Abstract
Deficiency in γδ T cells aggravates colitis in animal models suggesting that γδ T cells have regulatory properties. Therefore, proliferation, suppression and cytokine secretion of human γδ T cells were determined in vitro. Human peripheral γδ T cells were isolated from the whole blood of healthy donors by magnetic antibody cell sorting technology. The proliferation after CD3/CD28 stimulation was measured by 3[H]thymidine incorporation. Interferon-γ (IFN-γ), interleukin-2 (IL-2), transforming growth factor-β (TGF-β) and IL-10 concentrations were measured by enzyme-linked immunosorbent assay; TGF-β messenger RNA was also measured by reverse transcription–polymerase chain reaction. The expression of latency associated peptide (LAP), a TGF-β complex component, intracellular cytokine content and T helper cell proliferation were measured by flow cytometry. Human γδ T cells showed poor proliferation upon CD3/CD28 stimulation and suppressed T helper cell growth stronger than CD4+ CD25+ T cells, although γδ T cells were FOXP3 negative. They secreted little IL-2 but high concentrations of IFN-γ, IL-10 and TGF-β. When looking at LAP expression the Vδ1 subset was found to be the main TGF-β producer compared to Vδ2 T cells. Taken together, peripheral γδ T cells have in vitro a more potent regulatory potential than CD4+ CD25+ cells regarding T helper cell suppression. This is most likely the result of strong TGF-β secretion, particularly by the Vδ1 subset.
Keywords: anergy, cytokines, regulatory T cells, suppression, transforming growth factor-β
Introduction
There are two T-cell subsets characterized by their expression of a T-cell receptor (TCR) α chain and a β chain (αβ T cells) or a γ chain and a δ chain (γδ T cells). These T-cell subsets do not work in parallel but they act together. For instance, γδ T cells regulate αβ T-cell activation via cytokine secretion1,2 and assist their local inflammatory function.3 Although γδ T cells have only a limited repertoire of TCR rearrangements they are capable of responding to various environmental insults, such as exposure to toxin (e.g. ozone),4 infections,3,4 inflammation,5–11 tumours,2,12,13 or epithelial injury.14–18 Therefore, they have a broad functional armamentarium including secretion of cytokines [e.g. interferon-γ (IFN-γ) or interleukin-10 (IL-10)], cytotoxicity, or secretion of growth factors [keratinocyte growth factor, transforming growth factor-β (TGF-β)] and chemokines. To exert these functions γδ T cells are mainly situated in the intestinal epithelium, although in humans not quite as frequently as in rodents.
In recent years our understanding of the interaction of γδ T cells with the epithelium has increased.18 We now know that γδ T cells not only support regeneration of epithelium but also attract neutrophils just after tissue injury so as to remove necrotic epithelial cells. In line with these findings, depletion of or deficiency in γδ T cells aggravate inflammation in colitis models that exhibit injury of the epithelial barrier, i.e. 2,4,6-trinitrobenzene sulphonic acid-induced and dextran sulphate sodium-induced colitis.19 However, depletion of γδ T cells also increases inflammation in intestinal inflammation in a model without epithelial injury, i.e. TNFΔARE mice.7 Similarly, reconstitution of thymectomized non-obese diabetic (NOD) mice with γδ T cells prevented diabetes in a similar manner to CD4+ CD25+ regulatory T cells (Treg).11 Using oral insulin in euthymic mice require γδ T cells to induce Treg.10 Therefore, γδ T cells seem to interact with αβ T cells supporting regulatory mechanisms in addition to homeostatic effects on the epithelial barrier.
The following study was conducted to elucidate the properties of peripheral human γδ T cells in vitro. Therefore, peripheral human γδ T cells were obtained from healthy donors and examined with respect to their proliferative and suppressive behaviour as well as their cytokine profile. Finally, peripheral γδ T-cell functions were compared with those of other regulatory T cells (CD4+ CD25+). In this study, we will show that γδ T cells have regulatory functions themselves, such as anergy and suppression of T helper cell proliferation. Importantly, γδ T cells, particularly the Vδ1 subset, were found to be strong TGF-β producers.
Materials and methods
Monoclonal antibodies
The following monoclonal antibodies (mAb) were used in vitro: OKT3 (anti-human CD3; American Type Culture Collection, Manassas, VA); BW828 (anti-human CD28; a gift of Dr Kurrle, Behringwerke AG, Marburg, Germany); B1-phycoerythrin- (PE), fluorescein isothiocyanate- (FITC), biotin-conjugated (anti-human TCR-γδ; BD Biosciences, Heidelberg, Germany); B3.1-PE (anti-human Vγ9; BD Biosciences); B6-PE, -FITC (anti-human Vδ2; BD Biosciences); TS-1-FITC (anti-human Vδ1; Endogen, Woburn, MA); 4E3-PE (anti-human CD25-PE; Miltenyi Biotec, Bergisch Gladbach, Germany); RPA-T4-PE, -FITC (anti-human CD4-PE (BD Biosciences); BW135/80-PE (anti-human CD8; Miltenyi Biotec); MQ1-17H12-allophycocyanin (APC; anti-human IL-2; BD Biosciences); MP4-25D2-APC (anti-human IL-4; BD Biosciences); JES3-19F1-APC (anti-human IL-10; BD Biosciences); B27-APC, -FITC (anti-human IFN-γ; BD Biosciences); 27232-PE [anti-human LAP (TGF-β1); R&D Systems, Wiesbaden, Germany]; 3G3-APC (anti-human FOXP3; Miltenyi Biotec). OKT3 was purified from supernatants using spinner flasks followed by affinity chromatography employing Protein G–Sepharose (Amersham Biosciences, Freiburg, Germany).
Isolation of peripheral γδ T cells and subsets
The γδ T cells were separated from peripheral blood mononuclear cells (PBMC) by magnetic antibody cell sorting (MACS) technology using the TCR-γδ+ T Cell Isolation kit according to the manufacturer’s instruction (Miltenyi Biotec). Mononuclear cells were enriched from 50–100 ml (experiments with γδ T cells) or 500 ml (experiments with Vδ1 and Vδ2 T cells) heparinized whole blood from female and male middle-aged healthy donors by Ficoll density centrifugation (Biocoll separating solution; Biochrom AG, Berlin, Germany). Informed consent was obtained from each blood donor. The PBMC were incubated (15 min, 4°) in optimal concentration with PE-labelled anti-γδ TCR mAb (clone GL3; BD Biosciences). After washing twice with MACS buffer [0·5% bovine serum albumin, 2 mm ethylenediaminetetraacetic acid in phosphate-buffered saline (PBS)] anti-PE MicroBeads (Miltenyi Biotec) were added (1 : 5 dilution) and γδ T cells were enriched following the manufacturer’s instructions. Purity of γδ T cells was determined by flow cytometry and only cells with > 98% purity were used. Unlabelled γδ T cells were further separated into Vδ1 and Vδ2 subsets by MACS technology. Vδ2 T cells were labelled with anti-human Vδ2-PE mAb (BD Biosciences) and subsequently with anti-PE beads (Miltenyi Biotec). The Vδ1 and Vδ2 subsets were separated using a mass spectrometry column (Miltenyi Biotec), which is passed by unlabelled Vδ1 T cells while the labelled Vδ2 T cells are retained. Vδ2 T cells were released after removing the column from the magnet.
These γδ T-cell subsets were stimulated for 3 days in 48-well plates with 5 μg/ml concanavalin A (Con A; Sigma, Deisenhofen, Germany). Thereafter, cells were washed three times in PBS, resuspended in RNeasy lysis buffer buffer, shock-frozen in liquid nitrogen, and stored at −80° until quantification of TGF-β messenger RNA (mRNA).
Isolation of human CD4+ CD25+ T cells
To compare regulatory properties of γδ T cells with those of other human regulatory cells, human CD4+ CD25+ T cells were isolated from PBMC of whole blood from healthy donors by MACS technology using a CD4+ CD25+ Regulatory T Cell Isolation kit (Miltenyi Biotec) following the manufacturer’s instructions. Purity of CD4+ CD25+ T cells was determined by flow cytometry and only cells with > 98% purity were used.
Isolation and labelling of human CD4+ T cells
For allogeneic coculture experiments, CD4+ T cells were isolated from the initial MACS step of the CD4+ CD25+ T-cell isolation, where CD4+ T cells pass the separation column unlabelled. An aliquot was taken and washed, and purity was determined by flow cytometry. Only cells with > 98% purity were used. An aliquot of 107 CD4+ T cells was resuspended in 1 ml PBS with 1 μm carboxyfluorescein succinimidyl ester (CFSE; Fluka, Seelze, Germany). After incubation (10 min, room temperature in the dark), cells were washed twice in complete medium (RPMI-1640 medium containing 10% fetal calf serum, 100 U/ml penicillin/streptomycin, and 3 mm glutamine; PAA, Cölbe, Germany).
For proliferation studies, human CD4+ and CD8+ T cells were isolated from PBMC of healthy donors by MACS technology using CD4 or CD8 beads according to the manufacturer’s instructions (Miltenyi Biotec).
Proliferation assay
The proliferation index (stimulated/unstimulated) of CD4+, CD8+, CD4+ CD25+ and γδ T cells was determined via incorporation of [6-3H]thymidine. Cells were incubated in triplicate for 96 hr at 5 × 104 cells/well in 96-well round-bottom plates (NUNC, Wiesbaden, Germany) at 37° in 5% CO2 humidified air. Each well received 0·5 μCi of [6-3H]thymidine (Amersham Pharmacia, Little Chalfont, UK) during the last 18 hr of the 96 hr of culture. Incorporated [6-3H]thymidine was harvested on a glass fibre membrane and detected by liquid scintillation counting (LKB Wallac, Turku, Finland). For standard TCR stimulation, cells were stimulated via plate-bound anti-human CD3 mAb OKT3 (10 μg/ml) in the presence of 1 μg/ml soluble anti-human CD28 mAb BW828 with or without 100 U/ml recombinant human IL-2 (Sigma). Additionally, proliferation of CD4+, CD8+, CD4+ CD25+ and γδ T cells was measured after stimulation with 5 μg/ml isopentenyl pyrophosphate (Sigma) and 100 U/ml recombinant human (rhu) IL-2.
For determining the suppressive capacity of regulatory T cells, proliferation of CFSE-labelled CD4+ T cells was measured in monoculture and coculture with allogeneic γδ T cells and CD4+ CD25+ T cells by flow cytometry. Therefore, CD4+ T cells were incubated in triplicates for 6 days at 2 × 105 cells/well in 96-well round-bottom plates (NUNC) coated with anti-CD3 mAb OKT3 (10 μg/ml) in the presence of 1 μg/ml anti-CD28 mAb BW828 with or without 100 U/ml rhuIL-2 (Sigma) at 37° in 5% CO2 humidified air. In coculture experiments 1 × 105 CD4+ T cells/well were incubated under the same conditions as mentioned above with 1 × 105 regulatory T cells/well.
Cytokine assay
CD4+, CD4+ CD25+ and γδ T cells were incubated at 106 cells/ml in 24-well plates (NUNC) coated with OKT3 (10 μg/ml) and BW828 (1 μg/ml) added at 37° in a humidified atmosphere with 5% CO2. Supernatants were taken after 48 hr and examined for cytokine secretion (IL-2, IL-10, TGF-β and IFN-γ) by sandwich enzyme-linked immunsorbent assay using antibodies as well as recombinant protein standards for IL-2, IL-10 and IFN-γ (BD Biosciences), and TGF-β (Promega, Mannheim, Germany) according to the manufacturer’s instructions.
Intracellular cytokines of Con A (Sigma) stimulated CD4+ CD25+ and γδ T cells were measured by flow cytometry. Therefore, cells were stimulated for 5 days (5 μg/ml Con A) and restimulated with 10 ng/ml phorbol 12-myristate 13-acetate (Sigma) and 1 μg/ml ionomycin (Calbiochem, Schwalbach, Germany) for 6 hr and 5 μg/ml brefeldin A (Sigma) for 3 hr. Subsequently, cells were fixed in 2% formalin (Roth, Karlsruhe, Germany) for 20 min at room temperature.
RNA isolation and complementary DNA synthesis
Total RNA was isolated according the manufacturer’s protocol (innuPrep RNA minikit; analyticJena, Jena, Germany). To increase the RNA concentration, the final volume of the extracted RNA was reduced (Speed-Vac) and treated with DNase I (Sigma-Aldrich, Munich, Germany). Complementary DNA (cDNA) synthesis was performed with 200 ng of random primer (Promega), 0·1 m dithiothreitol 5 × reaction buffer, 0·5 mm dNTP (each obtained from Promega), and 100 U reverse transcriptase Superscript II RNase H (Invitrogen Life Technologies, Karlsruhe, Germany) in a total volume of 20 μl. Samples were incubated at 42° for 50 min.
TGF-β mRNA quantification
To analyse the expression of TGF-β genes in human peripheral blood lymphocytes, the RNA was extracted from cells snap frozen in Lysis solution and reverse transcribed as described above. The cDNA was added to the 2 × Taqman-Mastermix (Eurogentec, Köln, Germany) and amplified. For signal detection, the ABI Prism 7000 sequence detector (Applied Biosystems, Darmstadt, Germany) was programmed to an initial step of 6 min at 95°, followed by 50 thermal cycles of 15 seconds at 95° and 1 min at 60°. The following housekeeping gene and TGF-β forward (for) and reverse (rev) primers and probes were designed by using the computer software Primer Express (Primer Express® software v2·0; Applied Biosystems): MLN51 for 5′-CTT CAT CTG CGG CGG GTG-3′, rev 5′-ACC TTC AAT GCC ATC TTC ACT CT-3′ and probe Fam 5′-ACT CCG ACT CCT CAG CAC TCT TGG CG -3′ Tamra; TGF-β for 5′-TCA GCT CCA CGG AGA AGA ACT-3′, TGF-β rev 5′-GTT GGC ATG GTA GCC CCT GG-3′ and probe Fam 5′-TCC ACT TCC AGC CGA GGT CCT TGC G-3′ Tamra.
The optimal primer concentrations used are 500 nm each for the forward and reverse primers and 250 nm for the TaqMan probes (IBA BioTAGnologies, Göttingen, Germany). The same batch of cDNA (20 μl) was used to determine the cycle of threshold of the TGF-β gene and MLN51 as housekeeping gene in triplicate reactions. Because the amplification efficiencies are close to 1 (as assessed by template dilution), it is possible to apply the following equation to relate the amount of the cytokine genes to MLN51: 2(ct cytokine – ct MLN51).20
Flow cytometry
For surface immunostaining PE-, APC-, and FITC-labelled mAb against human CD3, CD4, CD8, TCR-γδ, Vγ9, Vδ1, Vδ2, CD25 and LAP, and respectively mouse and rat isotype controls as well as human serum were employed. Inactivated normal human serum was used for blocking unspecific binding. Cells were washed with fluorescence-activated cell sorting (FACS) buffer (0·5% bovine serum albumin in PBS) and stained on ice for 10 min with optimal dilution. After washing, cells were analysed by flow cytometry (FACS Calibur and CellQuest software; Becton/Dickinson, Heidelberg, Germany) using a live gate set around viable lymphocytes based on their forward scatter/side scatter (FSC/SSC) characteristics. 50 000 cells per FSC/SCC gate were measured.
FoxP3 expression of purified CD4+ T cells and γδ T cells was analysed by flow cytometry using APC-labelled anti-human FoxP3 antibody and the FoxP3 staining buffer set according to the manufacturer’s instruction (Miltenyi Biotec). Before FoxP3 staining, the unlabelled CD4+ T-cell fraction was stained with anti-human CD4-FITC mAb (BD Bioscience) and anti-human CD25-PE mAb (BD Bioscience). The γδ T-cell fraction was already labelled and was counterstained with FITC-labelled Streptavidin (BD Bioscience).
For intracellular staining cells were stained with APC-labelled mAb against human IL-2, IL-10 and IFN-γ as well as surface markers as described above in 0·5% saponin buffer (Sigma) for 30 min in the dark at room temperature. Cells were washed in 0·5% saponin and resuspended in FACS buffer and analysed by flow cytometry using a gate set around the lymphocyte population based on their FSC/SSC characteristics. 50 000 cells per FSC/SCC gate were measured.
To determine the proliferation of CFSE-labelled CD4+ T cells in mono- and coculture by flow cytometry the basic setting of the FACS was adjusted after 1 day of culture. A live gate was set around viable lymphocytes based on their FSC/SSC characteristics. Additionally, directly before measuring 1 μl propidium iodide (Sigma) was added to exclude necrotic cells (exclusion of PI+ cells). 50 000 cells per FSC/SCC gate were measured. The proliferation (reduction of CFSE intensity, CFSEdim) was determined on day 6. Data analysis was carried out using FlowJo (version 8.5.2; Tree Star Inc., Olten, Switzerland). To exclude cocultured cells, γδ T cells or CD4+ CD25+ T cells, a gate was set on CFSE-positive (labelled) cells before the evaluation of proliferating (CFSEdim) CD4+ T cells.
Immunofluorescence
CD4+ T cells and γδ T cells were isolated by MACS technology and thereafter cytospins were prepared from 4 × 105 cells per slide. These cytospins were air-dried overnight at room temperature and formalin-fixed before staining. Fixed cytospins were subjected to a heat-induced epitope retrieval step (2 min in sodium citrate buffer solution, pH 6·0) before incubation with antibodies.21 Cytospins were washed in Tris-buffered saline (TBS) with 0·5% human serum after each antibody incubation. Cytospins were incubated with the primary antibody against human FOXP3 (PCH101; eBiosciences, San Diego, CA, 1 : 200). Then, cytospins were incubated with secondary Alexa 488-labelled anti-rat antibody (Invitrogen, Carlsbad, CA, 1 : 100). Nuclei were counterstained with DAPI (Sigma, 1 : 1500) and slides were mounted in Fluoromount (DAKO, Hamburg, Germany). Images were acquired using a fluorescence microscope (AxioImager Z1) equipped with a CCD camera (AxioCam MRm) and processed with Axiovision software (Carl Zeiss MicroImaging, Inc., Göttingen, Germany).
Statistics
For statistical analysis Mann–Whitney U-test was used and calculations were made using spss for Windows SPSS, Chicago, IL. Values were expressed as mean (95% confidence intervals) and standard error of the mean (SEM). Differences were considered statistically significant for P < 0·05.
Results
Human γδ T cells are unresponsive to standard TCR stimulation
Human γδ T cells were isolated from PBMC of whole blood of healthy donors by MACS technology. The purity of these cells was determined by flow cytometry and only γδ T cells with > 98% purity were used in in vitro assays (Fig. 1). Human γδ T cells showed in vitro significantly less proliferation upon T-cell stimulation with anti-CD3/CD28 mAb than CD4+ T cells (Fig. 2a). However, proliferation indices of anti-CD3/CD28 ± rhuIL-2-stimulated γδ T cells were comparable to those of CD8+ T cells and CD4+ CD25+ T cells (Fig. 2a). Furthermore, proliferation of γδ T cells was induced by stimulation with the mycobacterial phosphoantigen isopentenyl pyrophosphate (IPP), which did not stimulate other T-cell populations (Fig. 2b).
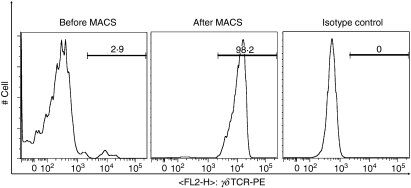
Figure 1.
Fluorescence-activated cell sorting analysis of γδ T-cell isolation of human peripheral blood mononuclear cells (PBMC) by magnetic antibody cell sorting (MACS). Histograms show human PBMC before MACS (left) and after MACS (middle) using phycoerythrin-labelled anti-T-cell receptor γδ monoclonal antibody as well as isotype control (mouse immunoglobulin G1, right).
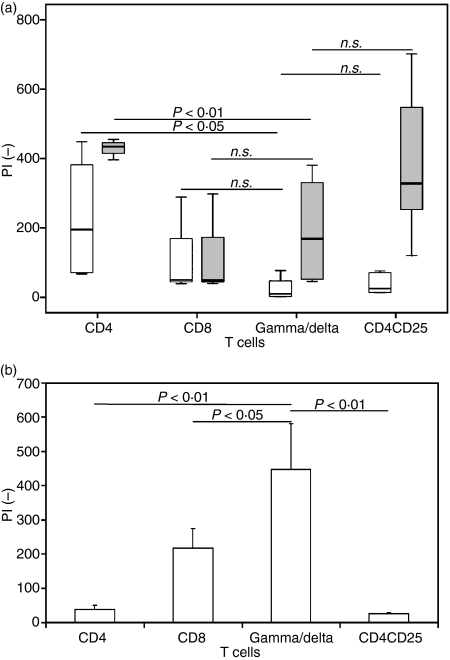
Figure 2.
γδ T cells are unresponsive to standard T-cell receptor stimulation via CD3/C28. (a) Box plot of proliferation index PI (−) of CD4+ T cells (n = 6), CD8+ T cells (n = 5), CD4+ CD25+ T cells (n = 5) and γδ T cells (n = 10) after stimulation via anti-CD3/CD28 (white boxes) and anti-CD3/CD28 + recombinant human interleukin-2 (rhuIL-2; grey boxes) and (b) bar chart (+ SEM) of PI (−) of CD4+, CD8+, γδ and CD4+ CD25+ T cells after stimulation with isopentenyl pyrophosphate + rhuIL-2. (NS, statistically not significant).
Human γδ T cells suppress CD4 T-cell proliferation
To investigate the suppressive potential of γδ T cells, the proliferation of cocultured CD4+ T cells was measured by CFSE labelling and flow cytometry. Figure 3 shows representative histograms of proliferating CD4+ T cells (CFSEdim) in monoculture and allogeneic coculture either with γδ T cells or CD4+ CD25+ T cells. To estimate the suppression of CD4+ T-cell growth the percentage of proliferating cells in monocultures, e.g. 70·6% in Fig. 3(a), was set to 100%. γδ T cells suppressed the growth of CD4+ T cells at cell ratios as low as 1 : 4 (Fig. 4a). Importantly, the suppressive capacity of γδ T cells remained after the addition of rhuIL-2 while CD4+ CD25+ T cells lost their suppressive capacity in the presence of rhuIL-2 (Fig. 4b). In contrast to CD4+ CD25+ Treg cells, unstimulated γδ T cells do not express CD25 (Fig. 5). Additionally, when looking at FOXP3 expression by immunofluorescence, γδ T cells did not show nuclear staining while 8% CD4+ T cells did (Fig. 6). In addition, FOXP3 expression analysed by flow cytometry was found to be negative in γδ T cells but positive in 5% of purified CD4+ T cells (data not shown).
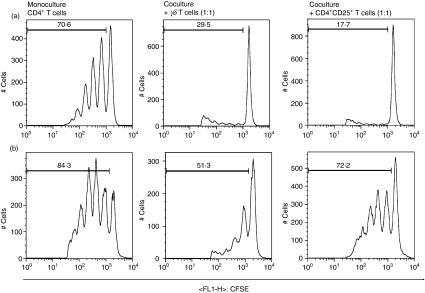
Figure 3.
Determination of CD4+ T-cell proliferation by flow cytometry. Representative histograms of anti-CD3/CD28 stimulated (a) and anti-CD3/CD28 + interleukin-2 (IL-2) stimulated (b) CD4+ T cells in monoculture (left row) and in allogeneic coculture with γδ T cells (middle row) and CD4+ CD25+ T cells (right row) at a cell ratio of 1 : 1 on day 6.
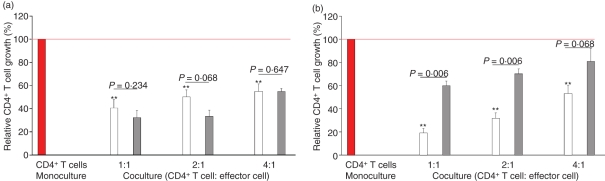
Figure 4.
γδ T suppress the growth of CD4+ T cells. Bar chart of mean (+ SEM) CD4+ T-cell growth (%) after stimulation via anti-CD3/CD28 (a) and via anti-CD3/CD28 + recombinant human interleukin-2 (rhulL-2) (b) in allogeneic coculture with γδT cells (white, n = 6) and CD4+ CD25+ T cells (grey, n = 5). The growth of the CD4+ T cell in monoculture (red, n = 7) as shown in Fig. 3(a) (e.g. 70.6% proliferating cells) was set to 100%. Asterisks indicate statistical significance (*P<0.05 and **P<0·01 compared with CD4+ T-cell growth in monoculture).
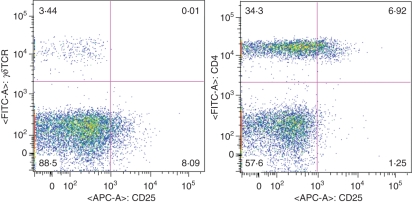
Figure 5.
γδ T cells express no CD25. Dot plots of freshly isolated peripheral blood mononuclear cells stained for CD25 expression in CD4+ and γδ T cells.
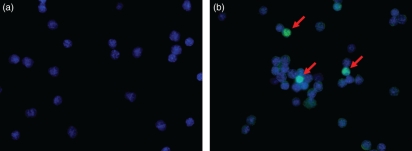
Figure 6.
γδ T cells are FOXP3 negative. Cytospins showing no expression of FOXP3 in the γδT-cell population (a) and some FOXP3-positive T cells among the CD4+ T cells labelled with Alexa Fluor 488 (green, red arrows) and counterstained with DAPI (blue nucleus) (magnification × 400).
γδ T cells secret both proinflammatory and anti-inflammatory cytokines
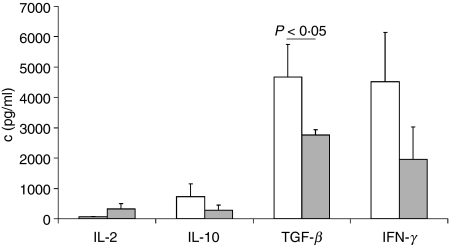
Anti-CD3/CD28 stimulated γδ T cells produced negligible amounts of IL-2 but high concentrations of both pro- and anti-inflammatory cytokines (Fig. 7). Similarly, FACS analysis of intracellular cytokines revealed high percentages of γδ T cells producing IFN-γ (48·5 ± 14·7%), IL-10 (13·3 ± 11·4%) and IL-4 (13·0 ± 5·3%), but only a few γδ T cells produced IL-2 (1·9 ± 1·8%). No coexpression of IFN-γ and IL-4 or IL-10 or IL-2 was found (data not shown). The cytokine profile of γδ T cells was comparable with that of CD4+ CD25+ T cells because there were no statistically significant differences regarding the secretion of IL-2, IL-10 and IFN-γ (Fig. 7). However, γδ T cells secreted more TGF-β than CD4+ CD25+ T cells (Fig. 7).
Figure 7.
γδ T cells secret both anti- and pro-inflammatory cytokines. Bar chart of mean (+ SEM) cytokine concentration (c, pg/ml) in 48 hr culture supernatants of anti-CD3/CD28-stimulated γδ T cells (white, n = 13) and CD4+ CD25+ T cells (grey, n = 13).
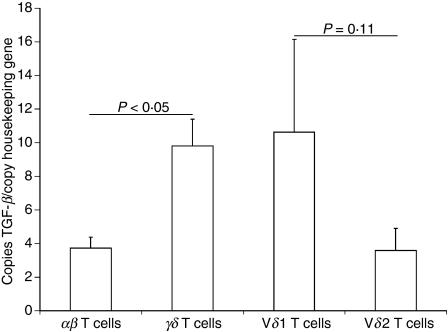
Next, we investigated whether increased TGF-β production originates from one γδ T-cell subset (Vδ1 or Vδ2). Therefore, we quantified the relative TGF-β mRNA content in the different γδ T-cell subsets isolated from peripheral blood (Vδ1 and Vδ2). Also, on the RNA level, TGF-β content was higher in γδ T cells than in αβ T cells (Fig. 8).
Figure 8.
Transforming growth factor-β (TGF-β) messenger RNA content is higher in γδ than in αβ T cells and higher in the Vδ1 than Vδ2 T-cell subset. Bar chart (+ SEM) of mean copies of TGF-β per copy of housekeeping gene MLN51 as quantified by reverse transcription–polymerase chain reaction.
The Vδ1 subset showed increased TGF-β mRNA content compared with Vδ2 T cells in six out of seven donors (Fig. 8). Furthermore, we compared the expression of the latency associated peptide (LAP), an indirect marker of TGF-β protein expression, by flow cytometry. As LAP expression is increased in the Vδ1 subset compared to Vδ2 T cells (45·0 ± 11·7% versus 8·8 ± 6·1%, P<0·05) the Vδ1 subset seems to be the main TGF-β producer.
Discussion
To the best of our knowledge, the present study provides the first evidence that human peripheral γδ T cells are a potent type of regulatory T cells. Although they are FOXP3 negative, they strongly suppress T helper cell proliferation in an IL-2-independent way and produce high amounts of TGF-β. Just as for CD4+ CD25+ T cells, human γδ T cells seem to be unresponsive to anti-CD3/CD28 stimulation in an IL-2-dependent manner, although CD8+ T cells were also unresponsive in the experiments presented here. Human γδ T cells did proliferate upon stimulation with the mycobacterial phosphoantigen IPP showing the proliferative potential of human γδ T cells.
Regulatory T cells are characterized by being anergic to anti-CD3/CD28 stimulation and acting suppressively. The most thoroughly investigated regulatory T cells are CD4+ CD25+ T cells, which lack IL-2 production.22 Anergy of CD4+ CD25+ T cells can be reversed by IL-2 stimulation. Like CD4+ CD25+ T cells, γδ T cells show negligible IL-2 production and the addition of IL-2 to anti-CD3/CD28 stimulation increases γδ T-cell proliferation though this increase is only small and the proliferation is still less than that of CD4+ T cells. Furthermore, IL-2 can not only reverse anergy but also suppressive behaviour of CD4+ CD25+ T cells.22,23 Surprisingly, we found suppression by γδ T cells to be IL-2 independent. In the context of inflammation, this IL-2 independency might be advantageous compared with CD4+ CD25+ T cells because other T cells might escape suppression by IL-2 secretion. The γδ T cells are quite unresponsive so the suppressive function of this regulatory cell population is probably restricted to epithelial surfaces where they are enriched.
As far as one can tell from in vitro experiments the suppressive power of γδ T cells is probably at least as strong as that of CD4+ CD25+ T cells. Like γδ T cells, CD4+ CD25+ T cells are also a small cell population in health and disease,24 but mediate potent suppression of CD4+ CD25− T cells.25,26 Additionally, intraepithelial γδ T cells from patients with coeliac disease were reported to suppress intraepithelial αβ T cells27 and tumour-infiltrating γδ1 T cells were shown to suppress proliferation of naïve T cells via CD3.28 However, in both studies neither healthy controls nor peripheral γδ T cells were investigated, raising the question whether these findings are a feature of all γδ T cells rather than the result of coeliac disease or tumour immunology.
In the present study we could show that human peripheral γδ T cells are FOXP3 negative, a well-known marker for regulatory T cells.29 However, FOXP3-negative regulatory T cells have also been described by others.30,31
Although the exact mechanism of suppression is neither fully understood for CD4+ CD25+ T cells nor for γδ T cells, the cytokines IL-10 and TGF-β have been proposed to play an important role. Increased TGF-β production by γδ T cells was previously reported in nephritic mice and patients with coeliac disease.27,32 Our data show that human γδ T cells are even stronger TGF-β producers than regulatory CD4+ CD25+ T cells. As the growth factor TGF-β is involved in the maintenance of epithelial integrity and intestinal immunological balance as well as in epithelial cell restitution,33–38γδ T cells might exert their regulatory and protective functions via TGF-β secretion. In addition, γδ T cells can increase TGF-β production indirectly via IL-10 secretion, which in turn can stimulate other T cells to produce more TGF-β.39
The Vδ1 T cells are mainly located in the epithelium, e.g. the intestinal epithelium, where they represent 70–90% of γδ T cells.21 The Vδ1 T-cell subset seems to be the major source of TGF-β production because the number of Vδ1 T cells which express LAP, a TGF-β complex component and thereby indirect marker of TGF-β expression, was found to be higher than among Vδ2 T cells. Besides LAP expression, the TGF-β mRNA content in Vδ1 and Vδ2 T cells was determined. However, the mean increase in TGF-β mRNA in Vδ1 T cells was not statistically significant because of high standard deviations. Still, six out of seven donors had increased TGF-β mRNA in Vδ1 T cells compared with Vδ2 T cells when looking at the Vδ1 : Vδ2 ratio. The Vδ1 T-cell subset was previously reported to have regulatory potential when looking at tumour-infiltrating lymphocyte clones. However, these so-called γδ1 Treg cells did not produce TGF-β or IL-10.28
The high production of IFN-γ by γδ T cells is probably important for defence against infectious and malignant diseases12,40,41 without necessarily triggering inflammation.6–9,19 Recently, γδ T cells have also been reported to be able to suppress tumour cytotoxicity in spite of being potent IFN-γ producers.28
In conclusion, human peripheral γδ T cells are unresponisve to standard TCR stimulation and showed in vitro suppressive behaviour as well as production of both pro- and anti-inflammatory cytokines. Their suppressive behaviour is IL-2 independent and is at least as powerful as that of CD4+ CD25+ T cells, making them key players in the regulation of other T cells as well as of epithelial homeostasis within mucosal surfaces. Still, their strong proliferation upon mycobacterial antigen stimulation as well as their marked secretion of IFN-γ shows their potential to counteract intestinal infections as well as to suppress inflammatory reactions using their regulatory repertoire. Importantly, they are not only strong IFN-γ and IL-10 producers but also very strong TGF-β producers. The strongest TGF-β production was found among the Vδ1 subset of γδ T cells.
Acknowledgments
The work of the authors was supported by SFB633/B3 and SFB 633/Z1, the German competence network on irritable bowel disease and by a grant from the German Crohn’s and Colitis foundation (DCCV).
Glossary
Abbreviations:
- FSC
forward scatter
- IBD
inflammatory bowel disease
- LAP
latency associated peptide
- PBMC
peripheral blood mononuclear cells
- SSC
side scatter
Disclosures
The authors report no conflict of interest.
References
- 1.Kaufmann SH, Blum C, Yamamoto S. Crosstalk between αβ T cells and γδ T cells in vivo: activation of αβ T-cell responses after γδ T-cell modulation with the monoclonal antibody GL3. Proc Natl Acad Sci USA. 1993;90:9620–4. doi: 10.1073/pnas.90.20.9620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pennington DJ, Silva-Santos B, Shires J, et al. The inter-relatedness and interdependence of mouse T cell receptor γδ + and αβ + cells. Nat Immunol. 2003;4:991–8. doi: 10.1038/ni979. [DOI] [PubMed] [Google Scholar]
- 3.Roberts SJ, Smith AL, West AB, Wen L, Findly RC, Owen MJ, Hayday AC. T-cell αβ + and γδ + deficient mice display abnormal but distinct phenotypes toward a natural, widespread infection of the intestinal epithelium. Proc Natl Acad Sci USA. 1996;93:11774–9. doi: 10.1073/pnas.93.21.11774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King DP, Hyde DM, Jackson KA, et al. Cutting edge: protective response to pulmonary injury requires γδ T lymphocytes. J Immunol. 1999;162:5033–6. [PubMed] [Google Scholar]
- 5.Hoffmann JC, Peters K, Henschke S, Herrmann B, Pfister K, Westermann J, Zeitz M. Role of T lymphocytes in rat 2,4,6-trinitrobenzene sulphonic acid (TNBS) induced colitis: increased mortality after γδ T cell depletion and no effect of alphabeta T cell depletion. Gut. 2001;48:489–95. doi: 10.1136/gut.48.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y, Chou K, Fuchs E, Havran WL, Boismenu R. Protection of the intestinal mucosa by intraepithelial γδ T cells. Proc Natl Acad Sci USA. 2002;99:14338–43. doi: 10.1073/pnas.212290499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kühl AA, Loddenkemper C, Westermann J, Hoffmann JC. Role of γδ T cells in inflammatory bowel disease. Pathobiology. 2002;70:150–5. doi: 10.1159/000068147. [DOI] [PubMed] [Google Scholar]
- 8.Tsuchiya T, Fukuda S, Hamada H, Nakamura A, Kohama Y, Ishikawa H, Tsujikawa K, Yamamoto H. Role of γδ T cells in the inflammatory response of experimental colitis mice. J Immunol. 2003;171:5507–13. doi: 10.4049/jimmunol.171.10.5507. [DOI] [PubMed] [Google Scholar]
- 9.Inagaki-Ohara K, Chinen T, Matsuzaki G, et al. Mucosal T cells bearing TCR γδ play a protective role in intestinal inflammation. J Immunol. 2004;173:1390–8. doi: 10.4049/jimmunol.173.2.1390. [DOI] [PubMed] [Google Scholar]
- 10.Harrison LC, Dempsey-Collier M, Kramer DR, Takahashi K. Aerosol insulin induces regulatory CD8 γδ T cells that prevent murine insulin-dependent diabetes. J Exp Med. 1996;184:2167–74. doi: 10.1084/jem.184.6.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Locke NR, Stankovic S, Funda DP, Harrison LC. TCR γδ intraepithelial lymphocytes are required for self-tolerance. J Immunol. 2006;176:6553–9. doi: 10.4049/jimmunol.176.11.6553. [DOI] [PubMed] [Google Scholar]
- 12.Gao Y, Yang W, Pan M, Scully E, Girardi M, Augenlicht LH, Craft J, Yin Z. γδ T cells provide an early source of interferon gamma in tumor immunity. J Exp Med. 2003;198:433–42. doi: 10.1084/jem.20030584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Girardi M, Glusac E, Filler RB, Roberts SJ, Propperova I, Lewis J, Tigelaar RE, Hayday AC. The distinct contributions of murine T cell receptor (TCR) γδ + and TCR αβ + T cells to different stages of chemically induced skin cancer. J Exp Med. 2003;198:747–55. doi: 10.1084/jem.20021282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haas W, Pereira P, Tonegawa S. γδ cells. Annu Rev Immunol. 1993;11:637–85. doi: 10.1146/annurev.iy.11.040193.003225. [DOI] [PubMed] [Google Scholar]
- 15.Boismenu R, Havran WL. Modulation of epithelial cell growth by intraepithelial γδ T cells. Science. 1994;266:1253–5. doi: 10.1126/science.7973709. [DOI] [PubMed] [Google Scholar]
- 16.Havran WL. A role for epithelial γδ T cells in tissue repair. Immunol Res. 2000;3:63–9. doi: 10.1385/IR:21:2-3:63. [DOI] [PubMed] [Google Scholar]
- 17.Havran WL, Jameson JM, Witherden DA. Epithelial cells and their neighbors. III. Interactions between intraepithelial lymphocytes and neighboring epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:G627–30. doi: 10.1152/ajpgi.00224.2005. [DOI] [PubMed] [Google Scholar]
- 18.Komori HK, Meehan TF, Havran WL. Epithelial and mucosal γδ T cells. Curr Opin Immunol. 2006;18:534–8. doi: 10.1016/j.coi.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Kuhl AA, Pawlowski NN, Grollich K, Loddenkemper C, Zeitz M, Hoffmann JC. Aggravation of intestinal inflammation by depletion/deficiency of γδ T cells in different types of IBD animal models. J Leukoc Biol. 2007;81:168–75. doi: 10.1189/jlb.1105696. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Deusch K, Luling F, Reich K, Classen M, Wagner H, Pfeffer K. A major fraction of human intraepithelial lymphocytes simultaneously expresses the γδ T cell receptor, the CD8 accessory molecule and preferentially uses the Vδ1 gene segment. Eur J Immunol. 1991;21:1053–9. doi: 10.1002/eji.1830210429. [DOI] [PubMed] [Google Scholar]
- 22.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 23.Vieira PL, Christensen JR, Minaee S, et al. IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+ CD25+ regulatory T cells. J Immunol. 2004;172:5986–93. doi: 10.4049/jimmunol.172.10.5986. [DOI] [PubMed] [Google Scholar]
- 24.Maul J, Loddenkemper C, Mundt P, Berg E, Giese T, Stallmach A, Zeitz M, Duchmann R. Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology. 2005;128:1868–78. doi: 10.1053/j.gastro.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 25.Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4+ CD25+ T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001;193:1285–94. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dieckmann D, Bruett CH, Ploettner H, Lutz MB, Schuler G. Human CD4+ CD25+ regulatory, contact-dependent T cells induce interleukin 10-producing, contact-independent type 1-like regulatory T cells [corrected] J Exp Med. 2002;196:247–53. doi: 10.1084/jem.20020642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhagat G, Naiyer AJ, Shah JG, Harper J, Jabri B, Wang TC, Green PH, Manavalan JS. Small intestinal CD8+ TCRγδ+ NKG2A+ intraepithelial lymphocytes have attributes of regulatory cells in patients with celiac disease. J Clin Invest. 2008;118:281–93. doi: 10.1172/JCI30989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng G, Wang HY, Peng W, Kiniwa Y, Seo KH, Wang RF. Tumor-infiltrating γδ T cells suppress T and dendritic cell function via mechanisms controlled by a unique toll-like receptor signaling pathway. Immunity. 2007;27:334–48. doi: 10.1016/j.immuni.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 29.Sakaguchi S, Ono M, Setoguchi R, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 30.Hillebrands JL, Whalen B, Visser JT, et al. A regulatory CD4+ T cell subset in the BB rat model of autoimmune diabetes expresses neither CD25 nor Foxp3. J Immunol. 2006;177:7820–32. doi: 10.4049/jimmunol.177.11.7820. [DOI] [PubMed] [Google Scholar]
- 31.Sun JB, Raghavan S, Sjoling A, Lundin S, Holmgren J. Oral tolerance induction with antigen conjugated to cholera toxin B subunit generates both Foxp3+ CD25+ and Foxp3− CD25− CD4+ regulatory T cells. J Immunol. 2006;177:7634–44. doi: 10.4049/jimmunol.177.11.7634. [DOI] [PubMed] [Google Scholar]
- 32.Wu H, Knight JF, Alexander SI. Regulatory gamma delta T cells in Heymann nephritis express an invariant Vγ6/Vδ1 with a canonical CDR3 sequence. Eur J Immunol. 2004;34:2322–30. doi: 10.1002/eji.200324780. [DOI] [PubMed] [Google Scholar]
- 33.Hahm KB, Im YH, Parks TW, Park SH, Markowitz S, Jung HY, Green J, Kim SJ. Loss of transforming growth factor β signalling in the intestine contributes to tissue injury in inflammatory bowel disease. Gut. 2001;49:190–8. doi: 10.1136/gut.49.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beck PL, Rosenberg IM, Xavier RJ, Koh T, Wong JF, Podolsky DK. Transforming growth factor-β mediates intestinal healing and susceptibility to injury in vitro and in vivo through epithelial cells. Am J Pathol. 2003;162:597–608. doi: 10.1016/s0002-9440(10)63853-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mammen JM, Matthews JB. Mucosal repair in the gastrointestinal tract. Crit Care Med. 2003;8(Suppl):S532–7. doi: 10.1097/01.CCM.0000081429.89277.AF. [DOI] [PubMed] [Google Scholar]
- 36.Dignass AU. Mechanisms and modulation of intestinal epithelial repair. Inflamm Bowel Dis. 2001;7:68–77. doi: 10.1097/00054725-200102000-00014. [DOI] [PubMed] [Google Scholar]
- 37.Babyatsky MW, Rossiter G, Podolsky DK. Expression of transforming growth factors α and β in colonic mucosa in inflammatory bowel disease. Gastroenterology. 1996;110:975–84. doi: 10.1053/gast.1996.v110.pm8613031. [DOI] [PubMed] [Google Scholar]
- 38.Thompson JS, Saxena SK, Sharp JG. Regulation of intestinal regeneration: new insights. Microsc Res Tech. 2000;51:129–37. doi: 10.1002/1097-0029(20001015)51:2<129::AID-JEMT4>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 39.Fuss IJ, Boirivant M, Lacy B, Strober W. The interrelated roles of TGF-γδ and IL-10 in the regulation of experimental colitis. J Immunol. 2002;168:900–8. doi: 10.4049/jimmunol.168.2.900. [DOI] [PubMed] [Google Scholar]
- 40.Lopez RD. Human γδ-T cells in adoptive immunotherapy of malignant and infectious diseases. Immunol Res. 2002;3:207–21. doi: 10.1385/IR:26:1-3:207. [DOI] [PubMed] [Google Scholar]
- 41.Ferrarini M, Ferrero E, Dagna L, Poggi A, Zocchi MR. Human γδ T cells: a nonredundant system in the immune-surveillance against cancer. Trends Immunol. 2002;23:14–8. doi: 10.1016/s1471-4906(01)02110-x. [DOI] [PubMed] [Google Scholar]