Abstract
Objective
A fundamental challenge of cartilage tissue engineering has been the inability to promote collagen synthesis up to native levels. In contrast, recent protocols have demonstrated that glycosaminoglycans (GAG) can be synthesized to native levels in 4–6 weeks of in vitro culture. We hypothesize that rapid GAG synthesis may be an impediment to collagen synthesis, possibly by altering transport pathways of nutrients or synthesis products. In this study, this hypothesis is tested by inducing enzymatic GAG loss in the early culture period of cartilage tissue constructs, and monitoring collagen content at various time points after cessation of enzymatic treatment.
Methods
In Study 1, to induce breakdown of proteoglycans, chondroitinase ABC (CABC, 0.002 U/mL) was continuously added into the culture media for the initial 4 weeks of culture or for 2 weeks starting on day 14 of culture. In Study 2, multiple transient CABC treatments (0.15 U/mL, for 2 days) were applied to the matured tissue-engineered constructs.
Results
Continuous and transient CABC treatments significantly increased the collagen concentration of the constructs, improving their tensile properties. The GAG content of the treated constructs recovered quickly to the pretreatment level after 2–3 weeks.
Conclusions
This study demonstrates that tissue-engineered cartilage constructs with improved tensile properties can be achieved by temporarily suppressing the GAG content enzymatically.
Introduction
Articular cartilage is the soft tissue lining the opposing surfaces of the articular joints, functioning as a highly wear-resistant and low-friction weight-bearing cushion.1,2 It has been long known that damaged articular cartilage has a limited capacity for self-repair. This clinical issue has motivated extensive research efforts aimed at developing functional tissue-engineered cartilage for implantation. A fundamental challenge of cartilage tissue engineering has been the inability to promote collagen synthesis up to native levels. In contrast, recent protocols have demonstrated that glycosaminoglycans can be synthesized to native levels in 4–6 weeks of in vitro culture.3,4
A number of studies have used chondroitinase ABC (CABC) to deplete cartilage of glycosaminoglycan (GAG) and to investigate the immediate effects on both mechanical properties and biologic responses to such perturbation of the tissue.5–7 CABC specifically depolymerizes chondroitin and dermatan sulfate,8 while collagen, the collagen network arrangement, keratan sulfate, and link protein remain unaffected by the enzyme.9,10 It has been shown that the compressive Young's modulus obtained from an indentation test decreases significantly after treatment with collagenase and CABC.9 Proteoglycan removal with CABC and other proteolytic enzymes increases the tissue's permeability11 and decreases the compressive stiffness12 and the shear modulus.13
Agarose has been used extensively in cartilage biology for maintaining long-term chondrocyte suspension cultures; its ability to promote and maintain the chondrocyte phenotype is well documented.14–18 Agarose is being used as a component of a next-generation autologous chondrocyte implantation therapy (Cartipatch) for repair of cartilage defects in humans.19,20 Our laboratory has grown chondrocyte-seeded agarose hydrogel constructs with some of the most native-like reported mechanical properties in the field.4,21 However, the nondegradable nature of agarose results in less optimal, inhomogeneous matrix deposition due to diminished nutrient diffusion into the constructs.22 Agarase hydrolyzes the linkage between d-galactose and 3,6-anhydro-l-galactose residues in agarose.23,24 In a previous study, digestion of the agarose scaffold with agarase was found to eventually improve the collagen content and dynamic compressive modulus of engineered cartilage constructs, despite a temporary degradation of mechanical properties and loss of GAG molecules immediately after agarase treatment.25 In these agarase studies, the role that the accompanying transient loss of GAG played in the elevation of collagen content was unclear.
Motivated by the above, we hypothesize that temporal treatment of engineered constructs with CABC will lead to increased collagen content and tensile properties. To test this hypothesis, we examined the effects of (1) continuous CABC treatment at a low concentration (0.002 U/mL) lasting for 2 or 4 weeks; (2) multiple transient CABC treatments at a higher concentration (0.15 U/mL) lasting for 2 days; or (3) multiple transient agarase (100 U/mL) treatments.
Material and Methods
Sample preparation and tissue culture
Chondrocyte-seeded agarose hydrogel disks were prepared as previously described.18 Briefly, primary chondrocytes were harvested from the carpometacarpal joint of 3–4-month-old calves via digestion in 0.05% (w/v) collagenase (Sigma Chemicals, St. Louis, MO) for 11 h. Cells were encapsulated in 2% (w/v) low-melt agarose (Type VII, Sigma Chemicals) in PBS at 30 × 106 cells/mL. Disks (Ø 4.00 mm) were cored from the slabs and cultured in defined serum-free chondrogenic medium (DMEM, 1% ITS + Premix, 50 μg/mL l-proline, 0.1 μM dexamethasone, 0.9 mM sodium pyruvate, and antibiotics), supplemented with ascorbate (50 μg/mL). rhTGF-β3 (10 ng/mL) (R&D Systems, Minneapolis, MN) was administered for the first 2 weeks of culture to help promote native levels of GAG synthesis.3 Media were changed three times a week.
CABC and agarase treatment
For Study 1, CABC (Sigma Chemicals) (0.002 U/mL) was added into the culture media for the initial 4 weeks of culture or for 2 weeks starting on day 14 of culture (after discontinuation of TGF β3 supplementation) (Fig. 1A). For Study 2, either fresh CABC (0.15 U/mL) or agarase (Sigma Chemicals) (100 U/mL) was added into the culture media from day 35 to day 37 (groups CABC1T and Agarase1T); in an additional set of specimens, this treatment was repeated from day 58 to day 60 (groups CABC2T and Agarase2T; Fig. 1B). In both studies, control groups received neither CABC nor agarase treatment.
FIG. 1.
Temporal application of CABC or agarase in the treatment groups in Study 1 (A) and Study 2 (B). Study 2 was extended to 105 days to permit recovery from the second enzymatic treatment. The thick line indicates transient TGF β3 administration.
Mechanical testing
The spatially averaged mechanical properties of construct disks were evaluated at selected time points using a custom table top testing device.18 The equilibrium Young's modulus (EY) was determined under unconfined compression at 10% strain, followed by tests for dynamic moduli at 0.1, 0.5, and 1 Hz and 1% strain amplitude. Tensile properties of the constructs were evaluated using a custom testing device. Briefly, a dumbbell-shaped specimen was cut out of the cylindrical construct and glued at both ends on a fixation device. During testing, one end of the sample was fixed in position and the other was pulled at a constant speed of 5 μm/s with tension force and sample elongation recorded simultaneously. The tensile strain was calculated by dividing the sample elongation by the initial length of sample. The tensile modulus was calculated from the slope of the stress–strain relationship before failure. The failure stress was calculated by dividing the failure force by the initial cross-sectional area of the gauge section of the dumbbell specimen.
Biochemical analysis
One-half of each construct was weighed wet, lyophilized, reweighed dry, and digested in 0.5 mg/mL Proteinase-K (Fisher Scientific, Pittsburgh, PA) at 56°C for 16 h. The PicoGreen assay (Invitrogen-Molecular Probes, Carlsbad, CA) was used to quantify the DNA content of the constructs with Lambda phage DNA (0–1 mg/mL) as a standard.26 The GAG content was measured using dimethylmethylene blue (DMMB; Sigma Chemicals) dye-binding assay with shark chondroitin sulfate (0–50 mg/mL) as a standard.27 The overall collagen content was assessed by measuring the orthohydroxyproline (OHP) content via dimethylaminobenzaldehyde and chloramine T assay. Collagen content was calculated by assuming a 1:7.5 OHP-to-collagen mass ratio.28 The collagen and GAG contents were normalized to the disk wet weight and DNA content.
Histological analysis
The other halves of the constructs were fixed in a fixative solution (5% acetic acid, 3.7% formaldehyde, and 70% ethanol) for 24 h and stored in 70% ethanol solution. After serial dehydration in ethanol, the constructs were embedded in paraffin (Fisher Scientific), sectioned to 8 μm, and mounted onto microscope slides. The samples were then dewaxed, rehydrated, and stained with Safranin-O (Sigma Chemicals) and Picrosirius Red (Sigma Chemicals) dyes to determine the distribution of GAG and collagen, respectively.
Statistical analysis
Statistica (Statsoft, Tulsa, OK) was used to perform statistical analyses using two-way ANOVA and the Tukey HSD post hoc test of the means (n = 4–6 samples per group) with culture duration and experimental groups as independent factors.
Results
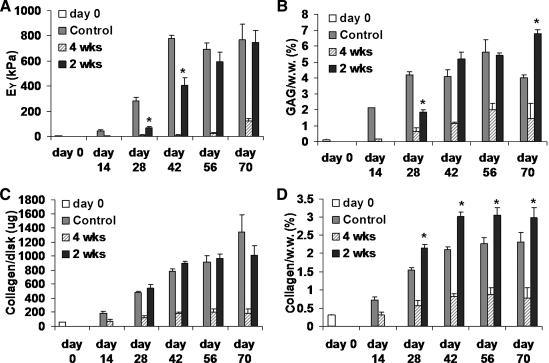
In Study 1, the mechanical properties and biochemical composition of the control group specimens rose until day 42 and plateaued subsequently (Fig. 2) to produce equilibrium and dynamic moduli around 700 kPa and 4 MPa, respectively (GAG/w.w. content of ∼6%, and collagen/w.w. content of ∼2.3%). For reference, GAG content is 6–8% w.w. and collagen content is 10–15% w.w. for similar immature cartilage from which the chondrocytes were isolated in this study.29,30 The 4-week CABC treatment group fared poorly in comparison, producing equilibrium and dynamic moduli of only ∼100 kPa and 1 MPa, respectively, by day 70, and similarly reduced GAG and collagen content (Fig. 2A). In contrast, the group that received the delayed CABC treatment (2 weeks), while exhibiting significantly lower equilibrium modulus than the control group on day 28 and day 42, recovered to comparable values by day 56 (Fig. 2A). The GAG content of the 2 weeks group was only about 40% of that of the control group on day 28 immediately after the treatment, but quickly recovered to the control level after only 14 days (day 42) (Fig. 2B). The absolute amount of collagen in the 2 weeks constructs is comparable to that of the control group starting from day 28 (Fig. 2C). The collagen content per wet weight of the 2 weeks group is significantly higher than that of the control beginning on day 28 because the control constructs swelled and exhibited significantly greater wet weight than the 2-week-treated constructs (Fig. 2D). DNA results indicate that CABC treatment suppressed cell proliferation and the degree of suppression increased with increasing treatment duration (data not shown). Safranin-O staining showed CABC-induced GAG loss (from the interterritorial regions) on day 28 and noticeable recovery of GAG content on day 56 (Fig. 3). On day 70 cracks were observed on the surface of the control constructs with a reduction in GAG content (Fig. 2B).
FIG. 2.
Study 1: (A) equilibrium modulus and (B) GAG content normalized to wet weight of the construct. (C) Total collagen content per construct. (D) Collagen content normalized to wet weight. *p < 0.005 versus Control, n = 4.
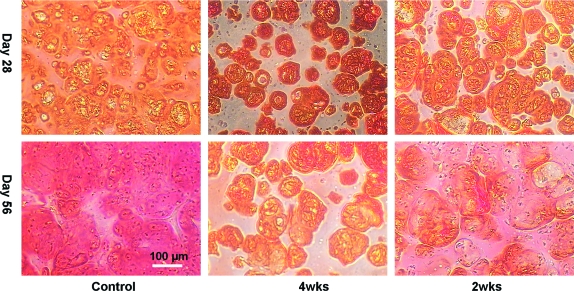
FIG. 3.
Study 1: Safranin-O staining of the histological sections on day 28 and day 56. Color images available online at www.liebertonline.com/ten.
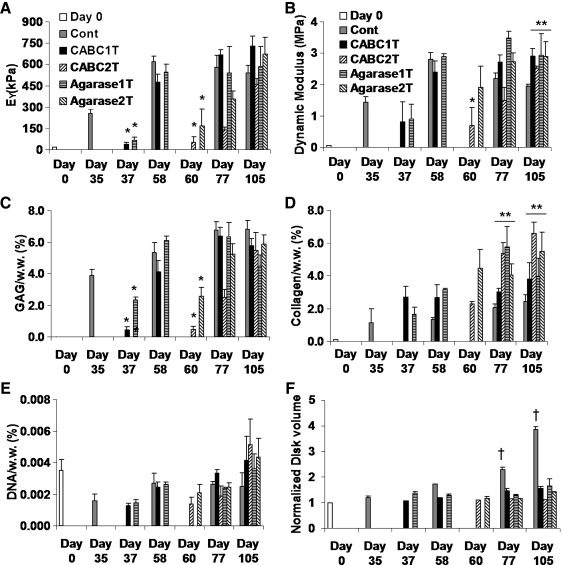
In Study 2, the equilibrium moduli of the CABC and agarase treatment groups all decreased significantly to about 20% of the pretreatment values immediately after treatment on day 37 and day 60, but eventually recovered to the level of the control group on day 58 (CABC1T, Agarase1T) and day 105 (CABC2T, Agarase2T) (Fig. 4A). Dynamic moduli were less affected by the enzyme treatments, and on day 105 all four treatment groups exhibited higher dynamic moduli than the control (Fig. 4B). The GAG content was significantly reduced, by around 90%, by the first and second CABC treatments, while the agarase treatments lowered the GAG content by around 40–50% (Fig. 4C). As observed in Study 1, the total amount of collagen in all treated constructs was not significantly different from that of the control (data not shown). However, the collagen content per wet weight of all treatment groups was significantly higher than that of the control on day 77 and day 105; the double treatment groups attained higher collagen content than the single treatment groups (CABC2T vs. CABC1T, and Agarase2T vs. Agarase1T) (Fig. 4D). The DNA content of the treatment groups was higher than that of the control on day 105 though not statistically significant (Fig. 4E). The volume of the control constructs swelled by almost 300% by day 105, significantly more than all of the treated constructs did, whereas constructs twice treated with CABC showed the least swelling (Fig. 4F).
FIG. 4.
Study 2: (A) equilibrium modulus and (B) dynamic modulus (0.1 Hz) of the constructs. (C) GAG content and (D) collagen content of the constructs (normalized to the wet weight of the constructs). (E) DNA content of the constructs. (F) Volume of the constructs normalized by the day 0 level. *p < 0.005 versus pretreatment level (day 35 Cont, and day 58 CABC1T and Agarase1T). **p < 0.005 versus control. †p < 0.005 versus all other groups (n = 4).
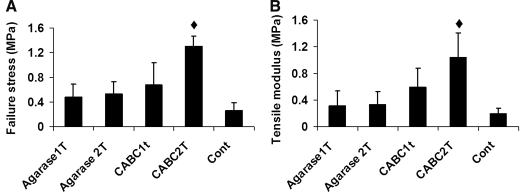
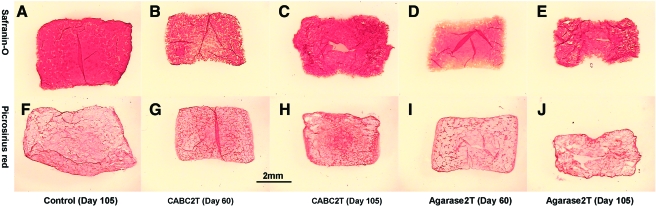
In general, the tensile modulus and failure stress of the treated constructs (CABC1T, Agarase1T/2T) were not significantly different than those of the control constructs, although they exhibited a trend toward higher values (Fig. 5). CABC2T constructs showed significantly higher tensile modulus and tensile failure stress than all other groups, reaching values of 1.04 ± 0.37 MPa and 1.30 ± 0.16 MPa, respectively (Fig. 5). Histological staining showed that CABC digestion removed GAG uniformly throughout the cross-sectional area of the constructs, while agarase preferentially induced GAG loss in the periphery of the constructs as the result of the removal of the agarose scaffold (Fig. 6B, D). The GAG staining eventually recovered on day 105 in the treated samples (Fig. 6C, E). Neither CABC nor agarase treatment affected the collagen distribution (Fig. 6G, I).
FIG. 5.
Study 2: failure stress (A) and tensile modulus (B) of the constructs on day 105. ♦p < 0.05 versus all other groups, n = 5.
FIG. 6.
Study 2: Safranin-O staining of the Control (A), CABCT2T (B, C), and Agarase2T (D, E). Picrosirius Red staining of the Control (F), CABCT2T (G, H), and Agarase2T (I, J). Color images available online at www.liebertonline.com/ten.
Discussion
Our results lead us to accept our hypothesis that temporal treatment of engineered constructs with CABC will lead to increased collagen content and tensile properties. Specifically, this study shows that temporarily suppressing the GAG content of engineered cartilage constructs with CABC can promote tissue formation having significantly higher collagen levels (Figs. 2D and 4D). This result is similar to that observed with agarase treatment of chondrocyte-seeded agarose constructs that led to transient tissue GAG loss with concomitant increased collagen levels.25 Interestingly, the (untreated) control constructs eventually expanded to the point of cracking, likely as a result of the supraphysiologic GAG levels and subphysiologic collagen levels forming a network insufficient to restrain the swelling force.31 While the absolute amount of collagen in Study 1 is statistically similar for the 2 weeks' group and control, the collagen content per wet weight is increased and reflects the lesser swelling in the treated samples, even after GAG content has recovered to control levels (Fig. 2B). In contrast, applying CABC in the first 4 weeks of the culture permanently stunted construct maturation. The latter may be due to inhibition of the beneficial effects of TGF β3 by CABC, or the protracted presence of CABC in the media. Further investigations are needed to examine the effects of extended CABC treatment (more than 2 weeks) after growth factor release. While the results are encouraging, suppression of GAG does not increase collagen content up to native levels and suggest that further optimization of this approach is required.
Previous studies showed that free-swelling growth of cartilage explants resulted in an expansive growth phenotype characterized by marked increase in tissue size and wet weight and a reduction in GAG and collagen concentration.5,32 On the other hand, removal of proteoglycans by CABC treatment restricted volumetric tissue swelling during subsequent culture and enhanced integrity of the collagen network, therefore resulting in improved tensile properties of the tissue.5 Similar results were observed in tissue-engineered cartilage as presented in Study 2. Tissue-engineered constructs grown under free-swelling condition (Control) swelled by almost 300% in size and wet weight, whereas constructs treated with CABC increased only slightly in size and wet weight (Fig. 4F). The marked increase in wet weight resulted in lower GAG/w.w. and collagen/w.w. content. The GAG content in the treated constructs recovered to its pretreatment level after about 2–3 weeks, resulting in similar equilibrium compressive modulus as compared to the no-treatment constructs. This outcome showed that temporary suppression by CABC may also switch the tissue-engineered constructs from an expansive growth phenotype to a maturation phenotype as seen in the cartilage explants, resulting in less tissue swelling, maintained compressive stiffness, and improved collagen content. Complementary studies of gene expression will be necessary to confirm this notion.
In Study 1, the collagen content of the constructs under the initial continuous CABC treatment plateaued at the end of the culture. In Study 2, two consecutive transient treatments of the CABC resulted in higher collagen content than that of the single treatment group, or the continuously treated constructs in Study 1. This suggests that further treatments with CABC after recovery of GAG content may increase the collagen content even more. We speculate that GAG synthesis or space exclusion by GAG may be an impediment to collagen synthesis and/or organization, possibly by altering transport pathways of nutrients or synthesis products.
While being able to withstand static compressive loading is a critical function of articular cartilage, the tensile properties of cartilage are also very important for regulating the response to dynamic compressive loading.33,34 The constructs treated with CABC and agarase exhibited higher tensile properties than the control constructs, and the CABC2T showed highest tensile properties. The higher tensile properties correlated with higher collagen content in the treated groups, consistent with expectations. By reducing the prestress due to osmotic swelling, temporary suppression of GAG content by CABC and agarase may have also created a more conducive environment for the elaboration and organization of the collagen network leading to better tensile properties. However, the methodology adopted in this study for tensile testing requires cutting the tissue-engineered cartilage into a dumbbell-shaped specimen. It should be noted that this sample preparatory procedure may have affected the intrinsic tensile properties of the constructs. Nevertheless, the higher tensile modulus observed in the treatment groups, relative to the control group (Fig. 5B), is consistent with the higher dynamic compressive modulus observed in the same groups (Fig. 4B).
Mechanical loading has been demonstrated in several in vivo35,36 and in vitro37–40 investigations to be important for the normal maintenance of articular cartilage and growth of tissue-engineered cartilage.16,18,41 Results from this study showed that enzymatic treatment can modulate the growth of tissue-engineered cartilage and enhance its functional properties. Therefore, there is reason to believe that combining mechanical loading with enzymatic treatment in cartilage tissue engineering could generate synergistic effects in further optimizing the functional properties, especially collagen-related properties of the tissue-engineered cartilage.
Conclusion
This study demonstrates that the collagen content of tissue-engineered cartilage constructs can be improved by enzymatically suppressing the GAG content temporarily through either continuous or transient application of CABC. Enzyme treatment of constructs with CABC or agarase yielded similar suppression of chondrocyte proliferation and a Young's modulus similar to controls, but with greater collagen content and dynamic compressive modulus (a measure of functional properties). As agarase's utility is specific to agarose hydrogel scaffolds,16,18,41 the finding that CABC treatment can increase collagen content via GAG removal is important as it can be applied to any scaffold or scaffold-free engineered cartilage system. Multiple transient treatments with CABC or agarase further increased the collagen content and tensile properties of engineered agarose hydrogel constructs. While many similarities exist between CABC and agarase treatment, the tensile properties were superior with CABC. As CABC does not affect agarose structure and content, our findings suggest that scaffold degradation may not be as critical as controlled removal of GAGs for elevating collagen content. In this respect, it is also possible that the maintenance of an interconnected scaffold (with accompanied GAG loss) is important for guiding matrix organization in the tissue and development of improved tensile properties. This strategy of altering the maturation patterns by using catabolic enzymes may prove to be an effective and promising methodology in achieving physiological functional properties in tissue-engineered cartilage with optimization of experimental parameters such as treatment duration and enzyme concentration.
Acknowledgment
This work was supported by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (AR46568, CTH) of the National Institutes of Health, USA.
Disclosure Statement
No competing financial interests exist.
References
- 1.Guilak F. Sah R.L. Setton L.A. Physical regulation of cartilage metabolism. In: Mow V.C., editor; Hayes W.C., editor. Basic Orthopaedic Biomechanics. Philadelphia: Lippincott-Raven; 1997. pp. 179–207. [Google Scholar]
- 2.Mow V.C. Bachrach N.M. Setton L.A. Guilak F. Stress, strain, pressure, and flow fields in articular cartilage and chondrocytes. In: Mow V.C., editor; Guilak F., editor; Hayes W.C., editor; Tran-Son-Tay R., editor; Hochmuth R.M., editor. Cell Mechanics and Cellular Engineering. New York: Springer-Verlag; 1994. pp. 345–79. [Google Scholar]
- 3.Byers B.A. Mauck R.L. Chiang I.E. Tuan R.S. Transient exposure to transforming growth factor beta 3 under serum-free conditions enhances the biomechanical and biochemical maturation of tissue-engineered cartilage. Tissue Eng. 2008;14:1821. doi: 10.1089/ten.tea.2007.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lima E.G. Bian L. Ng K.W. Mauck R.L. Byers B.A. Tuan R.S. Ateshian G.A. Hung C.T. The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF-beta3. Osteoarthritis Cartilage/OARS, Osteoarthritis Res Soc. 2007;15:1025. doi: 10.1016/j.joca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asanbaeva A. Masuda K. Thonar E.J. Klisch S.M. Sah R.L. Mechanisms of cartilage growth: modulation of balance between proteoglycan and collagen in vitro using chondroitinase ABC. Arthritis Rheum. 2007;56:188. doi: 10.1002/art.22298. [DOI] [PubMed] [Google Scholar]
- 6.Katta J. Stapleton T. Ingham E. Jin Z.M. Fisher J. The effect of glycosaminoglycan depletion on the friction and deformation of articular cartilage. Proc Inst Mech Eng. 2008;222:1. doi: 10.1243/09544119JEIM325. [DOI] [PubMed] [Google Scholar]
- 7.Otsuki S. Brinson D.C. Creighton L. Kinoshita M. Sah R.L. D'Lima D. Lotz M. The effect of glycosaminoglycan loss on chondrocyte viability: a study on porcine cartilage explants. Arthritis Rheum. 2008;58:1076. doi: 10.1002/art.23381. [DOI] [PubMed] [Google Scholar]
- 8.Yamagata T. Saito H. Habuchi O. Suzuki S. Purification and properties of bacterial chondroitinases and chondrosulfatases. J Biol Chem. 1968;243:1523. [PubMed] [Google Scholar]
- 9.Lyyra T. Arokoski J.P. Oksala N. Vihko A. Hyttinen M. Jurvelin J.S. Kiviranta I. Experimental validation of arthroscopic cartilage stiffness measurement using enzymatically degraded cartilage samples. Phys Med Biol. 1999;44:525. doi: 10.1088/0031-9155/44/2/017. [DOI] [PubMed] [Google Scholar]
- 10.Nahir A.M. Shomrat D. Awad M. Chondroitinase ABC affects the activity of intracellular enzymes in rabbit articular cartilage chondrocytes. J Rheumatol. 1995;22:702. [PubMed] [Google Scholar]
- 11.Lotke P.A. Granda J.L. Alterations in the permeability of articular cartilage by proteolytic enzymes. Arthritis Rheum. 1972;15:302. doi: 10.1002/art.1780150312. [DOI] [PubMed] [Google Scholar]
- 12.Bonassar L.J. Frank E.H. Murray J.C. Paguio C.G. Moore V.L. Lark M.W. Sandy J.D. Wu J.J. Eyre D.R. Grodzinsky A.J. Changes in cartilage composition and physical properties due to stromelysin degradation. Arthritis Rheum. 1995;38:173. doi: 10.1002/art.1780380205. [DOI] [PubMed] [Google Scholar]
- 13.Zhu W. Mow V.C. Koob T.J. Eyre D.R. Viscoelastic shear properties of articular cartilage and the effects of glycosidase treatments. J Orthop Res. 1993;11:771. doi: 10.1002/jor.1100110602. [DOI] [PubMed] [Google Scholar]
- 14.Benya P.D. Shaffer J.D. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 15.Aydelotte M.B. Schleyerbach R. Zeck B.J. Kuettner K.E. Articular chondrocytes cultured in agarose gel for study of chondrocytic chondrolysis. In: Kuettner K., editor. Articular Cartilage Biochemistry. New York: Raven Press; 1986. pp. 235–256. [Google Scholar]
- 16.Buschmann M.D. Gluzband Y.A. Grodzinsky A.J. Kimura J.H. Hunziker E.B. Chondrocytes in agarose culture synthesize a mechanically functional extracellular matrix. J Orthop Res. 1992;10:745. doi: 10.1002/jor.1100100602. [DOI] [PubMed] [Google Scholar]
- 17.Lee D.A. Bader D.L. The development and characterization of an in vitro system to study strain-induced cell deformation in isolated chondrocytes. In Vitro Cell Dev Biol Anim. 1995;31:828. doi: 10.1007/BF02634565. [DOI] [PubMed] [Google Scholar]
- 18.Mauck R.L. Soltz M.A. Wang C.C-B. Wong D.D. Chao P-H.G. Valhmu W.B. Hung C.T. Ateshian G.A. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122:252. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 19.Ait Si Selmi T. Neyret P.h. Verdonk P.C.M. Barnouin L. Autologous chondrocyte transplantation in combination with an alginate-agarose based hydrogel (Cartipatch) Techn Knee Surg. 2007;6:253. [Google Scholar]
- 20.Selmi T.A. Verdonk P.C.M. Chambat P. Dubrana F. Potel J.F. Barnouin L. Neyret P. Autologous chondrocyte implantation in a novel alginate-agarose hydrogel: outcome at two years. J Bone Joint Surg. 2008;90:597. doi: 10.1302/0301-620X.90B5.20360. [DOI] [PubMed] [Google Scholar]
- 21.Lima E.G. Tan A.R. Tai T. Bian L. Stoker A.M. Ateshian G.A. Cook J.L. Hung C.T. Differences in interleukin-1 response between engineered and native cartilage. Tissue Eng. 2008;14:1721. doi: 10.1089/ten.tea.2007.0347. [DOI] [PubMed] [Google Scholar]
- 22.Kelly T.A. Ng K.W. Wang C.C. Ateshian G.A. Hung C.T. Spatial and temporal development of chondrocyte-seeded agarose constructs in free-swelling and dynamically loaded cultures. J Biomech. 2006;39:1489. doi: 10.1016/j.jbiomech.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 23.Morrice L.M. McLean M.W. Williamson F.B. Long W.F. beta-Agarases I and II from Pseudomonas atlantica. Purifications and some properties. Eur J Biochem/FEBS. 1983;135:553. doi: 10.1111/j.1432-1033.1983.tb07688.x. [DOI] [PubMed] [Google Scholar]
- 24.Duckworth M. Turvey J.R. The action of a bacterial agarase on agarose, porphyran and alkali-treated porphyran. Biochem J. 1969;113:687. doi: 10.1042/bj1130687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ng K.W. Kugler L.E. Doty S.B. Ateshian G.A. Hung C.T. Scaffold degradation elevates the collagen content and dynamic compressive modulus in engineered articular cartilage. Osteoarthritis Cartilage/OARS, Osteoarthritis Res Soc. [DOI] [PMC free article] [PubMed]
- 26.McGowan K.B. Kurtis M.S. Lottman L.M. Watson D. Sah R.L. Biochemical quantification of DNA in human articular and septal cartilage using PicoGreen and Hoechst 33258. Osteoarthritis Cartilage/OARS, Osteoarthritis Res Soc. 2002;10:580. doi: 10.1053/joca.2002.0794. [DOI] [PubMed] [Google Scholar]
- 27.Farndale R.W. Buttle D.J. Barrett A.J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 28.Hollander A.P. Heathfield T.F. Webber C. Iwata Y. Bourne R. Rorabeck C. Poole A.R. Increased damage to type II collagen in osteoarthritic articular cartilage detected by a new immunoassay. J Clin Invest. 1994;93:1722. doi: 10.1172/JCI117156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bian L. Kaplun M. Williams D.Y. Xu D. Ateshian G.A. Hung C.T. Influence of chondroitin sulfate on the biochemical, mechanical and frictional properties of cartilage explants in long term culture. J Biomech. [DOI] [PMC free article] [PubMed]
- 30.Bian L. Lima E.G. Angione S.L. Ng K.W. Williams D.Y. Xu D. Stoker A.M. Cook J.L. Ateshian G.A. Hung C.T. Mechanical and biochemical characterization of cartilage explants in serum-free culture. J Biomech. 2008;41:1153. doi: 10.1016/j.jbiomech.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bian L. Angione S.L. Ng K.W. Lima E.G. Williams D.Y. Mao D.Q. Ateshian G.A. Hung C.T. Influence of decreasing nutrient path length on the development of engineered cartilage. Osteoarthritis Cartilage/OARS, Osteoarthritis Res Soc. [DOI] [PMC free article] [PubMed]
- 32.Williamson A.K. Masuda K. Thonar E.J. Sah R.L. Growth of immature articular cartilage in vitro: correlated variation in tensile biomechanical and collagen network properties. Tissue Eng. 2003;9:625. doi: 10.1089/107632703768247322. [DOI] [PubMed] [Google Scholar]
- 33.Park S. Ateshian G.A. Dynamic response of immature bovine articular cartilage in tension and compression, and nonlinear viscoelastic modeling of the tensile response. J Biomech Eng. 2006;128:623. doi: 10.1115/1.2206201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang C.Y. Stankiewicz A. Ateshian G.A. Mow V.C. Anisotropy, inhomogeneity, and tension–compression nonlinearity of human glenohumeral cartilage in finite deformation. J Biomech. 2005;38:799. doi: 10.1016/j.jbiomech.2004.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palmoski M. Perricone E. Brandt K.D. Development and reversal of a proteoglycan aggregation defect in normal canine knee cartilage after immobilization. Arthritis Rheum. 1979;22:508. doi: 10.1002/art.1780220511. [DOI] [PubMed] [Google Scholar]
- 36.Setton L.A. Mow V.C. Howell D.S. Mechanical behavior of articular cartilage in shear is altered by transection of the anterior cruciate ligament. J Orthop Res. 1995;13:473. doi: 10.1002/jor.1100130402. [DOI] [PubMed] [Google Scholar]
- 37.Guilak F. Meyer B.C. Ratcliffe A. Mow V.C. The effects of matrix compression on proteoglycan metabolism in articular cartilage explants. Osteoarthritis Cartilage. 1994;2:91. doi: 10.1016/s1063-4584(05)80059-7. [DOI] [PubMed] [Google Scholar]
- 38.Sah R.L. Kim Y.J. Doong J.Y. Grodzinsky A.J. Plaas A.H. Sandy J.D. Biosynthetic response of cartilage explants to dynamic compression. J Orthop Res. 1989;7:619. doi: 10.1002/jor.1100070502. [DOI] [PubMed] [Google Scholar]
- 39.Sah R.L. Doong J.Y. Grodzinsky A.J. Plaas A.H. Sandy J.D. Effects of compression on the loss of newly synthesized proteoglycans and proteins from cartilage explants. Arch Biochem Biophys. 1991;286:20. doi: 10.1016/0003-9861(91)90004-3. [DOI] [PubMed] [Google Scholar]
- 40.Buschmann M.D. Kim Y.J. Wong M. Frank E. Hunziker E.B. Grodzinsky A.J. Stimulation of aggrecan synthesis in cartilage explants by cyclic loading is localized to regions of high interstitial fluid flow. Arch Biochem Biophys. 1999;366:1. doi: 10.1006/abbi.1999.1197. [DOI] [PubMed] [Google Scholar]
- 41.Lee D.A. Bader D.L. Compressive strains at physiological frequencies influence the metabolism of chondrocytes seeded in agarose. J Orthop Res. 1997;15:181. doi: 10.1002/jor.1100150205. [DOI] [PubMed] [Google Scholar]