Abstract
We have characterized a Leishmania protein belonging to the silent information regulator 2 (SIR2) family [SIR2 related protein 1 (SIR2RP1)] that might play an immunoregulatory role during infection through its capacity to trigger B-cell effector functions. We report here that SIR2RP1 leads to the proliferation of activated B cells, causing increased expression of major histocompatibility complex (MHC) II and the costimulatory molecules CD40 and CD86, which are critical ligands for T-cell cross-talk during the development of adaptive immune responses. In contrast, B cells isolated from Toll-like receptor 2 (TLR2) knockout mice were unable to respond to the SIR2RP1 stimulus. Similarly, SIR2RP1 induced the maturation of dendritic cells (DCs) in a TLR2-dependent manner with the secretion of pro-inflammatory cytokines [interleukin (IL)-12 and tumour necrosis factor (TNF)-α] and enhanced the costimulatory properties of DCs. Nevertheless, immunization assays demonstrated that TLR2-deficient mice were able to mount a specific humoral response to SIR2RP1. Interestingly, further investigations showed that macrophages were activated by SIR2RP1 even in the absence of TLR2. Therefore, a different type of interplay between SIR2RP1 and the major antigen-presenting cells in vivo could explain the immune response observed in TLR2-deficient mice. Together, these results demonstrate that TLR2 signalling contributes to SIR2RP1 recognition by innate immune host cells.
Keywords: B cells, cytokines, innate immunity, Leishmania spp. (leishmaniasis), Toll receptors/Toll-like receptors
Introduction
Toll-like receptors (TLRs) are key components of effective innate immunity. They perform a vital role in mediating the innate recognition of pathogens and orchestrating the acquired immune response to bacteria, viruses and parasites. The family of TLRs is highly expressed in cells of the innate immune system, such as macrophages, dendritic cells (DCs), B cells and natural killer (NK) cells, and is responsible for recognizing conserved motifs, termed pathogen-associated molecular patterns (PAMPs), that are unique to invasive pathogens and not normally found in the host cells.1,2
All the members of the Toll family are transmembrane proteins containing an extracellular domain composed of leucine-rich repeats and a cytoplasmic domain homologous to the cytoplasmic region of the interleukin (IL)-1 receptor, known as the Toll/Interleukin-1 receptor (TIR) domain, which is required for downstream signalling.3 The identification of mammalian TLRs represented an important advance in our understanding of innate immunity to pathogenic micro-organisms. To date, 11 human and 13 mouse TLRs have been identified,4 and each TLR appears to respond to distinct PAMPs, leading to the activation of specific signalling pathways.5 TLR2 recognizes a myriad of unrelated molecules, including lipopeptides,6 peptidoglycans,7 outer membrane proteins,8 a Trypanosoma cruzi protein belonging to the thiol-disulfide oxidoreductase family9 and porins from a broad spectrum of Gram-negative bacteria.10–12 This diversity is a result of heterodimerization with TLR1 or TLR613 and/or accessory molecules, such as CD1414 and CD36.15 The activation of TLR2 by ligands triggers several intracellular signalling responses, including the activation of nuclear factor (NF)-κB and the induction of pro- and anti-inflammatory cytokines.16 One of the most extensively studied pathways involves signalling through MyD88.17 In fact, MyD88 is involved in NF-κB activation by every TLR with the exception of TLR3.18 Engagement of TLRs by PAMPs mediates downstream signalling that leads to up-regulation of both major histocompatibility complex (MHC) and costimulatory molecules, such as CD80 and CD86, which are involved in the optimal activation of naïve T cells, strengthening the adaptive immune response.19
Although the majority of experimental studies have indicated an essential role for MyD88 signalization in resistance against several intracellular infections,20–26 the role of TLR2 is still controversial. A protective role for TLR2 has been described for several infections, such as infections with Staphilococcus aureus,27Streptococcus pneumonia,28,29Toxoplasma gondii,30Mycobacterium bovis,31Mycobacterium avium32,33 and Mycobacterium tuberculosis.34,35 However, other studies have suggested that TLR2-dependent mechanisms might also contribute to the evasion or inhibition of an effective immune response in organisms such as Yersinia enterocolitica, Aspergillus fumigates and Candida albicans.36–39 Although TLR2 has been proposed to play a role during infection, as it is one of the molecules involved in Leishmania phagocytosis,40 the absence of TLR2 did not modify the course of visceral leishmaniasis (VL) in either the spleen or the liver.41 Nevertheless, very few studies have so far explored the interaction of Leishmania constituents with the family of Toll-like receptors. Lipophosphoglycan (LPG), a major surface promastigote phosphoglycan, can be considered as an exception, as it was demonstrated to behave as a TLR2 agonist activating mouse macrophage and human NK cells.22,42 This highlights the need for a thorough dissection of the parasite factors involved in TLR activation. In addition, TLR-based therapeutic or prophylactic strategies are currently measures used to fight infectious diseases. Indeed, TLRs have been implicated in the mechanism of adjuvanticity of many immunostimulants used in clinical or experimental vaccination. In this context, several bacterial proteins have recently been reported to mediate adjuvanticity by activating antigen-presenting cells (APCs) via TLR2. This has led to the assumption of multicomponent vaccines containing TLR2 agonists.43
Human leishmaniasis and experimental murine leishmaniasis have clearly demonstrated a central role for T lymphocytes in the immunological mechanisms of resistance against leishmaniasis. The contribution of B cells during the infectious process, either as APCs or as immunoglobulin-secreting cells, is more controversial. Some reports dissected the role of B cells and antibodies (Abs) in assisting cell-mediated responses during host defence against leishmaniasis. Although some reports failed to show evidence of a contribution of B cells to the development of polarized T-cell responses,44–46 others demonstrated a correlation between B-cell depletion and enhanced resistance to the disease.47,48 In this context, B lymphocytes were shown to contribute to susceptibility to Leishmania donovani infection unrelated to the presence or absence of immunoglobulin.49 Nevertheless, antigen-specific as well as natural immunoglobulin G (IgG) molecules are responsible for the opsonization of the parasite, so that components of the innate immune system will recognize the parasites as foreign.50 Recently, Woelbing et al.51 have reaffirmed the role of B cell-derived Abs in the development of adaptive immune responses during leishmaniasis. They reported that resistant mice without B cells or deficient in the Fcγ receptor became susceptible to disease. This was attributed to decreased numbers of infected CD11c+ DCs in vivo, which led to impaired T-cell priming and dramatically reduced interferon (IFN)-γ production. These observations suggest that parasite-reactive IgG and DC Fcγ receptors I and III are essential for optimal development of protective immunity.51 Overall, we face the challenge to identify parasite antigens that could be involved in such mechanisms.
The protein encoded by the SIR2 gene belongs to a highly conserved family of proteins, with NAD-dependent class III deacetylases, found in organisms from bacteria to humans and named homologous to SIR two (Hst) proteins or sirTuins.52 Several roles have been attributed to this protein family, including cell cycle progression and chromosome stability,53 DNA damage repair,54 and life-span extension in yeast55 and in Caenorhabditis elegans.56 We have detected the presence of a SIR2-like gene in Leishmania major and Leishmania infantum termed SIR2RP1.57,58 In addition, two other homologous sequences (SIR2RP2 and 3) can be found in the Leishmania genome database. Immunological investigations suggested an immunoregulatory role for SIR2RP1 during infection through its capacity to trigger specifically B-cell effector functions.59 In an attempt to unravel the molecular mechanism of its activity, we investigated the direct effect of SIR2RP1 on the spleen B-lymphocyte population. Our results showed that SIR2RP1 activates B cells via a TLR2-dependent mechanism. The activated B cells up-regulated expression of both MHC II and costimulatory molecules CD40 and CD86, and secreted tumour necrosis factor (TNF)-α. Similarly, SIR2RP1 induced the maturation of bone marrow-derived dendritic cells (BMDCs). Nevertheless, TLR2-deficient mice were capable of secreting Ab specific to SIR2RP1 after a classical immunization procedure. This could be explained by the interaction of SIR2RP1 with macrophages even in the absence of TLR2. Overall, the data presented in this study clearly demonstrate the importance of TLR2 in SIR2RP1 recognition by the innate immune system.
Materials and methods
Mice and immunization protocol
Male BALB/c and wild-type C57BL/6 mice were obtained from Harlan Iberica (Barcelona, Spain). TLR2-deficient mice with a C57BL/6 background60 were obtained from Dr S. Akira (Osaka University, Japan) via Dr Salomé Gomes (IBMC, Porto University, Portugal). Animal experiments were carried out in the approved facilities of Instituto de Biologia Molecular e Celular (IBMC) (licence no. 34-18 and 06016 to A. Ouaissi and A. Cordeiro-da-Silva, respectively). Defined groups of each strain of mice were injected three times intraperitoneally at 7-day intervals with 50 μg of SIR2RP1 in 300 μl of phosphate-buffered saline (PBS). As a control, mice received 300 μl of PBS. The sera were collected 2 weeks after the final immunization.
Purification of SIR2RP1 and thiol-dependent reductase 1 (TDR1)
The L. infantum SIR2RP1 and TDR1 proteins were obtained as recombinant proteins containing six-histidine residues at their N-terminals, as previously described.61 The purity of SIR2RP1 and TDR1 was determined by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) followed by staining with Coomassie blue. The proteins were dialysed against PBS using PD10 desalting columns (Amersham, Carnaxide, Portugal). To eliminate endotoxins, the recombinant proteins were passed through an EndoTrap®red column (Profos, Regensburg, Germany) following the manufacturer’s instructions. The concentration of both recombinant proteins was determined using the Folin procedure.
B-cell isolation, T-cell enrichment and generation of BMDCs and macrophages
After cervical dislocation, the spleens were removed and homogenized in Petri dishes to obtain a single-cell suspension. After two washes in RPMI-1640 culture medium (Cambrex, East Rutherford, NJ, USA), the cell concentrations were adjusted to 107 cells/ml in complete culture medium (RPMIc medium), consisting of RPMI-1640 culture medium supplemented with 10% fetal calf serum (FCS), 2 mm glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 20 mm HEPES and 0·05 μmβ-mercaptoethanol. Spleen B-cell isolation was carried out using the B-Cell Isolation Kit MACS® (Miltenyi Biotec, Bergisch Gladbach, Germany) as described by the manufacturer. The effectiveness of B-cell purification was determined by double labelling with specific mAbs (anti-μ+, anti-CD4+ and anti-CD8+) and further fluorescence-activated cell sorter (FACS) analysis. B-cell purity above 97% was obtained after the purification process. An enriched T-cell population was obtained from the spleen suspension depleted of B cells. Hence, the B cell depleted spleen cell suspension was left in a six-well flat-bottomed plate for 8 hr at 37° for macrophage and DC adherence. The non-adherent cells were then recovered and FACS analysis was performed, indicating T-cell enrichment between 85 and 90% in all cell preparations from BALB/c as well as C57BL/6 and TLR2(−/−) mice, with a B-cell percentage below 0·5%. BMDCs and bone marrow-derived macrophages (BMMφ) were generated as described in previous reports.62–64 Briefly, bone marrow cells were obtained by flushing the femurs of the mice with RPMI medium. The resulting cell suspension was centrifuged and the cells were re-suspended in RPMI (for BMDCs) or Dulbecco’s modified Eagle’s minimal essential medium (DMEM) (for BMMφ) supplemented with 10 mm glutamine, 10 mm HEPES, 1 mm sodium pyruvate and 10% FCS. BMDCs and BMMφ were obtained by culturing bone marrow cells from each mouse strain with recombinant granulocyte–macrophage colony-stimulating factor (GM-CSF) (Peprotech, Rocky Hill, NJ, USA) for 14 days or with L929 supernatant as source of macrophage colony-stimulating factor (M-CSF) for 10 days, respectively. After 14 days of culture, BMDCs were submitted to flow cytometry expression sorting. DCs were > 95% pure based on flow cytometry analysis of the CD11c integrin. Mφ were phenotypically characterized as F4/80+ and CD11b+ (Mac1+).
RNA isolation and semiquantitative reverse transcription–polymerase chain reaction (RT-PCR)
Isolated spleen B cells were stimulated or not with SIR2RP1 (10 μg/ml) and incubated at 37° in 5% CO2. After 20 hr of stimulation, total RNA (from 4 × 106 cells per stimulus) was isolated using a guanidium thiocyanate–phenol–chloroform single-step method.65 Reverse transcription was performed with equal amounts of total extracted RNA (1 μg) obtained from cells treated with or without SIR2RP1 using Superscript II RT (Gibco BRL, Grand Island, NY, USA) and random primers (Promega, Charbonnieres, France). An aliquot of the first strand was amplified by PCR in a thermocycler using a Taq polymerase (Gibco BRL). Specific primers for TLR1 (forward: 5′-ACATCAAGTGTGTGCTTGAA-3′; reverse: 5′-CCCCATCACTGTACCTTAGA-3′) (GenBank accession number AF316985), TLR2 (forward: 5′-ACAGCTACTGTGTGACTCTCCGCC-3′; reverse: 5′-GGTCTTGGTGTTCATTATCTTGCGC-3′) (GenBank accession number AF124741), TLR4 (forward: 5′-GACCTCAGCTTCAATGGTGC-3′; reverse: 5′-TATCAGAAATGCTACAGTGGATACC-3′) (GenBank accession number AF095353), TLR6 (forward: 5′-ACATCTCTTGCTGCCCTATG-3′; reverse: 5′-GAGGAACACTTGGTTTTTGAC-3′) (GenBank accession number AF314636), MyD88 (forward: 5′-CACCTGTGTCTGGTCCATT-3′; reverse: 5′-CGCAGGATACTGGGAAAGT-3′) (GenBank accession number MMU84409), NF-κB (forward: 5′-TGGCTACTATGAGGCTGACC-3′; reverse: 5′-GTTGATGGTGCTGAGGGAT-3′) (GenBank accession number AF199371) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (forward: 5′-CCATGGAGAAGGCTGGGG-3′; reverse: 5′-CAAAGTTGTCATGGATGACC-3′) were used for amplification. After a hot start at 94° for 3 min, a PCR cycle consisted of a denaturation step at 94° for 30 seconds, an annealing step at 55° for GAPDH, 56° for TLR1, 57° for TLR6 and NF-κB and 59° for TLR2, TLR4 and MyD88 for 30 seconds, and an elongation step for 72° for 30 seconds. For the last cycle, the elongation step was extended to 10 min at 72°. Reactions were carried out for 25–30 cycles in a thermocycler. PCR products were analysed by electrophoresis on a 1·0% agarose gel and visualized and photographed on an ultraviolet (UV) transilluminator (Vilber Lourmat, Eberhardzell, Germany). PCR band densities were determined using the bio-profil bio-1d software (Vilber Lourmat).
Measurement of cellular proliferation
Single-cell suspensions (2 × 105 cells/well) of spleen cells or isolated B cells from normal BALB/c, C57BL/6 wild-type or TLR2 knockout mice were cultured in triplicate in 96-well flat-bottom plates in a final volume of 200 μl. Cells were stimulated with lipopolysaccharide (LPS; 10 μg/ml) or SIR2RP1 (0·1, 1, 10 or 50 μg/ml) and incubated at 37° in 5% CO2. After 72 hr of incubation, [3H]thymidine (1 μCi/well) (Amersham Corp, Arlington, IL) was added for the last 8 hr. Pulsed cells were harvested on a glass filter using an automated multiple-sample harvester and dried. Incorporation of radioactive thymidine was then determined by liquid scintillation as specified in the standard protocol. Results are expressed as mean counts per minute (c.p.m.) for stimulated cells subtracted from background values for non-stimulated cells cultured in RPMIc medium.
Flow cytometry determinations
SIR2RP1 or TDR1 stimulated and non-stimulated cells (total spleen and isolated spleen B cells, BMDCs and BMMφ) from BALB/c, C57BL/6 wild-type or TLR2 knockout mice were washed by centrifugation and re-suspended in PBS supplemented with 2% FCS. For CD69 labelling, a total of 2 × 105 viable cells were incubated for 30 min at 4° with saturating concentrations of phycoerythrin (PE)-conjugated mAb to CD69 plus fluorescein isothiocyanate (FITC)-conjugated mAbs to CD4, CD8α or IgM (anti-μ) from BD Pharmingen (Le Pont de Claix, France). For other labelling, a total of 106 viable cells were incubated for 30 min at 4° in the dark with saturating concentrations of FITC-conjugated mAbs to CD40, CD80, CD86, I-Ab or I-Ad (both MHC class II) from BD Pharmingen. The mouse isotype controls IgG1 (FITC) and IgG2a (FITC) were purchased from Immunotools (Friesoythe, Germany). After two washing steps with PBS–2% FCS, the cells were analysed by flow cytometry in a FACS Scan equipped with cellquest pro software (Becton Dickinson, Pont-de-Claix, France). Lymphocytes were selected on the basis of forward-scatter/side-scatter values and dead cells were excluded from all samples by propidium iodide labelling.
Enzyme-linked immunosorbent assay (ELISA) for immunoglobulins
Sera from all of the immunized groups of mice were analysed by ELISA for the presence of anti-SIR2RP1 specific Abs. The ELISA technique used was adapted from the protocol described elsewhere.59 Briefly, 96-well flat-bottomed microtitre plates (Grainer, Courtaboeuf, France) were coated overnight at 4° with the recombinant SIR2RP1 (5 μg/ml) in 0·01 m carbonate/bicarbonate buffer, pH 8·5. After blocking and several washing steps, the plates were incubated at 37° with serial dilutions of each serum for 2 hr, followed by 30 min of incubation with peroxidase-labelled goat anti-mouse immunoglobulin isotypes (anti-IgG, anti-IgG1 and anti-IgG2a). The reactions were developed with the addition of 0·5 mg/ml of o-phenylenediamine dihydrochloride (OPD; Sigma, Sintra, Portugal) with 10 μl of H2O2 in citrate buffer. Absorbance values were read at 492 nm in an automatic ELISA reader (Power Wave XS; Bio-Tek, Winooski, VT, USA).
Cytokine ELISA
Isolated spleen B cells, BMDCs and BMMφ from BALB/c, C57BL/6 wild-type or TLR2 knockout mice were stimulated or not with LPS (1 or 10 μg/ml for APCs or B cells, respectively), SIR2RP1 (1, 5 or 10 μg/ml) or TDR1 (10 μg/ml) for 24 hr (BMDCs and BDMφ) or 72 hr (B cells). The levels of IL-10, IL-12p40, IL-12p70 and TNF-α were measured in the culture supernatants by ELISA following the manufacturer’s recommendations (BD Pharmingen for IL-10 and IL-12p70; BioLegend (San Diego, CA, USA) for IL12p40 and TNF-α). Minimum detection levels were 7·8 pg/ml for IL-12p40 and TNF-α, 31·3 pg/ml for IL-10 and 62·5 pg/ml for IL-12p70. Samples were assayed in triplicate, and the data are expressed as the mean and standard deviation (SD) for each cytokine examined.
Primary allogenic mixed lymphocyte reaction (MLR)
Immature DCs generated from mouse C57BL/6 WT or C57BL/6 TLR2(−/−) bone marrow cells were stimulated or not with SIR2RP1 (10 μg/ml) or LPS (1 μg/ml). After 4 days, DCs were washed twice with RPMIc, treated with mitomycin C (Sigma) for 20 min at 37°, washed three times with RPMIc and cultured in quadruplicate at 5 × 103 or 2 × 104 cells/200 μl/well in 96-well flat-bottomed cultured plates with 2 × 105 allogeneic T cells. Proliferation of T cells in the allogeneic MLR was measured by [3H]thymidine incorporation for the final 16 hr of the culture period of 5 days. Results are expressed as the mean (± SD) counts per minute (c.p.m.) of quadruplicate values.
Statistical analysis
Statistical analysis were performed using GraphPad Prism 5 (GraphPad Software Inc., La Jolla, CA, USA). The data were analysed using two-sided unpaired Student’s t-test. A one-way analysis of variance (anova), with a Bonferroni multiple comparison post-test, was used to assess the significance of the differences among various groups. A P-value < 0·05 was considered significant.
Results
SIR2RP1 treatment of B cells led to up-regulation of TLR2, TLR6, MyD88 and NF-κB gene transcription
In previous studies, we characterized the effect of SIR2 proteins from L. major59 and L. infantum (unpublished observations) on the spleen lymphocyte population. These proteins were found to trigger specifically B-cell effector functions, as demonstrated by CD69 up-regulation on the B-cell surface and secretion of significant levels of specific antibodies. Thus, it was of major relevance to explore the molecular basis of the SIR2RP1/B-cell interaction.
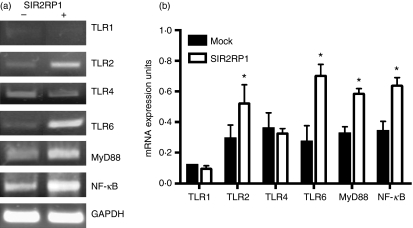
B cells possess functional characteristics of innate immune cells, as they can present antigen to T cells and can be stimulated with microbial molecules such as TLR ligands. Indeed, innate cell activation by pathogens often involves members of the TLR family.66 Therefore, we tested the involvement of these signalling molecules in SIR2RP1-mediated B-cell activation. Total RNA was isolated from purified spleen B cells cultured for 20 hr in the presence or absence of SIR2RP1 (10 μg/ml). The reverse-transcribed cDNA was submitted to PCR analysis using specific primers for TLRs, the accessory adaptor protein MyD88 and the transcription factor NF-κB (Fig. 1a). SIR2RP1 stimulus induced increased TLR2 mRNA expression compared with the unstimulated cells (Fig. 1b). As TLR2 has a unique ability to heterodimerize with TLR1 or TLR6,13 we further explored the expression of these signalling molecules following SIR2RP1 stimulus. As shown in Fig. 1b, increased TLR6 mRNA expression was observed after 20 hr of SIR2RP1 stimulus compared with unstimulated cells. However, SIR2RP1 had no effect on the expression of TLR1. Also, TLR4, which is known to recognize primarily LPS from Gram-negative bacteria,67 remained unaffected after SIR2RP1 stimulus. Recently, it has been proposed that the activation of membrane-bound TLR2 is followed by different signalling pathways. Among the signalling pathways, MyD88, an adaptor molecule, was shown to be involved in this process and upon stimulation, NF-κB, which is present in an inactive heterodimeric form in the cytoplasm, is translocated to the nucleus where it interacts with κB-responsive elements mediating transcriptional gene activation.68,69 Isolated B cells cultured in the presence of SIR2RP1 showed increased MyD88 and NF-κB transcripts, respectively, compared with unstimulated cells (Fig. 1b). Taken together, these data indicate that TLR2 and TLR6, but not TLR1, are co-expressed and might work in tandem with MyD88 and NF-κB to mediate SIR2RP1-dependent signal transduction.
Figure 1.
The effect of SIR2RP1 on mRNA expression of Toll-like receptor and downstream signaling molecules. (a) 2·5 × 105 spleen isolated B-cells were cultured with or without 2 μg of SIR2RP1 for 20 hr. Total RNA was extracted from the B cells and reverse transcribed to cDNA. The cDNA sample was used as a template for 25–30 cycles of PCR using primers for TLR1, TLR2, TLR4, TLR6, MyD88 and NF-κB. GAPDH was used as control housekeeping gene to assure the samples homogeneity. Ethidium bromide-stained PCR products were photographed, and the images were digitized and analyzed. (b) The PCR products were quantified and expressed as the ratio of each product regarding the GAPDH band density (mRNA expression units). The data represent the mean ± standard deviation of three independent experiments. *P < 0·05.
Proliferation of B cells in response to SIR2RP1 depends on TLR2 expression
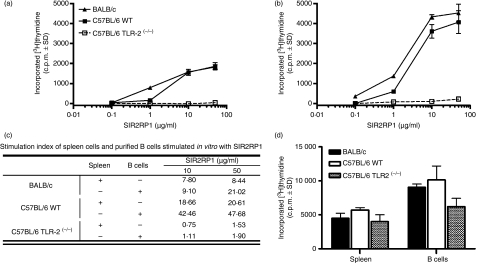
We had previously observed that SIR2RP1 triggers the direct activation of BALB/c B cells, as shown by up-regulation of the CD69 marker.59 The above data confirm and further characterize SIR2RP1-induced B-cell activation. To further examine the stimulatory activity of SIR2RP1 on the spleen cell populations, we measured proliferation by the incorporation of [3H]thymidine in total spleen and isolated B cells, when submitted to an in vitro SIR2RP1 stimulus. As shown in Fig. 2a, SIR2RP1 stimulated [3H]thymidine incorporation in spleen cells from BALB/c or C57BL/6 mice in a dose-dependent manner. The proliferating effect of SIR2RP1 was already evident at 1 μg/ml and reached a plateau at a concentration of 10 μg/ml. Interestingly, spleen cells from C57BL/6 TLR2(−/−) mice did not respond to any concentration tested. Complementary experiments were conducted to determine whether the SIR2RP1 proliferative effect on B cells was induced directly or, instead, if the presence of accessory cells, such as macrophages and DCs present in total spleen cell culture, was required. To this end, the proliferative capacity of isolated B cells purified from spleens was assessed. Similarly to total spleen cell cultures, a dose-dependent proliferative effect on B cells was observed upon SIR2RP1 treatment (Fig. 2b). Stimulation with 10 or 50 μg/ml of SIR2RP1 resulted in a significant proliferative response, when compared with SIR2RP1-stimulated total spleen cell cultures, as confirmed by an increase in the stimulatory index (SI) (using 50 μg/ml of SIR2RP1 and a 72-hr incubation period the SI reached 21·02 and 47·68 in isolated B cells versus 8·44 and 20·61 in total spleen cell cultures of BALB/c and C57BL/6 mice, respectively) (Fig. 2c). In order to rule out the possibility that LPS contaminating the SIR2RP1 preparation was responsible, at least in part, for the cell stimulation, isolated B cells from C3H/HeJ mice (LPS non-responder)67 were cultured with or without SIR2RP1. The proliferation of C3H/HeJ isolated B cells induced by the SIR2RP1 stimulus reached similar levels to that observed with BALB/c isolated B cells, as revealed by the corresponding SIs: 6·79 and 8·09 for C3H/HeJ mice and 7·80 and 8·44 for BALB/c mice, after 48 hr of stimulation with 10 and 50 μg/ml of SIR2RP1, respectively.
Figure 2.
Cell proliferation responses upon SIR2RP1 treatment. Total spleen cells (a) or purified spleen B-cells (b) 2·5 × 105 cells per well from BALB/c, C57BL/6 WT and C57BL/6 TLR2(−/−) mice were incubated with increasing concentrations of SIR2RP1 (0·02, 0·2, 2, 10 μg per well) for 72 hr. The corresponding stimulation index was determined by dividing the c.p.m. value for a given set of SIR2RP1-stimulated cells by the c.p.m. of the associated SIR2RP1-untreated cell (c) Proliferative response was also assessed using a LPS stimulus (d) Proliferation was assessed by [3H]-thymidine as described in Material and Methods section. Data are presented as mean c.p.m. and standard deviations subtracted from background values from unstimulated cells. The data are representative of three independent experiments.
The above observations suggest that B cells are the major targets of SIR2RP1. However, this does not necessarily rule out a potential role for accessory cells in vivo. In addition, isolated B cells from TLR2-deficient C57BL/6 mice have been shown to be unresponsive to SIR2RP1 stimulation. Given that the TLR2 knockout mice are known to respond similarly to LPS as C57BL/6 WT, in terms of both proliferating capacity and secretion of cytokines,60 we conducted in vitro studies using LPS as a triggering agent. As shown in Fig. 2d, C57BL/6 TLR2(−/−) mice responded in a similar manner to LPS as C57BL/6 WT or BALB/c mice, suggesting that the direct proliferative effect of SIR2RP1 on B cells specifically depends on TLR2, reinforcing the notion that SIR2RP1 is free of endotoxin contaminants.
SIR2RP1 up-regulates CD40, CD86 and MHC class II on isolated spleen B cells
To further characterize the stimulatory activity of SIR2RP1 on isolated B cells, we evaluated whether SIR2RP1 affects the expression pattern of costimulatory ligands which are critical for B-cell interaction with other immune cells, such as T lymphocytes. Therefore, 2 × 105 isolated spleen B cells were stimulated with 2 μg of SIR2RP1 for 20 hr and the expression of the accessory ligands was analysed by flow cytometry. Analysis of the relative fluorescence intensity demonstrated that SIR2RP1 only induced CD40, CD86 and class II MHC surface expression on B cells isolated from TLR2-competent mice (Table 1). The cell surface expression of CD80 (Table 1) and MHC I (data not shown) remained unaffected after SIR2RP1 stimulation. As a positive control, LPS was used, which induced up-regulation of all costimulatory molecules. The above data underline the essential role of TLR2 in regulating B-cell proliferative responses to SIR2RP1. Taken together, these observations indicate that the TLR2-dependent stimulatory activity of SIR2RP1 on B cells induces the expression of costimulatory molecules, which are known to play a crucial role in the initiation and maintenance of an immune response.
Table 1.
Increased expression of costimulatory molecules on isolated B cells and bone marrow-derived macrophages upon incubation with silent information regulator 2 related protein 1 (SIR2RP1)
| Unstimulated |
SIR2RP1 (10 μg/ml) |
Lipopolysaccharide |
|||||
|---|---|---|---|---|---|---|---|
| Cell type | Assay | C57BL/6 WT | C57BL/6 TLR2(−/−) | C57BL/6 WT | C57BL/6 TLR2(−/−) | C57BL/6 WT | C57BL/6 TLR2(−/−) |
| B cells | CD40 | 76 ± 8 | 83 ± 12 | 98 ± 12* | 86 ± 10 | 164 ± 21 | 183 ± 30 |
| CD80 | 10 ± 3 | 10 ± 1 | 9 ± 2 | 10 ± 3 | 26 ± 8 | 32 ± 11 | |
| CD86 | 6 ± 1 | 9 ± 2 | 23 ± 8** | 10 ± 3 | 42 ± 5 | 31 ± 10 | |
| MHC class II (IAb) | 18 ± 3 | 23 ± 7 | 76 ± 16** | 31 ± 9 | 256 ± 32 | 182 ± 41 | |
| Macrophages | CD40 | 6 ± 1 | 5 ± 2 | 30 ± 8** | 12 ± 3* | 35 ± 5 | 26 ± 3 |
| CD80 | 15 ± 2 | 17 ± 3 | 18 ± 2 | 20 ± 4 | 20 ± 3 | 20 ± 2 | |
| CD86 | 10 ± 2 | 9 ± 3 | 16 ± 3* | 14 ± 2* | 32 ± 7 | 32 ± 4 | |
| MHC class II (IAb) | 8 ± 2 | 7 ± 1 | 10 ± 4 | 9 ± 2 | 31 ± 9 | 28 ± 7 | |
Results are expressed as mean fluorescence intensity (MFI); mean ± standard deviation of three independent experiments is shown.
*P<0·05; **P<0·01, for comparison between SIR2RP1-stimulated and unstimulated cells.
TLR, Toll-like receptor; WT, wild type.
B cells secrete TNF-α in response to an SIR2RP1 stimulus
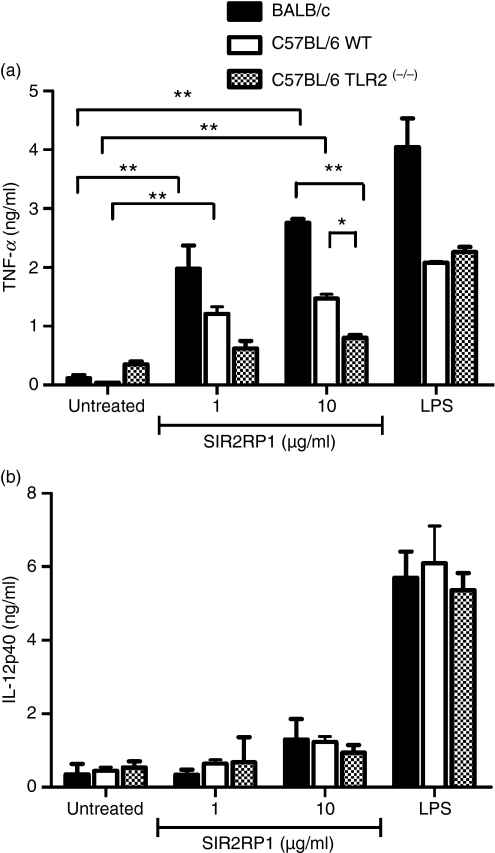
Complementary experiments were performed to further examine the effect of SIR2RP1 on the B-cell response, namely cytokine production. Hence, isolated B cells from BALB/c, C57BL/6 WT and C57BL/6 TLR2(−/−) mice were cultured for 72 hr in the presence or absence of SIR2RP1. As a positive control, isolated B cells were cultured with LPS (10 μg/ml). SIR2RP1 induced the secretion of large amounts of TNF-α even at a low dose, although this induction was higher in BALB/c than in C57BL/6 mice (Fig. 3a; P <0·01, for BALB/c and C57BL/6 mice with 1 or 10 μg/ml). Although IL-10 production by B cells upon stimulation with SIR2RP1 has been investigated, the results obtained were not conclusive. No changes were seen in the pro-inflammatory cytokine IL-12p40 (Fig. 3b). In order to confirm the specificity of this response, we performed complementary experiments with another unrelated excreted/secreted Leishmania protein named TDR1. Similar to SIR2RP1, TDR1 was obtained as an endotoxin-free recombinant protein produced in a bacterial system containing a six-histidine residue at its N-terminal. TDR1 is capable of activating B cells, but not CD4 or CD8 T cells, even in the absence of accessory cells (Fig. S1a,b). Nevertheless, the mechanism of B-cell activation was shown to be TLR2-independent, as isolated B cells purified from the spleens of C57BL/6 TLR2(−/−) mice were able to respond to TDR1 in a similar manner to TLR2-competent B cells, whatever the parameter considered: surface expression of the CD69 marker or pro-inflammatory cytokine production (IL-12p40 and TNF-α) (Fig. S1b and Table S1). Taken together, these observations support the notion of TLR2 signalling being involved in SIR2RP1-induced B-cell activation.
Figure 3.
TLR2-dependent secretion of interleukins by B cells. Purified spleen B-cells derived from BALB/c (black bars), C57BL/6 WT (white bars) or C57BL/6 TLR2(−/−) (dashed bars) mice were cultured in 96-well plates at 1 × 106/ml in the absence or the presence of LPS (1 μg/ml) or SIR2RP1 (1 or 10 μg/ml). After 72 hr of incubation, the levels of TNF-α (a) and IL-12p40 (b) were measured in the culture supernatants by ELISA. The results shown are one representative of two different experiments that yielded similar results. *P < 0·05, **P < 0·01.
TLR2 knockout mice secrete specific anti-SIR2RP1 Abs after immunization
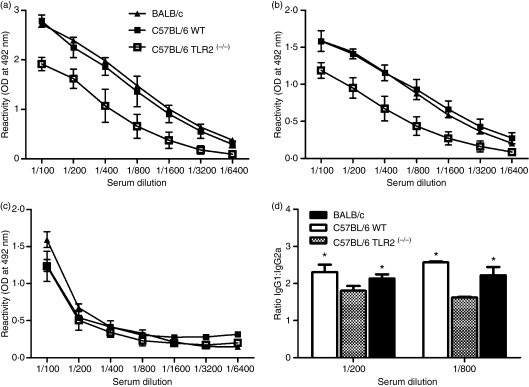
We had previously demonstrated that immunization of TLR2-competent mice (BALB/c) with SIR2RP1 induced a strong humoral response with the production of specific antibodies.59 To analyse in vivo the TLR2 dependence of the secretion of antibodies, similar immunization experiments were performed in C57BL/6 TLR2(−/−) and respective control mice (C57BL/6). We determined the levels of specific IgG and different subclasses of IgG (IgG1 and IgG2a) in the sera of mice that received three i.p. injections of SIR2RP1 at 7-day intervals. Surprisingly, TLR2-deficient mice were still able to produce specific antibodies against SIR2RP1 (Fig. 4a). Nevertheless, the humoral response was found to be lower in C57BL/6 TLR2(−/−) mice than in TLR2-competent mice, which could in part be explained by the secretion of lower amounts of IgG1 (Fig. 4b), as the levels of specific IgG2a antibodies in the serum of TLR2-deficient or -competent mice were found to be similar (Fig. 4c). A careful analysis of the SIR2RP1-specific Ab response indicated a statistically significant decrease in the SIR2RP1-specific IgG1:IgG2a ratio in C57BL/6 TLR2(−/−) mice compared with TLR2-competent mice (Fig. 4d; P <0·05).
Figure 4.
Immunization of C57BL/6 TLR2(−/−) mice with SIR2RP1 induced a decrease of IgG1/IgG2a ratio. Groups of 3 mice were immunized i.p. with SIR2RP1. Fifteen days after the last immunization, the levels of SIR2RP1-specific IgG (a) IgG1 (b) and IgG2 (c) antibodies were quantified in the sera of C57BL/6 WT, C57BL/6 TLR2(−/−), and BALB/c mice by ELISA-limiting dilution. The ratio between SIR2RP1-specific IgG1 and IgG2a levels is shown for 1/200 and 1/800 sera dilutions (d) The asterisk indicates a statistically significant difference (P < 0·05) between C57BL/6 WT or BALB/c mice and TLR2-deficient C57BL/6 mice. The data represent the mean and the standard deviation of three animals analyzed individually and representative of two independent experiments.
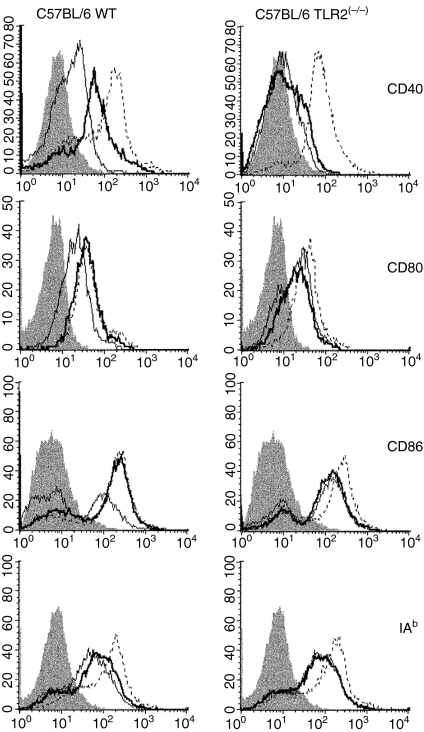
SIR2RP1 induces DC maturation in a TLR2-dependent manner
The above data therefore led us to investigate whether other TLR2-presenting cells, such as DCs or macrophages, are among the potential targets for SIR2RP1 in vivo, which may suggest a wider role for the Leishmania SIR2RP1 during the infectious process. Complementary experiments were performed in order to elucidate the role of these cells, which form a bridge between the innate and adaptive immune systems, in the recognition and processing of Leishmania SIR2RP1.
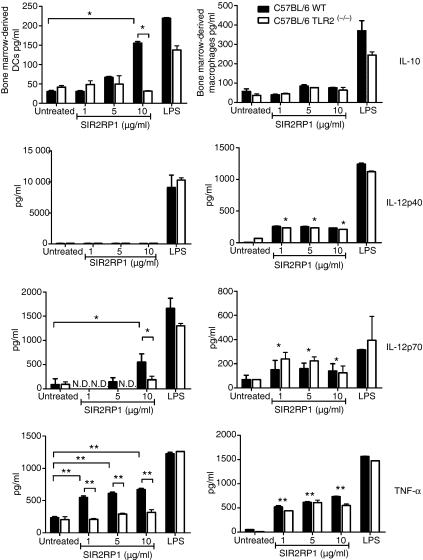
To evaluate the involvement of TLR2, BMDCs from TLR2-competent and TLR2-deficient C57BL/6 mice were stimulated for 24 hr with SIR2RP1 and the cell surface expression of activation markers was analysed by FACS. As shown in Fig. 5, SIR2RP1 induced up-regulation of surface CD40, CD80 and CD86 and a slight increase in class II MHC molecules in C57BL/6 mice. Moreover, stimulation of BMDCs with SIR2RP1 had no effect on class I MHC levels (data not shown). However, stimulation of BMDCs from TLR2-deficient mice with SIR2RP1 did not lead to up-regulation of any of these markers, although small increases were found in the CD40 molecule (Fig. 5). As a positive control, LPS induced the up-regulation of all evaluated markers in both TLR2-competent and -deficient mice. To further characterize the functionality of DCs induced to mature by SIR2RP1, we examined the ability of SIR2RP1 to induce the secretion of cytokines by DCs. In BMDCs, while LPS induced abundant secretion of both anti-inflammatory (IL-10) and pro-inflammatory (IL-12p40, IL-12p70 and TNF-α) cytokines in the presence or absence of TLR2, SIR2RP1 induced barely detectable levels of IL-12p40 and low amounts of IL-10 and IL-12p70, but abundant levels of TNF-α, in a TLR2-dependent manner (Fig. 6). Interestingly, the profile of the secreted cytokines closely resembles those induced by SIR2RP1 on isolated B cells, which confirms the requirement of TLR2 signalling for SIR2RP1-induced BMDC and B-cell activation.
Figure 5.
SIR2RP1 do not stimulate TLR2-deficient BM-DC cells. C57BL/6 WT and C57BL/6 TLR2(−/−) mice BM-DC were incubated for 24 hr with medium (thin solid line), 10 μg/ml of SIR2RP1 (thick solid line) or 1 μg/ml of LPS (dotted line) and the surface expression of CD40, CD80, CD86 and class II MHC was determined by flow cytometry analysis. The shaded line represents the correspondent isotypic control. Results are representative of one of three independent experiments.
Figure 6.
Different outcome in the SIR2RP1-APC interaction. BM-Dendritic cells and BM-Macrophage derived from C57BL/6 WT (black bars) or C57BL/6 TLR2(−/−) (white bars) mice were cultured in 24-well plates at 1 × 106/ml in the absence or the presence of LPS (1 μg/ml) or SIR2RP1 (1, 5 or 10 μg/ml). After 24 hr the levels of IL-10, IL-12p40, IL-12p70 and TNF-α were measured in the culture supernatants by ELISA. The results shown are one representative of three different experiments that yielded similar results. N.D. – below the detection limit; For BM-dendritic cells and BM-Macrophage, *P < 0·05, **P < 0·01 between SIR2RP1 stimulated and unstimulated C57Bl/6 WT or C57BL/6 TLR2(−/−) mice.
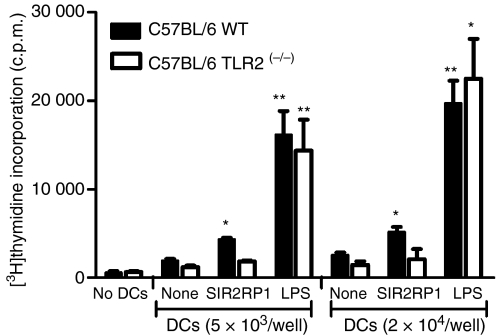
SIR2RP1 enhances the T-cell stimulatory properties of DCs
DCs induced to mature by SIR2RP1 expressed high levels of accessory and costimulatory molecules and hence should be primed to induce activation of T cells. Therefore, we tested the allostimulatory activity of SIR2RP1-stimulated DCs. In primary MLR assays, an MHC-restricted BALB/c enriched T-cell fraction (H-2d) was co-cultured with SIR2RP1-stimulated DCs from C57BL/6 WT or C57BL/6 TLR2(−/−) (H-2b) mice, and the proliferation of alloreactive T cells was quantified by the incorporation of [3H]thymidine. As shown in Fig. 7, TLR2-competent BMDCs stimulated with SIR2RP1 exhibited enhanced allostimulatory capacity compared with unstimulated DCs (SI of 2·1 ± 0·2, with 2 × 104 cells; n=4). As expected, BMDCs from C57BL/6 TLR2(−/−) mice presented low to zero levels of allostimulatory activity. As controls, BMDCs or T cells incubated alone showed an absence of proliferation and LPS-stimulated BMDCs induced high levels of proliferation of allogenic T cells.
Figure 7.
Allostimulatory capacity of BM-DC cultured with SIR2RP1. Immature DCs generated from C57Bl/6 WT or C57BL/6 TLR2(−/−) mice were stimulated with SIR2RP1 (10 μg/ml) or LPS (1 μg/ml). After 4 days, DC were treated with mitomycin C, washed, and then cultured with freshly isolated allogeneic T cells from BALB/c mice. Proliferation of T cells in the allogeneic MLR was measured by [3H]-thymidine incorporation on the final 16 hr of the culture period of 5 days. Results are expressed as mean c.p.m. of quadruplicate values. *P < 0·05 and **P < 0·01 compared to the unstimulated control.
Macrophage activation by SIR2RP1 is TLR2-independent
The above findings demonstrate the requirement for the TLR2-signalling pathway in SIR2RP1-induced B-cell and DC activation. Similar experiments were performed using BMMφ. Thus, 5 × 105 BMMφ were stimulated with 5 μg or 500 ng of SIR2RP1 or LPS, respectively. Analysis of relative fluorescence intensity after 24 hr of incubation demonstrated that SIR2RP1 induced increased expression of CD40 and CD86 compared with unstimulated cells (Table 1). In contrast, no significant increases in CD80 and class II MHC were observed. Surprisingly, TLR2-deficient BMMφ responded similarly to SIR2RP1 as TLR2-competent BMMφ. As a positive control, LPS induced up-regulation of all tested markers in both TLR2-competent and -deficient BMMφ. Moreover, cell-free supernatants of these cultures were harvested and examined by ELISA for cytokine production. SIR2RP1-induced BMMφ activation led to preferential secretion of the pro-inflammatory cytokines IL-12p40, IL-12p70 and TNF-α (Fig. 6). However, no significant differences were found in anti-nflammatory IL-10 secretion between SIR2RP1-stimulated and unstimulated cells. Interestingly, no dose-dependent response was observed for any cytokine tested and the BMMφ recovered from C57BL/6 TLR2(−/−) mice showed similar levels of cytokine secretion to C57BL/6 WT BMMφ. As a control, TDR1 stimulation of both BMMφ cultures showed an overall increase in IL-12p40 and TNF-α in both TLR2-competent and -deficient mice (Table S1). Together, these data suggest that a different type of interplay between SIR2RP1 and the major APCs might occur in vivo and probably explain the antibody response observed in TLR2-deficient mice.
Discussion
We previously reported a role for Leishmania SIR2RP1 in host B-cell activation and differentiation.59 In an attempt to explore the molecular basis of this activity, we decided to evaluate the direct effect of SIR2RP1 on the spleen B-lymphocyte population. In this paper we demonstrated that SIR2RP1 induced TLR2-dependent B-lymphocyte activation and proliferation. The TLR signalling pathway is involved in the initial recognition of Leishmania parasites by the innate immune system of the host. It has been suggested that the activation of TLRs leads to early resistance to infection by inducing IFN-γ cellular mediated immunity and helps the development of acquired immunity.70 In the past few years, several members of the TLR family and their respective adaptor molecules have been identified as key players in the resistance mechanism to a Leishmania spp. infection. However, the most convincing data indicating the importance of TLR in resistance to leishmaniasis are those obtained from infections with MyD88-deficient mice. Mice lacking the TLR adaptor protein MyD88 have increased susceptibility to infection in comparison with WT C57BL/6 mice, correlated with high levels of IL-4 and low levels of IFN-γ and IL-12.22 Although the increased susceptibility of MyD88 knockout mice does not necessarily imply a role for TLRs, as members of the IL-1 receptor subfamily also signal through the same pathway, it strongly suggests a contribution of TLR to disease outcome. In this context, experiments with TLR4-deficient mice showed that lack of this receptor led to higher parasite burdens and less efficient resolution of cutaneous lesions caused by the L. major strain, which suggests a role for TLR4 in host defence against Leishmania.71 Furthermore, using an RNA interference approach to eliminate the expression of TLRs, it was demonstrated that secretion of nitric oxide (NO) and TNF-α by infected macrophages is, at least in part, TLR2- and TLR3-dependent.40 All these observations have driven researchers to identify parasite factors that interact with TLRs. De Veer et al. and Becker et al. have focused their efforts on one of the major constituents of the promastigote surface: LPG.22,42 This molecule, but not other surface glycolipids, behaves as a TLR2 agonist capable of activating mouse macrophages and human NK cells, in a TLR2- and MyD88-dependent manner.
In several organisms, the role of TLRs in the innate and adaptive immune responses has been examined in detail. The activation of B cells, macrophages and DCs in response to different microbial products has been correlated to the presence of different TLRs on the cell surface, particularly TLR2 and TLR4. For example, structures such as Neisseria spp. and Shigella dysenteriae porins, macrophage-activating lipopeptide-2 (MALP-2) of Mycoplasma fermentans and Staphilococcus aureus protein A were shown to stimulate or sensitize B cells via TLR2.10,72–74 In a general sense, TLR activation leads to the initiation of signalling pathways, which will affect the immune response via the nuclear translocation of the transcription factor NF-κB. The data presented in this study have demonstrated that the in vitro stimulation of isolated B cells with SIR2RP1 up-regulated the mRNA level of constitutively expressed TLR2 (Fig. 1a,b). In addition to TLR2, expression of TLR6 but not TLR1 or TLR4 was enhanced, suggesting that co-expression of TLR2 and TLR6 is essential as a combinatory repertoire to detect the SIR2RP1 protein (Fig. 1a,b). Also, the mRNA for MyD88, the major effector molecule associated with the TLR-mediated response in immune cells, and NF-κB were up-regulated in SIR2RP1-stimulated B cells (Fig. 1a,b). The analysis of mRNA levels in B cells after SIR2RP1 stimulation suggests that the signalling pathway may go from the surface receptor TLR2 through the adaptor molecule MyD88, mediating its effects via the transcription factor NF-κB.
Isolated spleen B cells proliferated upon SIR2RP1 stimulation, whereas isolated B cells from TLR2-deficient mice were unresponsive (Fig. 2b). Two major conclusions may be drawn from this finding. First, SIR2RP1 directly stimulates the proliferation of B cells in vitro without the need for accessory cells. Secondly, TLR2 seems to be essential for SIR2RP1-induced B-cell activation. This conclusion was later confirmed by analysis of costimulatory molecule expression at the surface of B cells. The SIR2RP1 stimulus induced up-regulation of MHC II, CD40 and CD86 expression at the B-cell surface, which was abolished in the absence of TLR2 (Table 1). Interestingly, slight differences were observed in the proliferative responses and costimulatory molecule expression of isolated B cells from BALB/c and C57Bl/6 mice over the range of LPS concentrations tested (Fig. 2d).
We have previously characterized the Leishmania SIR2RP1 as an antigen capable of playing a dual role in vivo, being able to act as a T-cell-independent or a T-cell-dependent antigen, which was suggested to contribute to its enhanced immunogenicity.59 Nevertheless, the mechanism by which this protein is capable of inducing immunoglobulin isotype switching in the presence of T cells but not in their absence (BALB/c mice and BALB.nude mice, respectively) is unknown. Vos et al.75 have proposed that, in immunocompetent mice, some T-cell-independent antigens require a ‘second signal’ for the development of an IgG response. This could be the case for the SIR2RP1 protein. Considering that TLR2 has an essential role in SIR2RP1 signalling, one might suggest that one or several of these antigen non-specific cells could be among the targets of SIR2RP1, and play a role in isotype switching. Indeed, DCs and macrophages have already been suggested to be involved in this process, through the release of cytokines after activation as well as through the mobilization of T cells and thereby their derived cytokines.76 Also, the up-regulation of ligands such as B-cell activation factor of the TNF family (BAFF) and a proliferation-inducing ligand (APRIL), which are expressed in macrophages, DCs and T cells, provides survival signals and also may contribute to class switch recombination in B cells activated by T-cell-independent antigens.77,78 Indeed, and despite some controversy, TLRs were recently described as essential, in addition to CD4 T-cell help, for the generation of an optimal T-cell-dependent response of B cells to an antigen.79,80 Therefore, on the one hand, the observed up-regulation of surface molecules, such as MHC class II, CD40 and CD86, on B cells after SIR2RP1 stimulus suggests an enhanced capacity of these cells to present antigen to T cells, which may explain the isotype switching described in immunocompetent mice. It is known that CD86 is expressed as an early response to antigen, well ahead of CD80 expression, which is involved in a delayed response.81 On the other hand, the essential role of TLR2 in B-cell activation sheds some light on the potential contributions of other APCs, such as DCs and macrophages, either to class switch recombination or even to alternative biological roles of SIR2RP1. These hypotheses were corroborated by the fact that intravenous injection of SIR2RP1 increases the total number of spleen cells in a greater quantity than that of B cells.59 In addition, TLR2-deficient mice were able to mount a specific humoral response towards SIR2RP1 after immunization (Fig. 4a). Therefore, other cell populations, such as macrophages and DCs, may act as potential targets of SIR2RP1.
To investigate the possibility of an interaction between APCs and SIR2RP1, we performed complementary in vitro experiments with BMDCs and BMMφ from TLR2-competent (C57BL/6 WT) and -deficient (C57BL/6 TLR2(−/−)) mice. SIR2RP1 was capable of directly activating BMDCs, as shown by their up-regulation of costimulatory markers and cytokine secretion (Figs 5 and 6). Similar to B cells, the activation was dependent on the presence of TLR2, as BMDCs derived from TLR2-deficient mice were unresponsive to SIR2RP1 stimulus. Moreover, SIR2RP1 increased the T-cell costimulatory capacity of BMDCs (Fig. 7). In fact, SIR2RP1-activated BMDCs displayed increased allostimulatory activity and enhanced ability to induce the proliferation of naïve T cells in a TLR2-dependent manner. Hence, the stimulatory ability of SIR2RP1 in potentiating the immune functions of DCs can be a powerful tool for the extrinsic manipulation of immune responses. The choice of adjuvant is an important factor influencing the efficacy of any vaccine. In recent years, TLRs have been implicated in the mechanism of adjuvanticity. For example, well-known adjuvants such as monophosphoryl lipid A (MPL), imiquimod and CpG-containing oligodeoxynucleotides (CpG-ODN) produce their effects by mimicking TLR4, TLR7/8 and TLR9 agonists, respectively.82 Recently, the role of TLR2 in the adaptive immune responses induced by potential vaccine adjuvants has also been directly demonstrated. The most remarkable examples are the Neisseria spp. and S. dysenteriae porins, which have been suggested to mediate adjuvanticity by activating APCs via TLR2.10,12,73 In the majority of cases, the capacity to induce B-cell and DC activation via TLR2, along with up-regulation of cell surface costimulatory molecules, confirmed the role of TLR2 agonists as potent adjuvants.83 These results suggest the use of TLR2 agonists in the improvement of vaccine formulations against intracellular pathogens. Indeed, Wang et al.,43 using an M. tuberculosis model of infection, have suggested that the construction of fusion proteins consisting of well-characterized immunogenic proteins and a potent TLR2 agonist is a feasible approach that can be applied to many parasite proteins to provide cheap and effective new vaccines. We have previously demonstrated that an infectious Leishmania challenge after SIR2RP1 immunization results in decreased infectivity in the acute phase,84 which was interpreted to be partially attributable to the secretion of lytic and neutralizing antibodies.59 Here we have shown that in vitro SIR2RP1 stimulation induced several potent leishmanicidal mechanisms, such as increased IL-12p70 and TNF-α secretion and activation of APCs with increased allostimulatory activity. In the light of these results, it is tempting to speculate about a possible contribution of SIR2RP1 as an adjuvant in a multicomponent vaccine.
The production of SIR2RP1-specific antibodies in C57BL/6 TLR2(−/−) mice was rather unexpected, given the dependence of SIR2RP1 activation of B cells and DCs on TLR2. Therefore, we extended our analysis to the macrophage population. BMMφ stimulated with SIR2RP1 displayed up-regulation of costimulatory molecules CD40 and CD86, but not CD80 or MHC class I or II (Table 1). Also, SIR2RP1 induced the release of pro-inflammatory cytokines, such as IL-12 (both p40 and p70) and TNF-α (Fig. 6). Remarkably, these modifications were independent of TLR2, as BMMφ recovered from C57BL/6 TLR2(−/−) mice showed a similar behaviour to TLR2-competent BMMφ. These observations might explain the fact that TLR2-deficient mice were able to mount a humoral response to SIR2RP1. Moreover, SIR2RP1-stimulated macrophages displayed a pro-inflammatory cytokine profile with increased IL-12 but no IL-10 secretion, which correlated with a decrease in the ratio of SIR2RP1-specific IgG1:IgG2a compared with TLR2-competent cells (Fig. 4d). It was shown that the ratio of IgG1:IgG2a Abs generally corresponded well with the ratio of Th2:Th1 cytokines, as IL-12(−/−) mice produced primarily IgG1 Abs.85 Thus, the decrease in the IgG1:IgG2a ratio in TLR2-deficient mice could be evidence of SIR2RP1 processing by macrophages in these mice which induced the preferential secretion of IL-12. The different type of interplay found between SIR2RP1 and macrophages, when compared to DCs, suggests that receptors other than TLR2 are involved in SIR2RP1 sensing. In addition to TLRs, many other surface receptors have been proposed to participate in pathogen recognition by innate immune cells. Thus, TLR signalling is subjected to cross-talk from other signals, and such collaborative recognition of distinct pathogen components by different classes of innate immune receptors is crucial in orchestrating inflammatory responses.86,87 The C-type lectin receptor dectin-1 has been described as an example of a TLR co-operative molecule that is expressed at low levels on macrophages and high levels on DCs.86 Moreover, it is present on neutrophils, B cells and a subpopulation of T cells.88 Further studies are needed to investigate the potential role of dectin-1 in the dual APC recognition of SIR2RP1.
In conclusion, all this evidence suggests that TLR2 plays a central but not essential role in SIR2RP1 recognition. This work demonstrates that SIR2RP1-induced proliferation and surface up-regulation of costimulatory molecules on B cells and DCs is TLR2-dependent. In contrast, SIR2RP1 sensing on macrophages is TLR2-independent, suggesting that more than one surface receptor is involved in SIR2RP1 recognition. Overall, B cell and DC stimulation by TLR2 ligands such as SIR2RP1 seems to be a basic mechanism, which can be exploited to improve the immunogenicity of vaccine formulations.
Acknowledgments
This work was supported by Fundação para a Ciência e Tecnologia (FCT), Programa Operacional Ciência e Inovação 2010 (POCI 2010) and FEDER (project number POCI/CVT/59840/2004). The work was also supported by FCT and POCI 2010 and was co-funded by FEDER (projects PTD/SAU-FCF/67351/2006 and PTD/CVT/65047/2006). RS and AMS are supported by fellowships from FCT and FEDER (SFRH/BPD/41476/2007 and SFRH/BD/28316/2006). The TDR1 recombinant protein used in this work was kindly provided by Dr G. H. Coombs.
Glossary
Abbreviations:
- SI
stimulatory index
- SIR2RP1
silent information regulator 2 related protein 1
- TDR1
thiol-dependent reductase
- VL
visceral leishmaniasis
Conflict of interest
The authors have no financial conflict of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. B cells express CD69 in response to TDR1. Expression of CD69 activation marker in spleen cells from BALB/c (A) and purified spleen B cells isolated from BALB/c, C57BL/6 and C57BL/6 TLR2(−/−) mice (B) after culture with TDR1. A total of 2.5 × 105 cells per well were cultured in the presence of TDR1 (10 μg/ml) or LPS (10 μg/ml) for 20 h. To determine the percentage of CD69 in B (▪) cells or CD4 (•) or CD8 (▴) T cells, the different cell populations were positively gated. The results are from a representative experiment of two carried out independently.
Table S1. Effect of thiol-dependent reductase 1 (TDR1) on cytokine secretion by isolated spleen B cells, bone marrow-derived macrophages and bone marrow-derived dendritic cells.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than about missing material) should be directed to the corresponding author for the article.
References
- 1.Medzhitov R, Janeway CA., Jr Innate immunity: impact on the adaptive immune response. Curr Opin Immunol. 1997;9:4–9. doi: 10.1016/s0952-7915(97)80152-5. [DOI] [PubMed] [Google Scholar]
- 2.Muzio M, Mantovani A. Toll-like receptors. Microbes Infect. 2000;2:251–5. doi: 10.1016/s1286-4579(00)00303-8. [DOI] [PubMed] [Google Scholar]
- 3.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 4.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Carpenter S, O’Neill LA. How important are Toll-like receptors for antimicrobial responses? Cell Microbiol. 2007;9:1891–901. doi: 10.1111/j.1462-5822.2007.00965.x. [DOI] [PubMed] [Google Scholar]
- 6.Aliprantis AO, Yang RB, Mark MR, et al. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285:736–9. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- 7.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem. 1999;274:17406–9. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 8.Jeannin P, Renno T, Goetsch L, et al. OmpA targets dendritic cells, induces their maturation and delivers antigen into the MHC class I presentation pathway. Nat Immunol. 2000;1:502–9. doi: 10.1038/82751. [DOI] [PubMed] [Google Scholar]
- 9.Ouaissi A, Guilvard E, Delneste Y, et al. The Trypanosoma cruzi Tc52-released protein induces human dendritic cell maturation, signals via Toll-like receptor 2, and confers protection against lethal infection. J Immunol. 2002;168:6366–74. doi: 10.4049/jimmunol.168.12.6366. [DOI] [PubMed] [Google Scholar]
- 10.Massari P, Henneke P, Ho Y, Latz E, Golenbock DT, Wetzler LM. Cutting edge: immune stimulation by neisserial porins is toll-like receptor 2 and MyD88 dependent. J Immunol. 2002;168:1533–7. doi: 10.4049/jimmunol.168.4.1533. [DOI] [PubMed] [Google Scholar]
- 11.Galdiero M, Finamore E, Rossano F, et al. Haemophilus influenzae porin induces Toll-like receptor 2-mediated cytokine production in human monocytes and mouse macrophages. Infect Immun. 2004;72:1204–9. doi: 10.1128/IAI.72.2.1204-1209.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burke JM, Ganley-Leal LM, Khatri A, Wetzler LM. Neisseria meningitidis PorB, a TLR2 ligand, induces an antigen-specific eosinophil recall response: potential adjuvant for helminth vaccines? J Immunol. 2007;179:3222–30. doi: 10.4049/jimmunol.179.5.3222. [DOI] [PubMed] [Google Scholar]
- 13.Ozinsky A, Underhill DM, Fontenot JD, et al. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci U S A. 2000;97:13766–71. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwaki D, Nishitani C, Mitsuzawa H, Hyakushima N, Sano H, Kuroki Y. The CD14 region spanning amino acids 57-64 is critical for interaction with the extracellular Toll-like receptor 2 domain. Biochem Biophys Res Commun. 2005;328:173–6. doi: 10.1016/j.bbrc.2004.12.162. [DOI] [PubMed] [Google Scholar]
- 15.Hoebe K, Georgel P, Rutschmann S, et al. CD36 is a sensor of diacylglycerides. Nature. 2005;433:523–7. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- 16.Kopp EB, Medzhitov R. The Toll-receptor family and control of innate immunity. Curr Opin Immunol. 1999;11:13–8. doi: 10.1016/s0952-7915(99)80003-x. [DOI] [PubMed] [Google Scholar]
- 17.Bowie A, O’Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: signal generators for pro-inflammatory interleukins and microbial products. J Leukoc Biol. 2000;67:508–14. doi: 10.1002/jlb.67.4.508. [DOI] [PubMed] [Google Scholar]
- 18.Akira S, Yamamoto M, Takeda K. Role of adapters in Toll-like receptor signalling. Biochem Soc Trans. 2003;31:637–42. doi: 10.1042/bst0310637. [DOI] [PubMed] [Google Scholar]
- 19.Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Microbes Infect. 2004;6:1382–7. doi: 10.1016/j.micinf.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 20.Chen M, Aosai F, Norose K, Mun HS, Takeuchi O, Akira S, Yano A. Involvement of MyD88 in host defense and the down-regulation of anti-heat shock protein 70 autoantibody formation by MyD88 in Toxoplasma gondii-infected mice. J Parasitol. 2002;88:1017–9. doi: 10.1645/0022-3395(2002)088[1017:IOMIHD]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 21.Scanga CA, Aliberti J, Jankovic D, et al. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J Immunol. 2002;168:5997–6001. doi: 10.4049/jimmunol.168.12.5997. [DOI] [PubMed] [Google Scholar]
- 22.de Veer MJ, Curtis JM, Baldwin TM, et al. MyD88 is essential for clearance of Leishmania major: possible role for lipophosphoglycan and Toll-like receptor 2 signaling. Eur J Immunol. 2003;33:2822–31. doi: 10.1002/eji.200324128. [DOI] [PubMed] [Google Scholar]
- 23.Muraille E, De Trez C, Brait M, De Baetselier P, Leo O, Carlier Y. Genetically resistant mice lacking MyD88-adapter protein display a high susceptibility to Leishmania major infection associated with a polarized Th2 response. J Immunol. 2003;170:4237–41. doi: 10.4049/jimmunol.170.8.4237. [DOI] [PubMed] [Google Scholar]
- 24.Campos MA, Closel M, Valente EP, et al. Impaired production of proinflammatory cytokines and host resistance to acute infection with Trypanosoma cruzi in mice lacking functional myeloid differentiation factor 88. J Immunol. 2004;172:1711–8. doi: 10.4049/jimmunol.172.3.1711. [DOI] [PubMed] [Google Scholar]
- 25.Fremond CM, Yeremeev V, Nicolle DM, Jacobs M, Quesniaux VF, Ryffel B. Fatal Mycobacterium tuberculosis infection despite adaptive immune response in the absence of MyD88. J Clin Invest. 2004;114:1790–9. doi: 10.1172/JCI21027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drennan MB, Stijlemans B, Van den Abbeele J, et al. The induction of a type 1 immune response following a Trypanosoma brucei infection is MyD88 dependent. J Immunol. 2005;175:2501–9. doi: 10.4049/jimmunol.175.4.2501. [DOI] [PubMed] [Google Scholar]
- 27.Takeuchi O, Hoshino K, Akira S. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J Immunol. 2000;165:5392–6. doi: 10.4049/jimmunol.165.10.5392. [DOI] [PubMed] [Google Scholar]
- 28.Echchannaoui H, Frei K, Schnell C, Leib SL, Zimmerli W, Landmann R. Toll-like receptor 2-deficient mice are highly susceptible to Streptococcus pneumoniae meningitis because of reduced bacterial clearing and enhanced inflammation. J Infect Dis. 2002;186:798–806. doi: 10.1086/342845. [DOI] [PubMed] [Google Scholar]
- 29.Koedel U, Angele B, Rupprecht T, Wagner H, Roggenkamp A, Pfister HW, Kirschning CJ. Toll-like receptor 2 participates in mediation of immune response in experimental pneumococcal meningitis. J Immunol. 2003;170:438–44. doi: 10.4049/jimmunol.170.1.438. [DOI] [PubMed] [Google Scholar]
- 30.Mun HS, Aosai F, Norose K, et al. TLR2 as an essential molecule for protective immunity against Toxoplasma gondii infection. Int Immunol. 2003;15:1081–7. doi: 10.1093/intimm/dxg108. [DOI] [PubMed] [Google Scholar]
- 31.Heldwein KA, Liang MD, Andresen TK, et al. TLR2 and TLR4 serve distinct roles in the host immune response against Mycobacterium bovis BCG. J Leukoc Biol. 2003;74:277–86. doi: 10.1189/jlb.0103026. [DOI] [PubMed] [Google Scholar]
- 32.Gomes MS, Florido M, Cordeiro JV, Teixeira CM, Takeuchi O, Akira S, Appelberg R. Limited role of the Toll-like receptor-2 in resistance to Mycobacterium avium. Immunology. 2004;111:179–85. doi: 10.1111/j.0019-2805.2003.01807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gomes MS, Sousa Fernandes S, Cordeiro JV, Silva Gomes S, Vieira A, Appelberg R. Engagement of Toll-like receptor 2 in mouse macrophages infected with Mycobacterium avium induces non-oxidative and TNF-independent anti-mycobacterial activity. Eur J Immunol. 2008;38:2180–9. doi: 10.1002/eji.200737954. [DOI] [PubMed] [Google Scholar]
- 34.Sugawara I, Yamada H, Li C, Mizuno S, Takeuchi O, Akira S. Mycobacterial infection in TLR2 and TLR6 knockout mice. Microbiol Immunol. 2003;47:327–36. doi: 10.1111/j.1348-0421.2003.tb03404.x. [DOI] [PubMed] [Google Scholar]
- 35.Reiling N, Holscher C, Fehrenbach A, Kroger S, Kirschning CJ, Goyert S, Ehlers S. Cutting edge: Toll-like receptor (TLR)2- and TLR4-mediated pathogen recognition in resistance to airborne infection with Mycobacterium tuberculosis. J Immunol. 2002;169:3480–4. doi: 10.4049/jimmunol.169.7.3480. [DOI] [PubMed] [Google Scholar]
- 36.Sing A, Rost D, Tvardovskaia N, et al. Yersinia V-antigen exploits toll-like receptor 2 and CD14 for interleukin 10-mediated immunosuppression. J Exp Med. 2002;196:1017–24. doi: 10.1084/jem.20020908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sing A, Reithmeier-Rost D, Granfors K, Hill J, Roggenkamp A, Heesemann J. A hypervariable N-terminal region of Yersinia LcrV determines Toll-like receptor 2-mediated IL-10 induction and mouse virulence. Proc Natl Acad Sci USA. 2005;102:16049–54. doi: 10.1073/pnas.0504728102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Netea MG, Warris A, Van der Meer JW, et al. Aspergillus fumigatus evades immune recognition during germination through loss of toll-like receptor-4-mediated signal transduction. J Infect Dis. 2003;188:320–6. doi: 10.1086/376456. [DOI] [PubMed] [Google Scholar]
- 39.Bellocchio S, Montagnoli C, Bozza S, et al. The contribution of the Toll-like/IL-1 receptor superfamily to innate and adaptive immunity to fungal pathogens in vivo. J Immunol. 2004;172:3059–69. doi: 10.4049/jimmunol.172.5.3059. [DOI] [PubMed] [Google Scholar]
- 40.Flandin JF, Chano F, Descoteaux A. RNA interference reveals a role for TLR2 and TLR3 in the recognition of Leishmania donovani promastigotes by interferon-gamma-primed macrophages. Eur J Immunol. 2006;36:411–20. doi: 10.1002/eji.200535079. [DOI] [PubMed] [Google Scholar]
- 41.Silvestre R, Santarém N, Tavares J, Silva AM, Cordeiro-da-Silva Recognition of Leishmania parasites by innate immunity. Immun Endoc & Metab Agents in Med Chem. 2009;9 (In Press) [Google Scholar]
- 42.Becker I, Salaiza N, Aguirre M, et al. Leishmania lipophosphoglycan (LPG) activates NK cells through toll-like receptor-2. Mol Biochem Parasitol. 2003;130:65–74. doi: 10.1016/s0166-6851(03)00160-9. [DOI] [PubMed] [Google Scholar]
- 43.Wang B, Henao-Tamayo M, Harton M, Ordway D, Shanley C, Basaraba RJ, Orme IM. A Toll-like receptor-2-directed fusion protein vaccine against tuberculosis. Clin Vaccine Immunol. 2007;14:902–6. doi: 10.1128/CVI.00077-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alexander J, Phillips RS. Leishmania mexicana and Leishmania tropica major: adoptive transfer of immunity in mice. Exp Parasitol. 1980;49:34–40. doi: 10.1016/0014-4894(80)90053-3. [DOI] [PubMed] [Google Scholar]
- 45.Brown DR, Reiner SL. Polarized helper-T-cell responses against Leishmania major in the absence of B cells. Infect Immun. 1999;67:266–70. doi: 10.1128/iai.67.1.266-270.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Varkila K, Chatelain R, Leal LM, Coffman RL. Reconstitution of C.B-17 scid mice with BALB/c T cells initiates a T helper type-1 response and renders them capable of healing Leishmania major infection. Eur J Immunol. 1993;23:262–8. doi: 10.1002/eji.1830230141. [DOI] [PubMed] [Google Scholar]
- 47.Sacks DL, Perkins PV. Identification of an infective stage of Leishmania promastigotes. Science. 1984;223:1417–9. doi: 10.1126/science.6701528. [DOI] [PubMed] [Google Scholar]
- 48.Hoerauf A, Solbach W, Lohoff M, Rollinghoff M. The Xid defect determines an improved clinical course of murine leishmaniasis in susceptible mice. Int Immunol. 1994;6:1117–24. doi: 10.1093/intimm/6.8.1117. [DOI] [PubMed] [Google Scholar]
- 49.Smelt SC, Cotterell SE, Engwerda CR, Kaye PM. B cell-deficient mice are highly resistant to Leishmania donovani infection, but develop neutrophil-mediated tissue pathology. J Immunol. 2000;164:3681–8. doi: 10.4049/jimmunol.164.7.3681. [DOI] [PubMed] [Google Scholar]
- 50.Ochsenbein AF, Zinkernagel RM. Natural antibodies and complement link innate and acquired immunity. Immunol Today. 2000;21:624–30. doi: 10.1016/s0167-5699(00)01754-0. [DOI] [PubMed] [Google Scholar]
- 51.Woelbing F, Kostka SL, Moelle K, et al. Uptake of Leishmania major by dendritic cells is mediated by Fcgamma receptors and facilitates acquisition of protective immunity. J Exp Med. 2006;203:177–88. doi: 10.1084/jem.20052288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brachmann CB, Sherman JM, Devine SE, Cameron EE, Pillus L, Boeke JD. The SIR2 gene family, conserved from bacteria to humans, functions in silencing, cell cycle progression, and chromosome stability. Genes Dev. 1995;9:2888–902. doi: 10.1101/gad.9.23.2888. [DOI] [PubMed] [Google Scholar]
- 54.Tsukamoto Y, Kato J, Ikeda H. Silencing factors participate in DNA repair and recombination in Saccharomyces cerevisiae. Nature. 1997;388:900–3. doi: 10.1038/42288. [DOI] [PubMed] [Google Scholar]
- 55.Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–80. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–30. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- 57.Vergnes B, Sereno D, Tavares J, et al. Targeted disruption of cytosolic SIR2 deacetylase discloses its essential role in Leishmania survival and proliferation. Gene. 2005;363:85–96. doi: 10.1016/j.gene.2005.06.047. [DOI] [PubMed] [Google Scholar]
- 58.Yahiaoui B, Taibi A, Ouaissi A. A Leishmania major protein with extensive homology to silent information regulator 2 of Saccharomyces cerevisiae. Gene. 1996;169:115–8. doi: 10.1016/0378-1119(95)00785-7. [DOI] [PubMed] [Google Scholar]
- 59.Silvestre R, Cordeiro-da-Silva A, Tavares J, Sereno D, Ouaissi A. Leishmania cytosolic silent information regulatory protein 2 deacetylase induces murine B-cell differentiation and in vivo production of specific antibodies. Immunology. 2006;119:529–40. doi: 10.1111/j.1365-2567.2006.02468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takeuchi O, Hoshino K, Kawai T, et al. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–51. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 61.Tavares J, Ouaissi A, Santarem N, Sereno D, Vergnes B, Sampaio P, Cordeiro-da-Silva A. The Leishmania infantum cytosolic SIR2 related protein 1 (LiSIR2RP1) is an NAD+-dependent deacetylase and ADP-ribosyltransferase. Biochem J. 2008;415:377–86. doi: 10.1042/BJ20080666. [DOI] [PubMed] [Google Scholar]
- 62.Celada A, Gray PW, Rinderknecht E, Schreiber RD. Evidence for a gamma-interferon receptor that regulates macrophage tumoricidal activity. J Exp Med. 1984;160:55–74. doi: 10.1084/jem.160.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Swanson MS, Isberg RR. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect Immun. 1995;63:3609–20. doi: 10.1128/iai.63.9.3609-3620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Inaba K, Inaba M, Romani N, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 66.Medzhitov R, Janeway C., Jr The Toll receptor family and microbial recognition. Trends Microbiol. 2000;8:452–6. doi: 10.1016/s0966-842x(00)01845-x. [DOI] [PubMed] [Google Scholar]
- 67.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–8. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 68.Arbibe L, Mira JP, Teusch N, et al. Toll-like receptor 2-mediated NF-kappa B activation requires a Rac1-dependent pathway. Nat Immunol. 2000;1:533–40. doi: 10.1038/82797. [DOI] [PubMed] [Google Scholar]
- 69.Zhong H, SuYang H, Erdjument-Bromage H, Tempst P, Ghosh S. The transcriptional activity of NF-kappaB is regulated by the IkappaB-associated PKAc subunit through a cyclic AMP-independent mechanism. Cell. 1997;89:413–24. doi: 10.1016/s0092-8674(00)80222-6. [DOI] [PubMed] [Google Scholar]
- 70.Gazzinelli RT, Denkers EY. Protozoan encounters with Toll-like receptor signalling pathways: implications for host parasitism. Nat Rev Immunol. 2006;6:895–906. doi: 10.1038/nri1978. [DOI] [PubMed] [Google Scholar]
- 71.Kropf P, Freudenberg MA, Modolell M, et al. Toll-like receptor 4 contributes to efficient control of infection with the protozoan parasite Leishmania major. Infect Immun. 2004;72:1920–8. doi: 10.1128/IAI.72.4.1920-1928.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Borsutzky S, Kretschmer K, Becker PD, Muhlradt PF, Kirschning CJ, Weiss S, Guzman CA. The mucosal adjuvant macrophage-activating lipopeptide-2 directly stimulates B lymphocytes via the TLR2 without the need of accessory cells. J Immunol. 2005;174:6308–13. doi: 10.4049/jimmunol.174.10.6308. [DOI] [PubMed] [Google Scholar]
- 73.Ray A, Biswas T. Porin of Shigella dysenteriae enhances Toll-like receptors 2 and 6 of mouse peritoneal B-2 cells and induces the expression of immunoglobulin M, immunoglobulin G2a and immunoglobulin A. Immunology. 2005;114:94–100. doi: 10.1111/j.1365-2567.2004.02002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bekeredjian-Ding I, Inamura S, Giese T, et al. Staphylococcus aureus protein A triggers T cell-independent B cell proliferation by sensitizing B cells for TLR2 ligands. J Immunol. 2007;178:2803–12. doi: 10.4049/jimmunol.178.5.2803. [DOI] [PubMed] [Google Scholar]
- 75.Vos Q, Lees A, Wu ZQ, Snapper CM, Mond JJ. B-cell activation by T-cell-independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganisms. Immunol Rev. 2000;176:154–70. doi: 10.1034/j.1600-065x.2000.00607.x. [DOI] [PubMed] [Google Scholar]
- 76.Mond JJ, Vos Q, Lees A, Snapper CM. T cell independent antigens. Curr Opin Immunol. 1995;7:349–54. doi: 10.1016/0952-7915(95)80109-x. [DOI] [PubMed] [Google Scholar]
- 77.Castigli E, Wilson SA, Scott S, et al. TACI and BAFF-R mediate isotype switching in B cells. J Exp Med. 2005;201:35–9. doi: 10.1084/jem.20032000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schneider P. The role of APRIL and BAFF in lymphocyte activation. Curr Opin Immunol. 2005;17:282–9. doi: 10.1016/j.coi.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 79.Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;438:364–8. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- 80.Lanzavecchia A, Sallusto F. Toll-like receptors and innate immunity in B-cell activation and antibody responses. Curr Opin Immunol. 2007;19:268–74. doi: 10.1016/j.coi.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 81.Constant SL, Bottomly K. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu Rev Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 82.O’Hagan DT, MacKichan ML, Singh M. Recent developments in adjuvants for vaccines against infectious diseases. Biomol Eng. 2001;18:69–85. doi: 10.1016/s1389-0344(01)00101-0. [DOI] [PubMed] [Google Scholar]
- 83.Massari P, Ram S, Macleod H, Wetzler LM. The role of porins in neisserial pathogenesis and immunity. Trends Microbiol. 2003;11:87–93. doi: 10.1016/s0966-842x(02)00037-9. [DOI] [PubMed] [Google Scholar]
- 84.Santarém N, Silvestre R, Tavares J, Silva M, Cabral S, Maciel J, Cordeiro-da-Silva A. Immune response regulation by Leishmania secreted and nonsecreted antigens. J Biomed Biotechnol. 2007;2007:85154. doi: 10.1155/2007/85154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kopf M, Le Gros G, Coyle AJ, Kosco-Vilbois M, Brombacher F. Immune responses of IL-4, IL-5, IL-6 deficient mice. Immunol Rev. 1995;148:45–69. doi: 10.1111/j.1600-065x.1995.tb00093.x. [DOI] [PubMed] [Google Scholar]
- 86.Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med. 2003;197:1107–17. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.O’Neill LA. When signaling pathways collide: positive and negative regulation of toll-like receptor signal transduction. Immunity. 2008;29:12–20. doi: 10.1016/j.immuni.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 88.Willment JA, Marshall AS, Reid DM, Williams DL, Wong SY, Gordon S, Brown GD. The human beta-glucan receptor is widely expressed and functionally equivalent to murine Dectin-1 on primary cells. Eur J Immunol. 2005;35:1539–47. doi: 10.1002/eji.200425725. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.