Abstract
Sigma-1 receptors (Sig-1Rs) are endoplasmic reticulum (ER)-resident proteins known to be involved in learning and memory. Dendritic spines in hippocampal neurons play important roles in neuroplasticity and learning and memory. This study tested the hypothesis that Sig-1Rs might regulate denritic spine formation in hippocampal neurons and examined potential mechanisms therein. In rat hippocampal primary neurons, the knockdown of Sig-1Rs by siRNAs causes a deficit in the formation of dendritic spines that is unrelated to ER Ca2+ signaling or apoptosis, but correlates with the mitochondrial permeability transition and cytochrome c release, followed by caspase-3 activation, Tiam1 cleavage, and a reduction in Rac1·GTP. Sig-1R-knockdown neurons contain higher levels of free radicals when compared to control neurons. The activation of superoxide dismutase or the application of the hydroxyl-free radical scavenger N-acetyl cysteine (NAC) to the Sig-1R-knockdown neurons rescues dendritic spines and mitochondria from the deficits caused by Sig-1R siRNA. Further, the caspase-3-resistant TIAM1 construct C1199DN, a stable guanine exchange factor able to constitutively activate Rac1 in the form of Rac1·GTP, also reverses the siRNA-induced dendritic spine deficits. In addition, constitutively active Rac1·GTP reverses this deficit. These results implicate Sig-1Rs as endogenous regulators of hippopcampal dendritic spine formation and suggest a free radical-sensitive ER-mitochondrion-Rac1·GTP pathway in the regulation of dendritic spine formation in the hippocampus.
Keywords: mitochondria, ROS, N-acetyl cyteine, learning and memory, caspase-3
Dendritic spines in the CNS are important for many functions. Dendritic atrophy in the neocortical region is related to aging-induced amnesia, and its reversal improves memory retention (1). Similarly, the loss of dendritic spine-related synapses is currently a strong pathologic correlate of cognitive decline, and synaptic dysfunction is evident long before synapses and neurons are lost (2). On the other hand, exposure to drugs of abuse including cocaine, nicotine, or morphine produces persistent changes, usually in the form of increased dendritic spines and arborizations, in cells in brain regions involved in incentive motivation and reward (3). These persistent changes are thought to represent the neuronal reorganization that contributes to some of the persistent sequelae associated with drug use, including the establishment of motivational conditioning and learning (3).
The morphology of dendritic spines and axons is determined by the dynamic cytoskeleton protein actin. Rho family small GTPases including Rho, Cdc42, and Rac1 regulate the dynamics of actin and are critical for neuronal polarization and morphogenesis (4–6). Rho proteins are regulated by guanine nucleotide exchange factors (GEFs). In early stages of neural morphogenesis, the activation of Cdc42 promotes the formation of filopodia, the long thin protrusions serving as primary precursors of axons and dendritic spines (7). However, Rac1 and its specific GEF TIAM1 regulate the late stage of neural morphogenesis by targeting themselves to dendritic spines and controlling the formation of mature forms of spines (8–10). TIAM1-Rac1·GTP signaling is also important for dendrite branching. Thus, Tiam1-Rac1·GTP signaling is critical for the morphogenesis of dendritic spines especially in the later stages of neuronal maturation.
Originally mistaken as a subtype of opioid receptors (11), the Sigma-1 receptor (Sig-1R) (12–15) is now known to be a nonopioid endoplasmic reticulum (ER)-resident protein (16–19). Sig-1Rs are postulated to be involved in neuropsychiatric diseases including amnesia and addiction (13, 20, 21). By using CHO cells, we recently (17) identified the Sig-1R as a ligand-operated ER receptor chaperone that regulates Ca2+ signaling specifically by chaperoning type 3 IP3 receptors. Importantly, Sig-1Rs regulate neuritogenesis in PC12 cells (22) and enhance cell differentiation in rat oligodendrocytes (23). Sig-1Rs also promote reconstitution of lipid rafts in plasma membranes (22). Taken together, these results suggest the possibility that Sig-1Rs might affect the morphogenesis of neurons in the CNS. However, this possibility has never been examined.
We tested if the ER Sig-1R might regulate neuronal morphology in this study, specifically dendritic spine formation in rat primary hippocampal neurons. We also examined potential underlying mechanisms of such effect, if present. We report here that Sig-1Rs regulate dendritic spine formation via a potential ER-mitochondrion-Rac·GTP pathway, apparently due to this receptor's ability to regulate the redox state of neurons.
Results
Sig-1Rs Regulate the Morphogenesis of Hippocampal Primary Neurons.
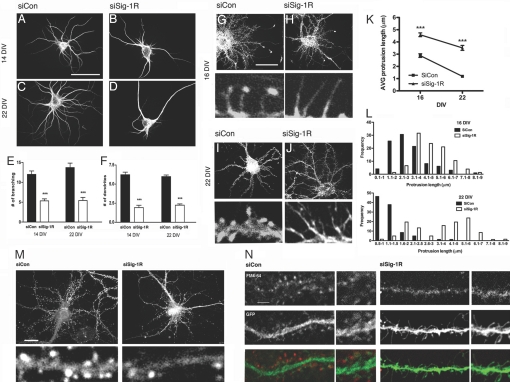
We examined the roles of Sig-1Rs in the morphogenesis of hippocampal primary neurons in vitro by silencing Sig-1R expression using siRNAs. Small hairpin RNAs, constructed in the pSIREN vector, were transfected into primary neurons by using the liposome-delivering system in this initial portion of the study. Rat hippocampal primary neurons were thus transfected with siRNAs (17, 23) together with pEGFP-N1 vectors on DIV 7. Morphologies were observed on DIV 14 or 21. Control neurons receiving transfection of control siRNAs (siCon-tf) on DIV 14 showed a typical morphology of a stage V neuron, having several long dendrites with tapering and branching characteristics (Fig. 1 A–F). The Sig-1R-siRNA-transfected (siSig-1R-tf) neurons, however, showed a reduction of extension and branching of dendrites. Long filopodia-like structures protruding from soma were often observed in siSig-1R-tf neurons. Similar effects were seen on DIV 21 in siSig-1R-tf neurons. Sig-1Rs also affected the formation and maturation of dendritic spines. Results are shown in Fig. 1 G–L. On DIV 16, control neurons formed filopodia-like long protrusions on dendrites, with majority of them (>60%) showing F-actin-enriched head structures at the tip. On DIV 22, control neurons formed clusters of stubby mushroom-shaped spine heads and possessed no filopodia, indicating the maturation of spines. In contrast, siSig-1R-tf neurons formed long and thin protrusions, and only few possessed spine heads at the tip. Further, F-actins were seen diffusing over the whole cytoplasm in the filopodia of siSig-1R-tf neurons, whereas F-actins discretely accumulated at the tip of filopodia in control neurons (Fig. 1 G–J). For siSig-1R-tf neurons, the longer the culture time, the more pronounced the abnormal phenotype was seen, such as increased numbers of elongated filopodia-like protrusions lacking head structures. In general, silencing of Sig-1R expression increased the length of protrusions by 59% on DIV 16 and 159% on DIV 22, respectively. These results suggest a dysregulation of stage-dependent signalings for actin compartmentalization/polymerization in Sig-1R-deficient neurons.
Fig. 1.
Inhibition of dendritic morphogenesis in Sig-1R-knockdown hippocampal neurons. (A–F) Effects of siSig-1R-tf on dendrite formation. Neurons were transfected with siRNAs on DIV 7 and were stained on 14 or 22 DIV with anti-MAP-2B antibodies. The number of established primary dendrites and branches were counted. Primary dendrites are defined as neurites originating from the neuronal soma and are at least longer than two times the diameter of the cell body. (Scale bar, 100 μm.) ***P < 0.001; n = 3, five to 10 neurons were quantified in each individual experiment. (G–J) Effect of siSig-1R-tf on spine formation. Neurons were transfected on DIV 7 with siCon (G and I) or siSig-1R (H and J) vectors in combination with EGFP. Neurons were stained with rhodamin phalloidin for F-actin. Note elongated filopodia in si-Sig-1R-tf neurons. (K and L) Quantitative assessments were made of the effects of siSig-1Rs on protrusion length, dendrite branching, and filopodium formation. The control neurons at DIV 22 had protrusions mostly <2 μm in length and mushroom shaped, which is characteristic of dendritic spines, whereas the siRNA neurons failed to retract protrusions (i.e., exhibited elongated filopodia). Protrusions equals dendritic spines plus filopodia. (Scale bar, 50 μm.) ***P = 0.0002; n = 2, at least 20 neurons were captured in each experiment, and two to three dendrites were randomly selected from each neuron for counting of protrusions. (M) Synaptophysin immunostaining. (N) Effects of siSig-1Rs on synaptic activity. FM4–64 (red)-labeled neurons were depolarized with KCl for measurement of synaptic activity. (Scale bars, M 20 μm and N 5 μm.)
Sig-1R-Knockdown Inhibits Synapse Formation.
Active synapses express specific proteins and receptors for proper function. We next asked if the knockdown of Sig-1Rs might affect the formation of synapses. In control neurons, staining for NR-1, GluR2/3, and PSD-95 showed puncta of strong immunoreactivities on dendritic spines. However, no such puncta were observed in the long filopodia-like protrusions in siSig-1R-tf neurons; these proteins remained in the dendritic shafts (Fig. S1 in SI Appendix). Axon terminals in siSig-1R-tf neurons also exhibited significantly less immunoreactivities for synaptophysin (Fig. 1M). Synaptic activities assessed by the depolarization-induced uptake of FM4–64 showed that the siSig-1R-tf-induced protrusions did not form functional synapses (Fig. 1N). These findings suggest that the filopodia-like long protrusions caused by siSig-1Rs failed to form functional synapses.
Sig-1R-Knockdown Inhibits Activation of Rac1 Rho GTPase.
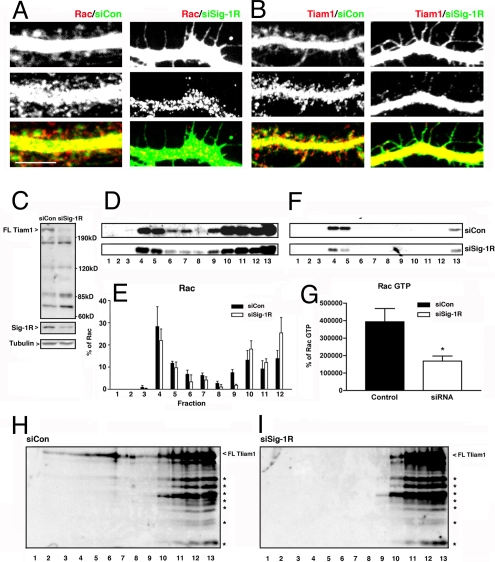
Rac1 regulates dendritic growth and spine maturation but not filopodium formation (8–10). Since the knockdown of Sig-1Rs stunt dendritic growth and synapse formation but not the formation of filopodia, we hypothesized that the Sig-1R might regulate Rac1 signaling in neurons. Both Rac1 and TIAM1 clustered in dendritic shafts and spines of control neurons (Fig. 2 A and B). However, intensities of Rac1 and TIAM1 in filopodium-like protrusions on the dendrites of siSig-1R-tf neurons were markedly decreased (Fig. 2 A and B). The expression levels of these proteins were examined by Western blotting, a technique that requires more protein to be analyzed. Since gene transfer using liposomes yields only a few percentage of successful transfection in primary neurons, we used an AAV-based gene delivery system that yields >70% efficacy in gene expression. Details of vector constructions are described in SI Methods and Fig. S2 in SI Appendix. Western blots showed that the knockdown of Sig-1Rs (i.e., AAV-siSig-1R transduced or abbreviated as AAV-siSig-1R-td) did not affect the total level of Rac1 protein in hippocampal neurons. However, the knockdown caused a down-regulation of full-length TIAM1, accompanied by increases in its proteolytic products (Fig. 2C).
Fig. 2.
Sig-1R-knockdown causes TIAM1 GEF cleavage and the inactivation of Rac. (A and B) Immunocytochemisty for Rac and Tiam1 in AAV-siCon-td or AAV-siSig-1R-td neurons at DIV 22. (Scale bar, 5 μm.) (C) TIAM1 cleavage detected by Western blots. Note the decrease of intact TIAM1 (200 kDa) with concomitant increases of cleaved TIAM1 (120, 85, and 74 kDa) in AAV-siSig-1R-td neurons. (D–G) Effect of AAV-siSig-1Rs on lipid raft distributions of total Rac (D–G) or Rac·GTP (F and G). *P < 0.05; n = 3. (H and I) Lipid raft distributions of TIAM1 and its proteolytic products.
Rac1 is compartmentalized in lipid rafts of postsynaptic membranes (24). We found (Fig. 2D) that Rac 1 existed in both raft (fractions 3, 4, and 5) and nonraft fractions (fractions 6 and higher). The knockdown of Sig-1Rs did not apparently affect the distribution pattern of Rac1 in raft or nonraft fractions (Fig. 2E). However, when active forms of GTP-binding Rac1 (i.e., Rac1·GTP) were measured by a pull-down assay, the Rac1·GTP in the raft fractions (fractions 4 and 5) was reduced in AAV-siSig-1R-td neurons (Fig. 2 F and G). The Sig-1R-knockdown also caused a decrease of intact TIAM1 in the raft fractions. The cleaved TIAMs, which were increased in AAV-si-Sig-1R-td neurons, were present only in nonraft fractions (Fig. 2 H and I). These data suggest that: (i) The active full-length TIAM1 preferentially exists at the raft where TIAM1 serves to activate Rac1 and (ii) the knockdown of Sig-1Rs decreases the amount of full-length TIAM1 at rafts, leading to a reduction of Rac1 activation. How the knockdown of Sig-1Rs might reduce the full-length TIAM1 is examined in the next section.
Knockdown of Sig-1Rs Activates Caspase-3 in an ER-Stress and Apoptosis-Independent Manner.
TIAM1, by activating Rac1, is known to maintain the structural configuration of dendritic spines (8–10). TIAM1 in the motor neuron is inactivated by caspase-dependent proteolysis when cells undergo stress (25). To determine whether increased cleavage of TIAM1, as seen above in AAV-siSig-1R-td neurons, is associated with caspase activation, active forms of several caspase members were analyzed by Western blots. As shown in Fig. S4 in SI Appendix, in the absence of ER stressors like thapsigargin, AAV-siSig-1R-td neurons showed a reduction of procaspase-3 with a concomitant increase in active caspase-3, seen as the cleaved form, when compared to the control (Fig. S4A, lane 2 vs. lane 1). There was no effect on the level of ER stress-responding proteins including caspase-12, GRP94, calnexin, ATF4, and BiP (Fig. S4 B and C, lane 2 vs. lane 1 in each panel). These results suggest that Sig-1R-knockdown did not elicit ER stress and that the knockdown of Sig-1Rs might have caused an increase in caspase-3 in an ER stress-independent manner. We thus decided to use thapsigargin, a well-known ER stressor that causes an increase in many of the ER stress-responding proteins detailed above. Inasmuch as the knockdown of Sig-1Rs did not cause ER stress, we postulated that the caspase-3 increase seen in AAV-siSig-1R-td neurons should continue to be present if neurons are challenged with thapsigargin, whereas the thapsigargin-elicited ER stress-responding proteins should not be affected, or more specifically enhanced, by the Sig-1R-knockdown. Indeed, we found that the caspase-3 activation continued to be seen in thapsigargin-treated Sig-1R-knockdown neurons (Fig. S4A), whereas no further enhancement of the ER stress-responding proteins was seen in thapsigargin-treated Sig-1R-knockdown neurons (Fig. S4C). Thus, the activation of capase-3 induced by the Sig-1R-knockdown may not involve ER stress but instead may involve other, as yet unidentified, pathway(s).
To ascertain whether the knockdown of Sig-1Rs would cause apoptosis, Hoechst 33342 staining was used to detect chromatin condensation in control and transfected or tranduced neurons. Staurosporine-treated (STS; 1 μM for 24 h) neurons served as positive controls for apoptosis. In both AAV-siCon-td and AAV-siSig-1R-td neurons, only a very small fraction of cells underwent apoptosis. No difference in the percentage of apoptotic cells was seen in AAV-siCon-td and AAV-siSig-1R-td neurons (Fig. S4D). Therefore, Sig-1R-knockdown in hippocampal primary neurons did not induce apoptosis.
Sig-1R-Knockdown Does Not Affect Ca2+ Dynamics in Hippocampal Neurons but Nevertheless Causes Mitochondrial Dysfunction.
Consistent with other reports (26, 27), we found that type-1 IP3 receptors, and not type-3 receptors, were the predominant IP3 receptors in hippocampal neurons. As Sig-1R chaperones are known to regulate only type-3 IP3 receptors but not the type-1 receptors (17), we found that the basal or activated level of mitochondrial or cytosolic Ca2+ transient/concentration in hippocampal neurons was not affected by Sig-1R-knockdown (Fig. S3 A–D in SI Appendix). We found, however, that Sig-1R-knockdown caused a disruption of mitochondrial membrane potential (28) as well as an enhanced release of cytochrome c from mitochondria (Fig. S3 E–L in SI Appendix). Thus, Sig-1R-knockdown caused the activation of caspase-3 by increasing mitochondrial cytochrome c release. However, this action of Sig-1Rs in this regard did not involve mitochondrial Ca2+.
Reduced Radical Scavenging Activity in Sig-1R-Knockdown Neurons.
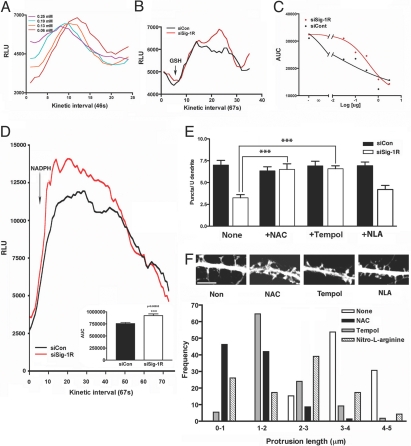
In addition to Ca2+ overload in mitochondria, free radical-related oxidative stress is also known to causes mitochondrial membrane disruption, which leads to mitochondrial permeability transition (MPT) (29–31). Thus, we next examined if free radicals are involved in the siSig-1R-induced MPT. First, we examined if the Sig-1R-knockdown affected the free radical scavenging activity of neurons. An in vitro test, employing the selenite-catalyzed superoxide generation system (32), was used. Selenite at 0.06 mM effectively induces superoxide anion generation upon the addition of glutathione (GSH; 0.2 mg/mL) (Fig. 3A). The superoxides (O2·−) thus generated were monitored by lucigenin-based chemiluminescence. Fig. 3B shows that lysates from AAV-siSig-1R-td neurons were less effective in scavenging O2·− when compared to control lysates. The scavenging activity was lysate protein-dose-dependent. Analyses of the area-under-the-curve (AUC) indicate an EC50 = 0.0144 μg protein for AAV-siCon-td controls and 0.3791 μg protein for AAV-siSig-1R-td neurons (Fig. 3C). We next examined if similar results might be seen if assays were done in the living system. Accordingly, NADPH was applied to living neurons to generate O2·−. NADPH induced a rapid generation of O2·− in both groups, but higher levels of detectable O2·− were seen in AAV-siSig-1R-td neurons (Fig. 3D). These results with the NADPH assay suggest that either AAV-siSig-1R-td neurons are less effective in scavenging O2·− or more O2·− are generated in those neurons.
Fig. 3.
Free radicals in Sig-1R-knockdown neurons. (A) Generation of superoxide anions by GSH and sodium selenite. Sodium selenite was mixed with GSH at 0.2 mg/mL. O2·− were monitored by lucigenin-based chemiluminescense (lucigenin at 50 μM). (B) Effect of hippocampal neuronal lysates on O2·− generated by GSH/selenite. Neurons were transduced with AAV-siCons or AAV-siSig-1Rs for 10 days before assay. (C) The AUC, calculated from assays in panel B (between the 10th and 25th intervals), seen with increasing lysates (0, 0.03, 0.1, 0.3, 1, and 3 μg protein). (D) Effect of AAV-siSig-1R-td on the NADPH-induced generation of O2·− in living hippocampal neurons. NADPH at 45 μM was applied to neurons in the presence of 50 μM lucigenin at the beginning of the fifth interval (AUC from 10th to 40th interval). (E) Inhibition of AAV-siSig-1R-td-induced mitochondrial permeability transition by free radical scavengers NAC and Tempol. The puncta of JC-1 positive mitochondria in dendrites were measured. ***P < 0.001; n = 5, each group in triplicates. (F) Effects of free radical scavengers on dendritic spine formation in AAV-siSig-1R-td neurons. Neurons were transduced with AAV-siSig-1Rs on DIV 13 when 50 μM Tempol, NAC, or NAL were also added. Protrusion lengths were measured on day 5 posttransduction. Frequencies of various protrusion lengths are shown; n = 3 independent experiments. Representative morphologies are shown at the Top. (Scale bar, 5 μm.)
Free Radical Scavengers Block Mitochondrial Dysfunction and Rescue Dendritic Spine Formation in Sig-1R-Knockdown Neurons.
To establish a causal relation between redox state and the alteration of mitochondrial function in AAV-siSig-1R-td neurons, we next examined if free radical scavengers might block the MPT in those neurons. N-acetyl cysteine (NAC) or 4-hydroxy-2,2,6,6-tetramethylpiperidinyloxy (Tempol), scavengers of hydroxyl or superoxide dismutase activator, respectively, successfully blocked the MPT caused by the knockdown of Sig-1Rs (Fig. 3E). The nitric oxide synthase inhibitor nitro-L-arginine (NAL) was, however, ineffective. Thus, siSig-1R-induced disruption of mitochondrial membrane potential is, at least, in part due to the neurons' deficiency in scavenging free radicals.
Free radical scavengers were also used to examine if they might block the aberrant spine development in AAV-siSig-1R-td neurons. Indeed, NAC and Tempol successfully rescued spine formation in Sig-1R-knockdown neurons (Fig. 3F). Specifically, NAC- or Tempol-treated AAV-siSig-1R-td neurons were able to retract filopodia and form dendritic spines. NAL was again less effective in this regard (Fig. 3F).
Caspase-3-Resistant TIAM1 Construct Rescues the Spine Formation in Sig-1R-Knockdown Neurons.
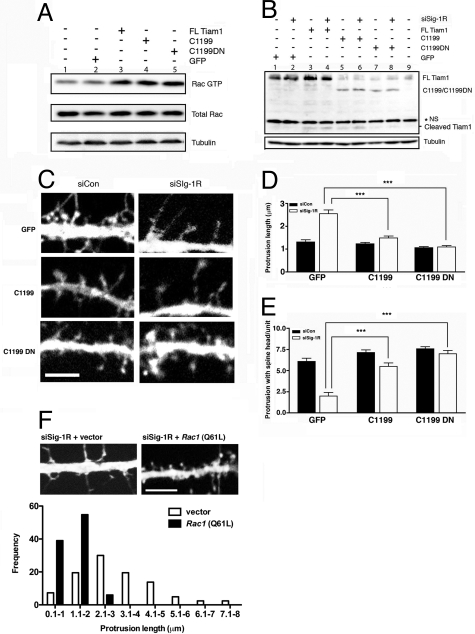
Our hypothesis suggests that the final pathway of Sig-1R-knockdown effects on dendritic spine formation is related to the inactivation of Rac1, which in turn is hinged upon TIAM1 cleavage by caspase-3. To provide evidence for the involvement of Sig-1Rs in this pathway, we examined if the deficit of spine formation caused by Sig-1R-knockdown might be rescued by a caspase-3-resistant TIAM1 construct. We therefore transfected siSig-1R-tf neurons with either an N-terminal-truncated TIAM1 construct called C1199, which is more stable when compared to full-length TIAM1 (10), or a construct derived from C1199, called C1199DN, that is fully resistant to caspase-3 (25). Initially, we used Neuro-2a cells, in lieu of primary hippocampal neurons, to characterize and confirm the properties of C1199 and C1199DN, because of the need for the abundant samples available from Neuro-2a cells for such an extensive characterization. We specifically examined the susceptibility of C1199 and C1199DN to siSig-1R-induced proteolysis. In Neuro-2a cells, both C1199 and C1199DN were able to increase the level of Rac·GTP (Fig. 4A). Both full-length TIAM1 and C1199 underwent slight cleavage in siCon-tf Neuro-2a cells (Fig. 4B, lanes 3 and 5). In siSig-1R-tf Neuro-2 cells, the cleaved form of full-length TIAM1 or C1199 increased, when compared to that seen in respective control cells (Fig. 4B, lanes 4 and 6 vs. lanes 3 and 5, respectively). However, the caspase-3-resistant construct C1199DN was resistant to cleavage both in control cells and in Sig-1R-null cells (Fig. 4B, lanes 7 and 8). These results indicated that C1199 and C1199DN would be useful tools to test our hypothesis.
Fig. 4.
Caspase-3-resistant TIAM1 construct C1199DN and its effects on Rac·GTP formation and dendritic spine maturation in Sig-1R-knockdown neurons. Effect of constitutively active Rac on dendritic spine formation in Sig-1R-knockdown neurons. (A) Characterizing the GEF activity between the full-length TIAM1, a more stable N terminus-truncated TIAM1 construct C1199, and a caspase-3-resistant TIAM1 construct C1199DN using Neuro-2a cells. Neuro-2a cells were transfected with various TIAM1 constructs: Full-length (FL) Tiam1, C1199, and C1199DN. All three TIAM1s equally increased the active form of Rac. (B) Resistance of C1199DN to proteolytic cleavage caused by siSig-1R-tf in Neuro-2a cells. The asterisk (*) indicates the nonspecific band. (C–E) In primary hippocampal neurons, overexpression of C1199 and C1199DN rescued aberrant dendritic spine morphologies caused by siSig-1R-tf. Note the greater potency of C1199DN in rescuing the morphologies. ***P < 0.001 (F) The effect of constitutively active Rac1 (Q61L) on dendritic spine formation in siSig-1R neurons. DIV 18 neurons were cotransfected with siRNA-Sig1R vectors together with constitutively active Rac1 or an empty vector. The morphologies were obtained at DIV 22; n = 3 experiments. At least 5–10 neurons were counted in each experiment. Scale bar, 5 μm.
Hippocampal primary neurons were subsequently transfected with a C1199 or C1199DN plasmid along with siSig-1Rs. To best monitor the effects of those constructs on the spine formation, plasmids were transfected into neurons specifically on DIV 14, a time when filopodia begin to form spines in primary cultures. Cytotoxicity caused by this late transfection was minimized by the following modifications in the protocol: (i) Neurons seeded at higher density (1 × 105 cells/well); (ii) pretreatment with kineuric acid and MgCl2 1 h before transfection; and (iii) neurobasal medium used as a diluent of the DNA-lipofectamine complexes. This modified protocol yielded no cytotoxicity. Our results showed that the overexpression of C1199 in siSig-1R-tf neurons slightly improved the maturation of dendritic spines, but filopodium-like protrusions still dominated the phenotype (Fig. 4C). This result is consistent with another report showing that branched filopodia and lamelapodia were occasionally observed in neurons overexpressing C1199 (10). The overexpression of caspase-3-resistant C1199DN in siSig-1R-tf neurons, however, successfully rescued dendritic spine formation in those neurons notably by decreasing the number of long filopodia while concomitantly increasing mature spines with mushroom-shaped head structures (Fig. 4 C–E).
Constitutively Active Rac Rescues the Spine Formation in Sig-1R-Knockdown Neurons.
To provide additional evidence for our hypothesis on the ER-mitochondrion- Rac1·GTP pathway, we examined if constitutively active Rac1 (i.e., Q61L) might rescue the deficit of dendritic spines caused by the Sig-1R-knockdown. Our results indicate that constitutively active Rac successfully rescued the formation of dendritic spines in siSig-1R-tf neurons. The siSig-1R-tf neurons cotransfected with Q61L on DIV 18 were able to form dendritic spines on DIV 22 (Fig. 4F).
Discussion
In this study we have demonstrated that a potential ER-mitochondrion-Rac·GTP pathway plays an important role in dendritic spine and synapse formation in hippocampal neurons and that the redox state of neurons is an integral and critical element in this pathway. Because Sig-1Rs are implicated in memory and cognition (33, 34), our findings also provide a mechanistic insight into the action of Sig-1Rs in neuroplasticity and cognition.
ER and mitochondria have been reported to participate in dendritic spine formation (35–39). Our results provide additional insights on how these two intracellular organelles may participate in dendritic spine formation in a unique fashion. Contrary to the notion that ER does so by regulating Ca2+ efflux via ryanodine or IP3 receptors on the ER membrane (35–37), our results indicate that the ER might regulate dendritic spine formation in hippocampal neurons via a free radical-related mechanism that might not involve Ca2+ signaling. Mitochondria supply energy for cells and are important in neural development (38, 39). Our results indicate that mitochondria might participate in dendritic formation via a TIAM1-Rac1·GTP signaling pathway.
Our results also indicate that caspase-3 plays an important role in the development of neurons. Although caspase-3 is recognized as a protease that executes death signals, growing evidence suggests that caspase-3 may also be important in synaptic plasticity and growth cone motility (40–43). We have thus added one more important physiological role to the action of caspase-3 and shown that it regulates dendritic spine formation in hippocampal neurons. Although Sig-1R-knockdown increased caspase-3 activity in primary neurons in this study, the knockdown did not cause apoptosis. As such, the increase in caspase-3 activity caused by Sig-1R-knockdown might represent an early stage of denritic spine dysfunction that is before apoptosis and might still be rescued by some therapeutic intervention including the use of free radical scavengers (see below).
One of the most important outcomes from this study is the demonstration that an ER protein can affect the activation of Rac1 and associated neuroplasticity by the level of free radicals in neurons. We do not know at present how Sig-1Rs modulate free radicals or reactive oxygen species (ROS) in the neuron. Mitochondria are the main source of free radicals/ROS levels in the cell, but the ER can also generate free radicals (44). The exact mechanism whereby free radicals are controlled by the ER Sig-1Rs warrants further investigation. Gene microarray and proteomic studies are being planned to provide some clues in this regard. Nevertheless, our results with free radical scavengers being able to rescue mitochondrial dysfunction and the aberrant morphologies of dendritic spines attest to the important role of redox state in the morphogenesis of neurons. Moreover, our results showing that the free radical scavenger NAC and the superoxide dismutase activator Tempol are able to rescue the dendritic arborization also suggest the possibility that such drugs might be of therapeutic potential in treating dendritic spine-related neurodegenerative diseases. Specifically, NAC is a mucolytic agent used in treating respiratory conditions. Our results suggest that NAC might represent an established drug with use in treating CNS diseases related to dendritic spine abnormalities, especially in the hippocampus, which is well-known to play an important role in learning and memory. In fact, several reports have demonstrated that NAC is neuroprotective and can be used to improve spatial working memory in rats (45, 46). Our results provide a potential mechanism for the memory-enhancing effect of NAC. Finally, because Sig-1Rs regulate the ganglioside composition in lipid rafts (22), the inability of Rac·GTP and TIAM1 to reside in the raft in Sig-1R-knockdown neurons (Fig. 2) suggests a dysregulation of ganglioside formation in those neurons.
Materials and Methods
Neuronal Culture and Viral Transduction.
For larger quantities of cell samples, primary neurons were prepared from whole brain in some experiments. Neurons at 10 DIV were transduced with AAV serotype 6 (AAV6) to knock down Sig-1Rs. Multiplicity of infection (MOI; 10,000 to ≈20,000) was used for neuronal transduction. For details, see SI Methods in SI Appendix.
Preparation of Detergent-Resistant Membrane.
Approximately 5 × 106 ≈1 × 107 cultured neurons (21 DIV) from whole brains were used. The remainder of experimental details can be found elsewhere (22).
Rac·GTP Pull Down Assay.
Procedures were performed according to the manufacturer's instructions (Upstate Biotechnology).
Assays for Superoxide Anions.
A chemiluminescence assay was used with modifications (32). Details of the assay are described in SI Methods in SI Appendix.
Free Radical Scavenging Experiments.
Neuronal cultures were infected routinely on DIV 10. One hundred micromolar hydroxyl free radical scavenger NAC, the superoxide dismutase activator Tempol, or the NO synthase inhibitor NAL were added to the cultures 24 h after infection. In “rescue” experiments, half of the culture medium was replaced daily with fresh medium supplemented with free radical scavengers. Neurons were subjected to JC-1 labeling for MPT analyses on DIV 15.
Supplementary Material
Acknowledgments.
The kindness and generosity of Dr. Margaret Chou in supplying various plasmids of the TIAM1 mutants is greatly appreciated. We thank Mr. Doug Howard for his technical assistance with the AAV vectors. This study is supported by the Intramural Research Programs of the National Institute on Drug Abuse and the National Institute on Aging, National Institutes of Health, Department of Health and Human Services.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0909089106/DCSupplemental.
References
- 1.Mervis RF, et al. Structural-functional correlates of neuroprotection in the aging rabbit by a calcium channel blocker. Nimodipine reverses neocortical dendritic atrophy and improves memory retention. Ann N Y Acad Sci. 1995;765:312–313. doi: 10.1111/j.1749-6632.1995.tb16596.x. [DOI] [PubMed] [Google Scholar]
- 2.Coleman P, Federoff H, Kurlan R. A focus on the synapse for neuroprotection in Alzheimer disease and other dementias. Neurology. 2004;63:1155–1162. doi: 10.1212/01.wnl.0000140626.48118.0a. [DOI] [PubMed] [Google Scholar]
- 3.Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 4.Bito H. Dynamic control of neuronal morphogenesis by rho signaling. J Biochem. 2003;134:315–319. doi: 10.1093/jb/mvg147. [DOI] [PubMed] [Google Scholar]
- 5.Arimura N, Kaibuchi K. Key regulators in neuronal polarity. Neuron. 2005;48:881–884. doi: 10.1016/j.neuron.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Watabe-Uchida M, Govek EE, Van Aelst L. Regulators of Rho GTPases in neuronal development. J Neurosci. 2006;26:10633–10635. doi: 10.1523/JNEUROSCI.4084-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanaka E, Sabry J. Making the connection: Cytoskeletal rearrangements during growth cone guidance. Cell. 1995;83:171–176. doi: 10.1016/0092-8674(95)90158-2. [DOI] [PubMed] [Google Scholar]
- 8.Lambert JM, et al. Tiam1 mediates Ras activation of Rac by a PI(3)K-independent mechanism. Nat Cell Biol. 2002;4:621–625. doi: 10.1038/ncb833. [DOI] [PubMed] [Google Scholar]
- 9.Tolias KF, et al. The Rac1-GEF Tiam1 couples the NMDA receptor to the activity-dependent development of dendritic arbors and spines. Neuron. 2005;45:525–538. doi: 10.1016/j.neuron.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H, Macara IG. The polarity protein PAR-3 and TIAM1 cooperate in dendritic spine morphogenesis. Nat Cell Biol. 2006;8:227–237. doi: 10.1038/ncb1368. [DOI] [PubMed] [Google Scholar]
- 11.Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine- and nalorphine-like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976;197:517–532. [PubMed] [Google Scholar]
- 12.Su TP, London ED, Jaffe JH. Steroid binding at sigma receptors suggest a link between nervous, endocrine, and immune systems. Science. 1988;240:219–221. doi: 10.1126/science.2832949. [DOI] [PubMed] [Google Scholar]
- 13.Snyder SH, Largent BL. Receptor mechanisms in antipsychotic drug action: Focus on sigma receptors. J Neuropsychiatry Clin Neurosci. 1989;1:7–15. doi: 10.1176/jnp.1.1.7. [DOI] [PubMed] [Google Scholar]
- 14.Graybiel AM, Besson MJ, Weber E. Neuroleptic-sensitive binding sites in the nigrostriatal system: Evidence for differential distribution of sigma sites in the subtantia nigra, pars compacta of the cat. J Neurosci. 1989;9:326–338. doi: 10.1523/JNEUROSCI.09-01-00326.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouchard P, Maurice T, St-Pierre S, Privat A, Quirion R. Neuropeptide Y and the calcitonin gene-related peptide attenuate learning impairments induced by MK-801 via a sigma receptor-related mechanism. Eur J Neurosci. 1997;9:2142–2151. doi: 10.1111/j.1460-9568.1997.tb01381.x. [DOI] [PubMed] [Google Scholar]
- 16.Aydar E, Palmer CP, Klyachko VA, Jackson MB. The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron. 2002;34:399–410. doi: 10.1016/s0896-6273(02)00677-3. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 18.Fontanilla D, et al. The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science. 2009;323:934–937. doi: 10.1126/science.1166127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fontanilla D, et al. Probing the steroid binding domain-like I (SBDLI) of the sigma-1 receptor binding site using N-substituted photoaffinity labels. Biochemistry. 2008;47:7205–7217. doi: 10.1021/bi800564j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maurice T, Phan VL, Privat A. The anti-amnesic effects of sigma1 (sigma1) receptor agonists confirmed by in vivo antisense strategy in the mouse. Brain Res. 2001;898:113–121. doi: 10.1016/s0006-8993(01)02152-7. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi T, Su TP. An update on the development of drugs for neuropsychiatric disorders: Focusing on the sigma 1 receptor ligand. Expert Opin Ther Targets. 2008;12:45–58. doi: 10.1517/14728222.12.1.45. [DOI] [PubMed] [Google Scholar]
- 22.Takebayashi M, Hayashi T, Su TP. Sigma-1 receptors potentiate epidermal growth factor signaling towards neuritogenesis in PC12 cells: Potential relation to lipid raft reconstitution. Synapse. 2004;53:90–103. doi: 10.1002/syn.20041. [DOI] [PubMed] [Google Scholar]
- 23.Hayashi T, Su TP. Sigma-1 receptors at galactosylceramide-enriched lipid microdomains regulate oligodendrocyte differentiation. Proc Natl Acad Sci USA. 2004;101:14949–14954. doi: 10.1073/pnas.0402890101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumanogoh H, Miyata S, Sokawa Y, Maekawa S. Biochemical and morphological analysis on the localization of Rac1 in neurons. Neurosci Res. 2001;39:189–196. doi: 10.1016/s0168-0102(00)00211-x. [DOI] [PubMed] [Google Scholar]
- 25.Qi H, et al. Caspase-mediated cleavage of the TIAM1 guanine nucleotide exchange factor during apoptosis. Cell Growth Differ. 2001;12:603–611. [PubMed] [Google Scholar]
- 26.Genazzani AA, Carafoli E, Guerini D. Calcineurin controls inositol 1,4,5-trisphosphate type 1 receptor expression in neurons. Proc Natl Acad Sci USA. 1999;96:5797–5801. doi: 10.1073/pnas.96.10.5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukatsu K, et al. Lateral diffusion of inositol 1,4,5-trisphosphate receptor type 1 is regulated by actin filaments and 4.1N in neuronal dendrites. J Biol Chem. 2004;279:48976–48982. doi: 10.1074/jbc.M408364200. [DOI] [PubMed] [Google Scholar]
- 28.Dubinsky JM, Levi Y. Calcium-induced activation of the mitochondrial permeability transition in hippocampal neurons. J Neurosci Res. 1998;53:728–741. doi: 10.1002/(SICI)1097-4547(19980915)53:6<728::AID-JNR10>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 29.Madesh M, Hajnoczky G. VDAC-dependent permeabilization of the outer mitochondrial membrane by superoxide induces rapid and massive cytochrome c release. J Cell Biol. 2001;155:1003–1015. doi: 10.1083/jcb.200105057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halestrap AP, McStay GP, Clarke SJ. The permeability transition pore complex: Another view. Biochimie. 2002;84:153–166. doi: 10.1016/s0300-9084(02)01375-5. [DOI] [PubMed] [Google Scholar]
- 31.Gunter TE, Yule DI, Gunter KK, Eliseev RA, Salter JD. Calcium and mitochondria. FEBS Lett. 2004;567:96–102. doi: 10.1016/j.febslet.2004.03.071. [DOI] [PubMed] [Google Scholar]
- 32.Davis RL, Spallholz JE. Inhibition of selenite-catalyzed superoxide generation and formation of elemental selenium (Se(o)) by copper, zinc, and aurintricarboxylic acid (ATA) Biochem Pharmacol. 1996;51:1015–1020. doi: 10.1016/0006-2952(95)02435-2. [DOI] [PubMed] [Google Scholar]
- 33.Iyo M, et al. Fluvoxamine as a sigma-1 receptor agonist improved cognitive impairments in a patient with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1072–1073. doi: 10.1016/j.pnpbp.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Maurice T, Lockhart BP. Neuroprotective and anti-amnesic potentials of sigma (sigma) receptor ligands. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:69–102. doi: 10.1016/s0278-5846(96)00160-1. [DOI] [PubMed] [Google Scholar]
- 35.Bardo S, Cavazzini MG, Emptage N. The role of the endoplasmic reticulum Ca2+ store in the plasticity of central neurons. Trends Pharmacol Sci. 2006;27:78–84. doi: 10.1016/j.tips.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 36.Hayashi Y, Majewska AK. Dendritic spine geometry: Functional implication and regulation. Neuron. 2005;46:529–532. doi: 10.1016/j.neuron.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 37.Penzes P, Cahill ME, Jones KA, Srivastava DP. Convergent CaMK and RacGEF signals control dendritic structure and function. Trends Cell Biol. 2008;18:405–413. doi: 10.1016/j.tcb.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 38.McBride HM, Neuspiel M, Wasiak S. Mitochondria: More than just a powerhouse. Curr Biol. 2006;16:R551–R560. doi: 10.1016/j.cub.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 39.Dietrich MO, Andrews ZB, Horvath TL. Exercise-induced synaptogenesis in the hippocampus is dependent on UCP2-regulated mitochondrial adaptation. J Neurosci. 2008;28:10766–10771. doi: 10.1523/JNEUROSCI.2744-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mashima T, et al. Actin cleavage by CPP-32/apopain during the development of apoptosis. Oncogene. 1997;14:1007–1012. doi: 10.1038/sj.onc.1200919. [DOI] [PubMed] [Google Scholar]
- 41.Diaz F, Bourguignon LY. Selective down-regulation of IP(3)receptor subtypes by caspases and calpain during TNF alpha-induced apoptosis of human T-lymphoma cells. Cell Calcium. 2000;27:315–328. doi: 10.1054/ceca.2000.0126. [DOI] [PubMed] [Google Scholar]
- 42.Glazner GW, Chan SL, Lu C, Mattson MP. Caspase-mediated degradation of AMPA receptor subunits: A mechanism for preventing excitotoxic necrosis and ensuring apoptosis. J Neurosci. 2000;20:3641–3649. doi: 10.1523/JNEUROSCI.20-10-03641.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gilman CP, Mattson MP. Do apoptotic mechanisms regulate synaptic plasticity and growth-cone motility? Neuromol Med. 2002;2:197–214. doi: 10.1385/NMM:2:2:197. [DOI] [PubMed] [Google Scholar]
- 44.Gorlach A, Klappa P, Kietzmann T. The endoplasmic reticulum: Folding, calcium homeostasis, signaling, and redox control. Antioxid Redox Signal. 2006;8:1391–1418. doi: 10.1089/ars.2006.8.1391. [DOI] [PubMed] [Google Scholar]
- 45.Jayalakshmi K, et al. Neuroprotective effect of N-acetyl cysteine on hypoxia-induced oxidative stress in primary hippocampal culture. Brain Res. 2005;1046:97–104. doi: 10.1016/j.brainres.2005.03.054. [DOI] [PubMed] [Google Scholar]
- 46.Jayalakshmi K, et al. N-acetyl cysteine supplementation prevents impairment of spatial working memory functions in rats following exposure to hypobaric hypoxia. Physiol Behav. 2007;92:643–650. doi: 10.1016/j.physbeh.2007.05.051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.