Abstract
Despite the recent advances in our understanding of the dynamics of the cellular interactions associated with the induction of immune responses, comparatively little is known about the in vivo behaviour of antigen-experienced T cells upon secondary antigen exposure in either priming or tolerance. Such information would provide an insight into the functional mechanisms employed by memory T cells of distinct phenotypes and provide invaluable knowledge of how a specific tolerogenic or immunogenic state is maintained. Using real-time imaging to follow the in vivo motility of naïve, primed and tolerized CD4+ T cells and their interactions with dendritic cells (DCs), we demonstrate that each of these distinct functional phenotypes is associated with specific patterns of behaviour. We show that antigen-experienced CD4+ T cells, whether primed or tolerized, display inherently slower migration, making many short contacts with DCs in the absence of antigen. Following secondary exposure to antigen, primed T cells increase their intensity or area of interaction with DCs whereas contacts between DCs and tolerized T cells are reduced. Importantly, this was not associated with alterations in the contact time between DCs and T cells, suggesting that T cells that have previously encountered antigen are more effective at surveying DCs. Thus, our studies are the first to demonstrate that naïve, primed and tolerized T cells show distinct behaviours before and after secondary antigen-encounter, providing a novel mechanism for the increased immune surveillance associated with memory T cells. These findings have important consequences for many immunotherapeutics, which aim to manipulate secondary immune responses.
Keywords: CD4+ T cell, imaging, priming, tolerance
Introduction
Although recent advances in imaging techniques have allowed visualization of the initial interactions involved in the induction of immune responses,1–5 relatively little is known about the dynamic behaviour of T cells upon secondary encounter with antigen. Naïve T cells migrate rapidly within the lymph node (LN) in a seemingly stochastic manner which may be a cell-intrinsic feature6 or dictated by the densely packed LN environment, including the reticular network,7,8 allowing naïve T cells to contact many dendritic cells (DCs).7,9 Upon recognition of cognate antigen on DCs, T-cell velocity is reduced and multiple short-lived contacts give way to long-lived interactions with the DC.2,4,5 Importantly, signals delivered to the T cell during this contact play an important role in determining the effector function of the T cell.10,11 Several reports have previously analysed the behaviour of CD4+ T cells during primary exposure to antigen in priming and tolerizing conditions.12,13 Whilst there are no differences in the velocities or meandering behaviour of T cells becoming primed or tolerized, the clustering behaviour of CD4+ T cells around DCs is altered, with large, stable clusters associated with immune priming whilst CD4+ T cells form a greater number of shorter-lived, smaller clusters during tolerance induction.12 Subsequently, T cells return to a rapid migration, although this may take longer under conditions of priming than following initiation of tolerance.13 Thus, subtle differences in the ability of T cells and DCs to interact can have profound consequences for the induction of immunity.
Whilst it is undoubtedly important to appreciate the in vivo dynamics of the events initiating primary immunogenic or tolerogenic immune responses, understanding the behaviour of antigen-experienced T cells and the effect of secondary exposure to antigen will be essential in designing therapies to manipulate immune function. Indeed, most patients with autoimmune diseases are encountered only after an autoimmune response is primed and the impact of vaccination or immunoregulation only revealed upon challenge with antigen. Thus, it will be important to understand the real-time, in vivo behaviour of T cells previously exposed to antigen such that they can be modulated to best effect.
Following antigen encounter, T cells display an altered profile of adhesion molecules and chemokine receptors,14 which may alter their migration within an LN as well as their ability to interact with antigen-presenting cells (APCs). Previously primed T cells have a decreased threshold for activation,15 requiring less costimulation and developing more robust, rapid effector responses.16–20 Static images of specific time-points have suggested that naïve, effector and memory T cells contact DCs in lymphoid organs, despite altered expression of various surface markers.21,22 However, whether the in vivo dynamics of the T cell–DC interaction is altered in CD4+ T cells that have previously encountered antigen is unclear. We have therefore employed multiphoton laser-scanning microscopy (MPLSM) to visualize naïve, primed and tolerized cells to determine their in situ behaviour in the absence of antigen and their ability to interact with antigen-presenting DCs. Importantly, we demonstrate for the first time that antigen-experienced CD4+ T cells, whether primed or tolerized, display inherently slower migration, with multiple short contacts with DCs. However, following antigen challenge, primed T cells increase the intensity of their interactions with DCs, whereas contacts between DCs and tolerized T cells are reduced. These findings could have important consequences for immunotherapeutics and vaccines that aim to manipulate secondary immune responses to antigen.
Materials and methods
Mice
BALB/c mice were purchased from Harlan-Olac (Bicester, UK). Ovalbumin (OVA)323–339-specific DO11.10 T-cell receptor (TCR) transgenic severe combined immunodeficiency (SCID) mice were used as donors.23 All mice were specific pathogen free and were maintained in accordance with local and UK Home Office regulations.
Cell preparation
DO11.10 SCID mice were primed by subcutaneous (s.c.) immunization with 150 μg of OVA (Sigma-Aldrich, Poole, UK) emulsified in complete Freund’s adjuvant (CFA) (Sigma-Aldrich) or tolerized by administration of 50 mg/ml/day OVA in drinking water for 10 days. Ten days later, peripheral and mesenteric LNs and spleens were harvested.
DCs were prepared from bone marrow of BALB/c mice as previously described.24 Cell suspensions were cultured in complete medium [RPMI-1640, 10% fetal calf serum (FCS), 2 mm l-glutamine, 100 U/ml penicillin and 100 mg/ml streptomycin; all obtained from Invitrogen, Paisley, UK] containing 10% culture supernatant from X63 myeloma cells transfected with mouse granulocyte–macrophage colony-stimulating factor (GM-CSF) cDNA. Fresh medium was added to the cell cultures every 3 days. On day 6, 1 μg/ml lipopolysaccharide (LPS) was added to each well and DCs were then harvested on day 7.
Cell suspensions were labelled with 8·5 μm carboxyfluorescein succinimidyl ester (CFSE) in Hanks’ balanced salt solution (HBSS) for 10 min at 37° or with 5 μm Cell Tracker Red (CMTPX) in CO2-independent medium (all from Invitrogen) for 40 min at 37°. Cells were then rested for 1 hr at 37° in cRPMI prior to intravenous (T cells) or subcutaneous (DCs) transfer into syngeneic BALB/c recipients.
In vitro antigen-specific cytokine production
Cytokine levels were determined in cultures of cell suspensions stimulated with or without 5 μm OVA323–339 peptide (Sigma-Aldrich) for 24 hr. Supernatants were assayed for levels of interleukin (IL)-2 by enzyme-linked immunosorbent assay (ELISA) (BD Pharmingen, Oxford, UK).
Fluorescence-activated cell sorter (FACS) analysis
Freshly isolated cells were re-suspended in FACS buffer containing Fc block (2.4G2 hybridoma supernatant) together with the appropriate combinations of the following antibodies: CD4-peridinin chlorophyll protein (PerCP), CD25-fluorescein isothiocyanate (FITC), programmed death-1 (PD-1)-phycoerythrin (PE), biotinylated KJ1.26 or Annexin V-FITC (all from BD Pharmingen except KJ1.26 which was from eBioscience, San Diego, CA). Intracellular staining for forkhead box p3 (Foxp3) was carried out using the PE anti-mouse/rat Foxp3 Staining Set (eBioscience) as stated in the manufacturer’s protocol. Intracellular staining for cytotoxic T-lymphocyte antigen (CTLA)-4 was carried out using an anti-CTLA-4-PE antibody (BD Pharmingen) with Cytofix/Cytoperm solution (BD Pharmingen). Data were obtained using FACSCanto (BD Bioscience) and analysed using FlowJO (Tree Star Inc., Stanford, CA).
In vivo challenge
One day after adoptive transfer of naïve, primed or tolerized T cells, recipient BALB/c mice were challenged in the footpad with 100 μg of OVA in CFA, or CFA only as an adjuvant control. Alternatively, recipients were challenged with 5 × 106 fluorescently labelled DCs pulsed with 1 mg/ml OVA or phosphate-buffered saline (PBS) for 90 min. Popliteal LNs were excised at 8 and 20 hr post challenge.
Multi-photon microscopy
To image cellular behaviour in LNs, excised LNs were transferred into CO2-independent medium at room temperature. The LN was bound with veterinary glue (Vetbond, 3M, St Paul, MN) onto a plastic coverslip that was then adhered with grease to the bottom of the imaging chamber which was continuously supplied with warmed (36·5°) and gassed (95% O2 and 5% CO2) RPMI before and throughout the period of microscopy. Excised LNs were imaged as previously described.12,25,26 The two-photon excitation source was a solid-state, tuneable titanium:sapphire laser system (5W Chameleon; Coherent Laser Group, Santa Clara, CA). The laser beam was routed into a multi-photon excitation laser scanning system (Radiance; Bio-Rad Laboratories, Hertfordshire, UK). The objective lens used for all imaging investigations was the CFi-60 Fluo-W 40X/0.8 water-dipping objective lens (Nikon, Surrey, UK). The sample was illuminated at 780–830 nm, with ∼ 210 fs pulse duration and 76 MHz repetition frequency. The emission spectrum was separated with a 550-nm dichroic mirror (Chroma Technologies, Rockingham, VT). The scans were acquired with 500 lps and 256 × 256-pixel boxes, for a frame rate of 1·95 fps. Each imaged volume consisted of between 11 and 18 planes 2·55 μm apart. Volumes were acquired every 18–38 seconds.
Analysis and statistics
Images were analysed using volocity software (Improvision, Coventry, UK). The location (centroid) of individual T cells within each three-dimensional image stack was determined by intensity threshold-based object detection. Objects were tracked for at least eight time-points and the mean velocity, displacement and meandering index calculated for each. Interaction between DCs and T cells was measured by quantifying the overlap of green-labelled DCs and red-labelled T cells. Thus, enumeration of red voxels which were also green was used to generate a colocalization coefficient – a measure of the proportion of T cells in contact with a DC, irrespective of T-cell density. These areas of colocalization were also tracked over time to determine the length of a T cell–DC interaction. Student’s independent t-test was performed on data and P-values of ≤ 0·05 were regarded as significant.
Results
Establishing tolerance model
Although many groups, including ourselves, have established models of in vivo tolerance induction using trackable populations of antigen-specific, CD4+ T cells,27–31 most have used flow cytometry or immunohistochemistry at specific time-points as a means of identifying transferred T cells. In contrast, we wanted to visualize fluorescent antigen-specific T cells migrating within the LN in real time. In order to ensure that the cells visualized by microscopy represented the antigen-specific T cells of interest, and to provide sufficient numbers of cells for visualization following adoptive transfer of equal numbers of naïve, primed and tolerized cells, we sought to establish methods of generating primed and tolerized OVA-specific T cells in DO11.10 mice on a SCID background. In these mice, all lymphocytes express the OVA-specific TCR and are naïve in the absence of antigen.
Previous studies have demonstrated the induction of oral tolerance or priming in intact TCR transgenic animals.32–34 However, it remained a possibility that the overrepresentation of a single clone of antigen-specific cells may alter the phenotype of cells recognizing antigen in the intact DO11.10 SCID mouse. We therefore initially confirmed the phenotype of cells isolated from the LNs of naïve DO11.10 SCID mice or DO11.10 SCID mice following exposure to either a priming (OVA/CFA s.c.) or a tolerizing (50 mg/ml OVA in drinking water) regimen. Ten days after priming or feeding, OVA-specific CD4+ T cells in peripheral and mesenteric LNs were analysed for a variety of surface and intracellular molecules and for functional characteristics.
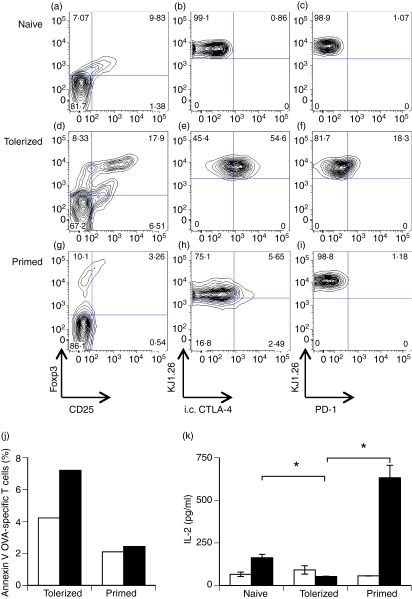
As demonstrated previously,29,32 OVA-specific T cells activated in vivo by orally delivered antigen showed increased levels of CD25 when compared with naïve or primed CD4+ T cells (Fig. 1a,d,g). Analysis of T cells isolated from the LNs of OVA-fed DO11.10 SCID mice showed a phenotype consistent with a tolerant phenotype. Thus, CD4+ KJ1.26+ cells from OVA-fed mice showed increased expression of the transcription factor Foxp3, together with elevated levels of intracellular CTLA-4 and the inhibitory surface molecule PD-1, compared with OVA-specific T cells from naïve or primed DO11.10 SCID mice (Fig. 1).30,32,35,36 The in vivo tolerized cells also showed enhanced Annexin-V binding relative to primed cells (Fig. 1j), supporting previous evidence that tolerant T cells are more susceptible to apoptosis.37 Importantly, these cells retained their phenotype following adoptive transfer into recipient mice and antigen challenge, with antigen-specific T cells isolated from tolerized DO11.10 SCID mice producing little IL-2 upon challenge (Fig. 1k), suggesting an anergic phenotype. These results demonstrate that we were able to generate distinct populations of phenotypically and functionally distinct cells, displaying the characteristics of primed and tolerized cells, respectively, which could be adoptively transferred to allow visualization of their behaviour upon secondary antigen challenge.
Figure 1.
Functional tolerance induction in intact DO11.10 severe combined immunodeficiency (SCID) mice. Ovalbumin (OVA)-specific (CD4+ KJ1.26+) cells from naïve (a–c), OVA-fed (d–f) or OVA-immunized (g–i) DO11.10 SCID mice were analysed by flow cytometry for the expression of the indicated molecules. Plots shown are gated on CD4+ KJ1.26+ (a, d, g) or CD4+ (b, c, e, f, h, i) lymphocytes. (j) OVA-specific cells isolated from OVA-fed (tolerized) or OVA-immunized (primed) DO11.10 SCID mice were transferred into naïve BALB/c recipients prior to immunization with OVA/complete Freund’s adjuvant (CFA) (filled bars) or CFA alone (empty bars). Apoptosis of OVA-specific cells was assessed 20 hr later by measuring Annexin-V binding. (k) Naïve, tolerized or primed OVA-specific T cells were transferred into BALB/c recipients prior to immunization with OVA/CFA. Twenty hours later, draining lymph nodes (LNs) were isolated and cells cultured alone (empty bars) or re-stimulated with 5 μm OVA323–339 (filled bars). Supernatants were analysed for interleukin (IL)-2 after 24 hr of stimulation. CTLA-4, cytotoxic T-lymphocyte antigen 4; PD-1, programmed death-1.
Characterizing the migratory behaviour of naïve, primed and tolerized T cells in the LN
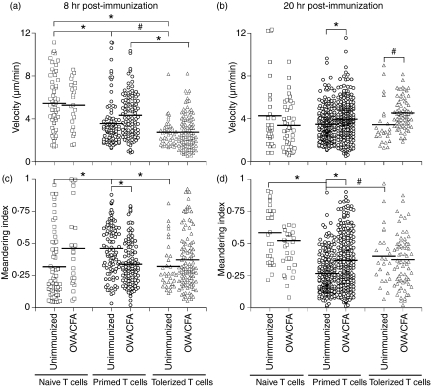
In order to analyse the response of CD4+ T cells to secondary encounter with antigen, OVA-specific CD4+ T cells were isolated from the LNs of naïve, primed or orally tolerized DO11.10 SCID mice, fluorescently labelled and adoptively transferred into naïve BALB/c recipients. These recipient mice were then challenged with OVA/CFA (or CFA alone) and the naïve, tolerized and primed cells in the draining popliteal LN visualized using MPLSM. As previously described, naïve T cells migrate around the LN in a seemingly random manner (Fig. 2), displaying relatively rapid migration. Interestingly, cells isolated from OVA-immunized, or OVA-fed, DO11.10 SCID mice displayed an inherently slower baseline velocity in the naïve recipient, together with a reduced meandering index (a measure of directed migration) compared with naïve T cells 20 hr after CFA or OVA/CFA challenge (Fig. 2). At earlier time-points, however, the meandering of antigen-experienced T cells increased relative to naïve T cells. Tolerized T cells had marginally slower velocities compared with primed T cells. Thus, primed and tolerized memory cells appear to show an inherent difference in their migration characteristics in the absence of antigen when compared with naïve T-cell migration.
Figure 2.
Motility of naïve, tolerized and primed antigen-specific T cells. Ovalbumin (OVA)-specific T cells from naïve (squares), OVA-fed (tolerized; triangles) or OVA-immunized (primed; circles) DO11.10 severe combined immunodeficiency (SCID) mice were carboxyfluorescein succinimidyl ester (CFSE)-labelled and adoptively transferred into naïve BALB/c mice. Recipients were subsequently challenged with either complete Freund’s adjuvant (CFA) only or OVA/CFA as indicated. T-cell velocity (a, b) and meandering (c, d) were analysed at 8 and 20 hr post-immunization. Data represent the migration of individual cells from several mice, with the mean indicated (#P<0·05; *P<0·005).
Following challenge with OVA in adjuvant, naïve T-cell velocity is reduced as the cells interact with APCs (Fig. 2).1,3 Surprisingly, the reduced baseline velocity of the tolerized and primed T cells in the absence of antigen increased upon subsequent encounter with antigen, with cells from OVA-immunized or OVA-fed mice increasing their velocity at both 8 and 20 hr after challenge (Fig. 2). Thus, whilst naïve T cells show one distinct change in behaviour upon antigen encounter, i.e. slowing down, secondary encounter with antigen increases the speed of the inherently slower primed and tolerized cells.
Interactions with DCs
One possible explanation for the different ‘baseline’ behaviour of primed and tolerized T cells (compared with naïve T cells) in the absence of antigenic challenge could lie in their relative abilities to interact with DCs. Thus, primed and tolerized T cells may spend longer surveying each individual DC, decreasing their mean velocity. We and others have used multicolour MPLSM to image the interactions between DCs and T cells in vivo,2,4,5,26 allowing measurement of the contact between the two populations. Following transfer of CMTPX-labelled naïve, primed or tolerized T cells into syngeneic mice, recipient mice were challenged with CFSE-labelled DCs pulsed with OVA. Cell interactions in the draining popliteal LN were imaged and the amount of DC–T cell contact determined as a colocalization coefficient and by measuring the contact duration. As described above, these measurements allow an accurate measurement of the proportion of the total T-cell volume in contact with DCs, and subsequent analysis of contact duration in an entirely automated, empirical manner, rather than subjective methods of analysis used in previous reports. As previously described,2,4,5,26 naïve T cells recognizing antigen on DCs increased interaction as they clustered on DCs, resulting in an increased colocalization coefficient and longer contact durations (Fig. 3). Importantly, in addition to showing inherently reduced velocities, T cells transferred from primed or tolerized DO11.10 SCID mice displayed increased interaction with unpulsed DCs, with a higher colocalization coefficient compared with naïve T cells (Fig. 3d). However, this was not associated with longer contacts between DCs and T cells (Fig. 3b,c), suggesting that T cells that have previously encountered antigen may be more effective at surveying DCs, spending less time surveying more DCs, or increasing their area of contact. Upon recognition of antigen, primed T cells dramatically increased their overall interaction with the OVA-pulsed DCs, forming multiple interactions, although these were of relatively short duration (Fig. 3). Conversely, tolerized T cells showed reduced interaction with OVA-pulsed DCs compared with unpulsed DCs (Fig. 3). Thus, tolerized and primed T cells show distinct behaviours following recognition of antigen, with primed cells increasing the intensity or area of contact with antigen-presenting DCs (although the contact is of short duration), whereas T cells from tolerized DO11.10 SCID mice increase in speed and have only few, short interactions with OVA-presenting DCs.
Figure 3.
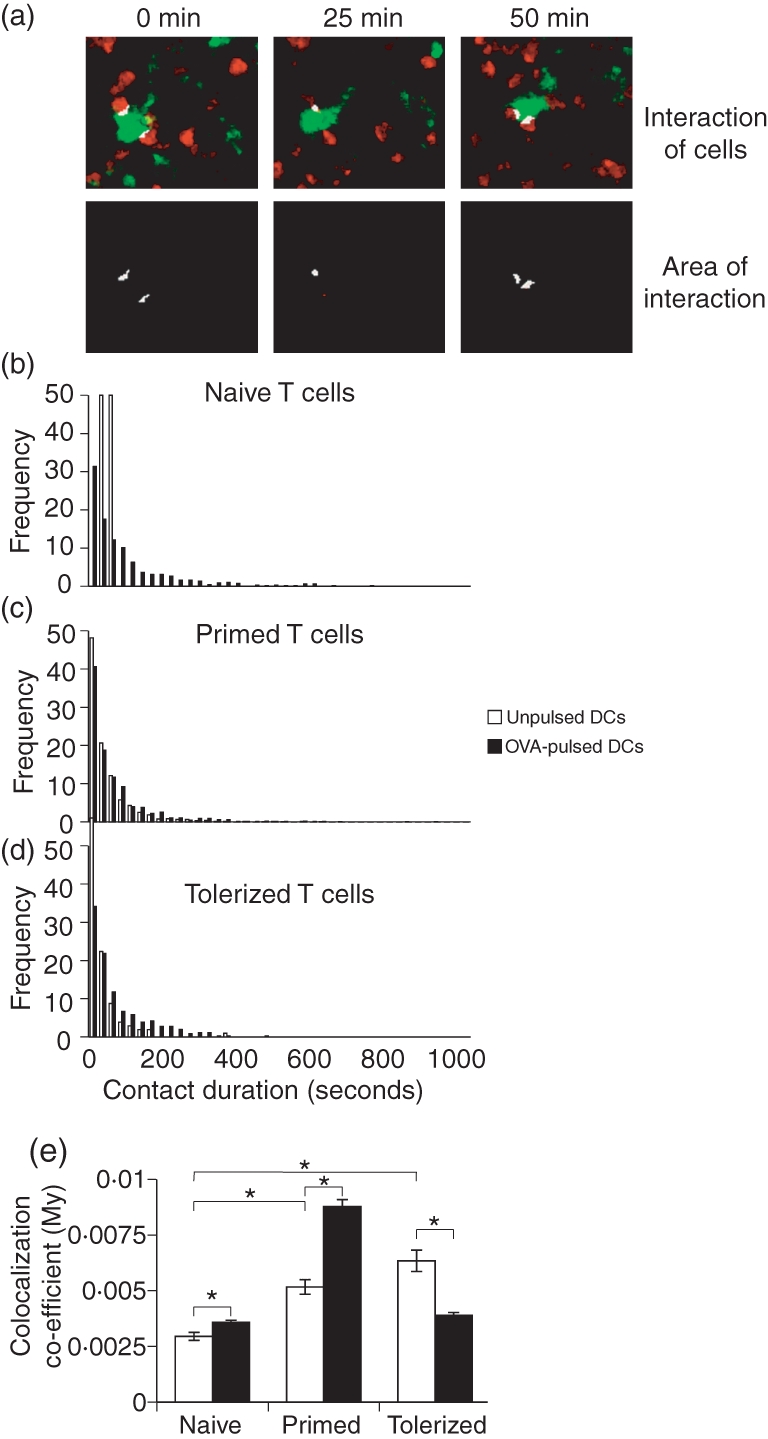
Interactions of naïve, tolerized and primed antigen-specific T cells with dendritic cells. Naïve, tolerized or primed DO11.10 severe combined immunodeficiency (SCID) cells were Cell Tracker Red™ (CMTPX)-labelled (red) and adoptively transferred into BALB/c recipients. Recipients were challenged with CFSE-labelled (green) unpulsed (empty bars) or ovalbumin (OVA)-pulsed (filled bars) bone marrow-derived dendritic cells (DCs) subcutaneously (s.c.) and the draining popliteal lymph node (LN) imaged 20 hr later. (a–d) Semi-automated estimation of contact between T cells and DCs was performed using volocity software to identify areas of interaction (identified as both green and red) within the imaging field (white), which were then tracked over time. (e) Total interaction of T cells with DCs was determined by measuring the proportion of red voxels overlapping with green, generating a colocalization coefficient, which is not affected by differences in T-cell densities between samples (#P<0·05).
Discussion
We have shown that, in the absence of antigen, T cells previously exposed to antigen moved with reduced velocities compared with naïve CD4+ T cells, although they only made transient contacts with DCs. Following secondary exposure to antigen, previously primed T cells increased their velocity and showed enhanced interactions with DCs, although these contacts were transient. As CD4+ memory T cells have low dependence on costimulation relative to naïve cells38 and exhibit accelerated antigen responsiveness,39 these frequent, short contacts with DCs may be sufficient to recall effector functions. Conversely, whilst tolerized CD4+ T cells migrated with similar velocities to primed cells in the absence of antigen and increased their velocity following antigen challenge, their degree of contact with APCs was not increased. The increase in velocity of tolerized T cells following antigen challenge may be related to the reported faster return to rapid migration of tolerized CD4+ T cells following an initial reduction in velocity in response to antigen exposure13 and is also comparable to the increased velocity of CD8+ T cells at 15–20 hr after tolerogenic antigen challenge.40 Previous work has visualized the migration and interactions of CD4+ CD25+ regulatory T cells and demonstrated the formation of stable interactions with DCs following antigen-loading.41 Further studies also suggested that the ability of CD4+ CD25+ regulatory T cells (Treg) to suppress bystander T-cell activation is mediated via the DC, rather than directly between T cells.41,42 Although oral tolerance has been suggested to involve CD4+ CD25+ Treg,29,32 we did not see stable interactions between tolerized OVA-specific T cells and antigen-presenting DCs, suggesting that the mechanism of tolerance may differ in our experimental system. Whether this reflects differences in the doses of antigen fed,43 or differences in the kinetics of the responses analysed remains to be resolved.
It remains a possibility that the differences in migratory behaviour among the populations of naïve, tolerized and primed CD4+ T cells represent alterations in the phenotype of cells observed in each group. Thus, in the tolerized population of T cells, a greater proportion of the cells imaged displayed a Foxp3+ CD25+ Treg phenotype (Fig. 1), and this may account for the differences observed upon imaging (Figs 2 and 3). Whilst previous reports have demonstrated stable interactions between Tregs and APCs, our data suggest that tolerized T cells have fewer, shorter interactions with antigen-loaded DCs compared with primed T cells. However, without suitable reporter systems or ‘markers’ of tolerance to define the phenotype of each individual cell imaged, it is not possible to characterize the different behaviours of individual cell types within this mixed, tolerized T-cell population, each potentially responding with different kinetics. Further work is therefore required to track and model the migration and interaction of individual T cells within these heterogeneous populations of antigen-experienced T cells.
Following tolerance induction, translocation of TCR and protein kinase C (PKCθ) to lipid rafts is impaired,34 with colocalization between the TCR and repressor activator protein-1 (Rap-1), which can antagonize phosphorylated extracellular signal-regulated kinase (pERK) signalling.44 As this may alter the ability of tolerized T cells to form a synapse, this could account for the failure of tolerized T cells to establish stable interactions with DCs. This once again suggests that the quality and duration of interactions may influence, as well as be influenced by, the activation state of the cells, with tolerant T cells having less contact with DCs associated with reduced signal transduction.
As highlighted above, T cells isolated from orally tolerized DO11.10 SCID mice expressed increased levels of intracellular CTLA-4, and it is possible that this may traffic to the cellular surface upon recognition of antigen. Previous studies have shown up-regulation of CTLA-4 on antigen-specific T cells after feeding,32,45 and inhibition of CTLA-4 reduces the induction of peripheral tolerance.30,35,36,46–48 Importantly, CTLA-4 has been demonstrated to play an important role in modulating T-cell motility, overriding the TCR-induced stop signal and reducing the stable interactions between DCs and T cells.25,49 Indeed, CTLA-4 engagement is associated with activation of Rap1 and activation of integrins, resulting in reduced dwell time on APCs, less clustering of zeta-chain-associated protein kinase-70 (ZAP-70) and reduced calcium flux.50 Thus, it may be that rapid and sustained mobilization of CTLA-4 to the cell surface of tolerized T cells recognizing antigen prevents stable interactions with DCs, resulting in a few, short contacts and failure to initiate T-cell activation. Conversely, transient expression of CTLA-4 on primed T cells may be associated with shortening of contact duration, but does not reduce the intensity or area of interaction with DCs.
The current study aimed to examine the migratory behaviour of primed or tolerized CD4+ T cells and their interaction with DCs following secondary exposure to antigen. These interactions are clearly important, as these two populations display distinct functions during primary and secondary antigen encounter and understanding the dynamics of these interactions provides a better understanding of vaccination strategies and the potential for manipulating the interactions for therapeutic benefit. Using MPLSM to follow the motility of naïve, primed and tolerized cells within the LN and their interaction with DCs, we have shown that each of these distinct functional phenotypes is associated with specific patterns of behaviour within the LN. Antigen-experienced T cells show an inherently slower motility, with more (short) contact with DCs. Upon antigen recognition, whilst previously primed T cells increase their interaction with DCs, contacts between tolerant T cells and DCs are reduced. Thus, these findings could have important implications for the design and application of vaccination and immunotherapeutic strategies in general, particularly those that aim to manipulate secondary immune responses to antigen.
Acknowledgments
This work was funded by a grant from the Wellcome Trust (grant number 068895/Z/02/Z) awarded to PG, JMB, A. M. Gurney and D. Wokosin. OM is an RCUK Academic Fellow.
Disclosures
There is no conflict of interest.
References
- 1.Miller MJ, Wei SH, Parker I, Cahalan MD. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 2002;296:1869–73. doi: 10.1126/science.1070051. [DOI] [PubMed] [Google Scholar]
- 2.Miller MJ, Safrina O, Parker I, Cahalan MD. Imaging the single cell dynamics of CD4+ T cell activation by dendritic cells in lymph nodes. J Exp Med. 2004;200:847–56. doi: 10.1084/jem.20041236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stoll S, Delon J, Brotz TM, Germain RN. Dynamic imaging of T cell-dendritic cell interactions in lymph nodes. Science. 2002;296:1873–6. doi: 10.1126/science.1071065. [DOI] [PubMed] [Google Scholar]
- 4.Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–9. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 5.Celli S, Garcia Z, Bousso P. CD4 T cells integrate signals delivered during successive DC encounters in vivo. J Exp Med. 2005;202:1271–8. doi: 10.1084/jem.20051018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dustin ML. Stop and go traffic to tune T cell responses. Immunity. 2004;21:305–14. doi: 10.1016/j.immuni.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 7.Beltman JB, Maree AF, Lynch JN, Miller MJ, de Boer RJ. Lymph node topology dictates T cell migration behavior. J Exp Med. 2007;204:771–80. doi: 10.1084/jem.20061278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bajenoff M, Egen JG, Koo LY, Laugier JP, Brau F, Glaichenhaus N, Germain RN. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity. 2006;25:989–1001. doi: 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller MJ, Hejazi AS, Wei SH, Cahalan MD, Parker I. T cell repertoire scanning is promoted by dynamic dendritic cell behavior and random T cell motility in the lymph node. Proc Natl Acad Sci U S A. 2004;101:998–1003. doi: 10.1073/pnas.0306407101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–48. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 11.Lenschow DJ, Herold KC, Rhee L, et al. CD28/B7 regulation of Th1 and Th2 subsets in the development of autoimmune diabetes. Immunity. 1996;5:285–93. doi: 10.1016/s1074-7613(00)80323-4. [DOI] [PubMed] [Google Scholar]
- 12.Zinselmeyer BH, Dempster J, Gurney AM, et al. In situ characterization of CD4+ T cell behavior in mucosal and systemic lymphoid tissues during the induction of oral priming and tolerance. J Exp Med. 2005;201:1815–23. doi: 10.1084/jem.20050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shakhar G, Lindquist RL, Skokos D, Dudziak D, Huang JH, Nussenzweig MC, Dustin ML. Stable T cell-dendritic cell interactions precede the development of both tolerance and immunity in vivo. Nat Immunol. 2005;6:707–14. doi: 10.1038/ni1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roman E, Miller E, Harmsen A, Wiley J, Von Andrian UH, Huston G, Swain SL. CD4 effector T cell subsets in the response to influenza: heterogeneity, migration, and function. J Exp Med. 2002;196:957–68. doi: 10.1084/jem.20021052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanchot C, Lemonnier FA, Perarnau B, Freitas AA, Rocha B. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science. 1997;276:2057–62. doi: 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- 16.Smith KM, McAskill F, Garside P. Orally tolerized T cells are only able to enter B cell follicles following challenge with antigen in adjuvant, but they remain unable to provide B cell help. J Immunol. 2002;168:4318–25. doi: 10.4049/jimmunol.168.9.4318. [DOI] [PubMed] [Google Scholar]
- 17.Dubey C, Croft M, Swain SL. Naive and effector CD4 T cells differ in their requirements for T cell receptor versus costimulatory signals. J Immunol. 1996;157:3280–9. [PubMed] [Google Scholar]
- 18.Iezzi G, Karjalainen K, Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8:89–95. doi: 10.1016/s1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- 19.Whitmire JK, Eam B, Whitton JL. Tentative T cells: memory cells are quick to respond, but slow to divide. PLoS Pathog. 2008;4:e1000041. doi: 10.1371/journal.ppat.1000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abraham C, Miller J. Molecular mechanisms of IL-2 gene regulation following costimulation through LFA-1. J Immunol. 2001;167:5193–201. doi: 10.4049/jimmunol.167.9.5193. [DOI] [PubMed] [Google Scholar]
- 21.Khanna KM, McNamara JT, Lefrancois L. In situ imaging of the endogenous CD8 T cell response to infection. Science. 2007;318:116–20. doi: 10.1126/science.1146291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Westermann J, Bode U, Sahle A, Speck U, Karin N, Bell EB, Kalies K, Gebert A. Naive, effector, and memory T lymphocytes efficiently scan dendritic cells in vivo: contact frequency in T cell zones of secondary lymphoid organs does not depend on LFA-1 expression and facilitates survival of effector T cells. J Immunol. 2005;174:2517–24. doi: 10.4049/jimmunol.174.5.2517. [DOI] [PubMed] [Google Scholar]
- 23.Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990;250:1720–3. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 24.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 25.Schneider H, Downey J, Smith A, et al. Reversal of the TCR stop signal by CTLA-4. Science. 2006;313:1972–5. doi: 10.1126/science.1131078. [DOI] [PubMed] [Google Scholar]
- 26.Millington OR, Gibson VB, Rush CM, Zinselmeyer BH, Phillips RS, Garside P, Brewer JM. Malaria impairs T cell clustering and immune priming despite normal signal 1 from dendritic cells. PLoS Pathog. 2007;3:1380–7. doi: 10.1371/journal.ppat.0030143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith KM, Davidson JM, Garside P. T-cell activation occurs simultaneously in local and peripheral lymphoid tissue following oral administration of a range of doses of immunogenic or tolerogenic antigen although tolerized T cells display a defect in cell division. Immunology. 2002;106:144–58. doi: 10.1046/j.1365-2567.2002.01427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Millington OR, Mowat AM, Garside P. Induction of bystander suppression by feeding antigen occurs despite normal clonal expansion of the bystander T cell population. J Immunol. 2004;173:6059–64. doi: 10.4049/jimmunol.173.10.6059. [DOI] [PubMed] [Google Scholar]
- 29.Thorstenson KM, Khoruts A. Generation of anergic and potentially immunoregulatory CD25+CD4 T cells in vivo after induction of peripheral tolerance with intravenous or oral antigen. J Immunol. 2001;167:188–95. doi: 10.4049/jimmunol.167.1.188. [DOI] [PubMed] [Google Scholar]
- 30.Fowler S, Powrie F. CTLA-4 expression on antigen-specific cells but not IL-10 secretion is required for oral tolerance. Eur J Immunol. 2002;32:2997–3006. doi: 10.1002/1521-4141(2002010)32:10<2997::AID-IMMU2997>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 31.Van Houten N, Blake SF. Direct measurement of anergy of antigen-specific T cells following oral tolerance induction. J Immunol. 1996;157:1337–41. [PubMed] [Google Scholar]
- 32.Zhang X, Izikson L, Liu L, Weiner HL. Activation of cd25(+)cd4(+) regulatory t cells by oral antigen administration. J Immunol. 2001;167:4245–53. doi: 10.4049/jimmunol.167.8.4245. [DOI] [PubMed] [Google Scholar]
- 33.Chung Y, Lee SH, Kim DH, Kang CY. Complementary role of CD4+CD25+ regulatory T cells and TGF-beta in oral tolerance. J Leukoc Biol. 2005;77:906–13. doi: 10.1189/jlb.1004599. [DOI] [PubMed] [Google Scholar]
- 34.Ise W, Nakamura K, Shimizu N, Goto H, Fujimoto K, Kaminogawa S, Hachimura S. Orally tolerized T cells can form conjugates with APCs but are defective in immunological synapse formation. J Immunol. 2005;175:829–38. doi: 10.4049/jimmunol.175.2.829. [DOI] [PubMed] [Google Scholar]
- 35.Samoilova EB, Horton JL, Zhang H, Khoury SJ, Weiner HL, Chen Y. CTLA-4 is required for the induction of high dose oral tolerance. Int Immunol. 1998;10:491–8. doi: 10.1093/intimm/10.4.491. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y, Ma Y. Roles of cytotoxic T-lymphocyte-associated antigen-4 in the inductive phase of oral tolerance. Immunology. 2002;105:171–80. doi: 10.1046/j.1365-2567.2002.01348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garside P, Steel M, Worthey EA, Kewin PJ, Howie SE, Harrison DJ, Bishop D, Mowat AM. Lymphocytes from orally tolerized mice display enhanced susceptibility to death by apoptosis when cultured in the absence of antigen in vitro. Am J Pathol. 1996;149:1971–9. [PMC free article] [PubMed] [Google Scholar]
- 38.Croft M, Bradley LM, Swain SL. Naive versus memory CD4 T cell response to antigen. Memory cells are less dependent on accessory cell costimulation and can respond to many antigen-presenting cell types including resting B cells. J Immunol. 1994;152:2675–85. [PubMed] [Google Scholar]
- 39.Garcia S, DiSanto J, Stockinger B. Following the development of a CD4 T cell response in vivo: from activation to memory formation. Immunity. 1999;11:163–71. doi: 10.1016/s1074-7613(00)80091-6. [DOI] [PubMed] [Google Scholar]
- 40.Hugues S, Fetler L, Bonifaz L, Helft J, Amblard F, Amigorena S. Distinct T cell dynamics in lymph nodes during the induction of tolerance and immunity. Nat Immunol. 2004;5:1235–42. doi: 10.1038/ni1134. [DOI] [PubMed] [Google Scholar]
- 41.Tang Q, Adams JY, Tooley AJ, et al. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tadokoro CE, Shakhar G, Shen S, Ding Y, Lino AC, Maraver A, Lafaille JJ, Dustin ML. Regulatory T cells inhibit stable contacts between CD4+ T cells and dendritic cells in vivo. J Exp Med. 2006;203:505–11. doi: 10.1084/jem.20050783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Friedman A, Weiner HL. Induction of anergy or active suppression following oral tolerance is determined by antigen dosage. Proc Natl Acad Sci USA. 1994;91:6688–92. doi: 10.1073/pnas.91.14.6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morton AM, McManus B, Garside P, Mowat AM, Harnett MM. Inverse Rap1 and phospho-ERK expression discriminate the maintenance phase of tolerance and priming of antigen-specific CD4+ T cells in vitro and in vivo. J Immunol. 2007;179:8026–34. doi: 10.4049/jimmunol.179.12.8026. [DOI] [PubMed] [Google Scholar]
- 45.Sun J, Dirden-Kramer B, Ito K, Ernst PB, Van Houten N. Antigen-specific T cell activation and proliferation during oral tolerance induction. J Immunol. 1999;162:5868–75. [PubMed] [Google Scholar]
- 46.Perez VL, Van Parijs L, Biuckians A, Zheng XX, Strom TB, Abbas AK. Induction of peripheral T cell tolerance in vivo requires CTLA-4 engagement. Immunity. 1997;6:411–7. doi: 10.1016/s1074-7613(00)80284-8. [DOI] [PubMed] [Google Scholar]
- 47.Greenwald RJ, Boussiotis VA, Lorsbach RB, Abbas AK, Sharpe AH. CTLA-4 regulates induction of anergy in vivo. Immunity. 2001;14:145–55. doi: 10.1016/s1074-7613(01)00097-8. [DOI] [PubMed] [Google Scholar]
- 48.Barone KS, Herms B, Karlosky L, Murray S, Qualls J. Effect of in vivo administration of anti-CTLA-4 monoclonal antibody and IL-12 on the induction of low-dose oral tolerance. Clin Exp Immunol. 2002;130:196–203. doi: 10.1046/j.1365-2249.2002.01961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Downey J, Smith A, Schneider H, Hogg N, Rudd CE. TCR/CD3 mediated stop-signal is decoupled in T-cells from Ctla4 deficient mice. Immunol Lett. 2008;115:70–2. doi: 10.1016/j.imlet.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schneider H, Smith X, Liu H, Bismuth G, Rudd CE. CTLA-4 disrupts ZAP70 microcluster formation with reduced T cell/APC dwell times and calcium mobilization. Eur J Immunol. 2008;38:40–7. doi: 10.1002/eji.200737423. [DOI] [PMC free article] [PubMed] [Google Scholar]