Abstract
Studies of the diffusion of proteins and lipids in the plasma membrane of cells have long pointed to the presence of membrane domains. A major challenge in the field of membrane biology has been to characterize the various cellular structures and mechanisms that impede free diffusion in cell membranes and determine the consequences that membrane compartmentalization has on cellular biology. In this review, we will provide a brief summary of the classes of domains that have been characterized to date, focusing on recent efforts to identify the properties of lipid rafts in cells through measurements of protein and lipid diffusion.
Keywords: membrane microdomains, lipid rafts, lateral diffusion, fluorescence recovery after photobleaching, fluorescence correlation spectroscopy, single particle tracking
Introduction
Since Frye and Edidin's groundbreaking paper in 1970 that demonstrated that membrane antigens are not static entities but rather can move within the plane of the membrane (1), scientists have sought to characterize the movement of membrane components. Over the years, a variety of methods have been developed to probe the diffusion of protein and lipid components in cell membranes. The most widely used approaches to date are fluorescence recovery after photobleaching (FRAP), fluorescence correlation spectroscopy (FCS), and single particle tracking (SPT) (Figure 1) [reviewed in (2-10)]. Measurements obtained using these techniques have revealed a number of features of cellular membranes, many of which are still not fully understood. Diffusion of molecules in model membranes is as much as 50 times faster than that of the same molecule in live cells (11). Diffusion measurements in cell membranes also suggest that membranes are heterogeneous, consisting of multiple classes of domains. A challenge in the field of membrane biology has been to characterize the various cellular structures and mechanisms that impede free diffusion in cell membranes and determine the consequences that membrane compartmentalization has on cellular biology. In this review, we will provide a brief summary of the classes of domains that have been characterized to date, focusing on recent efforts to identify the properties of lipid rafts in cells through measurements of protein and lipid diffusion.
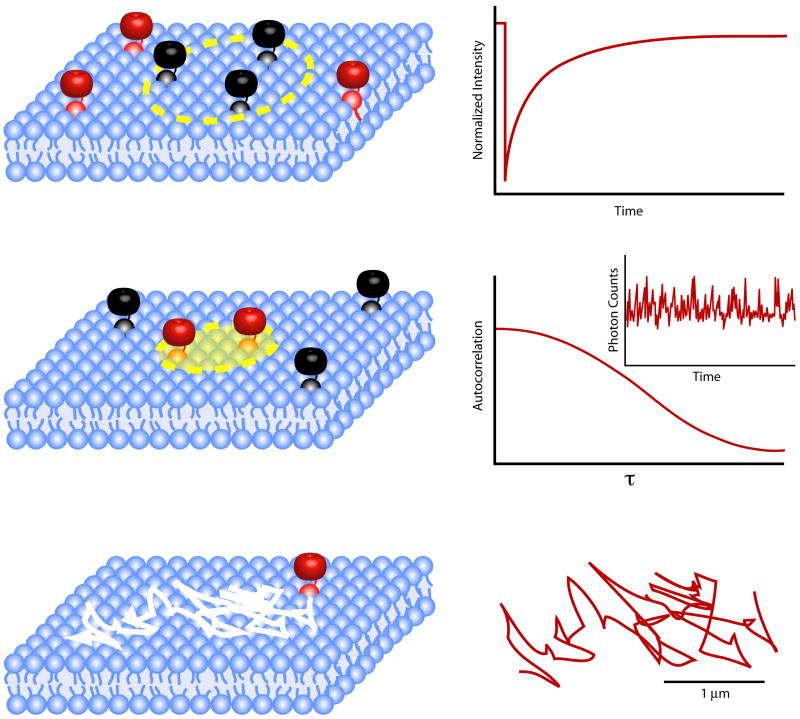
Figure 1. Diffusion-based biophysical methods used to study the membrane domains.
(A) Fluorescence recovery after photobleaching (FRAP). This method involves the labeling of a protein or lipid of interest with a fluorescent tag (red molecules). Then, using a focused laser spot, the fluorophores in a small patch of membrane, referred to as the bleach region of interest (ROI) (yellow circle), are irreversibly bleached with a brief pulse at high intensity (black molecules). Using a low intensity laser excitation, the subsequent lateral diffusion of unbleached fluorophores from the surrounding membrane into the ROI, and corresponding movement of bleached molecules out of the ROI, can be monitored. By plotting the change in fluorescence intensity in the ROI versus time (right panel) and then fitting the curve to an appropriate equation, an average rate of diffusion (D) can be calculated. A second kinetic parameter, the mobile fraction (Mf) can also be acquired from a FRAP experiment. This value is a percentage of the recovered fluorescence in the ROI compared to the fluorescence intensity lost during bleaching. Typically bleach ROI's in the μm range are used and diffusion coefficients obtained range from ∼0.01 to 1 μm2s-1 for membrane proteins and fluorescent lipid analogues. See (2, 3) for further information. (B) FCS measures the fluctuations of photons arising from fluorescent molecules (red molecules) contained within a very small three-dimensional volume (∼1 fmol) over time. In the case of membrane protein or lipid this 3 dimensional area is further reduced to a transverse plane of the imaging region at the laser beam waist (yellow circle), often 0.2 to 1 μm in radius. By constant illumination at the excitation wavelength of the fluorophore in a fixed beam waist size, changes in emitted photons can be measured as a function of time (inset, right panel). These intensity traces are used to calculate an autocorrelation curve (right panel). Through fitting the autocorrelation curve with the appropriate models a diffusion coefficient (D) can be calculated. Note that FCS typically can only measure D's > 0.1 μm2s-1 and thus is not useful for analyzing slowly diffusing membrane proteins. For recent reviews of this approach see (4, 5). (C) In single particle tracking (SPT) a molecule of interest is tagged, either genetically with a fluorescent protein or with antibodies conjugated to latex microspheres, colloidal gold, quantum dots or traditional fluorophores, and then imaged as it diffuses in the membrane. Using a fast camera and computer assisted analysis, the location of the molecule can be measured with high spatial accuracy (≤10 nm) and high temporal resolution (from 30 frames/s up to 50,000 frames per second (56)) (right panel). The resulting trajectories are then used to generate plots of the mean squared displacement of the diffusing molecule versus time, which can in turn be classified into different modes of diffusion (ex. free diffusion versus confined diffusion). See (2, 6) for more information about this technique.
Diffusion and domains in biological membranes
Diffusion measurements of proteins and lipids pointed to the presence of membrane domains prior to the development of the lipid raft hypothesis (12-15). A role of the cytoskeleton in slowing protein diffusion was recognized in early FRAP studies (16, 17). FRAP measurements as a function of bleach spot size revealed evidence for heterogeneities that impede free diffusion (13, 18-20). In a study examining the dependence of diffusion on bleach spot size (18), the mobility of several proteins and fluorescent lipid analogs showed a decrease in Mf with increasing spot size. The diffusion coefficient was also heterogeneous across the cell surface. The suggested explanation for this finding was the presence of a mixture of micrometer-size protein-rich and protein-poor domains (18). Subsequent work revealed that the ability of proteins to sense these domains depended on whether they were attached to the membrane by a transmembrane domain or GPI-anchor (19). The location of the barriers detected by transmembrane proteins was later identified as lying 2-3 nm beneath the plasma membrane surface (21). The diffusion of ligand-coated gold particles bound to plasma membrane receptors was also shown to be consistent with the presence of compartments that could be influenced by partial disruption of the cytoskeleton, leading to the development of the membrane skeleton fence model (22). Yet other single particle tracking experiments demonstrated that molecules undergo periods in which they appear to be transiently confined (23). Furthermore, transient interactions of proteins with coated pits could also be detected by FRAP (24). Thus, there is ample evidence that the diffusion of molecules in cell membranes is affected by interactions with membrane components. Before we consider the effects of lipid rafts on membrane protein and lipid dynamics, it is worth briefly reviewing how the lipid raft model evolved to its current state.
The emergence of lipid rafts as a novel class of domains
The discovery of lipid rafts was motivated by the question of how GPI-anchored proteins and glycosphingolipids are sorted to the apical surface of polarized epithelial cells (25, 26). Brown and Rose showed that fractionating membranes with detergent results in the solubilization of much of the membrane and the isolation of GPI and sphingomyelin enriched membrane sections, referred to as detergent resistant membranes (DRMs), that may serve as the sorting mechanism (27). The notion that the DRMs may be intact complexes native to the cell membrane spawned the lipid raft hypothesis (28). According to this hypothesis, specific proteins along with cholesterol and sphingomyelin assemble into complexes, or rafts, within the membrane. The physical basis for the formation of rafts is often attributed to the ability of lipid mixtures containing unsaturated phospholipid, sphingomyelin and cholesterol to spontaneously segregate into two distinct phases. In one phase, the lipids assume a liquid disordered state (Ld) characterized by both highly flexible acyl chains and highly mobile lipid molecules. In the other phase, sphingomyelin and cholesterol molecules are tightly packed and exhibit more restricted motion. This phase has been called the liquid ordered or Lo phase (29). These Lo phases produced in model membranes, along with DRMs, have been equated with lipid rafts (30). Membrane proteins are, in turn, hypothesized to preferentially associate with either Lo or Ld regions of the membrane.
Biochemical detergent extraction and biophysical studies in model membranes have generated an enormous body of literature detailing the function of lipid rafts and physical mechanisms underlying their formation. Yet, with few notable exceptions (31-33), most attempts to visualize large-scale phase separation in vivo have been unsuccessful. Even some biophysical measurements with a spatial resolution of better than 100 Å have shown that the majority of raft proteins appear to exist as monomers and only a small portion organized into clusters (34, 35). This has lead to the conclusion that if rafts exist in vivo they are generally small and also likely highly dynamic. Thus, recent efforts have focused on the use of biophysical techniques with exquisite sensitivity to protein and lipid dynamics to better understand the nature of these elusive domains. Several excellent reviews have discussed how such methods are currently being used to study lipid rafts and membrane domains (7-10, 36, 37). In addition, a number of models have been put forth in an effort to reconcile the sometimes conflicting data on raft structure and dynamics (9, 38-41). Here, we highlight several recent studies that have systematically compared the properties of “raft” and “non-raft” markers using diffusion-based measurements in an effort to define the properties of rafts.
Dynamics of rafts as a function of space and time
In order to understand the stability and dynamics of lipid rafts, it is important to consider how both raft and non-raft markers behave over all accessible time- and length-scales. Toward this end, a study from our group investigated the large-scale movements of rafts and raft proteins (42). By performing confocal FRAP measurements with a bleach region much larger (∼4 μm) than the suggested size of lipid rafts, kinetic measurements could be obtained that should reflect the diffusion of multiple rafts. Four lipid raft models differing in their predicted effects on Mf and D were tested using this technique: (i) stable, immobile rafts; (ii) stable, mobile rafts; (iii) dynamic partitioning; and (iv) no rafts. If rafts are stable and immobile complexes, we predicted that a low Mf and/or a low D value should be observed for all proteins located in the same raft. Likewise, for the stable, mobile model we predicted that all raft proteins diffuse over long distances at the rate of diffusion of the raft, thus producing similar D values for all raft components. This is in contrast to the dynamic partition model where D is proportional to the time spent inside and the time spent outside the raft, which could vary from one protein to another. D values would be unique for different proteins in the absence of rafts as well. In comparing FRAP measurements for putative raft and non-raft proteins, we observed that all proteins tested had high Mf and significantly different D values. This observation allows us to rule out both the stable, immobile and the stable, mobile models, as D was not equal for all proteins.
In an attempt to distinguish between the partitioning and no-raft model, we turned to conditions that should either drive dynamically partitioning proteins into rafts or decrease the number of rafts present at the cell surface (42). To do so, we took advantage of the reported sensitivity of rafts to temperature and cholesterol levels and repeated the experiment at different temperatures and following cholesterol depletion and cholesterol loading. Cholesterol depletion did not affect Mf values for either raft or nonraft proteins, but diffusion rates were significantly reduced following cholesterol depletion for both groups of proteins. Cholesterol loading, on the other hand, did not affect the rate of diffusion of the lipid raft markers examined. Finally, reducing temperature lowered D to a similar extent for both raft and nonraft proteins. At first glance, these results appear to be at odds with the view that decreased temperature and increased cholesterol levels stabilize and enlarge rafts. However, another interpretation of these findings is that the interaction of proteins with rafts is not the major factor that determines their diffusional mobility. They also point to the possibility that cholesterol depletion may have other effects on membranes besides disrupting rafts.
In fact, several groups have now reported that cholesterol depletion either has no effect on, or decreases the diffusion coefficient or mobile fraction of membrane components in live cells (Table 1). One suggestion is that this type of behavior is the result of phospholipids forming solid-like domains in the absence of cholesterol. These domains would exclude proteins and therefore act as impermeable barriers to the diffusion to both raft and nonraft proteins (43). Still others have observed an actin cytoskeleton-mediated decrease in mobile fraction as a result of cholesterol depletion (44). This implies that by depleting cholesterol, the effects of other barriers to diffusion become enhanced.
Table 1. Effects of cholesterol depletion on diffusion of proteins and fluorescent lipid analogs in the plasma membrane.
| Reference | Cell Type | Marker | Depletion Method | Experimental Method | Effect of Chol Depletion |
|---|---|---|---|---|---|
| Adkins et al. (90) | N2a | YFP-Dopamine transporter | MβCD | FRAP | ↑ D |
| EGFP-EGFR | MβCD | FRAP | no change | ||
| Crane et al. (91) | Cos7 | aquaporin1 | MβCD | SPT | ↓ D |
| MDCKII | aquaporin1 | MβCD | SPT | ↓ D | |
| MDCK | aquaporin1 | MβCD | SPT | ↓ D | |
| Ewers et al. (92) | 3T6 | murine polyoma virus-like particles (MPV) | MβCD | SPT | ↓ D |
| MPV | nystatin | SPT | ↓ D | ||
| MPV | progesterone | SPT | ↓ D | ||
| Goodwin et al (45) | Cos-7 | GFP-HRas | MβCD | FRAP | ↓ D |
| GFP-NRas | MβCD | FRAP | ↓ D | ||
| GFP-KRas | MβCD | FRAP | ↓ D | ||
| DiIC16 | MβCD | FRAP | no change | ||
| DiIC18 | MβCD | FRAP | no change | ||
| GFP-HRas | Lipoprotein depleted serum (LPDS) & compactin | FRAP | Slight ↑ D | ||
| GFP-NRas | LPDS & compactin | FRAP | Slight ↑ D | ||
| GFP-KRas | LPDS & compactin | FRAP | no change | ||
| DiIC16 | LPDS & compactin | FRAP | Slight ↑ D | ||
| Kenworthy et al. (42) | Cos7 | Cy3-CTXB | MβCD | FRAP | ↓ D |
| GFP-GPI | MβCD | FRAP | ↓ D | ||
| YFP-GL-GPI | MβCD | FRAP | ↓ D | ||
| Fyn-GFP | MβCD | FRAP | ↓ D | ||
| YFP-GT46 | MβCD | FRAP | ↓ D | ||
| GFP-VSVGsp | MβCD | FRAP | ↓ D | ||
| Lenne et al. (52) | Cos7 | GFP-GPI | Cholesterol Oxidase (COase) | FCS | ↓ D |
| GFP-Thy1 | COase | FCS | ↓ D | ||
| Fluorescent GM1 | COase | FCS | ↓ D | ||
| Fluorescent phosphatidylcholine | COase | FCS | ↓ D | ||
| Fluorescent sphingomyelin | COase | FCS | ↓ D | ||
| Fluorescent phosphatidylethanolamine | COase | FCS | ↓ D | ||
| Transferrin receptor-GFP | COase | FCS | no change | ||
| DPPIV-GFP | COase | FCS | ↓ D | ||
| Lommerse et al. (93) | 3T3-A14 | YFP-C-terminus of HRas | MβCD | SPT | no change |
| Nishimura et al. (43) | CHO | MHCII I-Ek | βCD | SPT | ↓ D |
| TRITC-DHPE (outer leaflet) | βCD | SPT | ↓ D | ||
| TRITC-DHPE (inner leaflet) | βCD | SPT | no change | ||
| DiIC18 | βCD | SPT | no change | ||
| Niv et al. (94) | Rat-1 | GFP-HRas | LPDS + Compactin | FRAP | ↑ D |
| GFP-HRas(12V) | LPDS + Compactin | FRAP | ↑ D | ||
| GFP-KRas | LPDS + Compactin | FRAP | no change | ||
| GFP-KRas(12V) | LPDS + Compactin | FRAP | no change | ||
| Orr et al. (94) | HME184A1 | EGF receptor | MβCD | SPT | ↓ D |
| HER2 | MβCD | SPT | ↓ D | ||
| Pucadyil and Chattopadhyay (95) | Hippocampal Neurons | DiIC18(3) | MβCD | FRAP | ↑ D |
| FAST DiI | MβCD | FRAP | ↑ D | ||
| Pucadyil and Chattopadhyay (96) | CHO | Serotonin1A-EYFP Receptor | MβCD | FRAP (small ROI) | ↓ D |
| Serotonin1A-EYFP Receptor | MβCD | FRAP (large ROI) | ↑ D | ||
| Shvartsman et al. (46) | Cos7 | Japan hemagglutinin (HA) (2A520) | MβCD | FRAP | ↓ D |
| Japan HA | MβCD | FRAP | no change | ||
| Japan HA (2A520) | LPDS + Compactin | FRAP | no change | ||
| Japan HA | LPDS + Compactin | FRAP | ↑ D | ||
| Rat-1 | GFP-HRas | MβCD | FRAP | ↓ D | |
| GFP-HRas(12V) | MβCD | FRAP | ↓ D | ||
| GFP-KRas(12V) | MβCD | FRAP | no change |
It is important to note however that the effects of cholesterol depletion appear to be strongly dependent on the method of depletion used (45, 46) (Table 1). In a study by Yoav Henis's group, the diffusion of both raft and non-raft forms of HA and Ras were measured following cholesterol depletion with methyl beta cyclodextrin (MβCD) or the statin compactin (46). Interestingly while both treatments lowered cholesterol to the same extent, they had opposing effects on diffusion. Cholesterol depletion with MβCD either did not effect diffusion or decreased diffusing of raft proteins, while compactin did not effect or increased D of the same molecules. The effect of MβCD was attributed to an undescribed function of MβCD, other than cholesterol depletion, as α-CD, which did not effect cholesterol levels, repeated had the same effects on diffusion as MβCD (46). Additional variables which were taken into account but which need to be recognized in cholesterol depletion studies are the differences in the time scale of depletion between enzymatic and cyclodextrin depletion methods and effects on lipid modification of proteins such as prenylation, and the effects on lipid concentrations besides cholesterol. Additionally, cholesterol depletion with MβCD is extremely sensitive to the cyclodextrin concentration, incubation time, and cell type (47). Although the intricacies of cholesterol depletion complicates the interpretation of diffusion measurements in cholesterol-depleted cells, it may provide important information about other changes that occur in cells that could account for some of the functional effects of these treatments, including for example blocks in endocytosis and signaling.
The idea that cell membranes may consist of a mixture of Lo and Ld domains has lead to the use of the term “partitioning” to describe the extent to which molecules are found in the raft versus non-raft region of the membrane (48). The partition coefficient provides a measure of the preference of a molecule for either the raft or non-raft regions of the membrane based on the ratio of its concentration in the two phases at equilibrium. This concept has been incorporated into a recent model of lipid rafts based on percolation theory (49, 50). A percolating system consists of two fluid phases, one of which is continuous or percolating, and the other is dispersed. Based on measurements of the diffusion of raft and non-raft proteins by confocal FRAP over minutes, the apical plasma membrane of a polarized epithelial cell line, MDCK, is proposed to teeter on a threshold between being a nearly continuous lipid raft dotted with small non-raft patches, or a nearly continuous non-raft membrane with small isolated rafts (49). Slight fluctuations in lipid levels or temperature can drive the membrane to either side of this threshold. At sub-physiological temperatures, which favor stabilization of Lo domains, the apical membrane is dominated by the raft phase. Under these conditions, proteins which partition exclusively to rafts are free to travel almost uninterrupted around the apical surface, while proteins that are immiscible in rafts are confined to small non-raft islands. However, at higher temperatures the percolation threshold is crossed, such that the non-raft phase becomes continuous and the non-raft proteins become free to move around the predominantly disordered membrane within which lipid raft proteins are confined. In this model, raft proteins are assumed to have a partition coefficient of one, denoting equal preference for raft and non-raft domains, and non-raft proteins have a partition coefficient much less than one, with partition coefficients closest to 0 having the greatest preference for the non-raft fraction. This raises an interesting question about what defines a raft protein; many models define them as having a distinct preference for rafts.
This model also suggests a strong dependence of the properties of rafts on cell type. When similar measurements were made in a fibroblast cell line, results similar to those reported previously (42), i.e. no discernable differences in the behavior of raft and non-raft proteins, were obtained. Why percolation-type behavior appears to be confined to polarized cells remains to be more fully explored. However, since the apical cell membrane of epithelial cells is highly enriched in sphingolipids as compared to the plasma membrane of non-polarized cells, one plausible explanation may be that only in apical membranes is the sphingolipid concentration sufficiently high enough for a percolating raft fraction to form.
It is also important to bear in mind that anchorage to the actin cytoskeleton or confinement in corrals can also slow diffusion. To distinguish such effects from those of lipid rafts, Wawrezinieck et al. have proposed a novel extension of the traditional FCS experiment for categorizing membrane domains, known as the FCS Diffusion Law (51-53) [reviewed in (4, 54)]. In this approach, two distinct classes of microdomains are considered. The first consists of a mesh-like series of diffusion barriers that mimics the actin cytoskeleton/corral model. Within each grid, molecules are free to diffuse, but diffusion between adjacent grids is controlled by the probability of the diffusing species crossing the diffusion barrier. The second class, modeling lipid rafts, comprises a series of isolated circular domains. Movement in and out of a given domain is controlled by the partition coefficient of the diffusing species for the domains, and molecules can also have different diffusion coefficients within domains and outside of domains. Based on numerical simulations, these two models can be differentiated on the basis of their dependence of diffusion times as a function of the area of observation. The theoretical basis for this behavior was recently determined for the mesh model (55).
Experimentally, the presence of these two classes of microdomains can be tested for by collecting a series of FCS measurements at different waist sizes. By graphing the diffusion time (τd) versus the radius squared of the waist (w2) a regression line can then be fit to the data, and a t0, or the theoretical diffusion time in a waist of zero nm, can be calculated (51). Using this method, a positive t0 is indicative of diffusing molecules that interact with isolated domains (lipid rafts), a negative t0 denotes mesh-like (actin cytoskeletal) constraints on diffusion, and a t0 equal to zero is associated with free diffusion (51, 52). Additionally, the slope of this graph will correspond to the diffusion coefficient D (52). By combining this method with treatments such as cholesterol depletion or cytoskeletal disruption, it is possible to test for shifts from confined to free diffusion thus allowing confirmation of the mechanism of diffusional trapping. By these criteria, a fluorescent sphingolipid analog and several GPI-anchored proteins were found to dynamically associate with lipid rafts, whereas the transferrin receptor was confined by a cytoskeletal meshwork (52). In contrast, fluorescent glycerophospholipid analogs showed no evidence of confinement by either mechanism. The authors estimate that the putative raft-associated proteins are confined in cholesterol- and sphingolipid- dependent regions of the membrane with a characteristic size of less than 120 nm (52).
Based on results obtained using extremely fast single molecule imaging approaches, Kusumi and coworkers argue that the diffusion of GPI-anchored proteins is in fact modulated by the cytoskeleton but not lipid rafts (56). Performing single particle tracking on transmembrane MHC II (TM-I-Ek) and a modified GPI anchored form (GPI-I-Ek), they demonstrated confinement of both proteins to corrals of ∼40 nm. Treatment with latrunculin A (an inhibitor of actin polymerization) caused increased compartment size in addition to increased macroscopic D, while cytochalasin D (an inducer of actin depolymerization) did not induce changes in the diffusion of either molecule. The authors suggest that discrepancy between the effects of latrunculin A and cytochalisin D can potentially be explained by the increased production of new actin filaments by the cell in response to the capping of barbed ends by cytochalisin, which would neutralize the effects of cytochalisin (56).
The effect seen with latrunculin A is consistent with a model in which the actin cytoskeleton forms fences along the inner leaflet of the plasma membrane (22, 57-59). These fences form corrals that can separate membrane regions, restricting the movement of transmembrane proteins as well as proteins anchored on the inner leaflet. In addition to physically impeding the movement of some membrane components, other proteins are bound directly to the actin cytoskeleton. Transmembrane proteins that are anchored directly to the actin cytoskeleton form pickets along the fences and impede the movement of molecules in both leaflets of the bilayer (60). While the corrals separated by pickets and fences segregate the plasma membrane, molecules are not completely confined to these corrals. For instance, hop diffusion, where a molecule jumps out of one corral into another has been recorded with single particle tracking (60). An important feature of the current study that enabled the visualization of hop diffusion is the time resolution of the measurements. Previous attempts to quantify the diffusion of these proteins performed at frame rates below 65 frames/s did not measure this confinement. In contrast, this latest study was done at 50,000 frames/s, allowing for the resolution of confinement and hops, which were blurred at the slower frame speeds. Indeed, the authors suggest that previous measurements of the diffusion of GPI-anchored proteins by single particle techniques may have missed seeing hop diffusion because of averaging effects at lower frame rates (56).
The finding by the Kusumi group that the diffusional mobility of GPI anchored proteins is affected by the cytoskeleton (56) is at odds with the FCS results of Lenne et al (52). As probed by FCS measurements as a function of spot size, GFP-GPI and GFP-Thy1 diffusion appeared to be controlled by lipid rafts as depletion of cholesterol and sphingomyelin reduced the diffusion rate consistent with lipid raft confinement (52). Additionally, confinement by the cytoskeleton was not detected, as disruption of the cytoskeleton by latrunculin B and cytochalasin D had no effect on their effective diffusion coefficient or intercept time, t0 (52). Finally, the SPT study measured the cytoskeletal domains to have an area of ∼40 nm2 (56), while rafts of ∼80 nm2 for GPI anchored proteins were measured by FCS (52). The reason these two studies reach opposite conclusions remains to be determined. The discrepancies may stem from differences in the cell type and specific GPI-anchored proteins used in each study.
The data outlined above emphasize that lipid rafts are not the only mechanism that can influence the mobility of putative raft proteins. At times, the association a specific protein with a specific type of domain has proven contentious as different experimental methods have lead to seemingly contradictory results. To explore further the complexity in the study of lipid rafts, we next consider a single raft marker and current evidence for how its diffusion is regulated in cells.
Cellular factors influencing the diffusion of a model raft protein, cholera toxin B subunit
One of the most studied markers of lipid rafts is the cholera toxin B subunit (CTXB). Native cholera toxin is composed of a single A subunit and five identical B subunits. The B subunit homopentamer binds 5 GM1 gangliosides on the extracellular leaflet of the cell membrane (61). Once anchored to the cell membrane, cholera toxin is endocytosed and transported via a retrograde pathway to the endoplamic reticulum where the A subunit is translocated into the cytoplasm, ultimately leading to disease (62). The ability of the B subunit to bind 5 GM1s with high specificity has lead to the idea that CTXB may be attaching to a lipid raft. Early evidence for this idea came from the observation that CTXB is associated with detergent resistant membranes (63, 64). CTXB can also be fluorescently labeled and directly visualized in both cells and model membranes containing GM1, making it an excellent reporter of lipid raft localization and dynamics. In addition, fluorescent analogs of GM1 also exist. Taking advantage of these probes, several recent studies have used FRAP and FCS to measure the diffusion of CTXB or fluorescent analogs of GM1 in cells (42, 52, 65-69).
In one such study, CTXB was reported to have a significantly slower rate of lateral diffusion than other putative raft membrane proteins (42). The slow diffusion of CTXB on the cell surface was speculated to reflect its interaction with immobile caveolae and/or trapping of cell surface proteins that interact with the cytoskeleton (42). In support of the former possibility, caveolin was recently shown to modulate CTXB diffusion (69). FCS measurements of CTXB in rat basophilic leukemia (RBL) cells also show the molecule to diffuse slowly, so much so as to appear immobile (65). However, in the same study CTXB diffused rapidly when incorporated into model membranes containing ganglioside GM1. Cholesterol depletion with MβCD did not affect CTXB diffusion in RBL cells. Yet, treatment with latrunculin A did cause an increase in CTXB D, demonstrating that the slow diffusion normally seen with CTXB is caused primarily by the actin cytoskeleton (65). Interestingly, a dramatically different result was seen for a fluorescent GM1 analog in COS-7 cells. By applying the FCS Diffusion Law it was found that BODIPY-C5-ganglioside-GM1 has a positive t0, consistent with lipid raft mediated diffusion (46). Further supporting this possibility, treatment with cholesterol oxidase or sphingomyelinase, but not jasplakinolide (an actin filament stabilizer), cytochalasin D, or latrunculin B (an actin polymerization inhibitor), induced free diffusion of the fluorescent GM1 analog (52).
It is not yet entirely clear why the diffusional mobility of CTXB and fluorescent GM1 differ. Although BODIPY-GM1 is often used to study the behavior of endogenous GM1, there may be intrinsic and yet undiscovered differences between the behavior of these two molecules as a result of the labeling with BODIPY on a shortened fatty acid chain. Alternatively, the slow diffusion of CTXB could also potentially result from several small GM1- containing rafts becoming crosslinked by CTXB. Both detergent resistant membrane assays and model membrane studies suggest such crosslinking may occur. The initial DRM work done with CTXB showed that CTXB treatment of cells produced a significant reduction in the amount of GM1 which became solubilized in 0.5% Triton X-100 (63). This would indicate that GM1 is being artificially clustered into detergent resistant fractions by CXTB. CTXB binding has also recently been shown to be able to modulate the phase behavior of Lo/Ld mixtures in model membranes (70). Giant unilamellar vesicles consisting of phosphatidylcholine, phosphatidylglycerol, sphingomyelin, cholesterol, and GM1 that are doped with fluorescent probes that preferentially segregate into the Lo or Ld fractions showed one homogenous bilayer. However, following CTXB treatment clear segregation of the membrane into two phases was seen (70). A similar result was recently reported where CTXB induced large scale separation of raft and non-raft fluorescent probes in cytoskeleton-free plasma membrane vesicles, which were still attached to live cells (68).
To more directly test the role of crosslinking of CTXB, a recent study evaluated the toxicity and raft association of cholera toxin chimeras containing a mutant form of CTXB that only binds 1 or 2 GM1's as compared to wild type holotoxin's 5 GM1 binding sites (66). The mutant toxin exhibited some toxicity, although with reduced potency compared to wild type. Endocytosis was also reduced. However, the mutant toxin had Mf and D values similar to wild type CTXB as assessed by FRAP. This suggests that the pentavalency of CTXB is important for efficient toxin uptake, but that it cannot explain the slow diffusion of the molecule while at the cell surface. An alternative explanation comes from work investigating the intra-endosomal mobility of CTXB and other cargo molecules that are internalized via caveolae such as the SV40 virus (67). In this study, enlarged endosomes were generated by expressing constitutively active Rab5a, enabling the visualization of endosomal subdomains. Caveolin-1-GFP and SV40 were immobilized within enlarged endosomes, whereas CTXB was not. However, when cells were incubated in the presence of NH4Cl to neutralize endosomal pH, CTXB became diffusionally restricted at sites enriched in caveolin-1-GFP. These findings (67) suggest that CTXB diffuses slowly at the cell surface as the result of its interaction with caveolar domains at neutral pH. The regulation of CTXB mobility by pH could help explain how endocytic sorting of CTXB from SV40 virus, which share the same receptor, occurs: CTXB can dissociate from caveolar domains at the low pH found in endosomes, whereas SV40 remains associated with caveolin in this compartment (67).
New directions in the study of microdomain dynamics
Most of the studies discussed above have investigated the mobility of a single type of protein or lipid at a time, comparing them against other molecules and across treatments. Until recently, a fundamental limitation of this approach is that the identity of cellular structures that impact diffusion, such as the cytoskeleton, has to be inferred indirectly. Furthermore, in order to better understand how domains influence various types of molecules, it would be useful to be able to determine the relationship between the movements of multiple molecules simultaneously within the same cell. Several recent studies have begun to explore approaches that make these types of measurements feasible.
One of the most powerful new developments in this area is the use of multiple labels for measurements of the mobility of two or more classes of molecules simultaneously (71-76). This has made it possible to examine the interaction of individual molecules with membrane domains in real time. For example, dual color FRAP and single molecule tracking studies have been instrumental in the identification of plasma membrane microdomains formed by the coclustering of CD2, LAT and Lck in T-cell plasma membranes during signaling (75). In this study, the authors used FRAP and SPT to examine the dynamics of a variety of GFP-tagged signaling proteins in resting and activated Jurkat T cells in order to gain insight into the nature of the membrane domains that form during T-cell signaling. These studies revealed that CD2, a costimulatory transmembrane protein, forms clustered membrane domains in activated cells. Cluster formation required functional LAT but not actin, a result that the authors suggest indicates a role for a network of protein-protein interactions. CD2 was largely immobilized within these clusters, allowing the authors to perform single molecule tracking of individual proteins relative to the clusters in dual color imaging experiments using CD2-mRFP and single GFP-tagged proteins. From these studies, it became clear that the clusters can act as barriers to the free diffusion of individual protein molecules. The authors conclude that the ability of these domains to exclude or trap signaling molecules contributes to the spatial organization of the T cell signaling machinery. Their results further suggest that lipid rafts are not a major contributor to the clustered domains observed in their studies, since two raft-associated but functionally inert proteins were not specifically enriched in the CD2 clusters.
In another study taking advantage of multiple labeling, single molecule tracking techniques were used to investigate signaling mechanisms of crosslinked GPI-anchored proteins (71, 72). In this comprehensive two-part study, colloidal gold molecules were used to both induce crosslinking and serve as fiduciary markers for the clusters of crosslinked proteins. The authors show that following crosslinking, the GPI-anchored receptor clusters alternatively become transiently immobilized and undergo simple diffusion. They then went on to investigate the recruitment of signaling proteins to these sites by simultaneously visualizing the clustered GPI-anchored proteins and GFP-tagged versions of intracellular signaling proteins. Importantly, this enabled them to determine not only what intracellular proteins were recruited to the GPI-anchored receptor clusters, but also the relationship between the recruitment of signaling proteins to the temporary immobilization of the GI-anchored proteins. In this way, the authors were able to trace out a likely order of events that occur during this process. They also tested for a role for lipid rafts in mediating protein recruitment to these sites by comparing the behavior of Lyn-GFP and LynN20-GFP, a construct consisting of the N-terminal 20 amino acid sequence of Lyn known to target the protein to lipid rafts. Interestingly, LynN20-GFP was recruited to the GPI-anchored proteins clusters more often than controls, although less frequently than Lyn-GFP itself, suggesting that lipid rafts may play a role in targeting it to the cluster but that protein-protein interactions are also important.
Although the cytoskeleton has long been known to impact the diffusion of cell surface proteins and lipids, only very recently has the exact relationship between protein dynamics and actin-defined domains been directly visualized (76). In this elegant study, the cell surface dynamics of FcεRI was visualized using quantum dot (QD)-labeled IgE simultaneously with the distribution of GFP-actin in order to address the role of the actin cytoskeleton in controlling receptor dynamics. In doing so, the authors could show that the receptor-QD diffusion was confined with actin-poor regions by overlaying the trajectory of the QD-IgE-FcεRI complex on images of actin distribution. Importantly, they also found that the location and size of the barriers formed by GFP-actin underwent reorganization over time. This indicates that the nature of the diffusion barriers formed by the cytoskeleton is time dependent.
Another class of evolving approaches that holds great promise in uncovering novel aspects of membrane microdomains is techniques that can be used to generate spatial maps of protein and lipid diffusion. Taking advantage of a recently described method known as PALM/FPALM to obtain super-resolution images (77, 78), several groups have now generated cellular maps of single molecule trajectories by serially activating and imaging groups of photoactivatable fluorescent proteins (79, 80). One of these studies (79) directly tested predictions of several different models of lipid rafts by evaluating the membrane organization of hemagglutinin (HA), one of the first transmembrane proteins shown to associate with lipid rafts. The motions of individual HA molecules appeared to be constrained, mapping out irregular shapes that were often elongated. Furthermore, HA is clustered over all length scales examined, indicating that no single characteristic raft size exists. In addition, the boundaries of the observed domains are not rounded, in contrast to the predictions of a fluid-fluid domain coexistence model. The observation that there is not one characteristic cluster dimension for HA is also very different from the distribution of GPI-anchored proteins and Ras, which exist as a combination of monomers and nanoclusters (34, 81). However, the authors indicate that the HA data could potentially be consistent with a model in which nanometer-size domains that are incorporated into clusters. They also raise the possibility that the complex membrane distribution and dynamics of HA uncovered in this study may explain why previous measurements of lipid raft dynamics over different time- and length-scales often yield such different results.
Yet other efforts have been directed on approaches that couple measurements of diffusion and either the oligomerization state or clustering of the diffusing species (82-89). For example, several studies have begun to tackle the question of how the oligomerization state of GPI-anchored proteins is regulated and whether different oligomeric forms have different dynamic properties (82, 84). One such study utilized a technique known as dynamic image correlation spectroscopy to show that a model GPI-anchored protein, GFP-GPI, exists as a mixture of monomers and clusters. The authors suggest that the clusters could potentially represent lipid rafts, as they were partially dispersed by cholesterol depletion. Interestingly, the clusters diffuse much more slowly (∼6 × 10-12 cm2/s) than the monomers, which diffused too rapidly to be resolved by this technique (>3.9 × 10-8 cm2/s). In addition, the tendency of GFP-GPI to cluster was highly temperature dependent, exhibiting a lower state of clustering at physiological temperature than at lower temperatures. Diffusion of the clusters was also temperature dependent, becoming essentially immobile at 4° C. Thus, although the exact nature of the clusters that these GPI-anchored proteins associate with is not entirely clear, they are both highly dynamic and temperature sensitive.
A much different picture of the regulation of GPI-anchored protein distribution and dynamics comes from a recent study using a combination of FRET, FCS, and photon counting histogram analysis to study the monomer-dimer dynamics of the GPI-anchored protein urokinase plasminogen activator receptor (uPAR) (84). Here, the authors show that uPAR dimerization is controlled by binding of the receptor to its ligand, the extracellular matrix protein vitronectin. In particular, vitronectin binding leads to partial immobilization and slow diffusion of uPAR on the basal surface of cells. In contrast, on the region of the membrane not in contact with the substrate, the receptor exists in a monomer-dimer equilibrium. Commitment of the receptor to undergo endocytosis also influences diffusion and the influences exchange between receptor monomers and dimers. The authors of this study conclude that it is important to consider the role of protein-protein interactions in controlling the dynamics of GPI-anchored proteins, and that at least for the case of uPAR, whether the interaction of the receptor with vitronectin increases uPAR's residence time within lipid rafts is not relevant to the control of uPAR dimerization.
In summary, the past few years have seen several tremendous advances in the new ways to study membrane domains. However, there is still much to be learned about membrane domains and how they control the distribution and dynamics of biomolecules. Particularly for the case of lipid rafts, much more work is needed to build a consensus viewpoint. While some studies point to the presence small, dynamic domains, others provide a picture of slowly diffusing, heavily clustered rafts. Importantly, many of the new techniques and approaches described have the potential to resolve these outstanding questions, and ultimately provide us with a better understanding of how membrane domains regulate cellular functions.
Acknowledgments
This work was supported by R01 GM073846 from the NIGMS. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIGMS or the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Frye LD, Edidin M. The rapid intermixing of cell surface antigens after formation of mouse-human heterokaryons. J Cell Sci. 1970;7:319–35. doi: 10.1242/jcs.7.2.319. [DOI] [PubMed] [Google Scholar]
- 2.Chen Y, Lagerholm BC, Yang B, Jacobson K. Methods to measure the lateral diffusion of membrane lipids and proteins. Methods. 2006;39:147–53. doi: 10.1016/j.ymeth.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Kenworthy AK. Fluorescence recovery after photobleaching studies of lipid rafts. In: McIntosh T, editor. Lipid Rafts. Humana Press; Towata, NJ: 2007. [DOI] [PubMed] [Google Scholar]
- 4.Marguet D, Lenne PF, Rigneault H, He HT. Dynamics in the plasma membrane: how to combine fluidity and order. EMBO J. 2006;25:3446–57. doi: 10.1038/sj.emboj.7601204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bacia K, Schwille P. Fluorescence correlation spectroscopy. Methods Mol Biol. 2007;398:73–84. doi: 10.1007/978-1-59745-513-8_7. [DOI] [PubMed] [Google Scholar]
- 6.Kusumi A, Ike H, Nakada C, Murase K, Fujiwara T. Single-molecule tracking of membrane molecules: plasma membrane compartmentalization and dynamic assembly of raft-philic signaling molecules. Semin Immunol. 2005;17:3–21. doi: 10.1016/j.smim.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Lommerse PH, Spaink HP, Schmidt T. In vivo plasma membrane organization: results of biophysical approaches. Biochim Biophys Acta. 2004;1664:119–31. doi: 10.1016/j.bbamem.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Lagerholm BC, Weinreb GE, Jacobson K, Thompson NL. Detecting microdomains in intact cell membranes. Annu Rev Phys Chem. 2005;56:309–36. doi: 10.1146/annurev.physchem.56.092503.141211. [DOI] [PubMed] [Google Scholar]
- 9.Jacobson K, Mouritsen OG, Anderson RG. Lipid rafts: at a crossroad between cell biology and physics. Nat Cell Biol. 2007;9:7–14. doi: 10.1038/ncb0107-7. [DOI] [PubMed] [Google Scholar]
- 10.Kenworthy AK. Fleeting glimpses of lipid rafts: how biophysics is being used to track them. J Investig Med. 2005;53:312–7. doi: 10.2310/6650.2005.53608. [DOI] [PubMed] [Google Scholar]
- 11.Kusumi A, Koyama-Honda I, Suzuki K. Molecular dynamics and interactions for creation of stimulation-induced stabilized rafts from small unstable steady-state rafts. Traffic. 2004;5:213–30. doi: 10.1111/j.1600-0854.2004.0178.x. [DOI] [PubMed] [Google Scholar]
- 12.Jacobson K, Sheets ED, Simson R. Revisiting the fluid mosaic model of membranes. Science. 1995;268:1441–2. doi: 10.1126/science.7770769. [DOI] [PubMed] [Google Scholar]
- 13.Edidin M. Patches, posts and fences: proteins and plasma membrane domains. Trends Cell Biol. 1992;2:376–80. doi: 10.1016/0962-8924(92)90050-w. [DOI] [PubMed] [Google Scholar]
- 14.Kusumi A, Sako Y. Cell surface organization by the membrane skeleton. Curr Opin Cell Biol. 1996;8:566–574. doi: 10.1016/s0955-0674(96)80036-6. [DOI] [PubMed] [Google Scholar]
- 15.Sheetz MP. Cellular plasma membrane domains. Mol Membr Biol. 1995;12:89–91. doi: 10.3109/09687689509038501. [DOI] [PubMed] [Google Scholar]
- 16.Sheetz MP, Schindler M, Koppel DE. Lateral mobility of integral membrane proteins is increased in spherocytic erythrocytes. Nature. 1980;285:510–1. doi: 10.1038/285510a0. [DOI] [PubMed] [Google Scholar]
- 17.Wu ES, Tank DW, Webb WW. Unconstrained lateral diffusion of concanavalin A receptors on bulbous lymphocytes. Proc Natl Acad Sci U S A. 1982;79:4962–6. doi: 10.1073/pnas.79.16.4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yechiel E, Edidin M. Micrometer-scale domains in fibroblast plasma membranes. J Cell Biol. 1987;105:755–60. doi: 10.1083/jcb.105.2.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edidin M, Stroynowski I. Differences between the lateral organization of conventional and inositol phospholipid-anchored membrane proteins. A further definition of micrometer scale membrane domains. J Cell Biol. 1991;112:1143–50. doi: 10.1083/jcb.112.6.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edidin M. Lipid microdomains in cell surface membranes. Curr Opin Struct Biol. 1997;7:528–532. doi: 10.1016/s0959-440x(97)80117-0. [DOI] [PubMed] [Google Scholar]
- 21.Edidin M, Zuniga MC, Sheetz MP. Truncation mutants define and locate cytoplasmic barriers to lateral mobility of membrane glycoproteins. Proc Natl Acad Sci U S A. 1994;91:3378–82. doi: 10.1073/pnas.91.8.3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sako Y, Kusumi A. Compartmentalized structure of the plasma membrane for receptor movements as revealed by a nanometer-level motion analysis. J Cell Biol. 1994;125:1251–64. doi: 10.1083/jcb.125.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simson R, Sheets ED, Jacobson K. Detection of temporary lateral confinement of membrane proteins using single-particle tracking analysis. Biophys J. 1995;69:989–93. doi: 10.1016/S0006-3495(95)79972-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fire E, Zwart DE, Roth MG, Henis YI. Evidence from lateral mobility studies for dynamic interactions of a mutant influenza hemagglutinin with coated pits. J Cell Biol. 1991;115:1585–94. doi: 10.1083/jcb.115.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simons K, van Meer G. Lipid sorting in epithelial cells. Biochemistry. 1988;27:6197–6202. doi: 10.1021/bi00417a001. [DOI] [PubMed] [Google Scholar]
- 26.Simons K, Wandinger-Ness A. Polarized sorting in epithelia. Cell. 1990;62:207–210. doi: 10.1016/0092-8674(90)90357-k. [DOI] [PubMed] [Google Scholar]
- 27.Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- 28.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 29.Brown DA, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed SN, Brown DA, London E. On the origin of sphingolipid/cholesterol-rich detergent-insoluble cell membranes: physiological concentrations of cholesterol and sphingolipid induce formation of a detergent-insoluble, liquid-ordered lipid phase in model membranes. Biochemistry. 1997;36:10944–10953. doi: 10.1021/bi971167g. [DOI] [PubMed] [Google Scholar]
- 31.Hao M, Mukherjee S, Maxfield FR. Cholesterol depletion induces large scale domain segregation in living cell membranes. Proc Natl Acad Sci U S A. 2001;98:13072–13077. doi: 10.1073/pnas.231377398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baumgart T, Hammond AT, Sengupta P, Hess ST, Holowka DA, Baird BA, Webb WW. Large-scale fluid/fluid phase separation of proteins and lipids in giant plasma membrane vesicles. Proc Natl Acad Sci U S A. 2007;104:3165–70. doi: 10.1073/pnas.0611357104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sengupta P, Baird B, Holowka D. Lipid rafts, fluid/fluid phase separation, and their relevance to plasma membrane structure and function. Semin Cell Dev Biol. 2007;18:583–90. doi: 10.1016/j.semcdb.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma P, Varma R, Sarasij RC, Ira, Gousset K, Krishnamoorthy G, Rao M, Mayor S. Nanoscale organization of multiple GPI-anchored proteins in living cell membranes. Cell. 2004;116:577–589. doi: 10.1016/s0092-8674(04)00167-9. [DOI] [PubMed] [Google Scholar]
- 35.Glebov OO, Nichols BJ. Lipid raft proteins have a random distribution during localized activation of the T-cell receptor. Nat Cell Biol. 2004;6:238–243. doi: 10.1038/ncb1103. [DOI] [PubMed] [Google Scholar]
- 36.Silvius JR, Nabi IR. Fluorescence-quenching and resonance energy transfer studies of lipid microdomains in model and biological membranes. Mol Membr Biol. 2006;23:5–16. doi: 10.1080/09687860500473002. [DOI] [PubMed] [Google Scholar]
- 37.Rao M, Mayor S. Use of Forster's resonance energy transfer microscopy to study lipid rafts. Biochim Biophys Acta. 2005;1746:221–33. doi: 10.1016/j.bbamcr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Hancock JF. Lipid rafts: contentious only from simplistic standpoints. Nat Rev Mol Cell Biol. 2006;7:456–62. doi: 10.1038/nrm1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mayor S, Rao M. Rafts: scale-dependent, active lipid organization at the cell surface. Traffic. 2004;5:231–40. doi: 10.1111/j.1600-0854.2004.00172.x. [DOI] [PubMed] [Google Scholar]
- 40.Kusumi A, Suzuki K. Toward understanding the dynamics of membrane-raft-based molecular interactions. Biochim Biophys Acta. 2005;1746:234–51. doi: 10.1016/j.bbamcr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 41.Maxfield FR. Plasma membrane microdomains. Curr Opin Cell Biol. 2002;14:483–487. doi: 10.1016/s0955-0674(02)00351-4. [DOI] [PubMed] [Google Scholar]
- 42.Kenworthy AK, Nichols BJ, Remmert CL, Hendrix GM, Kumar M, Zimmerberg J, Lippincott-Schwartz J. Dynamics of putative raft-associated proteins at the cell surface. J Cell Biol. 2004;165:735–46. doi: 10.1083/jcb.200312170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nishimura SY, Vrljic M, Klein LO, McConnell HM, Moerner WE. Cholesterol depletion induces solid-like regions in the plasma membrane. Biophys J. 2006;90:927–38. doi: 10.1529/biophysj.105.070524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kwik J, Boyle S, Fooksman D, Margolis L, Sheetz MP, Edidin M. Membrane cholesterol, lateral mobility, and the phosphatidylinositol 4,5-bisphosphate-dependent organization of cell actin. Proc Natl Acad Sci U S A. 2003;100:13964–9. doi: 10.1073/pnas.2336102100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goodwin JS, Drake KR, Remmert CL, Kenworthy AK. Ras diffusion is sensitive to plasma membrane viscosity. Biophys J. 2005;89:1398–410. doi: 10.1529/biophysj.104.055640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shvartsman DE, Gutman O, Tietz A, Henis YI. Cyclodextrins but not compactin inhibit the lateral diffusion of membrane proteins independent of cholesterol. Traffic. 2006;7:917–26. doi: 10.1111/j.1600-0854.2006.00437.x. [DOI] [PubMed] [Google Scholar]
- 47.Zidovetzki R, Levitan I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: Evidence, misconceptions and control strategies. Biochim Biophys Acta. 2007;1768:1311–24. doi: 10.1016/j.bbamem.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silvius JR. Partitioning of membrane molecules between raft and non-raft domains: insights from model-membrane studies. Biochim Biophys Acta. 2005;1746:193–202. doi: 10.1016/j.bbamcr.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 49.Meder D, Moreno MJ, Verkade P, Vaz WL, Simons K. Phase coexistence and connectivity in the apical membrane of polarized epithelial cells. Proc Natl Acad Sci U S A. 2006;103:329–34. doi: 10.1073/pnas.0509885103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vaz WL. Percolation properties of two-component, two-phase phospholipid bilayers. Mol Membr Biol. 1995;12:39–43. doi: 10.3109/09687689509038493. [DOI] [PubMed] [Google Scholar]
- 51.Wawrezinieck L, Rigneault H, Marguet D, Lenne PF. Fluorescence correlation spectroscopy diffusion laws to probe the submicron cell membrane organization. Biophys J. 2005;89:4029–42. doi: 10.1529/biophysj.105.067959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lenne PF, Wawrezinieck L, Conchonaud F, Wurtz O, Boned A, Guo XJ, Rigneault H, He HT, Marguet D. Dynamic molecular confinement in the plasma membrane by microdomains and the cytoskeleton meshwork. EMBO J. 2006;25:3245–56. doi: 10.1038/sj.emboj.7601214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wenger J, Conchonaud F, Dintinger J, Wawrezinieck L, Ebbesen TW, Rigneault H, Marguet D, Lenne PF. Diffusion analysis within single nanometric apertures reveals the ultrafine cell membrane organization. Biophys J. 2007;92:913–9. doi: 10.1529/biophysj.106.096586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He HT, Marguet D. T-cell antigen receptor triggering and lipid rafts: a matter of space and time scales. Talking Point on the involvement of lipid rafts in T-cell activation. EMBO Rep. 2008;9:525–30. doi: 10.1038/embor.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Destainville N. Theory of fluorescence correlation spectroscopy at variable observation area for two-dimensional diffusion on a meshgrid. Soft Matter. 2008;4:1288–1301. doi: 10.1039/b718583a. [DOI] [PubMed] [Google Scholar]
- 56.Umemura YM, Vrljic M, Nishimura SY, Fujiwara TK, Suzuki KG, Kusumi A. Both MHC class II and its GPI-anchored form undergo hop diffusion as observed by single-molecule tracking. Biophys J. 2008;95:435–50. doi: 10.1529/biophysj.107.123018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sako Y, Kusumi A. Barriers for lateral diffusion of transferrin receptor in the plasma membrane as characterized by receptor dragging by laser tweezers: fence versus tether. J Cell Biol. 1995;129:1559–74. doi: 10.1083/jcb.129.6.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ritchie K, Iino R, Fujiwara T, Murase K, Kusumi A. The fence and picket structure of the plasma membrane of live cells as revealed by single molecule techniques (Review) Mol Membr Biol. 2003;20:13–8. doi: 10.1080/0968768021000055698. [DOI] [PubMed] [Google Scholar]
- 59.Kusumi A, Sako Y, Yamamoto M. Confined lateral diffusion of membrane receptors as studied by single particle tracking (nanovid microscopy). Effects of calcium-induced differentiation in cultured epithelial cells. Biophys J. 1993;65:2021–40. doi: 10.1016/S0006-3495(93)81253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fujiwara T, Ritchie K, Murakoshi H, Jacobson K, Kusumi A. Phospholipids undergo hop diffusion in compartmentalized cell membrane. J Cell Biol. 2002;157:1071–1081. doi: 10.1083/jcb.200202050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Middlebrook JL, Dorland RB. Bacterial toxins: cellular mechanisms of action. Microbiol Rev. 1984;48:199–221. doi: 10.1128/mr.48.3.199-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chinnapen DJ, Chinnapen H, Saslowsky D, Lencer WI. Rafting with cholera toxin: endocytosis and trafficking from plasma membrane to ER. FEMS Microbiol Lett. 2007;266:129–37. doi: 10.1111/j.1574-6968.2006.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hagmann J, Fishman PH. Detergent extraction of cholera toxin and gangliosides from cultured cells and isolated membranes. Biochim Biophys Acta. 1982;720:181–7. doi: 10.1016/0167-4889(82)90010-6. [DOI] [PubMed] [Google Scholar]
- 64.Wolf AA, Jobling MG, Wimer-Mackin S, Ferguson-Maltzman M, Madara JL, Holmes RK, Lencer WI. Ganglioside structure dictates signal transduction by cholera toxin and association with caveolae-like membrane domains in polarized epithelia. J Cell Biol. 1998;141:917–927. doi: 10.1083/jcb.141.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bacia K, Scherfeld D, Kahya N, Schwille P. Fluorescence correlation spectroscopy relates rafts in model and native membranes. Biophys J. 2004;87:1034–43. doi: 10.1529/biophysj.104.040519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wolf AA, Jobling MG, Saslowsky DE, Kern E, Drake KR, Kenworthy AK, Holmes RK, Lencer WI. Attenuated endocytosis and toxicity of a mutant cholera toxin with decreased ability to cluster GM1. Infect Immun. 2008;76:1476–84. doi: 10.1128/IAI.01286-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pelkmans L, Burli T, Zerial M, Helenius A. Caveolin-stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic. Cell. 2004;118:767–80. doi: 10.1016/j.cell.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 68.Lingwood D, Ries J, Schwille P, Simons K. Plasma membranes are poised for activation of raft phase coalescence at physiological temperature. Proc Natl Acad Sci U S A. 2008;105:10005–10. doi: 10.1073/pnas.0804374105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lajoie P, Partridge EA, Guay G, Goetz JG, Pawling J, Lagana A, Joshi B, Dennis JW, Nabi IR. Plasma membrane domain organization regulates EGFR signaling in tumor cells. J Cell Biol. 2007;179:341–56. doi: 10.1083/jcb.200611106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hammond AT, Heberle FA, Baumgart T, Holowka D, Baird B, Feigenson GW. Crosslinking a lipid raft component triggers liquid ordered-liquid disordered phase separation in model plasma membranes. Proc Natl Acad Sci U S A. 2005;102:6320–5. doi: 10.1073/pnas.0405654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suzuki KG, Fujiwara TK, Edidin M, Kusumi A. Dynamic recruitment of phospholipase C gamma at transiently immobilized GPI-anchored receptor clusters induces IP3-Ca2+ signaling: single-molecule tracking study 2. J Cell Biol. 2007;177:731–42. doi: 10.1083/jcb.200609175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Suzuki KG, Fujiwara TK, Sanematsu F, Iino R, Edidin M, Kusumi A. GPI-anchored receptor clusters transiently recruit Lyn and G alpha for temporary cluster immobilization and Lyn activation: single-molecule tracking study 1. J Cell Biol. 2007;177:717–30. doi: 10.1083/jcb.200609174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Larson DR, Gosse JA, Holowka DA, Baird BA, Webb WW. Temporally resolved interactions between antigen-stimulated IgE receptors and Lyn kinase on living cells. J Cell Biol. 2005;171:527–36. doi: 10.1083/jcb.200503110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pelkmans L, Kartenbeck J, Helenius A. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat Cell Biol. 2001;3:473–83. doi: 10.1038/35074539. [DOI] [PubMed] [Google Scholar]
- 75.Douglass AD, Vale RD. Single-molecule microscopy reveals plasma membrane microdomains created by protein-protein networks that exclude or trap signaling molecules in T cells. Cell. 2005;121:937–50. doi: 10.1016/j.cell.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Andrews NL, Lidke KA, Pfeiffer JR, Burns AR, Wilson BS, Oliver JM, Lidke DS. Actin restricts FcepsilonRI diffusion and facilitates antigen-induced receptor immobilization. Nat Cell Biol. 2008;10:955–63. doi: 10.1038/ncb1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hess ST, Girirajan TP, Mason MD. Ultra-high resolution imaging by fluorescence photoactivation localization microscopy. Biophys J. 2006;91:4258–72. doi: 10.1529/biophysj.106.091116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Betzig E, Patterson GH, Sougrat R, Lindwasser OW, Olenych S, Bonifacino JS, Davidson MW, Lippincott-Schwartz J, Hess HF. Imaging intracellular fluorescent proteins at nanometer resolution. Science. 2006;313:1642–5. doi: 10.1126/science.1127344. [DOI] [PubMed] [Google Scholar]
- 79.Hess ST, Gould TJ, Gudheti MV, Maas SA, Mills KD, Zimmerberg J. Dynamic clustered distribution of hemagglutinin resolved at 40 nm in living cell membranes discriminates between raft theories. Proc Natl Acad Sci U S A. 2007;104:17370–5. doi: 10.1073/pnas.0708066104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Manley S, Gillette JM, Patterson GH, Shroff H, Hess HF, Betzig E, Lippincott-Schwartz J. High-density mapping of single-molecule trajectories with photoactivated localization microscopy. Nat Methods. 2008;5:155–7. doi: 10.1038/nmeth.1176. [DOI] [PubMed] [Google Scholar]
- 81.Plowman SJ, Muncke C, Parton RG, Hancock JF. H-ras, K-ras, and inner plasma membrane raft proteins operate in nanoclusters with differential dependence on the actin cytoskeleton. Proc Natl Acad Sci U S A. 2005;102:15500–5. doi: 10.1073/pnas.0504114102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nohe A, Keating E, Fivaz M, van der Goot FG, Petersen NO. Dynamics of GPI-anchored proteins on the surface of living cells. Nanomedicine. 2006;2:1–7. doi: 10.1016/j.nano.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 83.Inoue M, Digman MA, Cheng M, Breusegem SY, Halaihel N, Sorribas V, Mantulin WW, Gratton E, Barry NP, Levi M. Partitioning of NaPi cotransporter in cholesterol-, sphingomyelin-, and glycosphingolipid-enriched membrane domains modulates NaPi protein diffusion, clustering, and activity. J Biol Chem. 2004;279:49160–71. doi: 10.1074/jbc.M408942200. [DOI] [PubMed] [Google Scholar]
- 84.Caiolfa VR, Zamai M, Malengo G, Andolfo A, Madsen CD, Sutin J, Digman MA, Gratton E, Blasi F, Sidenius N. Monomer dimer dynamics and distribution of GPI-anchored uPAR are determined by cell surface protein assemblies. J Cell Biol. 2007;179:1067–82. doi: 10.1083/jcb.200702151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brown CM, Dalal RB, Hebert B, Digman MA, Horwitz AR, Gratton E. Raster image correlation spectroscopy (RICS) for measuring fast protein dynamics and concentrations with a commercial laser scanning confocal microscope. J Microsc. 2008;229:78–91. doi: 10.1111/j.1365-2818.2007.01871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dalal RB, Digman MA, Horwitz AF, Vetri V, Gratton E. Determination of particle number and brightness using a laser scanning confocal microscope operating in the analog mode. Microsc Res Tech. 2008;71:69–81. doi: 10.1002/jemt.20526. [DOI] [PubMed] [Google Scholar]
- 87.Digman MA, Dalal R, Horwitz AF, Gratton E. Mapping the number of molecules and brightness in the laser scanning microscope. Biophys J. 2008;94:2320–32. doi: 10.1529/biophysj.107.114645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Briddon SJ, Gandia J, Amaral OB, Ferre S, Lluis C, Franco R, Hill SJ, Ciruela F. Plasma membrane diffusion of g protein-coupled receptor oligomers. Biochim Biophys Acta. 2008 doi: 10.1016/j.bbamcr.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 89.Sieber JJ, Willig KI, Kutzner C, Gerding-Reimers C, Harke B, Donnert G, Rammner B, Eggeling C, Hell SW, Grubmuller H, Lang T. Anatomy and dynamics of a supramolecular membrane protein cluster. Science. 2007;317:1072–6. doi: 10.1126/science.1141727. [DOI] [PubMed] [Google Scholar]
- 90.Adkins EM, Samuvel DJ, Fog JU, Eriksen J, Jayanthi LD, Vaegter CB, Ramamoorthy S, Gether U. Membrane mobility and microdomain association of the dopamine transporter studied with fluorescence correlation spectroscopy and fluorescence recovery after photobleaching. Biochemistry. 2007;46:10484–97. doi: 10.1021/bi700429z. [DOI] [PubMed] [Google Scholar]
- 91.Crane JM, Tamm LK. Role of cholesterol in the formation and nature of lipid rafts in planar and spherical model membranes. Biophys J. 2004;86:2965–79. doi: 10.1016/S0006-3495(04)74347-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ewers H, Smith AE, Sbalzarini IF, Lilie H, Koumoutsakos P, Helenius A. Single-particle tracking of murine polyoma virus-like particles on live cells and artificial membranes. Proc Natl Acad Sci U S A. 2005;102:15110–5. doi: 10.1073/pnas.0504407102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lommerse PH, Blab GA, Cognet L, Harms GS, Snaar-Jagalska BE, Spaink HP, Schmidt T. Single-molecule imaging of the H-ras membrane-anchor reveals domains in the cytoplasmic leaflet of the cell membrane. Biophys J. 2004;86:609–16. doi: 10.1016/S0006-3495(04)74139-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Niv H, Gutman O, Kloog Y, Henis YI. Activated K-Ras and H-Ras display different interactions with saturable nonraft sites at the surface of live cells. J Cell Biol. 2002;157:865–872. doi: 10.1083/jcb.200202009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pucadyil TJ, Chattopadhyay A. Effect of cholesterol on lateral diffusion of fluorescent lipid probes in native hippocampal membranes. Chem Phys Lipids. 2006;143:11–21. doi: 10.1016/j.chemphyslip.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 96.Pucadyil TJ, Chattopadhyay A. Cholesterol depletion induces dynamic confinement of the G-protein coupled serotonin(1A) receptor in the plasma membrane of living cells. Biochim Biophys Acta. 2007;1768:655–68. doi: 10.1016/j.bbamem.2007.01.002. [DOI] [PubMed] [Google Scholar]