Abstract
Gangliosides are considered to be essential in the maintenance and repair of nervous tissues; however, the mechanisms for neurodegeneration caused by ganglioside defects are unknown. We examined gene expression profiles in double knockout (DKO) mice of GM2/GD2 synthase and GD3 synthase genes and showed that the majority of complement genes and their receptors were up-regulated in cerebellum in DKO mice. Inflammatory reactions were demonstrated in those tissues by measuring up-regulated inflammatory cytokines, indicating the presence of complement activation and inflammation as reported in Alzheimer's disease. Immunoblotting of fractionated membrane extracts by sucrose density gradient revealed that complement-regulatory molecules such as decay-accelerating factor and CD59 were dispersed from glycolipid-enriched microdomain/rafts in DKO cerebellum. Immunohistostaining of these molecules showed disordered membrane localization. These results suggested that dysfunction of complement-regulatory molecules may be due to abnormal glycolipid-enriched microdomain/rafts that triggered complement activation, subsequent inflammation, and neurodegeneration in DKO mice. Generation of the triple KO mice lacking complement activity in addition to the two glycosyltransferases suggested that complement activation is involved in the inflammatory reactions and neurodegeneration caused by the ganglioside deficiency.
Keywords: cerebellum, degeneration, inflammation, knockout, lipid raft
Gangliosides, sialic acid-containing glycosphingolipids, have been historically considered to be involved in the development, differentiation, and function of nervous systems in vertebrates (1). It has been recently clarified, however, based on studies of glycosylation-mutant animals, that gangliosides may mainly play roles in the maintenance and repair of nervous tissues (2, 3). Mice deficient in glycosyltransferases responsible for synthesis of gangliosides have exhibited degenerative changes mainly in the peripheral and central nervous systems (4, 5). GM2/GD2 synthase knockout (KO) mice showed mild neurological dysfunction at birth and progressive neurodegenerative changes (4–6). In contrast, combined KO of GM2/GD2 synthase and GD3 synthase (7) (double KO, hereafter abbreviated DKO) exhibited severe neurodegeneration with earlier onset and wider pathology distribution (8, 9). KO mice of glucosylceramide synthase, in which Glc-Cer synthase was conditionally deleted in the brain after birth, also exhibited neurodegenerative changes (10). All of these observations suggested that a lack of gangliosides causes defects in maintenance of integrity of nervous tissues. While deficiency of some groups of glycolipids was shown to be associated with neurodegenerative changes, the mechanism of degeneration caused by the absence of certain glycolipids has not been demonstrated.
In various neurodegenerative diseases, particularly in Alzheimer's disease (AD), one important factor considered to be involved in the death of neurons is local inflammation (11, 12). All components of the classical complement pathway have been identified in neurons (13), and this pathway has been reported to be activated in AD by fibrillar β amyloid peptide (14) or neurofibrillary tangles (15). The detection and activation of the alternative pathway in AD has also been reported (16). These results suggest that complement activation and inflammation is one of the mechanisms that may damage the brain in AD.
By analyzing gene expression in mice with KO of glycosyltransferases responsible for ganglioside synthesis we found that complement genes were up-regulated in nerve tissues. Further investigation revealed that both complement activation and inflammatory reactions are observed in the mutant mice, possibly based on disruption of membrane microdomains, that is, lipid rafts (17) or glycolipid-enriched microdomains (hereafter GEM/rafts). GEM/rafts have recently gained the interest of many biological researchers (18) for their putative roles in various biological processes (19).
We report here that neurodegeneration is associated with activated complement systems and subsequent inflammatory reactions in ganglioside-lacking mutant mice, which may be based on disrupted GEM/rafts. Involvement of the complement system in the inflammatory reaction and neurodegeneration was supported by mating DKO mice and C3-deficient mutant mice.
Results
Neurodegeneration Associated with Simultaneous Deletion of GM2/GD2 Synthase and GD3 Synthase.
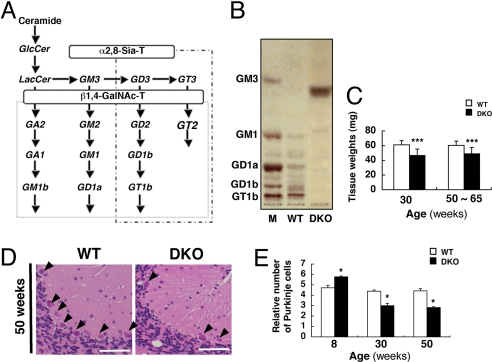
Double KO mice were generated lacking GM2/GD2 synthase and GD3 synthase genes (Fig. 1A). The cerebellum, containing only GM3 in TLC (Fig. 1B), weighed less in DKO mice at both 30 weeks (WT: 61.0 ± 5.6 mg, vs. DKO: 46.5 ± 8.7 mg) and 50–65 weeks (WT: 60.4 ± 5.7 mg vs. DKO: 48.9 ± 8.6 mg) (Fig. 1C). DKO mice displayed staggering gait (Movie S1). There was a clear loss of Purkinje cells in 30- (WT: 4.4 ± 0.1 vs. DKO: 3.0 ± 0.2) and 50-week-old (WT: 4.4 ± 0.2 vs. DKO: 2.8 ± 0.1) DKO cerebellum, although Purkinje cells in the 8-week-old DKO mice somewhat increased (WT: 4.7 ± 0.2 vs. DKO: 5.8 ± 0.1) (Fig. 1 D and E). Moreover, progressive tremor and abnormal gait were observed in the DKO mice, suggesting that neurodegeneration occurred in the cerebellum.
Fig. 1.
Synthetic pathway of gangliosides and phenotypes of DKO mice. (A) A synthetic pathway of gangliosides. Ganglioside species that are absent in the KO mice for GM2/GD2 synthase and GD3 synthase genes are indicated. Consequently, structures boxed in two squares were depleted in the DKO mice. (B) TLC of gangliosides from cerebella of 15-week-old WT and DKO mice. Resorcinol was used for the detection. (C) Wet weights of the cerebella of 30- and 50- to 65-week-old WT and DKO mice. Cerebella were cleaved at upper edge of inferior cerebellar peduncle after PBS perfusion. Numbers of tissues examined were: 30-week-old WT n = 15, DKO n = 14; 50- to 65-week-old WT n = 13, DKO n = 11; data are presented as mean ± SD. (D) Cerebellum sections of 50-week-old mice stained with HE. Arrowheads indicate Purkinje cells. (E) The numbers of Purkinje cells were counted and presented as the number per 9,000-μm range of the Purkinje layer. The numbers of mice examined were: 8-, 30-, and 50-week-old WT n = 2, DKO n = 2; data are presented as mean ± SD. [Scale bar, 50 μm (D).] *, P < 0.05; ***, P < 0.001.
Genes of Complement Components Were Up-Regulated in the Cerebellum of DKO Mice.
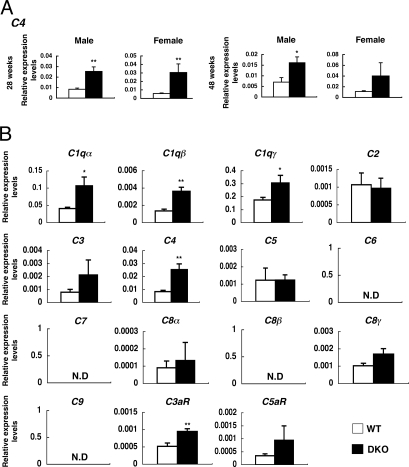
To investigate the mechanisms for neurodegeneration in the DKO mice, a DNA microarray analysis were performed with mRNAs from cerebellum of 28- and 48-week-old mice. Among genes differentially expressed between WT and DKO mice, complement genes (C1qα, C1qγ, C3, C4, and C3aR) were found to be up-regulated in the nervous tissues (Table S1). C4 expression was markedly up-regulated in the cerebellum of 48-week-old DKO mice (WT: 1.37 vs. DKO: 5.00).
To precisely determine gene expression profiles, real time RT-PCR was performed with mRNAs from individual mice. Consequently, expression levels of C4 and C3aR were up-regulated in DKO mice compared to WT at all ages examined, except in 48-week-old females (Fig. 2A and Fig. S1).
Fig. 2.
mRNA levels of complement components were up-regulated in the cerebellum of DKO mice. mRNA levels of complement genes in the cerebellum of individual mice were analyzed. (A) Expression of the C4 gene. (B) mRNA levels of 15 complement-related genes in the cerebellum of 28-week-old male mice were analyzed by real time RT-PCR and presented after correction with GAPDH gene. The number of mice examined was: 28-week-old male WT n = 3, DKO n = 3; 28-week-old female WT n = 3, DKO n = 6; 48-week-old male WT n = 3, DKO n = 3; 48-week-old female WT n = 3, DKO n = 5; data are presented as mean ± SD. *, P < 0.05; **, P < 0.01; ***, P < 0.001. N.D., not detectable.
The complement system functions in innate immunity and inflammatory reactions with sequential involvement of diverse components. Real time RT-PCR was performed for all complement system genes using mRNAs from the cerebellum. Expression levels of C1qα, C1qβ, C1qγ, C4 and C3aR were significantly up-regulated in DKO mice (Fig. 2B), suggesting that a majority of complement genes were up-regulated.
Complement Gene Expression Was Up-Regulated Increasing with Time in the DKO Mice.
Because the pathology of DKO mice was exacerbated with aging, we examined chronological changes in the expression levels of C1qα, C3, C4, C3aR, and C5aR using mRNAs from mouse cerebellum and liver at various ages (Fig. 3). Expression levels of those genes clearly increased in DKO mice at 15 weeks or thereafter, and significant differences between the DKO and the WT became definite with aging (Fig. 3 Left). Expression levels of complement genes were generally higher in the liver than in nerve tissues (Fig. 3 Right). These results suggested that up-regulation of complement genes took place during neurodegeneration in DKO mice. Actual protein levels and deposits of C4 in cerebellum were demonstrated (Fig. S2).
Fig. 3.
Complement genes were up-regulated with aging in the cerebellum of DKO mice. mRNAs from the cerebella of 4-, 15-, 28-, and 48-week-old mice and from livers of 4-, 15-, and 28-week-old mice were analyzed for the expression levels of five complement genes with real time RT-PCR and presented after correction by the GAPDH gene. Left column, expression levels of C1qα, C3, C4, C3aR, and C5aR in the cerebellum. Right column, expression levels in the liver. Open diamond, WT; closed square, DKO. The number of mice examined was: WT n = 3, DKO n = 3; data are presented as mean ± SD. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Secretion of Inflammatory Cytokines in the Cerebellum of DKO Mice.
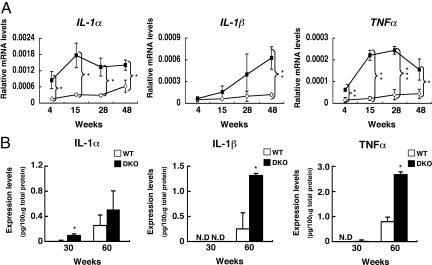
To investigate whether inflammation occurred in the cerebellum, mRNA expression levels of inflammatory cytokines (IL-1α, IL-1β, and TNFα) were measured in the nerve tissues. Expression of IL-1α was up-regulated in DKO mice at all ages (Fig. 4A). Expression of IL-1β and TNFα was also up-regulated in DKO mice, showing a tendency to increase with age. mRNAs of IL-6 and IFNγ were not detected in either type of mice.
Fig. 4.
Inflammatory cytokines increased in the cerebellum of DKO mice with aging. (A) mRNAs from the cerebellum of individual mice were analyzed for the expression levels of cytokine genes with real time RT-PCR, and corrected by the mouse GAPDH gene. Open diamond, WT; closed square, DKO. The number of mice examined was: 4-, 15-, 30-, and 60-week-old WT n = 3, DKO n = 3. (B) The protein levels of IL-1α, IL-1β, and TNFα in the cerebellum of 30- and 60-week-old mice as analyzed with ELISA. The number of mice examined was: 30- and 60-week-old WT n = 3, DKO n = 2. *, P < 0.05; **, P < 0.01; ***, P < 0.001. N.D., not detectable.
In ELISA for the inflammatory cytokines, IL-1α showed an approximate 10-fold increase in 30-week-old and 2-fold increase in 60-week-old DKO mice (Fig. 4B). IL-1β increased approximately 6-fold in 60-week-old DKO mice, and TNFα increased approximately 3.4-fold in 60-week-old DKO mice. These results suggested that the cerebellum of DKO mice had an inflammatory reaction, exacerbated with age.
DAF and GEM/Raft Markers Dispersed from GEM/Rafts in the Cerebellum of DKO Mice.
Complement-regulatory proteins such as decay-accelerating factor (DAF, or CD55) or CD59 are GPI-anchored proteins present in GEM/rafts, along with gangliosides. Therefore, it was predicted that GPI-anchored proteins undergo disorders due to altered GEM/rafts in the DKO mice.
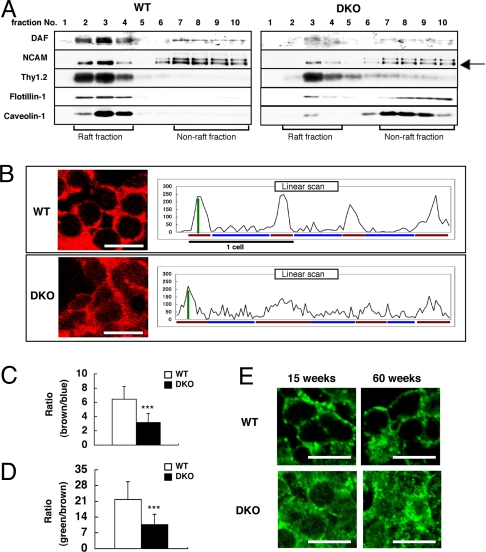
The total protein levels of DAF were not different in the cerebellum of WT and DKO mice (shown in Fig. S3). Therefore, we examined alterations of GPI-anchored proteins as well as GEM/raft markers in the floating patterns inside/outside of GEM/rafts in the DKO mice. Triton X-100 extracts from cell membranes of the cerebellum were separated by sucrose density gradient centrifugation. We found that DAF, N-CAM, flotillin-1, and caveolin-1 largely dispersed from the fractions reported to contain GEM/rafts in the DKO mice (Fig. 5A).
Fig. 5.
Altered distribution of GPI-anchored complement-regulatory proteins in the cerebellum of DKO mice. (A) Floatation patterns of DAF, N-CAM (a GPI-anchored isoform is indicated by an arrow), Thy1.2, flotillin-1, and caveolin-1 in the cerebellum from 46-week-old mice were analyzed by immunoblotting using fractions separated by sucrose density gradient centrifugation. GEM/rafts were located in fractions 2–4. (B–D) Confocal microscopic image of DAF (B) in the granular cells of the 60-week-old mouse cerebellum. Cellular distribution of DAF was analyzed by scanning images, and the ratios of fluorescence intensities between membrane and cytoplasm (brown/blue bar of B) were calculated (C). The sharpness of the peaks of fluorescence (green/brown bar of B) was also calculated (D). The number of neurons examined was: WT n = 20, DKO n = 20 (D and E); data are presented as mean ± SD. (E) Confocal microscopic images of flotillin-1 in the granular cells of 15- and 60-week-old cerebellum. [Scale bar,10 μm (B and E).] ***, P < 0.001.
Furthermore, we performed immunohistostaining to examine distribution patterns of DAF, CD59, and flotillin-1 in nerve cells. Staining of DAF in the granular cells in WT mice was mainly detected on plasma membranes. On the other hand, DAF in the granular cells of DKO mice was also present in the cytoplasm (Fig. 5B Left). The ratios of fluorescence intensities between membrane and cytoplasm (Fig. 5C) and the sharpness of the peaks (Fig. 5D) significantly decreased in the DKO mice (Fig. 5B Right). These results suggested that DAF existed in both plasma membranes and cytoplasm in DKO mice. Flotillin-1 and CD59 also stained distinctly when comparing DKO and the WT mice (Fig. 5E and Fig. S4, respectively).
Alleviation of the Inflammatory Reaction and Neurodegeneration in the DKO Mice by Genetic Disruption of Complement C3.
To investigate whether the inflammatory reaction and neurodegeneration in DKO mice associated with complement systems, we generated triple KO (TKO) mice by mating DKO with C3 KO mice. In this study, 15- and 30-week-old mice were used, where the inflammatory reaction and neurodegeneration were already obvious.
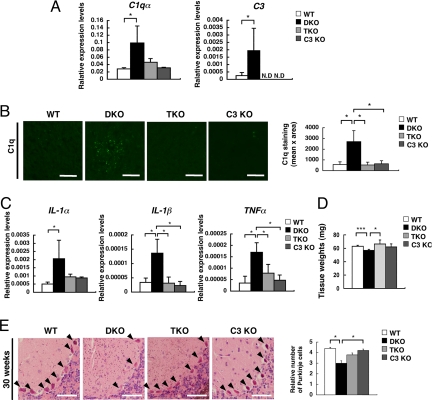
As expected, expression levels of C3 were null in the TKO mice (Fig. 6A). Although expression levels of C1qα were significantly higher in DKO mice, they became almost equivalent in TKO and WT mice. Deposits of C1q and C3b/iC3b/C3c (active forms of C3) were examined by immunohistostaining. TKO mice showed no deposits of any component (Fig. 6B and Fig. S5), indicating that complement activation was completely suppressed in TKO mice.
Fig. 6.
Alleviation of the complement activation, inflammation, and neurodegeneration in the DKO mice by disruption of complement C3 gene. (A) Expression levels of complement mRNAs from the cerebella of 15-week-old mice were analyzed for C1qα and C3 genes with real time RT-PCR. Relative expression levels are presented after correction by the GAPDH gene. The numbers of mice examined were: 15-week-old WT n = 5, DKO n = 4, TKO n = 3, C3 KO n = 3. (B) Deposits of C1q in the cerebella of 15-week-old WT, DKO, TKO, and C3 KO mice were analyzed with anti-C1q mAb in combination with Alexa Flour 488-conjugated anti-rat IgG. Fluorescence intensity (mean pixel × total area) of complement staining was measured by digital image analysis. Number of section area examined was: 15-week-old WT n = 6, DKO n = 6 TKO n = 6, C3 KO n = 6; data are presented as mean ± SD. The thickness of sections was 7 μm. (Scale bar, 20 μm.) (C) Expression levels of cytokine mRNAs (IL-1α, IL-1β, and TNFα) were analyzed with real time RT-PCR using RNAs from the cerebellum of individual mice. Relative expression levels are presented after correction by the mouse GAPDH gene. The number of mice examined was: 15-week-old WT n = 4, DKO n = 4, TKO n = 3, C3 KO n = 3. [Scale bar, 50 μm (A and B).] Thickness of sections, 7 μm. (D) Wet weights of the cerebella of 15-week-old WT, DKO and TKO mice as measured in Fig. 1. Numbers of tissues examined were: WT n = 6, DKO n = 5, TKO n = 3, C3 KO n = 3; data are presented as mean ± SD. (E) Cerebellar sections of 30-week-old mice stained with HE. Arrowheads indicate Purkinje cells. The numbers of Purkinje cells were counted and presented as in Fig. 1. The numbers of mice examined were: 30-week-old WT n = 2, DKO n = 2 TKO n = 2, C3 KO n = 2. Data are presented as mean ± SD. (Scale bar, 50 μm.) *, P < 0.05; ***, P < 0.001.
By measuring mRNA expression levels of inflammatory cytokines (IL-1α, IL-1β, and TNFα), expression of IL-1β and TNFα was down-regulated in TKO mice (Fig. 6C), while IL-1α tended to decrease. Inflammatory reactions observed in DKO mice were absent in TKO mice. The weights of cerebellum, which were significantly lower in the DKO mice than in the WT mice, were normal in the TKO mice (WT: 63.0 ± 1.4 mg vs. DKO: 56.8 ± 1.7 mg vs. TKO: 66.3 ± 6.0 mg vs. C3 KO: 62.0 ± 4.6 mg) (Fig. 6D), suggesting the lack of neurodegeneration. The number of Purkinje cells in the cerebellum restored in the TKO mice, although there was a clear loss of Purkinje cells in DKO mice compared with WT mice at 30-week-old (WT: 4.4 ± 0.1 vs. DKO: 3.0 ± 0.2 vs. TKO: 3.8 ± 0.2 vs. C3 KO: 4.2 ± 0.1) (Fig. 6E).
These results suggested that the deficiency of gangliosides were associated with disorganized GEM/rafts and dysfunctions of complement-regulatory proteins in DKO mice, leading to abnormal activation of the complement system and enhanced inflammatory reaction, which may be linked to neurodegeneration.
Discussion
Besides KO mice of GM2/GD2 synthase, a number of mutant mice of glycosyltransferase genes responsible for the synthesis of glycolipids or their precursors have been reported in ref. 2. These KO mice were found to have almost normal brains and histological architectures when they were born, except KO mice of GlcCer synthase (20). The majority of them exhibited degenerative changes in peripheral and central nervous systems after birth, indicating that gangliosides play roles in the maintenance of structures and functions of nervous tissues. However, definite mechanisms for neurodegeneration due to the loss of glycolipids have not been reported.
To investigate mechanisms for neurodegeneration caused by the lack of gangliosides, gene expression profiles in DKO nerve tissues were analyzed. Among genes showing up-regulation >3-fold in DKO significant numbers of inflammation- or immune-function-related genes such as S100A8 and Lrg1 as well as C4 and C3aR were identified. mRNAs of the majority of complement components showed up-regulation in the cerebellum of the DKO mice, while those in liver showed different behaviors (Fig. 3). These results suggested that the complement system in nerve tissues undergoes regulation distinct from that of systemic organs. Protein levels of complement components, however, did not clearly increase as the mRNAs did. Complement proteins in nerve tissues rather decreased with aging, suggesting that there is extensive consumption of complement components based on complement activation (21), and this effect appeared to become more serious with aging.
Basically, the complement system protects host health by attacking exogenous pathogens with or without association of antibodies (21). In addition to the systemic complement system, there are also complete features of the complement system in CNS; that is, complement factors, complement receptors (22, 23), and complement regulators (24). Not only glial cells such as astrocytes and microglia, but also neurons can generate these molecules (13, 22, 23).
In the last few decades, it has been demonstrated that the complement system is involved in diverse human neurodegenerative disorders such as AD (22). Complement activation has been considered to be positively involved in the progress of these diseases. In amyloid plaques and neurofibrill tangles, C1q and other components are deposited (14, 15), leading to complement activation. mRNAs of complement components are largely increased at the diseased regions (16, 25). Furthermore, an inhibitor of C1q attenuates AD pathology (26), suggesting that C1q exerts a detrimental effect on neuronal integrity, and activation of complement systems plays a central role in inflammation and subsequent neurodegeneration (11, 12).
In the DKO mice, almost all complement components underwent up-regulation, and the presence of obvious inflammatory reaction in the brain was demonstrated. The behavior of the complement system appeared similar to that found in AD. Thus, activation of the complement system and subsequent inflammation should be a critical factor exacerbating neurodegeneration in the DKO mice. However, the mechanisms for activation of the complement system leading to inflammatory reaction in the DKO mice were not clear, while it is well-known that accumulated Aβ causes astrocyte activation, cytokine production, and complement activation in AD (14, 15). Namely, the intermediate process between ganglioside defects and activation of the complement system and inflammation might be a key issue in the mechanism for neurodegeneration in the DKO mice.
One of the most important factors affecting complement activation and resulting neuronal damages are complement-regulatory proteins. In particular, CD59 has been well examined concerning its expression and functions in neuronal tissues. In fact, it is well known that expression levels of CD59 are largely suppressed in the diseased areas of AD (24). DAF is also a critical molecule for maintaining the integrity of tissues (27). Many of these complement-regulatory proteins are GPI-anchored proteins, and are enriched in GEM/raft fractions (28). Drastic changes in ganglioside compositions in the DKO mice might have induced profound modulation in the architectures and functions of GEM/rafts. Actually, even raft marker molecules such as flotillin-1 and caveolin-1 were found to be dispersed outside of GEM/rafts in the cerebellum of the DKO mice (Fig. 5).
Disorder of GEM/rafts in DKO brain tissues could bring about a disturbance in GPI-anchored protein functions. Consequently, the complement systems might not be well controlled, triggering proinflammatory reactions. Increased levels of proinflammatory cytokines in DKO nervous tissues further proved that inflammatory processes progressively occurred due to complement activation based on the disrupted regulatory system against self-attack. Why complement activation was induced mainly in nerve tissues is a most intriguing issue in need of clarification. First, the original ganglioside levels expressed should be exceptionally high in nervous tissues compared to other tissues, resulting in more serious damage by withdrawal mechanisms. Second, nervous tissues might be more susceptible to a lack of complement-regulatory molecules as shown in human fetal neurons (29). Furthermore, disturbed distribution of GEM/raft-residing molecules was demonstrated mainly by biochemical ways. Coexistence of these molecules on the living cells or tissues should be proved by direct methods in the future as suggested in ref. 30.
As a double-edged sword, the complement system has been considered to play both positive and negative roles (31) in terms of disease control. Besides roles in the induction of inflammation with complement activation and eventual neurodegeneration (23, 25), functions in development (32, 33), neurogenesis in adults (34), and protection in disease-susceptible mutant mice like Tg/Aβ have been reported in ref. 35. In particular, physiological roles of complements such as the elimination of unnecessary degraded cell components or the refinement of the inflammatory reaction have been reported in ref. 32. In these cases, inhibition of the complement system results in prominent neurodegeneration. Thus, the complement system functions in the clearance of cell debris, and refinement of aberrant innervation might be important in protection from neurodegeneration.
These contradictory functions of complement systems in nervous tissues have been explained based on differences in disease phases or spatiotemporal differences of the pathological processes observed (22, 36). The causes of complement activation might be critical. Many complement inhibitors have been applied to treat acute inflammation or brain injury, suggesting beneficial roles of the complement system in chronic disorders (36).
In the case of the DKO mice, complement activation and subsequent inflammatory reactions due to reduced functions of regulatory proteins may be a causal factor bringing about progressive neurodegeneration. To clarify this issue, TKO mice of GM2/GD2 synthase, GD3 synthase and C3 genes were successfully generated. Resulting phenotypes of the TKO mice demonstrated that complement activation may be involved in the complement deposits, increased secretion of inflammatory cytokines and reduced cerebellum weights (degeneration).
Although there have been a number of reports on the dynamic, quantitative, and qualitative changes in GEM/rafts during stimulation of cells, cell–cell interaction, infection of microorganisms, and endocytosis (37), disordered and altered distribution of GEM/rafts has never been reported to date. As for caveolae, the lack of its main component, caveolin-1, results in the disappearance of the characteristic concave morphology (38) and functional defects (39). In the case of GEM/rafts, there are no definite morphological features, and ganglioside GM1 has been used as a specific raft marker (19). In our recent studies, GM1 seemed to be a functional molecule to regulate signals via PDGF or NGF (40). In the DKO mice analyzed here, all gangliosides other than GM3 were lost. Therefore, the effects on the membrane environment should be very serious. In addition to the effects of altered GEM/rafts on GPI-anchored proteins, other functional proteins such as neurotrophic factor receptors (3) and integrins (41) might be also affected by the altered GEM/rafts.
In the DKO mice, changes in the floatation patterns of DAF and flotillin-1 suggested that microdomains as a stable platform for anchoring raft-residing molecules were disrupted. Ambiguous staining patterns of these molecules in tissue sections also suggested that the membrane structure underwent fundamental transfiguration in the DKO mice. However, correlation between intensity of the transfiguration of GEM/rafts and degree of ganglioside defects needs to be clarified. Fine structural disorders in the membrane, including GEM/rafts, in the DKO tissues also remain to be investigated.
Experimental Procedures
Mice.
The mutant mice used in this study were generated and maintained in our laboratory. GM2/GD2 synthase KO (6) and GD3 synthase KO (7) mice were mated; that is, heterozygotes of individual mutants were mated, and genotypes of the offspring were screened for the two genes as described in ref. 8. All experimental protocols were approved by the animal experimental committee of the Graduate School of Medicine in Nagoya University along the guidelines of Japanese government, and were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (1966).
Antibodies.
Antibodies used for Western immunoblotting and those used for immunohistochemistry were described in the SI Text. Chemiluminescence detection and immunofluorescence detection reagents were also in SI Text.
Primers.
Primers used for real time RT-PCR were designed according to Primer 3 Input as shown in Table S2.
Real Time RT-PCR.
WT and DKO mice were perfused with PBS. The cerebella and livers were isolated from mice and homogenized in TRIzol (Invitrogen). Total RNA was extracted following the manufacturer's protocol. RT-PCR was performed as described in ref. 3.
Western Immunoblotting.
The cerebellum and spinal cord were isolated after perfusion with PBS from mice and were homogenized in lysis buffer (10 mM Tris·HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 5 mM MgCl2, 50 mM NaF, 1 mM NaVO4, 1% Triton X-100, 200 mM PMSF, and 0.01–0.02 TIU/mL aprotinin). The lysates were pelleted by centrifugation at 8,000 × g for 60 min at 4 °C. The supernatant was centrifuged at 18,000 × g for 90 min at 4 °C, and clarified lysates were used for immunoblotting.
Immunohistostaining.
WT and DKO mice were perfused with PBS and then 4% paraformaldehyde in 0.1 M phosphate buffer. The cerebellum and spinal cord were removed, postfixed with 4% paraformaldehyde in 0.1 M phosphate buffer overnight at 4 °C, then replaced with 10% sucrose for 6 h, 15% sucrose for 6 h, 20% sucrose for 6 h, and embedded in O.C.T. compound (Sakura Finetechnical) and frozen in liquid nitrogen. Frozen sections were set at longitudinal orientation for the cerebellum and at dorso-ventral orientation for the spinal cord, and were cut into 7- to 10-μm-thick sections on a cryostat (LEICA, CM3050S) at −20 °C. For immunohistochemistry, tissue slices were stained by antibodies and were sealed with PermaFlour aqueous mounting medium (Thermo). Coverslips were mounted and observed by light microscopy or confocal fluorescence microscopy (FLUOVIEW FV500; Olympus).
ELISA.
ELISA measurement of levels of cytokines in tissues and fractionation of extracts with sucrose density gradient ultracentrifugation are described in SI Text.
Statistical Analysis.
Values obtained in the experiments were examined for significance with Student's t test. When P values were <0.05, they were considered significant.
Supplementary Material
Acknowledgments.
We thank Dr. T. Fujita at Fukushima University for valuable discussion, Dr. K. O. Lloyd at Memorial Sloan-Kettering Cancer Center for carefully reading the manuscript, and Ms. T. Mizuno and Y. Nakayasu for technical assistance. This work was supported by Grants-in-Aid from the Ministry of Education, Culture, Sports and Technology in Japan, and by a Grant-in-Aid from Japan Science and Technology Agency.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0912336106/DCSupplemental.
References
- 1.Wiegandt H. Gangliosides. In: Wiegandt H, editor. Glycolipids. New York: Elsevier; 1985. pp. 199–260. [Google Scholar]
- 2.Furukawa K, Tajima O, Okuda T, Tokuda N, Furukawa K. Knockout mice and glycolipids. In: Kamerling JP, et al., editors. Comprehensive Glycoscience. From Chemistry to Systems Biology. Oxford, UK: Elsevier; 2007. pp. 149–157. [Google Scholar]
- 3.Kittaka D, et al. Impaired hypoglossal nerve regeneration in complex ganglioside-lacking mutant mice: Down-regulation of neurotrophic factors and receptors as possible mechanisms. Glycobiology. 2008;18:509–516. doi: 10.1093/glycob/cwn032. [DOI] [PubMed] [Google Scholar]
- 4.Sugiura Y, et al. Sensory nerve-dominant nerve degeneration and remodeling in the mutant mice lacking complex gangliosides. Neuroscience. 2005;135:1167–1178. doi: 10.1016/j.neuroscience.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 5.Sheikh KA, et al. Mice lacking complex gangliosides develop Wallerian degeneration and myelination defects. Proc Natl Acad Sci USA. 1999;96:7532–7537. doi: 10.1073/pnas.96.13.7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takamiya K, et al. Mice with disrupted GM2/GD2 synthase gene lack complex gangliosides, but exhibit only subtle defects in their nervous system. Proc Natl Acad Sci USA. 1996;93:10662–10667. doi: 10.1073/pnas.93.20.10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okada M, et al. b-series ganglioside deficiency exhibits no definite changes in the neurogenesis and the sensitivity to Fas-mediated apoptosis, but impairs regeneration of the lesioned hypoglossal nerve. J Biol Chem. 2000;277:1633–1636. doi: 10.1074/jbc.C100395200. [DOI] [PubMed] [Google Scholar]
- 8.Inoue M, et al. Refractory skin injury in the complex knock-out mice expressing only GM3 ganglioside. J Biol Chem. 2002;277:29881–29888. doi: 10.1074/jbc.M201631200. [DOI] [PubMed] [Google Scholar]
- 9.Tajima O, et al. Reduced motor and sensory functions and emotional response in GM3-only mice: Emergence from early stage of life and exacerbation with aging. Behav Brain Res. 2009 doi: 10.1016/j.bbr.2008.10.024. In press. [DOI] [PubMed] [Google Scholar]
- 10.Jennemann R, et al. Cell-specific deletion of glucosyl- ceramide synthase in brain leads to severe neural defects after birth. Proc Natl Acad Sci USA. 2005;102:12459–12464. doi: 10.1073/pnas.0500893102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGeer EG, McGeer PL. The importance of inflammatory mechanisms in Alzheimer disease. Exp Gerontol. 1998;33:371–378. doi: 10.1016/s0531-5565(98)00013-8. [DOI] [PubMed] [Google Scholar]
- 12.Rogers J, Shen Y. A perspective on inflammation in Alzheimer's disease. Ann N Y Acad Sci. 2000;924:132–135. doi: 10.1111/j.1749-6632.2000.tb05571.x. [DOI] [PubMed] [Google Scholar]
- 13.Terai K, Walker DG, McGeer EG, McGeer PL. Neurons express proteins of the classical complement pathway in Alzheimer disease. Brain Res. 1997;769:385–390. doi: 10.1016/s0006-8993(97)00849-4. [DOI] [PubMed] [Google Scholar]
- 14.Rogers J, et al. Complement activation by beta-amyloid in Alzheimer disease. Proc Natl Acad Sci USA. 1992;89:10016–10020. doi: 10.1073/pnas.89.21.10016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen Y, et al. Complement activation by neurofibrillary tangles in Alzheimer's disease. Neurosci Lett. 2001;305:165–168. doi: 10.1016/s0304-3940(01)01842-0. [DOI] [PubMed] [Google Scholar]
- 16.Strohmeyer R, Shen Y, Rogers J. Detection of complement alternative pathway mRNA and proteins in the Alzheimer's disease brain. Brain Res Mol Brain Res. 2000;81:7–18. doi: 10.1016/s0169-328x(00)00149-2. [DOI] [PubMed] [Google Scholar]
- 17.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 18.Michel V, Bakovic M. Lipid rafts in health and disease. Biol Cell. 2007;99:129–140. doi: 10.1042/BC20060051. [DOI] [PubMed] [Google Scholar]
- 19.Ikonen E. Roles of lipid rafts in membrane transport. Curr Opin Cell Biol. 2001;13:470–477. doi: 10.1016/s0955-0674(00)00238-6. [DOI] [PubMed] [Google Scholar]
- 20.Yamashita T, et al. A vital role for glycosphingolipid synthesis during development and differentiation. Proc Natl Acad Sci USA. 1999;96:9142–9147. doi: 10.1073/pnas.96.16.9142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Truedsson L, Bengtsson AA, Sturfelt G. Complement deficiencies and systemic lupus erythematosus. Autoimmunity. 2007;40:560–566. doi: 10.1080/08916930701510673. [DOI] [PubMed] [Google Scholar]
- 22.Gasque P, et al. Complement components of the innate immune system in health and disease in the CNS. Immuno-pharmacology. 2000;49:171–186. doi: 10.1016/s0162-3109(00)80302-1. [DOI] [PubMed] [Google Scholar]
- 23.Barnum SR. Complement in central nervous system inflammation. Immuol Res. 2002;26:7–13. doi: 10.1385/IR:26:1-3:007. [DOI] [PubMed] [Google Scholar]
- 24.Yang LB, et al. Deficiency of complement defense protein CD59 may contribute to neurodegeneration in Alzheimer's disease. J Neurosci. 2000;20:7505–7509. doi: 10.1523/JNEUROSCI.20-20-07505.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yasojima K, Schwab C, McGeer EG, McGeer PL. Up-regulated production and activation of the complement system in Alzheimer's disease brain. Am J Pathol. 1999;154:927–936. doi: 10.1016/S0002-9440(10)65340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farkas I, et al. Complement C5a receptor-mediated signaling may be involved in neurodegeneration in Alzheimer's disease. J Immunol. 2003;170:5764–5771. doi: 10.4049/jimmunol.170.11.5764. [DOI] [PubMed] [Google Scholar]
- 27.Mason JC, et al. Induction of decay-accelerating factor by cytokines or the membrane-attack complex protects vascular endothelial cells against complement deposition. Blood. 1999;94:1673–1682. [PubMed] [Google Scholar]
- 28.Kinoshita T, Fujita M, Maeda Y. Biosynthesis, remodelling and functions of mammalian GPI-anchored proteins: Recent progress. J Biochem. 2008;144:287–294. doi: 10.1093/jb/mvn090. [DOI] [PubMed] [Google Scholar]
- 29.Singhrao SK, et al. Spontaneous classical pathway activation and deficiency of membrane regulators render human neurons susceptible to complement lysis. Am J Pathol. 2000;157:905–918. doi: 10.1016/S0002-9440(10)64604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munro S. Lipid rafts: Elusive or illusive? Cell. 2003;115:377–388. doi: 10.1016/s0092-8674(03)00882-1. [DOI] [PubMed] [Google Scholar]
- 31.Tenner AJ. Complement in Alzheimer's disease: Opportunities for modulating protective and pathogenic events. Neurobiol Aging. 2001;22:849–861. doi: 10.1016/s0197-4580(01)00301-3. [DOI] [PubMed] [Google Scholar]
- 32.Stevens B, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 33.Bénard M, et al. Characterization of C3a and C5a receptors in rat cerebellar granule neurons during maturation. Neuroprotective effect of C5a against apoptotic cell death. J Biol Chem. 2004;279:43487–43496. doi: 10.1074/jbc.M404124200. [DOI] [PubMed] [Google Scholar]
- 34.Rahpeymai Y, et al. Complement: A novel factor in basal and ischemia-induced neurogenesis. EMBO J. 2006;25:1364–1374. doi: 10.1038/sj.emboj.7601004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wyss-Coray T, et al. Prominent neurodegeneration and increased plaque formation in complement-inhibited Alzheimer's mice. Proc Natl Acad Sci USA. 2002;99:10837–10842. doi: 10.1073/pnas.162350199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Beek J, Elward K, Gasque P. Activation of complement in the central nervous system: Roles in neurodegeneration and neuroprotection. Ann NY Acad Sci. 2003;992:56–71. doi: 10.1111/j.1749-6632.2003.tb03138.x. [DOI] [PubMed] [Google Scholar]
- 37.Pike LJ. Rafts defined: A report on the Keystone Symposium on Lipid Rafts and Cell Function. J Lipid Res. 2006;47:1597–1598. doi: 10.1194/jlr.E600002-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Zhao YY, et al. Defects in caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension in knockout mice. Proc Natl Acad Sci USA. 2002;99:11375–11380. doi: 10.1073/pnas.172360799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fernández MA, et al. Caveolin-1 is essential for liver regeneration. Science. 2006;313:1628–1632. doi: 10.1126/science.1130773. [DOI] [PubMed] [Google Scholar]
- 40.Nishio M, et al. Overexpressed GM1 suppresses nerve growth factor (NGF) signals by modulating the intracellular localization of NGF receptors and membrane fluidity in PC12 cells. J Biol Chem. 2004;279:33368–33378. doi: 10.1074/jbc.M403816200. [DOI] [PubMed] [Google Scholar]
- 41.Toledo MS, Suzuki E, Handa K, Hakomori S. Effect of ganglioside and tetraspanins in microdomains on interaction of integrins with fibroblast growth factor receptor. J Biol Chem. 2005;280:16227–16234. doi: 10.1074/jbc.M413713200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.