Abstract
Protein tyrosine phosphorylation is an important early event in the signal transduction of numerous cell receptors involved in the immune response. The implication of protein tyrosine kinases in allergic asthma is well recognized, but the role of protein tyrosine phosphatases (PTPs) remains poorly understood. However, we recently reported that global inhibition of PTPs during either the allergen-sensitization phase or the allergen-challenge phase reduced the development of asthma and that this correlated with an increased T helper 1 (Th1) response in both lung and spleen tissues. Therefore, in this study we investigated individual roles of PTPs involved in regulating the immune response. We observed that genetic deficiency for PTP-1B resulted in increased recruitment of lung inflammatory cells, while protein tyrosine phosphatase-phosphatase and tensin homologue deleted (PTP-PEST)-deficient mice exhibited a phenotype similar to that of wild-type mice. Importantly, we found that a heterozygous mutation of T cell PTP (TC-PTP) dramatically abrogates immunoglobulin E production and reduces the recruitment of inflammatory cells to the lung, conferring an important role for TC-PTP in the development of allergic asthma. As opposed to other studies on Src homology phosphatase-1 (SHP-1) deficiency, specific acute SHP-1 inhibition during allergen challenge did not affect disease outcome. Collectively, our results underscore the importance of PTPs in the development of allergic asthma.
Keywords: asthma, protein tyrosine phosphatases, signal transduction
Introduction
The development of immune responses is dependent on the cellular activation of individual cells. In turn, the cell response to its environment depends on the signalling pathways triggered upon engagement of cell-surface receptors. One of the most prominent mechanisms of signal transmission involves tyrosine phosphorylation, required for the signalling of critical immune receptors including the T-cell receptor (TCR),1,2 interleukin (IL) receptors3 and Fcε receptor 1 (FcεR1).4 This protein tyrosine phosphorylation is mediated by protein tyrosine kinases (PTKs), while protein tyrosine phosphatases (PTPs) dephosphorylate phosphotyrosine residues.5 Interestingly, dephosphorylation can also activate signalling pathways through the dephosphorylation of inhibitory residues on kinases, such as the role of CD45 in TCR signalling.6 An optimal equilibrium between PTK and PTP activities is therefore necessary for fine-tuning the cell response to signals received in vivo and, consequently, this equilibrium is vital for the proper outcome of immune responses.5
In the context of allergic asthma, tyrosine phosphorylation is a crucial signalling event for disease development and the use of PTK inhibitors has been extensively studied (reviewed in ref. 7). For example, genistein,8 a general inhibitor of PTKs, as well as of many kinase-specific inhibitors targeting Lyn,9 Janus kinase 2 (JAK2)10 and Syk11 was shown to reduce the cardinal features of asthma. While the role of kinases in asthma have been investigated in detail,12 the role of PTPs in this disease remains largely unexplored.
The mouse genome contains 105 PTPs,13 but studies on their role in allergic diseases involved very few PTPs. Previous work on the phosphatase and tensin homologue (PTEN) in asthma revealed that the PTEN protein level is reduced in asthmatic lung upon allergen challenge, allowing the production of a stronger signal by phosphoinositide 3-kinase (PI3K), its opposed kinase.14 Overexpression of PTEN in this context prevented the development of asthma features. Another research group reported that a reduction of Src homology phosphatase-1 (SHP-1) activity in motheaten/+ (me/+) mice exacerbated asthma development, conferring a role for SHP-1 in the down-regulation of events leading to asthma development.15 This was confirmed in a second study on SHP-1, in which motheaten viable (mev/mev) mice showed similar results. Of interest, our laboratory previously noted that inhibition of global PTP activity [with bis peroxovanadium 1,10-phenanthroline [bpV (phen)] in mice resulted in a preferential increase of T helper 1 (Th1) cytokines in the spleen.16 As Th1 cytokines can favour protection in asthma allergic reactions, this led us to investigate the role of pharmacological inhibition of PTP in an asthma model. Using bpV(phen) we showed that intact PTP activity is necessary during both allergen-sensitization and allergen-challenge phases in order to observe asthma development.17 The inhibition of PTP activity reduced inflammatory cell recruitment to the lung, prevented the development of airway hyper-responsiveness (AHR) and reduced immunoglobulin E (IgE) production in mice treated during allergen sensitization. Here, we investigated the role of individual PTPs in allergic asthma development.
Given their importance in the modulation of the immune response, our attention was drawn to four specific PTPs: PTP-1B, protein tyrosine phosphatase-phosphatase and tensin homologue deleted (PTP-PEST), T cell PTP (TC-PTP) and SHP-1. The small PTPs, TC-PTP, PTP-1B18–20 and PTP-PEST,21 are known as important modulators of JAK family kinases activity and show a potent regulatory capacity on large-scale inflammatory events.22 This might confer them with the capacity to regulate complex immune responses such as allergic asthma. Another PTP, SHP-1, is also recognized to control c-Jun N-terminal kinase (JNK) activation,23–27 and is renowned for a more general implication in the control of inflammation. SHP-1 controls inflammation through a variety of actions, from its implication in TCR signalling,28 through a control of the Toll-like receptor (TLR) signal to inhibition of interlukin-1 receptor-associated kinase 1 (IRAK-1) activation.29 Given its importance in inflammation, other groups studied the role of SHP-1 in asthma, but their use of mice mutant for this PTP15,16 might hamper the conclusions drawn from their work. Indeed, mice deficient for SHP-1 develop severe autoimmunity and chronic inflammation.15,30 We decided to study the role of SHP-1 only at the allergen-challenge phase, by acute inactivation of SHP-1 through adenovirus-delivered short hairpins RNAs (shRNAs) in wild-type mice.
Here, we report that mice deficient for PTP-1B exhibit an increased recruitment of inflammatory cells to the lung. By contrast, mice heterozygous for a TC-PTP mutation exhibit a weaker recruitment of inflammatory cells to the lungs, as well as less inflammation in lung tissue, and produce fewer allergen-specific IgEs. Heterozygous expression of PTP-PEST did not alter asthma development, and acute inhibition of SHP-1 activity before allergen challenge by an adenovirus-delivered shRNA showed no increase in disease severity.
Materials and methods
Chemicals and reagents
Ovalbumin (OVA) grade V and aluminium hydroxide gel were purchased from Sigma-Aldrich (Sigma-Aldrich, Oakville, ON, Canada).
Animals and sensitization protocol
BALB/c mice, 6–8 weeks of age, were purchased from Charles River Canada (Saint-Constant, QC, Canada) and housed in the McGill University animal facility, in accordance with the Canadian Council on Animal Care guidelines. Mice with homozygous deletion of PTP-1B31 or heterozygous deletion of TC-PTP32 or yet PTP-PEST33 were generated as reported previously and were backcrossed four times to a BALB/c background (animals generously provided by Dr Michel L. Tremblay, McGill University). In all experiments, mutant animals were compared with their wild-type (WT) littermate controls to ensure adequate comparison (as opposed to comparison with a common WT control) given the fact that the genetic background will be slightly different in each mutant strain as a result of differing recombination events. Mice were injected intraperitoneally (i.p.) on days 0 and 7 with 40 μg of OVA and 2.6 mg of aluminium hydroxide in saline for a total volume of 200 μl. Allergen challenges were performed by nebulization with 5% OVA in saline for 5 min on days 21, 22 and 23, and the mice were killed on day 25.
Adenovirus preparation and treatment
Adenoviruses encoding SHP-1-specific shRNA and green fluorescent protein (GFP) were prepared using the AdEasy system, as reported previously.34 On day 18 of the sensitization protocol, 6 × 1010 virus particles/100 μl were injected into the tail vain of animals to establish an inhibition of SHP-1 messenger RNA (mRNA) expression during allergen challenge.
Animal lung function assessement
Forty-eight hours after the last allergen challenge, mice were put in a whole-body plethysmograph chamber (Buxco Research Systems, Willington, NC, USA). Enhanced pause (Penh) was measured after administration of increasing doses of nebulized metacholine and this measure was used to evaluate lung reactivity. The animals were killed, after taking Penh measurements, in order to evaluate other parameters.
Measurement of serum IgE
Serum was obtained from mice and was used to measure the concentration of IgE. Total IgEs were measured using the enzyme-linked immunosorbent assay (ELISA) technique with BD Pharmingen antibodies according to the recommended procedure (capture antibody clone R35-72 and detection antibody clone R35-118) (BD Biosciences, Missisauga, ON, Canada). The specific IgE titre was measured using the same capture antibody, but the detection antibody was replaced with 10 μg/ml of biotinylated OVA. OVA grade 5 was obtained from Sigma-Aldrich and conjugated to biotin using the biotin conjugation kit from Sigma-Aldrich. A ratio of biotin/OVA of 4 was achieved, as calculated by the extinction coefficient of avidin-4′-hydroxyazobenzene-2-carboxylic acid (HABA).
Bronchoalveolar lavage procedure
Lungs were lavaged with 1 ml of saline. Bronchoalveolar lavage fluids (BALFs) were centrifuged (400 g, 7 min at 4°), the cell pellet was resuspended in 100 μl of phosphate-buffered saline (PBS), a cell count was made and the cell suspension applied onto a microscope glass-slide using a Cytospin apparatus. The slide was then stained using the Diff-Quik stain and blind differential counting was performed on these slides. After the bronchoalveolar lavage (BAL) procedure, the lung was inflated with paraformaldehyde at a pressure of 25 cm H2O and allowed to absorb paraformaldehyde for 48 hr to achieve tissue fixation. After fixation, the lung was processed in paraffin, cut into 5-μm sections and mounted on slides before staining with haematoxylin & eosin (H & E) using a standard procedure. Inflammation was evaluated on a scale of 0–4 for recruitment of perialveolar/peribronchial and perivascular inflammatory cells.
Western blotting
Western blotting was performed as previously described.35 Briefly, organs were extracted, flash-frozen in liquid nitrogen and kept at −80° until required. Then, tissues were homogenized using a PRO 200 tissue homogenizer (Pro Scientific Inc., Oxford, CT, USA) in ice-cold lysis buffer [20 mm Tris–HCl (pH 8·0), 0·14 m NaCl, 10% glycerol (v/v), 1 mm phenylmethylsulphonyl fluoride (PMSF), 1 mm sodium orthovanadate (Na3VO4), 1 μm NaF and protease inhibitors (40 μg/ml of aprotinin and 20 μg/ml of leupeptin)] without Nonidet P-40 (NP-40); 1% NP-40 (v/v) was added to the lysate after homogenization. The lysates (30 μg/lane) were separated by sodium dodecyl suphate–polyacrylamide gel electrophoresis (SDS-PAGE) and the proteins were transferred to poly(vinylidene difluoride) (PVDF) membranes (GE Healthcare, Baie d’Urfé, QC, Canada). The membranes were blocked in Tris-buffered saline/0·1% Tween containing 5% milk for 1 hr at room temperature. Then, the membranes were washed and incubated for 1 hr with α-SHP1 monoclonal antibody (Cell Signalling, New England Biolabs, Pickering, ON, Canada). After washing, membranes were incubated with anti-rabbit horse reddish peroxidase (HRP) donkey Ig (GE Healthcare) for 1 hr and proteins were visualized with the use of the ECL Plus Western Blotting reagent (GE Healthcare). The membranes were stripped using the Restore Western Blot Stripping Buffer (Pierce, Nepean, ON, Canada) and reblotted using α-actin antibody (Sigma-Aldrich).
Statistical analysis
Statistically significant differences were identified using the analysis of variance (anova) module of StatView from the sas institute (version 5; SAS Institute, Cary, NC). P values of ≤ 0·05 were considered statistically significant. All data were presented as mean ± standard error of the mean (SEM).
Results
Allergen sensitization in PTP-deficient mice
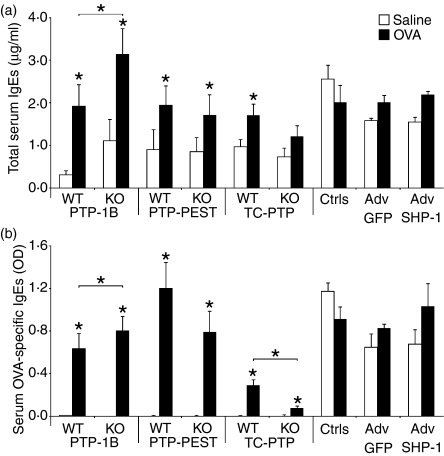
Allergen-specific IgE production is a reliable measure of the status of the allergic sensitization in animals injected with OVA/Alum. Therefore, the production of IgE was investigated in the different mice genotypes. As PTP-1B, PTP-PEST and TC-PTP mouse models are mutants, their deficiency in PTP activity is lifelong. Hence, the effect of PTP deficiency can be seen during allergen sensitization (Fig. 1a). By contrast, in the experiments where we inhibited SHP-1 activity by administration of an adenovirus encoding an shRNA to SHP-1 (the adenovirus was delivered i.v. 3 days before allergen challenge for optimal abolition of SHP-1 expression during allergen challenge), the sensitization was performed without PTP inhibition. As observed in Fig. 1a, Total serum IgEs were increased in PTP-1B mice by comparison with their WT littermate controls. Interestingly, this was also observed for OVA-specific IgEs (Fig. 1b), confirming that the increased level of IgEs observed in the absence of PTP-1B is caused by the allergen sensitization itself and is not a result of other, non-specific, mechanisms. Of interest, in the case of the heterozygous mutation of the PTP-PEST gene, the allergen sensitization resulted in an increase of both total and OVA-specific IgEs (Fig. 1a,b), but the levels did not differ between WT littermates and heterozygous animals. However, in heterozygous mice mutant for TC-PTP, the level of total serum IgEs was significantly increased by OVA sensitization only in WT littermate animals and not in heterozygous animals (Fig. 1a). Furthermore, the levels of OVA-specific IgEs were significantly different between the two groups of animals (Fig. 1b), clearly showing that TC-PTP activity is involved in the process of IgE production upon allergen sensitization. As expected in the experimental groups treated (or not) with the adenoviruses, the levels of serum IgEs were high, as a result of allergen sensitization, but no difference was observed between the groups, given that they were similarly treated for allergen sensitization with no PTP inhibition at this step (Fig. 1).
Figure 1.
Serum immunoglobulin E (IgE) levels. Blood was collected after the mice were killed and the serum was obtained. (a) Total serum IgE levels and (b) ovalbumin-specific serum IgE levels were measured using sandwich enzyme-linked immunosorbent assays (ELISAs). Inhibition of Src homology phosphatase-1 (SHP-1) activity was performed at allergen challenge, not at allergen sensitization. Data shown represent the average of 15–16 [protein tyrosine phosphatase-1B (PTP-1B)], 7–8 protein tyrosine phosphatase-phosphatase and tensin homologue deleted (PTP-PEST), 9–12 T cell PTP (TC-PTP) and 3–4 (SHP-1) animals per group. *Significant difference compared with the appropriate control or identified sample (P ≤ 0.05). A, absorbance; adenovirus (Adv); controls (Ctrls); GFP, green fluorescent protein; KO, knockout; OVA, ovalbumin; WT, wild type.
Lung inflammation of PTP-deficient mice
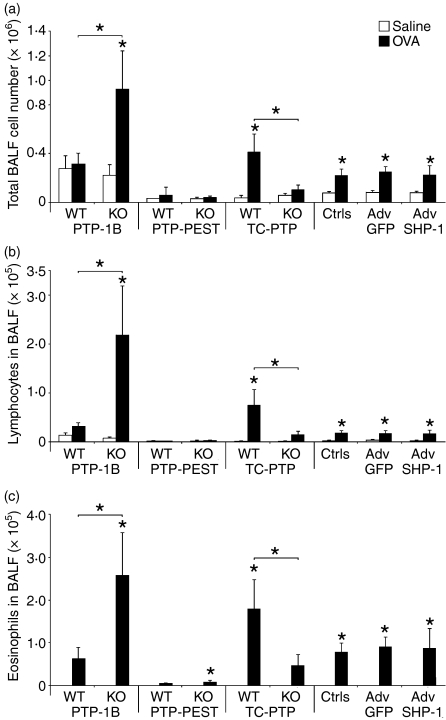
The next step of our investigation was to monitor the effects of allergen challenge in animals previously sensitized to the allergen (OVA). Typically, exposure of presensitized animals to allergen results in an increase of inflammatory cells recruitment to the lung, which is reflected by an increase in the number of cells retrieved in the BALF.17 This effect is particularly important for lymphocytes and eosinophils, the latter representing a major hallmark of the asthmatic lung.36 Therefore, we investigated the presence of total inflammatory cells, as well as lymphocytes and eosinophils, in the BALF. Figure 2 shows that PTP-1B WT littermate controls challenged with OVA show a very small increase (not significant) in the recruitment of total inflammatory cells as well as in the recruitment of lymphocytes and eosinophils (Fig. 2a–c, respectively). In stark contrast, knockout (KO) animals challenged with OVA show a marked increase in cellular recruitment for total cells, lymphocytes and eosinophils (Fig. 2a–c). The difference between WT and KO animals challenged with OVA was statistically significant, establishing that the absence of PTP-1B results in an increased recruitment of inflammatory cells.
Figure 2.
Inflammatory cells of the bronchoalveolar lavage fluid (BALF). The recruitment of inflammatory cells to the BALF compartment was evaluated using differential counts and total cell counts. Total cell counts (a), lymphocyte counts (b) and eosinophil counts (c) are presented. Data shown represent the average of 11–14 [protein tyrosine phosphatase-1B (PTP-1B)], 5–7 protein tyrosine phosphatase-phosphatase and tensin homologue deleted (PTP-PEST), 5–10 T cell PTP (TC-PTP) and 8 Src homology phosphatase-1 (SHP-1) animals per group. *Significant difference compared with the appropriate control or identified sample (P ≤ 0.05). adenovirus (Adv); controls (Ctrls); GFP, green fluorescent protein; KO, knockout; OVA, ovalbumin; WT, wild type.
In the case of PTP-PEST, no difference was observed between the heterozygous mice and their WT littermate counterparts, and the global cellular recruitment remained generally low (Fig. 2). The heterozygous mutation of TC-PTP, however, revealed that the activity of this PTP is required for inflammatory processes leading to recruitment of cells to the lung in animals sensitized to OVA. Indeed, the lungs of the WT littermate controls recruited more total cells, lymphocytes and eosinophils than the TC-PTP mutant lungs (Fig. 2). In the case of SHP-1 experiments, OVA challenge did induce an increased recruitment of all cell types, and this was similar in all groups, suggesting that SHP-1 inhibition by shRNA did not affect the exacerbation of the disease.
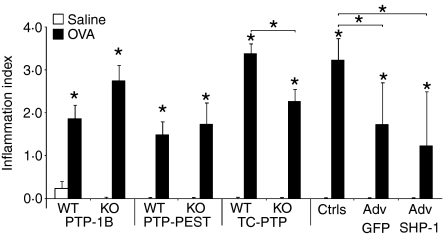
Subsequently, we investigated the status of tissue inflammation on lung histological sections. OVA-challenged mice exhibited a higher degree of tissue inflammation in all groups (Fig. 3), similarly to the data obtained in the BALF (Fig. 2). In mice KO for PTP-1B, there was a trend for exacerbation of the tissue inflammation, but this was not significant, unlike in the BALF (Fig. 2). The experiments involving PTP-PEST deficiency did not reveal any difference with the WT littermate controls, also coherent with our previous results (Figs 1 and 2). The heterozygous mutation of TC-PTP significantly reduced the inflammation in the lung tissue (Fig. 3), paralleling the results of Fig. 2. Very interestingly, administration of the shRNA adenovirus to SHP-1 reduced lung tissue inflammation. However, a similar result was also observed following administration of the control GFP-encoding adenovirus, suggesting that this decrease can probably be attributed to the adenovirus injection itself rather than to the absence of SHP-1.
Figure 3.
Lung tissue inflammatory cells. Local tissue inflammation was evaluated on histology cuts, of 5-μm thickness, stained with haematoxylin and eosin. Observations were made at 400× magnification on 7–9 [protein tyrosine phosphatase-1B (PTP-1B)], 4 protein tyrosine phosphatase-phosphatase and tensin homologue deleted (PTP-PEST), 4–7 T cell PTP (TC-PTP) and 4 Src homology phosphatase-1 (SHP-1) animals per group. *Significant difference compared with the appropriate control or identified sample (P ≤ 0.05). adenovirus (Adv); controls (Ctrls); KO, knockout; OVA, ovalbumin; WT, wild type.
SHP-1 inhibition and its effect on allergen challenge
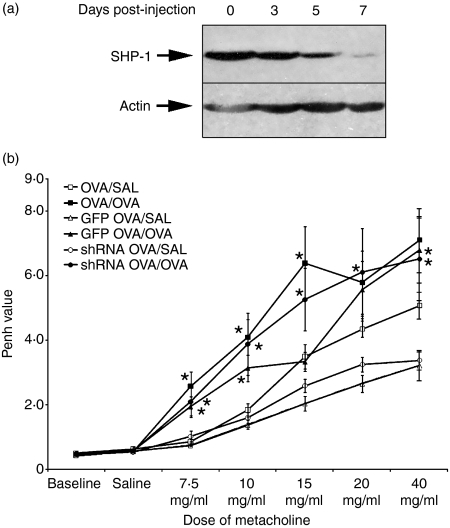
Before conducting experiments with the adenovirus encoding the shRNA to SHP-1, we were interested in ascertaining the time-dependent depletion of SHP-1. As seen in Fig. 4a, administration of the shRNA-encoding adenovirus i.v. to the mice resulted in a reduction of SHP-1 protein in the spleen from day 3, lasting at least until day 7. Previous experiments revealed that the depletion was optimal in the liver by day 3,34 and we also observed similar depletion in other organs (data not shown). Injection of the GFP-encoding adenovirus did not affect SHP-1 expression (data not shown and ref. 34). Therefore, we established that administration of the adenovirus 3 days before (day 18) the first allergen challenge (day 21) would mediate a depletion of SHP-1 protein from the first allergen challenge (day 21) to the measurement of lung reactivity (day 25).
Figure 4.
Inhibition of Src homology phosphatase-1 (SHP-1) activity and lung reactivity. (a) Following intravenous injection of the adenovirus expressing a short hairpins RNAs (shRNAs) to SHP-1, inhibition of SHP-1 protein expression was investigated in the spleen on days 3, 5 and 7. The injection of the control green fluorescent protein (GFP)-expressing adenovirus did not affect the expression of (SHP-1) (data not shown). Data shown are representative of three individual experiments. (b) The lung reactivity was evaluated using the Enhanced pause (Penh) measure. Administration of the adenovirus was performed 3 days before allergen challenge to ensure inhibition of SHP-1. Data shown represent the average of 8 animals per group. *Significant difference with appropriate control or identified sample (P ≤ 0.05). SAL, saline.
As SHP-1 was not inhibited during the sensitization (as is the case in other publications using me or mev mice),15,16 but only during allergen challenge, we aimed to investigate the effect of SHP-1 during this period of the disease. In addition to what we studied in our scan of asthma features in these PTP-deficient mice, we decided to investigate the lung reactivity in SHP-1-inhibited mice as this is a phenomenon highly dependent on the inflammation induced by exposure to allergen. Figure 4b shows these results. We observed that all OVA-challenged groups had significantly higher lung reactivity, but inhibition of SHP-1 did not affect this characteristic of asthma, suggesting that SHP-1 does not play a critical role in this acute exacerbation of asthma.
Discussion
Tyrosine phosphorylation is an important intracellular signalling event regulating cell responses to various environmental stimulations. The balance of functional activities between PTKs and PTPs in the modulation of protein tyrosine phosphorylation is important for the outcome of the cell response.5 The necessity of PTK activity in asthma has been recently documented,7–10,37 but the implication of PTP activity remains obscure except for a few reports.14,15 We previously established that abolition of PTP activity during either allergen sensitization or allergen challenge resulted in a decreased allergic asthma phenotype in our mouse model.17 In the present study, we analyzed this observation in more detail by assessing the implication of selected PTPs in an asthma model by taking advantage of mutant mouse models.
PTP-1B is now established as a potent regulator of insulin signalling,38 but its implication in the regulation of the immune system is still confused. It has been previously reported that PTP-1B can negatively modulate integrin signalling upon fibronectin binding.39 However, other experiments did not support this first observation.40 A definitive role for PTP-1B in cell adhesion still needs to be established. Our results may imply an involvement of PTP-1B in terminating the signal from integrins, as the absence of PTP-1B increased cellular infiltration in our model.
PTP-1B has also been reported to be a regulator of JAK219,20 and Tyk220 in fibroblasts stimulated with interferon (IFN)-α, IFN-γ or insulin. It is also known that the absence of PTP-1B in macrophages increases their sensitivity to lipopolysaccharide (LPS), resulting in an increase in the production of nitric oxide (NO) in vitro, or of IL-12 and IFN-γin vivo.41 These findings are also interesting in the context of the present study as JAK signalling is utilized downstream of many cytokine receptors and the inhibition of PTP-1B activity might result in a stronger or more sustained signal emanating from these receptors, potentially increasing the cell response to the stimulation. This could also partly explain our observed increased infiltration of cells in the BALF compartment.
PTP-PEST KO mice exhibit embryonic lethality,33 which prevented the use of full KO mice in our scan of PTPs. PTP-PEST was very recently shown to regulate a T-cell response upon TCR activation.42 PTP-PEST was also reported to affect TCR signalling through an inhibition of immune synapse formation43 and through an inhibition of Ras signalling.44 In the context of the present study, PTP-PEST did not appear to modulate asthma development, although allergen sensitization is expected to rely strongly on TCR signalling. As this is in contrast with its expected role in TCR regulation, we propose that the heterozygous expression of PTP-PEST is sufficient for its function in the TCR signal. We are actually attempting to generate mice with a conditional deletion of PTP-PEST in order to avoid the lethal effects of the homozygous deletion.
TC-PTP−/− mice can survive after birth, but rapidly exhibit serious health problems and die by 25 days of age.32 Among the defects noted is a gradual amplification of inflammatory disorders.22 These disorders result in an increased infiltration of tissues by mononuclear cells and a marked increase in inflammatory mediators such as IFN-γ, tumour necrosis factor-α (TNF-α) and NO.22
In our model, TC-PTP+/− mice exhibited a marked reduction in asthma development, as we noted a reduction in the level of IgEs, recruitment of inflammatory cells to the BALf compartment and lung tissue inflammation. Our results seem to indicate an obligatory role for TC-PTP in the establishment of allergic disease, possibly during both allergen sensitization and allergen challenge. Deficiency in TC-PTP is only partial, which could explain that lung inflammation and IgE production are not completely abolished. We suggest that the low level of IgE production is nonetheless dependent on this TC-PTP activity, but we could not assess it in these heterozygous mice. However, our data clearly illustrate the need for TC-PTP in the development of allergic asthma. This correlates well with a previously described reduction in TCR and B-cell receptor (BCR) signals in TC-PTP−/− mice.32,45
It was impossible to analyze lung reactivity on the mutant mice strains because reactivity to methacholine is a phenomenon highly dependent on genetic factors and is greatly affected by the mouse strain background.46 Therefore, we did not obtain consistent results in our mice where the backcross to the BALB/c background was incomplete (see the Materials and methods). Whole-body plethysmograph and invasive lung resistance methods were both unsuccessful in monitoring a reliable pattern of lung reactivity (data not shown).
SHP-1 is known for its regulation of immune responses in many contexts,47,48 and it is recognized as an important regulator of the TCR threshold.47,49,50 It has also been found to be the effector phosphatase that mediates TCR antagonism in the context of altered peptide ligands.51 More precisely in the context of asthma, Kamata and colleagues reported that reduction of SHP-1 activity exacerbated the severity of asthma.15 They used me/+ mice (in which they reported that SHP-1 activity is one-third of that of the WT mice) and observed increased asthmatic features (eosinophilia, mucus production and airway hyper-responsiveness).15 A recently published study showed similar observations in the mev mice, where an asthma-like disease spontaneously develops (without antigen sensitization or challenge).16 The major problem with these studies is the fact that the motheathen mutation, if homozygous, results in severe chronic inflammation and autoimmune disease, just as for the mev mice.48 According to the investigators, lymphocyte development appeared normal in me/+ mice. However, it is almost impossible to rule out the likelihood that chronic inflammation is already programmed in these mice and that the induction of allergic asthma is actually a trigger for this inflammation. Their results clearly show that SHP-1 is important in the control of inflammation, but one should be cautious regarding its significance in allergic asthma. We therefore investigated the effect of acute SHP-1 inhibition upon allergen challenge. To achieve this, we used adenoviruses encoding shRNA directed at SHP-1 and their control adenovirus encoding GFP, as previously reported.34 We knew from this previous work that SHP-1 inhibition is optimal at day 3 after adenovirus injection in most organs, such as the liver. In the present study, we wanted to confirm SHP-1 inhibition in lymphoid organs such as the spleen. We noted SHP-1 protein expression to be reduced between days 3 and 7 after i.v. injection with adenovirus. We also verified the expression of SHP-1 in the lung, but a basal level of SHP-1 in this organ was not detected by Western blotting (data not shown). Our results indicate that the inhibition of SHP-1 during allergen challenge does not exacerbate asthmatic features; in fact, inhibition of SHP-1 during this phase does not appear to modulate the outcome of the disease. Lung tissue inflammation is significantly different in adenovirus-injected OVA-sensitized mice, but is similar between GFP- and shRNA-encoding adenoviruses, suggesting that this observation is an artefact of the technique. One limitation of our approach is that freshly recruited cells might not be inhibited for SHP-1 activity as adenoviruses were administered 3 days before the first challenge and these non-replicative adenoviruses are present in finite numbers. A second limitation is that the reduced SHP-1 levels might still be sufficient to limit inflammation and prevent the exacerbation observed when SHP-1 is genetically deleted. But then, in this context, it was impossible to study its role only following allergen challenge, a feature that our approach was able to investigate. In regard to the previously discussed data,15,16 our results suggest that inhibition of SHP-1 at allergen challenge does not appear to support asthma development as much as permanent inhibition of SHP-1 (which should be associated with allergen sensitization).
Collectively, our results show that PTPs are important for the unfolding of immune events leading to allergic asthma. While in this study the inhibition of PTP-PEST and SHP-1 were not observed to modulate asthma development, TC-PTP activity appeared to be critical for the full development of allergic asthma, and inhibition of PTP-1B activity allowed a stronger recruitment of inflammatory cells to the BALF compartment. These data reveal important PTP-specific roles in asthma development and suggest that therapeutic approaches aimed at inhibiting specific PTPs could be envisaged in the treatment of allergic disorders such as asthma.
References
- 1.Mustelin T, Coggeshall KM, Isakov N, Altman A. T cell antigen receptor-mediated activation of phospholipase C requires tyrosine phosphorylation. Science. 1990;247:1584–7. doi: 10.1126/science.2138816. [DOI] [PubMed] [Google Scholar]
- 2.Stanley JB, Gorczynski R, Huang CK, Love J, Mills GB. Tyrosine phosphorylation is an obligatory event in IL-2 secretion. J Immunol. 1990;145:2189–98. [PubMed] [Google Scholar]
- 3.Ellery JM, Kempshall SJ, Nicholls PJ. Activation of the interleukin 2 receptor: a possible role for tyrosine phosphatases. Cell Signal. 2000;12:367–73. doi: 10.1016/s0898-6568(00)00085-1. [DOI] [PubMed] [Google Scholar]
- 4.Siraganian RP. Mast cell signal transduction from the high-affinity IgE receptor. Curr Opin Immunol. 2003;15:639–46. doi: 10.1016/j.coi.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Mustelin T, Vang T, Bottini N. Protein tyrosine phosphatases and the immune response. Nat Rev Immunol. 2005;5:43–57. doi: 10.1038/nri1530. [DOI] [PubMed] [Google Scholar]
- 6.Yanagi S, Sugawara H, Kurosaki M, Sabe H, Yamamura H, Kurosaki T. CD45 modulates phosphorylation of both autophosphorylation and negative regulatory tyrosines of Lyn in B cells. J Biol Chem. 1996;271:30487–92. doi: 10.1074/jbc.271.48.30487. [DOI] [PubMed] [Google Scholar]
- 7.Wong WS, Tsang F, Li H, Ma B. Effects of inhibitors of the tyrosine kinase signaling cascade on an in vitro model of allergic airways. Asian Pac J Allergy Immunol. 1999;17:229–37. [PubMed] [Google Scholar]
- 8.Duan W, Kuo IC, Selvarajan S, Chua KY, Bay BH, Wong WS. Antiinflammatory effects of genistein, a tyrosine kinase inhibitor, on a guinea pig model of asthma. Am J Respir Crit Care Med. 2003;167:185–92. doi: 10.1164/rccm.200205-420OC. [DOI] [PubMed] [Google Scholar]
- 9.Adachi T, Stafford S, Sur S, Alam R. A novel Lyn-binding peptide inhibitor blocks eosinophil differentiation, survival, and airway eosinophilic inflammation. J Immunol. 1999;163:939–46. [PubMed] [Google Scholar]
- 10.Kumano K, Nakao A, Nakajima H, et al. Blockade of JAK2 by tyrphostin AG-490 inhibits antigen-induced eosinophil recruitment into the mouse airways. Biochem Biophys Res Commun. 2000;270:209–14. doi: 10.1006/bbrc.2000.2403. [DOI] [PubMed] [Google Scholar]
- 11.Matsubara S, Li G, Takeda K, et al. Inhibition of spleen tyrosine kinase prevents mast cell activation and airway hyperresponsiveness. Am J Respir Crit Care Med. 2006;173:56–63. doi: 10.1164/rccm.200503-361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adcock IM, Chung KF, Caramori G, Ito K. Kinase inhibitors and airway inflammation. Eur J Pharmacol. 2006;533:118–32. doi: 10.1016/j.ejphar.2005.12.054. [DOI] [PubMed] [Google Scholar]
- 13.Alonso A, Sasin J, Bottini N, et al. Protein tyrosine phosphatases in the human genome. Cell. 2004;117:699–711. doi: 10.1016/j.cell.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 14.Kwak YG, Song CH, Yi HK, et al. Involvement of PTEN in airway hyperresponsiveness and inflammation in bronchial asthma. J Clin Invest. 2003;111:1083–92. doi: 10.1172/JCI16440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamata T, Yamashita M, Kimura M, et al. src homology 2 domain-containing tyrosine phosphatase SHP-1 controls the development of allergic airway inflammation. J Clin Invest. 2003;111:109–19. doi: 10.1172/JCI15719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oh SY, Zheng T, Kim YK, et al. A critical role of SHP-1 in regulation of Type 2 inflammation in the lung. Am J Respir Cell Mol Biol. 2008;40:568–74. doi: 10.1165/rcmb.2008-0225OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pouliot P, Camateros P, Radzioch D, Lambrecht BN, Olivier M. Protein tyrosine phosphatases regulate asthma development in a murine asthma model. J Immunol. 2009;182:1334–40. doi: 10.4049/jimmunol.182.3.1334. [DOI] [PubMed] [Google Scholar]
- 18.Simoncic PD, Lee-Loy A, Barber DL, Tremblay ML, McGlade CJ. The T cell protein tyrosine phosphatase is a negative regulator of janus family kinases 1 and 3. Curr Biol. 2002;12:446–53. doi: 10.1016/s0960-9822(02)00697-8. [DOI] [PubMed] [Google Scholar]
- 19.Gu F, Dube N, Kim JW, et al. Protein tyrosine phosphatase 1B attenuates growth hormone-mediated JAK2-STAT signaling. Mol Cell Biol. 2003;23:3753–62. doi: 10.1128/MCB.23.11.3753-3762.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myers MP, Andersen JN, Cheng A, et al. TYK2 and JAK2 are substrates of protein-tyrosine phosphatase 1B. J Biol Chem. 2001;276:47771–4. doi: 10.1074/jbc.C100583200. [DOI] [PubMed] [Google Scholar]
- 21.Horsch K, Schaller MD, Hynes NE. The protein tyrosine phosphatase-PEST is implicated in the negative regulation of epidermal growth factor on PRL signaling in mammary epithelial cells. Mol Endocrinol. 2001;15:2182–96. doi: 10.1210/mend.15.12.0743. [DOI] [PubMed] [Google Scholar]
- 22.Heinonen KM, Nestel FP, Newell EW, et al. T-cell protein tyrosine phosphatase deletion results in progressive systemic inflammatory disease. Blood. 2004;103:3457–64. doi: 10.1182/blood-2003-09-3153. [DOI] [PubMed] [Google Scholar]
- 23.Blanchette J, Dayyeh IA, Hassani K, Whitcombe L, Olivier M. Regulation of macrophage nitric oxide production by the protein tyrosine phosphatase Src homology 2 domain phosphotyrosine phosphatase 1 (SHP-1) Immunology. 2008;127:123–33. doi: 10.1111/j.1365-2567.2008.02929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rinna A, Forman HJ. SHP-1 inhibition by 4-hydroxynonenal activates Jun N-terminal kinase and glutamate cysteine ligase. Am J Respir Cell Mol Biol. 2008;39:97–104. doi: 10.1165/rcmb.2007-0371OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mizuno K, Tagawa Y, Mitomo K, et al. Src homology region 2 domain-containing phosphatase 1 positively regulates B cell receptor-induced apoptosis by modulating association of B cell linker protein with Nck and activation of c-Jun NH2-terminal kinase. J Immunol. 2002;169:778–86. doi: 10.4049/jimmunol.169.2.778. [DOI] [PubMed] [Google Scholar]
- 26.Mizuno K, Tagawa Y, Mitomo K, et al. Src homology region 2 (SH2) domain-containing phosphatase-1 dephosphorylates B cell linker protein/SH2 domain leukocyte protein of 65 kDa and selectively regulates c-Jun NH2-terminal kinase activation in B cells. J Immunol. 2000;165:1344–51. doi: 10.4049/jimmunol.165.3.1344. [DOI] [PubMed] [Google Scholar]
- 27.Xie ZH, Zhang J, Siraganian RP. Positive regulation of c-Jun N-terminal kinase and TNF-alpha production but not histamine release by SHP-1 in RBL-2H3 mast cells. J Immunol. 2000;164:1521–8. doi: 10.4049/jimmunol.164.3.1521. [DOI] [PubMed] [Google Scholar]
- 28.Brockdorff J, Williams S, Couture C, Mustelin T. Dephosphorylation of ZAP-70 and inhibition of T cell activation by activated SHP1. Eur J Immunol. 1999;29:2539–50. doi: 10.1002/(SICI)1521-4141(199908)29:08<2539::AID-IMMU2539>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 29.Abu-Dayyeh I, Shio MT, Sato S, Akira S, Cousineau B, Olivier M. Leishmania-induced IRAK-1 inactivation is mediated by SHP-1 interacting with an evolutionarily conserved KTIM motif. PLoS Negl Trop Dis. 2008;2:e305. doi: 10.1371/journal.pntd.0000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matte C, Marquis JF, Blanchette J, et al. Peroxovanadium-mediated protection against murine leishmaniasis: role of the modulation of nitric oxide. Eur J Immunol. 2000;30:2555–64. doi: 10.1002/1521-4141(200009)30:9<2555::AID-IMMU2555>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 31.Elchebly M, Payette P, Michaliszyn E, et al. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283:1544–8. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- 32.You-Ten KE, Muise ES, Itie A, et al. Impaired bone marrow microenvironment and immune function in T cell protein tyrosine phosphatase-deficient mice. J Exp Med. 1997;186:683–93. doi: 10.1084/jem.186.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sirois J, Cote JF, Charest A, et al. Essential function of PTP-PEST during mouse embryonic vascularization, mesenchyme formation, neurogenesis and early liver development. Mech Dev. 2006;123:869–80. doi: 10.1016/j.mod.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dubois MJ, Bergeron S, Kim HJ, et al. The SHP-1 protein tyrosine phosphatase negatively modulates glucose homeostasis. Nat Med. 2006;12:549–56. doi: 10.1038/nm1397. [DOI] [PubMed] [Google Scholar]
- 35.Jaramillo M, Godbout M, Naccache PH, Olivier M. Signaling events involved in macrophage chemokine expression in response to monosodium urate crystals. J Biol Chem. 2004;279:52797–805. doi: 10.1074/jbc.M403823200. [DOI] [PubMed] [Google Scholar]
- 36.Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol. 1999;17:255–81. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- 37.Malaviya R, Chen CL, Navara C, et al. Treatment of allergic asthma by targeting janus kinase 3-dependent leukotriene synthesis in mast cells with 4-(3′, 5′-dibromo-4′-hydroxyphenyl)amino-6,7-dimethoxyquinazoline (WHI-P97) J Pharmacol Exp Ther. 2000;295:912–26. [PubMed] [Google Scholar]
- 38.Cheng A, Dube N, Gu F, Tremblay ML. Coordinated action of protein tyrosine phosphatases in insulin signal transduction. Eur J Biochem. 2002;269:1050–9. doi: 10.1046/j.0014-2956.2002.02756.x. [DOI] [PubMed] [Google Scholar]
- 39.Liu F, Sells MA, Chernoff J. Protein tyrosine phosphatase 1B negatively regulates integrin signaling. Curr Biol. 1998;8:173–6. doi: 10.1016/s0960-9822(98)70066-1. [DOI] [PubMed] [Google Scholar]
- 40.Cheng A, Bal GS, Kennedy BP, Tremblay ML. Attenuation of adhesion-dependent signaling and cell spreading in transformed fibroblasts lacking protein tyrosine phosphatase-1B. J Biol Chem. 2001;276:25848–55. doi: 10.1074/jbc.M009734200. [DOI] [PubMed] [Google Scholar]
- 41.Heinonen KM, Dube N, Bourdeau A, Lapp WS, Tremblay ML. Protein tyrosine phosphatase 1B negatively regulates macrophage development through CSF-1 signaling. Proc Natl Acad Sci U S A. 2006;103:2776–81. doi: 10.1073/pnas.0508563103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arimura Y, Vang T, Tautz L, Williams S, Mustelin T. TCR-induced downregulation of protein tyrosine phosphatase PEST augments secondary T cell responses. Mol Immunol. 2008;45:3074–84. doi: 10.1016/j.molimm.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Badour K, Zhang J, Shi F, Leng Y, Collins M, Siminovitch KA. Fyn and PTP-PEST-mediated regulation of Wiskott–Aldrich syndrome protein (WASp) tyrosine phosphorylation is required for coupling T cell antigen receptor engagement to WASp effector function and T cell activation. J Exp Med. 2004;199:99–112. doi: 10.1084/jem.20030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davidson D, Veillette A. PTP-PEST, a scaffold protein tyrosine phosphatase, negatively regulates lymphocyte activation by targeting a unique set of substrates. EMBO J. 2001;20:3414–26. doi: 10.1093/emboj/20.13.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simoncic PD, McGlade CJ, Tremblay ML. PTP1B and TC-PTP: novel roles in immune-cell signaling. Can J Physiol Pharmacol. 2006;84:667–75. doi: 10.1139/y06-012. [DOI] [PubMed] [Google Scholar]
- 46.De Sanctis GT, Daheshia M, Daser A. Genetics of airway hyperresponsiveness. J Allergy Clin Immunol. 2001;108:11–20. doi: 10.1067/mai.2001.116429. [DOI] [PubMed] [Google Scholar]
- 47.Gregory DJ, Godbout M, Contreras I, Forget G, Olivier M. A novel form of NF-kappaB is induced by Leishmania infection: involvement in macrophage gene expression. Eur J Immunol. 2008;38:1071–81. doi: 10.1002/eji.200737586. [DOI] [PubMed] [Google Scholar]
- 48.Zhang J, Somani AK, Siminovitch KA. Roles of the SHP-1 tyrosine phosphatase in the negative regulation of cell signalling. Semin Immunol. 2000;12:361–78. doi: 10.1006/smim.2000.0223. [DOI] [PubMed] [Google Scholar]
- 49.Carter JD, Neel BG, Lorenz U. The tyrosine phosphatase SHP-1 influences thymocyte selection by setting TCR signaling thresholds. Int Immunol. 1999;11:1999–2014. doi: 10.1093/intimm/11.12.1999. [DOI] [PubMed] [Google Scholar]
- 50.Johnson KG, LeRoy FG, Borysiewicz LK, Matthews RJ. TCR signaling thresholds regulating T cell development and activation are dependent upon SHP-1. J Immunol. 1999;162:3802–13. [PubMed] [Google Scholar]
- 51.Kilgore NE, Carter JD, Lorenz U, Evavold BD. Cutting edge: dependence of TCR antagonism on Src homology 2 domain-containing protein tyrosine phosphatase activity. J Immunol. 2003;170:4891–5. doi: 10.4049/jimmunol.170.10.4891. [DOI] [PubMed] [Google Scholar]