Abstract
Toll-like receptors (TLRs) are key receptors of the innate immune system and show cell subset-specific expression. We investigated the messenger RNA (mRNA) expression of TLR genes in human haematopoietic stem cells (HSC), in naïve B cells, in memory B cells, in plasma cells from palatine tonsils and in plasma cells from peripheral blood. HSC and plasma cells showed unrestricted expression of TLR1–TLR9, in contrast to B cells which lacked TLR3, TLR4 and TLR8 but expressed mRNA of all other TLRs. We demonstrated, for the first time, that TLR triggering of terminally differentiated plasma cells augments immunoglobulin production. Thus, boosting the immediate antibody response by plasma cells upon pathogen recognition may point to a novel role of TLRs.
Keywords: B-cell subpopulations, immunoglobulin production, mRNA expression, plasma cells, toll-like receptors
Introduction
Toll-like receptors (TLRs) are key recognition structures of the innate immunity.1 They trigger antimicrobial responses by conserved pathogen-associated molecular patterns. The signalling cascade culminates, among others, in the activation of nuclear factor-κB (NF-κB), which results in the expression of pro-inflammatory cytokines that are critical for the innate as well as for the adaptive immune responses.1 Furthermore, TLRs play a role in autophagy,2 haematopoiesis3 and neutrophil activation.4 For each of the 10 known human TLRs (TLR1–TLR10), at least one distinct ligand has been identified, except for TLR10.1
Cell subpopulations exhibit specific TLR expression patterns,5,6 indicating that TLR expression is tailored to distinct cellular functions. Furthermore, the TLR expression pattern depends on the developmental stage, as exemplified by the developmental-dependent degree of TLR1–TLR5 expression in dendritic cells7 and of TLR9 in B cells.8 Delineation of the expression of TLRs during human B-cell development is so far incomplete.9 Data on TLR expression in human B cells at their maturation stages and in terminally differentiated plasma cells from the same donors and from the same secondary lymphatic organ where differentiation actually takes place (e.g. tonsils), have not yet been presented, and such data form the basis for functional studies.
Here, we hypothesized that the expression and function of TLRs are tailored to stages of B-cell development and differentiation. Therefore, we investigated the quantitative expression of TLR1–TLR10 in haematopoietic stem cells (HSC) as well as in naïve B cells, memory B cells and plasma cells from the same lymphoid tissue. Furthermore, we addressed the as-yet uninvestigated issue of whether TLR triggering affects plasma cell function, and we showed that TLR triggering increases the production and secretion of immunoglobulin from plasma cells.
Materials and methods
Cells and subpopulations
Cells were isolated from cord blood or tonsils, as described previously.10–12 The study was approved by the local ethics committee, and written informed consent was obtained for all tissue obtained. Cord blood HSC, B cells and plasma cells were isolated using CD34 microbeads, the B-cell isolation kit II and CD138 microbeads, respectively, according to the instructions of the manufacturer (Miltenyi Biotech, Bergisch Gladbach, Germany). Further separation of B cells into naïve and memory B cells was performed using the naïve-B-cell isolation kit (Miltenyi Biotech) or CD27 microbeads (Miltenyi Biotech).12 Isolated cell populations used for experiments were always > 95% pure, as determined by flow cytometry.
Quantitative real-time polymerase chain reaction
Quantitative real-time polymerase chain reaction (PCR) was performed for TLR9 and the housekeeping gene hydroxymethylbilane-synthase (HMBS), as described previously.10,12TLR10 was analyzed using primer/probe on demand (Hs01935337_s1, Assay-on-demand gene expression product; Applied Biosystems, Foster City, CA). SYBR Green primers for HMBS and TLR1–TLR8 were as described previously.13
Flow cytometry
Flow cytometry using fluorochrome-conjugated monoclonal antibodies to human CD34, CD19, CD27, CD138, IgM or IgG (BD Biosciences, Basel, Switzerland) was executed on a Cytomics FC500 instrument (Beckman Coulter, Nyon, Switzerland); data were analyzed using flowjo software (Treestar, Ashland, OR).
Intracellular immunoglobulin staining and enzyme-linked immunosorbent assay
Tonsillar plasma cells were either untreated or were stimulated with 10 μg/ml of peptidoglycan (TLR1/2 ligand; Fluka, Buchs, Switzerland), 1 μg/ml of poly(I:C) (TLR3 ligand; InvivoGen, San Diego, CA), 10 ng/ml of lipopolysaccharide (LPS) (TLR4 ligand; Sigma-Aldrich, Buchs, Switzerland), 10 ng/ml of flagellin (TLR5 ligand; InvivoGen), 3 μm R-848 (TLR7/8 ligand; InvivoGen), or 2 μm cytosine–phosphate–guanosine (CpG) oligonucleotide (ODN) 2006 (TLR9 ligand; Eurogentec, Köln, Germany). Seventy-two hours after stimulation, cells were harvested, fixed, permeabilized and stained. Intracellular staining of IgM and IgG on plasma cells was performed using fluorochrome-conjugated monoclonal antibodies and the BD Cytofix/Cytoperm kit (both from BD Biosciences), according to the manufacturer’s instructions.
The total amount of secreted immunoglobulin was determined using an in-house enzyme-linked immunosorbent assay: briefly, 96-well microtitre plates were coated with 10 μg/ml of Protein G (Calbiochem, Dietlikon, Switzerland) diluted in a carbonate–bicarbonate buffer (pH 9·6) and stored overnight at room temperature in a humid chamber. The plates were washed four times with phosphate-buffered saline (PBS) and incubated for 1 hr at room temperature with 200 μl per well of 3% bovine serum albumin in PBS. After discarding the blocking buffer, 50 μl of supernatant of the plasma cell samples, or serial dilutions of human immunoglobulin (NIBSC, Hertfordshire, UK) as a reference, were added to each well and allowed to react for 30 min at 37°. After three washing steps, peroxidase-labelled sheep anti-human immunoglobulin (Millipore, Munich, Germany) was incubated for 30 min at 37°. After three washing steps, 100 μl of 3,3′,5,5′-tetramethyl-benzidine substrate (Mabtech, Hamburg, Germany) was added and incubated for 30 min at 37° in the dark. The reactions were stopped by the addition of 50 μl of 1 m citrate. The absorbance was determined photometrically at 450 nm, with 620 nm as the reference filter.
Results
Pattern of TLR mRNA expression changes during B-cell development
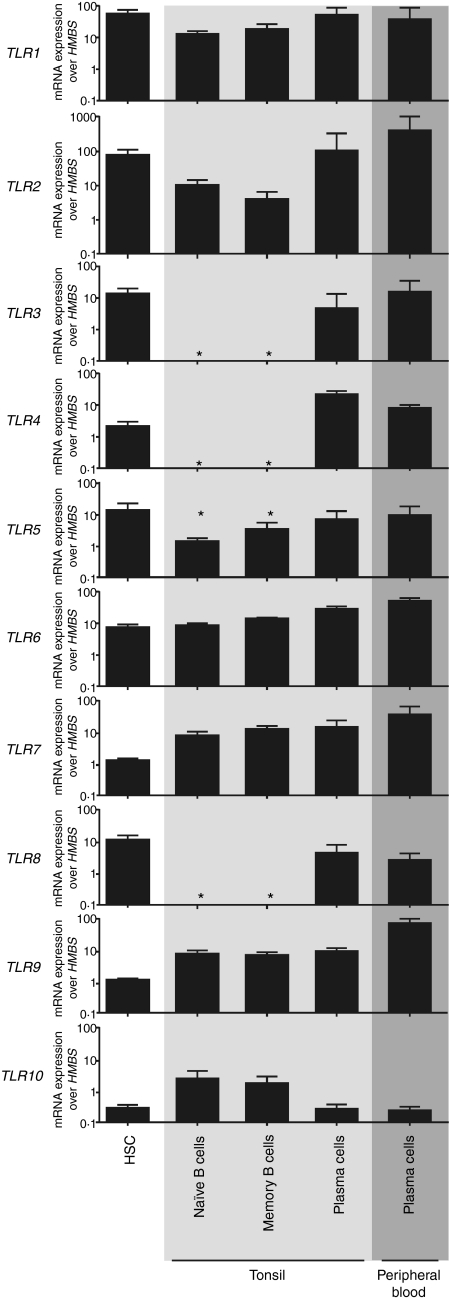
We aimed to assess the TLR expression patterns of distinct human B-cell subpopulations, including HSC and naïve and memory B cells, as well as plasma cells (Fig. S1). TLR protein levels may correlate with mRNA expression for cell-surface TLRs14,15 but in our hands commercially available TLR antibodies failed to reproduce previously described results on the subcellular localization of TLRs (data not shown). Thus, we quantified TLR expression using real-time PCR to TLR mRNA. Indeed, TLR expression patterns display notable changes during B-cell development. HSC and tonsillar plasma cells expressed all TLRs with the exception of TLR10 (Fig. 1). In contrast to HSC and plasma cells, we found a complete absence of TLR3, TLR4 and TLR8 expression in naïve and memory B cells, which is in agreement with published data,5,6,16 while the other TLRs were expressed to varying degrees (Fig. 1). Next, we investigated whether circulating plasma cells differ in their TLR expression from their tonsillar counterparts to exclude the possibility plasma cells exhibit distinct TLR expression patterns after exiting secondary lymphoid organs. We found that plasma cells from the peripheral blood exhibited the same expression pattern as tonsillar plasma cells but that expression of TLR9 was around 10-fold higher (Fig. 1). The cell subset-specific TLR expression observed here suggests that HSC and periphery terminally differentiated plasma cells need the expression of TLR1–TLR9, whereas B cells from secondary lymphoid organs need expression of TLR1, TLR2, TLR5–TLR7, TLR9 and TLR10.
Figure 1.
Messenger RNA (mRNA) expression levels of toll-like receptor (TLR)1–TLR10 show distinct regulation patterns during B-cell development. mRNA expression profiling is shown of TLR1–TLR10 in haematopoietic stem cells (HSC) isolated from cord blood, in naïve B cells, in memory B cells, in terminally differentiated plasma cells from tonsils and in terminally differentiated plasma cells from peripheral blood. HSC and plasma cells were isolated by positive selection using CD34 microbeads and CD138 microbeads, respectively. Naïve B cells and memory B cells were isolated using the naïve B-cell isolation kit and a combination of the B-cell isolation kit II and CD27 microbeads, respectively. Expression of TLR1–TLR10 and of the housekeeping gene hydroxymethylbilane-synthase (HMBS) mRNA was monitored by quantitative real-time polymerase chain reaction (PCR). Results shown are the means ± standard deviation (SD) of three biological replicates of one out of three representative experiments. *Denotes not detectable.
Engagement of TLRs on terminally differentiated plasma cells increases immunoglobulin production and secretion
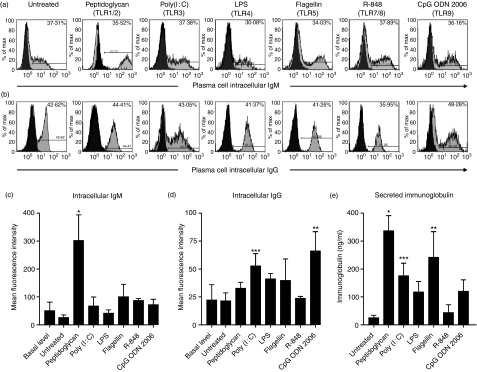
Based on the constitutively high TLR1–TLR9 expression by plasma cells (i.e. of the TLRs for which ligands are known), we wondered whether TLR triggering affects the function of these cells. Indeed, plasma cell TLR triggering resulted in significantly increased production of intracellular IgM following triggering of TLR1/2 (Fig. 2a,c) and of IgG following triggering of TLR3 or TLR9 (Fig. 2b,d) as well as secretion of total immunoglobulin following triggering of TLR1–TLR3 and TLR5 (Fig. 2e), when compared with freshly ex vivo isolated plasma cells.
Figure 2.
Triggering of toll-like receptors (TLRs) on plasma cells isolated from tonsils induces increased production of immunoglobulin. Intracellular (a) IgM and (b) IgG expression in plasma cells, mean fluorescence intensity of (c) IgM and (d) IgG in plasma cells and (e) secretion of immunoglobulin by plasma cells isolated from tonsils. Plasma cells were isolated from palatine tonsils by positive selection using CD138 microbeads. The viability of plasma cells after 72 hr in culture was always above 75%. Black histograms indicate isotype-control staining. The results shown are the means ± standard deviation of three biological replicates of one out of three representative experiments. *P< 0·001; **P< 0·01; ***P< 0·05, by Kruskall–Wallis and Dunn’s multicomparison test. CpG ODN, cytosine–phosphate–guanosine oligonucleotide 2006; LPS, lipopolysaccharide.
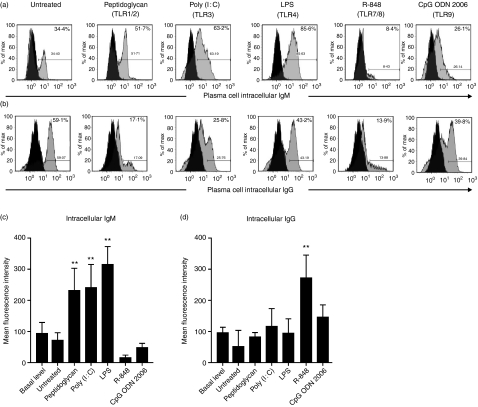
Next, we evaluated whether the increased TLR9 expression by plasma cells from peripheral blood compared with that from tonsils influences immunoglobulin production upon TLR triggering (Fig. 3). We observed a significant increase of intracellular IgM production following triggering of TLR1–TLR4 (Fig. 3a,c), and a significant increase of intracellular IgG production following triggering of TLR7/8 (Fig. 3b,d), when compared with freshly isolated plasma cells ex vivo.
Figure 3.
Triggering of toll-like receptors (TLRs) on plasma cells isolated from peripheral blood induces increased production of immunoglobulin. Intracellular (a) IgM and (b) IgG expression, and mean fluorescence intensity of (c) IgM and (d) IgG, in plasma cells isolated from peripheral blood. Plasma cells were isolated from peripheral blood by positive selection using CD138 microbeads. The viability of plasma cells after 72 hr in culture was always above 75%. Black histograms indicate isotype-control staining. The results shown are the means ± standard deviation of three biological replicates of one out of three representative experiments. **P< 0·01, by Kruskall–Wallis and Dunn’s multicomparison test. CpG ODN, cytosine–phosphate–guanosine oligonucleotide 2006; LPS, lipopolysaccharide.
This increased immunoglobulin production/secretion suggests that terminally differentiated plasma cell TLRs have functions beyond mere innate immunity pathogen sensing and thus may be critical in regulating the strength of adaptive antibody-mediated immune responses. The omnipresent TLR1–TLR9 mRNA expression by plasma cells seems to contrast the selective, strong responses to TLR1–TLR3, TLR5, or TLR9 triggering in tonsillar plasma cells and to TLR1–TLR4 and TLR7/8 triggering in peripheral blood plasma cells. One possible explanation for this could be that different TLRs drive different classes of antibodies. Nevertheless, the selective responses observed may depend on the specific adaptive immunity repertoire of the plasma cell donor, as indicated by the divergent results obtained for TLR3 and TLR7/8 triggering of tonsillar versus peripheral blood plasma cells, which were obtained from different donors.
Discussion
In the present study we aimed to assess the TLR expression patterns in human B cells (including HSC, naïve and memory B cells, as well as plasma cells) at distinct developmental stages and to analyze the impact of plasma cell TLR engagement. Indeed, we demonstrated that TLR expression patterns display notable changes during peripheral B-cell development, that plasma cells exhibit TLR expression distinct from naïve and memory B cells but similar to HSC, and that engagement of plasma cell TLRs results in augmented immunoglobulin production and secretion by these cells. These results suggest that TLR expression is tailored to cellular developmental stage function.
In agreement with published data,5,6,16 we found a complete absence of TLR3, TLR4 and TLR8 expression in naïve and memory B cells, while varying levels of the other TLRs were expressed in those cells. We complemented the characterization of the TLR profile in the B-cell lineage by examining terminally differentiated plasma cells: we found that plasma cells expressed all TLRs, with the exception of TLR10 which is very weakly expressed. By contrast, TLR10 was expressed very strongly in naïve and memory B cells. Strikingly, HSC expressed virtually no TLR10. Lack of acceptable specificity of antibodies to TLRs (data not shown) precluded analysis at the protein level. Triggering TLRs resulted in increased production of IgM and IgG as well as in the secretion of soluble immunoglobulin by plasma cells, which also speaks in favour of the functional integrity of TLRs and their immunological significance. This increased immunoglobulin production/secretion points to a novel role of TLRs: TLRs are known for their role in the innate immune response as well as for linking the innate and adaptive immune responses by enhancing cellular activation, up-regulation of the major histocompatibility complex (MHC) class molecules and release of cytokines; the fact that TLR agonists directly engage in the generation and strength of antibody production uncovers a novel role that may have therapeutic application (e.g. boosting an antibody response). Noteworthy, we observed preferential production of IgM following triggering of TLR1/2 and of IgG following triggering of TLR3 or TLR9, as well as a preferential secretion of immunoglobulin following triggering of TLR1/2, TLR3, or TLR5 by terminally differentiated plasma cells isolated from tonsils. By contrast, we observed preferential production of IgM following triggering of TLR1–TLR4, and of IgG following triggering of TLR7/8, by terminally differentiated plasma cells isolated from peripheral blood. This may be a result either of preferential recognition and specialized function of TLRs in terminally differentiated plasma cells in response to distinct conserved motifs or because of the preferential isotype immunoglobulin responses of the plasma cells we purified. The latter is dependent on the donor’s immunity repertoire. Previous analyses of TLR function in B-cell lineages have shown that naïve and memory B cells respond to TLR agonists by sustaining or increasing their TLR expression or by inducing the expression of pro-inflammatory cytokines such as interleukin-8 (IL-8).5,6,16 Thus, the data we report outline a higher complexity of TLR function in B-cell immunology when expanded to terminal plasma cell differentiation.
Our observation of TLR expression by plasma cells from tonsils or peripheral blood is in contrast to that reported for plasma cells from the bone marrow, which shows largely the absence of TLR expression.17 It is generally agreed that the bone marrow is the primary site of long-lived plasma cells which are responsible for at least some persistent antibodies, and that occur independently of memory B cells and antigen.18 Thus, given their continuous antibody production, long-lived plasma cells in the bone marrow may not need to be boosted via TLR triggering and therefore TLR expression by bone marrow plasma cells may be dispensable. Intriguingly, we found that peripheral blood plasma cells expressed TLR9 at approximately 10-fold higher levels than their tonsillar counterparts. Nevertheless, triggering of TLR9 in peripheral blood plasma cells did not result in increased intracellular IgG production, as observed in tonsillar plasma cells. Thus, the level of TLR9 expression seems not to correlate to the level of augmented immunoglobulin production upon triggering. A possible explanation for this may be that only a small portion of TLR9 is cleaved to the active form.19
The cell subset-specific TLR expression observed here suggests that HSC and periphery plasma cells need the expression of TLR1–TLR9, whereas B cells from secondary lymphoid organs need the expression of TLR1, TLR2, TLR5–TLR7, TLR9 and TLR10. We speculate that the distinct TLR profile of HSC is critical for their differentiation towards a specific cell subset and dependent upon the encountered pathogen recognition pattern to optimize pathogen-driven immune responses. Indeed, recent work in mice3 and humans20 has demonstrated that TLR signals bias HSC towards myelopoiesis directly by replacing endogenous cytokines normally required for the survival, proliferation and development of haematopoietic progenitors. In naïve and memory B cells, TLR triggering seems to be restricted to sustaining or increasing their TLR expression or inducing the expression of pro-inflammatory cytokines such as IL-8.5,6,16 Finally, as we show, peripheral plasma cell TLR engagement augments immunoglobulin release. Nevertheless, other so far unidentified TLR functions in these cell subsets may exist.
In conclusion, we report that TLR expression differs according to the B-cell developmental stage. TLR1–TLR9 may promote augmentation of antibody production by plasma cells, which may have therapeutic application. By contrast, TLR10, for which no ligand has so far been recognized and which is virtually unexpressed in HSC or in plasma cells, may functionally be linked to specific naïve and memory B-cell actions rather than simple pathogen sensing. It is tempting to speculate that TLR10 mediates a unique function (e.g. interaction with self-proteins and thereby homing of naïve and memory B cells to secondary lymphoid organs). This speculation is based on TLR10 being expressed at the cell surface1 and not by cell lineages other than B cells,5,6,9,16 B-cell differentiation to plasma cells, requiring (among others) changes in cell-surface proteins and homing,18 on findings of TLR4 recognizing host structures21 and of toll guiding dorsal-ventral cell orientation in Drosophila.22
Acknowledgments
The authors thank Prof. Adriano Fontana (University of Zurich, Zurich, Switzerland) for helpful suggestions on the manuscript. This work was supported by grants from the Swiss National Science Foundation (no. 10040–114118) (D.N.), the Cancer League of the Kanton of Zurich (D.N.), the Hartmann–Müller Foundation (D.N.) and the Forschungskredit der Universität Zürich (M.D.).
Disclosure
There are no financial disclosures for this work.
Supporting Information
The following supplementary material is available for this article:
Figure S1. Schematic flow-chart of cell isolations and cell purities.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Ishii KJ, Koyama S, Nakagawa A, Coban C, Akira S. Host innate immune receptors and beyond: making sense of microbial infections. Cell Host Microbe. 2008;3:352–63. doi: 10.1016/j.chom.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Delgado MA, Elmaoued RA, Davis AS, Kyei G, Deretic V. Toll-like receptors control autophagy. EMBO J. 2008;27:1110–21. doi: 10.1038/emboj.2008.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, Takatsu K, Kincade PW. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24:801–12. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayashi F, Means TK, Luster AD. Toll-like receptors stimulate human neutrophil function. Blood. 2003;102:2660–9. doi: 10.1182/blood-2003-04-1078. [DOI] [PubMed] [Google Scholar]
- 5.Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdorfer B, Giese T, Endres S, Hartmann G. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531–7. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 6.Bourke E, Bosisio D, Golay J, Polentarutti N, Mantovani A. The toll-like receptor repertoire of human B lymphocytes: inducible and selective expression of TLR9 and TLR10 in normal and transformed cells. Blood. 2003;102:956–63. doi: 10.1182/blood-2002-11-3355. [DOI] [PubMed] [Google Scholar]
- 7.Visintin A, Mazzoni A, Spitzer JH, Wyllie DH, Dower SK, Segal DM. Regulation of toll-like receptors in human monocytes and dendritic cells. J Immunol. 2001;166:249–55. doi: 10.4049/jimmunol.166.1.249. [DOI] [PubMed] [Google Scholar]
- 8.Bernasconi NL, Onai N, Lanzavecchia A. A role for toll-like receptors in acquired immunity: up-regulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells. Blood. 2003;101:4500–4. doi: 10.1182/blood-2002-11-3569. [DOI] [PubMed] [Google Scholar]
- 9.Chiron D, Bekeredjian-Ding I, Pellat-Deceunynck C, Bataille R, Jego G. Toll-like receptors: lessons to learn from normal and malignant human B cells. Blood. 2008;112:2205–13. doi: 10.1182/blood-2008-02-140673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ladell K, Dorner M, Zauner L, et al. Immune activation suppresses initiation of lytic Epstein–Barr virus infection. Cell Microbiol. 2007;9:2055–69. doi: 10.1111/j.1462-5822.2007.00937.x. [DOI] [PubMed] [Google Scholar]
- 11.Giger B, Bonanomi A, Odermatt B, et al. Human tonsillar tissue block cultures differ from autologous tonsillar cell suspension cultures in lymphocyte subset activation and cytokine gene expression. J Immunol Methods. 2004;289:179–90. doi: 10.1016/j.jim.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 12.Dorner M, Zucol F, Berger C, Byland R, Melroe GT, Bernasconi M, Speck RF, Nadal D. Distinct ex vivo susceptibility of B-cell subsets to Epstein–Barr virus infection according to differentiation status and tissue origin. J Virol. 2008;82:4400–12. doi: 10.1128/JVI.02630-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mempel M, Voelcker V, Kollisch G, et al. Toll-like receptor expression in human keratinocytes: nuclear factor kappaB controlled gene activation by Staphylococcus aureus is toll-like receptor 2 but not toll-like receptor 4 or platelet activating factor receptor dependent. J Invest Dermatol. 2003;121:1389–96. doi: 10.1111/j.1523-1747.2003.12630.x. [DOI] [PubMed] [Google Scholar]
- 14.Beklen A, Hukkanen M, Richardson R, Konttinen YT. Immunohistochemical localization of toll-like receptors 1–10 in periodontitis. Oral Microbiol Immunol. 2008;23:425–31. doi: 10.1111/j.1399-302X.2008.00448.x. [DOI] [PubMed] [Google Scholar]
- 15.Kajita K, Honda T, Amanuma R, et al. Quantitative messenger RNA expression of toll-like receptors and interferon-alpha1 in gingivitis and periodontitis. Oral Microbiol Immunol. 2007;22:398–402. doi: 10.1111/j.1399-302X.2007.00377.x. [DOI] [PubMed] [Google Scholar]
- 16.Mansson A, Adner M, Hockerfelt U, Cardell LO. A distinct toll-like receptor repertoire in human tonsillar B cells, directly activated by PamCSK, R-837 and CpG-2006 stimulation. Immunology. 2006;118:539–48. doi: 10.1111/j.1365-2567.2006.02392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bohnhorst J, Rasmussen T, Moen SH, Flottum M, Knudsen L, Borset M, Espevik T, Sundan A. Toll-like receptors mediate proliferation and survival of multiple myeloma cells. Leukemia. 2006;20:1138–44. doi: 10.1038/sj.leu.2404225. [DOI] [PubMed] [Google Scholar]
- 18.Shapiro-Shelef M, Calame K. Regulation of plasma-cell development. Nat Rev Immunol. 2005;5:230–42. doi: 10.1038/nri1572. [DOI] [PubMed] [Google Scholar]
- 19.Ewald SE, Lee BL, Lau L, Wickliffe KE, Shi GP, Chapman HA, Barton GM. The ectodomain of toll-like receptor 9 is cleaved to generate a functional receptor. Nature. 2008;456:658–62. doi: 10.1038/nature07405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sioud M, Floisand Y. TLR agonists induce the differentiation of human bone marrow CD34+ progenitors into CD11c+ CD80/86+ DC capable of inducing a Th1-type response. Eur J Immunol. 2007;37:2834–46. doi: 10.1002/eji.200737112. [DOI] [PubMed] [Google Scholar]
- 21.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 22.Anderson KV, Bokla L, Nusslein-Volhard C. Establishment of dorsal–ventral polarity in the Drosophila embryo: the induction of polarity by the toll gene product. Cell. 1985;42:791–8. doi: 10.1016/0092-8674(85)90275-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.