralized bone nodules formed in vitro from enzymatically released rat calvaria cell populations. Calcif Tissue Int. 1986;38:143. doi: 10.1007/BF02556874. [DOI] [PubMed] [Google Scholar]
- 38.Sakamoto S. Sakamoto M. Goldberg L. Colarusso L. Gotoh Y. Mineralization induced by beta-glycerophosphate in cultures leads to a marked increase in collagenase synthesis by mouse osteogenic MC3T3-E1 cells under subsequent stimulation with heparin. Biochem Biophys Res Commun. 1989;162:773. doi: 10.1016/0006-291x(89)92377-2. [DOI] [PubMed] [Google Scholar]
- 39.Thies R.S. Bauduy M. Ashton B.A. Kurtzberg L. Wozney J.M. Rosen V. Recombinant human bone morphogenetic protein-2 induces osteoblastic differentiation in W-20-17 stromal cells. Endocrinology. 1992;130:1318. doi: 10.1210/endo.130.3.1311236. [DOI] [PubMed] [Google Scholar]
- 40.Ratner B.D. Bryant S.J. Biomaterials: where we have been and where we are going. Annu Rev Biomed Eng. 2004;6:41. doi: 10.1146/annurev.bioeng.6.040803.140027. [DOI] [PubMed] [Google Scholar]
- 41.Langer R. Tirrell D.A. Designing materials for biology and medicine. Nature. 2004;428:487. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 42.Leong K.W. Brott B.C. Langer R. Bioerodible polyanhydrides as drug-carrier matrices. 1. Characterization, degradation, and release characteristics. J Biomed Mater Res. 1985;19:941. doi: 10.1002/jbm.820190806. [DOI] [PubMed] [Google Scholar]