Abstract
Mutations of peptides to generate altered peptide ligands, capable of switching immune responses from T helper 1 (Th1) to T helper 2 (Th2), are promising candidates for the immunotherapy of autoimmune diseases such as multiple sclerosis (MS). We synthesized two mutant peptides from myelin basic protein 87–99 (MBP87–99), an immunodominant peptide epitope identified in MS. Mutations of residues K91 and P96, known to be critical T-cell receptor (TCR) contact sites, resulted in the mutant peptides [R91, A96]MBP87–99 and [A91, A96]MBP87–99. Immunization of mice with these altered peptide ligands emulsified in complete Freund’s adjuvant induced both interferon-γ (IFN-γ) and interleukin-4 (IL-4) responses compared with only IFN-γ responses induced to the native MBP87–99 peptide. It was of interest that [R91, A96]MBP87–99 conjugated to reduced mannan induced 70% less IFN-γ compared with the native MBP87–99 peptide. However, [A91, A96]MBP87–99 conjugated to reduced mannan did not induce IFN-γ-secreting T cells, but elicited very high levels of interleukin-4 (IL-4). Furthermore, antibodies generated to [A91, A96]MBP87–99 peptide conjugated to reduced mannan did not cross-react with the native MBP87–99 peptide. By molecular modelling of the mutant peptides in complex with major histocompatibility complex (MHC) class II, I-As, novel interactions were noted. It is clear that the double-mutant peptide analogue [A91, A96]MBP87–99 conjugated to reduced mannan is able to divert immune responses from Th1 to Th2 and is a promising mutant peptide analogue for use in studies investigating potential treatments for MS.
Keywords: altered peptide ligand, autoimmunity, I-As, mutant analogue, myelin basic protein
Introduction
Myelin basic protein (MBP)a is a major autoantigen in the autoimmune disease, multiple sclerosis (MS).1 Consequently, T cells specific for the MBP87–99 epitope (VHFFKNIVTPRTP) have been detected in the blood or cerebrospinal fluid of MS patients2–5 and are related to the induction of MS in humans.6,7 Immunization with the MBP87–99 epitope induces experimental autoimmune encephalomyelitis (EAE) in SJL/J mice8,9 and in Lewis rats.10
Studies have shown that amino acids F90, N92, I93 and V94 interact with major histocompatibility complex (MHC), whilst amino acids K91, T95 and P96 interact with the T-cell receptor (TCR).10–12 Single alanine substitutions at positions 91, 95 or 96 of MBP87–99 were able to antagonize T-cell responses in vitro, with [A95]MBP87–99 and [A96]MBP87–99 being the most effective.10 However, in Lewis rats, [A91]MBP87–99 was able to prevent and reverse the clinical signs of EAE whilst [A95]MBP87–99 and [A96]MBP87–99 did not.10 In addition, administration of the [A91]MBP87–99 peptide analogue reduced the production of tumour necrosis factor-α (TNF-α) and interferon-γ (IFN-γ), cytokines responsible for inflammation.10
In another study, a single alanine substitution at position 96 to [A96]MBP87–99 prevented and reversed EAE. It was shown that [A96]MBP87–99 bound weakly to I-As and induced a lower response to the L10C1 clone, a T-cell line specific for the native MBP87–99 peptide, which induces severe EAE.13 Single mutant analogues ([A91]MBP87–99, [A92]MBP87–99 and [A93]MBP87–99) were not beneficial for the treatment of EAE in (PL/JxSJL/J)F1 mice.13 The mechanism of tolerance of the [A91]MBP87–99 peptide analogue showed that in vitro it acts as a partial agonist for the L10C1 clone, and its therapeutic effect for EAE cannot be attributed to MHC or TCR antagonism.13 Furthermore, EAE induced from T cells specific to the MBP1–11 epitope could be ameliorated when mice were injected with [A96]MBP87–99 and this effect was related to interleukin (IL)-4 secretion.13 Neutralization of IL-4 using an antibody to IL-4 was shown to block the therapeutic effect of the [A96]MBP87–99 peptide analogue.13 Moreover, the [A96]MBP87–99 peptide increased the IL-4 : TNF-α ratio.13 IL-4 has been found to be a potent inhibitor of TNF-α and is responsible for the inhibition of EAE.14,15 Additionally, IL-10 and IL-13 can inhibit TNF-α production and EAE.
The single-mutant [A91]MBP87–99 and [A97]MBP87–99 peptide analogues could block the development of EAE in SJL/J mice, ameliorate the symptoms of EAE and reduce the proliferation of a T-cell clone specific for the MBP87–99 epitope by 70%. The non-encephalitogenic [A91]MBP87–99 peptide analogue could also increase the IL-4 : IFN-γ or the IL-4 : IL-2 ratios.16 Conversely, the superagonist [A97]MBP87–99 peptide caused deletion of MBP87–99-responding cells. Thus, a single substitution at different positions of the MBP87–99 epitope plays an important role in the modulation of the immune response and could inhibit EAE.
We designed and synthesized linear MBP87–99 peptide and double-mutant analogues containing substitutions of critical TCR contact residues.17–20 Positions K91 and P96 were replaced with alanine/alanine ([A91, A96]MBP87–99) or with arginine/alanine ([R91, A96]MBP87–99), respectively. The K91 and P96 residues have also been predicted to be TCR contact residues when bound to H2 I-As.21 Molecular modelling studies with I-As suggested that the side-chains of the mutated residues at positions 91 and 96 were exposed to make contact with the TCR,11,12 which is similar to HLA-DR2. In addition, similarly to MS, EAE susceptibility is dependent on the MHC background of the mouse, and different peptides are immunogenic and induce EAE in different mouse strains. The SJL/J mouse strain (H2s haplotype) is commonly used for EAE, as numerous histopathological, clinical and immunological features resemble those of human MS. SJL/J mice do not express the H2-E-α chain, and therefore the only functional MHC class II molecule in this strain is H2-As (I-As). In the SJL/J mouse strain, residues from the encephalitogenic epitope MBP81–100 have been shown to bind with high affinity. The minimum epitopes required for binding are represented by the peptides MBP83–99 and MBP87–99.21 Thus, the MBP87–99 peptide is a potential candidate for the design of peptide analogues, which could be used to alter T-cell responses in the SJL/J mouse model.
In our previous studies, native linear MBP87–99 peptide induced EAE in Lewis rats, which was inhibited by the linear double-mutant analogue [R91, A96]MBP87–99. In addition, [R91, A96]MBP87–99 increased the T helper 2(Th2) : T helper 1 (Th1) cytokine ratio in blood from MS patients in vitro and suppressed proliferation of the CD4+ T-cell line from MS patients.22 Single or double mutants of the longer MBP83–99 peptide epitope conjugated to reduced mannan could divert immune responses from Th1 to Th2 in SJL/J mice.1,11,12,23,24 Reduced mannan targets C-type lectin receptors, including the mannose receptor on dendritic cells, and generates Th2 responses.25–32
Herein, we examined cytokine induction by two double-mutant peptide analogues of the minimal MBP87–99 peptide ([R91, A96]MBP87–99 and [A91, A96]MBP87–99) compared with the native peptide, when emulsified in complete Freund’s adjuvant (CFA) or when conjugated to reduced mannan. The [A91, A96]MBP87–99 peptide analogue conjugated to a suitable carrier (reduced mannan) did not induce IFN-γ-secreting T cells, but elicited very high levels of IL-4, and thus the diversion of the cytokine profile. As determined by structural analysis, the [A91, A96]MBP87–99 peptide showed the greatest differences in intermolecular hydrogen-bond interactions compared with [R91, A96]MBP87–99 and native MBP87–99 peptides.
Materials and methods
Solid-phase peptide synthesis of peptide analogues
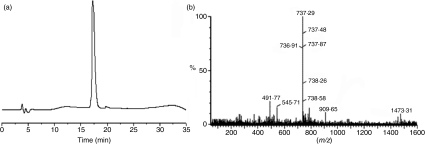
Peptides MBP87–99 (VHFFKNIVTPRTP), [R91, A96]MBP87–99 (VHFFRNIVTARTP) and [A91, A96]MBP87–99 (VHFFANIVTARTP) were prepared on 2-chlorotrityl chloride resin (CLTR-Cl) using Fmoc/tBu methodology.22,33–36. Preparative high-performance liquid chromatography (HPLC) for MBP87–99, [R91, A96]MBP87–99 and [A91, A96]MBP87–99 peptide analogues was performed using a Lichrosorb RP-18 reverse-phase semipreparative column with 7 μm packing material. The peptides were > 95% pure, as analyzed by analytical reverse-phase (RP)-HPLC and electrospray ionization-mass spectrometry (ESI-MS) (Scheme 1).
Scheme 1.
(a) Analytical reverse-phase high-performance liquid chromatography (RP-HPLC) of purified linear [A91, A96]MBP87–99 analogue after its purification by semipreparative RP-HPLC and lyophilization. Column: Nucleosil C18, 250 × 4·6 mm, 5 μm. TR: 17·3 min. Conditions: gradient 5% (B)–100% (B) in 35 min; flow rate 1 ml/min. [Eluents (A): Solution trifluoroacetic acid (TFA) in H2O 0·08% (v/v), (B): Solution TFA in acetyl-nitrile (AcN) 0·08% (v/v)]. (b) Electrospray ionization-mass spectrometry (ESI-MS) of linear [A91, A96]MBP87–99 analogue. M+ : 1473·31, M+2H+/2 : 737·29.
Conjugation of reduced mannan to MBP87–99 peptides via a keyhole limpet haemocyanin linker
MBP87–99, [R91, A96]MBP87–99 or [A91, A96]MBP87–99 peptides were conjugated to keyhole limpet haemocyanin (KLH) via glutaraldehyde, which acts as a linker between mannan and peptide.27 Mannan (14 mg from Saccharomyces cerevisiae; Sigma, Melbourne, Vic., Austraila) was dissolved in 1 ml of sodium phosphate buffer (pH 6·0), then 100 μl of 0·1 m sodium periodate (dissolved in pH 6·0 phosphate buffer) was added and the mixture was incubated at 4° for 1 hr in the dark. Ethanediol (10 μl) was added to the mixture, which was then incubated for a further 30 min at 4°. The resultant mixture (oxidized mannan) was passed through a PD-10 column (Sephadex G-25 M column; Amersham Biosciences, Melbourne, Vic., Austraila) pre-equilibrated in phosphate buffer (pH 9·0) and 2 ml of solution comprising oxidized mannan was collected. One milligram each of MBP87–99–KLH, [A91, A96]MBP87–99–KLH or [R91, A96]MBP87–99–KLH peptides were added to 2 ml of an oxidized mannan solution and incubated overnight at room temperature in the dark. Conjugation occurs via Schiff base formation between free amino groups of KLH and oxidized mannan. Reduced mannan–KLH–MBP87–99, reduced mannan–KLH–[R91, A96]MBP87–99 or reduced mannan–KLH–[A91, A96]MBP87–99 complexes were prepared by adding 1·0 mg of sodium borohydride for 6–8 hr at room temperature in the dark and were used without further purification, as previously described.12,23,30,31 MBP peptide analogues were previously characterized by capillary electropheresis for conjugation to mannan37 and by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE), followed by staining with Coomassie Brilliant Blue, Silver stain or Schiff’s reagent (data not shown). Peptides were 100% conjugated to reduced mannan.
Mice and immunizations
Female, 6–8-week-old SJL/J mice, used in this study, were purchased from the Walter and Eliza Hall Institute (Vic., Australia) and housed at the Biological Research Laboratory at Burnet Institute (Austin campus, Heidelberg, Australia) in accordance with the guidelines of the National Health and Medical Research Council (NIMRC) of Australia. For EAE experiments, mice were purchased from the Hellenic Pasteur Institute (Athens, Greece) and housed in the P3 facility of the B’ Neurological department of the AHEPA University Hospital, Aristotle University Medical School, in accordance with the National Institute of Health (NIH) guidelines. MBP87–99, [R91, A96]MBP87–99 or [A91, A96]MBP87–99 peptides were dissolved in phosphate-buffered saline (PBS) and emulsified in an equal volume of CFA (Sigma). SJL/J mice were given one subcutaneous injection containing 50 μg of peptide. For immunizations with peptide–KLH–reduced mannan, SJL/J mice were immunized twice on days 0 and 14, intradermally (at the base of the tail), with 50 μg of MBP87–99–KLH–reduced mannan, [R91, A96]MBP87–99–KLH–reduced mannan or [A91, A96]MBP87–99–KLH–reduced mannan conjugates. For EAE experiments, seven mice per group ([A91, A96]MBP87–99–KLH–reduced mannan, reduced mannan alone or the control PBS group) were given three intradermal injections in the base of the tail.
Enzyme-linked immunospot assay
The enzyme-linked immunospot (ELISpot) assay detects specific T-cell responses to antigens by measuring the secretion of specific cytokines from individual cells. Spleen cells from immunized SJL/J mice were isolated 28 days after immunization with CFA or 14 days after the last immunization with reduced mannan-peptides and were assessed using ELISpot assays for IFN-γ and IL-4 secretion by T cells. The IFN-γ ELISpot assay was performed on a MultiScreen-IP Filter Plate (MAIP S4510) with hydrophobic poly(vinylidene difluoride) (PVDF) filters (Millipore, Melbourne, Vic., Australia), while IL-4 ELISpot assays were performed on a MultiScreen-HA Filter Plate (MAHA S4510) with mixed cellulose-ester filters (Millipore). MAIP S4510 plates were prewetted with 50 μl of 70% ethanol, washed five times with 200 μl of sterile PBS and coated with 70 μl of 5 μg/ml of anti-IFN-γ capture antibody, AN18 (Mabtech, Melbourne, Vic., Australia) in PBS and incubated overnight at 4°. Seventy microlitres of 5 μg/ml anti-IL-4 capture antibody (Mabtech) was added directly to MAHA S4510 plates and incubated overnight at 4° without treatment with 70% ethanol. Following five washes with PBS, plates were blocked by the addition of 200 μl of culture medium (supplemented with 2·5% fetal calf serum) and incubated for 2 hr at 37°. The blocking medium was discarded and 10 μg/ml of MBP87–99, [R91, A96]MBP87–99 or [A91, A96]MBP87–99 recall peptides were added into each defined well. Concanavalin A (ConA) (1·0 μg/ml) was used as an internal positive control and no peptide (cells alone) was used as a negative control. Triplicate wells were set up for each condition. Spleen cells (0·5 million cells) in 100 μl of culture medium were seeded into each well and incubated at 37° for 18 hr for IFN-γ production or at 37° for 24 hr for IL-4 production. The plates were washed five times with PBS/0·05% Tween 20, five times with PBS and then incubated for 2 hr at room temperature with anti-mouse IFN-γ or IL-4 monoclonal antibody–biotin. Plates were washed and streptavidin–alkaline phosphate (ALP) conjugate was added at 1·0 μg/ml and incubated for a further 2 hr at room temperature. Spots of activity were detected using a colorimetric AP kit (Bio-Rad, Hercules, CA) and counted using an AID ELISpot plate reader (Autoimmun Diagnostika GmbH, Heidelberg, Germany). Data are presented as mean spot-forming units (SFU) per 0·5 million cells ± standard error of the mean (SEM).
Enzyme-linked immunosorbent assay
Blood was collected and sera isolated from mice before and 4 weeks after immunization with CFA, or 14 days after the last immunization with reduced mannan conjugates. MBP87–99 or [A91, A96]MBP87–99 peptides conjugated to bovine serum albumin (BSA) were coated onto polyvinyl chloride (PVC) microtiter plates at 10 μg/ml in 0·2 m NaHCO3 buffer, pH 9·6, overnight at 4°. Non-specific binding was blocked with 2% BSA for 1·0 hr at room temperature. After washing (0·05% Tween-20/PBS), serial dilutions of sera were added and incubated for a further 2 hr at room temperature. The plates were washed and bound antibody was detected using horseradish peroxidase (HRP)-conjugated sheep anti-mouse IgG (1 : 1000 dilution in PBS) (Amersham, Melbourne, Vic., Australia) and developed using 2,2’-azino-di(3-ethylbenzthiazoline)6-sulfonic acid (ABTS) (Sigma, UK). Absorbance at 405 nm was recorded using a Fluostar Optima microplate reader (BMG Labtech, Offenburg, Germany).
EAE induction and clinical evaluation
EAE was induced in SJL mice after two immunizations. On the day of EAE induction, a further immunization with the conjugates was performed. EAE was induced with a subcutaneous injection of 150 μg of proteolipid protein (PLP) (Day 0) emulsified in 100 μl of an emulsion composed of 50 μl of PBS and 50 μl of CFA containing 2 mg/ml of Mycobacterium tuberculosis H37RA. One-hundred microlitres of the emulsion was injected into each hind-flank of each animal. In addition, mice were injected intraperitoneally with 200 ng of pertussis toxin (Sigma) diluted in 0·5 ml of filtered PBS (day 0) and with another 100 ng of pertussis toxin diluted in 0·5 ml of filtered PBS on day 2 post-induction. Mice were clinically evaluated and weighed daily. The clinical status of each mouse was graded using the following scale: 0, normal; 1, flail tail; 2, tail paralysis; 3, hind limb weakness sufficient to impair righting; 4, paraplegia; 5, paraplegia with forelimb paresis or plegia; 6, death from EAE.
Statistical analysis
Mean values were compared using the Student’s two-tailed t-test for all immunological analyses. A P-value threshold of < 0·01 indicates a statistically significant difference. For EAE experiments, statistical analysis of the data were performed using the spss 11·5 software (Gainesville, FL). Values are expressed as mean ± standard deviation (SD).
Molecular modeling
Molecular modelling was carried out using the HyperChem modelling package (version 7·52; HyperCube Inc. Gainesville, FL) as previously described.12 The optimized potentials for liquid simulations (OPLS) force field was used for molecular mechanics geometry optimization. The MHC–peptide complexes were generated based on the crystal structure of the I-Au complex with MBP1–11 peptide [protein data bank (PDB) code 1K2D]. This template was chosen upon the consideration of nine relevant crystal structures, based on the combination of sequence identity to the target, crystal structure, resolution, and the degree of disruption to the peptide interaction residues, upon mutation.11,12 Alignment of the MBP peptide within the MHC cleft was carried out based on the analysis of all possible MBP positions, and the following preferred binding register was deduced.11,12
To produce mutated MBP peptides (see below), the lysine residue at position 91 and the proline residue at position 96 were mutated to A91 and A96 ([A91, A96]MBP87–96) and R91 and A96 ([R91, A96]MBP87–96), respectively. The complexes were then optimized and the intermolecular interactions in the complexes were studied using the program LigPlot.38
| 1K2D | (G | G) | A1 | S | Q | Y | R | P | S | Q8 |
| MBP | V87 | H | F | F | K | N | I | V | T | P96 |
Results
[R91, A96]MBP87–99 peptide conjugated to reduced mannan decreases IFN-γ production and generates high levels of IL-4
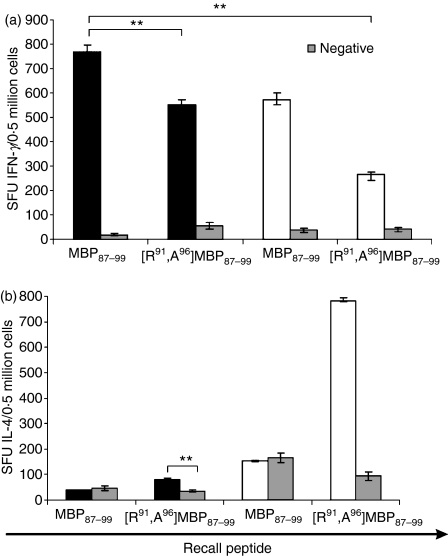
The ability of the native MBP87–99 peptide and the double mutant analogue [R91, A96]MBP87–99 to induce immune responses in SJL/J mice was assessed using IFN-γ and IL-4 ELISpot assays. High levels of IFN-γ were generated to MBP87–99 peptide, which were decreased by 30% (P< 0·01) when mice were immunized with [R91, A96]MBP87–99 peptide emulsified in CFA (Fig. 1a). However, the levels of IFN-γ were further decreased (70%) when [R91, A96]MBP87–99 peptide was conjugated to reduced mannan (P < 0·01) (Fig. 1a). Furthermore, immunization of mice with the native MBP87–99 peptide either emulsified in CFA or conjugated to reduced mannan did not induce IL-4 cytokine-secreting T cells (Fig. 1b). It was of interest, however, that immunization of mice with [R91, A96]MBP87–99 peptide emulsified in CFA induced low levels of IL-4 (significantly above background, P < 0·01) and very high IL-4 levels when conjugated to reduced mannan (P < 0·01) (Fig. 1b). It is clear that [R91, A96]MBP87–99 significantly decreases IFN-γ levels and generates high levels of IL-4 when conjugated to reduced mannan.
Figure 1.
(a) Interferon-γ (IFN-γ; upper panel) and (b) interleukin-4 (IL-4; lower panel) responses in SJL/J mice immunized with either MBP87–99 or [R91, A96]MBP87–99 peptides. Immunization with 50 μg of peptide emulsified in complete Freund’s adjuvant (CFA) (black bar) or conjugated to reduced mannan (white bar). Negative (background levels) are indicated as grey bars. IFN-γ or IL-4 responses are shown as spot-forming units (SFU) per 0·5 million cells ± standard error of mean. The results shown are representative of two experiments with three mice per group. MBP, myelin basic protein. **(P< 0·01).
[A91, A96]MBP87–99 peptide conjugated to reduced mannan diverts immune responses from Th1 (IFN-γ) to Th2 (IL-4)
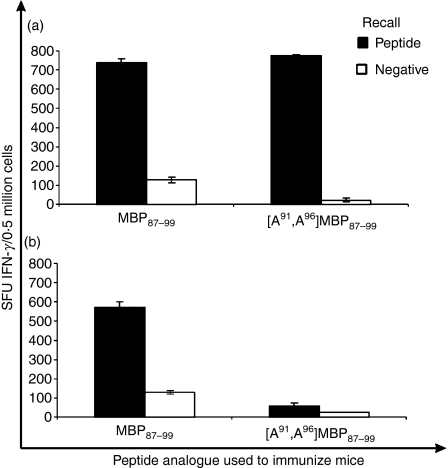
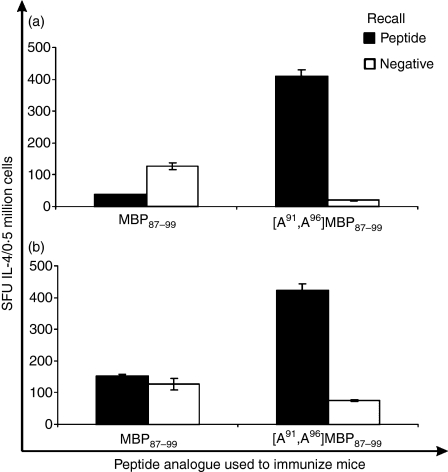
The ability of the linear MBP87–99 or [A91, A96]MBP87–99 peptides either emulsified in CFA or conjugated to reduced mannan, to induce T-cell responses after one or two immunizations, was assessed for IFN-γ and IL-4 secretion using ELISpot analysis. Mice immunized with MBP87–99 peptide (either emulsified in CFA or conjugated to reduced mannan) produced high levels of IFN-γ-secreting T cells (P < 0·01) (Fig. 2a,b). The cytokine IL-4 was not induced after immunization with MBP87–99 peptide prepared in either conjugate (Fig. 2a,b). Mice immunized with linear [A91, A96]MBP87–99 emulsified in CFA produced high levels of IL-4 (P< 0·01) (Fig. 3); however, high levels of IFN-γ were also produced (P < 0·01) (Fig. 2a). Interestingly, when [A91, A96]MBP87–99 peptide was conjugated to reduced mannan, high levels of IL-4 were induced (Fig. 3b) and no IFN-γ was detected (P < 0·01) (Fig. 2b). Overall, [A91, A96]MBP87–99 emulsified in CFA generated both Th1 and Th2 responses; however, the use of reduced mannan conjugated to [A91, A96]MBP87–99 was able to divert immune responses from Th1 to Th2. Peptide was omitted to serve as a negative control and ConA was used as an internal positive control, which consistently induced > 1000 SFU/0·5 million cells for both IFN-γ and IL-4 (data not shown).
Figure 2.
Interferon-γ (IFN-γ) responses in SJL/J mice immunized with either MBP87–99 or [A91, A96]MBP87–99 peptides. (a) Immunization with 50 μg of peptide emulsified in complete Freund’s adjuvant (CFA) and (b) immunization with 50 μg of peptide conjugated to reduced mannan. IFN-γ responses are shown as spot-forming units (SFU) per 0·5 million cells ± standard error of mean. The results shown are representative of two experiments with three mice per group. MBP, myelin basic protein.
Figure 3.
Interleukin-4 (IL-4) responses in SJL/J mice immunized with either MBP87–99 or [A91, A96]MBP87–99 peptides. (a) Immunization with 50 μg of peptide emulsified in complete Freund’s adjuvant (CFA) and (b) immunization with 50 μg of peptide conjugated to reduced mannan. IL-4 responses are shown as spot-forming units (SFU) per 0·5 million cells ± standard error of mean. The results shown are representative of two experiments with three mice per group. MBP, myelin basic protein.
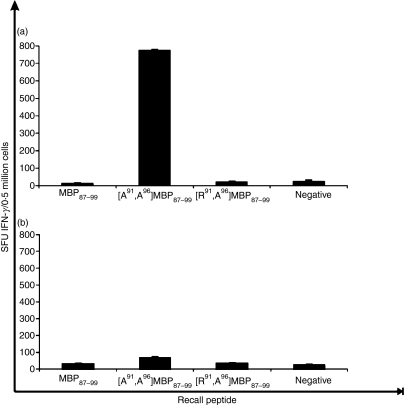
T cells from SJL/J mice immunized with [A91, A96]MBP87–99 emulsified in CFA or conjugated to reduced mannan do not cross-stimulate with the native MBP87–99 peptide
After immunization with the linear mutant [A91, A96]MBP87–99 peptide, either emulsified in CFA or conjugated to reduced mannan, T cells were examined (using an ELISpot assay) to determine whether they cross-stimulated the native MBP87–99 peptide. T cells from mice immunized with linear [A91, A96]MBP87–99 peptide emulsified in CFA (Fig. 4a) or conjugated to reduced mannan (Fig. 4b) did not cross-stimulate with the native MBP87–99 peptide (Fig. 4a,b). Peptide was omitted to serve as a negative control and ConA was used as an internal positive control, which consistently induced > 1000 SFU/0·5 million cells for both IFN-γ and IL-4 (data not shown). In addition, T cells from [A91, A96]MBP87–99 did not cross-stimulate with [R91, A96]MBP87–99 peptide, which acted as an additional negative (background) control (Fig. 4).
Figure 4.
Interferon-γ (IFN-γ) production by T cells from SJL/J mice immunized with the mutant peptide [A91, A96]MBP87–99 (a) emulsified in complete Freund’s adjuvant (CFA) or (b) conjugated to reduced mannan. Recall peptides were MBP87–99, [A91, A96]MBP87–99 or [R91, A96]MBP87–99, or no peptide (negative) (x-axis). IFN-γ production is shown as spot-forming units (SFU)/0·5 million cells ± standard error of mean. The results shown are representative of two experiments with three mice per group. MBP, myelin basic protein.
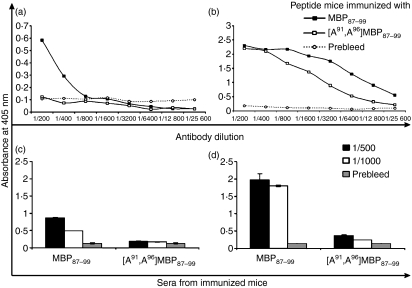
Antibody responses to MBP87–99 or [A91, A96]MBP87–99 peptides emulsified in CFA or conjugated to reduced mannan
The production of total IgG responses in mice immunized with MBP87–99 or [A91, A96]MBP87–99 peptide analogues, either emulsified in CFA (Fig. 5a) or conjugated to reduced mannan (Fig. 5b), were measured using enzyme-linked immunosorbent assays (ELISAs). No IgG was generated in mice immunized with [A91, A96]MBP87–99 peptide emulsified in CFA, and only very low levels of IgG were generated to the native MBP87–99 (Fig. 5a). However, high antibody levels were induced to both MBP87–99 and [A91, A96]MBP87–99 when they were conjugated to reduced mannan (titre > 1 : 16 400) (Fig. 5b). In previous studies using MBP83–99 modified peptides or MUC1 peptides conjugated to reduced mannan, the antibody isotype induced was of the IgG1 subtype. Conjugation of peptides to oxidized mannan induced antibodies of the IgG2a subtype. Hence, reduced mannan is a strong inducer of antibodies of the IgG1 subtype.11,12,23,25–32,39
Figure 5.
SJL/J mice immunized with (a) MBP87–99 or [A91, A96]MBP87–99 peptide analogues emulsified in complete Freund’s adjuvant (CFA), or, (b) MBP87–99 or [A91, A96]MBP87–99 peptide analogues conjugated to reduced mannan. Total immunoglobulin G (IgG) levels were measured by enzyme-linked immunosorbent assay (ELISA) coating with each respective peptides conjugated to bovine serum albumin (BSA) or keyhole limpet haemocyanin (KLH). (c,d) Cross-reactive IgG levels were measured coating with native MBP87–99 peptide and using sera from SJL/J mice immunized with MBP87–99 or [A91, A96]MBP87–99 peptides (c) emulsified in CFA or (d) conjugated to reduced mannan. Error bars depict the standard error of the mean. The results shown are representative of two experiments with three mice per group. MBP, myelin basic protein.
Because higher levels of antibodies were generated to linear [A91, A96]MBP87–99 peptide when conjugated to reduced mannan, it was investigated whether sera from these mice cross-reacted with the native MBP87–99 peptide. Sera from mice immunized with MBP87–99 reacted with MBP87–99 peptide (positive control), while antibodies from mice immunized with linear [A91, A96]MBP87–99 conjugated to reduced mannan did not cross-react with the native MBP87–99 peptide at 1 : 500 and 1 : 1000 dilutions of sera (Fig. 5d). Moreover, mice immunized with [A91, A96]MBP87–99 emulsified in CFA also did not cross-react with the native MBP87–99 peptide (Fig. 5c). Overall, while IgG antibodies were noted for [A91, A96]MBP87–99 conjugated to reduced mannan, they did not cross-react with the native MBP87–99 peptide.
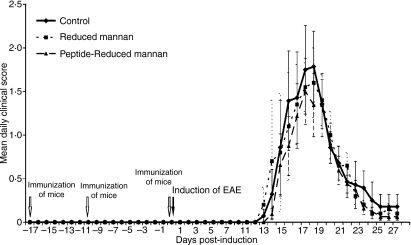
The altered responses induced by the [A91, A96]MBP87-99–KLH–reduced mannan complex do not protect mice against EAE via a bystander effect
All mice were clinically followed up to day 27 post-immunization in order to study both the first relapse and the remission phases of the disease. The clinical course of acute EAE is presented in Fig. 6. The mean daily clinical scores and body weights (data not shown) did not statistically differ at any time-point (P < 0·05). The mean maximal score for the PBS control group was 2·0 ± 1·4, that for the reduced mannan alone group was 2·2 ± 1·0 and that for the [A91, A96]MBP87–99–-KLH–-reduced mannan group was 1·7 ± 1·0, indicating a similar severity of disease in control and immunized mice. In addition, disease onset was not statistically different (Kaplan–Meier survival analysis, log rank P> 0·05) between the three groups (data not shown), indicating that immunization did not affect the onset of EAE. Overall, our data indicate that immunization with [A91, A96]MBP87–99–KLH–reduced mannan did not have any effect on the clinical course of PLP-induced EAE in SJL mice. These results imply that the [A91, A96]MBP87–99–KLH–reduced mannan-induced Th2 responses had no bystander effects on the PLP-induced immune responses in the SJL mice. Of note, we recently demonstrated that immunization of C57BL/6 mice with myelin oligodendrocyte glycoprotein35–55–reduced mannan and induction of EAE with MOG35–55 peptide protected mice against EAE (Tseveleki et al., submitted for publication). Likewise, immunization of PLP139–151–reduced mannan and induction of EAE in SJL mice using PLP139–151 peptide also protected against EAE symptoms. In addition, immunization of Lewis rats with MBP83–99-–reduced mannan conjugates, and induction of EAE using an irrelevant peptide MBP74–85 did not protect animals against EAE (Tseveleki et al., submitted for publication). In that study it was shown that the most likely mechanism of immunity and protection against EAE was via the induction of T-cell tolerance and not via a bystander effect. As previously demonstrated, native MBP87–99 peptide induced EAE in Lewis rats, which was inhibited by the double mutant analogue [R91, A96]MBP87–99 [22]. In addition, [R91, A96]MBP87–99 increased the Th2/Th1 cytokine ratio in blood from MS patients in vitro and suppressed proliferation of the CD4+ T-cell line from MS patients.22 We are currently analyzing the mechanism of action of [A91, A96]MBP87–99–KLH–reduced mannan peptide conjugates in human T cells from MS patients (manuscript in preparation), which will lead to further information on the mechanism of action and on its in vivo effects. Furthermore, we are testing the in vivo effects of [A91, A96]MBP87–99-–KLH–reduced mannan peptide conjugates on EAE in Lewis rats using the native MBP87–99 peptide to induce EAE.40,41 A study by Weissert et al.,42 demonstrated that a single amino acid substitution at position 79 of the peptide MBP68–85 dramatically altered protection against EAE in Lewis rats and hence was able to modulate disease. They showed that protection was not caused by a bystander effect and the mechanism of protection was not clear.
Figure 6.
SJL/J mice were immunized twice with [A91, A96]MBP87–99–keyhole limpet haemocyanin (KLH)–reduced mannan. On day 0, mice were immunized again and experimental autoimmune encephalomyelitis (EAE) was induced using PLP139–151 peptide. The clinical score was measured until day 27.
Interactions of [A91, A96]MBP87–99 and [R91, A96]MBP87–99 peptides in complex with MHC class II, I-As
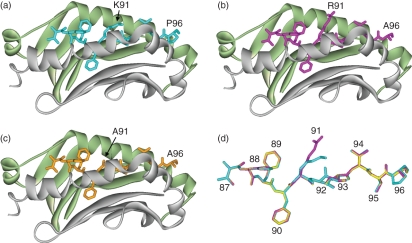
In the modelled H2 I-As complex of the MBP peptide, the residues V87, F90, N92 and T95 are anchored in MHC pockets P1, P4, P6 and P9, respectively.11,12. The residue K91 is pointing up from the MHC groove. The models indicate that mutations of the K91 residue do not cause major disruptions to the structures or to the intermolecular interactions between the peptide and the MHC cleft (Fig. 7). The effect of mutation at position 91 has been studied by analyzing intermolecular interactions in the mutated complexes. These are summarized in Tables 1 and 2. The intermolecular interactions in the modelled complex with ([R91, A96]MBP87–96) have been described previously.12 The analogue ([A91, A96]MBP87–96) shows similar trends, as described below (Fig. 7).
Figure 7.
Models of major histocompatibility complex (MHC) class II I-As with bound myelin basic protein (MBP) peptide ligands. MHC-binding grooves are shown as ribbons (α-chain, pale green; β-chain, grey) with the bound peptides as stick representations: (a) MBP wild-type (K91) peptide in cyan; (b) [R91, A96]MBP87–99 mutant analogue in magenta; (c) [A91, A96]MBP87–99 mutant analogue in orange. (d) Peptides are shown as overlays with the corresponding MBP residue positions (87–96) indicated.
Table 1.
Hydrogen-bonding interactions in the modelled complexes of MBP87–96 peptide and mutant analogues with H2 I-As
| Peptide |
MHC |
|||||
|---|---|---|---|---|---|---|
| Residue | Atom | Residue | Atom | MBP87–96 | [A91,A96]MBP87–96 | [R91,A96]MBP87–96 |
| V87 | N | S53(A)1 | O | + | + | + |
| H88 | N | N82(B)2 | OD1 | + | − | + |
| H88 | NE2 | E74(B) | OE2 | + | + | + |
| H88 | O | N82(B) | ND2 | + | + | + |
| H88 | NE2 | T77(B) | OG1 | − | + | − |
| F90 | N | Y9(A) | O | + | − | + |
| K91/A91/R91 | N | E74(B) | OE1 | + | − | + |
| K91 | NZ | Q70(B) | OE1 | + | n/a3 | +4 |
| N92 | N | N62(A) | OD1 | + | + | + |
| N92 | ND2 | N62(A) | O | + | + | + |
| I93 | N | Y30(B) | OH | + | + | + |
| V94 | N | Y67(B) | OH | + | − | + |
| V94 | O | Y67(B) | OH | + | − | + |
| T95 | O | Y68(A) | OH | + | − | + |
| P96/A96 | N | D57(B) | OD1 | − | + | − |
α-chain,
β-chain,
not applicable,
the NH1 atom of R91 in the peptide makes this contact.
MBP, myelin basic protein.
Table 2.
Van der Waals interactions in the modelled complexes of MBP87–96 peptide and mutant analogues with H2 I-As
| Peptide residue | MHC residue | MBP87–96 | [A91,A96]MBP87–96 | [R91,A96]MBP87–96 |
|---|---|---|---|---|
| V87 | Y9(A)1 | + | − | + |
| V87 | F54(A) | + | + | + |
| V87 | H81(B)2 | + | − | − |
| V87 | N82(B) | + | − | + |
| H88 | E74(B) | + | + | + |
| H88 | T77(B) | + | + | + |
| H88 | V78(B) | + | + | + |
| F89 | Y9(A) | + | − | + |
| F89 | F54(A) | + | + | + |
| F89 | G58(A) | + | + | + |
| F89 | V78(B) | − | + | − |
| F90 | F11(B) | + | + | + |
| F90 | G13(B) | + | + | + |
| F90 | E14(B) | + | + | + |
| F90 | C15(B) | + | + | + |
| F90 | V78(B) | + | + | − |
| F90 | C79(B) | − | + | − |
| K91 | Q70(B) | + | n/a3 | n/a |
| R91/A91 | F11(B) | n/a | + | + |
| R91 | Y67(B) | n/a | n/a | + |
| N92 | T65(A) | + | + | + |
| N92 | F11(B) | + | + | + |
| I93 | T65(A) | + | + | + |
| I93 | Y61(B) | + | + | + |
| I93 | Y67(B) | + | + | + |
| V94 | T65(A) | + | − | + |
| V94 | Y61(B) | − | + | − |
| V94 | Y68(A) | + | + | + |
| T95 | D57(B) | + | + | + |
| T95 | T69(A) | − | + | + |
| T95 | Y61(B) | − | + | − |
| P96 | Y60(B) | + | n/a | n/a |
α-chain,
β-chain,
not applicable.
MHC, major histocompatibility complex.
With respect to hydrogen-bonding interactions, the most pronounced effect includes the loss of contact made by the nitrogen atoms of K91 and F90 (MBP). Also, [A91, A96]MBP87–96 does not show the contacts to the MHC residues N82(B), Y67(B) and Y68(A) that are observed in the complex with the wild-type peptide. However, compensatory interactions are observed to the MHC residues T77(B) and D57(B). It must be noted that [A91, A96]MBP87–96 displays the greatest deviation from the intermolecular hydrogen-bond interactions of the wild-type complex, compared with all other mutants studied previously.11,12. Interestingly, the differences in the van der Waals interactions made by the native peptide versus [A91, A96]MBP87–96 were less pronounced than observed with other analogues.11,12 In this case, the loss of contact made by K91 is once more observed. To balance, the analogue exhibits the A91 (MBP) interactions with F11(B) (MHC). Other losses of contacts by [A91, A96]MBP87–96 include V87 to Y9(A), H81(B) and N82(B), F89 to Y9(A), and V94 to T65(A). All of these are compensated for by the interactions observed in the mutant complex, but not exhibited by the native MBP peptide itself: V87 to F54(A), F89 to V78(B), and V94 to Y61(B), respectively.
Discussion
Activation of CD4+ T cells is initiated by the interaction between the TCR and a peptide antigen that is presented by MHC class II molecules, and the engagement of costimulatory molecules of antigen-presenting cells.43 This process is followed by T-cell proliferation, stimulation of reactive T cells specific to the antigen and the secretion of relevant cytokines. Many studies have shown that peptides with mutations at critical TCR contact residues result in altered T-cell function.44–46 In particular, altered peptide ligands (or mutant peptides) have been found to shift the balance of immune responses from Th1 to Th2.44,47,48 Th1 responses (IFN-γ) involve pro-inflammatory cytokines that mediate autoimmune diseases, and Th2 responses (IL-4, IL-10) reduce IFN-γ secretion and other inflammatory cytokines, preventing autoimmunity.15,49,50
The development of safe and effective vaccines and immunotherapeutic approaches against MS are being studied actively. To date, a number of clinical trials, investigating altered peptide ligands, have been undertaken in MS patients.51,52 Even though the peptides were demonstrated to induce appropriate responses in preclinical studies, these clinical trials were discontinued because of adverse reactions.51,52 In some patients, unanticipated cross-reactions were stimulated by the peptide analogues against the native peptide/protein.51,52 Thus, further preclinical testing is required and new modified peptides need to be designed in order to develop an effective vaccine for MS. In the cancer setting, it was demonstrated that the mutation of the HLA-A2-derived peptide (from carcinoembryonic antigen) at position 6 generated a superagonist. It induced CD8+ T cells that cross-reacted with high concentrations of the native peptide but which, however, did not recognize carcinoembryonic antigen-expressing cancer cells.53 Thus, there is a need for extensive analysis of tumour cross-recognition before any clinical use can be permitted of altered peptide ligands as vaccines. Therefore, we synthesized two linear mutant peptides, based on the short immunodominant epitope, MBP87–99, with mutations at positions K91 and P96 (K91 was modified to R91 or A91 and P96 was modified to A96). The mutant analogues were injected into SJL/J mice in order to examine their ability to shift immune responses from Th1 to Th2, and to investigate whether T cells and antibodies cross-reacted with the native peptide. An adjuvant (CFA) or a suitable carrier (reduced mannan) was used.
Immunization of SJL/J mice with native MBP87–99 peptide emulsified in CFA generated high levels of IFN-γ, whilst the double-mutant analogue [R91, A96]MBP87–99 decreased IFN-γ secretion by 30%. These findings are similar to those previously published for the longer double-mutant peptide [R91, A96]MBP83–99.11 It is of interest that the levels of IFN-γ were further decreased (70%) when [R91, A96]MBP87–99 peptide was conjugated to the carrier, reduced mannan. Furthermore, immunization of mice with the native MBP87–99 peptide, which was either emulsified in CFA or conjugated to reduced mannan, did not induce IL-4 cytokine-secreting T cells. Thus, substitution of K91 and P96 with R91 and A96, respectively, could decrease IFN-γ levels, generate high levels of IL-4 when [R91, A96]MBP87–99 peptide was conjugated to reduced mannan, but there was still secretion of IFN-γ. By contrast, immunization with the double-mutant analogue [A91, A96]MBP87–99 conjugated to reduced mannan induced high levels of IL-4 and no IFN-γ was detected. Substitution of residues 91 and 96 with Ala ([A91, A96]MBP87–99) has the ability to generate IL-4; however, with the appropriate carrier, it is able to divert immune responses from IFN-γ to IL-4. Overall, the use of adjuvant (CFA) generates both Th1 and Th2 responses and does not seem to be beneficial, but the use of an appropriate carrier (such as reduced mannan) conjugated to [A91, A96]MBP87–99 was able to divert immune responses from Th1 to Th2. Most importantly, T cells secreting IFN-γ and IgG generated to the double mutant [A91, A96]MBP87–99 peptide did not cross-react with the native MBP87–99 peptide. It is clear that the double mutant [A91, A96]MBP87–99 peptide analogue is a promising candidate for further studies for use in the immunotherapy of MS. Likewise, we recently demonstrated, using single amino acid mutations of the longer peptide MBP83–99, that a single amino acid change of K91 to Y91 ([Y91]MBP83–99), when conjugated to reduced mannan, also diverted immune responses from Th1 to Th2,12 similarly to the [A91, A96]MBP87–99 peptide conjugated to reduced mannan. It is clear that the peptide sequence and length, and the type of adjuvant/carrier used, are important for eliciting the desired immune response to a peptide-based vaccine. The mechanism by which reduced mannan–MBP87–99 analogues switch immune responses is currently under investigation and preliminary studies suggest that tolerance is induced and responses are modulated by IL-17 and regulatory T cells (Treg cells) after immunization (manuscript in preparation). Furthermore, using MUC1 peptides conjugated to mannan, the addition of other adjuvants, such as muramyl dipeptide (MDP), glutaminyl-muramyl dipeptide (GMDP), aluminium hydroxide and adjuprime had no effect on the immune responses induced. Hence, mannan is an effective carrier for immune induction.54
Modelling of the ([A91, A96]MBP87−96) and ([R91, A96]MBP87–96) analogues, in complex with H2 I-As, revealed that the side-chains of the mutated residues are exposed to make contacts with the TCR and the mutations do not cause major disruptions to interactions between the peptides and the MHC cleft. It was noted that the backbone of [R91, A96]MBP87–96, [A91, A96]MBP87–96 and native MBP87–96 peptides overlapped very closely and only minor conformational changes were observed in the amino acids at positions 91 and 96 (Fig. 1d). Not unexpectedly, certain specific contacts observed in the complex with the native peptide were lost in the complexes with the mutants (Tables 1 and 2). These were compensated for by novel interactions with MHC residues. Most notably, the ([A91, A96]MBP87–96) analogue displayed the greatest reduction of intermolecular hydrogen bond interactions with respect to the native complex, when compared with all other mutants previously studied.11,12 This is not surprising given the extent of side-chain modification when an alanine residue is used instead of arginine and proline.
Peptides causing antagonism have been shown to have fewer hydrogen bond contacts between the peptide side-chains and the CDR3 loops of the TCR.55 Loss of hydrogen bond contact can cause agonist or super-agonist peptides (hyper-stimulatory altered peptide ligands) to become antagonists. For example, a single amino acid mutation in vesicular stomatitis virus peptide (VSV8) (RGYVYQGL to RGYVYEGL) leads to antagonism of T-cell hybridomas specific to native VSV8. The crystal structure of this altered peptide ligand with H-2Kb demonstrated that a minor peptide modification induced a large biological effect.56 The TCR, which recognizes VSV8 (RGYVYQGL) peptide and its altered peptide ligand (RGYVYEGL), was mutated by a single amino acid at the CDR3β loop, and this was able to modulate the TCR-antagonistic properties of an altered peptide ligand.57 These examples validate the results of our modelling studies with respect to MBP mutant peptides, namely that even subtle conformational changes are sufficient to have profound biological effects.
Acknowledgments
MK was supported by the Ministry of Development Secretariat of Research and Technology of Greece (Grant Aus. 005) and Du Pré grant from MSIF. VA (223316) and PAR (365209) were supported by NH&MRC of Australia R. Douglas Wright Fellowships, and this work was supported in part by NH&MRC project grant 223310.
Glossary
Abbreviations:
- CFA
complete Freund’s adjuvant
- ConA
concanavalin A
- EAE
experimental autoimmune encephalomyelitis
- ELISpot
enzyme-linked immunospot
- HPLC
high-performance liquid chromatography
- IFN-γ
interferon-γ
- IL
interleukin
- KLH
keyhole limpet haemocyanin
- MBP
myelin basic protein
- MHC
major histocompatibility complex
- MS
multiple sclerosis
- PBS
phosphate-buffered saline
- SEM
standard error of the mean
- SFU
spot-forming units
- TCR
T-cell receptor
- Th1
T helper 1
- Th2
T helper 2
- TNF-α
tumour necrosis factor-α
Disclosures
The authors have nothing to disclose.
References
- 1.Katsara M, Matsoukas J, Deraos G, Apostolopoulos V. Towards immunotherapeutic drugs and vaccines against multiple sclerosis. Acta Biochim Biophys Sin. 2008;40:636–42. doi: 10.1111/j.1745-7270.2008.00444.x. [DOI] [PubMed] [Google Scholar]
- 2.Martin R, Howell MD, Jaraquemada D, et al. A myelin basic protein peptide is recognized by cytotoxic T cells in the context of four HLA-DR types associated with multiple sclerosis. J Exp Med. 1991;173(1):19–24. doi: 10.1084/jem.173.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oksenberg JR, Baranzini SE, Barcellos LF, Hauser SL. Multiple sclerosis: genomic rewards. J Neuroimmunol. 2001;113:171–84. doi: 10.1016/s0165-5728(00)00444-6. [DOI] [PubMed] [Google Scholar]
- 4.Ota K, Matsui M, Milford EL, Mackin GA, Weiner HL, Hafler DA. T-cell recognition of an immunodominant myelin basic protein epitope in multiple sclerosis. Nature. 1990;346:183–7. doi: 10.1038/346183a0. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Markovic-Plese S, Lacet B, Raus J, Weiner HL, Hafler DA. Increased frequency of interleukin 2-responsive T cells specific for myelin basic protein and proteolipid protein in peripheral blood and cerebrospinal fluid of patients with multiple sclerosis. J Exp Med. 1994;179:973–84. doi: 10.1084/jem.179.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hafler DA, Slavik JM, Anderson DE, O’Connor KC, De Jager P, Baecher-Allan C. Multiple sclerosis. Immunol Rev. 2005;204:208–31. doi: 10.1111/j.0105-2896.2005.00240.x. [DOI] [PubMed] [Google Scholar]
- 7.Steinman L. Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system. Cell. 1996;85:299–302. doi: 10.1016/s0092-8674(00)81107-1. [DOI] [PubMed] [Google Scholar]
- 8.Sakai K, Sinha AA, Mitchell DJ, Zamvil SS, Rothbard JB, McDevitt HO, Steinman L. Involvement of distinct murine T-cell receptors in the autoimmune encephalitogenic response to nested epitopes of myelin basic protein. Proc Natl Acad Sci USA. 1988;85:8608–12. doi: 10.1073/pnas.85.22.8608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakai K, Zamvil SS, Mitchell DJ, Lim M, Rothbard JB, Steinman L. Characterization of a major encephalitogenic T cell epitope in SJL/J mice with synthetic oligopeptides of myelin basic protein. J Neuroimmunol. 1988;19:21–32. doi: 10.1016/0165-5728(88)90032-x. [DOI] [PubMed] [Google Scholar]
- 10.Karin N, Mitchell DJ, Brocke S, Ling N, Steinman L. Reversal of experimental autoimmune encephalomyelitis by a soluble peptide variant of a myelin basic protein epitope: T cell receptor antagonism and reduction of interferon gamma and tumor necrosis factor alpha production. J Exp Med. 1994;180:2227–37. doi: 10.1084/jem.180.6.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katsara M, Yuriev E, Ramsland PA, Deraos G, Tselios T, Matsoukas J, Apostolopoulos V. A double mutation of MBP(83–99) peptide induces IL-4 responses and antagonizes IFN-gamma responses. J Neuroimmunol. 2008;200:77–89. doi: 10.1016/j.jneuroim.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Katsara M, Yuriev E, Ramsland PA, Deraos G, Tselios T, Matsoukas J, Apostolopoulos V. Mannosylation of mutated MBP(83–99) peptides diverts immune responses from Th1 to Th2. Mol Immunol. 2008;45:3661–70. doi: 10.1016/j.molimm.2008.04.024. [DOI] [PubMed] [Google Scholar]
- 13.Brocke S, Gijbels K, Allegretta M, et al. Treatment of experimental encephalomyelitis with a peptide analogue of myelin basic protein. Nature. 1996;379:343–6. doi: 10.1038/379343a0. [DOI] [PubMed] [Google Scholar]
- 14.Cannella B, Raine CS. The adhesion molecule and cytokine profile of multiple sclerosis lesions. Ann Neurol. 1995;37:424–35. doi: 10.1002/ana.410370404. [DOI] [PubMed] [Google Scholar]
- 15.Racke MK, Bonomo A, Scott DE, Cannella B, Levine A, Raine CS, Shevach EM, Rocken M. Cytokine-induced immune deviation as a therapy for inflammatory autoimmune disease. J Exp Med. 1994;180:1961–6. doi: 10.1084/jem.180.5.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaur A, Boehme SA, Chalmers D, et al. Amelioration of relapsing experimental autoimmune encephalomyelitis with altered myelin basic protein peptides involves different cellular mechanisms. J Neuroimmunol. 1997;74:149–58. doi: 10.1016/s0165-5728(96)00220-2. [DOI] [PubMed] [Google Scholar]
- 17.Gauthier L, Smith KJ, Pyrdol J, Kalandadze A, Strominger JL, Wiley DC, Wucherpfennig KW. Expression and crystallization of the complex of HLA-DR2 (DRA, DRB1*1501) and an immunodominant peptide of human myelin basic protein. Proc Natl Acad Sci USA. 1998;95:11828–33. doi: 10.1073/pnas.95.20.11828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Huang Y, Lue J, Quandt JA, Martin R, Mariuzza RA. Structure of a human autoimmune TCR bound to a myelin basic protein self-peptide and a multiple sclerosis-associated MHC class II molecule. EMBO J. 2005;24:2968–79. doi: 10.1038/sj.emboj.7600771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith KJ, Pyrdol J, Gauthier L, Wiley DC, Wucherpfennig KW. Crystal structure of HLA-DR2 (DRA*0101, DRB1*1501) complexed with a peptide from human myelin basic protein. J Exp Med. 1998;188:1511–20. doi: 10.1084/jem.188.8.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wucherpfennig KW, Hafler DA, Strominger JL. Structure of human T-cell receptors specific for an immunodominant myelin basic protein peptide: positioning of T-cell receptors on HLA-DR2/peptide complexes. Proc Natl Acad Sci USA. 1995;92:8896–900. doi: 10.1073/pnas.92.19.8896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalbus M, Fleckenstein BT, Offenhausser M, et al. Ligand motif of the autoimmune disease-associated mouse MHC class II molecule H2-A(s) Eur J Immunol. 2001;31:551–62. doi: 10.1002/1521-4141(200102)31:2<551::aid-immu551>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 22.Matsoukas J, Apostolopoulos V, Kalbacher H, et al. Design and synthesis of a novel potent myelin basic protein epitope 87–99 cyclic analogue: enhanced stability and biological properties of mimics render them a potentially new class of immunomodulators. J Med Chem. 2005;48:1470–80. doi: 10.1021/jm040849g. [DOI] [PubMed] [Google Scholar]
- 23.Katsara M, Deraos G, Tselios T, Matsoukas J, Apostolopoulos V. Design of novel cyclic altered peptide ligands of myelin basic protein MBP83–99 that modulate immune responses in SJL/J mice. J Med Chem. 2008;51:3971–8. doi: 10.1021/jm8000554. [DOI] [PubMed] [Google Scholar]
- 24.Katsara M, Tselios T, Deraos S, Deraos G, Matsoukas MT, Lazoura E, Matsoukas J, Apostolopoulos V. Round and round we go: cyclic peptides in disease. Curr Med Chem. 2006;13:2221–32. doi: 10.2174/092986706777935113. [DOI] [PubMed] [Google Scholar]
- 25.Acres B, Apostolopoulos V, Balloul JM, et al. MUC1-specific immune responses in human MUC1 transgenic mice immunized with various human MUC1 vaccines. Cancer Immunol Immunother. 2000;48:588–94. doi: 10.1007/PL00006677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Apostolopoulos V, Barnes N, Pietersz GA, McKenzie IF. Ex vivo targeting of the macrophage mannose receptor generates anti-tumor CTL responses. Vaccine. 2000;18:3174–84. doi: 10.1016/s0264-410x(00)00090-6. [DOI] [PubMed] [Google Scholar]
- 27.Apostolopoulos V, Karanikas V, Haurum JS, McKenzie IF. Induction of HLA-A2-restricted CTLs to the mucin 1 human breast cancer antigen. J Immunol. 1997;159:5211–8. [PubMed] [Google Scholar]
- 28.Apostolopoulos V, McKenzie IF. Role of the mannose receptor in the immune response. Curr Mol Med. 2001;1:469–74. doi: 10.2174/1566524013363645. [DOI] [PubMed] [Google Scholar]
- 29.Apostolopoulos V, Pietersz GA, Gordon S, Martinez-Pomares L, McKenzie IF. Aldehyde-mannan antigen complexes target the MHC class I antigen-presentation pathway. Eur J Immunol. 2000;30:1714–23. doi: 10.1002/1521-4141(200006)30:6<1714::AID-IMMU1714>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 30.Apostolopoulos V, Pietersz GA, Loveland BE, Sandrin MS, McKenzie IF. Oxidative/reductive conjugation of mannan to antigen selects for T1 or T2 immune responses. Proc Natl Acad Sci USA. 1995;92:10128–32. doi: 10.1073/pnas.92.22.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Apostolopoulos V, Pietersz GA, McKenzie IF. Cell-mediated immune responses to MUC1 fusion protein coupled to mannan. Vaccine. 1996;14:930–8. doi: 10.1016/0264-410x(95)00258-3. [DOI] [PubMed] [Google Scholar]
- 32.Sheng KC, Pouniotis DS, Wright MD, Tang CK, Lazoura E, Pietersz GA, Apostolopoulos V. Mannan derivatives induce phenotypic and functional maturation of mouse dendritic cells. Immunology. 2006;118:372–83. doi: 10.1111/j.1365-2567.2006.02384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tselios T, Apostolopoulos V, Daliani I, et al. Antagonistic effects of human cyclic MBP(87–99) altered peptide ligands in experimental allergic encephalomyelitis and human T-cell proliferation. J Med Chem. 2002;45:275–83. doi: 10.1021/jm0102147. [DOI] [PubMed] [Google Scholar]
- 34.Tselios T, Daliani I, Deraos S, et al. Treatment of experimental allergic encephalomyelitis (EAE) by a rationally designed cyclic analogue of myelin basic protein (MBP) epitope 72–85. Bioorg Med Chem Lett. 2000;10:2713–7. doi: 10.1016/s0960-894x(00)00556-4. [DOI] [PubMed] [Google Scholar]
- 35.Tselios T, Daliani I, Probert L, et al. Treatment of experimental allergic encephalomyelitis (EAE) induced by guinea pig myelin basic protein epitope 72-85 with a human MBP(87–99) analogue and effects of cyclic peptides. Bioorg Med Chem. 2000;8:1903–9. doi: 10.1016/s0968-0896(00)00134-6. [DOI] [PubMed] [Google Scholar]
- 36.Tselios T, Probert L, Daliani I, et al. Design and synthesis of a potent cyclic analogue of the myelin basic protein epitope MBP72–85: importance of the Ala81 carboxyl group and of a cyclic conformation for induction of experimental allergic encephalomyelitis. J Med Chem. 1999;42:1170–7. doi: 10.1021/jm980250e. [DOI] [PubMed] [Google Scholar]
- 37.Tselios TV, Lamari FN, Karathanasopoulou I, Katsara M, Apostolopoulos V, Pietersz GA, Matsoukas JM, Karamanos NK. Synthesis and study of the electrophoretic behavior of mannan conjugates with cyclic peptide analogue of myelin basic protein using lysine-glycine linker. Anal Biochem. 2005;347:121–8. doi: 10.1016/j.ab.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 38.Wallace AC, Laskowski RA, Thornton JM. LIGPLOT: a program to generate schematic diagrams of protein-ligand interactions. Protein Eng. 1995;8:127–34. doi: 10.1093/protein/8.2.127. [DOI] [PubMed] [Google Scholar]
- 39.Apostolopoulos V, Yuriev E, Ramsland PA, et al. A glycopeptide in complex with MHC class I uses the GalNAc residue as an anchor. Proc Natl Acad Sci USA. 2003;100:15029–34. doi: 10.1073/pnas.2432220100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Graaf KL, Weissert R, Kjellen P, Holmdahl R, Olsson T. Allelic variations in rat MHC class II binding of myelin basic protein peptides correlate with encephalitogenicity. Int Immunol. 1999;11:1981–8. doi: 10.1093/intimm/11.12.1981. [DOI] [PubMed] [Google Scholar]
- 41.Stepaniak JA, Gould KE, Sun D, Swanborg RH. A comparative study of experimental autoimmune encephalomyelitis in Lewis and DA rats. J Immunol. 1995;155:2762–9. [PubMed] [Google Scholar]
- 42.Weissert R, Lobell A, de Graaf KL, Eltayeb SY, Andersson R, Olsson T, Wigzell H. Protective DNA vaccination against organ-specific autoimmunity is highly specific and discriminates between single amino acid substitutions in the peptide autoantigen. Proc Natl Acad Sci USA. 2000;97:1689–94. doi: 10.1073/pnas.030390097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harding CV, Leyva-Cobian F, Unanue ER. Mechanisms of antigen processing. Immunol Rev. 1988;106:77–92. doi: 10.1111/j.1600-065x.1988.tb00774.x. [DOI] [PubMed] [Google Scholar]
- 44.Evavold BD, Allen PM. Separation of IL-4 production from Th cell proliferation by an altered T cell receptor ligand. Science. 1991;252:1308–10. doi: 10.1126/science.1833816. [DOI] [PubMed] [Google Scholar]
- 45.Evavold BD, Sloan-Lancaster J, Hsu BL, Allen PM. Separation of T helper 1 clone cytolysis from proliferation and lymphokine production using analog peptides. J Immunol. 1993;1:3131–40. (Pt 1) [PubMed] [Google Scholar]
- 46.Sloan-Lancaster J, Evavold BD, Allen PM. Induction of T-cell anergy by altered T-cell-receptor ligand on live antigen-presenting cells. Nature. 1993;363:156–9. doi: 10.1038/363156a0. [DOI] [PubMed] [Google Scholar]
- 47.Nicholson LB, Greer JM, Sobel RA, Lees MB, Kuchroo VK. An altered peptide ligand mediates immune deviation and prevents autoimmune encephalomyelitis. Immunity. 1995;3:397–405. doi: 10.1016/1074-7613(95)90169-8. [DOI] [PubMed] [Google Scholar]
- 48.Windhagen A, Scholz C, Hollsberg P, Fukaura H, Sette A, Hafler DA. Modulation of cytokine patterns of human autoreactive T cell clones by a single amino acid substitution of their peptide ligand. Immunity. 1995;2:373–80. doi: 10.1016/1074-7613(95)90145-0. [DOI] [PubMed] [Google Scholar]
- 49.Street NE, Mosmann TR. Functional diversity of T lymphocytes due to secretion of different cytokine patterns. FASEB J. 1991;5:171–7. doi: 10.1096/fasebj.5.2.1825981. [DOI] [PubMed] [Google Scholar]
- 50.Swain SL. IL4 dictates T-cell differentiation. Res Immunol. 1993;144:616–20. doi: 10.1016/s0923-2494(05)80013-6. [DOI] [PubMed] [Google Scholar]
- 51.Bielekova B, Goodwin B, Richert N, et al. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83–99) in multiple sclerosis: results of a phase II clinical trial with an altered peptide ligand. Nature Med. 2000;6:1167–75. doi: 10.1038/80516. [DOI] [PubMed] [Google Scholar]
- 52.Kappos L, Comi G, Panitch H, Oger J, Antel J, Conlon P, Steinman L. Induction of a non-encephalitogenic type 2 T helper-cell autoimmune response in multiple sclerosis after administration of an altered peptide ligand in a placebo-controlled, randomized phase II trial. The Altered Peptide Ligand in Relapsing MS Study Group. Nature Med. 2000;6:1176–82. doi: 10.1038/80525. [DOI] [PubMed] [Google Scholar]
- 53.Iero M, Squarcina P, Romero P, et al. Low TCR avidity and lack of tumor cell recognition in CD8(+) T cells primed with the CEA-analogue CAP1-6D peptide. Cancer Immunol Immunother. 2007;56:1979–91. doi: 10.1007/s00262-007-0342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pietersz GA, Li W, Popovski V, Caruana JA, Apostolopoulos V, McKenzie IF. Parameters for using mannan-MUC1 fusion protein to induce cellular immunity. Cancer Immunol Immunother. 1998;45:321–6. doi: 10.1007/s002620050449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Degano M, Garcia KC, Apostolopoulos V, Rudolph MG, Teyton L, Wilson IA. A functional hot spot for antigen recognition in a superagonist TCR/MHC complex. Immunity. 2000;12:251–61. doi: 10.1016/s1074-7613(00)80178-8. [DOI] [PubMed] [Google Scholar]
- 56.Thomson CT, Kalergis AM, Sacchettini JC, Nathenson SG. A structural difference limited to one residue of the antigenic peptide can profoundly alter the biological outcome of the TCR-peptide/MHC class I interaction. J Immunol. 2001;166:3994–7. doi: 10.4049/jimmunol.166.6.3994. [DOI] [PubMed] [Google Scholar]
- 57.Kalergis AM, Nathenson SG. Altered peptide ligand-mediated TCR antagonism can be modulated by a change in a single amino acid residue within the CDR3 beta of an MHC class I-restricted TCR. J Immunol. 2000;165:280–5. doi: 10.4049/jimmunol.165.1.280. [DOI] [PubMed] [Google Scholar]