Abstract
The objectives of this study were to determine how tensile stimulation delivered up to 14 days in culture influenced type I collagen gene expression in stem cells cultured in collagen sponges, and to establish if gene expression, measured using a fluorescence method, correlates with an established method, real-time quantitative reverse transcriptase polymerase chain reaction (qRT-PCR). Using a novel model system, mesenchymal stem cells were harvested from six double transgenic mice in which the type I and type II collagen promoters were linked to green fluorescent protein-topaz and enhanced cyan fluorescent protein, respectively. Tissue-engineered constructs were created by seeding 0.5 × 106 mesenchymal stem cells onto type I collagen sponge scaffolds in a silicone dish. Constructs were then transferred to a custom pneumatic mechanical stimulation system housed in a standard incubator and stimulated for 5 h/day in tension for either 7 or 14 days using a repeated profile (2.4% peak strain for 20 s at 1 Hz followed by a rest period at 0% strain for 100 s). Control specimens were exposed to identical culture conditions but without mechanical stimulation. At three time points (0, 7, and 14 days), constructs were then prepared for evaluation of gene expression using fluorescence analysis and qRT-PCR, and the remaining constructs were failed in tension. Both analytical methods showed that constructs stimulated for 7 and 14 days showed significantly higher collagen type I gene expression than nonstimulated controls at the same time interval. Gene expression measured using qRT-PCR and fluorescence analysis was positively correlated (r = 0.9). Linear stiffness of stimulated constructs was significantly higher at both 7 and 14 days than that of nonstimulated controls at the same time intervals. Linear stiffness of the stimulated constructs at day 14 was significantly different from that of day 7. Future studies will vary the mechanical signal to optimize type I collagen gene expression to improve construct biomechanics and in vivo tendon repair.
Introduction
Injuries to soft tissues (tendon, ligament, and meniscus) represent almost half of the 33 million musculoskeletal injuries occurring in the United States each year1–6 and often lead to surgery.7,8 Inadequate healing of these injuries places patients at risk to dysfunction and disability. Tissue engineering9 is an appealing conceptual alternative when conventional repair techniques (autografts, allografts, xenografts, and prostheses10–18) prove unsatisfactory.
Tissue-engineered constructs made by seeding mesenchymal stem cells (MSCs) in scaffolds are being used to repair soft tissue defects,19–24 but these are usually vulnerable early after surgery because they lack the stiffness and strength of native tissue structures.22,24 To address this problem, investigators have been applying principles of functional tissue engineering25,26 to use recorded in vivo tissue forces27,28 as design parameters for new generations of reparative tissue constructs. Some in functional tissue engineering25,26 have also delivered aspects of these in vivo mechanical signals to precondition constructs while still in culture. Such preconditioning improves the material properties of constructs for soft tissue engineering by increasing the synthesis of extracellular matrix proteins such as collagen.29–32
Unfortunately, any mechanical and biological benefits of mechanical stimulation are usually not assessed until the end of mechanical stimulation in culture.23,33,34 Typically, 4 or more weeks may be required before a destructive test is performed to judge whether a stimulus upregulates gene expression or increases protein accumulation. This delay hampers the ability of the investigator to quickly optimize the stimulus in culture. A strategy that permits investigators to monitor near real-time gene expression throughout the tissue engineering process could allow them to either modify the stimulus or terminate an experiment leading to an undesirable outcome. Unfortunately, no such method currently exists for tissue engineers to rapidly assess how mechanical (chemical, etc.) stimuli affect near real-time gene expression.
To address this need, we bred double transgenic mice having type I and type II collagen promoters linked to green fluorescent protein-topaz (GFP-T) and enhanced cyan fluorescent proteins (ECFP), respectively. These intracellular proteins are expressed when the type I and type II collagen genes are activated, respectively. Such fluorescent proteins have recently served as visual reporters for transgene activity and can be viewed in real-time in living tissues.35–39 Investigators have used these promoter-fluorescent protein reporters to (1) examine early embryonic development, (2) conduct cell culture studies, and (3) identify cells within a defined lineage in primary cell culture.35–39 Another value of this technology, when applied to tissue engineering, could be the ability to track how mechanical stimulation affects near real-time collagen gene expression during maturation of constructs in culture.
The first objective of this study was to determine how a controlled mechanical stimulus applied to a stem cell–collagen sponge construct in culture influences the expression of the type I collagen gene as well as linear stiffness. The second objective was to establish if gene expression, measured using a fluorescence method, correlates with an established method, real-time quantitative reverse transcriptase polymerase chain reaction (qRT-PCR). We hypothesized that (1) mechanical stimulation would increase type I collagen gene expression as well as linear stiffness and (2) the fluorescence data and qRT-PCR data would be positively correlated.
Experimental Design
Bone marrow was harvested from both the femur and tibia of six 6–8-week-old double transgenic mice as previously described.40,41 After isolating and expanding the MSCs to second or third passage using a previously published protocol,40 15 constructs were created from each animal by seeding the cells at a concentration of 0.5 × 106 cells/construct in a type I collagen sponge (P1076; Kensey Nash Corporation, Exton, PA). Hence, a total of 90 samples were created using cells from six animals. For each animal, the resulting 15 constructs were assigned to five treatment groups: day 0 nonstimulated, day 7 stimulated, day 7 nonstimulated, day 14 stimulated, and day 14 nonstimulated (Table 1). Thus, three constructs were available per animal from each treatment group. One construct was assigned to evaluate GFP-T fluorescence in a spectrophotometer (measured in relative fluorescence units [RFU]),42 one construct was assigned to evaluate changes in type I collagen and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene expression using real-time qRT-PCR,43 and the remaining construct was failed in tension to determine its linear stiffness.44 Stimulated (S) constructs were stretched between 0% and 2.4% peak strain27 at 1 Hz for 20 s followed by a 100-s rest period. This pattern was repeated for 5 h/day. Peak amplitude, frequency, and duty cycle were chosen based on studies performed in our lab on the effects of tensile stimulation on rabbit MSCs seeded in collagen scaffolds.43,44 The nonstimulated (NS) constructs served as controls. The sample size of six animals was sufficient to detect a 30% treatment effect with an 80% power. Differences were considered significant for p < 0.05.
Table 1.
Treatment Conditions, Response Measures, and Assignment of Constructs
| Treatment conditions | Constructs/treatment condition/animal | Response measures (assignment of constructs) |
|---|---|---|
| Day 0 nonstimulated | 3 | Gene expression by qRT-PCR (n = 1) |
| Gene expression by RFUs (n = 1) | ||
| Linear stiffness (n = 1) | ||
| Day 7 stimulated | 3 | Gene expression by qRT-PCR (n = 1) |
| Gene expression by RFUs (n = 1) | ||
| Linear stiffness (n = 1) | ||
| Day 7 nonstimulated | 3 | Gene expression by qRT-PCR (n = 1) |
| Gene expression by RFUs (n = 1) | ||
| Linear stiffness (n = 1) | ||
| Day 14 stimulated | 3 | Gene expression by qRT-PCR (n = 1) |
| Gene expression by RFUs (n = 1) | ||
| Linear stiffness (n = 1) | ||
| Day 14 nonstimulated | 3 | Gene expression by qRT-PCR (n = 1) |
| Gene expression by RFUs (n = 1) | ||
| Linear stiffness (n = 1) |
Materials
Double transgenic mice
The mouse containing the transgene pOBCol3.6GFPtpz was acquired courtesy of David Rowe, University of Connecticut Health Center. This transgene contains a 3.6 kb fragment of the rat col1a1 promoter, enhancer sequence, and GFP-T. GFP-T expression in these transgenic mice is evident in skin, tendon, and osseous tissues.37
Plasmid pCol2-ECFP was derived by replacing the -gal gene with ECFP (Clontech, Palo Alto, CA), in the expression gene containing the mouse type II collagen promoter and enhancer45 (provided by W. Horton, Northeastern Ohio College of Medicine). This pCol2-ECFP construct was injected into mice blastocysts. Founder mice showed high levels of ECFP expression in cartilaginous tissue.
Mice transgenic for either pOBCol3.6GFPtpz or pCol2-ECFP were then bred to produce double transgenic animals. No apparent phenotypic differences were observed between the double and nontransgenic mice. Both GFP-T and ECFP have a 24-h half-life, which indicates that fluorescence decays to half of its original by 24 h.
Mesenchymal stem cells
Mice were euthanized by CO2 according to Institutional Animal Care and Use Committee (IACUC) protocols. Their long bones were excised, transected, and placed in adapted centrifuge tubes.40 Marrow was extracted by centrifugation for 1 min at 400 g. Extracted cells were plated at 7.5 × 106 cells/100 mm dishes and fed media with supplements (MesenCult™ Basal Medium for Mouse Mesenchymal Stem Cells and Mouse Mesenchymal Stem Cell Stimulatory Supplements; Stem Cell Technologies, Vancouver, Canada). Cells were allowed to attach for 2 days, washed with phosphate-buffered saline (PBS; Gibco BRL/Life Technologies, Gaithersburg, MD) to remove nonadherent cells, and fed fresh media. Adherent cells were allowed to grow for 7–10 days before first passage. Cells were then trypsinized and replated at 1 × 106 cells/100 mm dishes and cultured for 1 additional week. Cells at P2 and P3 were then subcultured at a density of 1 × 106 cells/100 mm dish and cultured for another week.
Scaffold and construct preparation
Sterilized collagen type I sponges (94% pore volume; 62 μm mean pore diameter) were provided by Kensey Nash Corporation. Scaffolds were cut from these sponges such that they fit in the wells of a silicone dish.44 Before seeding with cells, each scaffold was soaked overnight in PBS and then placed in each well of the dish. MSCs were suspended in media at a concentration of 2 × 106 cells/mL. Two hundred fifty microliters of this cell suspension was pipetted on top of each scaffold. All constructs were placed in an incubator (Steri-Cult Model 3033; Forma Scientific, Marietta, OH; 37°C, 5% CO2, and 95% relative humidity) for 2 weeks and fed three times weekly with advanced DMEM, 1% antibiotic/antimycotic, 1% glutamax, and 10% FBS.
Methods
Mechanical stimulation
After 2 days of incubation, the silicone dishes containing the constructs to be stimulated were placed into a computer-controlled five-station pneumatic mechanical stimulation system housed within an incubator (Steri-Cult Model 3033; Forma Scientific)44 and stretched using the pattern described in Experimental Design section. Dishes were removed from the incubator after either 7 or 14 days, and constructs were then prepared for either evaluation of gene expression using fluorescence analysis and qRT-PCR or failure testing in tension.
Fluorescence microscopy
Each construct was washed in PBS for 1 h with gentle shaking to remove media and then visualized for GFP-T fluorescence in a fluorescence microscope (Axiovert 25; Carl Zeiss, Göttingen, Germany) equipped with filter sets for visualizing GFP-T and ECFP (XF104-2 and XF114-2, respectively; Omega, Brattleboro, VT). To rule out cell auto-fluorescence, each construct was also visualized for rhodamine using a specific filter set (11002VZ; Chroma, Rockingham, VT).
Spectrophotometric analysis
After imaging, constructs were digested for 40 min in 4 mL of 100 U/mL type I collagenase (Sigma Chemical, St. Louis, MO) in an incubator with gentle shaking. The resulting collagen fragments were further digested for 20 min in 2 mL of trypsin (Invitrogen–Gibco BRL/Life Technologies, Gaithersburg, MD). Digests were then centrifuged at 2000 rpm for 6 min, and the supernatant was discarded. The remaining pellets were re-suspended in 1 mL of PBS and filtered using a 100 μm nylon mesh (BD Falcon, Bedford, MA). These digests were then pipetted into a black-bottom microplate (200 μL per well in three wells). GFP-T fluorescence in these digests was quantified in RFUs42 by reading the microplate in a spectrophotometer (Spectra Max M2; Molecular Devices, Sunnyvale, CA) using an excitation wavelength of 491 nm and an emission wavelength of 529 nm, with a cut off filter of 519 nm. Pilot studies on GFP-T expressing fibroblasts harvested from the ribs of new born mice did not show any qualitative differences in GFP-T fluorescence when subjected to the above-mentioned collagenase, trypsin digestion and filtration steps.
RNA extraction and conventional gene expression analysis
RNA extraction and conventional gene expression analysis were performed according to previously published protocols.43 Briefly, the constructs were stored in RNAlater (Qiagen, Valencia, CA) for 2 days at −4°C. The RNAlater was aspirated carefully, and the constructs were frozen in liquid nitrogen to prevent RNA degradation. RNA from each construct was extracted using an RNeasy mini kit (Qiagen). Conventional RT reaction (MuLV reverse transcriptase; Applied Biosystems, Foster City, CA) was performed43 to create first-strand complementary deoxyribonucleic acid (cDNA). Mouse-specific primers were used for type I collagen and GAPDH gene expression. The forward and reverse primer sequences and the resultant products are summarized in Table 2. Before use in the experiment, all primers were tested under conventional and real-time qRT-PCR conditions to ensure specificity with only one band by electrophoresis. The conventional PCR of the reverse-transcribed RNA was performed according to previously published protocols.43 The amplified products were verified by 2% agarose gel electrophoresis in Tris-acetate-EDTA and SYBR safe DNA gel stain (Invitrogen–Molecular Probes, Eugene, OR).
Table 2.
Sequence of Primers Used for Gene Expression Analysis and Product Size in Base Pairs
| Gene | Primer sequence | Product size (bp) | Annealing temperature (°C) |
|---|---|---|---|
| Collagen I | TGT GTG CGA TGA CGT GCA AT GGG TCC CTC GAC TCC TAC A | 132 | 58 |
| GAPDH | AAT GGT GAA GGT CGG TGT G CCT TCG GGT AGT GGT AGA AG | 200 | 55 |
Real-time qRT-PCR
Real-time qRT-PCR was performed to quantify mRNA levels of the genes according to previously published protocols.43 qRT-PCR was performed by monitoring SYBR Green fluorescent dye (SYBR Green PCR master mix; Applied Biosystems) bound to double-strand DNA with a continuous fluorescence detector (DNA Engine Opticon 2 System; MJ Research Incorporated, Waltham, MA). All samples were run in duplicate since differences in cycle number values between samples were less than 0.3 cycles. Standard curves were created for each target gene to quantify gene expression for each cell–sponge construct. The absolute amount of the corresponding gene mRNA in each construct was obtained from the corresponding gene standard curve. Gene expression was normalized by calculating the ratio between type I collagen and GAPDH genes for each sample.
Biomechanical evaluation of the constructs
After 0, 7, or 14 days in culture, constructs for biomechanical analysis were placed in cryovials and frozen at −80°C. On the day of testing, constructs were removed from the freezer and thawed to room temperature. Small squares of gauze were used to cover the postholes in each end of the constructs. These gauzes provided a surface that would minimize slippage and the premature failure of the specimens in the grips. Constructs were inserted into custom grips in a materials testing system (100R6; Testresources, Shakopee, MN) and fixed at a gauge length of 12 mm to minimize Saint Venant's gripping effects on specimen properties.46 The specimens were then failed under displacement control at a rate of 10%/s in a PBS bath at room temperature.20 Linear stiffness was calculated from the linear region of the force–elongation curve generated by the constructs during failure testing.
Statistical analysis
The duplicate gene expression measures and triplicate RFU measures for each cell line from each of the six mice were averaged before statistical analysis. Gene expression and mechanical properties of the stimulated versus nonstimulated stem cell–collagen sponge constructs were compared using a mixed-effects model (SAS proc mixed) with culture time and stimulation as fixed factors and animal as a random factor.47 All conclusions regarding the significance of mechanical stimulation on gene expression and mechanical properties were made for p < 0.05.
Results
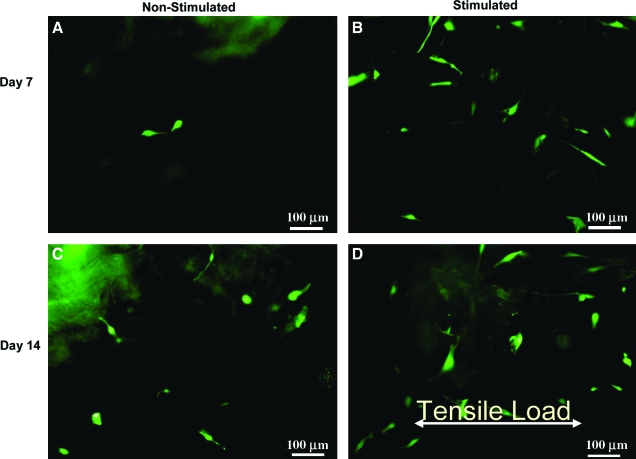
Cells did not fluoresce in any of the constructs at day 0. However, GFP-T fluorescent cells were found in all 7- and 14-day constructs. No fluorescence signal was evident when using the ECFP and rhodamine filter sets, indicating the specificity of the GFP-T signal and the lack of detectable levels of type II collagen gene expression, even in the end regions where the construct attaches to the posts. The fluorescing cells appeared elongated in all constructs at both time periods of stimulation (Fig. 1A–D). Elongated cells in both the controls (Fig. 1A, C) and the S constructs (Fig. 1B, D) were randomly oriented with respect to the direction of applied tensile strain.
Fig. 1.
Elongated GFP-T fluorescent cells in (A) nonstimulated constructs and (B) stimulated constructs at day 7 as well as (C) nonstimulated constructs and (D) stimulated constructs at day 14. Original magnification, × 10. Greater fluorescence was observed in the stimulated versus nonstimulated control constructs at 7 days. Color images available online at www.liebertonline.com/ten.
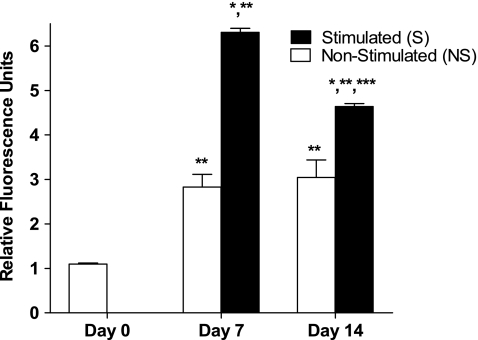
Tensile stimulation significantly increased measured RFU values at both time points. At day 7, RFU values in the S constructs increased by 2.2-fold compared to those for the NS constructs (p = 0.0002) (Fig. 2). At day 14, RFU values in the S constructs increased by 1.5-fold compared to those for the NS constructs (p = 0.01) (Fig. 2). RFU values for the S versus NS construct groups averaged 6.3 ± 0.22 versus 2.8 ± 0.69 at day 7 and 4.63 ± 0.16 versus 3.04 ± 0.97 at day 14 (mean ± SD). No difference was seen between NS constructs at days 7 and 14 (p > 0.5) (Fig. 2). There was a significant decrease in RFUs in S constructs between days 7 and 14 (p = 0.0001) (Fig. 2).
Fig. 2.
Tensile stimulation increased GFP-T expression by RFUs. *Significantly different from nonstimulated (NS) controls at same time interval (p < 0.02). **Significantly different from day 0 (p < 0.00001). ***Significantly different from day 7 stimulated (S) constructs (p < 0.0002). Data represented as mean ± standard deviations; n = 6 for all groups.
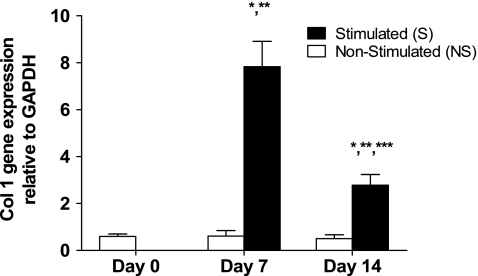
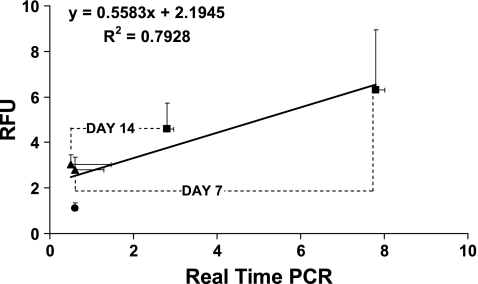
Seven and 14 days of in vitro mechanical stimulation significantly increased type I collagen gene expression in stem cell–collagen sponge constructs measured using qRT-PCR. S constructs showed a 12-fold increase in type I collagen gene expression (p = 0.001) relative to NS controls at day 7 and a 5-fold increase at day 14 (p = 0.01) (Fig. 3). Type I collagen gene expression as measured by qRT-PCR for the S versus NS construct groups averaged 7.8 ± 2.6 versus 0.6 ± 0.5 at day 7 and 2.8 ± 1.1 versus 0.5 ± 0.4 at day 14 (mean ± SD). No difference was seen between NS constructs at days 7 and 14 (p > 0.5) (Fig. 3), but there was a significant decrease in type I collagen gene expression in the S constructs between days 7 and 14 (p = 0.005) (Fig. 3). Mechanical stimulation did not significantly increase GAPDH gene expression (p > 0.5) at day 7 or 14 in culture. GAPDH values for the S versus NS construct groups averaged 5.8 E-07 ± 1.7 E-07 versus 5.6 E-07 ± 1.3 E-07 at day 7 and 5.4 E-07 ± 1.4 E-07 versus 5.3 E-07 ± 1.2 E-07 at day 14 (mean ± SD). The GAPDH values for day 0 constructs were 5.8 E-07 ± 1.7 E-07. The results from qRT-PCR and fluorescence analysis were positively correlated (R2 = 0.79; Fig. 4). The slope of the linear regression curve was 0.56.
Fig. 3.
Tensile stimulation increased type I collagen gene expression by qRT-PCR. *Significantly different from nonstimulated (NS) controls at same time interval (p < 0.02). **Significantly different from day 0 (p < 0.0003). ***Significantly different from day 7 stimulated (S) constructs (p < 0.006). Data represented as means ± standard deviations; n = 6 for all groups.
Fig. 4.
Type I collagen gene expression as measured by RFUs and by qRT-PCR were positively correlated (R2 = 0.79). Each point represents the average of six samples (means ±standard deviations). Circle denotes day-0 constructs, triangles denote nonstimulated constructs, and squares denote stimulated constructs.
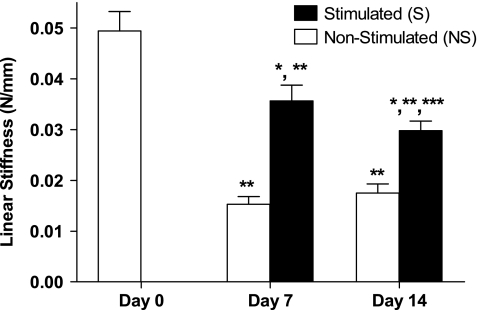
Both 7 and 14 days of tensile stimulation increased the construct's linear stiffness compared to NS constructs, but had no effect on dimensions (p > 0.05; Table 3). S constructs showed a 2.3-fold increase in linear stiffness (p = 0.0006) relative to NS controls at day 7 and a 1.6-fold increase at day 14 (p = 0.003) (Fig. 5). Linear stiffness for the S versus NS construct groups averaged 0.035 ± 0.006 N/mm versus 0.015 ±0.003 N/mm at day 7 and 0.029 ± 0.004 N/mm versus 0.0175 ±0.4 N/mm at day 14 (mean ± SD). No difference was seen between NS constructs at days 7 and 14 (p = 0.1) (Fig. 5). Tensile stiffness values of both 7- and 14-day constructs were significantly less than the corresponding stiffness values of day 0 constructs (p = 0.005) (Fig. 5).
Table 3.
Tensile Stimulation Did Not Significantly Affect Construct Dimensions (p > 0.05)
| Evaluation time points | Length (mm) | Width (mm) | Thickness (mm) |
|---|---|---|---|
| Day 0 | 23.2 ± 0.5 | 8.5 ± 0.7 | 2.7 ± 0.1 |
| Day 7 stimulated | 23.8 ± 0.4 | 8.3 ± 0.5 | 2.6 ± 0.4 |
| Day 7 nonstimulated | 23.1 ± 0.6 | 8.1 ± 0.5 | 2.5 ± 0.5 |
| Day 14 stimulated | 23.6 ± 0.3 | 8.0 ± 0.6 | 2.5 ± 0.5 |
| Day 14 nonstimulated | 23.2 ± 0.4 | 8.1 ± 0.9 | 2.4 ± 0.5 |
Shown are means ± standard deviations for all three parameters.
Fig. 5.
Tensile stimulation increased linear stiffness. *Significantly different from nonstimulated controls at same time interval (p < 0.004). **Significantly different from day 0 (p < 0.0005). ***Significantly different from day 7 stimulated (p < 0.05). Data represented as means ± standard deviations; n = 6 for all groups.
Discussion
Understanding how to control matrix production and assembly during culture is one of the primary objectives in developing functional tissue-engineered replacements for load-bearing connective tissues.25 Although externally applied deformations have been shown to strengthen tissue-engineered constructs,29–32,48 long culture times are typically required for cells to synthesize enough matrix to improve resulting mechanical properties that can remotely approach the levels of the native tissue. This slow process could reflect the fact that mechanical stimuli have yet to be optimized for inducing such cellular-based benefits. In particular, we still know little about how various components of the stimulation profile (e.g., strain amplitude, frequency, and duty cycle) affect construct quality and functionality in culture and after surgery. We do know, however, that determining the importance of each component can be a very expensive and time-consuming process. Therefore, a strategy to evaluate how these factors influence the production of extracellular matrix components like type I (tendon and ligament midsubstance) or type II collagen (soft tissue insertions, articular cartilage, meniscus, and intervertebral disc) would appear to be a very beneficial step in this development effort. It was for these reasons that we bred the double transgenic mouse model for the current study. By using this fluorescence technology, we could repeatedly track changes in gene expression during rest periods between bouts of mechanical stimulation in culture rather than use destructive microarray or qRT-PCR techniques that could only detect final changes after stimulation. Also, this technology provides a unique way to detect near real-time changes in expression in culture by one or both of these genes and to readjust the mechanical signal applied to the evolving construct to obtain the desired spatial and temporal gene expression patterns.
This study utilizing MSCs harvested from these mice was designed to evaluate the effects of uniaxial tension on gene expression and biomechanics in cell–collagen constructs. We hypothesized that (1) mechanical stimulation would increase type I collagen gene expression as well as linear stiffness and (2) the fluorescence data and qRT-PCR data would be positively correlated. In our study, we found that tensile stimulation increased GFP-T fluorescence and type I collagen gene expression compared to controls, thus validating the first part of our hypothesis. The increases in both measures (Figs. 2 and 3) that we observed in S constructs reinforce the importance of stimulating a cell–scaffold construct with a defined mechanical signal in a cell culture system. The fact that the tensile stimulus chosen in this study significantly increased GFP-T fluorescence in the construct's cells without affecting ECFP activity suggests that tensile stimulation did not affect collagen type II gene expression.
Several reasons could explain the increases in GFP-T fluorescence (type I collagen gene expression) we observed after 7 and 14 days of tensile stimulation. Tensile strains are known to trigger the creation of cell–surface stretch receptors and integrins, activating a cascade of genes responsible for the synthesis and secretion of extracellular matrix components.49 Of course, the stimulus we applied may have also produced other cellular changes (cell proliferation, mass transfer rates of nutrients, metabolites, and waste materials,50 and expression and synthesis of various growth factors and cytokines), some of which are known to be sensitive to mechanical stimulation.51–54 Several of these factors will be examined in greater detail now that we have demonstrated a significant positive effect of mechanical stimulation on gene expression in this model system.
Our findings are generally consistent with previous reports examining how tensile stimulation affects gene expression of cells and cell-based constructs, although the specific treatments, conditions, and response measures vary greatly among studies. Investigators using human fibroblasts32,55,56 as well as human mesenchymal23,57 and human and bovine stromal cells50 have demonstrated positive effects of mechanical stimulation on type I collagen gene expression and protein synthesis. However, these studies were conducted either using cells on monolayer57 or after placing cells in three-dimensional polyurethanes32 and collagen gels.23,50 These differences in local matrix environment can be important to driving cell phenotype and gene expression patterns. The 2.4% peak strain that we delivered in the current study is far less than the 5% and 10%32,55–57 peak strain treatments used by other groups and not as complex as reported biaxial strain patterns.50 While the studies by Park et al.57 and Noth et al.23 matched the frequency (1 Hz) delivered in our study and the 14-day treatment assessment23,50 was identical to our time interval, other aspects of these studies differed markedly. In fact, direct comparison of our results to those used by other investigators was difficult given the broad range of frequencies (0.0167 Hz,50 0.25 Hz,55 and 0.167 Hz56) and time assessment periods (from 6 to 24 h)32,55–57 reported in the literature. Studies will ultimately need to either limit these stimulation conditions across studies or examine the interactive effects of these treatment components if we are to understand how to control and optimize gene and protein expression in tissue engineering.
Our findings are also different from a previous study in the literature.50 Although Altman et al.50 examined mechanically induced changes in gene expression using stem cells over the same time interval used in the current study, they used collagen gels rather than sponges, and found that tensile stimulation significantly increased type I collagen gene expression at day 14 but not at day 7. Such differences between our two outcomes could be due to different cell sources (bovine vs. murine) or mechanical strain patterns (combined axial and torsional strains vs. uniaxial tensile strains), although other differences may have also contributed. For example, a cell's biosynthetic activity is strongly related to its ability to attach to its collagen scaffold.58–63 Once accomplished, subsequent collagen synthesis within these three-dimensional collagen lattices is then regulated by transcriptional and posttranscriptional mechanisms mediated by α and β integrins.61 Similarly, synthetic scaffolds can also provide a conducive environment, as shown by Chastain et al.,64 who found increases in α6 and β6 gene expression over a 5-week culture period using MSCs deposited in PLGA scaffolds. Regardless of scaffold type, increases in type I collagen gene expression will clearly require the appropriate signals and microenvironment for effective cell-based therapy.
We also found in this study that collagen type I gene expression measured using real-time qRT-PCR and fluorescence analysis were positively correlated (Fig. 4), thus validating our second hypothesis. This correlation serves as a validation for using fluorescence as an indicator of average gene expression within tissue-engineered constructs. Additional correlations of this type may soon allow investigators to use fluorescence as a reasonable estimate of expression of genes like type II collagen than using more traditional approaches like qRT-PCR. These correlations may also be possible at even finer levels in the tissue-engineered constructs, including expression by groups of cells or even individual cells in the future.
We also found that applying both 7 and 14 days of tensile stimulation increased the construct's linear stiffness compared to NS constructs in culture (Fig. 5) validating the last part of our first hypothesis. Other investigators have noted positive effects of stimulating collagen-based constructs on biomechanics as well as gene and protein expression using different cell types (tendon fibroblasts,65 smooth muscle cells,53 ligament fibroblasts,66 and MSCs50,57) and methods of stimulation.50,53,55,57,65,66 Our results are also similar to another study in our laboratory where mechanical stimulation of rabbit MSCs in collagen sponge constructs not only increased construct stiffness but also produced increases in type I and III collagen mRNA expression using qRT-PCR.43
Day-7 and day-14 constructs showed significantly lower stiffness than day-0 constructs (Fig. 5). It has been shown that when chondrocytes67 and fibroblasts68 are seeded in collagen-based constructs, they produce matrix metallo proteinase (MMPs) that degrade the collagen scaffold. It is plausible that in our study the mouse MSCs also produced MMPs that degraded the collagen scaffold and, in turn, decreased its stiffness. Such decreases in stiffness are likely offset by tensile stimulation. In the future, we plan also to monitor MMP production in our stimulated and control constructs.
The stiffness of our constructs is still orders of magnitude less than that of that of native tendons44 and hence cannot currently replace the entire ruptured tendon. Instead, we have been using these constructs for biological augmentation in a load-protected regime to repair central defect injuries to the rabbit patellar tendon. Implanting such stimulated cell–scaffolds in vivo can speed up the repair process as the cells are already acclimatized in vitro to some of the deformations that they might experience in vivo. Further, these cells are already synthesizing type I collagen in vitro and might fill the repair site much faster. We have shown that such compliant constructs produce rabbit patellar tendon repairs whose average stiffness is three orders of magnitude greater than the stiffness of initial constructs at surgery and capable of matching the stiffness of normal patellar tendon up to 150% of peak in vivo force values.44
The results obtained from this study have the potential to be translated to higher species such as humans. If we can demonstrate through in vitro studies that murine cell-based constructs respond to mechanical stimuli (as well as chemical stimuli) in ways similar to rabbit cell-based constructs by a range of response measures, it is then our hope to be able to apply the results of genetically based experiments in the mouse to new therapies in larger animals where such tools do not yet exist. The benefits that we formulate and test in lower species and then validate in larger preclinical models can then be translated to improved repair in humans.
Our study has limitations. (1) In this study we recorded nondestructively the average effects of uniaxial tension on GFP-T expression in MSCs. We are currently examining techniques to measure fluorescence without destroying the constructs. (2) We did not quantify the number of fluorescing cells. This prevented us from distinguishing whether the increases in fluorescence were due to increased numbers of fluorescing cells and/or increased production of GFP-T by already fluorescing cells. Determining which of these effects predominates will require more local recordings of gene expression at the individual cell level combined with in situ hybridization. Fluorescing cells could also be counted in a nondestructive way by applying statistical methods if we had the optical systems that could scan through the entire depth of the opaque constructs. (3) We selected each fluorescent protein to have a 24-h half-life, thus providing near, rather than actual, real-time gene expression. While it would have been ideal to have instantaneous feedback of gene upregulation much like a conventional transducer, the longer period ensured that we could detect measurable changes in fluorescence before signal dissipation. (4) Cell viability within the constructs was not determined. Diminished cell viability in individual constructs could have adversely affected our RFU results for GFP-T fluorescence and thus increased interspecimen variability. (5) This method of fluorescence analysis is limited to the specific transgenic species described with two modified fluorescent proteins. (6) We chose to measure only the construct's linear stiffness because we were concerned that the lower aspect ratio of 2:1 (less than the ideal ratio of 3:1 to avoid St. Venant effects) might alter the structure's failure properties. (7) These constructs can only be used to repair tendons in a load-protected regime. We are currently investigating ways to further improve the stiffness of our constructs.
In conclusion, this study has demonstrated that short-term tensile stimulation increases type I collagen gene expression and linear stiffness of murine stem cell–collagen sponge constructs, and that two measures used to track changes in type I collagen gene expression are correlated. Future studies will focus on optimizing the effects of these in vitro signal components on expression of the type I collagen gene and the degree to which they affect construct and then repair biomechanics. Given that construct stiffness is positively correlated with repair stiffness after implanting rabbit autologous MSC–collagen constructs in patellar tendon defects,44 our efforts to optimize stiffness in culture might also positively impact tendon repair biomechanics and gene expression across multiple species and injury scenarios. We also envision that once optical technologies have been developed to visualize fluorescence in opaque constructs in a bioreactor at magnifications consistent with light microscopy, we and others should be capable of tracking real-time, local changes in gene fluorescence. Ultimately, we hope to speed tendon repair and match the tangent stiffness of normal tendons to levels well above peak in vivo force levels.44
Acknowledgments
This study was supported in part by NIH Grants EB002361-02 from NIBIB and AR46574-06 from NIAMS.
Disclosure Statement
No competing financial interests exist.
References
- 1.Huang H.H. Qureshi A.A. Biundo J.J., Jr. Sports and other soft tissue injuries, tendinitis, bursitis, and occupation-related syndromes. Curr Opin Rheumatol. 2000;12:150. doi: 10.1097/00002281-200003000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Liu S. Nguyen T. Ankle sprains and other soft tissue injuries. Curr Opin Rheumatol. 1999;11:132. doi: 10.1097/00002281-199903000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Bennett W.F. Arthroscopic repair of anterosuperior (supraspinatus/subscapularis) rotator cuff tears: a prospective cohort with 2- to 4-year follow-up. Classification of biceps subluxation/instability. Arthroscopy. 2003;19:21. doi: 10.1053/jars.2003.50023. [DOI] [PubMed] [Google Scholar]
- 4.Praemer A. Furner S. Rice D.P. Parke Ridge, IL: American Academy of Orthopaedic Surgeons; 1999. Musculoskeletal Condition in the United States. [Google Scholar]
- 5.Bey M.J. Ramsey M.L. Soslowsky L.J. Intratendinous strain fields of the supraspinatus tendon: effect of a surgically created articular-surface rotator cuff tear. J Shoulder Elbow Surg. 2002;11:562. doi: 10.1067/mse.2002.126767. [DOI] [PubMed] [Google Scholar]
- 6.Soslowsky L.J. Thomopoulos S. Tun S. Flanagan C.L. Keefer C.C. Mastaw J. Carpenter J.E. Neer Award 1999. Overuse activity injures the supraspinatus tendon in an animal model: a histologic and biomechanical study. J Shoulder Elbow Surg. 2000;9:79. [PubMed] [Google Scholar]
- 7.Albright J.C. Carpenter J.E. Graf B.K. Richmond J.C. Knee and leg: soft-tissue trauma. Orthop Knowledge Update. 1999;20:281. [Google Scholar]
- 8.McNicholas M. Rowley D. McGurty D. Adalberth T. Abdon P. Lindstrand A. Lohmander L. Total meniscectomy in adolescence. A thirty-year follow-up. J Bone Joint Surg Br. 2000;82:217. [PubMed] [Google Scholar]
- 9.Langer R. Vacanti J.P. Tissue engineering. Science. 1993;260:920. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 10.Richmond J.C. Manseau C.J. Patz R. McConville O. Anterior cruciate reconstruction using a Dacron ligament prosthesis: a long-term study. Am J Sports Med. 1992;20:24. doi: 10.1177/036354659202000107. [DOI] [PubMed] [Google Scholar]
- 11.Moyen B.J.L. Jenny J.-Y. Mandrino A.H. Lerat J.-L. Comparison of reconstruction of the anterior cruciate ligament with and without a Kennedy ligament-augmentation device. A randomized, prospective study. J Bone Joint Surg A. 1992;74:1313. [PubMed] [Google Scholar]
- 12.Amiel D. Billings E.J. Akeson W.H. Ligament structure, chemistry and physiology. In: Daniel D., editor; Akeson W., editor; O'Connor J., editor. Knee Ligaments: Structure, Function, Injury and Repair. New York: Raven Press; 1990. p. 77. [Google Scholar]
- 13.Jorgensen U. Bak K. Ekstrand J. Scavenius M. Reconstruction of the anterior cruciate ligament with the iliotibial band autograft in patients with chronic knee instability. Knee Surg Sports Traumatol Arthrosc. 2001;9:137. doi: 10.1007/s001670000163. [DOI] [PubMed] [Google Scholar]
- 14.Steenbrugge F. Verdonk R. Vorlat P. Mortier F. Verstraete K. Repair of chronic ruptures of the anterior cruciate ligament using allograft reconstruction and a ligament augmentation device. Acta Orthop Belg. 2001;67:252. [PubMed] [Google Scholar]
- 15.Stone K.R. Meniscus replacement. Clin Sports Med. 1996;15:557. [PubMed] [Google Scholar]
- 16.Bruns J. Kahrs J. Kampen J. Behrens P. Plitz W. Autologous perichondral tissue for meniscal replacement. J Bone Joint Surg Br. 1998;80B:918. doi: 10.1302/0301-620x.80b5.8023. [DOI] [PubMed] [Google Scholar]
- 17.Kohn D. Wirth C.J. Reiss G. Plitz W. Maschek H. Erhardt W. Wulker N. Medial meniscus replacement by a tendon autograft—experiments in sheep. J Bone Joint Surg Br. 1992;74:910. doi: 10.1302/0301-620X.74B6.1447257. [DOI] [PubMed] [Google Scholar]
- 18.Messner K. Meniscal substitution with a Teflon periosteal composite graft—a rabbit experiment. Biomaterials. 1994;15:223. doi: 10.1016/0142-9612(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 19.Ouyang H.W. Goh J.C. Thambyah A. Teoh S.H. Lee E.H. Knitted poly-lactide-co-glycolide scaffold loaded with bone marrow stromal cells in repair and regeneration of rabbit Achilles tendon. Tissue Eng. 2003;9:431. doi: 10.1089/107632703322066615. [DOI] [PubMed] [Google Scholar]
- 20.Awad H.A. Boivin G.P. Dressler M.R. Smith F.N. Young R.G. Butler D.L. Repair of patellar tendon injuries using a cell-collagen composite. J Orthop Res. 2003;21:420. doi: 10.1016/S0736-0266(02)00163-8. [DOI] [PubMed] [Google Scholar]
- 21.Young R.G. Butler D.L. Weber W. Caplan A.I. Gordon S.L. Fink D.J. Use of mesenchymal stem cells in a collagen matrix for Achilles tendon repair. J Orthop Res. 1998;16:406. doi: 10.1002/jor.1100160403. [DOI] [PubMed] [Google Scholar]
- 22.Juncosa-Melvin N. Boivin G.P. Galloway M.T. Gooch C. West J.R. Sklenka A.M. Butler D.L. Effects of cell to collagen ratio in mesenchymal stem cell-seeded implants on tendon repair biomechanics and histology. Tissue Eng. 2005;11:448. doi: 10.1089/ten.2005.11.448. [DOI] [PubMed] [Google Scholar]
- 23.Noth U. Schupp K. Heymer A. Kall S. Jakob F. Schutze N. Baumann B. Barthel T. Eulert J. Hendrich C. Anterior cruciate ligament constructs fabricated from human mesenchymal stem cells in a collagen type I hydrogel. Cytotherapy. 2005;7:447. doi: 10.1080/14653240500319093. [DOI] [PubMed] [Google Scholar]
- 24.Walsh C.J. Goodman D. Caplan A.I. Goldberg V.M. Meniscus regeneration in a rabbit partial meniscectomy model. Tissue Eng. 1999;5:327. doi: 10.1089/ten.1999.5.327. [DOI] [PubMed] [Google Scholar]
- 25.Butler D.L. Goldstein S.A. Guilak F. Functional tissue engineering: the role of biomechanics. J Biomech Eng. 2000;122:570. doi: 10.1115/1.1318906. [DOI] [PubMed] [Google Scholar]
- 26.Guilak F. Butler D.L. Goldstein S.A. Mooney D. Functional Tissue Engineering. New York: Springer-Verlag; 2003. [Google Scholar]
- 27.Juncosa N. West J.R. Galloway M.T. Boivin G.P. Butler D.L. In vivo forces used to develop design parameters for tissue engineered implants for rabbit patellar tendon repair. J Biomech. 2003;36:483. doi: 10.1016/s0021-9290(02)00459-1. [DOI] [PubMed] [Google Scholar]
- 28.West J. Juncosa N. Galloway M. Boivin G. Butler D. Characterization of in-vivo Achilles tendon forces in rabbits during treadmill locomotion at varying speeds and inclinations. J Biomech. 2004;37:1647. doi: 10.1016/j.jbiomech.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 29.Mauck R.L. Seyhan S.L. Ateshian G.A. Hung C.T. Influence of seeding density and dynamic deformational loading on the developing structure/function relationships of chondrocyte-seeded agarose hydrogels. Ann Biomed Eng. 2002;30:1046. doi: 10.1114/1.1512676. [DOI] [PubMed] [Google Scholar]
- 30.Lo S.S. Mauck R.L. Seyhan S.L. Palmer G.D. Mow V.C. Hung C.T. American Society of Mechanical Engineers, Bioengineering Division (Publication) BED; NY: 2001. Mechanical Loading Modulates Gene Expression in Chondrocyte-Seeded Agarose Hydrogels; pp. 291–292. [Google Scholar]
- 31.Shearn J.T. Juncosa-Melvin N. Butler D.L. Boivin G.P. Galloway M.T. Goodwin W. Gooch C. Mechanical stimulation of tendon tissue engineered constructs: effects on construct stiffness, repair biomechanics, and their correlation. J Biomech Eng. 2007;129:848. doi: 10.1115/1.2800769. [DOI] [PubMed] [Google Scholar]
- 32.Webb K. Hitchcock R.W. Smeal R.M. Li W. Gray S.D. Tresco P.A. Cyclic strain increases fibroblast proliferation, matrix accumulation, and elastic modulus of fibroblast-seeded polyurethane constructs. J Biomech. 2006;39:1136. doi: 10.1016/j.jbiomech.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 33.Butler D.L. Shearn J.T. Juncosa N. Dressler M.R. Hunter S.A. Functional tissue engineering parameters toward designing repair and replacement strategies. Clin Orthop Relat Res. 2004;427S:S190. doi: 10.1097/01.blo.0000144858.65450.d2. [DOI] [PubMed] [Google Scholar]
- 34.Butler D.L. Juncosa N. Dressler M.R. Functional efficacy of tendon repair processes. Annu Rev Biomed Eng. 2004;6:303. doi: 10.1146/annurev.bioeng.6.040803.140240. [DOI] [PubMed] [Google Scholar]
- 35.Grant T.D. Cho J. Ariail K.S. Weksler N.B. Smith R.W. Horton W.A. Col2-GFP reporter marks chondrocyte lineage and chondrogenesis during mouse skeletal development. Dev Dyn. 2000;218:394. doi: 10.1002/(SICI)1097-0177(200006)218:2<394::AID-DVDY12>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 36.Harvey K.J. Lukovic D. Ucker D.S. Membrane-targeted green fluorescent protein reliably and uniquely marks cells through apoptotic death. Cytometry. 2001;43:273. doi: 10.1002/1097-0320(20010401)43:4<273::aid-cyto1059>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 37.Kalajzic I. Kalajzic Z. Hurley M.M. Lichtler A.C. Rowe D.W. Stage specific inhibition of osteoblast lineage differentiation by FGF2 and noggin. J Cell Biochem. 2003;88:1168. doi: 10.1002/jcb.10459. [DOI] [PubMed] [Google Scholar]
- 38.Ma X. Robin C. Ottersbach K. Dzierzak E. The Ly-6A (Sca-1) GFP transgene is expressed in all adult mouse hematopoietic stem cells. Stem Cells. 2002;20:514. doi: 10.1634/stemcells.20-6-514. [DOI] [PubMed] [Google Scholar]
- 39.Mignone J.L. Kukekov V. Chiang A.-S. Steindler D. Enikolopov G. Neural stem and progenitor cells in Nestin-GFP transgenic mice. J Comp Neurol. 2004;469:311. doi: 10.1002/cne.10964. [DOI] [PubMed] [Google Scholar]
- 40.Peister A. Mellad J.A. Larson B.L. Hall B.M. Gibson L.F. Prockop D.J. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- 41.Dobson K.R. Reading L. Haberey M. Marine X. Scutt A. Centrifugal isolation of bone marrow from bone: an improved method for the recovery and quantitation of bone marrow osteoprogenitor cells from rat tibiae and femurae. Calcif Tissue Int. 1999;65:411. doi: 10.1007/s002239900723. [DOI] [PubMed] [Google Scholar]
- 42.Molecular Devices Spectramax Gemini-XS Application Note 44. Product Literature from Molecular Devices. http://www.moleculardevices.com/product_literature/family_links.php?prodid=63. Jun 1, 2008. http://www.moleculardevices.com/product_literature/family_links.php?prodid=63
- 43.Juncosa-Melvin N. Matlin K.S. Holdcraft R.W. Nirmalanandhan V.S. Butler D.L. Mechanical stimulation increases collagen type I and collagen type III gene expression of stem cell-collagen sponge constructs for patellar tendon repair. Tissue Eng. 2007;13:1219. doi: 10.1089/ten.2006.0339. [DOI] [PubMed] [Google Scholar]
- 44.Juncosa-Melvin N. Shearn J.T. Boivin G.P. Gooch C. Galloway M.T. West J.R. Nirmalanandhan V.S. Bradica G. Butler D.L. Effects of mechanical stimulation on the biomechanics and histology of stem cell-collagen sponge constructs for rabbit patellar tendon repair. Tissue Eng. 2006;12:2291. doi: 10.1089/ten.2006.12.2291. [DOI] [PubMed] [Google Scholar]
- 45.Tsumaki N. Tanaka K. Arikawa-Hirasawa E. Nakase T. Kimura T. Terrig Thomas J. Ochi T. Luyten F.P. Yamada Y. Role of CDMP-1 in skeletal morphogenesis: promotion of mesenchymal cell recruitment and chondrocyte differentiation. J Cell Biol. 1999;144:161. doi: 10.1083/jcb.144.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Popov E.P. Engineering Mechanics of Solids. Englewood Cliffs, NJ: Prentice Hall; 1990. [Google Scholar]
- 47.Montgomery D. Design and Analysis of Experiments. fifth. New York: John Wiley and Sons; 2001. [Google Scholar]
- 48.Juncosa-Melvin N. Boivin G.P. Gooch C. Galloway M.T. West J.R. Dunn M.G. Butler D.L. The effect of autologous mesenchymal stem cells on the biomechanics and histology of gel-collagen sponge constructs used for rabbit patellar tendon repair. Tissue Eng. 2006;12:369. doi: 10.1089/ten.2006.12.369. [DOI] [PubMed] [Google Scholar]
- 49.Toyoda T. Matsumoto H. Fujikawa K. Saito S. Inoue K. Tensile load and the metabolism of anterior cruciate ligament cells. Clin Orthop Relat Res. 1998;353:247. doi: 10.1097/00003086-199808000-00029. [DOI] [PubMed] [Google Scholar]
- 50.Altman G.H. Horan R.L. Martin I. Farhadi J. Stark P.R. Volloch V. Richmond J.C. Vunjak-Novakovic G. Kaplan D.L. Cell differentiation by mechanical stress. FASEB J. 2002;16:270. doi: 10.1096/fj.01-0656fje. [DOI] [PubMed] [Google Scholar]
- 51.Archambault J. Tsuzaki M. Herzog W. Banes A.J. Stretch and interleukin-1β induce matrix metalloproteinases in rabbit tendon cells in vitro. J Orthop Res. 2002;20:36. doi: 10.1016/S0736-0266(01)00075-4. [DOI] [PubMed] [Google Scholar]
- 52.Riser B.L. Cortes P. Heilig C. Grondin J. Ladson-Wofford S. Patterson D. Narins R.G. Cyclic stretching force selectively up-regulates transforming growth factor-β isoforms in cultured rat mesangial cells. Am J Pathol. 1996;148:1915. [PMC free article] [PubMed] [Google Scholar]
- 53.Sumpio B.E. Banes A.J. Link W.G. Johnson G., Jr. Enhanced collagen production by smooth muscle cells during repetitive mechanical stretching. Arch Surg. 1988;123:1233. doi: 10.1001/archsurg.1988.01400340059010. [DOI] [PubMed] [Google Scholar]
- 54.Zeichen J. van Griensven M. Bosch U. The proliferative response of isolated human tendon fibroblasts to cyclic biaxial mechanical strain. Am J Sports Med. 2000;28:888. doi: 10.1177/03635465000280061901. [DOI] [PubMed] [Google Scholar]
- 55.Howard P.S. Kucich U. Taliwal R. Korostoff J.M. Mechanical forces alter extracellular matrix synthesis by human periodontal ligament fibroblasts. J Periodontal Res. 1998;33:500. doi: 10.1111/j.1600-0765.1998.tb02350.x. [DOI] [PubMed] [Google Scholar]
- 56.Kim S.-G. Akaike T. Sasagawa T. Atomi Y. Kurosawa H. Gene expression of type I and type III collagen by mechanical stretch in anterior cruciate ligament cells. Cell Struct Funct. 2002;27:139. doi: 10.1247/csf.27.139. [DOI] [PubMed] [Google Scholar]
- 57.Park J.S. Chu J.S.F. Cheng C. Chen F. Chen D. Li S. Differential effects of equiaxial and uniaxial strain on mesenchymal stem cells. Biotechnol Bioeng. 2004;88:359. doi: 10.1002/bit.20250. [DOI] [PubMed] [Google Scholar]
- 58.Paye M. Nusgens B.V. Lapiere C.M. Modulation of cellular biosynthetic activity in the retracting collagen lattice. Eur J Cell Biol. 1987;45:44. [PubMed] [Google Scholar]
- 59.Nakagawa S. Pawelek P. Grinnell F. Long-term culture of fibroblasts in contracted collagen gels: effects on cell growth and biosynthetic activity. J Investig Dermatol. 1989;93:792. doi: 10.1111/1523-1747.ep12284425. [DOI] [PubMed] [Google Scholar]
- 60.Thie M. Schlumberger W. Rauterberg J. Robenek H. Mechanical confinement inhibits collagen synthesis in gel-cultured fibroblasts. Eur J Cell Biol. 1989;48:294. [PubMed] [Google Scholar]
- 61.Langholz O. Rockel D. Mauch C. Kozlowska E. Bank I. Krieg T. Eckes B. Collagen and collagenase gene expression in three-dimensional collagen lattices are differentially regulated by α1β1 and α2β 1 integrins. J Cell Biol. 1995;131:1903. doi: 10.1083/jcb.131.6.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rikonen T. Westermarck J. Koivisto L. Broberg A. Kahari V.-M. Heino J. Integrin α2β1 is a positive regulator of collagenase (MMP-1) and collagen α1(I) gene expression. J Biol Chem. 1995;270:13548. doi: 10.1074/jbc.270.22.13548. [DOI] [PubMed] [Google Scholar]
- 63.Xu J. Clark R.A.F. A three-dimensional collagen lattice induces protein kinase C-zeta activity: role in β2 integrin and collagenase mRNA expression. J Cell Biol. 1997;136:473. doi: 10.1083/jcb.136.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chastain S.R. Kundu A.K. Dhar S. Calvert J.W. Putnam A.J. Adhesion of mesenchymal stem cells to polymer scaffolds occurs via distinct ECM ligands and controls their osteogenic differentiation. J Biomed Mater Res A. 2006;78:73. doi: 10.1002/jbm.a.30686. [DOI] [PubMed] [Google Scholar]
- 65.Zeichen J. van Griensven M. Bosch U. The proliferative response of isolated human tendon fibroblasts to cyclic biaxial mechanical strain. Am J Sports Med. 2000;28:888. doi: 10.1177/03635465000280061901. [DOI] [PubMed] [Google Scholar]
- 66.Kim S.G. Akaike T. Sasagaw T. Atomi Y. Kurosawa H. Gene expression of type I and type III collagen by mechanical stretch in anterior cruciate ligament cells. Cell Struct Funct. 2002;27:139. doi: 10.1247/csf.27.139. [DOI] [PubMed] [Google Scholar]
- 67.Ronziere M.C. Roche S. Gouttenoire J. Demarteau O. Herbage D. Freyria A.M. Ascorbate modulation of bovine chondrocyte growth, matrix protein gene expression and synthesis in three-dimensional collagen sponges. Biomaterials. 2003;24:851. doi: 10.1016/s0142-9612(02)00418-0. [DOI] [PubMed] [Google Scholar]
- 68.Karamichos D. Brown R.A. Mudera V. Collagen stiffness regulates cellular contraction and matrix remodeling gene expression. J Biomed Mater Res A. 2007;3:887. doi: 10.1002/jbm.a.31423. [DOI] [PubMed] [Google Scholar]